Volume 15, Issue 4 (2023)
Iran J War Public Health 2023, 15(4): 415-420 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/07/5 | Accepted: 2024/01/18 | Published: 2024/01/21
Received: 2023/07/5 | Accepted: 2024/01/18 | Published: 2024/01/21
How to cite this article
Hussein K, Madhi K, Al-Mayyahi Z, Al-Ammar N, Abbas B, Faraj A. Cytotoxin Production and Slim Layer Formation by Methicillin-Resistant Staphylococcus auras Isolated from Diabetic Patients. Iran J War Public Health 2023; 15 (4) :415-420
URL: http://ijwph.ir/article-1-1367-en.html
URL: http://ijwph.ir/article-1-1367-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Medical Sciences, College of Nursing, University of Basrah, Basrah, Iraq
2- Department of Human Anatomy, College of Medicine, University of Basrah, Basrah, Iraq
3- Department of Medicine, College of Medicine, University of Basrah, Basrah, Iraq
4- Department of Microbiology, College of Medicine, University of Basrah, Basrah, Iraq
5- Department of Microbiology, College of Veterinary Medicine, University of Basrah, Basrah, Iraq
6- Public Health Laboratory, Sulaymaniyah, Iraq
2- Department of Human Anatomy, College of Medicine, University of Basrah, Basrah, Iraq
3- Department of Medicine, College of Medicine, University of Basrah, Basrah, Iraq
4- Department of Microbiology, College of Medicine, University of Basrah, Basrah, Iraq
5- Department of Microbiology, College of Veterinary Medicine, University of Basrah, Basrah, Iraq
6- Public Health Laboratory, Sulaymaniyah, Iraq
Full-Text (HTML) (549 Views)
Introduction
Foot infections are a major cause of morbidity in diabetes patients and the most common cause of diabetes-related hospitalization and lower limb amputation [1]. The International Diabetes Foundation reports a significant increase in the number of individuals afflicted with diabetic foot ulcers (DFUs), estimated to be between 40 to 60 million people. This figure represents a notable surge compared to the range of cases reported in 2015, which was between 9 and 26 million [2, 3].
The physiopathology of diabetic foot infections (DFI) is complex, but its severity and prevalence result from host-related disorders and pathogens-factors, such as virulence and antibiotic resistance traits [2]. Several studies have shown that DFUs are classically polymicrobial-infected [3], characterized by several pathological sequelae, such as neuropathy and peripheral vascular disease [4, 5]. Staphylococcus epidermidis, Staphylococcus aureus, and Propionibacterium acnes are a few examples of pathogenic bacteria that can cause infection. They can also be present in the skin's physiological microflora. Almost any germ that touches the surface of an ulcer is likely to colonize it. Medical personnel, supplies, and drugs used for therapy frequently unwittingly spread harmful microflora. Various bacteria strains are often present in the mixed flora of ulcers [6, 7]. Staphylococcus aureus (S. aureus) is frequently identified as the predominant pathogen in these cases. Infections in these ulcers are characterized by disruptions in extracellular matrix remodeling, abnormal angiogenesis-related growth factors, and excessive inflammatory responses, all hindering wound healing [8]. However, if S. aureus bacteria develop antibiotic resistance, they can lead to severe opportunistic infections or diseases. According to a US Centers for Disease Control and Prevention (CDC) survey, approximately 5% of the population carries the MRSA strain [9]. Diabetic foot ulcers (DFUs) caused by antibiotic-resistant bacteria, particularly Methicillin-Resistant Staphylococcus aureus (MRSA), are associated with more severe infections [10]. In addition to producing a slim layer, S. aureus is involved in the infection of soft tissues and bones. The slim layer (glycocalyx or biofilm) forms bacteria that infect foot ulceration, which is the cause of 80% of lower-limb amputations. Toxins, which can cause tissue necrosis, progression, and spread of infection in DFI patients, can also be released by S. aureus in addition to basic adhesion mechanisms [10-12]. Methicillin-resistant S. aureus (MRSA), which accounts for most S. aureus infections, is present in 10-40% of diabetic wounds and produces Panton-valentine leukocidin (PVL). This cytotoxin is crucial to methicillin-resistant Staphylococcus aureus virulence [13, 14].
MRSA was initially identified in England 1961, shortly after introducing methicillin, the first penicillinase-resistant semi-synthetic penicillin [15]. Since then, MRSA has emerged as the predominant pathogen responsible for hospital-acquired infections worldwide, with its incidence still on the rise in many countries [16]. MRSA exhibits resistance to multiple antibiotics, including methicillin, penicillin, oxacillin, cloxacillin, cefazolin, cefoxitin, and other commonly used antibiotics [17]. Transmission of MRSA can occur through close contact with infected individuals or via contaminated objects. In healthcare settings, infections acquired in hospitals or other healthcare facilities are called nosocomial infections [9].
Once Staphylococcus aureus adheres to host tissues, it can create biofilms. These biofilms facilitate its persistence by allowing bacteria to evade host defenses, obstructing access to certain immune cells like macrophages. These immune cells struggle to penetrate the biofilm matrix fully, leading to a phenomenon known as frustrated phagocytosis [2]. Additionally, cells within biofilms exhibit heightened antibiotic tolerance, making it challenging to treat S. aureus infections that have formed biofilms. The fibronectin-binding proteins (FnBPs), including FnBPA and FnBPB, play a role in stimulating biofilm formation by clinically relevant MRSA strains [18].
