Volume 15, Issue 4 (2023)
Iran J War Public Health 2023, 15(4): 337-345 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/07/5 | Accepted: 2023/11/5 | Published: 2023/11/16
Received: 2023/07/5 | Accepted: 2023/11/5 | Published: 2023/11/16
How to cite this article
Aqeel Abdul Munem H. Effect of Silymarin Extract in Prevention of Intoxication of the reproductive system Induced by Nanoparticles of Cadmium in Rats. Iran J War Public Health 2023; 15 (4) :337-345
URL: http://ijwph.ir/article-1-1369-en.html
URL: http://ijwph.ir/article-1-1369-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
H. Aqeel Abdul Munem *
Department of Biology, College of Science, Al-Muthanna University, Samawah, Iraq
Full-Text (HTML) (1879 Views)
Introduction
Silybum marianum L., known as milk thistle, has been used for more than 2000 years for different diseases and has a long history as a medicinal plant in folk medicine against liver disorders, kidney problems, rheumatism, gastronomic disturbances, cardiac disorders, and gall bladder-related disorders, such as jaundice, hepatitis, and cirrhosis [1].
The oldest reported use of milk thistle was by Dioscorides, who recommended the herb as a treatment for serpent bites. Pliny the Elder (AD 23-79) reported that the juice of the plant mixed with honey was indicated for “carrying off bile”. Milk thistle was first revered as an antidote for liver toxins in the Middle Ages3,4 and was later used by the British herbalist Culpepper to relieve obstructions of the liver [2, 3]. In 1898, Eclectic physicians Felter and Lloyd recognized that the herb was good for “congestion” of the liver, spleen, and kidney [2, 3]. Native Americans have used milk thistle to treat boils and other skin diseases. Homeopathic practitioners have used preparations from the seeds to treat a variety of illnesses, including jaundice, gallstones, peritonitis, hemorrhage, bronchitis, and varicose veins [3], and currently use milk thistle to treat liver dysfunction. The German Commission E recommends its use primarily for dyspeptic complaints and liver conditions, including toxin-induced liver damage and hepatic cirrhosis, and as a supportive therapy for chronic inflammatory liver conditions [4].
Milk thistle is a tall, biennial herb that grows up to 5 to 10 feet. It is also characterized by big prickly leaves, large purple flowering heads, and strong spinescent stems. Milk thistle is named for its milky veins on the leaves [5]. The plant is indigenous to South and North America, Australia, Southern Europe, North Africa, and some regions in Asia [6]. It is traditionally used in Europe as a vegetable in salads, and the seeds are used as a galactagogue for breastfeeding mothers [7]. S. marianum has protective effects against different biological poisons (such as mycotoxins, snake venoms, and bacterial toxins) and chemical poisons (such as metals, fluoride, pesticides, cardiotoxic, neurotoxic, hepatotoxic, and nephrotoxic agents) [8].
Milk thistle contains the flavonoid silymarin. Seventy to eighty percent of the silymarin flavonolignans and 20% to 30% of a chemically undefined fraction composed primarily of polymeric and oxidized polyphenolic compounds are isolated from a standardized extract of S. marianum seeds [9]. Isosilybin accounts for 5%, silychristin for 20%, and silydianin for 10% of the silymarin complex [10]. Only by their relationship to coniferyl alcohol does the taxifolin moiety of silybin, silychristin, and silydianin differ from one another as isomers [11].
Silymarin has been shown to significantly reduce lipid peroxidation and exhibit anti-oxidant, antihypertensive, antidiabetic, and hepatoprotective effects [12, 13]. Previous research projects disclosed that S. marianum reduces the viability, adhesion, and migration of tumor cells by induction of apoptosis and formation of Reactive Oxygen Species (ROS), reducing glutathione levels, B-cell lymphoma 2 (Bcl-2), survivin, cyclin D1, Notch 1 Intracellular Domain (NICD), as well as enhancing the amount of Bcl-2-associated X protein (Bax) level [14, 15].
Recently, therapeutic preparations have been developed for the treatment of liver diseases, jaundice, and gallstones using purified silymarin extracted from the seeds, together with its main isomer silybin [11]. The powerful antioxidant silymarin may protect liver cells (as well as other cells in the body and brain) against noxious chemicals, as has been hypothesized, in such a way that glutathione oxidation is reduced and protein synthesis in liver cells is boosted [16]. Besides preventing free radical damage and promoting cellular renewal and protein production [17, 18], it also has antioxidant effects [18].
Milk thistle is available as capsules, tablets, tinctures, and intravenous solutions. Its drug interaction is low, and it has no severe effects on cytochromes P-450 [19]. Different clinical trials have shown that silymarin is safe for pharmaceutical use and bioavailable [20, 21]. Silymarin has demonstrated no significant toxicity in animals [22]. Silybin, silydianin, and silychristin have no cytotoxicity or genotoxic effects at 100μM [23]. Silymarin is also safe for humans. Hence, at therapeutic doses patients demonstrated no negative effects at the high dose of 700mg, three times a day, for 24 weeks [1]. There have been gastrointestinal discomforts such as nausea and diarrhea [24].
Based on the World Health Organization definition, infertility is defined as “a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse.” It is defined as a disability, and in global ranking for severe disability, female infertility is in the fifth rank [25]. In every four couples, one couple suffered from disability to have a child in developing countries [26]. Approximately 48.5 million couples (15% of couples) are affected by infertility worldwide. The highest infertility rate was observed in Africa and Central/Eastern Europe. 20%–30% of infertility is attributed to male factors, and 50% of them are due to female factors. Although, the percentage range of male infertility can be varied from 20-70% in different countries, but overally 20-30 % of infertility is attributed to male factors, and 50% of them are due to female factors [27].
The etiology and pathogenesis of male infertility have not been identified. Beside anatomical abnormalities and neurological disorders, some constant and environmental factors may influence male fertility. Alcohol abuse, smoking, obesity, chronic stress, urogenital trauma, reproductive system inflammation, chemicals, heavy metals, pesticides, heat, and electromagnetic radiation – these factors by triggering oxidative stress process may impact on spermatogenesis and induce infertility [28].
A great number of factors may affect sperm motility, numbers, DNA structure, and ultimately fertility. Last but not least factor is oxidative stress. Destructive environmental factors, inflammation, and infections trigger ROS generation by white blood cells and immature sperm cells in the semen. ROS dysregulates cell signaling, and it can be harmful to cellular functions, cell proliferation, and finally increase apoptosis. Enzymatic and nonenzymatic antioxidant systems protect cells against oxidative stress. Glutathione (GSH), pantothenic acid, coenzyme Q-10, carnitine, zinc, selenium, copper, and vitamins (A, E, C, and B complex) are nonenzymatic defenses. Many surveys indicate and recommend that antioxidant consumption can improve fertility [28].
Because "toxic heavy metals" such as lead, cadmium (Cd), and mercury have no known biological function in humans, they are generally avoided. These harmful metals accumulate in the environment over a long period of time because they are non-biodegradable and persistent and can disrupt several physiological systems in animals, even in very small amounts [18]. Animals exposed to heavy metals suffer from a wide range of diseases, including cancer, hepatotoxicity, nephrotoxicity, poor cognitive consequences, and altered reproductive processes [29, 30].
Environmental factors, such as expo-sure to environmental toxicants, are responsible for the remaining 77% of male infertility cases [31, 32], while pathologic diseases such varicocele and other reproductive problems account for only 23%. Oxidative stress, apoptosis, and necrosis in spermatogonial germ cells, altered steroidogenesis and morphology of testicular tissue, and decreased number and motility of spermatozoa have all been linked to Cd-induced reproductive toxicity in males, leading to decreased fertility [33, 34].
