Volume 13, Issue 4 (2021)
Iran J War Public Health 2021, 13(4): 283-288 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/12/19 | Accepted: 2021/12/31 | Published: 2022/02/5
Received: 2021/12/19 | Accepted: 2021/12/31 | Published: 2022/02/5
How to cite this article
Ali Al Shammary S, Ibrahim Hussein I. Guillain-Barré Syndrome; a Neurological Disease during Convalescence Period. Iran J War Public Health 2021; 13 (4) :283-288
URL: http://ijwph.ir/article-1-1084-en.html
URL: http://ijwph.ir/article-1-1084-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
S.H. Ali Al Shammary *1, I. Ibrahim Hussein1
1- Physiology Department, Al Mustansiriya College of Medicine, Al Mustansiriya University, Baghdad, Iraq
Full-Text (HTML) (630 Views)
Introduction
Coronavirus disease (COVID-19) is a pandemic severe acute respiratory syndrome caused by a coronavirus (nCov), which can be presented with extra-pulmonary manifestations, including preference of the central and peripheral nervous systems [1]. nCov can attack the central nervous system (CNS) because it has a spike protein that binds to the angiotensin-converting enzyme receptor-2 (ACE-R2) that is available in the CNS, particularly in the areas that regulate respiration [2, 3].
Coronavirus can attack the nervous system through different mechanisms. The virus can enter the CNS directly across the cribriform plate, which explains the higher percentage of patients who complained of smell and taste dysfunction [4]. The other possible direct invasion is through blood circulation, in which the virus enters into neuronal cells via ACE-R2; in addition, coronavirus can directly damage the blood-brain barrier [5]. Indirect mechanisms are also involved in the pathogenesis of neurological manifestations, which include hypoxia, coagulopathy, and cytokines storm [6-9]. Several case-report studies showed that nCov could cause a broad spectrum of neurological manifestations, including headache, fatigue, cloudy consciousness, confusion, ataxia, stroke, seizures, hyposmia, hypogeusia, and neuralgias, which occurred during the acute phase of infection [10-13]. In addition, there is evidence showing that nCov is implicated in the development of multiple sclerosis, acute disseminated encephalomyelitis, and necrotizing hemorrhagic encephalitis [14-16]. An association between Guillain-Barré syndrome (GBS) and COVID-19 was reported in case-report studies proved with an electrophysiological investigation showing segmental demyelinating polyneuropathy cerebrospinal fluid biochemistry showed an albumin-cytologic dissociation [17]. Delayed distal latencies and absent F waves are features of the nerve conduction studies during the infected phase, which indicated demyelinating neuropathy [18].
This study aimed to summarize the clinical neurological features and electrophysiological studies of COVID-19 patients found during the recovery phase, which suggested GBS.
Patients and Methods
Setting and Design
This prospective, observational non-consecutive case series study was done in the Department of Physiology, College of Medicine at Al-Mustansiriya University in cooperation with Al-Yarmouk Teaching Hospital in Bagdad from 1st June to 31 December 2020. The study was approved by the ethical and scientific committees in the College of Medicine.
Participants
Patients who had a history of proved COVID-19 diagnosed with polymerase chain reaction (PCR) test recovered from the illnesses and complaining from neurological signs and symptoms were included in this study. Forty-four patients (25 males and 19 females) were referred by neurologists and neurosurgeons for electromyography (EMG) and nerve conduction study (NCS) to identify the diagnosis of their illnesses. The latent period from their COVID-19 recovery to the neurological manifestations ranged from 1-3 months. All the patients were subjected to electrophysiological studies.
