Volume 15, Issue 1 (2023)
Iran J War Public Health 2023, 15(1): 43-48 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/06/29 | Accepted: 2023/01/30 | Published: 2023/03/1
Received: 2022/06/29 | Accepted: 2023/01/30 | Published: 2023/03/1
How to cite this article
Mezban J, Abbas B, Khudor M. Erythromycin Resistance Genes among Coagulase-negative Staphylococci Isolated from Humans in Basrah, Iraq. Iran J War Public Health 2023; 15 (1) :43-48
URL: http://ijwph.ir/article-1-1193-en.html
URL: http://ijwph.ir/article-1-1193-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Veterinary Microbiology and Parasitology, College of Veterinary Medicine, University of Basrah, Iraq
Full-Text (HTML) (1037 Views)
Introduction
Antimicrobial resistance (AMR) poses a major threat to human health around the world. Many studies have estimated the effect of AMR on incidence, deaths, hospital length of stay, and health-care costs for specific pathogen–drug combinations in select locations. Bacterial AMR occurs when changes in bacteria cause the drugs used to treat infections to become less effective. The Review on Antimicrobial Resistance, commissioned by the UK Government, argued that AMR could kill 10 million people per year by 2050. Although these forecasts have been criticized by some, World Health Organization (WHO) and numerous other groups and researchers agree that the spread of AMR is an urgent issue requiring a global, coordinated action plan to address. Information about the current magnitude of the burden of bacterial AMR, trends in different parts of the world, and the leading pathogen–drug combinations contributing to bacterial AMR burden is crucial. If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today [1].
The use of antibiotics in humans, to treat infections, and in animals, to promote growth and prevent colonization by pathogenic bacteria, has led to an increased resistance among bacteria [2]. The resistance often is transferable at interspecies and intergeneric levels [3]. The relative ease with which bacteria become resistant to currently used antimicrobial agents is of concern to public health officials [4].
In recent years, the global incidence of infections caused by gram-negative bacteria resistant to antibiotics has increased. It has been predicted that up to two million people in the United States will contract an antibiotic-resistant bacterial infection each year, resulting in over 23,000 fatalities [5].
Staphylococci are gram-positive, non-motile, non-spore-producing bacteria that are ubiquitous and include various opportunistic/pathogenic species responsible for human and animal infections. This group of microorganisms colonizes the skin, hair, nose, and throat of humans and animals, and from these sources, they can be transferred to food because both organisms are the main reservoir [6]. On the basis of the ability to clot blood plasma, Staphylococci are divided into two groups: coagulase negative, and coagulase positive staphylococci [6].
The spread of resistance to antimicrobial agents in staphylococci is largely due to the acquisition of plasmids and/or transposons [7]. In staphylococci, the conjugative transfer of resistance determinants is usually mediated by conjugative plasmids, which spread resistance determinants between species and genera [8]. Besides transferring the resistance determinants, they can mobilize non-conjugative plasmids, recombine with non-conjugative plasmids to form new plasmids, or acquire and transfer resistance transposons [9]. The spread of antibiotic resistance among Coagulase-Negative Staphylococci (CoNS), containing resistance genes, from animal products may represent a hazard to human health through the transfer of resistance genes between staphylococcal species and direct transmission of resistant pathogens to humans [10, 11].
Several previous studies have been conducted on Staphylococcus aureus bacteria in the same area [12-17]. Studies with human staphylococcal strains indicate that Staphylococcus epidermidis is a reservoir of antibiotic resistance genes that can be transferred to S. aureus under in vitro and in vivo conditions [18]. Studies of drug resistance transfer between staphylococcal strains have been done mostly on human isolates; studies of transfer between animal and human staphylococcal strains are rare [19].
This study aimed to investigate the presence and frequency of CoNS in contact between animals and people and determine the phenotypic antimicrobial resistance profiles of CoNS isolates from these sources.
Materials and Methods
Samples collection
To obtain Staphylococcus spp., different samples were collected from several regions in Basrah province, Iraq. Forty hand swabs of milker people were collected by moistening the sterilized cotton swab with Buffered Peptone Water (BPW). Swabs were rolled over the palm of the hands, the area between fingers tips, and nails, then incubated for 24 hrs at 37°C. Forty nasal swabs were also collected using sterile swabs. The swab samples were enriched in the appropriate amount of BPW in a 1:9 ratio and incubated at 37°C for 24 hrs according to the standard methods.
