Volume 15, Issue 1 (2023)
Iran J War Public Health 2023, 15(1): 35-42 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/12/27 | Accepted: 2023/02/20 | Published: 2023/03/11
Received: 2022/12/27 | Accepted: 2023/02/20 | Published: 2023/03/11
How to cite this article
Abd Uljaleel A, Hassan E, Mohammad A, Hadi N. Protective Effect of Dulaglutide on Lung Injury in Endotoxemia Mouse Model. Iran J War Public Health 2023; 15 (1) :35-42
URL: http://ijwph.ir/article-1-1289-en.html
URL: http://ijwph.ir/article-1-1289-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Pharmacology & Therapeutics, Faculty of Medicine, University of Kufa, Najaf, Iraq
Full-Text (HTML) (474 Views)
Introduction
According to World Health Organization estimates, sepsis affects 30 million or more people each year, resulting in 6 million deaths worldwide and 1 million in newborns [1]. Endotoxemia is defined as a systemic response to inflammation as a result of infection with gram-negative and gram-positive bacteria. In intensive care units, it was considered the major disease that can occur in patients [2]. It is a series of combined effects related to infectious agents, microbiologic poisons, the response of the patient to inflammation, and endogenous mediator's motivation that can cause severe hypotension, Acute Respiratory Distress Syndrome (ARDS), Acute Lung Injury (ALI), and systemic multi-organ [3].
The lungs are among the major organs that are most susceptible to damage from sepsis. Acute lung injury or acute respiratory distress syndrome evolves in more than 50% of septic patients [4]. It is manifested by tachypnea, hypoxia, and metabolic acidosis [5]. Some patients will need intubation and mechanical ventilation due to fatigue that occurs in respiratory muscles [6]. Infiltration of neutrophils in the lungs is considered a main pathophysiological feature of ALI. Unrestrained emigration of neutrophils into the lungs leads to excessive production of myeloperoxidase, reactive oxygen species, chemokines, cytokines, nitric oxide, and neutrophils. The extracellular traps result in uncontrolled inflammation, impairment of lung function, and death [7].
Cytokine-associated lung injury leads to increased alveolar and capillary endothelium permeability and non-cardiac pulmonary edema that damages oxygenation and ventilation [8].
Induction of sepsis in animals occurs either by injection of Lipopolysaccharide (LPS) or live bacteria systemically or by generating an endogenous infection by Cecal Ligation and Puncture process (CLP) [9], which results in lung injury due to peritonitis [10]. CLP causes the deliverance of LPS, a gram-negative bacteria element, which is a basic regulator of bacterial infection pathogenesis and is involved in endotoxic crises [11]. Lung injury secondary to CLP matures during 18-24 hours and is distinguished by hypoxemia, neutrophilic inflammation, and edema in interstitial spaces and alveoli [9].
Immunological processes are stimulated in response to the entry of pathogens. The antigen-presenting cells are activated to cause up regulation of many cells, such as macrophages, monocytes, endothelial and dendritic cells [12]. Dysregulation of the patient's immune system during sepsis makes the pro-inflammatory signaling quickens dysfunction of vascular endothelium. After that, they support entry of additional inflammatory cells as macrophages, monocytes, lymphocytes, and neutrophils to produce the so-called vicious pro-inflammatory ring. In acute systemic inflammation, increased permeability of lung microvasculature is characterized as a main injury resulting by sepsis during the development of ARDS, and so plasma exudate fills the alveoli [13]. Furthermore, besides the damage of alveolar epithelial cells, as a result of apoptosis and necrosis encouraged by sepsis, the high exudates that fill alveoli, cause alveolar edema and then the hyaline membrane is developed [14]. In severe inflammation, as in sepsis, the stimulation of inflammatory process by pathogen results in cytokines storm such as Interleukin 1, Interleukin 8, Interleukin 6 (IL-6), Tumour Necrosis Factor- Alpha (TNF-α) to amplify the response to inflammation [15].
Toll like Receptor family (TLR) is one of the Pattern Recognition Receptors (PRRs) which have an essential role in stimulating intracellular signal pathways and provoking pro-inflammatory cytokines after recognition of exogenous and endogenous ligands [16]. The most imperative among this family is TLR4; it can spot LPS and thus stimulates immune response [17, 18] by translocation of Nuclear Factor-κB (NF-κB), which in turn stimulates pulmonary cytokines such as IL-6, IL-1β, and TNF-alpha inducing ALI [19]. The severity of the inflammatory response is linked with the expression level of TLR4. The harm of microarchitecture and damage to alveolar epithelium and vascular endothelium tissue seem to be attached to the TLR4 gene [20].
TNF-α as an inflammatory mediator can also initiate comparable mechanisms resulting in nuclear translocation of NF-κB and thus increase production of cytokines [21].
IL-6 is a main pro-inflammatory mediator in sepsis as it initiates acute phase response [22] and is considered an early marker of lung injury by its levels in broncho-alveolar fluid, plasma, and pulmonary tissues [23] also its prognostic factor for continued mechanical ventilation, morbidity and death in ARDS [24].
IL-1β has a significant role in pulmonary inflammation caused by bacteria and bacterial products. It is secreted in lungs after induction by LPS during CLP [25].
Macrophage migration inhibitory factor (MIF) is a pro inflammatory cytokine. Immune cells such as macrophage, monocytes, B-cells and T-cells are the main source of this marker. It releases in response to microbial products, hypoxia and proliferative signs [26]. MIF level will be noticeably higher in patients with sepsis, and it can be utilized as a diagnostic marker in this condition [27].
Mitochondria is the main organelle that maintains cellular function through ATP production. Pathological conditions such as sepsis can harm mitochondria, which lead to energy stress and production of reactive oxygen species due to lipid peroxidation [28]. Shifting of the metabolic pathway from aerobic to anaerobic condition that occurs during sepsis as a result of tissue hypoxia will lead to a reduction in ATP production and accumulation of acid lactate, thus intracellular acidosis occurs [29]. 8-iso-Prostaglandin F2-alpha (8-isoPGF2α), the Lipid peroxidation product of arachidonic acid, is considered the greatest useful oxidative stress biomarker in vivo [30]. It can be counted in all biological tissues and fluids such as Cerebrospinal Fluid (CSF), plasma, urine, pulmonary epithelial lining, and fluid of broncho-alveolar lavage [31].
Approaches that target sepsis advancement and break the linking of the inflammatory net at the correct time to preserve vascular barrier integrity are instantly required [15].
Dulaglutide is a Glucagon-Like Peptide-1 agonist (GLP-1) used for the management of patients with diabetes mellitus type II [32]. It is anti-inflammatory and is demonstrated in a variety of animal species to lower pro-inflammatory cytokine levels. Dulaglutide lowered interleukin-1 beta levels and TNF alpha in the LPS-induced sepsis model in mice [33].
In contrast, despite numerous spans of effort, there is yet a shortage of active pharmacologic approaches against ARDS [34]. Neuromuscular blocking agents are among the most widely used treatments for ARDS, which only act as an adjuvant to avoid lung injury due to ventilation [35]. So there is an insistent requirement to detect particular therapeutic drugs for treating ARDS.
This study aimed to examine if dulaglutide may help in keeping the lungs from polymicrobial sepsis by modulating the pathways of inflammation and oxidative stress.
Materials and Methods
The study was conducted in the Department of Pharmacology and Therapeutics, the Faculty of Medicine, University of Kufa, as well as the Middle Euphrates Centre for Cancer Research in the period between 5 February and 30 August 2022.