In contrast to heritable antibiotic resistance mechanisms, biofilm-associated tolerance represents a temporary state wherein bacteria typically susceptible to antibiotics undergo physiological changes that reduce their sensitivity. When these cells disperse and return to a planktonic state, they regain their normal susceptibility profile [19]. Bacteria entrenched within biofilms pose a challenge for eradication due to various nutrient gradients that hinder or halt bacterial growth, protein synthesis, and other physiological activities. Bacteria within biofilms are less responsive to antibiotics due to their reduced growth rates [20]. Additional factors contributing to biofilm-mediated antimicrobial resistance include inefficient diffusion or entrapment of the antibiotic within the biofilm matrix, the presence of "persister" cells, and other unidentified phenotypic differences [20].
This study aimed to identify methicillin-resistant Staphylococcus aureus genetically from DFU patients and discuss its function in Biofilm formation. It also identified the genes that are the primary contributors to delayed healing and lower limb amputations, produced the cytotoxin (pvl), the primary cause of tissue necrosis, and assessed the frequency of its genes in MRSA isolated from infected DFU patients.
Materials and Methods
Morphological and Biochemical Characteristics
A total of 80 swabs from the necrotic lesions of patients with DFUs, whose cause was complex diabetic foot syndrome (DFS), were obtained and evaluated under the supervision of a doctor. In Al-Basrah, southern Iraq, the main hospitals (Al-Faiha General Teaching Hospital and Al-Mawanaa General Teaching Hospital) treated and admitted patients to the Diabetes Endocrinology Center for the 2019-2020 academic year. All of the patients had type 2 diabetes mellitus (T2DM), which had an average duration of 14.5±2.5 years. The patients were between 40 and 55, plus or minus five years. These swabs were cultured in sterile tubes with 5ml of Brain Heart Infusion Broth-BHIB (HIMEDIA; India) and then moved to the laboratory, incubated for 24h at 37°C. The Staphylococcus Chromogeneic Agar Media (CONDA Pronadisa; Spain) was streaked after the broth media showed positive growth. Colonies developed after being cultivated on nutrient agar (HIMEDIA; India) for 24 hours at 37°C [21]. Gram staining was done to identify the colonies.
Congo Red Agar (CRA) method
According to Freeman [22], this agar was made by mixing blood agar base with 0.8gm congo red, 50gm sucrose, and 1-liter distilled water. The pH was then adjusted to 8, and the agar was autoclaved at 121C for 15 minutes.
Bacteria 16srDNA Genotyping
Extraction of DNA
The DNA extraction was done using a genomic DNA micro kit (Geneaid; Taiwan).
I6srDNA amplification and sequencing
To identify the bacterial strains, the extracted DNA was processed through PCR to amplify the universal bacterial 16SrDNA gene, which is represented by the sequences B 27F (5'-AGAGTTTG ATCCTGGC-3') and U 1492R (5'-GGTTACCT TGTTACGACTT-3').92°C for 2 minutes, then 30 cycles of 94°C for 30 seconds, 51°C for 45 seconds, 72°C for 1.5 minutes, and 72°C for 5 minutes [23].
The positive samples of PCR products for the universal 16srDNA gene were sent to Bioneer Company (Korea) for sequencing to identify them further.
16srDNA identification
The 16srDNA sequences of the bacterium isolates were compared to the Genbank 16srDNA reference database (http://blast.ncbi.nlm.nih.gov/).
Detection of the mecA, icaA, and pvl genes
The mecA gene is used to identify methicillin-resistant Staphylococcus aureus species using the primers MecA1 and MecA2 [24] and Luk-PV-2, depending on [25]. The slime layer is encoded by the icaA gene, which has the primers icaA1 and icaA2, according to [25]. The pvl genes use the primers Luk-PV-1 and Luk-PV-2 to detect the PVL S/F bicomponent proteins, depending on [26].
MecA genes were subjected to a set of thermocycling conditions that included three minutes at 95°C followed by 30 cycles of one minute each at 94°C, 53°C, one minute at 72°C, and six minutes at 72°C IcaA genes were subjected to a set of conditions that included five minutes at 95°C. followed by fifty cycles of one minute each at 94°C five minutes at 55.5°C, one minute at 72°C, and one minute at 95°C.
Findings
Morphological and Biochemical Characteristics
Staphylococcus aureus was isolated and found to be dependent on its color (pink to mauve) on Staphylococcus Chromogenic agar media. Out of 80 swab samples, only 31 isolates, including 12 (38.7%) isolates, were identified as Staphylococcus aureus, while the other 19 (61.3%) isolates were identified as Staphylococcus spp.
I6srDNA amplification and sequencing
The PCR products for the extracted DNA from 12 isolates resulted in (100%) positive results, and when they were seen under a UV light source and compared to a DNA ladder, they showed a single band at a location of 1500bp on an agarose gel. Using the Basic Local Alignment Search Tool (BLAST), 7 (87.5%) strains of S. aureus from 12 isolates were found. These strains were then compared to a reference strain from Gene Bank (Table 1), which was the same strain. These include the S. aureus strains pak2 (n=5), MR30 (n=3), CFSAN007896 (n=3), and ST4 (n=1).
