Volume 13, Issue 3 (2021)
Iran J War Public Health 2021, 13(3): 195-198 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/08/16 | Accepted: 2021/08/5 | Published: 2021/11/10
Received: 2021/08/16 | Accepted: 2021/08/5 | Published: 2021/11/10
How to cite this article
Zaki Jafar Al-Asady A, Ali Hussein Al-Hilaly I. Using Vascular Cell Adhesion Molecule-1 as a Biomarker for Detecting Progression of Different Types of Malignant Tumors. Iran J War Public Health 2021; 13 (3) :195-198
URL: http://ijwph.ir/article-1-993-en.html
URL: http://ijwph.ir/article-1-993-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
A. Zaki Jafar Al-Asady1, I. Ali Hussein Al-Hilaly *2
1- Pharmacology and Toxicology Department, Faculty of Pharmacy, University of Kufa, Kufa, Iraq
2- Laboratory Investigation Department, Faculty of Science, University of Kufa, Kufa, Iraq
2- Laboratory Investigation Department, Faculty of Science, University of Kufa, Kufa, Iraq
Full-Text (HTML) (674 Views)
Introduction
Biomarkers are molecules that signal the presence of cancer or provide information about cancer (e. g., progression or response to treatments). In recent years, biomarkers have begun to play an increasingly essential role in cancer research, particularly in patients' detection, therapy, and follow-up [1].
Vascular cell adhesion molecule-1 (VCAM-1) is a transmembrane glycoprotein closely related to tumorigenicity and tumor metastasis. It is also a well-known candidate for detecting tumors [2]. Mostly expressed in endothelial cells. VCAM-1 was discovered as an endothelial cell surface glycoprotein in 1989. Its production is triggered by pro-inflammatory cytokines TNF and ROS, oxidized low-density lipoprotein, elevated glucose levels, toll-like receptor agonists, and shear stress, all of which have a role [3]. VCAM-1 is a type I transmembrane protein containing seven homologous immunoglobulin (Ig)-like domains on the extracellular domain, a transmembrane domain, and a cytosolic domain. Both the disulfide-linked loops and the N-glycosylation site that binds to galectin-3 on eosinophils are found in the extracellular domain's Ig-like domains. In addition to galectin-3, VCAM -1's Ig-like domain 1 and/or 4 is involved in ligand binding, as are α4β1 and α4β7 integrin integrins. VCAM-1–mediated rolling and firm adherence of leukocytes to the endothelium, as well as leukocyte transmigration processes, are all mediated by α4β1 integrin [3]. VCAM-1 is an adhesion protein that induces cytokine expression on the surface of cancer cells and is engaged in interactions with immune cells [4]. In cancer cells, VCAM-1 binds to α4β1 integrin expressed on macrophages in the lungs, activating the PI3K/Akt survival pathway [5]. VCAM-1 expression on tumor cells is a key player for metastatic colonization of the lungs and bones in breast cancer in breast cancer VCAM-1) as a critical participant in the colonization of metastatic sites [5].
VCAM-1 is overexpressed in breast cancer cells and can bind to its natural ligand α4β1integrin, also known as very late antigen 4 (VLA-4). Breast cancer cells traveling to the lungs, bones, and brain seems to be caused by this binding. The α4β1 integrin - VCAM-1 interaction. As a result, VCAM-1 interaction may be a therapeutic option for metastatic breast cancer cells. The discovery of inhibitors of this interaction may be useful in the clinical treatment of patients with breast cancer [6].
Due to the lack of studies in this field and the importance of on-time detecting different types of cancer as a very common consequence of war-related symptoms, this study aimed to investigate the probability of using VCAM-1 as an indicator for the progression of breast, lung, and bladder cancers.