Animals
Twenty Swiss albino male mice with an average weight of 25-35 g and mature at 8-12 weeks were acquired from the Faculty of Science, University of Kufa (Animal Resource Center), which were kept at a special temperature of 25°C and humidity of 60-65%, with a 12 h light: 12 h dark cycle.
Study design
In this study, mice were divided into 4 groups of 5 as follows:
• Sham group: mice in this group were anesthetized and exposed to laparotomy surgery without CLP.
• Sepsis group (CLP): mice cecum of this group was ligated and punctured.
• Vehicle group: all mice in this group were given an equal volume of Normal Saline (NS) subcutaneously (S.C) before CLP.
• Dulaglutide pre-treated group: mice in this group received 0.6mg per kg dulaglutide twice weekly S.C. for 2 weeks before CLP [36].
Experimental procedure
Mice were anesthetized intraperitoneally with 1:1 75 mg/kg ketamine and 15 mg/kg xylazine. The cecum was identified after a 1-2cm median abdominal laparotomy. After that, the cecum was ligated just below the ileocecal valve to be punctured twice with a G-20 needle and then returned to its normal position. Then, the abdomen was sutured with 5.0 medical sutures. Mice were monitored every 4 h for 1 day for any signs of illness in their cages with freely reaching for food and drink. The The sham group is the surgical control group, in which mice without CLP undergo anesthesia and laparotomy [37, 38].
Preparation of dulaglutide
Dulaglutide pen was purchased (Eli Lilly and Company, Indianapolis, USA) and diluted with its vehicle (NS) to be administered in a dose of 0.6 mg/kg twice weekly S.C. for 2 weeks before CLP [36].
Tissue homogenization for IL-6, IL-1β, TNF-α, MIF, TLR4, and F2-isoprostane measurement
The lung was cleaned from Red Blood Cells (RBCs) and clots with a 0.9% sodium chloride solution before being maintained in a deep freeze at -80°C. The lung sections were then homogenized employing an elevated ultrasonic liquid processing in 1:10 [weight/volume] phosphate buffer saline (PBS saltwater with 1% Triton X-100 and 1% protease inhibitor cocktail) [39]. Then the lysates were centrifuged for 10 min at 4°C and 10,000 rpm, and the supernatants were applied to measure IL-6, IL-1β, TNF-α, MIF, TLR4, and F2-isoprostane [40] by Elisa kit for mice (Bioassay®, china), specific for each mediator.
Tissue preparation for histopathology
The other area of the lung tissue sample was immersed in 10% formalin, dehydrated in various alcohols, cleaned in xylene, and embedded in a paraffin block. Tissue slide slices were subsequently cut into 5 mm-thick horizontal lines, stained with Hematoxylin and Eosin (H&E), and then sent to a histopathologist for histological examination [41]. Lung tissue injury was assessed blindly by competent pathologists using four randomly selected areas. The sections were graded using a scale constructed to determine the degree of lung injury.
To assess variation in lung impairment, histological slices were examined for all groups under original magnification from X100 –X400, and results scored as follows [42]:
• Normal texture, without damage (Score 0)
• Score 1, with less than 25% damage (mild)
• Score 2, with 25-50% damage (moderate)
• Score 3, with 50-75% damage (severe)
• Score 4, with 75-100% damage (highly severe)
Statistical analysis
SPSS 26 software was used for the statistical analysis of data. Data were expressed as mean±SEM. To compare different categories, one-way Analysis of Variance (ANOVA) with Bonferroni's post hoc test was used. According to the histopathological alterations in the lung tissues, the Kruskal-Wallis test was used to determine the statistically significant difference between the groups.
Findings
Effect of dulaglutide on IL-6, IL-1β, TNF-α, MIF, and TLR4
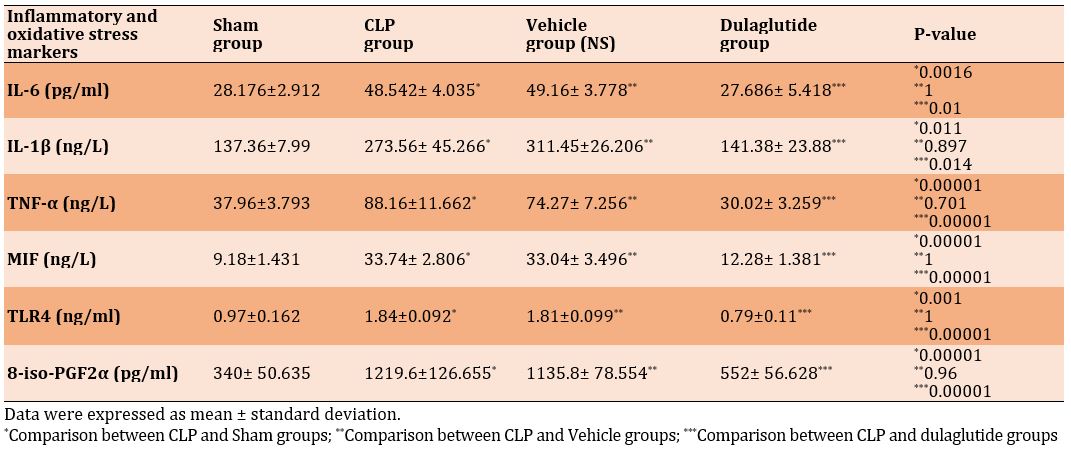
When the CLP and vehicle groups were matched to the sham group, the lung levels of inflammatory cytokines and TLR4 were significantly greater in CLP and vehicle groups (p<0.05), while their levels were significantly lower in the dulaglutide group (p<0.05; Table 1).
Dulaglutide attenuates oxidative stress (8-iso-PGF2α) in lung tissue
Mice in the CLP group showed a significant increment in lung tissue level of 8-iso-PGF2α than in the sham group. Lung level of 8-iso-PGF2α was decreased significantly in the dulaglutide treatment group compared to CLP one (Table 1).
Table 1) Comparison of mean inflammatory mediators and oxidative stress of lung tissue in different groups
Effect of dulaglutide on lung histopathology
The results indicated a significant improvement in the histopathological features of the lung in the group pretreated with dulaglutide, where the histopathological score of sepsis was significantly reduced compared to the CLP and vehicle groups. Therefore, the dulaglutide-pretreated group showed a mild form of inflammation, summarized by the mild accumulation of macrophages and neutrophils in the alveoli with focal hyperemia and vascular congestion (Diagram 1 and Figure 1).


Figure 1) Histopathological examination of lung sections
A: Photomicrograph shows normal histology, and no damage is observed in the lung tissue of sham group mice (H&E; 400X).
B: Photomicrograph shows lung tissue of mice with severe mixed inflammatory cells infiltration rich in neutrophils and macrophages, involving 60% of the examined lung tissue (red arrows) with hyperemia, congestion (blue arrows), and hemorrhage; CLP group (H&E; 100X).
C: Photomicrograph shows lung tissue with severe interstitial, perivascular, and intra-alveolar mixed inflammation (red arrows), involving 80% of the examined tissue with hyperemia (blue arrows) and extravasation of RBCs; vehicle group (H&E; 100X).
D: Photomicrograph shows lung tissue of mice with focal very mild interstitial inflammation, involving 3% of tissue with vascular congestion; dulaglutide group
(H&E; 400X).

Diagram 1) Mean histopathological score of lung tissue Sham vs. CLP and vehicle group (p<0.05), dulaglutide vs. CLP and vehicle group (p<0.05)
Discussion
The studies are still insufficient to determine the role of pharmacological therapies in cases of sepsis [43]. In the current study, for the first time, we estimated the protective effect of dulaglutide on improving lung function following endotoxemia induced by the CLP model in mice.
