Volume 15, Issue 2 (2023)
Iran J War Public Health 2023, 15(2): 167-175 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/04/13 | Accepted: 2023/05/23 | Published: 2023/06/10
Received: 2023/04/13 | Accepted: 2023/05/23 | Published: 2023/06/10
How to cite this article
Abdulhafedh H, Al-Saadoon A, Abu-Mejdad N. Efficiency of Fungal β-carotene Against Some Causative Agents of Dermatomycoses. Iran J War Public Health 2023; 15 (2) :167-175
URL: http://ijwph.ir/article-1-1339-en.html
URL: http://ijwph.ir/article-1-1339-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Biology, College of Science, University of Basrah, Basrah, Iraq
2- Department of Pathological Analyses, College of Science, University of Basrah. Basrah, Iraq
2- Department of Pathological Analyses, College of Science, University of Basrah. Basrah, Iraq
Full-Text (HTML) (1836 Views)
Introduction
Dermatomycoses are infections of the skin, hair and nails, which are caused in most cases by dermatophytes, and in rarer cases by yeasts and molds. Fungal skin infections have been recognized as the fourth most common health disorder globally (after dental caries, tension‐type headaches and migraine) [1], and dermatophyte infections of the skin, hair and nails are the most common fungal infections [2].
Dermatomycosis is characterized by both superficial and subcutaneous infections of keratinous tissues and mucous membranes caused by a variety of fungal agents, the two most common classes being dermatophytes and yeasts. Overall, the stepwise process of host infection is similar among the main dermatomycotic species; however, the species-specific ability to elicit a host reaction upon infection is distinct. Yeasts such as Candida albicans elicit a relatively low level of host tissue damage and inflammation during pathogenic infection, while dermatophytes may induce a higher level of tissue damage and inflammatory reaction. Both pathogens can, however, manipulate the host's immune response, ensuring survival and prolonging chronic infection [3].
The genus Candida includes about 200 different species, but only a few species are human opportunistic pathogens and cause infections when the host becomes debilitated or immunocompromised. Candida infections can be superficial or invasive. Superficial infections often affect the skin or mucous membranes and can be treated successfully with topical antifungal drugs. However, invasive fungal infections are often life-threatening, probably due to inefficient diagnostic methods and inappropriate initial antifungal therapies [4].
Although the antifungal drugs used in clinical treatments appear to be diverse and numerous, only a few classes of antifungal agents are currently available to treat infections with Candida spp. [5, 6].
The emergence of antifungal resistance, with an increasing number of reports of difficult-to-treat infections [7-9], has been highlighted as an issue of growing concern. Antifungal resistance is based on different mechanisms, namely, (i) reduced drug intracellular accumulation, (ii) decreased target affinity/processivity for the drug, and (iii) counteraction of the drug effect. Particularly, the mechanism of resistance will be different depending on the mode of action of antifungal compounds [10].
Fungal infections caused by Candida spp. are deemed increasingly challenging in human medicine, despite the availability of anti-fungal medications [11]. The antifungal medicine effect is not a new problem, even among persons who did not expose to antifungal medicine, it is reported the resistance of unsaturated hydrocarbon carotenoid dissolved in ether petroleum [12].
Pigments are colorants that are produced from plants, animals, and microorganisms and can also be synthesized by chemicals synthetically. The pigments have many applications in industries (food, textile, paper, cosmetics, plastic, paint) [13], agriculture, biology (antibiotics, antimicrobial agents, anticancer agents) [14, 15], etc.
Nowadays, pigments produced from living organisms gained more importance as synthetic pigments are toxic and show harmful effects [16]. The microbial productions of pigments are in many ways superior to the pigments produced from the plants and animal sources due to several reasons such as their rapid growth rate, easy downstream processing, cost-effectiveness, independent of season and geographical conditions, controllable, more stable and safe to use [17, 18]. Microbial pigments can be produced from bacteria, algae, fungi, and protozoa.
Carotenoids are a large group of organic, pigmented, isoprenoid-type compounds that play biological activities in plants and microorganisms (yeasts, bacteria, and microalgae). Carotenoids also can act as antimicrobial agents, and few reports showed quantitative measurements of Minimal Inhibitory Concentrations against different pathogens. The demand for scale-up of different naturally obtained carotenoids has increased due to the concern about the detrimental health effects caused by synthetic molecules and antimicrobial resistance [19].
Carotenoid is produced in nature from different microbial and plant sources such as bacteria, molds, yeasts, and fungi [20, 21]. This pigment is applied in different fields so that it come very interested [22]. In the past years, the interest in this pigment has increased due to its many benefits and importance for human health because it is a source of vitamin A in the body and it is converted into a vitamin in the intestine, which is very necessary to maintain vision. It is also anti-tumor and anti-oxidation and protects the body against free radical initiators [23], and strengthens the immune system [24].
The production of carotenoids from plants has become limited due to the huge cost of production in comparison with revenues. Therefore, many researches and studies are conducted to obtain carotenoids from microorganisms, which have become an alternative source instead of plants due to their low production cost and limited impact on the environment [25]. In addition, carotenoids produced by these microorganisms are never affected by the seasonal and geographical changes that plants face [26].
Yeasts, due to their nature, are the most applied for carotenoid production compared to other microorganisms, as they are unicellular organisms in addition to overgrowth. Yeasts that produce carotenoids are Rhodotorula, sporpbolomyces, Phaffia, Cryptococcus, and Rhodosporidium [27].
There are few studies on the antifungal activity of β-carotene. According to our knowledge, this is the first study in Iraq on extracting carotenoids from yeasts and testing their activity.
This study aimed to extract and purify β-carotenoid from two types of Rhodotorula diobovata and Rhodotorula mucilaginosa and test its reactivity toward some yeasts isolated from dermatomycoses infection.
Materials and Methods
Sampling
Six soil samples were collected from the extreme environments inside and outside Basra Governorate, including Jabal Sanam (Safwan Hill), Sawa Lake, Al-Faw Sediment, Al-Nasiriyah Desert, and Sulfur Lake in Heet. The samples were collected using a clean spatula after removing the surface layer of the soil to a depth of about 5-10cm and from several places so that the weight of each sample reached 500g. The samples were diluted in concentrations of 0.1, 0.01, and 0.001g/ml and cultured on Potato Dextrose Agar (PDA) medium at 25°C for seven days. After that, pigmented colonies of yeasts were isolated in pure cultures and apparently diagnosed [28].
Genetic diagnosis
The genetic diagnosis was performed in the genome extraction steps using the Presto Mini gDNA Yeast Kit (Geneaid; South Korea). ITS region was amplified using universal primers ITS1 and ITS4 using thermal Cycler (Bioneer Corporation; South Korea). The total volume of 25µl consisted of 3µl DNA Form, 1µl F. Primer, 1µl R. Primer, 12.5µl Mastermix, and 7.5µl Nuclease free water.
The PCR program was as follows: 94°C for 3min, followed by 35 cycles at 94°C for 45s, 52°C for 1min, 72°C for 1min, with a delay at 72°C for 7min.
PCR products were analyzed by 2% agarose gel electrophoresis alongside DNA Ladder (100 bp) after RedSafe™ staining. Sanger gene sequencing was performed at Macrogen (South Korea). A homology search was conducted using the Basic Local Alignment Search Tool (BLAST) program that is available at NCBI (online at https://www.ncbi.nlm.nih.gov), and the sequences saved at the Gene bank under Code No. LC472129.1 and LC463094.1.