Electrophysiological studies
The EMG system (NIHONKOHDEN MEB – 9400 A/K EMG) recorded sensory and motor nerves and muscle activity electrophysiology. This system has four-channel preamplifiers and two isolated stimulators with separate jacks (A and B). Stimulus intensity can be adjusted between 1-100 mA, and the evoked responses can be displayed on the LCD monitor, on which the four channels can be displayed simultaneously. The sensory potential is detected by applying a surface stimulation. The stimulus intensity was 20-30% above the current necessary to evoke a maximal sensory nerve action potential. The supramaximal stimulating current was kept below the threshold for motor fibers, especially in the mixed nerves, since sensory fibers generally have a lower stimulation threshold than motor fibers. The latency, amplitude, conduction velocity, and response pattern were recorded. For motor nerve conduction study, fibular and posterior tibial nerves (of both sides) were stimulated at two points along its course by applying stimuli at distal and proximal sites of the nerve and recording the muscle activity innervated by the stimulated nerve. The stimulus intensity is higher than that used with sensory nerves because all the nerve fibers during stimulation should be activated. Distal motor latency, motor nerve conduction velocity, F-wave latency, amplitude, conduction block, and temporal dispersion are recorded. The Electrophysiological diagnostic criteria for acute inflammatory demyelinating polyneuropathy are the presence of at least three of the following in the motor nerves [19]:
1. Prolonged distal latency (DL) of two or more nerves, not at entrapment sites, which is >115% of the upper limit normal for normal compound muscle action potentials amplitudes (CMAP); and DL > 125% of the normal upper limit for CMAP which is less than lower limit normal.
2. Slowing conduction velocity (CV) of two or more nerves not across entrapment sites. A CV of <90% of lower limit normal for CMAP amplitudes of >50% lower limit normal; CV of <80% lower limit normal for CMAP amplitudes of <50% lower limit normal.
3. Prolonged late responses: F response and H reflexes of one or more nerves by >125% upper-lower normal; or absent F responses.
4. Conduction block/temporal dispersion of one or more nerves, which showed:
(a) Unequivocal conduction block: proximal/distal CMAP area ratio <0.50;
(b) Possible conduction block: proximal/distal CMAP amplitude ratio <0.70; and
(c) Temporal dispersion: proximal/distal CMAP duration ratio >1.15.
Moreover, the sensory action potential of the sural nerve is normal (sural sparing pattern) compared with abnormal or absent action potentials of the median and ulnar sensory nerve [20, 21].
Findings
According to the electrophysiological diagnostic criteria of GBS, seven patients (3 males and 4 females) were fulfilled these criteria (Table 1). All the patients were presented clinically with a rapidly progressive symmetrical ascending weakness of both lower followed by upper limbs. Lower limbs are affected more than upper limbs, and the proximal muscles are affected more than the distal ones, showing grades 0 to 2. All patients show diffuse, hypo-or-areflexia of the deep tendon reflexes, especially at the ankle and knee joints. The sensory symptoms were variably present, including pain, paresthesia, numbness, hotness, and occasionally tightness of the upper limbs. All the patients had negative PCR tests, and the complete blood picture showed a normal blood cellular pattern except three patients were presented with neutrophilia (>70.05), lymphocytopenia (<25%), high serum C-reactive protein (>6mg/L), and normal platelet indices (platelet count ranged between 185,000 and 260,000/mm3).
Coronavirus disease (COVID-19) is a pandemic severe acute respiratory syndrome caused by a coronavirus (nCov), which can be presented with extra-pulmonary manifestations, including preference of the central and peripheral nervous systems [1]. nCov can attack the central nervous system (CNS) because it has a spike protein that binds to the angiotensin-converting enzyme receptor-2 (ACE-R2) that is available in the CNS, particularly in the areas that regulate respiration [2, 3].
