Volume 15, Issue 4 (2023)
Iran J War Public Health 2023, 15(4): 421-428 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/07/5 | Accepted: 2024/01/18 | Published: 2024/01/28
Received: 2023/07/5 | Accepted: 2024/01/18 | Published: 2024/01/28
How to cite this article
Saeed Z, Jaafar J, Najim R. Comparing the Effect of Ketamine and Fentanyl in Emergence Agitation under Sevoflurane Anesthesia in Tonsillectomy. Iran J War Public Health 2023; 15 (4) :421-428
URL: http://ijwph.ir/article-1-1368-en.html
URL: http://ijwph.ir/article-1-1368-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Faculty of Pharmacy, University of Kufa, Kufa, Iraq
2- Faculty of Medicine, University of Kufa, Kufa, Iraq
3- Ministry of Health, Al-Sadar Teaching Hospital, Kufa, Iraq
2- Faculty of Medicine, University of Kufa, Kufa, Iraq
3- Ministry of Health, Al-Sadar Teaching Hospital, Kufa, Iraq
Full-Text (HTML) (962 Views)
Introduction
Emergence agitation (EA) is an acute confusion state during recovery from anesthesia, characterized by disorientation, hallucination, restlessness, and purposeless hyperactive physical behavior. The exact mechanism of EA after general anesthesia is unclear [1-4].
In children, causes of EA include a high degree of anxiety related to surgery, full bladder (or obstructed urinary catheter), new surroundings, separation from parents, and encounter with foreign medical staff. These may increase sympathetic activity and prolong the excited state during anesthesia recovery [5-7]. It can be harmful to the child, like bleeding, self-injury, parents' distress, risks of falling, injury to the operative site, delayed medical treatments, the stress caused to both health professionals and families, and the need for continuous monitoring of patients by recovery room staff, and physical checking of patient, delay discharge and supplemental sedative and analgesic drugs [8]. It is more common after certain anesthetic agents (sevoflurane-desflurane-isoflurane>halothane-TIVA), lasts 10 to 15 minutes, and is discontinued spontaneously or after an IV dose of propofol, ketamine, opioids, midazolam, dexmedetomidine, clonidine, or any other medications [9].
Risk factors of EA include age, male gender, type of surgical procedure (hernia repair, genitourinary procedure), emergency surgery, use of volatile anesthetics with low blood solubility, long duration of surgery, use of anticholinergic drugs, phenothiazine, too rapid awakening, voiding surgery, post-operative pain, and the presence of invasive devices. No sole factor can explain the etiology of emergence agitation. Although pain is a major cause of emergence agitation, screaming due to pain should be distinguished from emergence agitation. However, especially in younger children, it is sometimes difficult to distinguish between them. It is believed reducing or eliminating pain might reduce the incidence of emergence agitation after sevoflurane anesthesia [5].
Differential diagnoses of EA include pain (most common), hypoxia, hypotension, hypocarbia, hypercarbia, hypothermia, hypoglycemia, full bladder, and raised intracranial pressure [10]. As critical physiological disorders have been ruled out in children, reassurance and kind words from a sympathetic attendant or the parents calm the pediatric patient [11]. Various methods are suggested to reduce the incidence and severity of EA, such as giving sedative drugs before induction, altered maintenance of anesthesia, or drugs administrated at the end of anesthesia [13, 14].
Among these methods, drug administration at the end of anesthesia is believed to be the most appropriate method in clinical circumstances because it doesn’t depend on the nature of the anesthetic drugs given during induction and maintenance or the duration of anesthesia [9, 13, 15]. Small doses of ketamine or fentanyl are effective in reducing the incidence of emergence agitation after sevoflurane anesthesia.