Culturing the samples and extraction of pigments
Yeasts were cultured on Potato Dextrose Agar (PDA) and incubated at 25°C for five days. Then 6.5g of the grown yeast was collected using a sterilized blade and placed on a 500ml glass beaker containing 250ml of acetone and 2g of glass balls with a magnetic rod for 36 hours on a magnetic motor to destroy walls of cells and release carotenoids. After that, destroyed cells were rejected using a centrifugal device. The settlement was neglected, and the filtrate was placed on a separatory funnel, and a 50:50 mixture of ether and methanol with a small amount of NaCl was added. The separatory funnel was strongly stirred for two minutes to form two polar and non-polar layers. The upper layer contained ether and carotenoid, and the lower layer was ignored. The upper layer was evaporated using glass dishes, and the product was kept in opaque bottles [29].
Total purification
In this method, a glass column was used whose dimensions were 30x30mm. In the end, there was glass wool, the height of which was 2.5cm. After that, the column was filled with mesh emulsion, silica gel 100-200µ, and a mixture of ether and acetone (200:800, respectively). 0.25g of carotenoid was dissolved in 1mL of distilled water and then smoothly added to the surface of the mesh, silica gel, evenly. After that, the mixture was added to the column until the end of the separation process with some modifications [30]. The amount of runoff at the column was 1ml/20sec, and separated patterns were received and collected from the end of the column through 1ml test tubes. Then components of each one were selected using a thin-layer chromatography technique as they were tested and exposed to Iodine steam [31]. Similar products were collected together.
Beta-carotene diagnosis
Spectroscopy UV absorption
Spectroscopy UV absorption, the measurement of vision, and UT regions are important tools to specify pigments, including carotenoids. These spectra were registered in the vision and UT regions using Heliosαv4.60U.V-visible Spectrophotometer, England, in the regions ranging from 200 cm-1 to 600 cm-1 [32].
Infrared spectra (IR)
IR spectra were registered applying KBr discs technology in the regions ranged from 400 cm-1 to 4000 cm-1 using Fourier Transform Infrared Spectrophotometer (FTIR)-84005 (Shimadzu; Japan).
Antifungal activity of β-carotene
• Beta-carotene activity was investigated in four isolated yeasts: Candida albicans HAM25, Candida dubliniensis, Candida tropicales HAM13, and Cutaneotrichosporon dermatis Judy 4.
Yeasts were isolated from superficial fungal infections in the Fungal Research Laboratory, College of Science, University of Basra University, and kept in the gene bank with numbers LC722508.1, LC722507.1, LC7261991, and LC731319.1. Then they were activated in PDA medium at 35±2°C for 24 hours, and a suspension of grown fungi was prepared according to McFarland's second standard with a cell concentration of 6x106 [33].
• The agar diffusion method was applied to evaluate the activity of β-carotene. 0.1ml of cell suspension (prepared in the first step) was diffused on a PDA medium. Three concentrations of β-carotene including 0.01, 0.02, and 0.03mg/ml, and volume 300µl at the same concentrations and volumes of Nystatin as a standard compound and by three repetitions per each isolate with each concentration were used and then incubated at 37°C for 48h [34].
Toxicity
The toxicity of purified β-carotene was tested on two red blood cell isolates in vitro with a concentration of 0.03mg/ml, and a volume of 200µl per isolate was added to 800µl of blood separately. The tubes were then incubated at 37°C for 24h, with phosphate-buffered saline as the positive control and tap water as the negative control, according to the technique of Nair et al. [35], with some changes.
Findings
There was little difference between R. diobovata and R. mucilaginosa isolates, with filter weights of 0.27g for R. diobovata and 0.15g for R. mucilaginosa, while the total purification product for R. diobovata extraction was 0.2g. One spot of purified β-carotene collected from tube 58-121 with Relative Flow (RF) on thin-layer chromatography was about 0.93, while the product of total purification of the extract of R. mucilaginosa was 0.1g. Purified β-carotene from one spot of tube 58-112 of relative flow was equal to ca.0.92. Upon verifying the present of β-carotene, thin-layer chromatography techniques were applied, and measured RF was equal to ca. standard RF 0.95, using movable phase 800 petroleum ether: 200 acetone.