This experimental research reported a significant elevation in IL- 6, TNF-α, IL-1β, MIF, TLR4, and 8-iso-PGF2α in the lung tissue in both sepsis and vehicle groups in comparison with the sham group after sepsis.
IL-6 is one of the most important inflammatory cytokines, which its level is markedly elevated in the majority, if not in all inflammatory situations [44]. IL-1β is a key player in the beginning of inflammatory response in lung diseases such as Chronic Obstructive Pulmonary Disease (COPD) [45]. TNF-α has a very important role in the progression of ALI induced by sepsis [46]. MIF is an innate cytokine that is expressed extensively with an essential role in the prognosis of septic shock [47]. Chao et al. found that the blocking of MIF was efficient in reducing mortality in mice with sepsis as it attenuates vascular permeability and leakage [48]. As a pattern recognition receptor (PRR), TLR4 instigates the immune response through recognition of LPS to keep the body from infection. So the high TLR4 activation by LPS results in the extensive release of pro-inflammatory cytokines that may lead to cytokine storm and severe sepsis [49]. NF-κB acts as a main transcription factor of non-specific immune response intermediated by TLR4 [49]. Our findings are consistent with many other studies. In a study conducted by Senousy et al. to investigate the protective effects of α-Chymotrypsin on the liver, lungs, and kidneys of septic rats with a CLP model, TLR4 was highly elevated [50].
In Ibrahim et al.’s study that dealt with the protective effects of tocilizumab on acute lung injury, IL-6 level obviously increased in the sepsis group compared to the sham group [51]. Additionally, Ali et al. stated that the levels of IL-6, IL-1β, and TNF- α were dramatically increased in the sepsis group by checking the anti-inflammatory effects of continentalic acid on mice's lungs [52]. High MIF serum level in the neonate with sepsis was also reported by Chen et al. when the expression of cytokines in the serum of septic infants was studied compared to control infants [53].
8-iso-PGF2-α is a biomarker of lipid peroxidation and oxidative stress, and high levels of this marker are associated with a greater risk of pulmonary disease [54]. Chen et al.’s study demonstrated that intestinal 8-iso-PGF2-α level increases after ischemia-reperfusion injury-induced sepsis [55].
The findings of this study demonstrated a substantial decrease in TLR4, pro-inflammatory cytokines (IL-6, TNF-α, IL-1β, MIF), and oxidative stress marker 8-iso-PGF2-α in the dulaglutide-treated group compared to the sepsis and vehicle groups. The initiation of inflammation and exaggerated construction of inflammatory elements are the direct manifestations of sepsis on lung tissues. TLR4 activation by PAMPs (e.g., LPS) results in the activation of the TLR4/Myd88 signaling pathway to encourage the phosphorylation of IκB kinase, which is an essential inhibitor of NF-κB. This will induce the dissociation of NF-κB from IκB and NF-κB complex by IκB phosphorylation, then NF-κB further transport and reaches the nucleus to stimulate transcription of inflammatory mediators [56, 57]. In our study, by dulaglutide drug, the activations of the TLR4/NF-κB pathway and inflammation were substantially alleviated, showing the noticeable inhibitory outcome of dulaglutide versus inflammation in lung tissues caused by LPS. Wang et al. presented the same outcomes with dulaglutide on LPS-induced cardio-myocytes injury in mice [33]. The exact mechanism under which dulaglutide decreases the production of these cytokines (IL-6, TNF-α, IL-1β, MIF) is poorly understood [58]. In the first period of infection-stimulated acute inflammation, the PAMPs (e.g., LPS) are recognized and react with PPRs (e.g., TLRs) by inflammatory cells [59]. After that, abroad range of inflammatory cytokines is released like TNF-a, IL-1B, and IL-6 [59]. However, the suppression of the TLR4/NF-κB pathway results in the inhibition of these cytokines secretion [33]. Zheng et al. also presented that the levels of IL-6, TNF-α, and IL-1β were depressed markedly in treatment with dulaglutide [60]. Unfortunately, there has been no study about the effect of dulaglutide on MIF to the best of our knowledge. Xu et al. showed that activation of the GLP-1 receptor improves ALI induced by LPS and results in the inhibition of IL-6 and MIF markers in pulmonary tissues of LPS-challenged mice [61]. Oxidative stress was linked with a variety of lung diseases, such as ALI [62] and COPD [63]. F2-isoprostane is commonly measured to give a figure about oxidative stress occurrence because it is specific and sensitive index to reflect oxidative stress [64]. Indeed, the probable antioxidant effects of dulaglutide and other GLP-1RA may be due to inhibition of arachidonic acid production, which is the main source of Prostaglandins (PGs) [65]. Li et al.’s study found that 8-iso-PGF2-a serum level was remarkably decreased with dulaglutide in patients with Type 2 Diabetes Mellitus (T2DM) [66]. The overall useful effects of GLP-1 agonist drugs in ALI mammal models and mice model of COPD [61, 67-69] indicated that there are possible ways to repurpose the GLP-1 drugs for the treatment of lung injury.
In the current study, we found that the sepsis and vehicle groups had significantly more lung tissue damage than the sham group. Sepsis and the vehicle group had the highest histopathological damage scores. Histopathological observations in the sepsis and vehicle groups were related to acute and extensive infiltration of inflammatory cells in the alveolar spaces and interstitium, in addition to congestion and extravasation of RBCs. The alveolar wall became thicker with edema and patchy hemorrhage, and also hyaline membrane was formed compared to the sham group. Our findings are consistent with the research of Zhou et al., who showed that LPS-challenged mice exhibited severe grades of inflammatory cell penetration, interstitial edema, and within the alveoli in addition to thickening in the septum of alveoli [70]. Dulaglutide pretreatment considerably decreased mononuclear inflammation and another pathological abrasion resulted by the LPS challenge. In the current study, the lung injury score in the CLP group was higher compared to the group treated with dulaglutide. This indicated that dulaglutide pretreatment efficiently attenuated the injury score of the lung. So the histopathological score was significantly reduced in mice receiving the drug. This diminished pathological score in the CLP+ dulaglutide group supported our novel finding that this drug potentially has lung protective effects where it decreased penetration of inflammatory cells into the lungs, hyperemia, edema, and congestion. As far as we know to date, there are no sufficient studies available about the protective effects of dulaglutide on lungs with sepsis. However, many GLP-1 agonists produced the same effects on the lungs as our drug. Zhou et al. showed that GLP-1 analog liraglutide effectively decreases histopathological lesions in lungs challenged with LPS and reduced lung injury score by 4 times [70].
This study used dulaglutide in a dose of 0.6 mg/kg for 2 weeks, so further researches with different doses and longer duration may be justified. The protective effect of lung that demonstrated in current study was mediated by TLR4 & NF-κB cascades. Additional researches for other possible signalling pathways are needed. Future studies must investigate diabetic patients with sepsis and treated by this drug.
Conclusion
Dulaglutide can attenuate acute lung injury during CLP-induced endotoxemia in mice through its modulating effects on TLR4 and oxidative stress, downstream signaling pathways, and subsequently decreased lung tissue levels of pro-inflammatory mediators.
Acknowledgments: The researchers would like to thank all the workers in the Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa, for their continuous support in completing the research requirements.
Ethical Permission: The study was authorized by the Bioethics Committee at the University of Kufa, as well as its representation in the Faculty of Medicine (Ethical approval. No. 2933 in 2/2/2022). Committee’s recommendations were followed throughout the proceedings of work.
Conflict of Interests: All authors declare that there is no conflict of interest in the study.