Nowadays, infertility problems impose a heavy burden on many developing countries. Consequently, many studies have focused on the effective treatment of infertility. The role of oxidative stress in both male and female infertility has been revealed. Many studies have shown protective and antioxidative properties of silymarin against adverse effects of chemotherapy medications and environmental toxins in sperms and oocytes [35]. In addition, earlier studies show that the testes are a main target organ of Cd poisoning, which may explain the recent decline in male fertility. Therefore, the present study aimed to examine the protective effect of silymarin against cadmium nanoparticle-induced toxicity in the reproductive system of rats.
Materials and Methods
Study design
In this experimental study, 60 male rats weighing 190 ±10g, aged 90 days, were selected and divided into 6 groups of 10 as follows:
• Control group: 10 male rats were gavaged distilled water for 28 days.
• T1 group: 10 male rats received cadmium (10mg/kg of body weight; Natures manufacturers; USA) for 28 days.
• T2 group: 10 male rats received silymarin (200mg/kg of body weight; Nanoshel a Nanotechnology Company; USA) for 28 days.
• T3 group: 10 male rats received cadmium (10mg/kg of body weight) for 14 days and then silymarin (200mg/kg of body weight) for 14 days.
• T4 group: 10 male rats received silymarin (200mg/kg of body weight) for 14 days and then cadmium (10mg/kg of body weight) for 14 days.
• T5 group: 10 male rats received a combination of cadmium and silymarin for 28 days.
After killing the male rats, blood tissue samples were collected, and their serum was separated. Then Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH), and testosterone were evaluated by the ELISA (Enzyme-Linked Immunosorbent Assay) method. Also, serum levels of antioxidants, including Malondialdehyde (MDA), Glutathione (GSH), and Superoxide Dismutase (SOD) were investigated.
Findings
Hormones profile
Luteinizing Hormone (LH): There was a significant decrease in the level of luteinizing hormone in male rats in the T1 group compared to the control group, while there was a significant increase in the level of luteinizing hormone in the T2 group compared to the T1 group and the control group. Also, there was an improvement in the level of hormone in the T3 group compared to the control group. The T4 group was better in the level of luteinizing hormone compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 1).
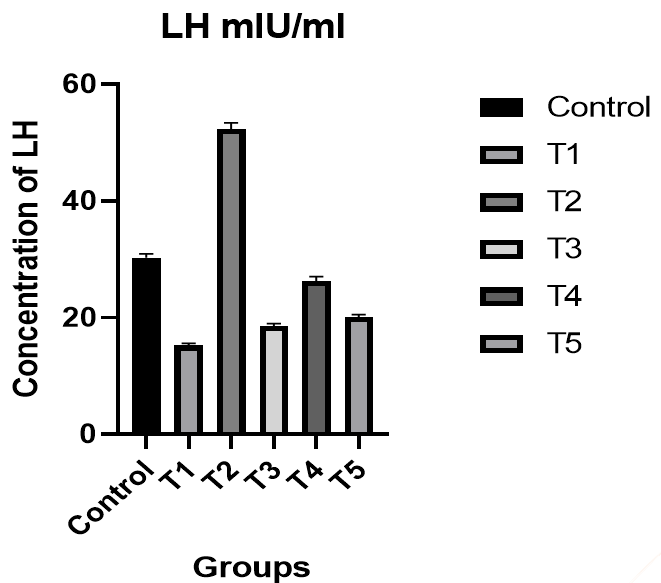
Figure 1. The effect of silymarin on the toxicity of nano-cadmium (Cd) on LH of male rats
Follicle-Stimulating Hormone (FSH): There was a significant decrease in the level of FSH in male rats in the T1 group compared to the control group, while there was a significant increase in the level of FSH in the T2 group compared to the T1 group and the control group. Also, an improvement was observed in the level of hormone in the T3 group compared to the control group. In the T4 group, the level of FSH was better compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 2).
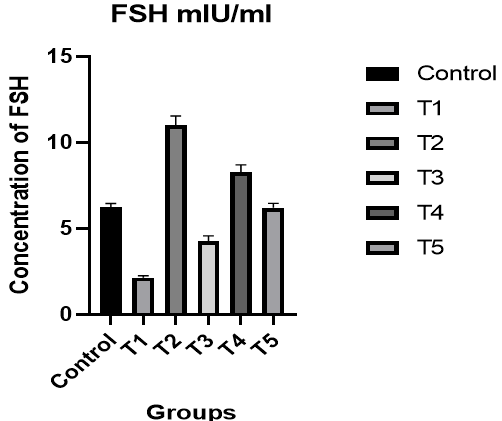
Figure 2. The effect of silymarin on the toxicity of nano-cadmium (Cd) on FSH of male rats
Testosterone: There was a significant decrease in the level of testosterone in male rats in the T1 group compared to the control group, while there was a significant increase in the level of testosterone in the T2 group compared to the T1 group and the control group. In addition, there was an improvement in the level of hormone in the T3 group compared to the control group. The level of testosterone was better in the T4 group compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 3).
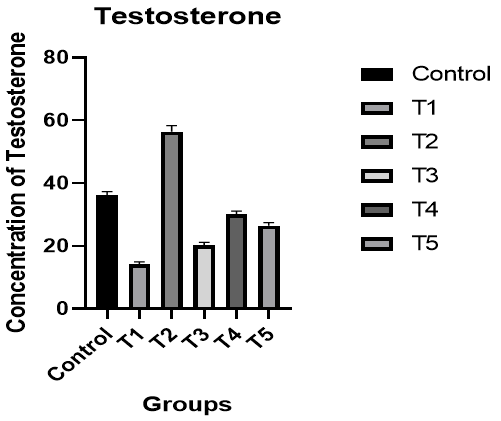
Figure 3. The effect of silymarin on the toxicity of nano-cadmium (Cd) on testosterone of male rats
Antioxidants profile
Superoxide Dismutase (SOD): A significant decrease was observed in the level of SOD in male rats of the T1 group compared to the control group, while there was a significant increase in the level of SOD in the T2 group compared to the T1 group and the control group. Additionally, there was an improvement in the level of SOD in the T3 group compared to the control group. In the T4 group, the level of SOD was better compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 4).
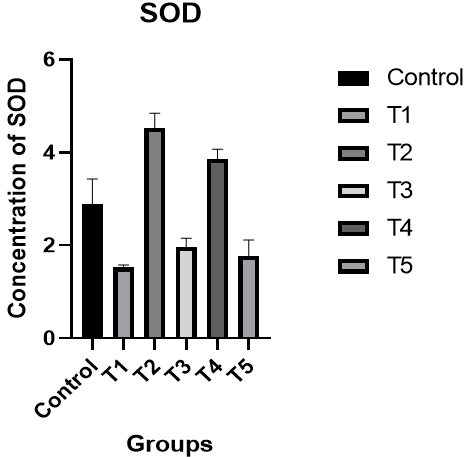
Figure 4. The effect of silymarin on the toxicity of nano-cadmium (Cd) on SOD of male rats
Glutathione (GSH): There was a significant decrease in the level of GSH in male rats in the T1 group compared to the control group, while there was a significant increase in the level of GSH in the T2 group compared to the T1 group and the control group. An improvement was shown in the level of GSH in the T3 group compared to the control group. In the T4 group, the level of GSH was better compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 5).
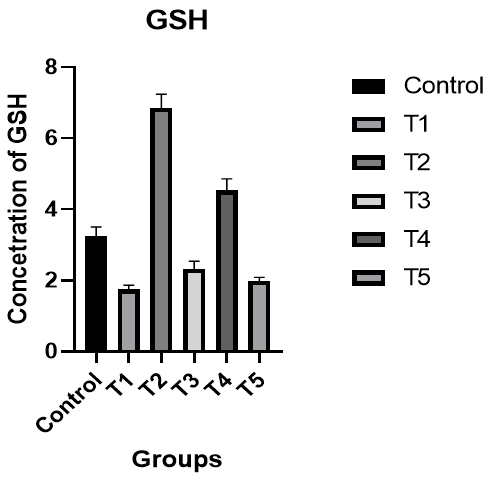
Figure 5. The effect of silymarin on the toxicity of nano-cadmium (Cd) on GSH of male rats
Malondialdehyde (MDA): There was a significant increase in the level of GSH in male rats in the T1 group compared to the control group, while there was a significant decrease in the level of MDA in the T2 group compared to the T1 group and the control group. There was an improvement in the level of MDA in the T3 group compared to the control group. In the T4 group, the level of MDA was better compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 6).