Adenoidectomy and tonsillectomy are among the most common surgical procedures performed for the pediatric population. EA in the post-operative period remains a major problem in PACU in children undergoing such procedures. This association may be related to the sensation of suffocation in adenotonsillectomy, which can contribute to emergence agitation. Airway obstruction, resulting in sleep-disordered breathing or obstructive sleep apnea (OSA), and frequent infections are the most established indicators for adenotonsillectomy. Most tonsillectomies are performed for infection; OSA, at present, is the most common indicator, accounting for up to 75% of cases [16]. Children with obstructive sleep apnea syndrome (OSAS) usually attract medical attention because of nighttime breathing problems. Parents display symptoms of snoring, apneic episodes, choking, gasping, making every effort to breathe, disturbed sleep, unfamiliar sleeping positions, and recurrent waking up [17, 18]. Even if daytime somnolence may exist, it is not a common symptom in children comparable to adults [19]. Pediatric OSAS has been linked with cor pulmonale, right-sided heart failure, systemic hypertension, failure to thrive, enuresis, and neurocognitive and behavioral problems [20, 21]. Surgery should be deferred whenever acute infection or doubt of a coagulation problem (such as current aspirin intake) is evident. The use of an anticholinergic medication might reduce pharyngeal secretions. A history of airway obstruction or apnea suggests an inhalational anesthetic induction without paralysis until the ability to ventilate with positive pressure is established [11]. The diagnosis of EA in pediatrics is difficult for numerous causes: 1. The presence of pain is a significant confounding element in confirming the diagnosis. 2. the diagnosis of EA has been challenging in the absence of a verified scale. To encounter this problem, the pediatric anesthesia emergence delirium (PAED) score was developed and verified as an objective measure of EA. A score above ten or, more recently, above 12 is considered strongly indicative of EA [22]. The score for the five listed behaviors is added to obtain an overall score of 20. A score of ≥10 shows 64% sensitivity and 86% specificity, and a score of >12 gives 100% sensitivity and 94.5% specificity for diagnosing EA [10].
Sevoflurane is an isopropyl ether volatile agent with less blood solubility than isoflurane and halothane but not desflurane. The lower solubility and lack of pungency facilitate rapid mask induction, and the low blood solubility accelerates washout and recovery from anesthesia. Sevoflurane has dose-dependent CNS, CVS, and respiratory depressant effects [24, 25]. Sevoflurane is broken down by carbon dioxide absorbents to nephrotoxic (compound A) even though renal toxicity does not occur in humans. Compared with other inhalation anesthetics, insignificant amounts of carbon monoxide are produced from the breakdown of sevoflurane by carbon dioxide absorbents [26]. Sevoflurane reduces both the respiratory rate and the tidal volume. Respirations may need to be assisted during the early induction stage in sevoflurane anesthetized children. Children over three years usually experience increased heart rate and no change in systolic blood pressure with sevoflurane [27]. Sevoflurane is a popular volatile anesthetic used to induce and maintain anesthesia in children because it maintains desired hemodynamic stability, and it is the preferred agent for mask induction due to less irritation to mucus membranes [28]. The causes of the high emergence agitation after sevoflurane are not understood. Sevoflurane, in special circumstances, exerts an irritating side effect on the CNS because epileptiform seizure activity in previously non-epileptic patients was observed with electroencephalography during sevoflurane anesthesia. The mechanism of cortical epileptogenicity with sevoflurane is unknown [29, 30].
Sevoflurane has a low blood/gas partition coefficient of 0.69 [31]. Alveolar equilibrium is rapid and convenient for rapid induction of anesthesia [32]. Undergoes lower biotransformation; only 5% is metabolized by cytochromeP450 CYP2E1 to hexafluoroisopropanol (HFIP) by releasing inorganic fluoride and CO2 [33]. Approximately 5% of the sevoflurane dose may be metabolized. Between 95% and 98% of sevoflurane is eliminated through the lungs [33].
Ketamine is a phencyclidine derivative that yields good flexibility in the clinical support of pediatric patients. This agent can be administrated as premedication (orally, nasally, rectally, or intramuscularly); general anesthetic induction agent (IV, IM); and maintenance agent as an infusion, as a sedative (IV, IM); or as a neuraxial analgesia (caudal-epidural) [34]. The exact mechanism by which ketamine exerts its effects is unknown. Ketamine occupies some m-opioid receptors in the brain and spinal cord, which may partially explain its analgesic effects. It has an antagonist effect at Nmethyl-D-aspartate (NMDA) receptors throughout the CNS, which mediate general anesthesia. It also acts on monoaminergic receptors, muscarinic receptors, and calcium ion channels. The most common commercially available preparation is a racemic mixture of two enantiomers: S(+) ketamine and R(–) ketamine. A single enantiomer preparation of S(+) ketamine is now in many countries. S(+) ketamine has four times the affinity of R(–) ketamine for the receptors. Its anesthetic potency is three times that of the racemic mixture [35].