Authors’ Contribution: Abd Uljaleel AQ (First Author), Introduction Writer/Main Researcher/Discussion Writer (30%); Hassan ES (Second Author), Methodologist/Assistant Researcher/Statistical Analyst (30%); Mohammad AR (Third Author), Assistant Researcher/Discussion Writer (20%); Hadi NR (Fourth Author), Introduction Writer/Assistant Researcher (20%)
Funding: This study is self-funded. All authors contributed to the costs.
According to World Health Organization estimates, sepsis affects 30 million or more people each year, resulting in 6 million deaths worldwide and 1 million in newborns [1]. Endotoxemia is defined as a systemic response to inflammation as a result of infection with gram-negative and gram-positive bacteria. In intensive care units, it was considered the major disease that can occur in patients [2]. It is a series of combined effects related to infectious agents, microbiologic poisons, the response of the patient to inflammation, and endogenous mediator's motivation that can cause severe hypotension, Acute Respiratory Distress Syndrome (ARDS), Acute Lung Injury (ALI), and systemic multi-organ [3].
The lungs are among the major organs that are most susceptible to damage from sepsis. Acute lung injury or acute respiratory distress syndrome evolves in more than 50% of septic patients [4]. It is manifested by tachypnea, hypoxia, and metabolic acidosis [5]. Some patients will need intubation and mechanical ventilation due to fatigue that occurs in respiratory muscles [6]. Infiltration of neutrophils in the lungs is considered a main pathophysiological feature of ALI. Unrestrained emigration of neutrophils into the lungs leads to excessive production of myeloperoxidase, reactive oxygen species, chemokines, cytokines, nitric oxide, and neutrophils. The extracellular traps result in uncontrolled inflammation, impairment of lung function, and death [7].
Cytokine-associated lung injury leads to increased alveolar and capillary endothelium permeability and non-cardiac pulmonary edema that damages oxygenation and ventilation [8].
Induction of sepsis in animals occurs either by injection of Lipopolysaccharide (LPS) or live bacteria systemically or by generating an endogenous infection by Cecal Ligation and Puncture process (CLP) [9], which results in lung injury due to peritonitis [10]. CLP causes the deliverance of LPS, a gram-negative bacteria element, which is a basic regulator of bacterial infection pathogenesis and is involved in endotoxic crises [11]. Lung injury secondary to CLP matures during 18-24 hours and is distinguished by hypoxemia, neutrophilic inflammation, and edema in interstitial spaces and alveoli [9].
Immunological processes are stimulated in response to the entry of pathogens. The antigen-presenting cells are activated to cause up regulation of many cells, such as macrophages, monocytes, endothelial and dendritic cells [12]. Dysregulation of the patient's immune system during sepsis makes the pro-inflammatory signaling quickens dysfunction of vascular endothelium. After that, they support entry of additional inflammatory cells as macrophages, monocytes, lymphocytes, and neutrophils to produce the so-called vicious pro-inflammatory ring. In acute systemic inflammation, increased permeability of lung microvasculature is characterized as a main injury resulting by sepsis during the development of ARDS, and so plasma exudate fills the alveoli [13]. Furthermore, besides the damage of alveolar epithelial cells, as a result of apoptosis and necrosis encouraged by sepsis, the high exudates that fill alveoli, cause alveolar edema and then the hyaline membrane is developed [14]. In severe inflammation, as in sepsis, the stimulation of inflammatory process by pathogen results in cytokines storm such as Interleukin 1, Interleukin 8, Interleukin 6 (IL-6), Tumour Necrosis Factor- Alpha (TNF-α) to amplify the response to inflammation [15].
Toll like Receptor family (TLR) is one of the Pattern Recognition Receptors (PRRs) which have an essential role in stimulating intracellular signal pathways and provoking pro-inflammatory cytokines after recognition of exogenous and endogenous ligands [16]. The most imperative among this family is TLR4; it can spot LPS and thus stimulates immune response [17, 18] by translocation of Nuclear Factor-κB (NF-κB), which in turn stimulates pulmonary cytokines such as IL-6, IL-1β, and TNF-alpha inducing ALI [19]. The severity of the inflammatory response is linked with the expression level of TLR4. The harm of microarchitecture and damage to alveolar epithelium and vascular endothelium tissue seem to be attached to the TLR4 gene [20].
TNF-α as an inflammatory mediator can also initiate comparable mechanisms resulting in nuclear translocation of NF-κB and thus increase production of cytokines [21].
IL-6 is a main pro-inflammatory mediator in sepsis as it initiates acute phase response [22] and is considered an early marker of lung injury by its levels in broncho-alveolar fluid, plasma, and pulmonary tissues [23] also its prognostic factor for continued mechanical ventilation, morbidity and death in ARDS [24].
IL-1β has a significant role in pulmonary inflammation caused by bacteria and bacterial products. It is secreted in lungs after induction by LPS during CLP [25].
Macrophage migration inhibitory factor (MIF) is a pro inflammatory cytokine. Immune cells such as macrophage, monocytes, B-cells and T-cells are the main source of this marker. It releases in response to microbial products, hypoxia and proliferative signs [26]. MIF level will be noticeably higher in patients with sepsis, and it can be utilized as a diagnostic marker in this condition [27].
Mitochondria is the main organelle that maintains cellular function through ATP production. Pathological conditions such as sepsis can harm mitochondria, which lead to energy stress and production of reactive oxygen species due to lipid peroxidation [28]. Shifting of the metabolic pathway from aerobic to anaerobic condition that occurs during sepsis as a result of tissue hypoxia will lead to a reduction in ATP production and accumulation of acid lactate, thus intracellular acidosis occurs [29]. 8-iso-Prostaglandin F2-alpha (8-isoPGF2α), the Lipid peroxidation product of arachidonic acid, is considered the greatest useful oxidative stress biomarker in vivo [30]. It can be counted in all biological tissues and fluids such as Cerebrospinal Fluid (CSF), plasma, urine, pulmonary epithelial lining, and fluid of broncho-alveolar lavage [31].
Approaches that target sepsis advancement and break the linking of the inflammatory net at the correct time to preserve vascular barrier integrity are instantly required [15].
Dulaglutide is a Glucagon-Like Peptide-1 agonist (GLP-1) used for the management of patients with diabetes mellitus type II [32]. It is anti-inflammatory and is demonstrated in a variety of animal species to lower pro-inflammatory cytokine levels. Dulaglutide lowered interleukin-1 beta levels and TNF alpha in the LPS-induced sepsis model in mice [33].
In contrast, despite numerous spans of effort, there is yet a shortage of active pharmacologic approaches against ARDS [34]. Neuromuscular blocking agents are among the most widely used treatments for ARDS, which only act as an adjuvant to avoid lung injury due to ventilation [35]. So there is an insistent requirement to detect particular therapeutic drugs for treating ARDS.
This study aimed to examine if dulaglutide may help in keeping the lungs from polymicrobial sepsis by modulating the pathways of inflammation and oxidative stress.
Materials and Methods
The study was conducted in the Department of Pharmacology and Therapeutics, the Faculty of Medicine, University of Kufa, as well as the Middle Euphrates Centre for Cancer Research in the period between 5 February and 30 August 2022.
Animals
Twenty Swiss albino male mice with an average weight of 25-35 g and mature at 8-12 weeks were acquired from the Faculty of Science, University of Kufa (Animal Resource Center), which were kept at a special temperature of 25°C and humidity of 60-65%, with a 12 h light: 12 h dark cycle.
Study design
In this study, mice were divided into 4 groups of 5 as follows:
• Sham group: mice in this group were anesthetized and exposed to laparotomy surgery without CLP.
• Sepsis group (CLP): mice cecum of this group was ligated and punctured.