Table 1. Alignments of PCR product for16SrDNA gene (all 100% identical with reference strain)


Results for the mecA, icaA, and pvl genes
Amplification of the mecA and icaA genes produced bands at 310bp and 188bp, respectively, as shown in Figure 1. Nine (75%) of the 12 isolates encode for the pvl S/F gene (Bicomponent proteins), which produced bands at position 433bp.
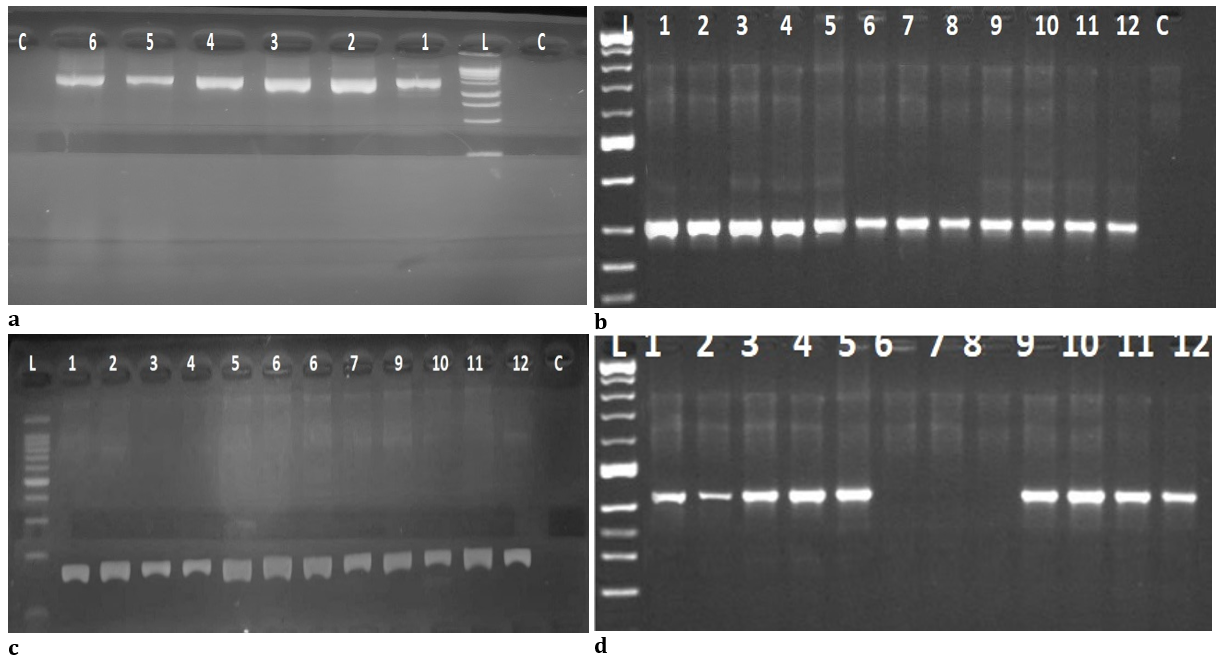
Figure 1. (a) Electrophoresis of agarose gel (1%) for Universal 16srDNA PCR products at position 1500bp from (1-6), L: (Ladder:250-10000bp); (b) Electrophoresis of agarose gel (1%) from (1-12) specific mecA gene products at position 310bp, L: (Ladder:100-1000bp); (c) Electrophoresis of agarose gel (1%) from (1-12) specific icaA gene products at position 188bp, L: (Ladder:100-1000bp); (d) Electrophoresis of agarose gel (1%) from (1-9) specific pvl S/F gene products at position 433bp, L: (Ladder:100-1000bp)
Discussion
The present work examined CHROMagar as a direct isolation medium for specimen isolation, enabling simple differentiation of bacterial colonies based on color and shape characteristics on CHROMagar. As a result, when mixed pathogens were grown on the medium, the medium's capacity to detect them was enhanced [27, 28]. The collected DNA was amplified with the help of universal 16srDNA primers (F27 and R1492). The primers avoid the loss of any potential or new bacterial strains by amplifying the 16srDNA (16s ribosomal DNA) gene for all bacterial strains [28-29].
Out of 80 patient swab samples, this study found that the prevalence of Staphylococcus aureus was 12 (38.7%), which is consistent with research done in Babylon, Iraq [30] and Bandar Abbas, southern Iran [31]. Additionally, this study's prevalence of MRSA was 100% of mecA in locations where methicillin-resistant S. aureus was found using molecular techniques. This percentage was found in the study, which is greater than the figures found in the United States (29.8%) and Portugal (24.5%) [32, 33].
The widespread and unchecked use of antibiotics in Iraq, which has led to significant levels of multi-drug resistance, is one of the country's biggest problems. Additionally, a lot of patients self-medicate with antibiotics, especially broad-spectrum antibiotics, without consulting a doctor. Therefore, we believe the high prevalence of MRSA in S. aureus isolates is the misuse of antibiotics, improper sterilization techniques, or the use of incorrect sterilizers to treat ulcerated wounds. Any diabetic foot infection treated with MRSA will likely lead to increased resistance and medical expenses. All 12 MRSA isolates formed biofilm (100%) after samples underwent icaA and pvl S/F gene amplification, and 9 (75%) of them produced the cytotoxin (PVL), both of which are crucial for MRSA pathogenicity. The production and secretion of glycocalyx are necessary for S. aureus to be harmful, and the neutrophil response is the first line of defense against S. aureus infection for strains derived from DFUs. Polysaccharide synthesis starts soon after attachment and starts to cover the bacteria. This is a crucial step in forming a biofilm that delays wound healing and increases biofilm thickness, especially in diabetic patients. Avoiding immune response by producing capsules or slime layers that conceal it and inhibiting phagocyte death after ingestion [7, 34, 35].