Laboratory diagnosis
The specimens were directly transported to the laboratory, then directly inoculated onto Mannitol Salt Agar (MSA) and blood agar, and then incubated at 37 ºC for 24 hrs. All colonies from primary cultures were purified by subculture onto MSA medium and incubated at 37ºC for 24-48 hrs. Gram stain and other biochemical tests were done, such as catalase test, oxidase test, coagulase test, clumping factor test, and hemolysin production. The VITEK 2 is an automated microbiology system utilizing growth-based technology.
Antibiotic Susceptibility Testing (AST)
Antibiotic susceptibility testing was performed according to Bauer et al. [20] using the disc diffusion method.
Molecular study using Polymerase chain reaction (PCR) technique
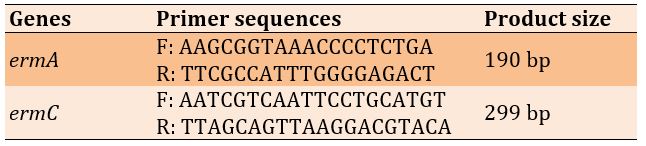
Bacterial DNA was extracted using the Geneaid™ DNA Isolation Kit (Bacteria). All staphylococcal isolates were grown in 5 mL of Lysogeny Broth (LB) overnight at 37°C for DNA extraction. Genomic DNA was amplified using the primers presented in Table 1. These primers were used to amplify the ermA and ermC genes. From each extracted sample, 5 μl of bacterial DNA was amplified by PCR with specific primers and cycling conditions as previously described [21]. PCR amplified product was detected by electrophoresis on 1% agarose gel. 4 µl of PCR product was inoculated in each well of agarose gel. The molecular weight of the PCR amplified product was determined using a 100 bp ladder after 60 min at 70V.
Table 1) Sequences of primers for ermA and ermC genes
Findings
Collection and processing of sample
Of the 80 samples collected, only 37 samples (46.25%) were positive for staphylococcal infection, which 19 samples (46.25%) and 18 samples (45.0%) were from human hand swabs and nasal swabs, respectively. There was no significant difference between the type of sample and isolates number (p>0.05; Table 2).
Table 2) Frequency of positive staphylococcal samples according to sample type

Cultural characteristics
Based on culture, suspected colonies of CoNS were smooth, round, raised, glistening, gray to deep golden yellow and white in the color plate, while the colonies on blood agar plates were large, round creamy/buff colored colonies with β or α-hemolysis (Figure 1).
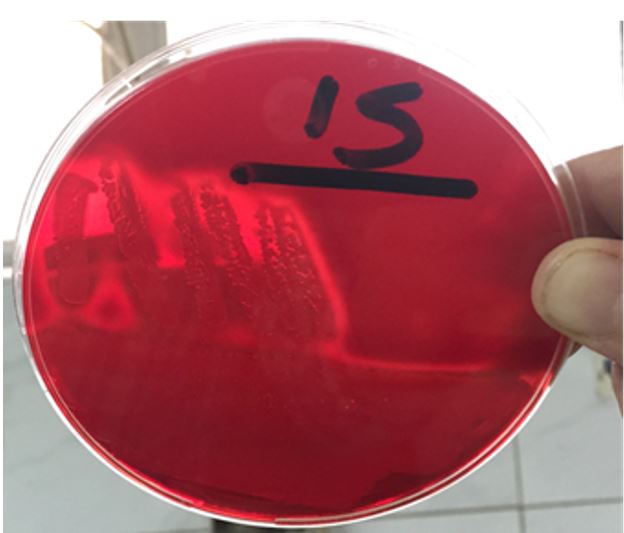
Figure 1) Hemolysis by CoNS on blood agar
With a gram stain, the smear of suspected colonies showed clusters or different irregular shapes of gram-positive cocci. All isolates were positive for the catalase test and negative for oxidase and coagulase tests. There were 5 species of CoNS according to Vitek 2 kit. Staphylococci fell into the following species: S. sciuri, S. lentus, S. gallinarum, S. chromogen, and S. haemolyticus.
Antibiotic sensitivity of CoNS isolates
37 CoNS from different sources were examined for their susceptibility to antibiotics using the agar dilution method according to NCCLS (National Committee for Clinical Laboratory Standards) guidelines (Figure 2; Table 3).

Figure 2) Antibiotic susceptibility test on CoNS
Molecular study
DNA extraction and PCR technique
DNA was extracted from CoNS isolates using conventional methods. The DNA extraction results were accepted, and the concentration and purity were determined using a Nanodrop 1000 spectrophotometer at 280/260 nm. The concentration of DNA was between 135.5-637.7 ng/μl, and the purity was between 1.49 and 2.2, as well as was observed by horizontal gel electrophoresis in 1% agarose (Figure 3).