• Vehicle group: all mice in this group were given an equal volume of Normal Saline (NS) subcutaneously (S.C) before CLP.
• Dulaglutide pre-treated group: mice in this group received 0.6mg per kg dulaglutide twice weekly S.C. for 2 weeks before CLP [36].
Experimental procedure
Mice were anesthetized intraperitoneally with 1:1 75 mg/kg ketamine and 15 mg/kg xylazine. The cecum was identified after a 1-2cm median abdominal laparotomy. After that, the cecum was ligated just below the ileocecal valve to be punctured twice with a G-20 needle and then returned to its normal position. Then, the abdomen was sutured with 5.0 medical sutures. Mice were monitored every 4 h for 1 day for any signs of illness in their cages with freely reaching for food and drink. The The sham group is the surgical control group, in which mice without CLP undergo anesthesia and laparotomy [37, 38].
Preparation of dulaglutide
Dulaglutide pen was purchased (Eli Lilly and Company, Indianapolis, USA) and diluted with its vehicle (NS) to be administered in a dose of 0.6 mg/kg twice weekly S.C. for 2 weeks before CLP [36].
Tissue homogenization for IL-6, IL-1β, TNF-α, MIF, TLR4, and F2-isoprostane measurement
The lung was cleaned from Red Blood Cells (RBCs) and clots with a 0.9% sodium chloride solution before being maintained in a deep freeze at -80°C. The lung sections were then homogenized employing an elevated ultrasonic liquid processing in 1:10 [weight/volume] phosphate buffer saline (PBS saltwater with 1% Triton X-100 and 1% protease inhibitor cocktail) [39]. Then the lysates were centrifuged for 10 min at 4°C and 10,000 rpm, and the supernatants were applied to measure IL-6, IL-1β, TNF-α, MIF, TLR4, and F2-isoprostane [40] by Elisa kit for mice (Bioassay®, china), specific for each mediator.
Tissue preparation for histopathology
The other area of the lung tissue sample was immersed in 10% formalin, dehydrated in various alcohols, cleaned in xylene, and embedded in a paraffin block. Tissue slide slices were subsequently cut into 5 mm-thick horizontal lines, stained with Hematoxylin and Eosin (H&E), and then sent to a histopathologist for histological examination [41]. Lung tissue injury was assessed blindly by competent pathologists using four randomly selected areas. The sections were graded using a scale constructed to determine the degree of lung injury.
To assess variation in lung impairment, histological slices were examined for all groups under original magnification from X100 –X400, and results scored as follows [42]:
• Normal texture, without damage (Score 0)
• Score 1, with less than 25% damage (mild)
• Score 2, with 25-50% damage (moderate)
• Score 3, with 50-75% damage (severe)
• Score 4, with 75-100% damage (highly severe)
Statistical analysis
SPSS 26 software was used for the statistical analysis of data. Data were expressed as mean±SEM. To compare different categories, one-way Analysis of Variance (ANOVA) with Bonferroni's post hoc test was used. According to the histopathological alterations in the lung tissues, the Kruskal-Wallis test was used to determine the statistically significant difference between the groups.
Findings
Effect of dulaglutide on IL-6, IL-1β, TNF-α, MIF, and TLR4
When the CLP and vehicle groups were matched to the sham group, the lung levels of inflammatory cytokines and TLR4 were significantly greater in CLP and vehicle groups (p<0.05), while their levels were significantly lower in the dulaglutide group (p<0.05; Table 1).
Dulaglutide attenuates oxidative stress (8-iso-PGF2α) in lung tissue
Mice in the CLP group showed a significant increment in lung tissue level of 8-iso-PGF2α than in the sham group. Lung level of 8-iso-PGF2α was decreased significantly in the dulaglutide treatment group compared to CLP one (Table 1).
Table 1) Comparison of mean inflammatory mediators and oxidative stress of lung tissue in different groups

Effect of dulaglutide on lung histopathology
The results indicated a significant improvement in the histopathological features of the lung in the group pretreated with dulaglutide, where the histopathological score of sepsis was significantly reduced compared to the CLP and vehicle groups. Therefore, the dulaglutide-pretreated group showed a mild form of inflammation, summarized by the mild accumulation of macrophages and neutrophils in the alveoli with focal hyperemia and vascular congestion (Diagram 1 and Figure 1).


Figure 1) Histopathological examination of lung sections
A: Photomicrograph shows normal histology, and no damage is observed in the lung tissue of sham group mice (H&E; 400X).
B: Photomicrograph shows lung tissue of mice with severe mixed inflammatory cells infiltration rich in neutrophils and macrophages, involving 60% of the examined lung tissue (red arrows) with hyperemia, congestion (blue arrows), and hemorrhage; CLP group (H&E; 100X).
C: Photomicrograph shows lung tissue with severe interstitial, perivascular, and intra-alveolar mixed inflammation (red arrows), involving 80% of the examined tissue with hyperemia (blue arrows) and extravasation of RBCs; vehicle group (H&E; 100X).
D: Photomicrograph shows lung tissue of mice with focal very mild interstitial inflammation, involving 3% of tissue with vascular congestion; dulaglutide group
(H&E; 400X).

Diagram 1) Mean histopathological score of lung tissue Sham vs. CLP and vehicle group (p<0.05), dulaglutide vs. CLP and vehicle group (p<0.05)
Discussion
The studies are still insufficient to determine the role of pharmacological therapies in cases of sepsis [43]. In the current study, for the first time, we estimated the protective effect of dulaglutide on improving lung function following endotoxemia induced by the CLP model in mice.
This experimental research reported a significant elevation in IL- 6, TNF-α, IL-1β, MIF, TLR4, and 8-iso-PGF2α in the lung tissue in both sepsis and vehicle groups in comparison with the sham group after sepsis.
IL-6 is one of the most important inflammatory cytokines, which its level is markedly elevated in the majority, if not in all inflammatory situations [44]. IL-1β is a key player in the beginning of inflammatory response in lung diseases such as Chronic Obstructive Pulmonary Disease (COPD) [45]. TNF-α has a very important role in the progression of ALI induced by sepsis [46]. MIF is an innate cytokine that is expressed extensively with an essential role in the prognosis of septic shock [47]. Chao et al. found that the blocking of MIF was efficient in reducing mortality in mice with sepsis as it attenuates vascular permeability and leakage [48]. As a pattern recognition receptor (PRR), TLR4 instigates the immune response through recognition of LPS to keep the body from infection. So the high TLR4 activation by LPS results in the extensive release of pro-inflammatory cytokines that may lead to cytokine storm and severe sepsis [49]. NF-κB acts as a main transcription factor of non-specific immune response intermediated by TLR4 [49]. Our findings are consistent with many other studies. In a study conducted by Senousy et al. to investigate the protective effects of α-Chymotrypsin on the liver, lungs, and kidneys of septic rats with a CLP model, TLR4 was highly elevated [50].
In Ibrahim et al.’s study that dealt with the protective effects of tocilizumab on acute lung injury, IL-6 level obviously increased in the sepsis group compared to the sham group [51]. Additionally, Ali et al. stated that the levels of IL-6, IL-1β, and TNF- α were dramatically increased in the sepsis group by checking the anti-inflammatory effects of continentalic acid on mice's lungs [52]. High MIF serum level in the neonate with sepsis was also reported by Chen et al. when the expression of cytokines in the serum of septic infants was studied compared to control infants [53].
8-iso-PGF2-α is a biomarker of lipid peroxidation and oxidative stress, and high levels of this marker are associated with a greater risk of pulmonary disease [54]. Chen et al.’s study demonstrated that intestinal 8-iso-PGF2-α level increases after ischemia-reperfusion injury-induced sepsis [55].