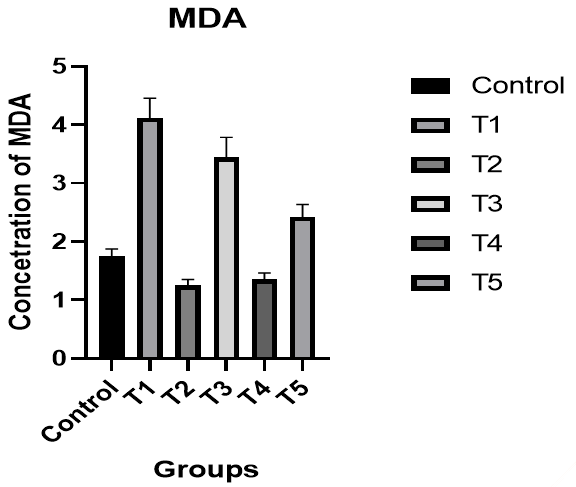
Figure 6. The effect of silymarin on the toxicity of nano-cadmium (Cd) on MDA of male rats
Discussion
In the present study, the protective effects of silymarin against nano-cadmium toxicity in the reproductive system of rats were demonstrated. Decreases in LH, FSH, and testosterone, as well as antioxidants were observed 14 and 28 days after Cd treatment. It was shown that after a longer period of time (14 and 28 days, respectively), the values of hormones and antioxidants recovered, suggesting that this effect is due to the natural defense of silymarin against nano-cd toxicity [36, 37].
In Trentacoste et al.'s study, mild food restriction resulted in reductions in serum testosterone concentration, in the weight of androgen-dependent organs, and in serum LH, FSH, and testosterone concentration [38]. This suggests that the highly significant reduction in testicular and seminal vesicle weights recorded in animals receiving Cd alone may also be related to decrease feeding. Our findings are consistent with these observations. The findings of this study confirm the results of previous studies showing that cadmium impairs testicular function [39, 40] in rats by reducing the levels of hormones and antioxidants, as well as the findings of Laskey et al. and Foote [41, 42] who observed a similar decline in progressive motility, sperm normality, and testicular structure.
Recent research has demonstrated that cadmium has an inhibitory effect on Sertoli cell function. Since this peptide is the primary inhibitory signal for FSH secretion [43], it may explain why plasma levels of FSH have been observed to rise. De Souza Predes et al. [44] also saw some shifts, and our findings corroborated them. Since cadmium accumulates in the testis, the drop-in testosterone levels seen after pre- and post-pubertal exposure may be due to direct effects of the metal there [45]. Variations in plasma LH levels may be caused by Cd's role as a major environmental endocrine disruptor [46], which suggests that the metal may act at the hypothalamic level, altering the activity of the endogenous clock, and causing a shift in the daily mean concentration of LH secreted by the pituitary gland [47].
In men, testosterone synthesis is boosted by the hypothalamic hormone Gonadotropin-Releasing Hormone (GnRH), which stimulates the anterior pituitary gland to secrete more follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This suggests that silymarin has an effect on the hypothalamus-pituitary-testis axis, as serum testosterone, LH, FSH, and GnRH all increased simultaneously. The hypothalamic-pituitary-testis axis can be affected by both positive and negative control elements. Studies reveal that the hormone norepinephrine influences the axis [48, 49]. It has been shown that silymarin raises levels of norepinephrine, serotonin, and dopamine in the brains of laboratory white mice [50]. Increased pituitary production of gonadotropin hormones has been associated to increased norepinephrine release, according to the studies. The central effects of silymarin on the hypothalamus-pituitary-testis axis, along with the synthesis and metabolism of this hormone, may account for the elevated testosterone levels seen in the present investigation. Silymarin is one of the most potent aromatase inhibitors [51]. Aromatase catalyzes the conversion of testosterone to estrogen. Blocking this enzyme has the unintended effect of increasing testosterone levels in the body [51]. Oufi et al. observed that Silybin (one of the structural isoforms of silymarin) increases sperm motility, sperm count, and sperm diameter in the testes of laboratory white mice [52]. Increased testosterone secretion is possible, too [52]. The number of spermatogenic cells in the blood is positively correlated with serum levels of the LH and FSH hormones. When FSH attaches to its receptor on the surface of Sertoli cells, cAMP levels rise due to the activation of the adenylyl cyclase enzyme, and Protein Kinase C (PKC) is activated in the cytoplasm [53]. The catalytic subunit of the ABP gene is nuclear-resident and commences transcription when the gene is activated. During the normal phase of spermatogenesis, the concentration of testosterone in the seminiferous tubules is maintained due to the increased synthesis and release of ABP (Androgen Binding Protein) induced by FSH [54]. When LH binds to Leydig cells, it induces the creation of testosterone [55]. The increase in testosterone levels, commonly known as a survival factor in spermatogenesis, is due to silymarin, which increases sperm density and quantity [56].
The use of antioxidant therapy as a diabetes treatment has shown encouraging results. However, conventional antioxidants like vitamins E and C don't appear to work [57]. We found that antioxidant molecules like superoxide dismutase and catalase were significantly elevated. Hence, the use of silymarin may counteract the pathways leading to the development of oxidative stress in diabetes. This suggests that silymarin may be able to inhibit the mechanism responsible for diabetes complications. Superoxide Dismutase (SOD), Catalase (CAT), and glutathione (non-enzymatic) antioxidants were studied as part of this investigation into the antioxidant defense system. The increased serum glutathione levels in the liver of treated rats is one putative mechanism by which silymarin reduces lipid peroxidation. Glutathione is an essential component of the cell's antioxidant defense system [58], where it serves to neutralize reactive oxygen species, detoxify hydrogen peroxide and lipid peroxides, and protect biomolecules from oxidation. Hyperlipidemia, which encourages lipid peroxidation, was associated with elevated serum MDA levels, while hypolipidemia was associated with decreased MDA levels. Therefore, the efficacy of silymarin can be assessed by comparing serum MDA levels in the control and treatment groups [59]. Silymarin's ability to reduce free radical load, boost GSH levels, and promote SOD activity suggests it can prevent the body's two principal detoxification mechanisms from being depleted. As a result, DNA and RNA are damaged, along with the rest of the cell contents [60]. The cytoprotective effects of silymarin can be attributed in great part to its antioxidant and free radical scavenging properties. Additionally, silymarin can facilitate communication between cellular membrane components and the lipid fraction crucial to maintaining fluid balance [61].
In a study by Eskandari and Momeni, oxidative stress signaling pathway induction and free radical species production were imposed on ram sperm by sodium arsenite, and it was shown that treated cells with silymarin had better motility. Silymarin, as an antioxidant, by scavenging free radicals and promoting antioxidant enzyme capacity, can improve sperm viability, motility, and mitochondrial membrane potential [62, 63]. In another study conducted by Eskandari and Momeni, the integrity of the plasma membrane and acrosome of ram epididymal spermatozoa exposed to arsenite increased significantly compared to the control group that was not treated with silymarin [64]. These two studies showed that strong antioxidant properties of silymarin protect ram sperm against the disruptive effects of arsenite. During sperm storage, lipid peroxidation (LPO) and ROS production increase, and detrimental products accumulation lead to sperm damage. Silymarin, as ROS scavenging polyphenols, can counteract with this destructive process. Addition of silymarin as a supplement for ram semen storage showed that sperm quality was improved. Supplementation with caproic acid had a better impact [65]. In another similar study, the addition of silymarin to sperm maintenance medium showed positive effects on bull sperm preservation, in both chilled and frozen condition [66].