Coronavirus can attack the nervous system through different mechanisms. The virus can enter the CNS directly across the cribriform plate, which explains the higher percentage of patients who complained of smell and taste dysfunction [4]. The other possible direct invasion is through blood circulation, in which the virus enters into neuronal cells via ACE-R2; in addition, coronavirus can directly damage the blood-brain barrier [5]. Indirect mechanisms are also involved in the pathogenesis of neurological manifestations, which include hypoxia, coagulopathy, and cytokines storm [6-9]. Several case-report studies showed that nCov could cause a broad spectrum of neurological manifestations, including headache, fatigue, cloudy consciousness, confusion, ataxia, stroke, seizures, hyposmia, hypogeusia, and neuralgias, which occurred during the acute phase of infection [10-13]. In addition, there is evidence showing that nCov is implicated in the development of multiple sclerosis, acute disseminated encephalomyelitis, and necrotizing hemorrhagic encephalitis [14-16]. An association between Guillain-Barré syndrome (GBS) and COVID-19 was reported in case-report studies proved with an electrophysiological investigation showing segmental demyelinating polyneuropathy cerebrospinal fluid biochemistry showed an albumin-cytologic dissociation [17]. Delayed distal latencies and absent F waves are features of the nerve conduction studies during the infected phase, which indicated demyelinating neuropathy [18].
This study aimed to summarize the clinical neurological features and electrophysiological studies of COVID-19 patients found during the recovery phase, which suggested GBS.
Patients and Methods
Setting and Design
This prospective, observational non-consecutive case series study was done in the Department of Physiology, College of Medicine at Al-Mustansiriya University in cooperation with Al-Yarmouk Teaching Hospital in Bagdad from 1st June to 31 December 2020. The study was approved by the ethical and scientific committees in the College of Medicine.
Participants
Patients who had a history of proved COVID-19 diagnosed with polymerase chain reaction (PCR) test recovered from the illnesses and complaining from neurological signs and symptoms were included in this study. Forty-four patients (25 males and 19 females) were referred by neurologists and neurosurgeons for electromyography (EMG) and nerve conduction study (NCS) to identify the diagnosis of their illnesses. The latent period from their COVID-19 recovery to the neurological manifestations ranged from 1-3 months. All the patients were subjected to electrophysiological studies.
Electrophysiological studies
The EMG system (NIHONKOHDEN MEB – 9400 A/K EMG) recorded sensory and motor nerves and muscle activity electrophysiology. This system has four-channel preamplifiers and two isolated stimulators with separate jacks (A and B). Stimulus intensity can be adjusted between 1-100 mA, and the evoked responses can be displayed on the LCD monitor, on which the four channels can be displayed simultaneously. The sensory potential is detected by applying a surface stimulation. The stimulus intensity was 20-30% above the current necessary to evoke a maximal sensory nerve action potential. The supramaximal stimulating current was kept below the threshold for motor fibers, especially in the mixed nerves, since sensory fibers generally have a lower stimulation threshold than motor fibers. The latency, amplitude, conduction velocity, and response pattern were recorded. For motor nerve conduction study, fibular and posterior tibial nerves (of both sides) were stimulated at two points along its course by applying stimuli at distal and proximal sites of the nerve and recording the muscle activity innervated by the stimulated nerve. The stimulus intensity is higher than that used with sensory nerves because all the nerve fibers during stimulation should be activated. Distal motor latency, motor nerve conduction velocity, F-wave latency, amplitude, conduction block, and temporal dispersion are recorded. The Electrophysiological diagnostic criteria for acute inflammatory demyelinating polyneuropathy are the presence of at least three of the following in the motor nerves [19]:
1. Prolonged distal latency (DL) of two or more nerves, not at entrapment sites, which is >115% of the upper limit normal for normal compound muscle action potentials amplitudes (CMAP); and DL > 125% of the normal upper limit for CMAP which is less than lower limit normal.
2. Slowing conduction velocity (CV) of two or more nerves not across entrapment sites. A CV of <90% of lower limit normal for CMAP amplitudes of >50% lower limit normal; CV of <80% lower limit normal for CMAP amplitudes of <50% lower limit normal.
3. Prolonged late responses: F response and H reflexes of one or more nerves by >125% upper-lower normal; or absent F responses.
4. Conduction block/temporal dispersion of one or more nerves, which showed:
(a) Unequivocal conduction block: proximal/distal CMAP area ratio <0.50;
(b) Possible conduction block: proximal/distal CMAP amplitude ratio <0.70; and
(c) Temporal dispersion: proximal/distal CMAP duration ratio >1.15.