Materials & Methods
This experimental study was conducted on 15 to 89 years-old patients who attended the Middle Euphrates cancer center and breast disease clinic in AL-Sadder Medical City teaching hospital in Al-Najaf Province, Iraq from September 2020 to January 2021. These patients were mostly from the middle and south regions of Iraq. According to similar studies [5, 7], 88 samples were selected by random sampling method; 22 patients for each breast, lung, and bladder cancer group and 22 healthy subjects as control.
A demographic questionnaire was used to collect the samples information; age, stage of disease, and the number of doses of treatments. An Enzyme-Linked Immunosorbent Assay (ELISA) method was used to evaluate the concentration of VCAM-1 by Human Cluster of differentiation 105 ELISA Kit Cat. No. E7306Hu (Bioassay Technology Laboratory; China).
The researchers attended the Middle Euphrates cancer center and breast disease clinic and started to select the samples. Five ml of venous blood were drawn from were resting patients and a healthy group from a cubital vein using disposable plastic syringes. After clotting the samples were isolated at room temperature by centrifuge at 3000 runs per minute (rpm) for 15 minutes and transported into Eppendorf tubes by micropipette and stored in freezing conditions at -20ᴼC for ELISA performance.
Data were analyzed by GraphPad Prism 5.00 software using one-way ANOVA and Tukey's multiple comparison tests. A p-value less than 0.05 was considered significant.
Findings
There was no significant difference in the levels of serum VCAM-1 according to gender, age, stage of disease, and the number of doses of treatments, but there was a significant negative correlation between VCAM-1 level in lung cancer patients and age (Table 1).
Table 1) Results of ANOVA test for cancers

Serum VCAM-1 levels increased significantly in all groups compared to the control group (p<0.05), except in breast cancer (p>0.05). The highest level of VCAM-1 was in the bladder cancer group.
The means±SE of VCAM-1 levels in the control group and breast cancer group were 42.29±3.928ng/ml, 58.12±2.357ng/ml, respectively, and there was no significant difference between the two groups (Diagram 1)
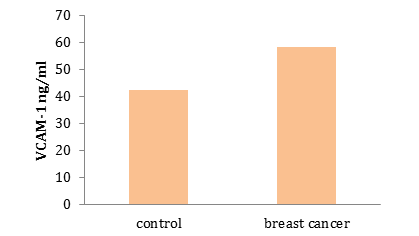
Diagram 1) Results of comparing the serum VCAM-1 level (ng/ml) in control and breast cancer
The level of VCAM-1 in lung cancer was 66.81±4.307ng/ml, and there was a significant difference in VCAM-1 level between control and lung cancer (p=0.0004; Diagram 2).

Diagram 2) Results of comparing the serum VCAM-1 level (ng/ml) in control and lung cancer patient
The level of serum VCAM-1 in bladder cancer was 82.85±7.292 ng/ml. there was a significant difference in VCAM-1 level between control and bladder cancer patients (p=0.0001; Diagram 3).
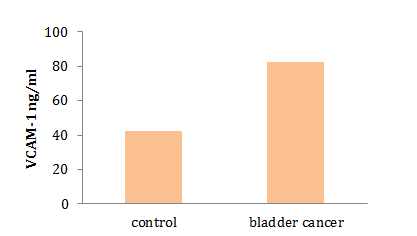
Diagram 3) Results of comparing the serum VCAM-1 level (ng/ml) in control and bladder cancer
Discussion
Our study showed no significant difference between control and breast cancer patients because of the chemotherapy effect. The study presented by [7] supports our findings. There were significantly lower VCAM-1 in subjects who had been treated with cyclophosphamide inflammatory cytokines, and cyclophosphamide is known to modulate lymphocyte function and suppress inflammation. In this way, it can be postulated that CYC assists in the healing of vascular endothelial cells and thereby reduces VCAM-1 levels. Our findings are in agreement with [8] findings which showed their levels tend to be significantly decreased (p<0.05) in postoperative patients compared to preoperative patients Since natalizumab has the potential to inhibit α4β1-cell adhesion molecule interactions.