Analysis of produced β-carotene
The pigment demonstrated maximum absorbance at 438,386nm for R. diobovata and R. mucilaginosa (Figures 1 & 2).
In addition, FT-IR showed the types of pigments similar to the chromatogram that are well-known as standard carotenes (Figures 3 & 4).

Figure 1) UV spectrum of β‐carotene purified from R. diobovata isolate
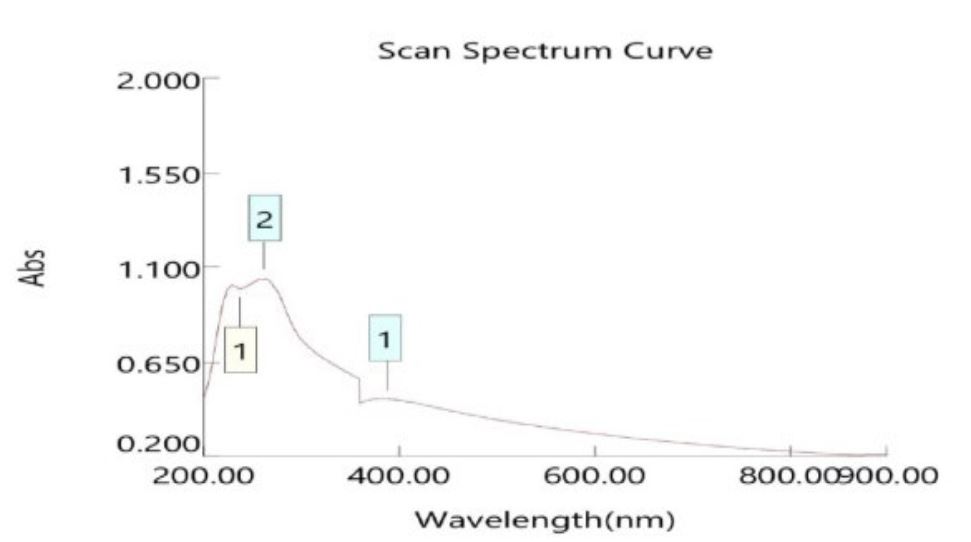
Figure 2) UV spectrum of β‐carotene purified from R. mucilaginosa isolate

Figure 3) Infrared spectrum of β‐carotene purified from R. diobovata isolate
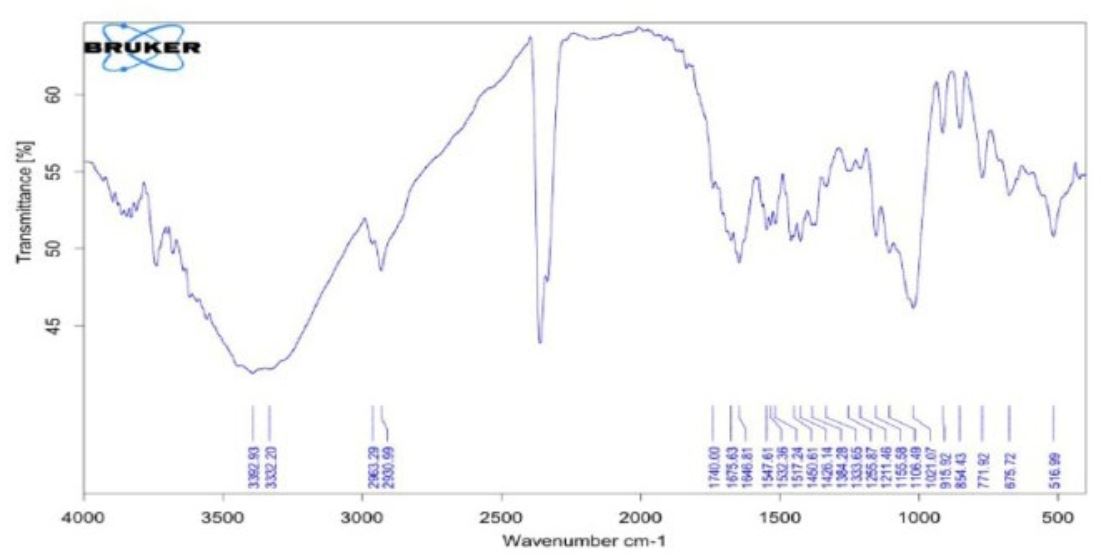
Figure 4) Infrared spectrum of β‐carotene purified from R. mucilaginosa isolate
Table 1) Average inhibition diameters (mm) of β‐carotene purified from R. diobovata (R1) against four yeast isolates at three different concentrations and compared with the standard compound Nystatin after 48h

Table 2) Average inhibition diameters (mm) of β‐carotene purified from R. mucilaginosa (R2) against four yeast isolates at three different concentrations, compared to the standard compound of Nystatin after 48h

Beta-carotene activity
All tested isolates, including Candida albicans HAM25, Candida dubliniensis, Candida tropicales HAM13, and Cutaneotrichosporon dermatis Judy 4, showed resistance against β-carotene purified from R. diobovata (R1) and Nystatin at the concentration 0.01mg/ml. However, β-carotene and Nystatin showed activity against all isolates with concentrations of 0.02 and 0.03mg/ml. The purified compound from R. mucilaginosa (R2) showed activity against all isolates in three concentrations, according to concentration and type. The highest amount was related to Candida albicans HAM 25, and the lowest was related to Cutaneotrichosporon dermatis Judy 4. There was a significant difference between the antifungal activity of both purified β-carotene and Nystatin (p<0.0001; Tables 1 & 2; Figures 5 & 6).
Toxicity of β-carotene
Purified β-carotene from two isolates showed no obvious changes or decay in blood cells in vitro.
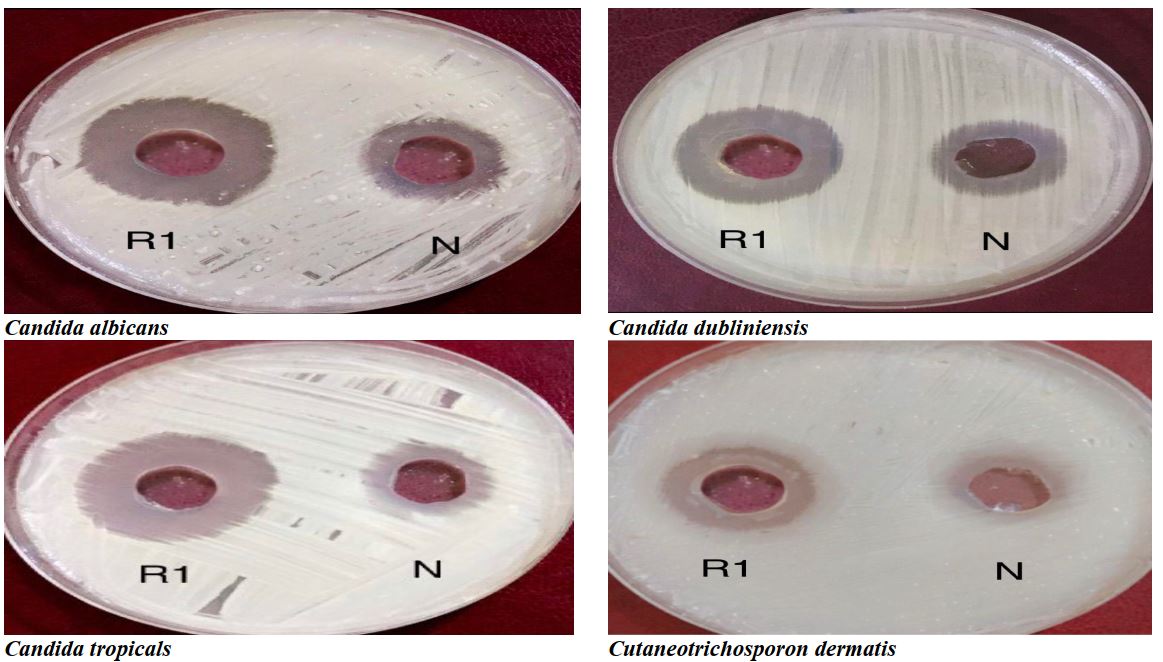
Figure 5) Antifungal activity of β‐carotene purified from R. diobovata (R1) and nystatin (N) against Candida albicans, C. dubliniensis, C. tropicalis, and Cutaneotrichosporon dermatis

Figure 6) Antifungal activity of β‐carotene purified from R. mucilaginosa (R2) and nystatin (N) against Candida albicans, C. dubliniensis, C. tropicalis, and Cutaneotrichosporon dermatis
Discussion
Dermatomycoses are a common category of fungal infections affecting the skin and subcutaneous tissues. Recent advances in topical and systemic antifungal chemotherapy have provided us with satisfactory results in most superficial fungal infections, both clinically and mycologically. Despite the efficacy of antifungal agents, however, some dermatomycoses do not respond to our current standard regimens of treatment [36]. These infections have become recalcitrant to treatment which can be due to antifungal resistance [37].
Carotenoids are yellow, orange, and red pigments that are found in several animals, plants, and microorganisms [38]. Recent studies have shown that some of these pigments have important biological functions such as antibiotic, antifungal, and antitumor activities, and many of them have potential chemotherapeutic effects [39]. Carotenoid production from yeasts has been considered a safe alternative to chemically synthesized carotenoids since plant-based carotenoids are expensive and an irregular source for obtaining pigments [40].
This study aimed to extract and purify β-carotenoid from two types of Rhodotorula diobovata and Rhodotorula mucilaginosa and test its reactivity toward some yeasts isolated from dermatomycoses infection.
The results of the present study stated that there was little difference between R. diobovata and R. mucilaginosa isolates, with filter weights of 0.27g for R. diobovata and 0.15g for R. mucilaginosa, while the total purification product for R. diobovata extraction was 0.2g. One spot of purified β-carotene collected from tube 58-121 of RF on thin-layer chromatography was about 0.93, while the product of total purification of the extract of R. mucilaginosa was 0.1g. Purified β-carotene from one spot of tube 58-112 with relative flow was equal to ca.0.92. Upon verifying the present of β-carotene, thin-layer chromatography techniques were applied, and measured RF was equal to ca. standard RF 0.95, using movable phase 800 petroleum ether: 200 acetone.