Antimicrobial resistance (AMR) poses a major threat to human health around the world. Many studies have estimated the effect of AMR on incidence, deaths, hospital length of stay, and health-care costs for specific pathogen–drug combinations in select locations. Bacterial AMR occurs when changes in bacteria cause the drugs used to treat infections to become less effective. The Review on Antimicrobial Resistance, commissioned by the UK Government, argued that AMR could kill 10 million people per year by 2050. Although these forecasts have been criticized by some, World Health Organization (WHO) and numerous other groups and researchers agree that the spread of AMR is an urgent issue requiring a global, coordinated action plan to address. Information about the current magnitude of the burden of bacterial AMR, trends in different parts of the world, and the leading pathogen–drug combinations contributing to bacterial AMR burden is crucial. If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today [1].
The use of antibiotics in humans, to treat infections, and in animals, to promote growth and prevent colonization by pathogenic bacteria, has led to an increased resistance among bacteria [2]. The resistance often is transferable at interspecies and intergeneric levels [3]. The relative ease with which bacteria become resistant to currently used antimicrobial agents is of concern to public health officials [4].
In recent years, the global incidence of infections caused by gram-negative bacteria resistant to antibiotics has increased. It has been predicted that up to two million people in the United States will contract an antibiotic-resistant bacterial infection each year, resulting in over 23,000 fatalities [5].
Staphylococci are gram-positive, non-motile, non-spore-producing bacteria that are ubiquitous and include various opportunistic/pathogenic species responsible for human and animal infections. This group of microorganisms colonizes the skin, hair, nose, and throat of humans and animals, and from these sources, they can be transferred to food because both organisms are the main reservoir [6]. On the basis of the ability to clot blood plasma, Staphylococci are divided into two groups: coagulase negative, and coagulase positive staphylococci [6].
The spread of resistance to antimicrobial agents in staphylococci is largely due to the acquisition of plasmids and/or transposons [7]. In staphylococci, the conjugative transfer of resistance determinants is usually mediated by conjugative plasmids, which spread resistance determinants between species and genera [8]. Besides transferring the resistance determinants, they can mobilize non-conjugative plasmids, recombine with non-conjugative plasmids to form new plasmids, or acquire and transfer resistance transposons [9]. The spread of antibiotic resistance among Coagulase-Negative Staphylococci (CoNS), containing resistance genes, from animal products may represent a hazard to human health through the transfer of resistance genes between staphylococcal species and direct transmission of resistant pathogens to humans [10, 11].
Several previous studies have been conducted on Staphylococcus aureus bacteria in the same area [12-17]. Studies with human staphylococcal strains indicate that Staphylococcus epidermidis is a reservoir of antibiotic resistance genes that can be transferred to S. aureus under in vitro and in vivo conditions [18]. Studies of drug resistance transfer between staphylococcal strains have been done mostly on human isolates; studies of transfer between animal and human staphylococcal strains are rare [19].
This study aimed to investigate the presence and frequency of CoNS in contact between animals and people and determine the phenotypic antimicrobial resistance profiles of CoNS isolates from these sources.
Materials and Methods
Samples collection
To obtain Staphylococcus spp., different samples were collected from several regions in Basrah province, Iraq. Forty hand swabs of milker people were collected by moistening the sterilized cotton swab with Buffered Peptone Water (BPW). Swabs were rolled over the palm of the hands, the area between fingers tips, and nails, then incubated for 24 hrs at 37°C. Forty nasal swabs were also collected using sterile swabs. The swab samples were enriched in the appropriate amount of BPW in a 1:9 ratio and incubated at 37°C for 24 hrs according to the standard methods.
Laboratory diagnosis
The specimens were directly transported to the laboratory, then directly inoculated onto Mannitol Salt Agar (MSA) and blood agar, and then incubated at 37 ºC for 24 hrs. All colonies from primary cultures were purified by subculture onto MSA medium and incubated at 37ºC for 24-48 hrs. Gram stain and other biochemical tests were done, such as catalase test, oxidase test, coagulase test, clumping factor test, and hemolysin production. The VITEK 2 is an automated microbiology system utilizing growth-based technology.
Antibiotic Susceptibility Testing (AST)
Antibiotic susceptibility testing was performed according to Bauer et al. [20] using the disc diffusion method.