Our results showed there was a significant difference between control and lung cancer patients in serum vcam1 level. A study [7] showed that these adhesion molecules can be detected in a circulating soluble form when overexpressed and are considered markers of underlying endothelial activity and damage. These findings support our result.
Vascular cell adhesion molecule-1 (VCAM-1) is closely associated with tumor progression and metastasis. However, the relevance and role of VCAM-1 in lung cancer have not been elucidated that VCAM-1 was highly overexpressed in lung cancer tissue compared with that of normal lung tissue, and high VCAM-1 expression correlated with poor survival in lung cancer patients. Gastric, pancreatic, breast, and ovarian cancers Moreover, VCAM-1 expression in breast cancer cells enhances their metastasis to the lungs by allowing them to interact with leukocytes that express α4 integrin counter-receptors, demonstrate an association between high VCAM-1 expression and poor survival and further suggest that increased VCAM-1 expression is closely associated with lung cancer tissue. Second, high VCAM-1 expression correlated with poor survival of lung cancer patients, implying that VCAM-1 has a key role in lung cancer progression. This result suggests that most VCAM-1-stained cells are cancer cells rather than endothelium. However, we cannot exclude the possibility that other cell types also express VCAM-1 in lung cancer tissue [10]. These findings agree with our findings
It is now different studies show that VCAM1 is closely related to the development of malignant tumors, such as breast cancer, ovarian cancer and clear cell renal carcinoma; researchers discovered that tumor cells' overexpression of VCAM1 accomplished lung or bone metastasis by recruiting monocytes or macrophages and forming a complex that facilitates circulating tumor cell evasion from immune system attack and transendothelial migration [9].
There was a significant difference in serum VCAM 1 level in control and bladder cancer patients; there is a relevance of VCAM to increase angiogenesis since there is evidence indicates increase VCAM in tissues rich with new vasculature (angiogenesis), and these levels become dropped in tissues without micro-vessels in gastric vessels. This indicates that the levels of intercellular adhesion molecules remain at a high level. However, surgical operation and chemotherapy so that these molecules may be confirmed remaining tumor cells invade the other tissues of the body study which are showed indicated a significant increase (p<0.05) in the levels of sVCAM -1, in all patient men affected with colon and urinary bladder cancer when compared with their counterparts of healthy men. Also, the findings of the current study are consistent with those of [11], who have shown that VCAM-1 is abnormally expressed in some tumors and plays an important role in tumor progression our search limited by the number of patients; therefore, urther research should performed on larger number of patients.
Conclusion
The highest level of VCAM-1 is in the bladder cancer group; therefore, evidence suggests that serum VCAM-1 can be used as an indicator for bladder cancer.
Acknowledgments: The authors did not respond.
Ethical Permissions: The authors did not respond.
Conflicts of Interests: The authors did not respond.
Authors' Contribution: Zaki Jafar Al-Asady A. (First Author), Introduction Writer/Original Researcher/Statistical Analyst (50%); Ali Hussein Al-Hilaly I. (Second Author), Methodologist/Original Researcher/Discussion Writer (50%).
Funding/Support: The authors did not respond.
Biomarkers are molecules that signal the presence of cancer or provide information about cancer (e. g., progression or response to treatments). In recent years, biomarkers have begun to play an increasingly essential role in cancer research, particularly in patients' detection, therapy, and follow-up [1].