This result complies with the findings of Latha & Jeebaratnam [31], who used a thin-layer chromatography technique and the same movable phase to obtain β-carotene from R. glutinis upon the same runoff rate of 0.92. Zeb & Murkovic [41] reported that petroleum ether, acetone, and Hexane are the main applied movable phases in the thin-layer chromatography technique, and it is proven that this mode is eligible to be the first choice to analysis carotenes in the biological samples.
The pigment demonstrated maximum absorbance at 438,386nm for R. diobovata and R. mucilaginosa. The maximum absorbance for the pigment is stated between 300-600nm, indicating the presence of carotenes [42]. Also, Madhukar stated that the maximum absorbance of β-carotene is 425-430nm and 470-489nm [43]. However, according to Finkel’shtein's study [44], most carotene pigments have maximum absorbance ranging between 375-505 nm.
According to the results of the present study, β-carotene of purified extracts had high activity against four yeast isolates, including C. albicans HAM25, C. dubliniensis, C. tropicals HAM13, and Cutaneotrichosporon dermatis Judy 4 on.
The activity of β-carotene has never been studied before except on C. albicans compared to echinocandins [45]. The results showed that with the increase in the concentration of β-carotene, its activity against all different isolates increases, so the combination of β-carotene has more antigenic activity than the standard antifungal nystatin, which may be the reason for its significant use.
Several studies reported that the carotenoids synthesized by yeasts of the Rhodotorula and Sporobolomyces genera show strong anti-microbial activity [46-49].
Manimala et al. [50] studied the activity of β-carotene produced from R. mucilaginosa YP 197 Yeast and stated that β-carotene shows anti-bacteria activity more than standard Chloramphenicol, and the most activity is appeared against Bacillus subtills and Staphylococcus aureus.
Manimala & Murugesan [46] reported similar action in carotene pigment extracted from Sporobolomyces sp. isolated from natural sources and stated that negative gram bacteria has higher level of resistance against β-carotene. Also, Madhukar [43] reported the antifungal activity of β-carotene pigment against some pathological fungi attack plants such as Aspergillus niger NCIM 1025, Penicillium chrusogenum NCIM 709, and Fusarium oxysposrum NCIM 1281, which its activity was at two concentrations of 50µl and 100µl.
The importance of β-carotene in inhibiting the growth of some pathological microorganisms, such as bacteria and fungi, is because it dissolves in the lipids of the protoplasm membrane and then increases its penetration through the membrane and stops the growth or kills the fungi.
Yoo et al. showed the antibacterial activity of glucosidal carotenoids from the yeast R. mucilaginosa AY-01 toward antibiotic-resistant bacteria isolated from the porcine semen [51]. In addition, the carotenoid contents of Rhodotorula glutinis M29 strain (1.07 mg L−1) showed antibacterial activity against a range of bacteria, e.g., Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Salmonella enteritidis, and Escherichia coli at 103CFU/mL [47].
Keceli et al. concluded that carotenoids extracted from some strains of Rhodotorula glutinis (M21, 22, 24, 28, 29, 30, 31, 35, 37, 39, 40, 42, and 47) can effectively be used to obtain both antioxidant and antibacterial effect. These strains can be isolated best from tree leaves, parsley, and carrots, and these strains produce high-quality carotenoids to be used as preservatives. The antioxidant or antibacterial effect of carotenoids significantly depends on source of isolation and carotenoid composition rather than carotenoid content [47].
Finally, the present study investigated the cellular toxicity of β-carotene. The cellular toxicity test is the most practical test used for bioassay and evaluation of blood cells, which observes the apparent effects of cells in the laboratory. In the present study, the purified β-carotene from two isolates showed no obvious changes or decay in blood cells in vitro, which is consistent with the findings of Burton et al. [52].
Conclusion
Both isolates of Rhodotorula diobovata and Rhodotorula mucilaginosa produce β-carotene, and the preference quantitatively is to isolate R. diobovata first. The activity of β-carotene against all tested yeast isolates are higher than the antifungal Nystatin. The non-toxicity of β-carotene was proven in the blood decay test in vitro.
Acknowledgements: Nothing reported by the authors.
Ethical Permission: Nothing reported by the authors.
Conflict of Interests: Nothing reported by the authors.
Authors’ Contribution: Abdulhafedh HM (First Author), Introduction Writer/Methodologist/Main Researcher/Statistical Analyst/Discussion Writer (40%); Al-Saadoon AH (Second Author), Introduction Writer/Methodologist/Main Researcher/Statistical Analyst/Discussion Writer (30%); Abu-Mejdad NM (Third Author), Methodologist/Main Researcher/Statistical Analyst/Discussion Writer (30%)
Funding: This study was self-funded.
Dermatomycoses are infections of the skin, hair and nails, which are caused in most cases by dermatophytes, and in rarer cases by yeasts and molds. Fungal skin infections have been recognized as the fourth most common health disorder globally (after dental caries, tension‐type headaches and migraine) [1], and dermatophyte infections of the skin, hair and nails are the most common fungal infections [2].
Dermatomycosis is characterized by both superficial and subcutaneous infections of keratinous tissues and mucous membranes caused by a variety of fungal agents, the two most common classes being dermatophytes and yeasts. Overall, the stepwise process of host infection is similar among the main dermatomycotic species; however, the species-specific ability to elicit a host reaction upon infection is distinct. Yeasts such as Candida albicans elicit a relatively low level of host tissue damage and inflammation during pathogenic infection, while dermatophytes may induce a higher level of tissue damage and inflammatory reaction. Both pathogens can, however, manipulate the host's immune response, ensuring survival and prolonging chronic infection [3].
The genus Candida includes about 200 different species, but only a few species are human opportunistic pathogens and cause infections when the host becomes debilitated or immunocompromised. Candida infections can be superficial or invasive. Superficial infections often affect the skin or mucous membranes and can be treated successfully with topical antifungal drugs. However, invasive fungal infections are often life-threatening, probably due to inefficient diagnostic methods and inappropriate initial antifungal therapies [4].
Although the antifungal drugs used in clinical treatments appear to be diverse and numerous, only a few classes of antifungal agents are currently available to treat infections with Candida spp. [5, 6].
The emergence of antifungal resistance, with an increasing number of reports of difficult-to-treat infections [7-9], has been highlighted as an issue of growing concern. Antifungal resistance is based on different mechanisms, namely, (i) reduced drug intracellular accumulation, (ii) decreased target affinity/processivity for the drug, and (iii) counteraction of the drug effect. Particularly, the mechanism of resistance will be different depending on the mode of action of antifungal compounds [10].
Fungal infections caused by Candida spp. are deemed increasingly challenging in human medicine, despite the availability of anti-fungal medications [11]. The antifungal medicine effect is not a new problem, even among persons who did not expose to antifungal medicine, it is reported the resistance of unsaturated hydrocarbon carotenoid dissolved in ether petroleum [12].
Pigments are colorants that are produced from plants, animals, and microorganisms and can also be synthesized by chemicals synthetically. The pigments have many applications in industries (food, textile, paper, cosmetics, plastic, paint) [13], agriculture, biology (antibiotics, antimicrobial agents, anticancer agents) [14, 15], etc.