The findings of this study demonstrated a substantial decrease in TLR4, pro-inflammatory cytokines (IL-6, TNF-α, IL-1β, MIF), and oxidative stress marker 8-iso-PGF2-α in the dulaglutide-treated group compared to the sepsis and vehicle groups. The initiation of inflammation and exaggerated construction of inflammatory elements are the direct manifestations of sepsis on lung tissues. TLR4 activation by PAMPs (e.g., LPS) results in the activation of the TLR4/Myd88 signaling pathway to encourage the phosphorylation of IκB kinase, which is an essential inhibitor of NF-κB. This will induce the dissociation of NF-κB from IκB and NF-κB complex by IκB phosphorylation, then NF-κB further transport and reaches the nucleus to stimulate transcription of inflammatory mediators [56, 57]. In our study, by dulaglutide drug, the activations of the TLR4/NF-κB pathway and inflammation were substantially alleviated, showing the noticeable inhibitory outcome of dulaglutide versus inflammation in lung tissues caused by LPS. Wang et al. presented the same outcomes with dulaglutide on LPS-induced cardio-myocytes injury in mice [33]. The exact mechanism under which dulaglutide decreases the production of these cytokines (IL-6, TNF-α, IL-1β, MIF) is poorly understood [58]. In the first period of infection-stimulated acute inflammation, the PAMPs (e.g., LPS) are recognized and react with PPRs (e.g., TLRs) by inflammatory cells [59]. After that, abroad range of inflammatory cytokines is released like TNF-a, IL-1B, and IL-6 [59]. However, the suppression of the TLR4/NF-κB pathway results in the inhibition of these cytokines secretion [33]. Zheng et al. also presented that the levels of IL-6, TNF-α, and IL-1β were depressed markedly in treatment with dulaglutide [60]. Unfortunately, there has been no study about the effect of dulaglutide on MIF to the best of our knowledge. Xu et al. showed that activation of the GLP-1 receptor improves ALI induced by LPS and results in the inhibition of IL-6 and MIF markers in pulmonary tissues of LPS-challenged mice [61]. Oxidative stress was linked with a variety of lung diseases, such as ALI [62] and COPD [63]. F2-isoprostane is commonly measured to give a figure about oxidative stress occurrence because it is specific and sensitive index to reflect oxidative stress [64]. Indeed, the probable antioxidant effects of dulaglutide and other GLP-1RA may be due to inhibition of arachidonic acid production, which is the main source of Prostaglandins (PGs) [65]. Li et al.’s study found that 8-iso-PGF2-a serum level was remarkably decreased with dulaglutide in patients with Type 2 Diabetes Mellitus (T2DM) [66]. The overall useful effects of GLP-1 agonist drugs in ALI mammal models and mice model of COPD [61, 67-69] indicated that there are possible ways to repurpose the GLP-1 drugs for the treatment of lung injury.
In the current study, we found that the sepsis and vehicle groups had significantly more lung tissue damage than the sham group. Sepsis and the vehicle group had the highest histopathological damage scores. Histopathological observations in the sepsis and vehicle groups were related to acute and extensive infiltration of inflammatory cells in the alveolar spaces and interstitium, in addition to congestion and extravasation of RBCs. The alveolar wall became thicker with edema and patchy hemorrhage, and also hyaline membrane was formed compared to the sham group. Our findings are consistent with the research of Zhou et al., who showed that LPS-challenged mice exhibited severe grades of inflammatory cell penetration, interstitial edema, and within the alveoli in addition to thickening in the septum of alveoli [70]. Dulaglutide pretreatment considerably decreased mononuclear inflammation and another pathological abrasion resulted by the LPS challenge. In the current study, the lung injury score in the CLP group was higher compared to the group treated with dulaglutide. This indicated that dulaglutide pretreatment efficiently attenuated the injury score of the lung. So the histopathological score was significantly reduced in mice receiving the drug. This diminished pathological score in the CLP+ dulaglutide group supported our novel finding that this drug potentially has lung protective effects where it decreased penetration of inflammatory cells into the lungs, hyperemia, edema, and congestion. As far as we know to date, there are no sufficient studies available about the protective effects of dulaglutide on lungs with sepsis. However, many GLP-1 agonists produced the same effects on the lungs as our drug. Zhou et al. showed that GLP-1 analog liraglutide effectively decreases histopathological lesions in lungs challenged with LPS and reduced lung injury score by 4 times [70].
This study used dulaglutide in a dose of 0.6 mg/kg for 2 weeks, so further researches with different doses and longer duration may be justified. The protective effect of lung that demonstrated in current study was mediated by TLR4 & NF-κB cascades. Additional researches for other possible signalling pathways are needed. Future studies must investigate diabetic patients with sepsis and treated by this drug.
Conclusion
Dulaglutide can attenuate acute lung injury during CLP-induced endotoxemia in mice through its modulating effects on TLR4 and oxidative stress, downstream signaling pathways, and subsequently decreased lung tissue levels of pro-inflammatory mediators.
Acknowledgments: The researchers would like to thank all the workers in the Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa, for their continuous support in completing the research requirements.
Ethical Permission: The study was authorized by the Bioethics Committee at the University of Kufa, as well as its representation in the Faculty of Medicine (Ethical approval. No. 2933 in 2/2/2022). Committee’s recommendations were followed throughout the proceedings of work.
Conflict of Interests: All authors declare that there is no conflict of interest in the study.
Authors’ Contribution: Abd Uljaleel AQ (First Author), Introduction Writer/Main Researcher/Discussion Writer (30%); Hassan ES (Second Author), Methodologist/Assistant Researcher/Statistical Analyst (30%); Mohammad AR (Third Author), Assistant Researcher/Discussion Writer (20%); Hadi NR (Fourth Author), Introduction Writer/Assistant Researcher (20%)
Funding: This study is self-funded. All authors contributed to the costs.