In addition to biofilm formation, S. aureus contains a wide range of cytotoxins, which significantly impact the progression and spread of the bacteria in DFUs and potentially result in tissue necrosis [36]. In this investigation, most isolates from DFU patients have icaA and pvl genes. This outcome aligns with research done in France and Iraq [12, 37].
The association between the MRSA strain's icaA and pvl genes and the severe infection of diabetic foot ulcers, however, has not been studied. All MRSA isolated (100%) developed the slime layer that retarded healing. Additionally, (75%) of the 9 MRSA isolated produced the cytotoxin pvl that causes tissue necrosis. We then go on to describe how this virulence might cause foot amputation as a result of disease progression and sluggish wound healing.
Conclusion
Panton-valentine leukocidin has a high prevalence among MRSA strains isolated from diabetic foot ulcer patients that form biofilms, causing patients to have significant inflammation, illness progression, and challenging wound healing, which may result in lower limb amputations.
Acknowledgments: We would like to thank all the patients who allowed me to take the swabs and assistance in conducting this study.
Ethical Permissions: Ethical Permissions were approved by ethical committee, college of Medicine, university of Basrah, Basrah, Iraq.
Conflicts of Interests: Authors are declare that there is no conflict of interest.
Authors’ Contribution: Hussein KA (First Author), Introduction Writer (15%); Madhi KS (Second Author), Methodologist (20%); Al-Mayyahi ZA (Third Author), Assistant Researcher (20%); Al-Ammar NA (Fourth Author), Main Researcher (20%); Abbas BA (Fifth Author), Discussion Writer (15%); Faraj AH (Sixth Author), Statistical Analyst (10%)
Funding/Support: The project is self funded.
Foot infections are a major cause of morbidity in diabetes patients and the most common cause of diabetes-related hospitalization and lower limb amputation [1]. The International Diabetes Foundation reports a significant increase in the number of individuals afflicted with diabetic foot ulcers (DFUs), estimated to be between 40 to 60 million people. This figure represents a notable surge compared to the range of cases reported in 2015, which was between 9 and 26 million [2, 3].
The physiopathology of diabetic foot infections (DFI) is complex, but its severity and prevalence result from host-related disorders and pathogens-factors, such as virulence and antibiotic resistance traits [2]. Several studies have shown that DFUs are classically polymicrobial-infected [3], characterized by several pathological sequelae, such as neuropathy and peripheral vascular disease [4, 5]. Staphylococcus epidermidis, Staphylococcus aureus, and Propionibacterium acnes are a few examples of pathogenic bacteria that can cause infection. They can also be present in the skin's physiological microflora. Almost any germ that touches the surface of an ulcer is likely to colonize it. Medical personnel, supplies, and drugs used for therapy frequently unwittingly spread harmful microflora. Various bacteria strains are often present in the mixed flora of ulcers [6, 7]. Staphylococcus aureus (S. aureus) is frequently identified as the predominant pathogen in these cases. Infections in these ulcers are characterized by disruptions in extracellular matrix remodeling, abnormal angiogenesis-related growth factors, and excessive inflammatory responses, all hindering wound healing [8]. However, if S. aureus bacteria develop antibiotic resistance, they can lead to severe opportunistic infections or diseases. According to a US Centers for Disease Control and Prevention (CDC) survey, approximately 5% of the population carries the MRSA strain [9]. Diabetic foot ulcers (DFUs) caused by antibiotic-resistant bacteria, particularly Methicillin-Resistant Staphylococcus aureus (MRSA), are associated with more severe infections [10]. In addition to producing a slim layer, S. aureus is involved in the infection of soft tissues and bones. The slim layer (glycocalyx or biofilm) forms bacteria that infect foot ulceration, which is the cause of 80% of lower-limb amputations. Toxins, which can cause tissue necrosis, progression, and spread of infection in DFI patients, can also be released by S. aureus in addition to basic adhesion mechanisms [10-12]. Methicillin-resistant S. aureus (MRSA), which accounts for most S. aureus infections, is present in 10-40% of diabetic wounds and produces Panton-valentine leukocidin (PVL). This cytotoxin is crucial to methicillin-resistant Staphylococcus aureus virulence [13, 14].
MRSA was initially identified in England 1961, shortly after introducing methicillin, the first penicillinase-resistant semi-synthetic penicillin [15]. Since then, MRSA has emerged as the predominant pathogen responsible for hospital-acquired infections worldwide, with its incidence still on the rise in many countries [16]. MRSA exhibits resistance to multiple antibiotics, including methicillin, penicillin, oxacillin, cloxacillin, cefazolin, cefoxitin, and other commonly used antibiotics [17]. Transmission of MRSA can occur through close contact with infected individuals or via contaminated objects. In healthcare settings, infections acquired in hospitals or other healthcare facilities are called nosocomial infections [9].