Nowadays, pigments produced from living organisms gained more importance as synthetic pigments are toxic and show harmful effects [16]. The microbial productions of pigments are in many ways superior to the pigments produced from the plants and animal sources due to several reasons such as their rapid growth rate, easy downstream processing, cost-effectiveness, independent of season and geographical conditions, controllable, more stable and safe to use [17, 18]. Microbial pigments can be produced from bacteria, algae, fungi, and protozoa.
Carotenoids are a large group of organic, pigmented, isoprenoid-type compounds that play biological activities in plants and microorganisms (yeasts, bacteria, and microalgae). Carotenoids also can act as antimicrobial agents, and few reports showed quantitative measurements of Minimal Inhibitory Concentrations against different pathogens. The demand for scale-up of different naturally obtained carotenoids has increased due to the concern about the detrimental health effects caused by synthetic molecules and antimicrobial resistance [19].
Carotenoid is produced in nature from different microbial and plant sources such as bacteria, molds, yeasts, and fungi [20, 21]. This pigment is applied in different fields so that it come very interested [22]. In the past years, the interest in this pigment has increased due to its many benefits and importance for human health because it is a source of vitamin A in the body and it is converted into a vitamin in the intestine, which is very necessary to maintain vision. It is also anti-tumor and anti-oxidation and protects the body against free radical initiators [23], and strengthens the immune system [24].
The production of carotenoids from plants has become limited due to the huge cost of production in comparison with revenues. Therefore, many researches and studies are conducted to obtain carotenoids from microorganisms, which have become an alternative source instead of plants due to their low production cost and limited impact on the environment [25]. In addition, carotenoids produced by these microorganisms are never affected by the seasonal and geographical changes that plants face [26].
Yeasts, due to their nature, are the most applied for carotenoid production compared to other microorganisms, as they are unicellular organisms in addition to overgrowth. Yeasts that produce carotenoids are Rhodotorula, sporpbolomyces, Phaffia, Cryptococcus, and Rhodosporidium [27].
There are few studies on the antifungal activity of β-carotene. According to our knowledge, this is the first study in Iraq on extracting carotenoids from yeasts and testing their activity.
This study aimed to extract and purify β-carotenoid from two types of Rhodotorula diobovata and Rhodotorula mucilaginosa and test its reactivity toward some yeasts isolated from dermatomycoses infection.
Materials and Methods
Sampling
Six soil samples were collected from the extreme environments inside and outside Basra Governorate, including Jabal Sanam (Safwan Hill), Sawa Lake, Al-Faw Sediment, Al-Nasiriyah Desert, and Sulfur Lake in Heet. The samples were collected using a clean spatula after removing the surface layer of the soil to a depth of about 5-10cm and from several places so that the weight of each sample reached 500g. The samples were diluted in concentrations of 0.1, 0.01, and 0.001g/ml and cultured on Potato Dextrose Agar (PDA) medium at 25°C for seven days. After that, pigmented colonies of yeasts were isolated in pure cultures and apparently diagnosed [28].
Genetic diagnosis
The genetic diagnosis was performed in the genome extraction steps using the Presto Mini gDNA Yeast Kit (Geneaid; South Korea). ITS region was amplified using universal primers ITS1 and ITS4 using thermal Cycler (Bioneer Corporation; South Korea). The total volume of 25µl consisted of 3µl DNA Form, 1µl F. Primer, 1µl R. Primer, 12.5µl Mastermix, and 7.5µl Nuclease free water.
The PCR program was as follows: 94°C for 3min, followed by 35 cycles at 94°C for 45s, 52°C for 1min, 72°C for 1min, with a delay at 72°C for 7min.
PCR products were analyzed by 2% agarose gel electrophoresis alongside DNA Ladder (100 bp) after RedSafe™ staining. Sanger gene sequencing was performed at Macrogen (South Korea). A homology search was conducted using the Basic Local Alignment Search Tool (BLAST) program that is available at NCBI (online at https://www.ncbi.nlm.nih.gov), and the sequences saved at the Gene bank under Code No. LC472129.1 and LC463094.1.
Culturing the samples and extraction of pigments
Yeasts were cultured on Potato Dextrose Agar (PDA) and incubated at 25°C for five days. Then 6.5g of the grown yeast was collected using a sterilized blade and placed on a 500ml glass beaker containing 250ml of acetone and 2g of glass balls with a magnetic rod for 36 hours on a magnetic motor to destroy walls of cells and release carotenoids. After that, destroyed cells were rejected using a centrifugal device. The settlement was neglected, and the filtrate was placed on a separatory funnel, and a 50:50 mixture of ether and methanol with a small amount of NaCl was added. The separatory funnel was strongly stirred for two minutes to form two polar and non-polar layers. The upper layer contained ether and carotenoid, and the lower layer was ignored. The upper layer was evaporated using glass dishes, and the product was kept in opaque bottles [29].
Total purification
In this method, a glass column was used whose dimensions were 30x30mm. In the end, there was glass wool, the height of which was 2.5cm. After that, the column was filled with mesh emulsion, silica gel 100-200µ, and a mixture of ether and acetone (200:800, respectively). 0.25g of carotenoid was dissolved in 1mL of distilled water and then smoothly added to the surface of the mesh, silica gel, evenly. After that, the mixture was added to the column until the end of the separation process with some modifications [30]. The amount of runoff at the column was 1ml/20sec, and separated patterns were received and collected from the end of the column through 1ml test tubes. Then components of each one were selected using a thin-layer chromatography technique as they were tested and exposed to Iodine steam [31]. Similar products were collected together.
Beta-carotene diagnosis
Spectroscopy UV absorption
Spectroscopy UV absorption, the measurement of vision, and UT regions are important tools to specify pigments, including carotenoids. These spectra were registered in the vision and UT regions using Heliosαv4.60U.V-visible Spectrophotometer, England, in the regions ranging from 200 cm-1 to 600 cm-1 [32].
Infrared spectra (IR)
IR spectra were registered applying KBr discs technology in the regions ranged from 400 cm-1 to 4000 cm-1 using Fourier Transform Infrared Spectrophotometer (FTIR)-84005 (Shimadzu; Japan).
Antifungal activity of β-carotene
• Beta-carotene activity was investigated in four isolated yeasts: Candida albicans HAM25, Candida dubliniensis, Candida tropicales HAM13, and Cutaneotrichosporon dermatis Judy 4.
Yeasts were isolated from superficial fungal infections in the Fungal Research Laboratory, College of Science, University of Basra University, and kept in the gene bank with numbers LC722508.1, LC722507.1, LC7261991, and LC731319.1. Then they were activated in PDA medium at 35±2°C for 24 hours, and a suspension of grown fungi was prepared according to McFarland's second standard with a cell concentration of 6x106 [33].
• The agar diffusion method was applied to evaluate the activity of β-carotene. 0.1ml of cell suspension (prepared in the first step) was diffused on a PDA medium. Three concentrations of β-carotene including 0.01, 0.02, and 0.03mg/ml, and volume 300µl at the same concentrations and volumes of Nystatin as a standard compound and by three repetitions per each isolate with each concentration were used and then incubated at 37°C for 48h [34].
Toxicity
The toxicity of purified β-carotene was tested on two red blood cell isolates in vitro with a concentration of 0.03mg/ml, and a volume of 200µl per isolate was added to 800µl of blood separately. The tubes were then incubated at 37°C for 24h, with phosphate-buffered saline as the positive control and tap water as the negative control, according to the technique of Nair et al. [35], with some changes.