Vascular cell adhesion molecule-1 (VCAM-1) is a transmembrane glycoprotein closely related to tumorigenicity and tumor metastasis. It is also a well-known candidate for detecting tumors [2]. Mostly expressed in endothelial cells. VCAM-1 was discovered as an endothelial cell surface glycoprotein in 1989. Its production is triggered by pro-inflammatory cytokines TNF and ROS, oxidized low-density lipoprotein, elevated glucose levels, toll-like receptor agonists, and shear stress, all of which have a role [3]. VCAM-1 is a type I transmembrane protein containing seven homologous immunoglobulin (Ig)-like domains on the extracellular domain, a transmembrane domain, and a cytosolic domain. Both the disulfide-linked loops and the N-glycosylation site that binds to galectin-3 on eosinophils are found in the extracellular domain's Ig-like domains. In addition to galectin-3, VCAM -1's Ig-like domain 1 and/or 4 is involved in ligand binding, as are α4β1 and α4β7 integrin integrins. VCAM-1–mediated rolling and firm adherence of leukocytes to the endothelium, as well as leukocyte transmigration processes, are all mediated by α4β1 integrin [3]. VCAM-1 is an adhesion protein that induces cytokine expression on the surface of cancer cells and is engaged in interactions with immune cells [4]. In cancer cells, VCAM-1 binds to α4β1 integrin expressed on macrophages in the lungs, activating the PI3K/Akt survival pathway [5]. VCAM-1 expression on tumor cells is a key player for metastatic colonization of the lungs and bones in breast cancer in breast cancer VCAM-1) as a critical participant in the colonization of metastatic sites [5].
VCAM-1 is overexpressed in breast cancer cells and can bind to its natural ligand α4β1integrin, also known as very late antigen 4 (VLA-4). Breast cancer cells traveling to the lungs, bones, and brain seems to be caused by this binding. The α4β1 integrin - VCAM-1 interaction. As a result, VCAM-1 interaction may be a therapeutic option for metastatic breast cancer cells. The discovery of inhibitors of this interaction may be useful in the clinical treatment of patients with breast cancer [6].
Due to the lack of studies in this field and the importance of on-time detecting different types of cancer as a very common consequence of war-related symptoms, this study aimed to investigate the probability of using VCAM-1 as an indicator for the progression of breast, lung, and bladder cancers.
Materials & Methods
This experimental study was conducted on 15 to 89 years-old patients who attended the Middle Euphrates cancer center and breast disease clinic in AL-Sadder Medical City teaching hospital in Al-Najaf Province, Iraq from September 2020 to January 2021. These patients were mostly from the middle and south regions of Iraq. According to similar studies [5, 7], 88 samples were selected by random sampling method; 22 patients for each breast, lung, and bladder cancer group and 22 healthy subjects as control.
A demographic questionnaire was used to collect the samples information; age, stage of disease, and the number of doses of treatments. An Enzyme-Linked Immunosorbent Assay (ELISA) method was used to evaluate the concentration of VCAM-1 by Human Cluster of differentiation 105 ELISA Kit Cat. No. E7306Hu (Bioassay Technology Laboratory; China).
The researchers attended the Middle Euphrates cancer center and breast disease clinic and started to select the samples. Five ml of venous blood were drawn from were resting patients and a healthy group from a cubital vein using disposable plastic syringes. After clotting the samples were isolated at room temperature by centrifuge at 3000 runs per minute (rpm) for 15 minutes and transported into Eppendorf tubes by micropipette and stored in freezing conditions at -20ᴼC for ELISA performance.
Data were analyzed by GraphPad Prism 5.00 software using one-way ANOVA and Tukey's multiple comparison tests. A p-value less than 0.05 was considered significant.
Findings
There was no significant difference in the levels of serum VCAM-1 according to gender, age, stage of disease, and the number of doses of treatments, but there was a significant negative correlation between VCAM-1 level in lung cancer patients and age (Table 1).
Table 1) Results of ANOVA test for cancers

Serum VCAM-1 levels increased significantly in all groups compared to the control group (p<0.05), except in breast cancer (p>0.05). The highest level of VCAM-1 was in the bladder cancer group.
The means±SE of VCAM-1 levels in the control group and breast cancer group were 42.29±3.928ng/ml, 58.12±2.357ng/ml, respectively, and there was no significant difference between the two groups (Diagram 1)

Diagram 1) Results of comparing the serum VCAM-1 level (ng/ml) in control and breast cancer
The level of VCAM-1 in lung cancer was 66.81±4.307ng/ml, and there was a significant difference in VCAM-1 level between control and lung cancer (p=0.0004; Diagram 2).