Polyunsaturated fatty acids, which are found abundantly in the mammalian spermatozoa cell membrane, provide a vulnerable state that results in ROS production by LPO and cell's detriment. Antioxidants neutralize ROS and defend cells against injury. By assuming antioxidant activity of silymarin, Oufi et al. investigated the impacts of silibinin, the most biologically active flavonoids of silymarin, on the testicular tissue of mice. With a dose-dependent manner, a significant improvement in testosterone level and diameter of spermatid, and testicular associated factor were observed [52].
In an animal study, which was conducted by Abedi et al., the effects of silymarin on spermatogenesis, changes of testicular tissue, and hormones of the hypothalamic–pituitary–gonadal axis (LH, FSH, GnRH, and testosterone) in male rats were evaluated. Compared to the control group, experimental groups, which were treated with silymarin, showed a significant increase in LH, FSH, gonadotropin-releasing hormone and testosterone levels, and the number of spermatids and spermatozoa cells [67].
In the study of El-hanbuli et al., male albino rats were treated with testosterone intramuscularly and silymarin orally to evaluate the protective effects of silymarin against testosterone damage in the reproductive system. In the testosterone group, caspase-3 and P53 overexpression were observed. Oral silymarin had a significant role in the prevention of testosterone biochemical and histopathological adverse reactions [68]. Based on the study results of Attia et al., which was conducted to evaluate the reproductive alteration of rabbits fed milk thistle seeds and rosemary leaves, in the silymarin group (10g/kg), the sperm concentration, total sperm output, live sperm, total live sperm, total motile sperm, testosterone level, and fertility rate were significantly improved [69].
In a review by Hosseinabadi et al., the results of studies showed that antioxidants in the male reproductive system reduce oxidative stress in the testis and improve spermatogenesis. Many studies have shown the protective and antioxidant properties of silymarin against damage from chemotherapeutic drugs and environmental toxins in sperm. Also, the main published studies showed the positive effects of silymarin on increasing the quality and quantity of sperm [70]. Therefore, it is recommended that silymarin be prescribed to treat diseases caused by the effects of oxidative stress on the male reproductive system and improve fertility.
Conclusion
Silymarin has a clear effect on removing the toxic effects caused by nano-cadmium through its activity as an antioxidant and also by eliminating the toxic effect of nano-cadmium on reproductive hormones.
Acknowledgements: Nothing reported by the author.
Ethical Permission: Nothing reported by the author.
Conflict of Interests: Nothing reported by the author.
Authors’ Contribution: Aqeel Abdul Munem H (First Author), Introduction Writer/Methodologist/Main Researcher/Statistical Analyst/Discussion Writer (100%)
Funding: Nothing reported by the author.
Silybum marianum L., known as milk thistle, has been used for more than 2000 years for different diseases and has a long history as a medicinal plant in folk medicine against liver disorders, kidney problems, rheumatism, gastronomic disturbances, cardiac disorders, and gall bladder-related disorders, such as jaundice, hepatitis, and cirrhosis [1].
The oldest reported use of milk thistle was by Dioscorides, who recommended the herb as a treatment for serpent bites. Pliny the Elder (AD 23-79) reported that the juice of the plant mixed with honey was indicated for “carrying off bile”. Milk thistle was first revered as an antidote for liver toxins in the Middle Ages3,4 and was later used by the British herbalist Culpepper to relieve obstructions of the liver [2, 3]. In 1898, Eclectic physicians Felter and Lloyd recognized that the herb was good for “congestion” of the liver, spleen, and kidney [2, 3]. Native Americans have used milk thistle to treat boils and other skin diseases. Homeopathic practitioners have used preparations from the seeds to treat a variety of illnesses, including jaundice, gallstones, peritonitis, hemorrhage, bronchitis, and varicose veins [3], and currently use milk thistle to treat liver dysfunction. The German Commission E recommends its use primarily for dyspeptic complaints and liver conditions, including toxin-induced liver damage and hepatic cirrhosis, and as a supportive therapy for chronic inflammatory liver conditions [4].
Milk thistle is a tall, biennial herb that grows up to 5 to 10 feet. It is also characterized by big prickly leaves, large purple flowering heads, and strong spinescent stems. Milk thistle is named for its milky veins on the leaves [5]. The plant is indigenous to South and North America, Australia, Southern Europe, North Africa, and some regions in Asia [6]. It is traditionally used in Europe as a vegetable in salads, and the seeds are used as a galactagogue for breastfeeding mothers [7]. S. marianum has protective effects against different biological poisons (such as mycotoxins, snake venoms, and bacterial toxins) and chemical poisons (such as metals, fluoride, pesticides, cardiotoxic, neurotoxic, hepatotoxic, and nephrotoxic agents) [8].
Milk thistle contains the flavonoid silymarin. Seventy to eighty percent of the silymarin flavonolignans and 20% to 30% of a chemically undefined fraction composed primarily of polymeric and oxidized polyphenolic compounds are isolated from a standardized extract of S. marianum seeds [9]. Isosilybin accounts for 5%, silychristin for 20%, and silydianin for 10% of the silymarin complex [10]. Only by their relationship to coniferyl alcohol does the taxifolin moiety of silybin, silychristin, and silydianin differ from one another as isomers [11].
Silymarin has been shown to significantly reduce lipid peroxidation and exhibit anti-oxidant, antihypertensive, antidiabetic, and hepatoprotective effects [12, 13]. Previous research projects disclosed that S. marianum reduces the viability, adhesion, and migration of tumor cells by induction of apoptosis and formation of Reactive Oxygen Species (ROS), reducing glutathione levels, B-cell lymphoma 2 (Bcl-2), survivin, cyclin D1, Notch 1 Intracellular Domain (NICD), as well as enhancing the amount of Bcl-2-associated X protein (Bax) level [14, 15].
Recently, therapeutic preparations have been developed for the treatment of liver diseases, jaundice, and gallstones using purified silymarin extracted from the seeds, together with its main isomer silybin [11]. The powerful antioxidant silymarin may protect liver cells (as well as other cells in the body and brain) against noxious chemicals, as has been hypothesized, in such a way that glutathione oxidation is reduced and protein synthesis in liver cells is boosted [16]. Besides preventing free radical damage and promoting cellular renewal and protein production [17, 18], it also has antioxidant effects [18].
Milk thistle is available as capsules, tablets, tinctures, and intravenous solutions. Its drug interaction is low, and it has no severe effects on cytochromes P-450 [19]. Different clinical trials have shown that silymarin is safe for pharmaceutical use and bioavailable [20, 21]. Silymarin has demonstrated no significant toxicity in animals [22]. Silybin, silydianin, and silychristin have no cytotoxicity or genotoxic effects at 100μM [23]. Silymarin is also safe for humans. Hence, at therapeutic doses patients demonstrated no negative effects at the high dose of 700mg, three times a day, for 24 weeks [1]. There have been gastrointestinal discomforts such as nausea and diarrhea [24].
Based on the World Health Organization definition, infertility is defined as “a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse.” It is defined as a disability, and in global ranking for severe disability, female infertility is in the fifth rank [25]. In every four couples, one couple suffered from disability to have a child in developing countries [26]. Approximately 48.5 million couples (15% of couples) are affected by infertility worldwide. The highest infertility rate was observed in Africa and Central/Eastern Europe. 20%–30% of infertility is attributed to male factors, and 50% of them are due to female factors. Although, the percentage range of male infertility can be varied from 20-70% in different countries, but overally 20-30 % of infertility is attributed to male factors, and 50% of them are due to female factors [27].
The etiology and pathogenesis of male infertility have not been identified. Beside anatomical abnormalities and neurological disorders, some constant and environmental factors may influence male fertility. Alcohol abuse, smoking, obesity, chronic stress, urogenital trauma, reproductive system inflammation, chemicals, heavy metals, pesticides, heat, and electromagnetic radiation – these factors by triggering oxidative stress process may impact on spermatogenesis and induce infertility [28].