Ketamine is highly lipophilic with a rapid onset of action within 30 seconds and a maximum effect of one minute; the half-time to equilibrate in the effect site ”brain” is eleven seconds [36]. The effective blood concentration of ketamine for anesthesia is 3μg/mL [37]. Clearance of ketamine is reduced in neonates but reaches adult levels by six months of age [38]. Ketamine is believed to cause selective depression of the projections from the thalamus to the limbic system and cortex. The anesthesia derived from the administration of ketamine has thus been termed dissociative anesthesia. For the induction of anesthesia, the intravenous dose of ketamine is 1 to 2mg/kg, whereas the intramuscular dose is 5 to 10mg/kg. The induction of anesthesia after intravenous administration is achieved within 60 seconds. The induction of anesthesia after intramuscular administration is achieved within 2 to 4 minutes. Return of consciousness after an intravenous dose of ketamine requires 10-20 minutes, whereas full orientation may take 60-90 minutes. Ketamine may also be administered orally or rectally. Ketamine is an effective agent for sedation, analgesia, and amnesia, and the effect of ketamine on decreasing the incidence of EA was confirmed by many studies. Intravenous ketamine 0.25mg/kg and 0.5mg/kg have been reported to have a similar decrease in the incidence of EA due to the combined analgesic and sedative effect, but less pain score was noted with the higher dose of ketamine; it is also suggested that an increase in the ketamine dose was effective in analgesic action, whereas increasing the dose does not affect the incidence of EA [15, 39, 42].
1-Phenethyl-4-(N-phenylpropionamido) piperidine synthetic lipophilic phenylpiperidine opioid agonist with analgesic and anesthetic effects. Fentanyl selectively binds to the mu receptor in the (CNS) to mimic the effects of endogenous opioids. Stimulation of the mu subtype opioid receptor stimulates the exchange of GTP for GDP on the G-protein complex and subsequently inhibits adenylate cyclase. This decreases intracellular cAMP and reduces the release of neurotransmitters such as P, GABA, dopamine, acetylcholine, and noradrenaline. The analgesic effect of fentanyl is likely to be due to its metabolite morphine, which causes the opening of G-protein coupled inwardly rectifying potassium (GIRK) channels and blocks the opening of N-type voltage-gated calcium channels, thereby resulting in hyperpolarization and decreased neuronal excitability [43]. Fentanyl may decrease the incidence of EA under sevoflurane anesthesia. Fentanyl is a potent opioid receptor agonist with sedative and analgesic properties. It is routinely used in the practice of pediatric perioperative medicine. Some clinical trials have shown that fentanyl can prevent EA under sevoflurane anesthesia in children [44].
The goal of this study was to determine the efficacy of giving ketamine or fentanyl in reducing the incidence of emergence agitation in children after general anesthesia maintained with sevoflurane.
Materials and Methods
This prospective clinical trial was conducted at Al Sader Medical City from January to December 2020. Sixty children were subjected to elective ENT surgery (tonsillectomy with or without adenoidectomy) in a single-blind clinical trial. Inclusion criteria included, 1. Children aged 3-10 years; 2. American Association of Anesthesia (ASA) class I to II; 3. Planned to undergo tonsillectomy with or without adenoidectomy; 4. Total anesthesia time 30-60 minutes; and 5. Both genders have average weight and age. Children with congenital anomalies (uncorrected CHD), with delayed mental development (e.g., CP, autism, down syndrome), with respiratory disease (e.g., untreated chest infection), with known allergy to the drugs used, and with prolonged surgery (more than 60 minutes) were not entered the study.
Assessment of emergence delirium and diagnosis
The incidence of agitation was assessed using the PAED scale [22] (Table 1).
Table 1. Pediatric anesthesia emergence delirium scale [23]
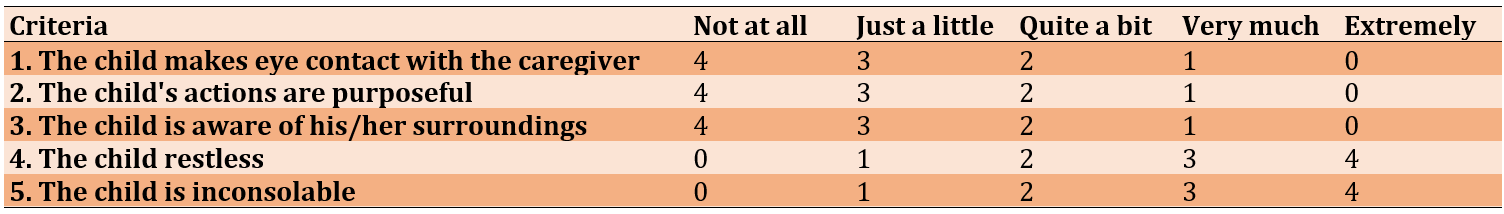
The score in the first and third articles explains the disturbance in the child’s consciousness. The second article, where the child's actions are purposeful, reveals an abnormality in the child’s cognition. However, all these do not explain pain. The fourth and fifth articles, “Restlessness and Inconsolability,” may reveal pain. PAED above 12 indicates the presence of emergence agitation.