Moreover, the sensory action potential of the sural nerve is normal (sural sparing pattern) compared with abnormal or absent action potentials of the median and ulnar sensory nerve [20, 21].
Findings
According to the electrophysiological diagnostic criteria of GBS, seven patients (3 males and 4 females) were fulfilled these criteria (Table 1). All the patients were presented clinically with a rapidly progressive symmetrical ascending weakness of both lower followed by upper limbs. Lower limbs are affected more than upper limbs, and the proximal muscles are affected more than the distal ones, showing grades 0 to 2. All patients show diffuse, hypo-or-areflexia of the deep tendon reflexes, especially at the ankle and knee joints. The sensory symptoms were variably present, including pain, paresthesia, numbness, hotness, and occasionally tightness of the upper limbs. All the patients had negative PCR tests, and the complete blood picture showed a normal blood cellular pattern except three patients were presented with neutrophilia (>70.05), lymphocytopenia (<25%), high serum C-reactive protein (>6mg/L), and normal platelet indices (platelet count ranged between 185,000 and 260,000/mm3).
Table 1) The electrophysiological findings
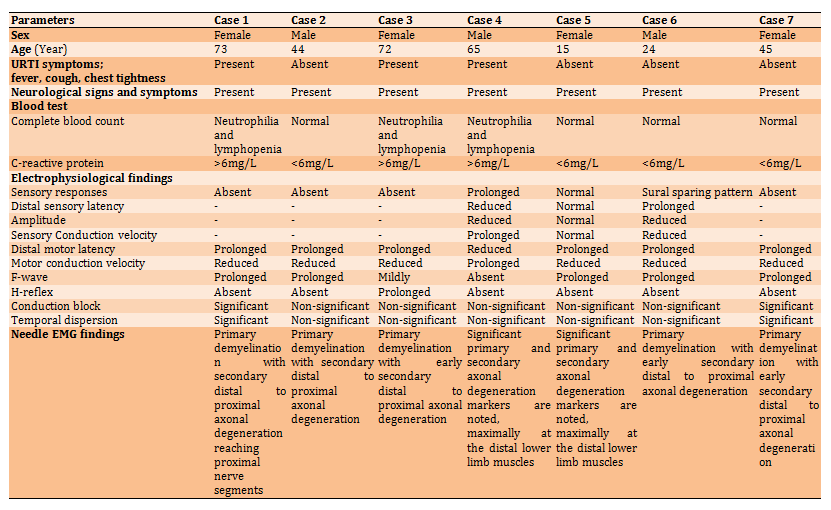
Case 1
A 73 years old woman presented with fever and dyspnea who recovered from Covid-19 with a latent period of three-month of nCoV infection. In addition to the above neurological manifestations, the patient had bilateral facial nerve palsy. Laboratory investigations showed neutrophilia, lymphocytopenia, and a significantly high serum level of C-reactive protein, but the PCR test was negative. NCS study showed absence sensory responses, prolonged distal motor latencies, reduced motor conduction velocities, prolonged F-latency, absence of H-reflex, and a markedly reduced amplitude of the CAMPs. Needle EMG study showed early superimposed secondary axonal degeneration, spontaneous activity with unobtainable to reduced recruitment pattern, and reduced firing motor units. These findings suggested a GBS subtype of acute inflammatory demyelinating polyradiculoneuropathy with bilateral facial nerve palsy.
Case 2
A 44 years old man without clinical features of upper respiratory tract infections who recovered from Covid-19 with a latent period of two-month of nCoV infection presented with the above-mentioned neurological manifestations. NCS study showed absence sensory response, prolonged distal motor latencies, reduced motor conduction velocities, and prolonged F-latency. Needle EMG study showed spontaneous activity (grade 1-3) in fibrillation, positive sharp waves, and reduced numbers of the firing motor units with unobtainable to reduced recruitment pattern. These findings suggested a GBS subtype of acute inflammatory demyelinating polyradiculoneuropathy.