A great number of factors may affect sperm motility, numbers, DNA structure, and ultimately fertility. Last but not least factor is oxidative stress. Destructive environmental factors, inflammation, and infections trigger ROS generation by white blood cells and immature sperm cells in the semen. ROS dysregulates cell signaling, and it can be harmful to cellular functions, cell proliferation, and finally increase apoptosis. Enzymatic and nonenzymatic antioxidant systems protect cells against oxidative stress. Glutathione (GSH), pantothenic acid, coenzyme Q-10, carnitine, zinc, selenium, copper, and vitamins (A, E, C, and B complex) are nonenzymatic defenses. Many surveys indicate and recommend that antioxidant consumption can improve fertility [28].
Because "toxic heavy metals" such as lead, cadmium (Cd), and mercury have no known biological function in humans, they are generally avoided. These harmful metals accumulate in the environment over a long period of time because they are non-biodegradable and persistent and can disrupt several physiological systems in animals, even in very small amounts [18]. Animals exposed to heavy metals suffer from a wide range of diseases, including cancer, hepatotoxicity, nephrotoxicity, poor cognitive consequences, and altered reproductive processes [29, 30].
Environmental factors, such as expo-sure to environmental toxicants, are responsible for the remaining 77% of male infertility cases [31, 32], while pathologic diseases such varicocele and other reproductive problems account for only 23%. Oxidative stress, apoptosis, and necrosis in spermatogonial germ cells, altered steroidogenesis and morphology of testicular tissue, and decreased number and motility of spermatozoa have all been linked to Cd-induced reproductive toxicity in males, leading to decreased fertility [33, 34].
Nowadays, infertility problems impose a heavy burden on many developing countries. Consequently, many studies have focused on the effective treatment of infertility. The role of oxidative stress in both male and female infertility has been revealed. Many studies have shown protective and antioxidative properties of silymarin against adverse effects of chemotherapy medications and environmental toxins in sperms and oocytes [35]. In addition, earlier studies show that the testes are a main target organ of Cd poisoning, which may explain the recent decline in male fertility. Therefore, the present study aimed to examine the protective effect of silymarin against cadmium nanoparticle-induced toxicity in the reproductive system of rats.
Materials and Methods
Study design
In this experimental study, 60 male rats weighing 190 ±10g, aged 90 days, were selected and divided into 6 groups of 10 as follows:
• Control group: 10 male rats were gavaged distilled water for 28 days.
• T1 group: 10 male rats received cadmium (10mg/kg of body weight; Natures manufacturers; USA) for 28 days.
• T2 group: 10 male rats received silymarin (200mg/kg of body weight; Nanoshel a Nanotechnology Company; USA) for 28 days.
• T3 group: 10 male rats received cadmium (10mg/kg of body weight) for 14 days and then silymarin (200mg/kg of body weight) for 14 days.
• T4 group: 10 male rats received silymarin (200mg/kg of body weight) for 14 days and then cadmium (10mg/kg of body weight) for 14 days.
• T5 group: 10 male rats received a combination of cadmium and silymarin for 28 days.
After killing the male rats, blood tissue samples were collected, and their serum was separated. Then Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH), and testosterone were evaluated by the ELISA (Enzyme-Linked Immunosorbent Assay) method. Also, serum levels of antioxidants, including Malondialdehyde (MDA), Glutathione (GSH), and Superoxide Dismutase (SOD) were investigated.
Findings
Hormones profile
Luteinizing Hormone (LH): There was a significant decrease in the level of luteinizing hormone in male rats in the T1 group compared to the control group, while there was a significant increase in the level of luteinizing hormone in the T2 group compared to the T1 group and the control group. Also, there was an improvement in the level of hormone in the T3 group compared to the control group. The T4 group was better in the level of luteinizing hormone compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 1).

Figure 1. The effect of silymarin on the toxicity of nano-cadmium (Cd) on LH of male rats
Follicle-Stimulating Hormone (FSH): There was a significant decrease in the level of FSH in male rats in the T1 group compared to the control group, while there was a significant increase in the level of FSH in the T2 group compared to the T1 group and the control group. Also, an improvement was observed in the level of hormone in the T3 group compared to the control group. In the T4 group, the level of FSH was better compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 2).

Figure 2. The effect of silymarin on the toxicity of nano-cadmium (Cd) on FSH of male rats
Testosterone: There was a significant decrease in the level of testosterone in male rats in the T1 group compared to the control group, while there was a significant increase in the level of testosterone in the T2 group compared to the T1 group and the control group. In addition, there was an improvement in the level of hormone in the T3 group compared to the control group. The level of testosterone was better in the T4 group compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 3).

Figure 3. The effect of silymarin on the toxicity of nano-cadmium (Cd) on testosterone of male rats
Antioxidants profile
Superoxide Dismutase (SOD): A significant decrease was observed in the level of SOD in male rats of the T1 group compared to the control group, while there was a significant increase in the level of SOD in the T2 group compared to the T1 group and the control group. Additionally, there was an improvement in the level of SOD in the T3 group compared to the control group. In the T4 group, the level of SOD was better compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 4).

Figure 4. The effect of silymarin on the toxicity of nano-cadmium (Cd) on SOD of male rats
Glutathione (GSH): There was a significant decrease in the level of GSH in male rats in the T1 group compared to the control group, while there was a significant increase in the level of GSH in the T2 group compared to the T1 group and the control group. An improvement was shown in the level of GSH in the T3 group compared to the control group. In the T4 group, the level of GSH was better compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 5).

Figure 5. The effect of silymarin on the toxicity of nano-cadmium (Cd) on GSH of male rats
Malondialdehyde (MDA): There was a significant increase in the level of GSH in male rats in the T1 group compared to the control group, while there was a significant decrease in the level of MDA in the T2 group compared to the T1 group and the control group. There was an improvement in the level of MDA in the T3 group compared to the control group. In the T4 group, the level of MDA was better compared to the T1 and T3 groups and the control group, while the results of the T5 group were better than the T1 and T3 groups (p<0.05; Figure 6).

Figure 6. The effect of silymarin on the toxicity of nano-cadmium (Cd) on MDA of male rats
Discussion
In the present study, the protective effects of silymarin against nano-cadmium toxicity in the reproductive system of rats were demonstrated. Decreases in LH, FSH, and testosterone, as well as antioxidants were observed 14 and 28 days after Cd treatment. It was shown that after a longer period of time (14 and 28 days, respectively), the values of hormones and antioxidants recovered, suggesting that this effect is due to the natural defense of silymarin against nano-cd toxicity [36, 37].
In Trentacoste et al.'s study, mild food restriction resulted in reductions in serum testosterone concentration, in the weight of androgen-dependent organs, and in serum LH, FSH, and testosterone concentration [38]. This suggests that the highly significant reduction in testicular and seminal vesicle weights recorded in animals receiving Cd alone may also be related to decrease feeding. Our findings are consistent with these observations. The findings of this study confirm the results of previous studies showing that cadmium impairs testicular function [39, 40] in rats by reducing the levels of hormones and antioxidants, as well as the findings of Laskey et al. and Foote [41, 42] who observed a similar decline in progressive motility, sperm normality, and testicular structure.
Recent research has demonstrated that cadmium has an inhibitory effect on Sertoli cell function. Since this peptide is the primary inhibitory signal for FSH secretion [43], it may explain why plasma levels of FSH have been observed to rise. De Souza Predes et al. [44] also saw some shifts, and our findings corroborated them. Since cadmium accumulates in the testis, the drop-in testosterone levels seen after pre- and post-pubertal exposure may be due to direct effects of the metal there [45]. Variations in plasma LH levels may be caused by Cd's role as a major environmental endocrine disruptor [46], which suggests that the metal may act at the hypothalamic level, altering the activity of the endogenous clock, and causing a shift in the daily mean concentration of LH secreted by the pituitary gland [47].