Study Protocol and procedures
After the agreement of the Scientific Council of Anesthesia and Intensive Care in Iraqi board for medical specializations in the Anesthesia and intensive care department, and after informed written signed consent was obtained from parents or patients’ relatives of participant children, Children were divided into three groups according to every other case manner; the patient’s age, gender, weight, and ASA state with complete medical history was recorded.
Twenty children were involved in the ketamine group, twenty children were involved in the fentanyl group, and twenty children were involved in the control group.
- All children were fasting for eight hours for solid food, four hours for milk, and two hours for water.
- All patients in three groups were prepared to undergo elective surgery
- Upon arrival at the operating room, patients were assessed by history, physical examination, chest auscultation, IV cannula was established, and patients were monitored by pulse oximetry.
- All patients given the same premedication (Atropine 0.01mg/kg, dexamethasone 1m/kg).
- Patients were received preoxygenation with 100% oxygen (6L/min) through a face mask.
- Anesthesia was induced with propofol (2mg/kg), [anesthetizing dose], neuromuscular blocking with traction or rocuronium (0.5mg/kg) to facilitate tracheal intubation.
- Maintenance of Anesthesia done with 2% sevoflurane.
In all patients, a 10mg/kg paracetamol infusion was administered prior to the start of the operation.
Patients received mechanical ventilation, and the following timings were recorded: time of induction, time of surgery, time of studied agent, and duration of anesthesia, defined as “time from the start of induction until the time of turning off inhalational agent.”
For patients in the ketamine group, 0.5mg/kg of IV ketamine was given 10 minutes before the end of the surgery.
- Patients with fentanyl group 1µg/kg of IV fentanyl were given 10 minutes before the end of the surgery.
In patients with the control group, nothing was given.
- At the end of the surgery, when hemostasis was achieved, sevoflurane was discontinued, the patient was placed in the lateral decubitus position, and spontaneous ventilation was performed with 100% oxygen.
Ventilation continued until the child’s spontaneous respiration resumed, and neuromuscular blocking was reversed with neostigmine (0.05mg/kg) and atropine (0.01mg/kg). Then, the patient was extubated.
- Patients were transported to the post-anesthesia care unit, and the incidence of agitation was assessed five minutes after arrival.
Emergence time was defined as the time from the first moment a child responds to a command by opening their eyes or making any other purposeful movement.
- Follow patients through recovery using the Pediatric Anesthesia Emergence Delirium Scale.
Statistical analysis
The data of the 60 patients were entered, processed, and analyzed with the statistical package for social sciences; all data were tested for error or inconsistency. Descriptive statistics reported as mean, standard deviation, range, median, interquartile range (IQR), and 95% confidence interval of the mean were calculated for the PAED score. Frequency and percentage were used to present the gender variable and the ED incidence. Scale (continuous) variables, including age, weight, total anesthesia time, operation time, and PAED score, were tested for statistical normal distribution; all variables did not follow the normal statistical distribution. Therefore, comparisons of these variables were performed using the non-parametric analysis. Gender was compared using the Chi-square test. All other variables were compared using the Kruskal–Wallis one-way analysis of variance test for multiple comparisons(pairwise) with the least significant difference (LSD) post hoc tests. Bivariate correlation analysis: Pearson’s and Spearman’s tests assessed the correlation between the PAED score and another variable.
Findings
60 child patients were enrolled in this study, and 60 surgeries were performed, including 47 adenoidectomies + tonsillectomy and 13 tonsillectomies.
Patients were equally assigned into three groups (each n=20); Ketamine (11 male+9 female), Fentanyl (13 male+7 female), and Control (10 male+10 female). Patients in all groups were almost matched for age and gender (p=0.622). The mean age and weight were not significantly different among the three groups (Table 2).
Table 2. Baseline pre-operative characteristics of the studied groups (each n=20)

The mean total anesthesia time was significantly (p<0.001) longer in Ketamine (51.5±8.6) than in Fentanyl (37.0±9.5) and also significantly (p=0.002) longer than in the Control (40.7±12.2) group. There was no significant difference between Fentanyl and Control groups (p=0.25).
The operation time was significantly (p<0.001) longer in Ketamine (39.0±8.2min) than in Fentanyl (26.0±9.4min) group and also significantly (p=0.008) longer than in the Control (30.7±10.8min) group. There was no significant difference between Fentanyl and Control groups.