Case 3
A 72 years old woman presented with fever and dyspnea who recovered from Covid-19 with a latent period of three-month of nCoV infection. Laboratory investigations showed neutrophilia, lymphocytopenia, and a significantly high serum level of C-reactive protein, but the PCR test was negative. NCSs showed absence of sensory responses, the median and ulnar nerves showed no motor responses, the responses obtained from both fibular and tibial nerves are of prolonged latencies to proximal muscles, and no responses were obtained from both extensor digitorum brevis muscle and abductor halluces muscle, and no evidence of conduction block. Needle EMG study showed spontaneous activity with unobtainable to reduced recruitment pattern. The right first dorsal interosseus, biceps, right, left vastus medialis, tibialis anterior, and extensor digitorum brevis muscles showed spontaneous 1-3 in fibrillation and positive sharpness waves without large motor units activity is noted by passive and volition. These findings suggested a GBS subtype of acute inflammatory demyelinating polyradiculoneuropathy.
Case 4
A 65 years old man presented with fever and dyspnea who recovered from COVID-19 with a latent period of three-month of nCoV infection. Laboratory investigations showed neutrophilia, lymphocytopenia, and a significantly high serum level of C-reactive protein, but the PCR test was negative. Nerve conduction study showed absent and dispersed sensory responses with prolonged distal sensory latencies; mildly prolonged distal motor latencies, normal proximal motor conduction velocities; and F–wave latencies in the testing the right median, ulnar, sural, and fibular nerves—reduced compound muscle action potentials amplitude on proximal and distal stimulation sites. The needle EMG study shows widespread spontaneous activity, large motor unit obtained of long duration, high amplitude, increased percentage of polyphagia with a reduced recruitment pattern. The right first dorsal interosseus, biceps, right and left vastus medialis, tibialis anterior. Extensor digitorum brevis muscles showed spontaneous activity, and the large motor units obtained of long duration and high amplitude. These findings suggested a GBS subtype of acute motor and sensory axonal neuropathy (AMSAN).
Case 5
A 15 years old girl without clinical features of upper respiratory tract infections, who recovered from Covid-19 with a latent period of two-month of nCoV infection, presented with rapidly progressive muscle weakness of upper and lower limbs. Nerve conduction study showed normal sensory; normal distal motor latencies, proximal motor conduction velocities and F –wave latencies; reduced amplitude of the distal lower limb compound of the muscle action potentials on proximal and distal stimulation sites with no evidence of conduction block. H reflex latencies were absent. Needle EMG study showed spontaneous activity with reduced recruitment pattern. Spontaneous activity grades 1-3 in fibrillation, positive sharp waves, complex repetitive discharges and increased mean potential duration, amplitude and percentage of polyphasic of the sampled units were observed. These findings suggested a GBS subtype of acute motor axonal neuropathy (AMAN).
Case 6
A 24 years old young man without clinical features of upper respiratory tract infections, who recovered from Covid-19 with a latent period of one month of nCoV infection, presented with the abovementioned neurological manifestations. The NCS of the right median, ulnar, sural, and fibular nerves showed absent median and ulnar with normal sural sensory responses (sural sparing pattern ); prolonged distal motor latencies; reduced motor conduction velocities, and prolonged F –wave latencies without evidence of conduction block was observed. Needle EMG study showed spontaneous activity; large motor units are obtained with reduced recruitment pattern. The right first dorsal interosseus biceps, right and left vastus medialis, tibialis anterior. Extensor digitorum brevis muscles showed spontaneous grade 1-2 in fibrillation and positive sharp waves, and the large motor units obtained of long duration and high amplitude. These findings suggested a GBS subtype of acute inflammatory demyelinating polyradiculoneuropathy with superimposed secondary axonal degeneration.