In men, testosterone synthesis is boosted by the hypothalamic hormone Gonadotropin-Releasing Hormone (GnRH), which stimulates the anterior pituitary gland to secrete more follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This suggests that silymarin has an effect on the hypothalamus-pituitary-testis axis, as serum testosterone, LH, FSH, and GnRH all increased simultaneously. The hypothalamic-pituitary-testis axis can be affected by both positive and negative control elements. Studies reveal that the hormone norepinephrine influences the axis [48, 49]. It has been shown that silymarin raises levels of norepinephrine, serotonin, and dopamine in the brains of laboratory white mice [50]. Increased pituitary production of gonadotropin hormones has been associated to increased norepinephrine release, according to the studies. The central effects of silymarin on the hypothalamus-pituitary-testis axis, along with the synthesis and metabolism of this hormone, may account for the elevated testosterone levels seen in the present investigation. Silymarin is one of the most potent aromatase inhibitors [51]. Aromatase catalyzes the conversion of testosterone to estrogen. Blocking this enzyme has the unintended effect of increasing testosterone levels in the body [51]. Oufi et al. observed that Silybin (one of the structural isoforms of silymarin) increases sperm motility, sperm count, and sperm diameter in the testes of laboratory white mice [52]. Increased testosterone secretion is possible, too [52]. The number of spermatogenic cells in the blood is positively correlated with serum levels of the LH and FSH hormones. When FSH attaches to its receptor on the surface of Sertoli cells, cAMP levels rise due to the activation of the adenylyl cyclase enzyme, and Protein Kinase C (PKC) is activated in the cytoplasm [53]. The catalytic subunit of the ABP gene is nuclear-resident and commences transcription when the gene is activated. During the normal phase of spermatogenesis, the concentration of testosterone in the seminiferous tubules is maintained due to the increased synthesis and release of ABP (Androgen Binding Protein) induced by FSH [54]. When LH binds to Leydig cells, it induces the creation of testosterone [55]. The increase in testosterone levels, commonly known as a survival factor in spermatogenesis, is due to silymarin, which increases sperm density and quantity [56].
The use of antioxidant therapy as a diabetes treatment has shown encouraging results. However, conventional antioxidants like vitamins E and C don't appear to work [57]. We found that antioxidant molecules like superoxide dismutase and catalase were significantly elevated. Hence, the use of silymarin may counteract the pathways leading to the development of oxidative stress in diabetes. This suggests that silymarin may be able to inhibit the mechanism responsible for diabetes complications. Superoxide Dismutase (SOD), Catalase (CAT), and glutathione (non-enzymatic) antioxidants were studied as part of this investigation into the antioxidant defense system. The increased serum glutathione levels in the liver of treated rats is one putative mechanism by which silymarin reduces lipid peroxidation. Glutathione is an essential component of the cell's antioxidant defense system [58], where it serves to neutralize reactive oxygen species, detoxify hydrogen peroxide and lipid peroxides, and protect biomolecules from oxidation. Hyperlipidemia, which encourages lipid peroxidation, was associated with elevated serum MDA levels, while hypolipidemia was associated with decreased MDA levels. Therefore, the efficacy of silymarin can be assessed by comparing serum MDA levels in the control and treatment groups [59]. Silymarin's ability to reduce free radical load, boost GSH levels, and promote SOD activity suggests it can prevent the body's two principal detoxification mechanisms from being depleted. As a result, DNA and RNA are damaged, along with the rest of the cell contents [60]. The cytoprotective effects of silymarin can be attributed in great part to its antioxidant and free radical scavenging properties. Additionally, silymarin can facilitate communication between cellular membrane components and the lipid fraction crucial to maintaining fluid balance [61].
In a study by Eskandari and Momeni, oxidative stress signaling pathway induction and free radical species production were imposed on ram sperm by sodium arsenite, and it was shown that treated cells with silymarin had better motility. Silymarin, as an antioxidant, by scavenging free radicals and promoting antioxidant enzyme capacity, can improve sperm viability, motility, and mitochondrial membrane potential [62, 63]. In another study conducted by Eskandari and Momeni, the integrity of the plasma membrane and acrosome of ram epididymal spermatozoa exposed to arsenite increased significantly compared to the control group that was not treated with silymarin [64]. These two studies showed that strong antioxidant properties of silymarin protect ram sperm against the disruptive effects of arsenite. During sperm storage, lipid peroxidation (LPO) and ROS production increase, and detrimental products accumulation lead to sperm damage. Silymarin, as ROS scavenging polyphenols, can counteract with this destructive process. Addition of silymarin as a supplement for ram semen storage showed that sperm quality was improved. Supplementation with caproic acid had a better impact [65]. In another similar study, the addition of silymarin to sperm maintenance medium showed positive effects on bull sperm preservation, in both chilled and frozen condition [66].
Polyunsaturated fatty acids, which are found abundantly in the mammalian spermatozoa cell membrane, provide a vulnerable state that results in ROS production by LPO and cell's detriment. Antioxidants neutralize ROS and defend cells against injury. By assuming antioxidant activity of silymarin, Oufi et al. investigated the impacts of silibinin, the most biologically active flavonoids of silymarin, on the testicular tissue of mice. With a dose-dependent manner, a significant improvement in testosterone level and diameter of spermatid, and testicular associated factor were observed [52].
In an animal study, which was conducted by Abedi et al., the effects of silymarin on spermatogenesis, changes of testicular tissue, and hormones of the hypothalamic–pituitary–gonadal axis (LH, FSH, GnRH, and testosterone) in male rats were evaluated. Compared to the control group, experimental groups, which were treated with silymarin, showed a significant increase in LH, FSH, gonadotropin-releasing hormone and testosterone levels, and the number of spermatids and spermatozoa cells [67].
In the study of El-hanbuli et al., male albino rats were treated with testosterone intramuscularly and silymarin orally to evaluate the protective effects of silymarin against testosterone damage in the reproductive system. In the testosterone group, caspase-3 and P53 overexpression were observed. Oral silymarin had a significant role in the prevention of testosterone biochemical and histopathological adverse reactions [68]. Based on the study results of Attia et al., which was conducted to evaluate the reproductive alteration of rabbits fed milk thistle seeds and rosemary leaves, in the silymarin group (10g/kg), the sperm concentration, total sperm output, live sperm, total live sperm, total motile sperm, testosterone level, and fertility rate were significantly improved [69].
In a review by Hosseinabadi et al., the results of studies showed that antioxidants in the male reproductive system reduce oxidative stress in the testis and improve spermatogenesis. Many studies have shown the protective and antioxidant properties of silymarin against damage from chemotherapeutic drugs and environmental toxins in sperm. Also, the main published studies showed the positive effects of silymarin on increasing the quality and quantity of sperm [70]. Therefore, it is recommended that silymarin be prescribed to treat diseases caused by the effects of oxidative stress on the male reproductive system and improve fertility.
Conclusion
Silymarin has a clear effect on removing the toxic effects caused by nano-cadmium through its activity as an antioxidant and also by eliminating the toxic effect of nano-cadmium on reproductive hormones.
Acknowledgements: Nothing reported by the author.
Ethical Permission: Nothing reported by the author.
Conflict of Interests: Nothing reported by the author.
Authors’ Contribution: Aqeel Abdul Munem H (First Author), Introduction Writer/Methodologist/Main Researcher/Statistical Analyst/Discussion Writer (100%)
Funding: Nothing reported by the author.