Once Staphylococcus aureus adheres to host tissues, it can create biofilms. These biofilms facilitate its persistence by allowing bacteria to evade host defenses, obstructing access to certain immune cells like macrophages. These immune cells struggle to penetrate the biofilm matrix fully, leading to a phenomenon known as frustrated phagocytosis [2]. Additionally, cells within biofilms exhibit heightened antibiotic tolerance, making it challenging to treat S. aureus infections that have formed biofilms. The fibronectin-binding proteins (FnBPs), including FnBPA and FnBPB, play a role in stimulating biofilm formation by clinically relevant MRSA strains [18].
In contrast to heritable antibiotic resistance mechanisms, biofilm-associated tolerance represents a temporary state wherein bacteria typically susceptible to antibiotics undergo physiological changes that reduce their sensitivity. When these cells disperse and return to a planktonic state, they regain their normal susceptibility profile [19]. Bacteria entrenched within biofilms pose a challenge for eradication due to various nutrient gradients that hinder or halt bacterial growth, protein synthesis, and other physiological activities. Bacteria within biofilms are less responsive to antibiotics due to their reduced growth rates [20]. Additional factors contributing to biofilm-mediated antimicrobial resistance include inefficient diffusion or entrapment of the antibiotic within the biofilm matrix, the presence of "persister" cells, and other unidentified phenotypic differences [20].
This study aimed to identify methicillin-resistant Staphylococcus aureus genetically from DFU patients and discuss its function in Biofilm formation. It also identified the genes that are the primary contributors to delayed healing and lower limb amputations, produced the cytotoxin (pvl), the primary cause of tissue necrosis, and assessed the frequency of its genes in MRSA isolated from infected DFU patients.
Materials and Methods
Morphological and Biochemical Characteristics
A total of 80 swabs from the necrotic lesions of patients with DFUs, whose cause was complex diabetic foot syndrome (DFS), were obtained and evaluated under the supervision of a doctor. In Al-Basrah, southern Iraq, the main hospitals (Al-Faiha General Teaching Hospital and Al-Mawanaa General Teaching Hospital) treated and admitted patients to the Diabetes Endocrinology Center for the 2019-2020 academic year. All of the patients had type 2 diabetes mellitus (T2DM), which had an average duration of 14.5±2.5 years. The patients were between 40 and 55, plus or minus five years. These swabs were cultured in sterile tubes with 5ml of Brain Heart Infusion Broth-BHIB (HIMEDIA; India) and then moved to the laboratory, incubated for 24h at 37°C. The Staphylococcus Chromogeneic Agar Media (CONDA Pronadisa; Spain) was streaked after the broth media showed positive growth. Colonies developed after being cultivated on nutrient agar (HIMEDIA; India) for 24 hours at 37°C [21]. Gram staining was done to identify the colonies.
Congo Red Agar (CRA) method
According to Freeman [22], this agar was made by mixing blood agar base with 0.8gm congo red, 50gm sucrose, and 1-liter distilled water. The pH was then adjusted to 8, and the agar was autoclaved at 121C for 15 minutes.
Bacteria 16srDNA Genotyping
Extraction of DNA
The DNA extraction was done using a genomic DNA micro kit (Geneaid; Taiwan).
I6srDNA amplification and sequencing
To identify the bacterial strains, the extracted DNA was processed through PCR to amplify the universal bacterial 16SrDNA gene, which is represented by the sequences B 27F (5'-AGAGTTTG ATCCTGGC-3') and U 1492R (5'-GGTTACCT TGTTACGACTT-3').92°C for 2 minutes, then 30 cycles of 94°C for 30 seconds, 51°C for 45 seconds, 72°C for 1.5 minutes, and 72°C for 5 minutes [23].
The positive samples of PCR products for the universal 16srDNA gene were sent to Bioneer Company (Korea) for sequencing to identify them further.
16srDNA identification
The 16srDNA sequences of the bacterium isolates were compared to the Genbank 16srDNA reference database (http://blast.ncbi.nlm.nih.gov/).
Detection of the mecA, icaA, and pvl genes
The mecA gene is used to identify methicillin-resistant Staphylococcus aureus species using the primers MecA1 and MecA2 [24] and Luk-PV-2, depending on [25]. The slime layer is encoded by the icaA gene, which has the primers icaA1 and icaA2, according to [25]. The pvl genes use the primers Luk-PV-1 and Luk-PV-2 to detect the PVL S/F bicomponent proteins, depending on [26].
MecA genes were subjected to a set of thermocycling conditions that included three minutes at 95°C followed by 30 cycles of one minute each at 94°C, 53°C, one minute at 72°C, and six minutes at 72°C IcaA genes were subjected to a set of conditions that included five minutes at 95°C. followed by fifty cycles of one minute each at 94°C five minutes at 55.5°C, one minute at 72°C, and one minute at 95°C.
Findings
Morphological and Biochemical Characteristics
Staphylococcus aureus was isolated and found to be dependent on its color (pink to mauve) on Staphylococcus Chromogenic agar media. Out of 80 swab samples, only 31 isolates, including 12 (38.7%) isolates, were identified as Staphylococcus aureus, while the other 19 (61.3%) isolates were identified as Staphylococcus spp.