Findings
There was little difference between R. diobovata and R. mucilaginosa isolates, with filter weights of 0.27g for R. diobovata and 0.15g for R. mucilaginosa, while the total purification product for R. diobovata extraction was 0.2g. One spot of purified β-carotene collected from tube 58-121 with Relative Flow (RF) on thin-layer chromatography was about 0.93, while the product of total purification of the extract of R. mucilaginosa was 0.1g. Purified β-carotene from one spot of tube 58-112 of relative flow was equal to ca.0.92. Upon verifying the present of β-carotene, thin-layer chromatography techniques were applied, and measured RF was equal to ca. standard RF 0.95, using movable phase 800 petroleum ether: 200 acetone.
Analysis of produced β-carotene
The pigment demonstrated maximum absorbance at 438,386nm for R. diobovata and R. mucilaginosa (Figures 1 & 2).
In addition, FT-IR showed the types of pigments similar to the chromatogram that are well-known as standard carotenes (Figures 3 & 4).

Figure 1) UV spectrum of β‐carotene purified from R. diobovata isolate

Figure 2) UV spectrum of β‐carotene purified from R. mucilaginosa isolate

Figure 3) Infrared spectrum of β‐carotene purified from R. diobovata isolate

Figure 4) Infrared spectrum of β‐carotene purified from R. mucilaginosa isolate
Table 1) Average inhibition diameters (mm) of β‐carotene purified from R. diobovata (R1) against four yeast isolates at three different concentrations and compared with the standard compound Nystatin after 48h

Table 2) Average inhibition diameters (mm) of β‐carotene purified from R. mucilaginosa (R2) against four yeast isolates at three different concentrations, compared to the standard compound of Nystatin after 48h

Beta-carotene activity
All tested isolates, including Candida albicans HAM25, Candida dubliniensis, Candida tropicales HAM13, and Cutaneotrichosporon dermatis Judy 4, showed resistance against β-carotene purified from R. diobovata (R1) and Nystatin at the concentration 0.01mg/ml. However, β-carotene and Nystatin showed activity against all isolates with concentrations of 0.02 and 0.03mg/ml. The purified compound from R. mucilaginosa (R2) showed activity against all isolates in three concentrations, according to concentration and type. The highest amount was related to Candida albicans HAM 25, and the lowest was related to Cutaneotrichosporon dermatis Judy 4. There was a significant difference between the antifungal activity of both purified β-carotene and Nystatin (p<0.0001; Tables 1 & 2; Figures 5 & 6).
Toxicity of β-carotene
Purified β-carotene from two isolates showed no obvious changes or decay in blood cells in vitro.

Figure 5) Antifungal activity of β‐carotene purified from R. diobovata (R1) and nystatin (N) against Candida albicans, C. dubliniensis, C. tropicalis, and Cutaneotrichosporon dermatis

Figure 6) Antifungal activity of β‐carotene purified from R. mucilaginosa (R2) and nystatin (N) against Candida albicans, C. dubliniensis, C. tropicalis, and Cutaneotrichosporon dermatis
Discussion
Dermatomycoses are a common category of fungal infections affecting the skin and subcutaneous tissues. Recent advances in topical and systemic antifungal chemotherapy have provided us with satisfactory results in most superficial fungal infections, both clinically and mycologically. Despite the efficacy of antifungal agents, however, some dermatomycoses do not respond to our current standard regimens of treatment [36]. These infections have become recalcitrant to treatment which can be due to antifungal resistance [37].
Carotenoids are yellow, orange, and red pigments that are found in several animals, plants, and microorganisms [38]. Recent studies have shown that some of these pigments have important biological functions such as antibiotic, antifungal, and antitumor activities, and many of them have potential chemotherapeutic effects [39]. Carotenoid production from yeasts has been considered a safe alternative to chemically synthesized carotenoids since plant-based carotenoids are expensive and an irregular source for obtaining pigments [40].
This study aimed to extract and purify β-carotenoid from two types of Rhodotorula diobovata and Rhodotorula mucilaginosa and test its reactivity toward some yeasts isolated from dermatomycoses infection.
The results of the present study stated that there was little difference between R. diobovata and R. mucilaginosa isolates, with filter weights of 0.27g for R. diobovata and 0.15g for R. mucilaginosa, while the total purification product for R. diobovata extraction was 0.2g. One spot of purified β-carotene collected from tube 58-121 of RF on thin-layer chromatography was about 0.93, while the product of total purification of the extract of R. mucilaginosa was 0.1g. Purified β-carotene from one spot of tube 58-112 with relative flow was equal to ca.0.92. Upon verifying the present of β-carotene, thin-layer chromatography techniques were applied, and measured RF was equal to ca. standard RF 0.95, using movable phase 800 petroleum ether: 200 acetone.
This result complies with the findings of Latha & Jeebaratnam [31], who used a thin-layer chromatography technique and the same movable phase to obtain β-carotene from R. glutinis upon the same runoff rate of 0.92. Zeb & Murkovic [41] reported that petroleum ether, acetone, and Hexane are the main applied movable phases in the thin-layer chromatography technique, and it is proven that this mode is eligible to be the first choice to analysis carotenes in the biological samples.
The pigment demonstrated maximum absorbance at 438,386nm for R. diobovata and R. mucilaginosa. The maximum absorbance for the pigment is stated between 300-600nm, indicating the presence of carotenes [42]. Also, Madhukar stated that the maximum absorbance of β-carotene is 425-430nm and 470-489nm [43]. However, according to Finkel’shtein's study [44], most carotene pigments have maximum absorbance ranging between 375-505 nm.
According to the results of the present study, β-carotene of purified extracts had high activity against four yeast isolates, including C. albicans HAM25, C. dubliniensis, C. tropicals HAM13, and Cutaneotrichosporon dermatis Judy 4 on.
The activity of β-carotene has never been studied before except on C. albicans compared to echinocandins [45]. The results showed that with the increase in the concentration of β-carotene, its activity against all different isolates increases, so the combination of β-carotene has more antigenic activity than the standard antifungal nystatin, which may be the reason for its significant use.
Several studies reported that the carotenoids synthesized by yeasts of the Rhodotorula and Sporobolomyces genera show strong anti-microbial activity [46-49].
Manimala et al. [50] studied the activity of β-carotene produced from R. mucilaginosa YP 197 Yeast and stated that β-carotene shows anti-bacteria activity more than standard Chloramphenicol, and the most activity is appeared against Bacillus subtills and Staphylococcus aureus.
Manimala & Murugesan [46] reported similar action in carotene pigment extracted from Sporobolomyces sp. isolated from natural sources and stated that negative gram bacteria has higher level of resistance against β-carotene. Also, Madhukar [43] reported the antifungal activity of β-carotene pigment against some pathological fungi attack plants such as Aspergillus niger NCIM 1025, Penicillium chrusogenum NCIM 709, and Fusarium oxysposrum NCIM 1281, which its activity was at two concentrations of 50µl and 100µl.
The importance of β-carotene in inhibiting the growth of some pathological microorganisms, such as bacteria and fungi, is because it dissolves in the lipids of the protoplasm membrane and then increases its penetration through the membrane and stops the growth or kills the fungi.