The mean PAED score was much higher in the Control group (13.5±1.5) compared to that in the Ketamine group (9.3±2.0) and (8.0±1.1) in the Fentanyl group (p<0.001). Furthermore, multiple pairwise comparisons (LSD) post hoc analysis for the mean PAED score across the three groups revealed a significant difference in all possible pairs of comparisons. The ketamine group had a higher PAED score than the fentanyl group, which had the lowest mean score compared to the other two groups in all comparisons (p<0.05; Figure 1).
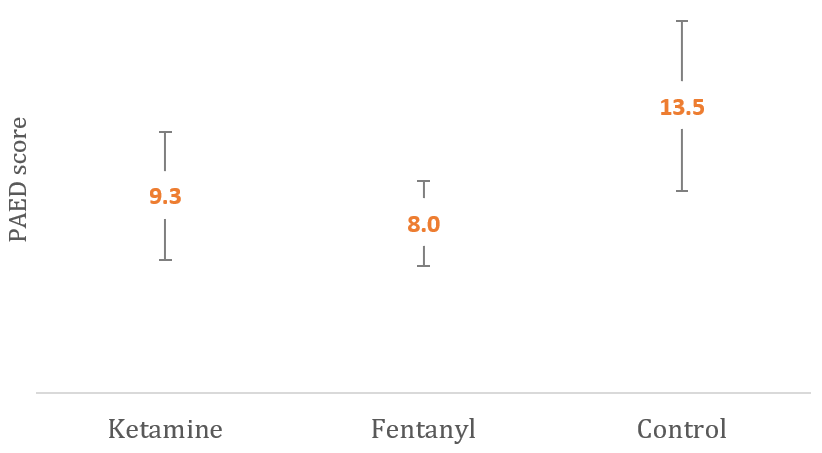
Figure 1. Graphical comparison of mean PAED score between the studied groups
From other points of view, according to the standard cutoff point of the PAED score of 12 used to diagnose ED, it had been found that only three patients with ketamine (15%) had ED. In contrast, all patients in the control group developed ED, and none of the patients in the Fentanyl group had (p<0.001).
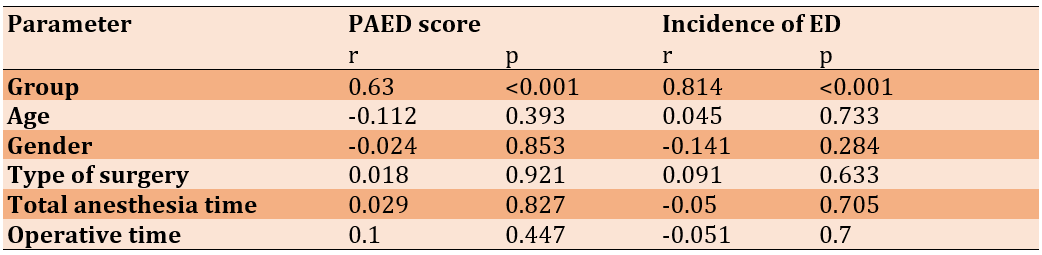
The PAED score was not significantly affected by the studied parameters, but a used agent significantly affected the lower PAED score and incidence of ED (Table 3).
Table 3. Correlation of PAED score and incidence of Emergence Delirium with other parameters
Discussion
Emergence agitation is a common problem in pediatric anesthesia and has increased in incidence with the use of inhalational anesthesia [8]. Many different factors have been associated with the emergence of agitation, including age, postoperative pain, psychological, social, and environmental factors related to the operation, type of surgical procedure, inhaled and intravenous anesthetics, anesthetic premedication, and adjuvant drugs [45]. Surgery is usually performed in the preschool period, and it can be an additional important factor associated with the highest incidence of EA after sevoflurane anesthesia, so it is important to provide effective prevention of this clinical dilemma, allowing early ambulation [8, 46].
It is often difficult to distinguish between post-operative pain and EA in younger children, as symptoms of both might be similar. Although postoperative pain is regarded as a contributing factor in the etiology of EA [8], there are more supporting reports of increased EA after sevoflurane in pain-free children, even if adequate analgesia was given intra-operatively or even if the regional block was applied [8, 46, 47]. Therefore, pain cannot be considered the sole contributing factor to EA.
Many different studies and meta-analyses have studied the effectiveness of ketamine and fentanyl on decreasing emergence agitation after sevoflurane. Still, the incidence of postoperative emergence agitation was significantly less in groups using intraoperative fentanyl compared to groups using ketamine, and this is consistent with Batarseh et al. [48], which studied the impact of intravenous administration of ketamine, fentanyl, and propofol in decreasing Pediatric post-tonsillectomy emergence agitation.