Molecular study using Polymerase chain reaction (PCR) technique
Bacterial DNA was extracted using the Geneaid™ DNA Isolation Kit (Bacteria). All staphylococcal isolates were grown in 5 mL of Lysogeny Broth (LB) overnight at 37°C for DNA extraction. Genomic DNA was amplified using the primers presented in Table 1. These primers were used to amplify the ermA and ermC genes. From each extracted sample, 5 μl of bacterial DNA was amplified by PCR with specific primers and cycling conditions as previously described [21]. PCR amplified product was detected by electrophoresis on 1% agarose gel. 4 µl of PCR product was inoculated in each well of agarose gel. The molecular weight of the PCR amplified product was determined using a 100 bp ladder after 60 min at 70V.
Table 1) Sequences of primers for ermA and ermC genes

Findings
Collection and processing of sample
Of the 80 samples collected, only 37 samples (46.25%) were positive for staphylococcal infection, which 19 samples (46.25%) and 18 samples (45.0%) were from human hand swabs and nasal swabs, respectively. There was no significant difference between the type of sample and isolates number (p>0.05; Table 2).
Table 2) Frequency of positive staphylococcal samples according to sample type

Cultural characteristics
Based on culture, suspected colonies of CoNS were smooth, round, raised, glistening, gray to deep golden yellow and white in the color plate, while the colonies on blood agar plates were large, round creamy/buff colored colonies with β or α-hemolysis (Figure 1).

Figure 1) Hemolysis by CoNS on blood agar
With a gram stain, the smear of suspected colonies showed clusters or different irregular shapes of gram-positive cocci. All isolates were positive for the catalase test and negative for oxidase and coagulase tests. There were 5 species of CoNS according to Vitek 2 kit. Staphylococci fell into the following species: S. sciuri, S. lentus, S. gallinarum, S. chromogen, and S. haemolyticus.
Antibiotic sensitivity of CoNS isolates
37 CoNS from different sources were examined for their susceptibility to antibiotics using the agar dilution method according to NCCLS (National Committee for Clinical Laboratory Standards) guidelines (Figure 2; Table 3).

Figure 2) Antibiotic susceptibility test on CoNS
Molecular study
DNA extraction and PCR technique
DNA was extracted from CoNS isolates using conventional methods. The DNA extraction results were accepted, and the concentration and purity were determined using a Nanodrop 1000 spectrophotometer at 280/260 nm. The concentration of DNA was between 135.5-637.7 ng/μl, and the purity was between 1.49 and 2.2, as well as was observed by horizontal gel electrophoresis in 1% agarose (Figure 3).

Figure 3) Gel electrophoresis of genomic DNA extraction from some staphylococci 1% agarose gel was observed under UV light at 5 V/cm for 30 minutes.
Detection of ermA and ermC genes
PCR was performed to detect the ermA gene region in 20 staphylococcal isolates, and out of 20 amplification samples, only 12 positive samples were purified for the ermA gene region with a PCR product of 190 bp. The results also showed the presence of an ermC band with a size of 299 bp, which represents the correct expected band in 8 isolates out of all isolates examined (Figure 4).
Detection of ermA and ermC genes
PCR was performed to detect the ermA gene region in 20 staphylococcal isolates, and out of 20 amplification samples, only 12 positive samples were purified for the ermA gene region with a PCR product of 190 bp. The results also showed the presence of an ermC band with a size of 299 bp, which represents the correct expected band in 8 isolates out of all isolates examined (Figure 4).