Keywords:
Milk Thistle [MeSH], Silymarin [MeSH], Cadmium [MeSH], Male Reproductive System [MeSH], Antioxidant [MeSH], Rat [MeSH]
References
1. Ansari D, Tingstedt B, Andersson B, Holmquist F, Sturesson C, Williamsson C, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. 2016;12(16):1929-46. [Link] [DOI:10.2217/fon-2016-0010]
2. Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93(2):139-43. [Link] [DOI:10.1111/j.1572-0241.1998.00139.x]
3. Pepping J. Milk thistle: Silybum marianum. Am J Health Syst Pharm. 1999;56(12):1195-7. [Link] [DOI:10.1093/ajhp/56.12.1195]
4. Blumenthal M, Busse WR, eds. The Complete German commission e monographs: Therapeutic guide to herbal medicines. Austin, Texas: American Botanical Council; 1999. [Link]
5. Becker-Schiebe M, Mengs U, Schaefer M, Bulitta M, Hoffmann W. Topical use of a silymarin-based preparation to prevent radiodermatitis: results of a prospective study in breast cancer patients. Strahlenther Onkol. 2011;187(8):485-91. [Link] [DOI:10.1007/s00066-011-2204-z]
6. Bhatia N, Agarwal C, Agarwal R. Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma A431 cells. Nutr Cancer. 2001;39(2):292-9. [Link] [DOI:10.1207/S15327914nc392_20]
7. Emadi SA, Ghasemzadeh Rahbardar M, Mehri S, Hosseinzadeh H. A review of therapeutic potentials of milk thistle ( Silybum marianum L.) and its main constituent, silymarin, on cancer, and their related patents. Iran J Basic Med Sci. 2022;25(10):1166-76. [Link]
8. Brandon-Warner E, Eheim AL, Foureau DM, Walling TL, Schrum LW, McKillop IH. Silibinin (Milk Thistle) potentiates ethanol-dependent hepatocellular carcinoma progression in male mice. Cancer Lett. 2012;326(1):88-95. [Link] [DOI:10.1016/j.canlet.2012.07.028]
9. Křen V, Walterová D. Silybin and silymarin - new effects and application. Biomedical Papers. 2005;149(1):29-41. [Link] [DOI:10.5507/bp.2005.002]
10. Radjabian T, Huseini HF. Anti-hyperlipidemic and anti-atherosclerotic activities of silymarins from cultivated and wild plants of Silybum marianum L. with different content of flavonolignans. Iran J Pharmacol Ther. 2010;9(2):63-0. [Link]
11. Hahn G, Lehmann HD, Kürten M, Uebel H, Vogel G. On the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) Gaertn. Arzneimittelforschung. 1968;18(6):698-704. [German] [Link]
12. Brantley SJ, Oberlies NH, Kroll DJ, Paine MF. Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations. J Pharmacol Exp Ther. 2010;332(3):1081-7. [Link] [DOI:10.1124/jpet.109.161927]
13. Chambers CS, Holečková V, Petrásková L, Biedermann D, Valentová K, Buchta M, et al. The silymarin composition… and why does it matter? Food Res Int. 2017;100(Pt 3):339-53. [Link] [DOI:10.1016/j.foodres.2017.07.017]
14. Chen CH, Huang TS, Wong CH, Hong CL, Tsai YH, Liang CC, et al. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem Toxicol. 2009;47(3):638-44. [Link] [DOI:10.1016/j.fct.2008.12.024]
15. Fehér P, Ujhelyi Z, Váradi J, Fenyvesi F, Róka E, Juhász B, et al. Efficacy of pre- and post-treatment by topical formulations containing dissolved and suspended Silybum marianum against UVB-induced oxidative stress in guinea pig and on HaCaT keratinocytes. Molecules. 2016;21(10):1269. [Link] [DOI:10.3390/molecules21101269]
16. Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035-63. [Link] [DOI:10.2165/00003495-200161140-00003]
17. Katiya SK, Korman NJ, Mukhtar H, Agrawal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89(8):556-66. [Link] [DOI:10.1093/jnci/89.8.556]
18. Manfo FP, Moundipa PF, Dechaud H, Tchana AN, Nantia EA, Zabot MT, et al. Effect of agropesticides use on male reproductive function: a study on farmers in Djutitsa (Cameroon). Environ Toxicol. 2012;27(7):423-32. [Link] [DOI:10.1002/tox.20656]
19. Gillessen A, Schmidt HHJ. Silymarin as supportive treatment in liver diseases: a narrative review. Adv Ther. 2020;37(4):1279-301. [Link] [DOI:10.1007/s12325-020-01251-y]
20. Deep G, Agarwal R. Chemopreventive efficacy of silymarin in skin and prostate cancer. Integr Cancer Ther. 2007;6(2):130-45. [Link] [DOI:10.1177/1534735407301441]
21. Hackett ES, Twedt DC, Gustafson DL. Milk thistle and its derivative compounds: a review of opportunities for treatment of liver disease. J Vet Intern Med. 2013;27(1):10-6. [Link] [DOI:10.1111/jvim.12002]
22. Hosseini S, Rezaei S, Moghaddam MRN, Elyasi S, Karimi G. Evaluation of oral nano-silymarin formulation efficacy on prevention of radiotherapy induced mucositis: A randomized, double-blinded, placebo-controlled clinical trial. PharmaNutrition. 2021;15:100253. [Link] [DOI:10.1016/j.phanu.2021.100253]
23. Bijak M, Synowiec E, Sitarek P, Sliwiński T, Saluk-Bijak J. Evaluation of the cytotoxicity and genotoxicity of flavonolignans in different cellular models. Nutrients. 2017;9(12):1356. [Link] [DOI:10.3390/nu9121356]
24. Soleimani V, Delghandi PS, Moallem SA, Karimi G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother Res. 2019;33(6):1627-38. [Link] [DOI:10.1002/ptr.6361]
25. World Health Organization. Infertility [Internet]. Geneva: World Health Organization;2023 [cited 2023 July 27]. Available from: https://www.who.int/news-room/fact-sheets/detail/infertility [Link]
26. World Health Organization. Infecundity, infertility, and childlessness in Developing Countries - DHS comparative reports No. 9 [Internet]. Geneva: World Health Organization;2004 [cited 2023 July 27]. Available from: https://www.who.int/publications/m/item/infecundity-infertility-and-childlessness-in-developing-countries---dhs-comparative-reports-no.-9 [Link]
27. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. [Link] [DOI:10.1186/s12958-015-0032-1]
28. Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66(1):60-7. [Link] [DOI:10.5173/ceju.2013.01.art19]
29. Lam HS, Kwok KM, Chan PH, So HK, Li AM, Ng PC, et al. Long-term neurocognitive impact of low dose prenatal methylmercury exposure in Hong Kong. Environ Int. 2013;54,59-64. [Link] [DOI:10.1016/j.envint.2013.01.005]
30. Rani A, Kumar A, Lal A, Pant M. Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res. 2014;24(4):378-99. [Link] [DOI:10.1080/09603123.2013.835032]
31. Bhardwaj JK, Kumari P, Saraf P, Yadav AS. Antiapoptotic effects of vitamins C and E against cypermethrin-induced oxidative stress and spermatogonial germ cell apoptosis. J Biochem Mol Toxicol. 2018;32(8):e22174. [Link] [DOI:10.1002/jbt.22174]
32. Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010;16(6):690-703. [Link] [DOI:10.1093/humupd/dmq018]
33. Cupertino MC, Novaes RD, Santos EC, Neves AC, Silva E, Oliveira JA, et al. Differential susceptibility of germ and Leydig cells to cadmium-mediated toxicity: impact on testis structure, adiponectin levels, and steroidogenesis. Oxid Medi Cell Longev. 2017;2017:3405089. [Link] [DOI:10.1155/2017/3405089]
34. Zhao LJ, Ru YF, Liu M, Tang JN, Zheng JF, Wu B, et al. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS One. 2017;12(11):e0186727. [Link] [DOI:10.1371/journal.pone.0186727]