The PAED was used in this study because it is valid and reliable. According to Sikich and Lerman, the PAED scale is a reliable and valid tool based on its reliability, content, and initial construct validity profile determined in their study [22]. Aouad and Nasr recommended using the PAED scale as a reliable and valid tool to minimize measurement error in the clinical evaluation of EA [49].
Patients in all three groups were matched for age, gender, and ASA status, and mean weight was not significantly different among the three groups. The mean total anesthesia time was relatively longer in Ketamine than in fentanyl and the control group, which was a statistically significant difference due to prolonged operative time with ketamine. Regarding the operation time, it was significantly longer in the ketamine group than in the fentanyl group and in controls, which was related to surgeon experience; however, no significant difference was found between the fentanyl and control groups in mean operative time.
The mean PAED score was much higher in the control group, compared to that in the ketamine group and in the fentanyl group, which was significant; the ketamine group appears to have a higher PAED score than the fentanyl group; this might be due to many factors; which include, longer anesthesia time in ketamine group and this may be associated with increased incidence of emergence agitation, may be due to circumstances of the research done. It is shown that ketamine increases overall activity in the prefrontal cortex, which may be due to preferential inhibition of NMDA receptors. This study appears to be inconsistent with Ashraf Arafat Abdelhalim et al. [50] in that the incidence of emergence is 15% with the ketamine group, 17.5% with the fentanyl group, and 42.5% with the control group because this study took a larger sample size (120 patients). Anesthesia was induced with sevoflurane, fentanyl, and rocuronium; in this study, the Children's Hospital of Eastern Ontario Pain Scale (CHEOPS) was used, which included the pain score, and the score was taken at different time intervals.
Also, the results are comparable with Kim et al. [52], comparing the effect of propofol and fentanyl on PAED scores in children undergoing inguinal hernia repair. In this study, anesthesia was induced and maintained by inhaling sevoflurane in oxygen via a face mask, and Spontaneous ventilation was maintained in all subjects. Furthermore, multiple pairwise comparisons (LSD) post hoc analysis for the mean PAED score across the three groups revealed a significant difference in all possible pairs of comparison and that the ketamine group had a higher PAED score than the fentanyl group, had the lowest mean score compared to the other two groups, in all comparisons.
According to the standard cutoff point of the PAED score of 12, used to diagnose ED, it had been found that only three patients with ketamine (15%) had ED. In contrast, all patients in the control group developed ED, and none of the patients in the Fentanyl group did have a highly significant difference.
Further analysis was performed to assess the effect of other variables on the PAED score among the studied groups. Whether the lower PAED score and lower incidence of ED could be attributed to the administration of ketamine or fentanyl or due to other factors, the results of these analyses demonstrated that where the PAED score was not significantly affected by these variables, indicating that ketamine and fentanyl had the good ameliorating effect that reduces the incidence of EA which is consistent with David Costi et al. 2014 [52], which compare the effect of sevoflurane versus other intravenous anesthetic agents (ketamine, opioids; especially fentanyl, alpha two agonist, propofol, and midazolam) and halothane in the incidence of ED, and found that the effect of IV anesthetic and halothane are superior to sevoflurane.
These are the recommendations:
1- Administration of ketamine in a dose of (0.5mg/kg), or fentanyl at a dose of (1μg/kg) at the end of inhalational anesthesia for children undergoing surgeries under general anesthesia to reduce ED.
2- Further studies with a larger sample size are highly suggested, considering the use of multiple centers and evaluating the effect of sedation as it is necessary when using the PAED scale.
3- By incorporating the use of ketamine and fentanyl in the anesthetic plan for pediatric patients, anesthesia providers will be able to decrease the incidence of EA and its associated adverse outcomes.
4- Take scoring at different time points.
5- Including pain scales in the analysis.
Conclusion
The intravenous administration of fentanyl before the end of surgery in sevoflurane anesthetized children undergoing tonsillectomy with or without adenoidectomy reduces the incidence of postoperative agitation more effectively than ketamine.
Acknowledgments: None declared.
Ethical Permissions: After agreement of the scientific council of anaesthesia and intensive care in Iraqi boardfor medical specializations Anesthesia and intensivecare department, and after informed written signedconsent was obtained from parents or patients’ relatives of participant children.
Conflicts of Interests: No conflict of interest.
Authors’ Contribution: M.Saeed ZM (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer (50%); Jaafar JH (Second Author), Assistant Researcher/Discussion Writer (25%); Najim RF (Third Author), Assistant Researcher/Statistical Analyst (25%)
Funding/Support: None declared.