Case 7
A 45 years old woman without clinical features of upper respiratory tract infections, who recovered from Covid-19 with a latent period of one month of nCoV infection, presented with the abovementioned neurological manifestations. Moreover, the electrophysiological findings suggested severe proximal demyelinating radicular lesions. Nerve conduction study showed the absence of sensory responses, prolongation of distal motor latencies, reducing motor conduction velocities, and prolonged F–wave latencies. Needle EMG study showed spontaneous activity grade 1-3 with reduced recruitment pattern. These findings suggested a GBS subtype of acute inflammatory demyelinating polyradiculoneuropathy.
Discussion
This study shows that the clinical manifestations and the electrophysiological studies suggest the patients developed GBS after a latent period of 1-3 months from their COVID-19 illness. The characteristics of the participants, the onset and the clinical manifestations, as well as the latent period of developing the disease, are similar to that reported by others [22]. The current case series study demonstrates six patients presenting acute inflammatory demyelinating polyradiculoneuropathy and one with acute motor axonal neuropathy subtypes. Moreover, Case 1 is a variant of GBS presented with facial nerve palsy and paresthesia, and it is less frequently reported [23]. Previous studies reported that neurological manifestations of complicated COVID-19 can precede upper respiratory tract infections, while the current study demonstrates the clinical manifestations followed the COVID-19 [24]. Paybast et al. [25] reported a patient presented with bilateral facial paralysis, areflexia, decreased sensation in the distal limbs, and a nerve conduction study suggested acute axonal demyelinating neuropathy during the outbreak of COVID-19 and a positive PCR test.
Moreover, three patients presented at the same time with fever and dyspnea (influenza-like symptoms) as well as with neurological manifestations, which is unlikely due to recurrent COVID-19 because PCR tests are negative. El Otmani et al. reported a 70-year-old woman with rheumatoid arthritis. They presented at the same time with acute motor and sensory axonal neuropathy subtype of GBS and COVID-19 disease with a positive PCR test [26]. Another case report demonstrated that two family members with COVID-19 developed ascending paraesthesia with bilateral facial nerve paralysis after five days of upper respiratory tract infection [25]. Accordingly, the neurological complications of GBS due to nCov can occur at any time and present with any subtype of GBS.
Furthermore, none of our case series had a history of vaccination. A recent study demonstrates atypical neurological manifestations of GBS with nerve conduction velocity matched with acute inflammatory demyelinating polyneuropathy [27]. Current studies demonstrate that nCov can damage the nervous system directly [28], which is confirmed by the presence of nCov in the cerebrospinal fluid [29], or indirectly due to hypoxia viremia and cytokines [8, 30]. The possibility of autoimmunity can be suggested, although it is still not confirmed. One important limitation of this case report is that the cerebrospinal fluid examination was not carried out because the diagnosis was based on the clinical background and electrophysiological studies.
Conclusion
Clinical manifestations of GBS confirmed by electrophysiological studies are associated with COVID-19, which show similarity with GBS due to the post-viral infections with the autoimmune background. Moreover, COVID-19 patients who recovered from their illness should be supervised to anticipate the latent complications.
Acknowledgments: The author expresses his gratitude to Emeritus Professor Marwan S. Al-Nimer for his kind advice.
Ethical Permissions: The identity of all patients remained private.
Conflicts of Interests: None declared by the author.
Authors’ Contributions: Safaa Hussein Ali (First Author), Introduction Writer/Main Researcher Discussion Writer (65%); Ismail Ibrahim Hussein (Second Author), IMethodologist (35%).
Funding/Support: Al-Mustansiriya University supported this work.