Yoo et al. showed the antibacterial activity of glucosidal carotenoids from the yeast R. mucilaginosa AY-01 toward antibiotic-resistant bacteria isolated from the porcine semen [51]. In addition, the carotenoid contents of Rhodotorula glutinis M29 strain (1.07 mg L−1) showed antibacterial activity against a range of bacteria, e.g., Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Salmonella enteritidis, and Escherichia coli at 103CFU/mL [47].
Keceli et al. concluded that carotenoids extracted from some strains of Rhodotorula glutinis (M21, 22, 24, 28, 29, 30, 31, 35, 37, 39, 40, 42, and 47) can effectively be used to obtain both antioxidant and antibacterial effect. These strains can be isolated best from tree leaves, parsley, and carrots, and these strains produce high-quality carotenoids to be used as preservatives. The antioxidant or antibacterial effect of carotenoids significantly depends on source of isolation and carotenoid composition rather than carotenoid content [47].
Finally, the present study investigated the cellular toxicity of β-carotene. The cellular toxicity test is the most practical test used for bioassay and evaluation of blood cells, which observes the apparent effects of cells in the laboratory. In the present study, the purified β-carotene from two isolates showed no obvious changes or decay in blood cells in vitro, which is consistent with the findings of Burton et al. [52].
Conclusion
Both isolates of Rhodotorula diobovata and Rhodotorula mucilaginosa produce β-carotene, and the preference quantitatively is to isolate R. diobovata first. The activity of β-carotene against all tested yeast isolates are higher than the antifungal Nystatin. The non-toxicity of β-carotene was proven in the blood decay test in vitro.
Acknowledgements: Nothing reported by the authors.
Ethical Permission: Nothing reported by the authors.
Conflict of Interests: Nothing reported by the authors.
Authors’ Contribution: Abdulhafedh HM (First Author), Introduction Writer/Methodologist/Main Researcher/Statistical Analyst/Discussion Writer (40%); Al-Saadoon AH (Second Author), Introduction Writer/Methodologist/Main Researcher/Statistical Analyst/Discussion Writer (30%); Abu-Mejdad NM (Third Author), Methodologist/Main Researcher/Statistical Analyst/Discussion Writer (30%)
Funding: This study was self-funded.
Keywords:
Dermatomycoses [MeSH], Beta-Carotene [MeSH], Rhodotorula [MeSH], Candida [MeSH], Antifungal Agents [MeSH]
References
1. Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743‐800. [Link]
2. Zhan P, Liu W. The changing face of dermatophytic infections worldwide. Mycopathologia. 2017;182(1-2):77‐86. [Link] [DOI:10.1007/s11046-016-0082-8]
3. Hube B, Hay R, Brasch J, Veraldi S, SchallerM. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J Mycol Med. 2015;25(1):e44-58. [Link] [DOI:10.1016/j.mycmed.2014.11.002]
4. Spampinato C, Leonardi D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed Res Int. 2013;2013:204237. [Link] [DOI:10.1155/2013/204237]
5. Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, et al. The biology and chemistry of antifungal agents: a review. Bioorg Med Chem. 2012;20(19):5678-98. [Link] [DOI:10.1016/j.bmc.2012.04.045]
6. Denning DW, Hope WW. Therapy for fungal diseases: opportunities and priorities. Trend Microbiol. 2010;18(5):195-204. [Link] [DOI:10.1016/j.tim.2010.02.004]
7. Tripathi R, Paudel V, Pradhan M, Pandey BR. Growing burden of dermatophytosis in southern region of Nepal. Br J Dermatol. 2021;185(S1):48. [Link] [DOI:10.1111/bjd.20013]
8. Chen E, Ghannoum M, Elewski BE. Treatment‐resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184(1):164‐5. [Link] [DOI:10.1111/bjd.19420]
9. Halvaee S, Daie‐Ghazvini R, Hashemi SJ, Khodavaisy S, Rahimi-Foroushani A, Bakhshi H, et al. A mycological and molecular epidemiologic study on onychomycosis and determination in vitro susceptibilities of isolated fungal strains to conventional and new antifungals. Front Cell Infect Microbiol. 202;15(11):693522. [Link] [DOI:10.3389/fcimb.2021.693522]
10. Powell J, Porter E, Field S, O'Connell NH, Carty K, Dunne CP. Epidemiology of dermatomycoses and onychomycoses in Ireland (2001-2020): A single‐institution review. Mycoses. 2022;65(7):770-9. [Link] [DOI:10.1111/myc.13473]
11. Mba IE, Nweze EI. Mechanism of Candida pathogenesis: revisiting the vital drivers. Eur J Clin Microbiol Infect Dis. 2020;39(10):1797-819. [Link] [DOI:10.1007/s10096-020-03912-w]
12. Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645-58. [Link] [DOI:10.2147/IDR.S173867]
13. Aberoumand A. A review article on edible pigments properties and sources as natural colorants in foodstuff and food industry. World J Dairy Food Sci. 2011;6(1):71-8. [Link]
14. Ferreira CV, Bos CL, Versteeg HH, Justo GZ, Durán N, Peppelenbosch MP. Molecular mechanism of violacein-mediated human leukemia cell death. Blood. 2004;104(5):1459-67. [Link] [DOI:10.1182/blood-2004-02-0594]
15. Kodach LL, Bos CL, Durán N, Peppelenbosch MP, Ferreira CV, Hardwick JCH. Violacein synergistically increases 5-fluorouracil cytotoxity, induces apoptosis and inhibits Akt-mediated signal transduction in human colorectal cancer cells. Carcinogenesis 2006;27(3):508-16. [Link] [DOI:10.1093/carcin/bgi307]
16. Koyyati R, Kudle KR, Padigya, PRM. Antibacterial, antioxidant and cytotoxic activity of bacterial carotenoids isolated from Rhodopseudomonas palustris KRPR01 and KRPR02. Int J Pharm Sci Res. 2019;10(10):4644-9. [Link]
17. Joshi V, Attri D, Bala A, Bhushan S. Microbial pigments. Indian J Biotechnol. 2003;2(3):362-9. [Link]
18. Manikprabhu D and Lingappa K. γ Actinorhodin a natural and attorney source for the synthetic dye to detect acid production of fungi. Saudi J Biol Sci. 2013;20(2):163-68. [Link] [DOI:10.1016/j.sjbs.2013.01.004]
19. Vargas-Sinisterra AF, Ramírez-Castrillón M. Yeast carotenoids: production and activity as antimicrobial biomolecule. Arch Microbiol. 2021;203:873-88. [Link] [DOI:10.1007/s00203-020-02111-7]
20. Choudhary MKN, Mallya R. Phytochemical investigation and antibacterial activity of a medicinal plant. International Journal of Pharmaceutical and Pharmacological Research. 2019;9(4):53-8. [Link]