Emergence agitation (EA) is an acute confusion state during recovery from anesthesia, characterized by disorientation, hallucination, restlessness, and purposeless hyperactive physical behavior. The exact mechanism of EA after general anesthesia is unclear [1-4].
In children, causes of EA include a high degree of anxiety related to surgery, full bladder (or obstructed urinary catheter), new surroundings, separation from parents, and encounter with foreign medical staff. These may increase sympathetic activity and prolong the excited state during anesthesia recovery [5-7]. It can be harmful to the child, like bleeding, self-injury, parents' distress, risks of falling, injury to the operative site, delayed medical treatments, the stress caused to both health professionals and families, and the need for continuous monitoring of patients by recovery room staff, and physical checking of patient, delay discharge and supplemental sedative and analgesic drugs [8]. It is more common after certain anesthetic agents (sevoflurane-desflurane-isoflurane>halothane-TIVA), lasts 10 to 15 minutes, and is discontinued spontaneously or after an IV dose of propofol, ketamine, opioids, midazolam, dexmedetomidine, clonidine, or any other medications [9].
Risk factors of EA include age, male gender, type of surgical procedure (hernia repair, genitourinary procedure), emergency surgery, use of volatile anesthetics with low blood solubility, long duration of surgery, use of anticholinergic drugs, phenothiazine, too rapid awakening, voiding surgery, post-operative pain, and the presence of invasive devices. No sole factor can explain the etiology of emergence agitation. Although pain is a major cause of emergence agitation, screaming due to pain should be distinguished from emergence agitation. However, especially in younger children, it is sometimes difficult to distinguish between them. It is believed reducing or eliminating pain might reduce the incidence of emergence agitation after sevoflurane anesthesia [5].
Differential diagnoses of EA include pain (most common), hypoxia, hypotension, hypocarbia, hypercarbia, hypothermia, hypoglycemia, full bladder, and raised intracranial pressure [10]. As critical physiological disorders have been ruled out in children, reassurance and kind words from a sympathetic attendant or the parents calm the pediatric patient [11]. Various methods are suggested to reduce the incidence and severity of EA, such as giving sedative drugs before induction, altered maintenance of anesthesia, or drugs administrated at the end of anesthesia [13, 14].
Among these methods, drug administration at the end of anesthesia is believed to be the most appropriate method in clinical circumstances because it doesn’t depend on the nature of the anesthetic drugs given during induction and maintenance or the duration of anesthesia [9, 13, 15]. Small doses of ketamine or fentanyl are effective in reducing the incidence of emergence agitation after sevoflurane anesthesia.
Adenoidectomy and tonsillectomy are among the most common surgical procedures performed for the pediatric population. EA in the post-operative period remains a major problem in PACU in children undergoing such procedures. This association may be related to the sensation of suffocation in adenotonsillectomy, which can contribute to emergence agitation. Airway obstruction, resulting in sleep-disordered breathing or obstructive sleep apnea (OSA), and frequent infections are the most established indicators for adenotonsillectomy. Most tonsillectomies are performed for infection; OSA, at present, is the most common indicator, accounting for up to 75% of cases [16]. Children with obstructive sleep apnea syndrome (OSAS) usually attract medical attention because of nighttime breathing problems. Parents display symptoms of snoring, apneic episodes, choking, gasping, making every effort to breathe, disturbed sleep, unfamiliar sleeping positions, and recurrent waking up [17, 18]. Even if daytime somnolence may exist, it is not a common symptom in children comparable to adults [19]. Pediatric OSAS has been linked with cor pulmonale, right-sided heart failure, systemic hypertension, failure to thrive, enuresis, and neurocognitive and behavioral problems [20, 21]. Surgery should be deferred whenever acute infection or doubt of a coagulation problem (such as current aspirin intake) is evident. The use of an anticholinergic medication might reduce pharyngeal secretions. A history of airway obstruction or apnea suggests an inhalational anesthetic induction without paralysis until the ability to ventilate with positive pressure is established [11]. The diagnosis of EA in pediatrics is difficult for numerous causes: 1. The presence of pain is a significant confounding element in confirming the diagnosis. 2. the diagnosis of EA has been challenging in the absence of a verified scale. To encounter this problem, the pediatric anesthesia emergence delirium (PAED) score was developed and verified as an objective measure of EA. A score above ten or, more recently, above 12 is considered strongly indicative of EA [22]. The score for the five listed behaviors is added to obtain an overall score of 20. A score of ≥10 shows 64% sensitivity and 86% specificity, and a score of >12 gives 100% sensitivity and 94.5% specificity for diagnosing EA [10].