35. Zarif-Yeganeh M, Rastegarpanah M. Clinical role of silymarin in oxidative stress and infertility: a short review for pharmacy practitioners. J Res Pharm Pract. 2019;8(4):181-8. [Link] [DOI:10.4103/jrpp.JRPP_18_100]
36. Gupta RS, Sharma R, Chaudhary R, Yadav RY, Khan TI. Effect of textile waste water on the spermatogenesis of male albino rats. J Appl Toxicol. 2003;23(3):171-5. [Link] [DOI:10.1002/jat.862]
37. El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food Chem Toxicol. 2004;42(10):1563-71. [Link] [DOI:10.1016/j.fct.2004.05.001]
38. Trentacoste SV, Friedman AS, Youker RT, Breckenridge CB, Zirkin BR. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Andro. 2001;22(1):142-8. [Link] [DOI:10.1002/j.1939-4640.2001.tb02164.x]
39. Lafuente A, Esquifino AI. Cadmium effects on hypothalamic activity and pituitary hormone secretion in the male. Toxicol Lett. 1999;110(3):209-18. [Link] [DOI:10.1016/S0378-4274(99)00159-9]
40. Garside DA, Harvey PW. Endocrine toxicology of the male reproductive system. In: Atterwill CK, Flack JD, editors. Endocrine Toxicology. Cambridge: Cambridge University Press; 1992. Pp.285-312. [Link]
41. Laskey JW, Rehnberg GL, Laws SC, Hein JF. Reproductive effects of low acute doses of cadmium chloride in adult male rats. Toxicol Appl Pharmacol. 1984;73(2):250-5. [Link] [DOI:10.1016/0041-008X(84)90330-2]
42. Foote RH. Cadmium affects testes and semen of rabbits exposed before and after puberty. Reprod Toxicol. 1999;13(4):269-77. [Link] [DOI:10.1016/S0890-6238(99)00019-2]
43. Manna P, Sinha M, Sil PC. Cadmium induced testicular pathophysiology: Prophylactic role of taurine. Reprod Toxicol. 2008;26(3-4):282-91. [Link] [DOI:10.1016/j.reprotox.2008.09.009]
44. de Souza Predes F, Diamante MAS, Dolder H. Testis response to low doses of cadmium in Wistar rats. Int J Exp Pathol. 2010;91(2):125-31. [Link] [DOI:10.1111/j.1365-2613.2009.00692.x]
45. Antonio MT, Corpas I, Leret ML. Neurochemical changes in newborn rat's brain after gestational cadmium and lead exposure. Toxicol Lett. 1999;104(1-4):1-9. [Link] [DOI:10.1016/S0378-4274(98)00125-8]
46. López-Artíguez M, Soria ML, Cameán A, Repetto M. Cadmium in the diet of the local population of Seville (Spain). Bull Environ Contam Toxicol. 1993;50(3):417-24. [Link] [DOI:10.1007/BF00197203]
47. Min L, Li-li L. Research progress on female reproductive toxicity of cadmium. J Environ Occup Med. 2019;36(1):57-62. [Japanese] [Link]
48. Shirama K, Furuya T, Takeo Y, Shimizu K, Maekawa A. Influence of pinealectomy on circadian patterns of plasma luteinizing hormone, follicle-stimulating hormone, testosterone and dihydrotestosterone in the male rat. J Endocrinol Invest. 1982;5(6):397-401. [Link] [DOI:10.1007/BF03350540]
49. Selvage DJ, Johnston CA. Interaction between norepinephrine, oxytocin, and nitric oxide in the stimulation of gonadotropin-releasing hormone release from proestrous rat basal hypothalamus explants. J Neuroendocrinol. 2004;16(10):819-24. [Link] [DOI:10.1111/j.1365-2826.2004.01235.x]
50. Całka J. The role of nitric oxide in the hypothalamic control of LHRH and oxytocin release, sexual behavior and aging of the LHRH and oxytocin neurons. Folia Histochem Cytobiol. 2006;44(1):3-12. [Link]
51. Osuchowski MF, Johnson VJ, He Q, Sharma RP. Alteration in regional brain neur-otransmitters by silymarin, a natural antioxidant flavonoid mixture, in BALB/c mice. Pharm Biol. 2004;42(4-5):384-9. [Link] [DOI:10.1080/13880200490519712]
52. Oufi HG, Al-Shawi NN, Hussain S. What are the effects of Silibin in on testicular tissue of mice? J Appl Pharm Sci. 2012;2(11):9-13. [Link]
53. Adam JA, Menhere PPCA, Van Dielen FM, Soeters PB. Decreased plasma Orexin A level in obese individuals. Int J Obes Relat Metab Disord. 2002;26(2):274-6. [Link] [DOI:10.1038/sj.ijo.0801868]
54. Walker S, Robison OW, Whisnant CS, CassadyS JP. Effect of divergent selection for testosterone production on testicular morphology and daily sperm production in boars. J Anim Sci. 2004;82(8):2259-63. [Link] [DOI:10.2527/2004.8282259x]
55. Carlson BM. Human embryology and developmental biology. 3rd Edition. Philadelphia: Mosby; 2004. [Link]
56. Guyton AC, Hall JE, editors. Text book of medical physiology. 11th Edition. New York: Elsevier Saunders; 2006. [Link]
57. Ceriello A, Testa R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S232-6. [Link] [DOI:10.2337/dc09-S316]
58. Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52(12):4026-37. [Link] [DOI:10.1021/jf049696w]
59. Shukla R, Gupta S, Shukla R, Bhatia S, Gambhir JK, Prabhu KM, et al. Antioxidant effect of aqueous extract of the bark of Ficus bengalensis in hypercholesterolemic rabbits. J Ethanopharmacol. 2004;92(1):47-51. [Link] [DOI:10.1016/j.jep.2004.01.020]
60. Wisemann H. Dietary influences on membrane function: Importance in protection against oxidative damage and disease. J Nutr Biochem. 1996;7(1):2-15. [Link] [DOI:10.1016/0955-2863(95)00152-2]
61. Muriel P, Mourelle M. The role of membrane composition in ATPase activities of cirrhotic rat liver: effect of silymarin. J Appl Toxicol. 1990;10(4):281-4. [Link] [DOI:10.1002/jat.2550100409]
62. Zarban A, Ziaee M. Evaluation of antioxidant properties of silymarin and its potential to inhibit peroxyl radicals in vitro. Pak J Pharm Sci. 2008;21(3):249-54. [Link]
63. Eskandari F, Momeni HR. Protective effect of silymarin on viability, motility and mitochondrial membrane potential of ram sperm treated with sodium arsenite. Int J Reprod Biomed. 2016;14(6):397-402. [Link] [DOI:10.29252/ijrm.14.6.397]
64. Eskandari F, Momeni HR. Silymarin protects plasma membrane and acrosome integrity in sperm treated with sodium arsenite. Int J Reprod Biomed. 2016;14(1):47-52. [Link] [DOI:10.29252/ijrm.14.1.47]
65. Roostaei-Ali Mehr M, Parisoush P. Effect of different levels of silymarin and caproic acid on storage of ram semen in liquid form. Reprod Domest Anim. 2016;51(4):569-74. [Link] [DOI:10.1111/rda.12721]
66. El-Sheshtawy R, El-Nattat W. Impact of silymarin enriched semen extender on bull sperm preservability. Asian Pac J Reprod. 2017;6(2):81-4. [Link] [DOI:10.12980/apjr.6.20170206]
67. Abedi H, Jahromi HK, Hashemi SA, Jashni HK, Jahromi ZK, Pourahmadi M. The effect of silymarin on spermatogenesis process in rats. Int Sci J Med Res Health Sci. 2016;5:146-50. [Link]
68. El-hanbuli HM, Abo-Sief AF, Mostafa T. Protective effect of silymarin on the testes of rats treated with anabolic androgenic steroid: A biochemical, histological, histochemical and immunohistochemical study. J Histol Histopathol. 2017;4(1):10. [Link] [DOI:10.7243/2055-091X-4-10]
69. Attia YA, Hamed RS, Bovera F, Abd El-Hamid AE, Al-Harthi MA, Shahba HA. Semen quality, antioxidant status and reproductive performance of rabbits bucks fed milk thistle seeds and rosemary leaves. Anim Reprod Sci. 2017;184:178-86. [Link] [DOI:10.1016/j.anireprosci.2017.07.014]
70. Hosseinabadi F, Faraji T, Malmir M, Mohamadi H. Ameliorative impact of silymarin on the male reproductive system: an updated systematic review. Jorjani Biomed J. 2022;10(2):10-23. [Link] [DOI:10.52547/jorjanibiomedj.10.2.10]