Table 3) Antibiotic susceptibility patterns of CoNS isolated during the present study
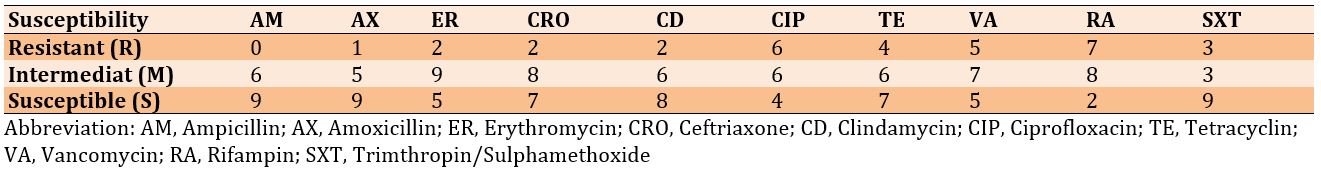
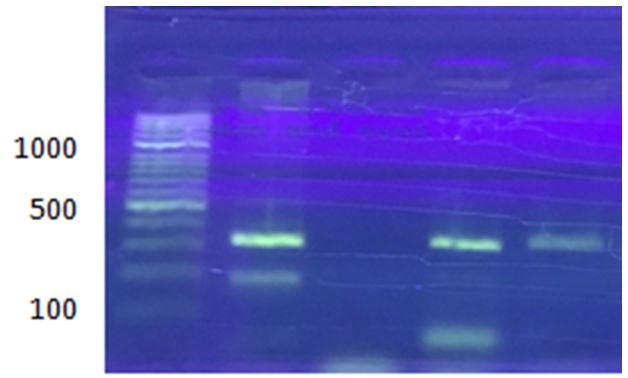


Figure 4) Gel electrophoresis of multiplex PCR products of ermA (190 bp) and ermC (299 bp) genes of CoNS on 1% agarose gel at 7 V/cm for 1 hour with 100 bp DNA ladder
Discussion
Culturing examination of Staphylococcus was performed by streaking all samples into nutrient agar, mannitol salt agar, and blood agar and incubating at 37ºC for 24 hours. The results showed that most bacteria colonies on nutrient agar, after 24 hours of incubation, were Staphylococcus with an appearance of slightly yellow, flat large, circular, and opaque. When the bacteria grow on the mannitol salt agar, the color of the medium changes. Isolates were gram-positive (purple color) spherical cells or cocci arranged in irregular grape-like clusters. This finding is correlated well with the finding of Fuchs and Sanyal [22], who found that gram-positive organisms have been increasingly identified as the source of acute clinical infection in animals and human.
The VITEK 2 system identify medically important Staphylococci in 15 hours due to a sensitive fluorescence-based technology and allows a result to be generated without the need for a morphological assessment [23].
Most of the isolates were sensitive, and some were intermediate to most of the antibiotics used in the study, as illustrated in Table 3. Resistance may be because some antibiotics cannot penetrate the outer membrane, which may reduce drug permeability.
In Ghostaslo et al.’s study [24], the most common organism causing neonatal sepsis was coagulase-negative staphylococci. Gram-negative organisms were isolated in 31.43% of cases, and the most common Gram-negative organism causing neonatal sepsis was Klebsiella pneumoniae. They reported that the resistance rates of K. pneumoniae to penicillin, ampicillin, cephalexin, chloramphenicol, gentamicin, co-trimoxazol, amikacin, and ciprofloxacin were 94.3%, 91.4%, 82.8%, 74.4%, 60.0%, 54.2%, 40.0%, and 2.8%, respectively.
However, all isolates of CoNS were resistant to ampicillin, corroborating the findings of Elliot et al. [25], who documented that CoNS often produce β-lactamases and are resistant to ampicillin and that multiply resistant strains may limit antibiotic choice. The resistance of CoNS to amoxicillin may be due to the common use of this antibiotic in the treatment to most clinical infections.
Six isolates (30%) out of 20 CoNS were positive for the ermC gene, and only one sample (5%) was positive for the ermA gene, and six isolates (30%) were positive for both ermA and ermC genes by multiplex PCR method.
Erytromycin resistance in staphylococci is encoded by erm genes (ermA, ermB, ermC and msrA) [26]. Lim et al. [27] reported that the ermA gene was more prevalent than the other erytromycin resistance genes in S. aureus isolates, and ermC gene was found mostly in CoNS. Similarly, in a study performed by Martineau et al. [28], the ermC gene has been reported to be more prevalent in CoNS. However, ermA has been reported to be the more common gene in CoNS in another study [29]. Taponen et al. [30] found that the presence of the ermA gene is detected in 53.9% of all strains, which is higher than our result (5%), and it disagrees with the present findings.
The ability of CoNS to adhere to extracellular matrix proteins is thought to be essential for the colonization and the establishment of infections [31]. CoNS possesses various adhesion genes, including the ermC gene [32].
PCR analysis of the other virulence genes revealed the ermC gene in 41 isolates. This finding suggests the important role of these elements in resistance and pathogenicity in bovines and humans. However, ermC was present among the strains. Our present results agreed with the combined occurrence of ermC genes that has been described by other investigators [33, 34].
Duran et al. [21] evaluated the association between antibiotic susceptibility patterns and the antibiotic resistance genes in staphylococcal isolates obtained from various clinical samples of patients attending a teaching hospital in Hatay, Turkey. A total of 298 staphylococci clinical isolates were subjected to antimicrobial susceptibility testing. The genes implicated in antimicrobial resistance were amplified using the multiplex PCR method, in which a total of 165 isolates were resistant to erythromycin and contained at least one of the erythromycin resistance genes (ermA, ermB, ermC, and msrA).