Keywords:
References
1. Wilcox, ME, Daou M, Dionne JC, Dodek P, Englesakis M, Garland A, et al. Protocol for a scoping review of sepsis epidemiology. Syst Rev. 2022;11(1):125. [Link] [DOI:10.1186/s13643-022-02002-6]
2. Hamza RT, Majeed SA. Nephroprotective effect of melatonin in sepsis induces renal injury : CLP Mice Model. Lat Am J Pharm. 2021;40(4):589-96. [Link]
3. Mahapatra S, Heffner AC, Atarthi-Dugan JM. Septic shock (nursing). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. [Link]
4. Gu WJ, Wan YD, Tie HT, Kan QC, Sun TW. Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: a meta-analysis. PLoS One. 2014;9(2):e90426. [Link] [DOI:10.1371/journal.pone.0090426]
5. Mikkelsen ME, Shah CV, Meyer NJ, Gaieski DF, Lyon S, Miltiades AN, et al. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock. 2013;40(5):375-81. [Link] [DOI:10.1097/SHK.0b013e3182a64682]
6. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. [Link] [DOI:10.1038/nrdp.2016.45]
7. Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol. 2017;8:81 [Link] [DOI:10.3389/fimmu.2017.00081]
8. Kim WY, Hong SB. Sepsis and acute respiratory distress syndrome: Recent update. Tuberc Respir Dis (Seoul). 2016;79(2):53-7. [Link] [DOI:10.4046/trd.2016.79.2.53]
9. Chimenti L, Morales-Quinteros L, Puig F, Camprubi-Rimblas M, Guillamat-Prats R, Gómez MN, et al. Comparison of direct and indirect models of early induced acute lung injury. Intensive Care Med Exp. 2020;8(Suppl 1):62. [Link] [DOI:10.1186/s40635-020-00350-y]
10. Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4(10):854-65. [Link] [DOI:10.1038/nrd1854]
11. Jawad AS, Hassan ES, Mohammad AR. Protective effect of empagliflozin from acute renal injury during endotoxemia in mice model. Lat Am J Pharm. 2022;41(4):463-71. [Link]
12. Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435-44. [Link] [DOI:10.2353/ajpath.2007.060872]
13. Chavez A, Smith M, Mehta D. New insights into the regulation of vascular permeability. Int Rev Cell Mol Biol. 2011;290:205-48. [Link] [DOI:10.1016/B978-0-12-386037-8.00001-6]
14. Bakowitz M, Bruns B, McCunn M. Acute lung injury and the acute respiratory distress syndrome in the injured patient. Scand J Trauma Resusc Emerg Med. 2012;20:54. [Link] [DOI:10.1186/1757-7241-20-54]
15. Dolmatova EV, Wang K, Mandavilli R, Griendling KK. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res. 2021;117(1):60-73. [Link] [DOI:10.1093/cvr/cvaa070]
16. Mohammad AR, Hadi AR, Hassan ES. Potential protective effect of Ibrutinib from acute brain injury during endotoxemia in mice. Lat Am J Pharm. 2022;41(2):472-80. [Link]
17. Hassan ES, Jawad AS, Mohammad AR. Protective effect of liraglutide from acute renal injury during endotoxemia in mice mode. Lat Am J Pharm. 2022;41(2):428-36. [Link]
18. Hussein SN, Majeed SA, Ghafil FA, Hassan ES, Hadi NR. Toll-like receptors 4 antagonist, Ibudilast, ameliorates acute renal impairment induced by sepsis in an experimental model. Bull National Instit Health. 2022;140(7):2900-09. [Link]
19. Hu R, Xu H, Jiang H, Zhang Y, Sun Y. The role of TLR4 in the pathogenesis of indirect acute lung injury. Front Biosci (Landmark Ed). 2013;18(4):1244-55. [Link] [DOI:10.2741/4176]
20. Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, et al. Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury. Int J Exp Pathol. 2007;88(6):387-91. [Link] [DOI:10.1111/j.1365-2613.2007.00566.x]
21. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [Link] [DOI:10.1038/sigtrans.2017.23]
22. Voiriot G, Razazi K, Amsellem V, Tran Van Nhieu J, Abid S, Adnot S, et al. Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respir Res. 2017;18(1):64. [Link] [DOI:10.1186/s12931-017-0553-6]
23. Vaporidi K, Voloudakis G, Priniannakis G, Kondili E, Koutsopoulos A, Tsatsanis C, et al. Effects of respiratory rate on ventilator-induced lung injury at a constant PaCO2 in a mouse model of normal lung. Crit Care Med. 2008;36(4):1277-83. [Link] [DOI:10.1097/CCM.0b013e318169f30e]
24. Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73(5):2751-7. [Link] [DOI:10.1128/IAI.73.5.2751-2757.2005]
25. Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol. 2020;11:1722. [Link] [DOI:10.3389/fimmu.2020.01722]
26. Grieb G, Merk M, Bernhagen J, Bucala R. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 2010;23(4):257-64. [Link] [DOI:10.1358/dnp.2010.23.4.1453629]
27. Meawed TE, Mansour MA, Mansour SA, Mohamed ML, Ibrahim EM, Ali AM. Functional and prognostic relevance of -173 G/C gene polymorphism of macrophage migration inhibitory factor in sepsis patients in Egyptian intensive care units. East Mediterr Health J. 2015;21(10):762-9. [Link] [DOI:10.26719/2015.21.10.762]
28. Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10 Suppl 1:S10-23. [Link] [DOI:10.1080/13547500500216546]
29. Granata S, Votrico V, Spadaccino F, Catalano V, Netti GS, Ranieri E, et al. Oxidative stress and ischemia/reperfusion injury in kidney transplantation: focus on ferroptosis, mitophagy and new antioxidants. Antioxidants (Basel). 2022;11(4):769. [Link] [DOI:10.3390/antiox11040769]
30. Kadiiska MB, Basu S, Brot N, Cooper C, Saari Csallany A, Davies MJ, et al. Biomarkers of oxidative stress study V: ozone exposure of rats and its effect on lipids, proteins, and DNA in plasma and urine. Free Radic Biol Med. 2013;61:408-15. [Link] [DOI:10.1016/j.freeradbiomed.2013.04.023]
31. Soffler C, Campbell VL, Hassel DM. Measurement of urinary F2-isoprostanes as markers of in vivo lipid peroxidation: a comparison of enzyme immunoassays with gas chromatography-mass spectrometry in domestic animal species. J Vet Diagn Invest. 2010;22(2):200-9. [Link] [DOI:10.1177/104063871002200205]
32. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-10. [Link] [DOI:10.2337/ds16-0026]
33. Wang R, Wang N, Han Y, Xu J, Xu Z. Dulaglutide alleviates LPS-induced injury in cardiomyocytes. ACS Omega. 2021;6(12):8271-8. [Link] [DOI:10.1021/acsomega.0c06326]
34. Hussain M, Xu C, Ahmad M, Majeed A, Lu M, Wu X, et al. Acute respiratory distress syndrome: Bench-to-bedside approaches to improve drug development. Clin Pharmacol Ther. 2018;104(3):484-94. [Link] [DOI:10.1002/cpt.1034]
35. Hurford WE. Neuromuscular blockade applicability in early acute respiratory distress syndrome. Anesthesiology. 2020;132(6):1577-84. [Link] [DOI:10.1097/ALN.0000000000003180]
36. Sanada J, Obata A, Obata Y, Fushimi Y, Shimoda M, Kohara K, et al. Dulaglutide exerts beneficial anti atherosclerotic effects in ApoE knockout mice with diabetes: the earlier, the better. Sci Rep. 2021;11(1):1425. [Link] [DOI:10.1038/s41598-020-80894-x]
37. Drosatos K, Khan RS, Trent CM, Jiang H, Son NH, Blaner WS, et al. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail. 2013;6(3):550-62. [Link] [DOI:10.1161/CIRCHEARTFAILURE.112.000177]
38. Wellington D, Mikaelian I, Singer L. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci. 2013;52(4):481-7. [Link]
39. Yousif NG, Hadi NR, Al-Amran FG, Zigam QA. The cardioprotective effect of irbesartan in polymicrobial sepsis : the role of the P38 MAPK/ NF- ĸB signaling pathway. Herz. 2018;43(2):140-5. [Link] [DOI:10.1007/s00059-017-4537-6]