Diagram 2) Results of comparing the serum VCAM-1 level (ng/ml) in control and lung cancer patient
The level of serum VCAM-1 in bladder cancer was 82.85±7.292 ng/ml. there was a significant difference in VCAM-1 level between control and bladder cancer patients (p=0.0001; Diagram 3).

Diagram 3) Results of comparing the serum VCAM-1 level (ng/ml) in control and bladder cancer
Discussion
Our study showed no significant difference between control and breast cancer patients because of the chemotherapy effect. The study presented by [7] supports our findings. There were significantly lower VCAM-1 in subjects who had been treated with cyclophosphamide inflammatory cytokines, and cyclophosphamide is known to modulate lymphocyte function and suppress inflammation. In this way, it can be postulated that CYC assists in the healing of vascular endothelial cells and thereby reduces VCAM-1 levels. Our findings are in agreement with [8] findings which showed their levels tend to be significantly decreased (p<0.05) in postoperative patients compared to preoperative patients Since natalizumab has the potential to inhibit α4β1-cell adhesion molecule interactions.
Our results showed there was a significant difference between control and lung cancer patients in serum vcam1 level. A study [7] showed that these adhesion molecules can be detected in a circulating soluble form when overexpressed and are considered markers of underlying endothelial activity and damage. These findings support our result.
Vascular cell adhesion molecule-1 (VCAM-1) is closely associated with tumor progression and metastasis. However, the relevance and role of VCAM-1 in lung cancer have not been elucidated that VCAM-1 was highly overexpressed in lung cancer tissue compared with that of normal lung tissue, and high VCAM-1 expression correlated with poor survival in lung cancer patients. Gastric, pancreatic, breast, and ovarian cancers Moreover, VCAM-1 expression in breast cancer cells enhances their metastasis to the lungs by allowing them to interact with leukocytes that express α4 integrin counter-receptors, demonstrate an association between high VCAM-1 expression and poor survival and further suggest that increased VCAM-1 expression is closely associated with lung cancer tissue. Second, high VCAM-1 expression correlated with poor survival of lung cancer patients, implying that VCAM-1 has a key role in lung cancer progression. This result suggests that most VCAM-1-stained cells are cancer cells rather than endothelium. However, we cannot exclude the possibility that other cell types also express VCAM-1 in lung cancer tissue [10]. These findings agree with our findings
It is now different studies show that VCAM1 is closely related to the development of malignant tumors, such as breast cancer, ovarian cancer and clear cell renal carcinoma; researchers discovered that tumor cells' overexpression of VCAM1 accomplished lung or bone metastasis by recruiting monocytes or macrophages and forming a complex that facilitates circulating tumor cell evasion from immune system attack and transendothelial migration [9].
There was a significant difference in serum VCAM 1 level in control and bladder cancer patients; there is a relevance of VCAM to increase angiogenesis since there is evidence indicates increase VCAM in tissues rich with new vasculature (angiogenesis), and these levels become dropped in tissues without micro-vessels in gastric vessels. This indicates that the levels of intercellular adhesion molecules remain at a high level. However, surgical operation and chemotherapy so that these molecules may be confirmed remaining tumor cells invade the other tissues of the body study which are showed indicated a significant increase (p<0.05) in the levels of sVCAM -1, in all patient men affected with colon and urinary bladder cancer when compared with their counterparts of healthy men. Also, the findings of the current study are consistent with those of [11], who have shown that VCAM-1 is abnormally expressed in some tumors and plays an important role in tumor progression our search limited by the number of patients; therefore, urther research should performed on larger number of patients.
Conclusion
The highest level of VCAM-1 is in the bladder cancer group; therefore, evidence suggests that serum VCAM-1 can be used as an indicator for bladder cancer.