I6srDNA amplification and sequencing
The PCR products for the extracted DNA from 12 isolates resulted in (100%) positive results, and when they were seen under a UV light source and compared to a DNA ladder, they showed a single band at a location of 1500bp on an agarose gel. Using the Basic Local Alignment Search Tool (BLAST), 7 (87.5%) strains of S. aureus from 12 isolates were found. These strains were then compared to a reference strain from Gene Bank (Table 1), which was the same strain. These include the S. aureus strains pak2 (n=5), MR30 (n=3), CFSAN007896 (n=3), and ST4 (n=1).
Table 1. Alignments of PCR product for16SrDNA gene (all 100% identical with reference strain)


Results for the mecA, icaA, and pvl genes
Amplification of the mecA and icaA genes produced bands at 310bp and 188bp, respectively, as shown in Figure 1. Nine (75%) of the 12 isolates encode for the pvl S/F gene (Bicomponent proteins), which produced bands at position 433bp.

Figure 1. (a) Electrophoresis of agarose gel (1%) for Universal 16srDNA PCR products at position 1500bp from (1-6), L: (Ladder:250-10000bp); (b) Electrophoresis of agarose gel (1%) from (1-12) specific mecA gene products at position 310bp, L: (Ladder:100-1000bp); (c) Electrophoresis of agarose gel (1%) from (1-12) specific icaA gene products at position 188bp, L: (Ladder:100-1000bp); (d) Electrophoresis of agarose gel (1%) from (1-9) specific pvl S/F gene products at position 433bp, L: (Ladder:100-1000bp)
Discussion
The present work examined CHROMagar as a direct isolation medium for specimen isolation, enabling simple differentiation of bacterial colonies based on color and shape characteristics on CHROMagar. As a result, when mixed pathogens were grown on the medium, the medium's capacity to detect them was enhanced [27, 28]. The collected DNA was amplified with the help of universal 16srDNA primers (F27 and R1492). The primers avoid the loss of any potential or new bacterial strains by amplifying the 16srDNA (16s ribosomal DNA) gene for all bacterial strains [28-29].
Out of 80 patient swab samples, this study found that the prevalence of Staphylococcus aureus was 12 (38.7%), which is consistent with research done in Babylon, Iraq [30] and Bandar Abbas, southern Iran [31]. Additionally, this study's prevalence of MRSA was 100% of mecA in locations where methicillin-resistant S. aureus was found using molecular techniques. This percentage was found in the study, which is greater than the figures found in the United States (29.8%) and Portugal (24.5%) [32, 33].
The widespread and unchecked use of antibiotics in Iraq, which has led to significant levels of multi-drug resistance, is one of the country's biggest problems. Additionally, a lot of patients self-medicate with antibiotics, especially broad-spectrum antibiotics, without consulting a doctor. Therefore, we believe the high prevalence of MRSA in S. aureus isolates is the misuse of antibiotics, improper sterilization techniques, or the use of incorrect sterilizers to treat ulcerated wounds. Any diabetic foot infection treated with MRSA will likely lead to increased resistance and medical expenses. All 12 MRSA isolates formed biofilm (100%) after samples underwent icaA and pvl S/F gene amplification, and 9 (75%) of them produced the cytotoxin (PVL), both of which are crucial for MRSA pathogenicity. The production and secretion of glycocalyx are necessary for S. aureus to be harmful, and the neutrophil response is the first line of defense against S. aureus infection for strains derived from DFUs. Polysaccharide synthesis starts soon after attachment and starts to cover the bacteria. This is a crucial step in forming a biofilm that delays wound healing and increases biofilm thickness, especially in diabetic patients. Avoiding immune response by producing capsules or slime layers that conceal it and inhibiting phagocyte death after ingestion [7, 34, 35].
In addition to biofilm formation, S. aureus contains a wide range of cytotoxins, which significantly impact the progression and spread of the bacteria in DFUs and potentially result in tissue necrosis [36]. In this investigation, most isolates from DFU patients have icaA and pvl genes. This outcome aligns with research done in France and Iraq [12, 37].
The association between the MRSA strain's icaA and pvl genes and the severe infection of diabetic foot ulcers, however, has not been studied. All MRSA isolated (100%) developed the slime layer that retarded healing. Additionally, (75%) of the 9 MRSA isolated produced the cytotoxin pvl that causes tissue necrosis. We then go on to describe how this virulence might cause foot amputation as a result of disease progression and sluggish wound healing.
Conclusion
Panton-valentine leukocidin has a high prevalence among MRSA strains isolated from diabetic foot ulcer patients that form biofilms, causing patients to have significant inflammation, illness progression, and challenging wound healing, which may result in lower limb amputations.
Acknowledgments: We would like to thank all the patients who allowed me to take the swabs and assistance in conducting this study.
Ethical Permissions: Ethical Permissions were approved by ethical committee, college of Medicine, university of Basrah, Basrah, Iraq.
Conflicts of Interests: Authors are declare that there is no conflict of interest.
Authors’ Contribution: Hussein KA (First Author), Introduction Writer (15%); Madhi KS (Second Author), Methodologist (20%); Al-Mayyahi ZA (Third Author), Assistant Researcher (20%); Al-Ammar NA (Fourth Author), Main Researcher (20%); Abbas BA (Fifth Author), Discussion Writer (15%); Faraj AH (Sixth Author), Statistical Analyst (10%)
Funding/Support: The project is self funded.