40. Hadi NR, Al-amran FG, Hussein AA. Effects of thyroid hormone analogue and a leukotrienes pathway-blocker on renal ischemia/reperfusion injury in mice. BMC Nephrol. 2011;12:70. [Link] [DOI:10.1186/1471-2369-12-70]
41. Chandrashekhar VM, Ranpariya VL, Ganapaty S, Parashar A, Muchandi AA. Neuroprotective activity of Matricaria recutita Linn against global model of ischemia in rats. J Ethnopharmacol. 2010;127(3):645-51. [Link] [DOI:10.1016/j.jep.2009.12.009]
42. Zahran R, Ghozy A, Elkholy SS, El-Taweel F, El-Magd MA. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia-reperfusion injury in a rat model. Int J Urol. 2020;27(11):1039-49. [Link] [DOI:10.1111/iju.14345]
43. Polat G, Ugan RA, Cadirci E, Halici Z. Sepsis and septic shock: Current treatment strategies and new approaches. Eurasian J Med. 2017;49(1):53-8. [Link] [DOI:10.5152/eurasianjmed.2017.17062]
44. Rose-John S. Local and systemic effects of interleukin-6 (IL-6) in inflammation and cancer. FEBS Lett. 2022;596(5):557-66. [Link] [DOI:10.1002/1873-3468.14220]
45. Yi G, Liang M, Li M, Fang X, Liu J, Lai Y, et al. A large lung gene expression study identifying IL1B as a novel player in airway inflammation in COPD airway epithelial cells. Inflamm Res. 2018;67(6):539-51. [Link] [DOI:10.1007/s00011-018-1145-8]
46. Xue H, Li M. Protective effect of pterostilbene on sepsis-induced acute lung injury in a rat model via the JAK2/STAT3 pathway. Ann Transl Med. 2020;8(21):1452. [Link] [DOI:10.21037/atm-20-5814]
47. Tilstam PV, Schulte W, Holowka T, Kim BS, Nouws J, Sauler M, et al. MIF but not MIF-2 recruits' inflammatory macrophages in an experimental polymicrobial sepsis model. J Clin Invest. 2021;131(23):e127171. [Link] [DOI:10.1172/JCI127171]
48. Chao C-H, Chen H-R, Chuang Y-C, Yeh T-M. Macrophage migration inhibitory factor-induced autophagy contributes to thrombin-triggered endothelial hyperpermeability in sepsis. Shock. 2018;50(1):103-11. [Link] [DOI:10.1097/SHK.0000000000000976]
49. Wu S, Lin C, Zhang T, Zhang B, Jin Y, Wang H, et al. Pentamidine alleviates inflammation and lipopolysaccharide- induced sepsis by inhibiting TLR4 activation via targeting MD2. Front Pharmacol. 2022;13:835081. [Link] [DOI:10.3389/fphar.2022.835081]
50. Senousy SR, Ahmed AF, Abdelhafeez DA, Khalifa MMA, Abourehab MAS, El-Daly M. Alpha-chymotrypsin protects against acute lung, kidney, and liver injuries and increases survival in CLP-induced sepsis in rats through inhibition of TLR4/NF-κB pathway. Drug Des Devel Ther. 2022;16:3023-39. [Link] [DOI:10.2147/DDDT.S370460]
51. Ibrahim YF, Moussa RA, Bayoumi AM, Ahmed A-SF. Inflammopharmacology. 2020;28(1):215-30. [Link] [DOI:10.1007/s10787-019-00628-y]
52. Ali H, Khan A, Ali J, Ullah H, Khan A, Ali H, et al. Attenuation of LPS-induced acute lung injury by continentalic acid in rodents through inhibition of inflammatory mediators correlates with increased Nrf2 protein expression. BMC Pharmacol Toxicol. 2020;21(1):81. [Link] [DOI:10.1186/s40360-020-00458-7]
53. Chen S, Kuang M, Qu Y, Huang S, Gong B, Lin S, et al. Expression of serum cytokines profile in neonatal sepsis. Infect Drug Resist. 2022;15:3437-45. [Link] [DOI:10.2147/IDR.S368772]
54. Chen J-X, O'Mara PW, Poole SD, Brown N, Ehinger NJ, Slaughter JC, et al. Isoprostanes as physiological mediators of transition to newborn life: novel mechanisms regulating patency of the term and preterm ductus arteriosus. Pediatr Res. 2012;72(2):122-8. [Link] [DOI:10.1038/pr.2012.58]
55. Chen S, Li X, Wang Y, Mu P, Chen C, Huang P, et al. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol Med Rep. 2019;19(5):3633-41. [Link] [DOI:10.3892/mmr.2019.10018]
56. Zi S-f, Li J-h, Liu L, Deng C, Ao X, Chen D-D, et al. Dexmedetomidine-mediated protection against septic liver injury depends on TLR4/MyD88/NF-kappaB signaling downregulation partly via cholinergic anti-inflammatory mechanisms. Int Immunopharmacol. 2019;76:105898. [Link] [DOI:10.1016/j.intimp.2019.105898]
57. Jin YH, Li ZT, Chen H, Jiang XQ, Zhang YY, Wu F. Effect of dexmedetomidine on kidney injury in sepsis rats through TLR4/MyD88/NF-kappaB/iNOS signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(11):5020-5. [Link]
58. Kim GW, Lee NR, Pi RH, Lim YS, Lee YM, Lee JM, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res. 2015;38(5):575-84. [Link] [DOI:10.1007/s12272-015-0569-8]
59. Herwald H, Egesten A. On PAMPs and DAMPs. J Innate Immun. 2016;8(5):427-8. [Link] [DOI:10.1159/000448437]
60. Zheng W, Pan H, Wei L, Gao F, Lin X. Dulaglutide mitigates inflammatory response in fibroblast-like synoviocytes. Int Immunopharmacol. 2019;74:105649. [Link] [DOI:10.1016/j.intimp.2019.05.034]
61. Xu J, Wei G, Wang J, Zhu J, Yu M, Zeng X, et al. Glucagon-like peptide-1 receptor activation alleviates lipopolysaccharide-induced acute lung injury in mice via maintenance of endothelial barrier function. Lab Invest. 2019;99(4): 577-87. [Link] [DOI:10.1038/s41374-018-0170-0]
62. Lei J, Wei Y, Song P, Li Y, Zhang T, Feng Q, et al. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur J Pharmacol. 2018;818:110-4. [Link] [DOI:10.1016/j.ejphar.2017.10.029]
63. Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144(1):266-73. [Link] [DOI:10.1378/chest.12-2664]
64. Zheng L, Fei J, Feng C-M, Xu Z, Fu L, Zhao H. Serum 8-iso-PGF2α predicts the severity and prognosis in patients with community-acquired pneumonia: a retrospective cohort study. Front Med. 2021;8:633442. [Link] [DOI:10.3389/fmed.2021.633442]
65. Reddy IA, Pino JA, Weikop P, Osses N, Sørensen G, Bering T, et al. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl Psychiatry. 2016;6(5):e809. [Link] [DOI:10.1038/tp.2016.86]
66. Li H, Xu X, Wang J, Kong X, Chen M, Jing T, et al. A randomized study to compare the effects of once-weekly dulaglutide injection and once-daily glimepiride on glucose fluctuation of type 2 diabetes mellitus patients: a 26-week follow-up. J Diabetes Res. 2019;2019:6423987. [Link] [DOI:10.1155/2019/6423987]
67. Balk-Moller E, Windelov JA, Svendsen B, Hunt J, Ghiasi SM, Sorensen CM et al. Glucagon-like peptide 1 and atrial natriuretic peptide in a female mouse model of obstructive pulmonary disease. J Endocr Soc. 2019;4(1):bvz034. [Link] [DOI:10.1210/jendso/bvz034]
68. Viby NE, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154(12):4503-11. [Link] [DOI:10.1210/en.2013-1666]
69. Zhu T, Li C, Zhang X, Ye C, Tang S, Zhang W, et al. GLP-1 analogue liraglutide enhances SP-A expression in LPS-induced acute lung injury through the TTF-1 signaling pathway. Mediators Inflamm. 2018;2018:3601454. [Link] [DOI:10.1155/2018/3601454]
70. Zhou W, Shao W, Zhang Y, Liu D, Liu M, Jin T. Glucagon-like peptide-1 receptor mediates the beneficial effect of Liraglutide in an acute lung injury mouse model involving the thioredoxin interacting protein. American Journal of Physiology-Endocrinology and Metabolism. Am J Physiol Endocrinol Metab. 2020;319(3):E568-78. [Link] [DOI:10.1152/ajpendo.00292.2020]