Conclusion
Gram-positive organisms are increasingly identified as the source of acute clinical infection in animals and humans. Some isolates are resistant to several different antibiotics. The ermC gene, ermA gene, and both ermA and ermC genes are present in the genome of these bacteria.
Acknowledgements: It is not applicable.
Ethical Permission: Nothing has been reported by the authors.
Conflict of Interests: Nothing has been reported by the authors.
Authors’ Contribution: Mezban JM (First Author), Introduction Writer/Main Researcher/Discussion Writer (40%); Abbas BA (Second Author), Methodologist/Assistant Researcher/Statistical Analyst/Discussion Writer (40%); Khudor MH (Third Author), Introduction Writer/Assistant Researcher (20%)
Funding: Nothing has been reported by the authors.
Discussion
Culturing examination of Staphylococcus was performed by streaking all samples into nutrient agar, mannitol salt agar, and blood agar and incubating at 37ºC for 24 hours. The results showed that most bacteria colonies on nutrient agar, after 24 hours of incubation, were Staphylococcus with an appearance of slightly yellow, flat large, circular, and opaque. When the bacteria grow on the mannitol salt agar, the color of the medium changes. Isolates were gram-positive (purple color) spherical cells or cocci arranged in irregular grape-like clusters. This finding is correlated well with the finding of Fuchs and Sanyal [22], who found that gram-positive organisms have been increasingly identified as the source of acute clinical infection in animals and human.
The VITEK 2 system identify medically important Staphylococci in 15 hours due to a sensitive fluorescence-based technology and allows a result to be generated without the need for a morphological assessment [23].
Most of the isolates were sensitive, and some were intermediate to most of the antibiotics used in the study, as illustrated in Table 3. Resistance may be because some antibiotics cannot penetrate the outer membrane, which may reduce drug permeability.
In Ghostaslo et al.’s study [24], the most common organism causing neonatal sepsis was coagulase-negative staphylococci. Gram-negative organisms were isolated in 31.43% of cases, and the most common Gram-negative organism causing neonatal sepsis was Klebsiella pneumoniae. They reported that the resistance rates of K. pneumoniae to penicillin, ampicillin, cephalexin, chloramphenicol, gentamicin, co-trimoxazol, amikacin, and ciprofloxacin were 94.3%, 91.4%, 82.8%, 74.4%, 60.0%, 54.2%, 40.0%, and 2.8%, respectively.
However, all isolates of CoNS were resistant to ampicillin, corroborating the findings of Elliot et al. [25], who documented that CoNS often produce β-lactamases and are resistant to ampicillin and that multiply resistant strains may limit antibiotic choice. The resistance of CoNS to amoxicillin may be due to the common use of this antibiotic in the treatment to most clinical infections.
Six isolates (30%) out of 20 CoNS were positive for the ermC gene, and only one sample (5%) was positive for the ermA gene, and six isolates (30%) were positive for both ermA and ermC genes by multiplex PCR method.
Erytromycin resistance in staphylococci is encoded by erm genes (ermA, ermB, ermC and msrA) [26]. Lim et al. [27] reported that the ermA gene was more prevalent than the other erytromycin resistance genes in S. aureus isolates, and ermC gene was found mostly in CoNS. Similarly, in a study performed by Martineau et al. [28], the ermC gene has been reported to be more prevalent in CoNS. However, ermA has been reported to be the more common gene in CoNS in another study [29]. Taponen et al. [30] found that the presence of the ermA gene is detected in 53.9% of all strains, which is higher than our result (5%), and it disagrees with the present findings.
The ability of CoNS to adhere to extracellular matrix proteins is thought to be essential for the colonization and the establishment of infections [31]. CoNS possesses various adhesion genes, including the ermC gene [32].
PCR analysis of the other virulence genes revealed the ermC gene in 41 isolates. This finding suggests the important role of these elements in resistance and pathogenicity in bovines and humans. However, ermC was present among the strains. Our present results agreed with the combined occurrence of ermC genes that has been described by other investigators [33, 34].
Duran et al. [21] evaluated the association between antibiotic susceptibility patterns and the antibiotic resistance genes in staphylococcal isolates obtained from various clinical samples of patients attending a teaching hospital in Hatay, Turkey. A total of 298 staphylococci clinical isolates were subjected to antimicrobial susceptibility testing. The genes implicated in antimicrobial resistance were amplified using the multiplex PCR method, in which a total of 165 isolates were resistant to erythromycin and contained at least one of the erythromycin resistance genes (ermA, ermB, ermC, and msrA).