Keywords:
References
1. Spichler A, Hurwitz BL, Armstrong DG, Lipsky BA. Microbiology of diabetic foot infections: from Louis Pasteur to crime scene investigation. BMC Med. 2015;13:1-13. [Link] [DOI:10.1186/s12916-014-0232-0]
2. Mottola C, Matias CS, Mendes JJ, Melo-Cristino J, Tavares L, Cavaco-Silva P, Oliveira M. Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections. BMC Microbiol. 2016;16:1-9. [Link] [DOI:10.1186/s12866-016-0737-0]
3. Richard J-L, Lavigne J-P, Got I, et al. Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study. Diabetes Metab. 2011;37(3):208-15. [Link] [DOI:10.1016/j.diabet.2010.10.003]
4. Bhowmik D, Chetri S, Das BJ, Chanda DD, Bhattacharjee A. Distribution of virulence genes and SCC mec types among methicillin-resistant Staphylococcus aureus of clinical and environmental origin: A study from community of Assam, India. BMC Res Notes. 2021;14(1):58. [Link] [DOI:10.1186/s13104-021-05473-3]
5. Heravi FS, Zakrzewski M, Vickery K, Armstrong DG, Hu H. Bacterial diversity of diabetic foot ulcers: Current status and future prospectives. J Clin Med. 2019;8(11):1935. [Link] [DOI:10.3390/jcm8111935]
6. Gad GFM, El-Feky MA, El-Rehewy MS, Hassan MA, Abolella H, Abd El-Baky RM. Detection of icaA, icaD genes and biofilm production by Staphylococcus aureus and Staphylococcus epidermidis isolated from urinary tract catheterized patients. J Infect Dev Ctries. 20149;3(05):342-51. [Link] [DOI:10.3855/jidc.241]
7. Pouget C, Dunyach-Remy C, Pantel A, Schuldiner S, Sotto A, Lavigne JP. Biofilms in diabetic foot ulcers: Significance and clinical relevance. Microorganisms. 2020;8(10):1580. [Link] [DOI:10.3390/microorganisms8101580]
8. Chang M, Nguyen TT. Strategy for treatment of infected diabetic foot ulcers. Acc Chem Res. 2021;54(5):1080-93. [Link] [DOI:10.1021/acs.accounts.0c00864]
9. Nandhini P, Kumar P, Mickymaray S, Alothaim AS, Somasundaram J, Rajan M. Recent developments in methicillin-resistant Staphylococcus aureus (MRSA) treatment: a review. Antibiotics. 2022;11(5):606. [Link] [DOI:10.3390/antibiotics11050606]
10. Hefni AA, Ibrahim AR, Attia KM, Moawad MM, El-ramah AF, Shahin MM, et al. Bacteriological study of diabetic foot infection in Egypt. J Arab Soc Med Res. 2018;8(1):26-32. [Link] [DOI:10.4103/1687-4293.132774]
11. Kareem KA, Shihab LAW. Genetic Study for icaAD gene to Staphylococcus aureus isolated from different part of computer. Int J Adv Res Comput Sci Software Eng. 2016;6(1):97-100. [Link]
12. Alhilfi WAH, Al-Tameemi KAH, Alasadi ITF, Alnajafe MTJ. Isolation and characterization of some clinical bacterial strains as a biofilm producers. J Pharm Sci Res. 2019;11(2):380-6. [Link]
13. Liang Y, Tu C, Tan C, El-Sayed Ahmed M, Dai M, Xia Y, et al. Antimicrobial resistance, virulence genes profiling and molecular relatedness of methicillin-resistant Staphylococcus aureus strains isolated from hospitalized patients in Guangdong Province, China. Infect Drug Resist. 2019;12:447-59. [Link] [DOI:10.2147/IDR.S192611]
14. Quadri SA, Al-Sultan AA, Al-Ramdan AM, Badger-Emeka LI, Ali SI. Frequency of panton-valentine leukocidin gene among clinical isolates of methicillin-resistant Staphylococcus aureus in Eastern Province of Saudi Arabia. J Glob Infect Dis. 2020;12(1):37-8. [Link] [DOI:10.4103/jgid.jgid_27_19]
15. Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. J Antimicrob Chemother. 2000;44(6):1549-55. [Link] [DOI:10.1128/AAC.44.6.1549-1555.2000]
16. Ayliffe GA. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1997;24(1):74-9. [Link] [DOI:10.1093/clinids/24.Supplement_1.S74]
17. Mickymaray S, Alturaiki W, Al-Aboody MS, Mariappan P, Rajenderan V, Alsagaby SA, Kalyanasundram U, Alarfajj AA. Anti-bacterial efficacy of bacteriocin produced by marine Bacillus subtilis against clinically important extended spectrum beta-lactamase strains and methicillin-resistant Staphylococcus aureus. Int J Med Res Health Sci. 2018;7(2):75-83. [Link]
18. Kamel Z, Helmy NA, Soliman MK, Mashahit MA. Impact of biofilm production in methicillin resistant Staphylococcus aureus among diabetic foot patient. Egypt J Med Microbiol. 2018;27(2):93-8. [Link] [DOI:10.21608/ejmm.2018.285547]
19. Lister JL, Horswill AR. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol. 2014;4:178. [Link] [DOI:10.3389/fcimb.2014.00178]