Acknowledgments: The authors did not respond.
Ethical Permissions: The authors did not respond.
Conflicts of Interests: The authors did not respond.
Authors' Contribution: Zaki Jafar Al-Asady A. (First Author), Introduction Writer/Original Researcher/Statistical Analyst (50%); Ali Hussein Al-Hilaly I. (Second Author), Methodologist/Original Researcher/Discussion Writer (50%).
Funding/Support: The authors did not respond.
Keywords:
References
1. Duffy MJ. Clinical use of tumor biomarkers: An overview. Klin Biochem Metab. 2017;25(4):157-61. []
2. Zhang X, Liu C, Hu F, Zhang Y, Wang J, Gao Y, et al. PET imaging of VCAM-1 expression and monitoring therapy response in tumor with a 68 Ga-labeled single chain variable fragment. Mol Pharm. 2018;15(2):609-18. [] [DOI:10.1021/acs.molpharmaceut.7b00961] [PMID]
3. Kong DH, Kim YK, Kim MR, Jang JH, Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int J Mol Sci. 2018;19(4):13-7. [] [DOI:10.3390/ijms19041057] [PMID] [PMCID]
4. Liu YS, Lin HY, Lai SW, Huang CY, Huang BR, Chen PY, et al. MIR-181b modulates EGFR-dependent VCAM-1 expression and monocyte adhesion in glioblastoma. Oncogene. 2017;36(35):5006-22. [] [DOI:10.1038/onc.2017.129] [PMID]
5. Montemagno C, Dumas L, Cavailles P, Ahmadi M, Bacot S, Debiossat M, et al. In vivo assessment of VCAM-1 expression by SPECT/CT imaging in mice models of human triple negative breast cancer. Cancers. 2019;11(7):1039. [] [DOI:10.3390/cancers11071039] [PMID] [PMCID]
6. Sharma R, Sharma R, Khaket TP, Dutta C, Chakraborty B, Mukherjee TK. Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol. 2017;40(3):199-208. [] [DOI:10.1007/s13402-017-0324-x] [PMID]
7. Thakkar V, Patterson KA, Stevens W, Wilson M, Roddy J, Sahhar J, et al. Increased serum levels of adhesion molecules ICAM-1 and VCAM-1 in systemic sclerosis are not specific for pulmonary manifestations. Clin Rheumatol. 2018;37(6):1563-71. [] [DOI:10.1007/s10067-018-4081-7] [PMID]
8. Mirdan RAAR. Levels of some cellular adhesion molecules (CEA, sICAM-1, sVCAM-, and E- selecting) in patients affected with colon and urinary bladder cancer during pre and post-operative surgery. Ann Trop Med Public Health. 2020;23(24):1-11. [] [DOI:10.36295/ASRO.2020.232439]
9. Zhang D, Bi J, Liang Q, Wang S, Zhang L, Han F, et al. VCAM1 promotes tumor cell invasion and metastasis by inducing EMT and Transendothelial migration in colorectal cancer. Front Oncol. 2020;10:1066. [] [DOI:10.3389/fonc.2020.01066] [PMID] [PMCID]
10. Kim MR, Jang JH, Park CS, Kim TK, Kim YJ, Chung J, et al. A human antibody that binds to the sixth Ig-like domain of VCAM-1 blocks lung cancer cell migration in vitro. Int J Mol Sci. 2017;18(3):566. [] [DOI:10.3390/ijms18030566] [PMID] [PMCID]
11. Chang AC, Chen PC, Lin YF, Su CM, Liu JF, Lin TH, et al. Osteoblast-secreted WISP-1 promotes adherence of prostate cancer cells to bone via the VCAM-1/integrin α4β1 system. Cancer Lett. 2018;426:47-56. [] [DOI:10.1016/j.canlet.2018.03.050] [PMID]