Conclusion
Gram-positive organisms are increasingly identified as the source of acute clinical infection in animals and humans. Some isolates are resistant to several different antibiotics. The ermC gene, ermA gene, and both ermA and ermC genes are present in the genome of these bacteria.
Acknowledgements: It is not applicable.
Ethical Permission: Nothing has been reported by the authors.
Conflict of Interests: Nothing has been reported by the authors.
Authors’ Contribution: Mezban JM (First Author), Introduction Writer/Main Researcher/Discussion Writer (40%); Abbas BA (Second Author), Methodologist/Assistant Researcher/Statistical Analyst/Discussion Writer (40%); Khudor MH (Third Author), Introduction Writer/Assistant Researcher (20%)
Funding: Nothing has been reported by the authors.
Keywords:
Erythromycin [MeSH], Antibiotic Resistance [MeSH], Bacterial Genes [MeSH], Staphylococcus [MeSH], Human [MeSH], PCR [MeSH]
References
1. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-55. [Link]
2. Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264(5157):375-82. [Link] [DOI:10.1126/science.8153624]
3. Noble W C, Virani Z, Cree R G. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;72(2):195-8. [Link] [DOI:10.1111/j.1574-6968.1992.tb05089.x]
4. Grabow WOK, Middendorf IG, Prozesky OW. Survival in maturation ponds of coliform bacteria with transferable drug resistance. Water Res. 1973;7(11):1589-97. [Link] [DOI:10.1016/0043-1354(73)90130-9]
5. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903-10. [Link] [DOI:10.2147/IDR.S234610]
6. Anacarso I, Condò C, Sabia C, Messi P, de Niederhausern S, Bondi M, et al. Antimicrobial resistance and other related virulence factors in Staphylococcus spp isolated from food, environmental and humans in Italy. Univ J Microbiol Res. 2013;1(1):1-9. [Link] [DOI:10.13189/ujmr.2013.010101]
7. Lozano C, Aspiroz C, Rezusta A, Gómez-Sanz E, Simon C, Gómez P, et al. Identification of novel vga(A)-carrying plasmids and a Tn5406-like transposon in meticillin-resistant Staphylococcus aureus and Staphylococcus epidermidis of human and animal origin. Int J Antimicrob Agents. 2012;40(4):306-12. [Link] [DOI:10.1016/j.ijantimicag.2012.06.009]
8. Malachowa N, DeLeo FR. Mobile genetic elements of Staphylococcus aureus. Cell Mol Life Sci. 2010;67(18):3057-71. [Link] [DOI:10.1007/s00018-010-0389-4]
9. Khan SA, Nawaz MS, Khan AA, Cerniglia CE. Transfer of erythromycin resistance from poultry to human clinical strains of phylococcus aureus. J Clin Microbiol. 2000;38(5):1832-8. [Link] [DOI:10.1128/JCM.38.5.1832-1838.2000]
10. Walther C, Perreten V. Methicillin-resistant Staphylococcus epidermidis in organic milk production. J Dairy Sci. 2007;90(12):5351. [Link] [DOI:10.3168/jds.2007-0547]
11. Mezban JM, Khudor MH, Abbas BA. Multiplex PCR detection of erythromycin resistance genes in coagulase negative Staphylococci isolated from cows in Basrah, Iraq. Bas J Vet Res. 2018;17(1):86-102. [Link] [DOI:10.33762/bvetr.2018.144911]
12. Khudor MH, Abbas BA, Idbeis HI. Detection of enterotoxin genes of Staphylococcus aureus isolates from raw milk. Bas J Vet Res. 2012;11(1):254. [Link] [DOI:10.33762/bvetr.2012.54852]
13. Abbas BA, Khudor, MH, Idbeis HI. Iinvestigation of the activityand pathogenecity of Staphylococcus aureus enterotoxin c by ligated ileal loopassay in rabbits. Bas J Vet Res. 2013;12(2):104-12. [Link] [DOI:10.33762/bvetr.2013.83629]
14. Khudaier BY, Abbas BA, Khudaier AM. Detection of methicillin resistant Staphylococcus aureus isolated from human and animals in Basrah Province/Iraq. MRVSA. 2013;2(3):12-21. [Link]
15. Abbas BA, Khudor MH, Hanoon BM. Isolation and identification of Staphylococcus aureus from bovine and the detection of its coagulase gene (Coa) using polymerase chain reaction (PCR). Sc Res Assays. 2014;9(20):864-8. [Link] [DOI:10.5897/SRE2014.6029]