20. Kaplan JB. Antibiotic-induced biofilm formation. Int J Artif Organs. 2011;34:737-51. [Link] [DOI:10.5301/ijao.5000027]
21. Al-Tameemi KAH. Colonization of pathogenic microbes on contaminated nebulizer devices for respiratory tract diseases at emergency department in hospitals. Int J Curr Res. 2018;8(14):13-6. [Link]
22. Freeman DJ, Falkner FR, Keane CT. New method for detecting slime production by coagulase-negative staphylococci. J Clin Pathol. 1989;42(8):872-4. [Link] [DOI:10.1136/jcp.42.8.872]
23. Miyoshi T, Iwatsuki T, Naganuma T. Phylogenetic characterization of 16S rRNA gene clones from deep-groundwater microorganisms that pass through 0.2-micrometer-pore-size filters. Appl Environ Microbiol. 2005;71(2):1084-8. [Link] [DOI:10.1128/AEM.71.2.1084-1088.2005]
24. Eed EM, Ghonaim MM, Hussein YM, Al-Shehri SS, Khalifa AS. Molecular characterisation of Panton-Valentine leucocidin-producing methicillin-resistant Staphylococcus aureus clones isolated from the main hospitals in Taif, KSA. Indian J Med Microbiol. 2016;34(4):476-82. [Link] [DOI:10.4103/0255-0857.195364]
25. Zhang K, Sparling J, Chow BL, Elsayed S, Hussain Z, Church DL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42(11);4947-55. [Link] [DOI:10.1128/JCM.42.11.4947-4955.2004]
26. Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001;39(6):2151-6. [Link] [DOI:10.1128/JCM.39.6.2151-2156.2001]
27. Mamdoh H, Hassanein KM, Eltoony LF, Khalifa WA, Hamed E, Alshammari TO, et al. Clinical and bacteriological analyses of biofilm-forming Staphylococci isolated from diabetic foot ulcers. Infect Drug Resist. 2023;16:1737-50. [Link] [DOI:10.2147/IDR.S393724]
28. Mellmann A, Backer K, Fiff C, Keckevoet U, Schumann P, Harmsen D. Sequencing and Staphylococci Identification. Emerg Infect Dis. 2005;12(2):333-6. [Link] [DOI:10.3201/eid1202.050962]
29. Ibraheim HK, Madhi KS, Baqer GK, Gharban HA. Effectiveness of raw bacteriocin produced from lactic acid bacteria on biofilm of methicillin-resistant Staphylococcus aureus. Veterinary World. 2023;16(3):491-9. [Link] [DOI:10.14202/vetworld.2023.491-499]
30. Al-Allak MH, Al-Khafaji NS, Al-Dahmoshi HO. Microbial and resistance profile among diabetic foot-infections. Drug Invention Today. 2019;13(2):1953-7. [Link]
31. Ahmadishooli A, Davoodian P, Shoja S, Ahmadishooli B, Dadvand H, Hamadiyan H, et al. Frequency and antimicrobial susceptibility patterns of diabetic foot infection of patients from Bandar Abbas District, Southern Iran. J Pathog. 2020;2020:1057167. [Link] [DOI:10.1155/2020/1057167]
32. Mendes JJ, Marques-Costa A, Vilela C, Neves J, Candeias N, Cavaco-Silva P, Melo-Cristino J. Clinical and bacteriological survey of diabetic foot infections in Lisbon. Diabetes Res Clin Pract. 2012;95(1):153-61. [Link] [DOI:10.1016/j.diabres.2011.10.001]
33. Lavery LA, Fontaine JL, Bhavan K, Kim PJ, Williams JR, Hunt NA. Risk factors for methicillin-resistant Staphylococcus aureus in diabetic foot infections. Diabet Foot Ankle. 2014;5(1):23575. [Link] [DOI:10.3402/dfa.v5.23575]
34. Ray RR, Lahiri D, Nag M, Dey S, Dutta B. Phytocompounds of Curcuma longa extract are more effective against bacterial biofilm than pure curcumin only-an in-vitro and in-silico analysis. Kuwait J Sci. 2021;48(2):8310. [Link] [DOI:10.48129/kjs.v48i2.8310]
35. Dashti NH, Al-Sarraf NYA, Cherian VM, Montasser MS. Isolation and characterization of novel plant growth-promoting rhizobacteria (PGPR)isolates from tomato (Solanum lycopersicum L.) rhizospherical soil: A novel IAA producing bacteria. Kuwait J Sci. 2021;48(2):8427. [Link] [DOI:10.48129/kjs.v48i2.8427]
36. Ambrosch A, Haefner S, Jude E, Lobmann R. Diabetic foot infections: microbiological aspects, current and future antibiotic therapy focusing on methicillin‐resistant Staphylococcus aureus. Int Wound J. 2011;8(6):567-77. [Link] [DOI:10.1111/j.1742-481X.2011.00849.x]
37. Naas T, Fortineau N, Spicq C, Robert J, Jarlier V, Nordmann P. Three-year survey of community-acquired methicillin-resistant Staphylococcus aureus producing Panton-Valentine leukocidin in a French university hospital. J Hosp Infect. 2005;61(4):321-9. [Link] [DOI:10.1016/j.jhin.2005.01.027]