Keywords:
References
1. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-60. [Link]
2. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281-92.e6. [Link] [DOI:10.1016/j.cell.2020.02.058] [PMID] [PMCID]
3. Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R373-81 [Link] [DOI:10.1152/ajpregu.00292.2006] [PMID] [PMCID]
4. Swanson PA 2nd, McGavern DB. Viral diseases of the central nervous system. Curr Opin Virol. 2015;11:44-54. [Link] [DOI:10.1016/j.coviro.2014.12.009] [PMID] [PMCID]
5. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-8. [Link] [DOI:10.1021/acschemneuro.0c00122] [PMID] [PMCID]
6. Abdennour L, Zeghal C, Dème M, Puybasset L. Interaction cerveau-poumon [Interaction brain-lungs]. Ann Fr Anesth Reanim. 2012;31(6):e101-7. [Link] [DOI:10.1016/j.annfar.2012.04.013] [PMID]
7. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020;126(10):1456-74. [Link] [DOI:10.1161/CIRCRESAHA.120.317015] [PMID] [PMCID]
8. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-34. [Link] [DOI:10.1016/S0140-6736(20)30628-0]
9. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. [Link] [DOI:10.3389/fncel.2018.00386] [PMID] [PMCID]
10. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-90. [Link] [DOI:10.1001/jamaneurol.2020.1127] [PMID] [PMCID]
11. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-9. [Link] [DOI:10.1001/jama.2020.1585] [PMID] [PMCID]
12. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-13. [Link] [DOI:10.1016/S0140-6736(20)30211-7]
13. Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115-9. [Link] [DOI:10.1016/j.bbi.2020.04.077] [PMID] [PMCID]
14. Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913-21. [Link] [DOI:10.1128/JVI.74.19.8913-8921.2000] [PMID] [PMCID]
15. Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1 Pt 1):e73-6. [Link] [DOI:10.1542/peds.113.1.e73] [PMID]
16. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119-20. [Link] [DOI:10.1148/radiol.2020201187] [PMID] [PMCID]
17. Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: A case report and review of recent literature. J Peripher Nerv Syst. 2020;25(2):204-7. [Link] [DOI:10.1111/jns.12382] [PMID] [PMCID]
18. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence?. Lancet Neurol. 2020;19(5):383-4. [Link] [DOI:10.1016/S1474-4422(20)30109-5]
19. Albers JW, Kelly JJ Jr. Acquired inflammatory demyelinating polyneuropathies: clinical and electrodiagnostic features. Muscle Nerve. 1989;12(6):435-51. [Link] [DOI:10.1002/mus.880120602] [PMID]
20. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388(10045):717-27. [Link] [DOI:10.1016/S0140-6736(16)00339-1]
21. Vucic S, Cairns KD, Black KR, Chong PS, Cros D. Neurophysiologic findings in early acute inflammatory demyelinating polyradiculoneuropathy. Clin Neurophysiol. 2004;115(10):2329-35. [Link] [DOI:10.1016/j.clinph.2004.05.009] [PMID]
22. Talukder RK, Sutradhar SR, Rahman KM, Uddin MJ, Akhter H. Guillian-Barre syndrome. Mymensingh Med J. 2011;20(4):748-56. [Link]
23. Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: A literature review. J Clin Neurosci. 2020;77:8-12. [Link] [DOI:10.1016/j.jocn.2020.05.017] [PMID] [PMCID]
24. Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671-83. [Link] [DOI:10.1038/s41582-019-0250-9] [PMID] [PMCID]
25. Paybast S, Gorji R, Mavandadi S. Guillain-barré syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurologist. 2020;25(4):101-3. [Link] [DOI:10.1097/NRL.0000000000000291] [PMID] [PMCID]
26. El Otmani H, El Moutawakil B, Rafai MA, El Benna N, El Kettani C, Soussi M, et al. Covid-19 and Guillain-Barré syndrome: More than a coincidence!. Rev Neurol. 2020;176(6):518-9. [Link] [DOI:10.1016/j.neurol.2020.04.007] [PMID] [PMCID]
27. Christensen SK, Ballegaard M, Boesen MS. Guillian Barré syndromeafter mRNA-1273 vaccination against COVID-19. Ugeskr Laeger. 2021;183(35):V05210455. [Danish] [Link]
28. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [Link] [DOI:10.1016/j.bbi.2020.03.031] [PMID] [PMCID]
29. Nooshin A, Zahra G. COVID-19 and central nervous system. Entry Routes and Basic and Clinical Neuroscience. 11(3.Covid19). 217-22. [Link]
30. Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. [Link] [DOI:10.1186/s40779-020-00240-0] [PMID] [PMCID]