21. Mansy W, Rathod S. Temporal association between antibiotic use and resistance in Gram-negative bacteria. Arch Pharm Pract. 2020;11(2):13-8. [Link]
22. Haddad M, Aghaei S, Zargar M. Antimicrobial and antioxidant activity of carotenoid pigment produced by native Rhodococcus spp. isolated from soil. Int J Mol Clin Microbiol. 2017;7(1):809-15. [Link]
23. Yolmeh M, Hamedi H, Khomeiri M. Antimicrobial activity of pigments extracted from Rhodotorula glutinis against some bacteria and fungi. Zahedan J Res Med Sci. 2016;18(12):e4954. [Link] [DOI:10.17795/zjrms-4954]
24. Afra S, Makhdoumi A, Matin MM, Feizy J. A novel red pigment from marine Arthrobacter sp. G20 with specific anticancer activity. J Appl Microbiol. 2017;123(5):1228-36. [Link] [DOI:10.1111/jam.13576]
25. Azman AS, Mawang CI, Abubakar S. Bacterial pigments and an alternative for therapeutic as applications. Natural Product Communications. 2018;13(12):1747-50. [Link] [DOI:10.1177/1934578X1801301240]
26. Venil CK, Zakaria ZA, Ahmad WA. Bacterial pigments and their applications. Process Biochem. 2013;48(7):1065-79. [Link] [DOI:10.1016/j.procbio.2013.06.006]
27. Caro Y, Venkatachalam M, Lebeau J, Fouillaud M, Dufossé L. Pigments and colorants from filamentous fungi. In Merillon JM, Ramawat K, editors. Fungal metabolites. 1st Edition. Chambridge, UK: Springer International Publishing; 2017. Pp: 499-568. [Link] [DOI:10.1007/978-3-319-25001-4_26]
28. Kurtzman CP, Fell JW, Boekhout T. The yeast. A taxonomic study. Volume 1-3. 5th Edition. San Diego, CA: Elsevier; 2010. [Link]
29. Buzzini P, Martini A, Gaetani M, Turchetti B, Pagnoni UM, Davoli P. Optimization of carotenoid production by Rhodotorula graminis DBVPG 7021 as a function of trace element concentration by means of response surface analysis. Enzyme Microb Technol. 2005;36(5-6):687-92. [Link] [DOI:10.1016/j.enzmictec.2004.12.028]
30. Somashekar D, Joseph R. Inverse relationship between carotenoid and lipid formation in Rhodotorula gracilis according to the C/N ratio of the growth medium. World J Microbiol Biotech. 2000;16:491-93. [Link] [DOI:10.1023/A:1008917612616]
31. Latha BV, Jeevaratnam K. Purification and characterization of the pigments from Rhodotorula glutinis DFR-PDY isolated from natural source. Glob J Biotechnol Biochem. 2010;5(3):166-74. [Link]
32. Silverstein RM, Webster FX, Kiemle DJ, Bryce DL. Spectrometric identification of organic compounds. 8th Edition. Wiley; 2014. [Link]
33. Macfaddin JF. Biochemical tests for identification of medical bacteria, 3rd Edition. London: The Williams and Wilkins Co.; 2000. [Link]
34. National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9th Edition. Wayne, Pa: Clinical and Laboratory Standards Institute; 2012. [Link]
35. Nair MG, Putnam AR, Mishra SK, Mulks MH, Taft WH, Keller JE, et al. Faeriefungin: a new board-spectrum antibiotic from streptomyces griseus var. autotrophicus. J Natural Prod. 1989;52(4):797-809. [Link] [DOI:10.1021/np50064a022]
36. Matsuda T, Matsumoto T. Treatment-resistant dermatomycoses. Nihon Ishinkin Gakkai Zasshi. 1998;39(4):219-23. [Japanese] [Link] [DOI:10.3314/jjmm.39.219]
37. Sardana K, Kaur R, Arora P, Goyal R, Ghunawat S. Is antifungal resistance a cause for treatment failure in dermatophytosis: a study focused on Tinea Corporis and Cruris from a Tertiary Centre? Indian Dermatol Online J. 2018;9(2):90-5. [Link] [DOI:10.4103/idoj.IDOJ_137_17]
38. Guerin M, Huntley ME, Olaizola M. Haematococcusastaxanthin: Applications for Human. Health and Nutrition. Trend Biotechnol. 2003;21(5):210-6. [Link] [DOI:10.1016/S0167-7799(03)00078-7]
39. Astuti W, Radjasa OK, Karwur FF, Rondonuwu FS. Identification of carotenoids in Halimeda macroloba reef associated bacteria. Indonesian J Marine.Sci. 2016;21(4):151-60. [Link]
40. Paul D, Kusuma Kumar Pi, Siddiqui N. Yeast carotenoids: cost-effective fermentation strategies for health care applications. Fermentation. 2023;9(2):147. [Link] [DOI:10.3390/fermentation9020147]
41. Zeb A, Murkovic M. Thin-layer chromatographic analysis of carotenoids in plant and animal samples. J Planar Chromatogr Mod TLC. 2010;23:94-103. [Link] [DOI:10.1556/JPC.23.2010.2.1]
42. Dawoud TM, Alharbi NS, Theruvinthalakal AM, Thekkangil A, Kadaikunnan S, Khaled J, et al. Characterization and antifungal activity of the yellow pigment produced by a Bacillus sp. DBS4 isolated from the lichen Dirinaria agealita. Saudi J Biol Sci. 2020;27(5):1403-11. [Link] [DOI:10.1016/j.sjbs.2019.11.031]
43. Madhukar CV. Antimicrobial and antioxidant potentials of carotenoid pigment produced by indigenous novel soil isolate Rhodococcus kroppenstedtii. World J Environ Biosci. 2021;10(1):29-34. [Link] [DOI:10.51847/9QrSrJyTN2]
44. Finkel'shtein EI. Modern methods of analysis of carotenoids. Pharm Chem J. 2016;50(2):96-107. [Link] [DOI:10.1007/s11094-016-1405-2]
45. Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20 Suppl 3:76-98. [Link] [DOI:10.1111/1469-0691.12360]
46. Manimala MRA, Murugesan R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J Appl Natural Sci. 2014;6(2):649-53. [Link] [DOI:10.31018/jans.v6i2.511]
47. Ungureanu C, Ferdes M. Evaluation of antioxidant and antimicrobial activities of torularhodin. Adv Sci Lett. 2012;18:50-3. [Link] [DOI:10.1166/asl.2012.4403]
48. Dumitriu C, Ungureanu C, Popescu S, Tofan V, Popescu M, Pirvu C. Ti surface modification with a natural antioxidant and antimicrobial agent. Surf Coat Technol. 2015;276:175-85. [Link] [DOI:10.1016/j.surfcoat.2015.06.063]
49. Keceli TM, Erginkaya Z, Turkkan E, Kaya U. Antioxidant and Antibacterial Effects of Carotenoids extracted from Rhodotorula glutinis Ssrains. Asian J Chem. 2013;25(1):42-6. [Link] [DOI:10.14233/ajchem.2013.12377]
50. Manimala MRA, Murugesan R, Gunasekaren S. Antimicrobial activity of carotenoid pigment produced from Yeast Rhodotorula mucilaginosa YP 187. Trend Biosci. 2014;7(9):769-72. [Link]
51. Yoo AY, Alnaeeli M, Park J. Production control and characterization of antibacterial carotenoids from yeast Rhodotorula mucilaginosa AY-01. Process Biochem. 2016;51:463-73. [Link] [DOI:10.1016/j.procbio.2016.01.008]
52. Burton GW, Mogg TJ, Stupak J, Stark FC, Twine SM, Li J. Safety and uptake of fully oxidized β-carotene. Food Chem Toxicol. 2022;168:113387. [Link] [DOI:10.1016/j.fct.2022.113387]