16. Abbas BA, Khudor MH, Hanoon B. The relationship between biotype, serotype, antibiotic susceptibility and Coa gene polymorphism in Staphylococcus aureus isolated from bovine. Vet Med Assiut Univ Egypt. 2016;17:33. [Link]
17. Abbas BA, Khudaier BY, Khudair AM. Studies on mecA gene in methicillin resistant Staphylococcus aureus isolates. Jokull J 2017;58-65. [Link]
18. Lyon BR, Skurray RA. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987;51(1):88-134. [Link] [DOI:10.1128/mr.51.1.88-134.1987]
19. Muhammad G, Hoblet KH, Jackwood DJ, Nielsen SB, Smith KL. Interspecific conjugal transfer of antibiotic resistance among staphylococci isolated from the bovine mammary gland. Am J Vet Res. 1993;54(9):1432-40. [Link]
20. Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493-6. [Link] [DOI:10.1093/ajcp/45.4_ts.493]
21. Duran N, Ozer B, Duran GG, Onlen Y, Demir C. Antibiotic resistance genes & susceptibility patterns in staphylococci. Indian J Med Res. 2012;135(3):389-96. [Link]
22. Fuchs M, Sanyal AJ. Sepsis and cholestasis. Clin Liver Dis. 2008;12(1):151-72. [Link] [DOI:10.1016/j.cld.2007.11.002]
23. Graf B, Thomas A, Edith Z, Göbel UB. Evaluation of the VITEK 2 system for rapid identification of yeasts and yeast-like organisms. J Clin Microbiol. 2000;38(5):1782-5. [Link] [DOI:10.1128/JCM.38.5.1782-1785.2000]
24. Ghostaslo R, Ghorashi Z, Nahaei MR. Klebsiella pneumoniae in neonatal sepsis: a 3-year-study in the pediatric hospital of Tabriz, Iran. Jpn J Infect Dis. 2007;60(2-3):126-8. [Link]
25. Elliot T, Hastings M, Desselberger U. Lecture notes on medical microbiology. 3rd Edition. London: Blackwell Science: 1997. [Link]
26. Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39(3):577-85. [Link] [DOI:10.1128/AAC.39.3.577]
27. Lim JA, Kwon AR, Kim SK, Chong Y, Lee K, Choi EC. Prevalence of resistance to macrolide, lincosamide and streptogramin antibiotics in Gram-positive cocci isolated in a Korean hospital. J Antimicrob Chemother. 2002;49(3):489-95. [Link] [DOI:10.1093/jac/49.3.489]
28. Martineau F, Picard FJ, Lansac N, Menard C, Roy PH, Ouellette M, et al. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic suseptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44(2):231-8. [Link] [DOI:10.1128/AAC.44.2.231-238.2000]
29. Thakker-Varia S, Jenssen WD, Moon-McDermott L, Weinstein MP, Dubin DT. Molecular epidemiology of macrolides-lincosamides-streptogramin B resistance in Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987;31(5):735-43. [Link] [DOI:10.1128/AAC.31.5.735]
30. Taponen S, Simojoki H, Haveri M, Larsen HD, Pyörälä S. Clinical characteristics and persistence of bovine mastitis caused by different species of coagulase-negative staphylococci identified with API or AFLP. Vet Microbiol. 2006;115(1-3):199-207. [Link] [DOI:10.1016/j.vetmic.2006.02.001]
31. Akineden, Annemüller C, Hassan AA, Lmmler C, Wolter W, Zschck M. Toxin genes and other characteristics of Staphylococcus aureus isolates from milk of cows with mastitis. Clin Diagn Lab Immunol. 2001;8(5):959-64. [Link] [DOI:10.1128/CDLI.8.5.959-964.2001]
32. El-Sayed A, Alber J, Lmmler C, Bonner B, Huhn A, Kaleta EF, et al. PCR-based detection of genes encoding virulence determinants in Staphylococcus aureus from birds. J Vet Med B Infect Dis Vet Public Health. 2005;52(1):38-44. [Link] [DOI:10.1111/j.1439-0450.2004.00814.x]
33. Stephan R, Annemüller C, Hassan AA, Lmmler C. Characterization of enterotoxigenic Staphylococcus aureus strains isolated from bovine mastitis in north-east Switzerland. Vet Microbiol. 2001;78(4):373-82. [Link] [DOI:10.1016/S0378-1135(00)00341-2]
34. Fitzgerald JR, Hartigan PJ, Meaney WJ, Smyth CJ. Molecular population and virulence factor analysis of Staphylococcus aureus from bovine intramammary infection. J Appl Microbiol. 2000;88(6):1028-37. [Link] [DOI:10.1046/j.1365-2672.2000.01071.x]