Sevoflurane is an isopropyl ether volatile agent with less blood solubility than isoflurane and halothane but not desflurane. The lower solubility and lack of pungency facilitate rapid mask induction, and the low blood solubility accelerates washout and recovery from anesthesia. Sevoflurane has dose-dependent CNS, CVS, and respiratory depressant effects [24, 25]. Sevoflurane is broken down by carbon dioxide absorbents to nephrotoxic (compound A) even though renal toxicity does not occur in humans. Compared with other inhalation anesthetics, insignificant amounts of carbon monoxide are produced from the breakdown of sevoflurane by carbon dioxide absorbents [26]. Sevoflurane reduces both the respiratory rate and the tidal volume. Respirations may need to be assisted during the early induction stage in sevoflurane anesthetized children. Children over three years usually experience increased heart rate and no change in systolic blood pressure with sevoflurane [27]. Sevoflurane is a popular volatile anesthetic used to induce and maintain anesthesia in children because it maintains desired hemodynamic stability, and it is the preferred agent for mask induction due to less irritation to mucus membranes [28]. The causes of the high emergence agitation after sevoflurane are not understood. Sevoflurane, in special circumstances, exerts an irritating side effect on the CNS because epileptiform seizure activity in previously non-epileptic patients was observed with electroencephalography during sevoflurane anesthesia. The mechanism of cortical epileptogenicity with sevoflurane is unknown [29, 30].
Sevoflurane has a low blood/gas partition coefficient of 0.69 [31]. Alveolar equilibrium is rapid and convenient for rapid induction of anesthesia [32]. Undergoes lower biotransformation; only 5% is metabolized by cytochromeP450 CYP2E1 to hexafluoroisopropanol (HFIP) by releasing inorganic fluoride and CO2 [33]. Approximately 5% of the sevoflurane dose may be metabolized. Between 95% and 98% of sevoflurane is eliminated through the lungs [33].
Ketamine is a phencyclidine derivative that yields good flexibility in the clinical support of pediatric patients. This agent can be administrated as premedication (orally, nasally, rectally, or intramuscularly); general anesthetic induction agent (IV, IM); and maintenance agent as an infusion, as a sedative (IV, IM); or as a neuraxial analgesia (caudal-epidural) [34]. The exact mechanism by which ketamine exerts its effects is unknown. Ketamine occupies some m-opioid receptors in the brain and spinal cord, which may partially explain its analgesic effects. It has an antagonist effect at Nmethyl-D-aspartate (NMDA) receptors throughout the CNS, which mediate general anesthesia. It also acts on monoaminergic receptors, muscarinic receptors, and calcium ion channels. The most common commercially available preparation is a racemic mixture of two enantiomers: S(+) ketamine and R(–) ketamine. A single enantiomer preparation of S(+) ketamine is now in many countries. S(+) ketamine has four times the affinity of R(–) ketamine for the receptors. Its anesthetic potency is three times that of the racemic mixture [35].
Ketamine is highly lipophilic with a rapid onset of action within 30 seconds and a maximum effect of one minute; the half-time to equilibrate in the effect site ”brain” is eleven seconds [36]. The effective blood concentration of ketamine for anesthesia is 3μg/mL [37]. Clearance of ketamine is reduced in neonates but reaches adult levels by six months of age [38]. Ketamine is believed to cause selective depression of the projections from the thalamus to the limbic system and cortex. The anesthesia derived from the administration of ketamine has thus been termed dissociative anesthesia. For the induction of anesthesia, the intravenous dose of ketamine is 1 to 2mg/kg, whereas the intramuscular dose is 5 to 10mg/kg. The induction of anesthesia after intravenous administration is achieved within 60 seconds. The induction of anesthesia after intramuscular administration is achieved within 2 to 4 minutes. Return of consciousness after an intravenous dose of ketamine requires 10-20 minutes, whereas full orientation may take 60-90 minutes. Ketamine may also be administered orally or rectally. Ketamine is an effective agent for sedation, analgesia, and amnesia, and the effect of ketamine on decreasing the incidence of EA was confirmed by many studies. Intravenous ketamine 0.25mg/kg and 0.5mg/kg have been reported to have a similar decrease in the incidence of EA due to the combined analgesic and sedative effect, but less pain score was noted with the higher dose of ketamine; it is also suggested that an increase in the ketamine dose was effective in analgesic action, whereas increasing the dose does not affect the incidence of EA [15, 39, 42].
1-Phenethyl-4-(N-phenylpropionamido) piperidine synthetic lipophilic phenylpiperidine opioid agonist with analgesic and anesthetic effects. Fentanyl selectively binds to the mu receptor in the (CNS) to mimic the effects of endogenous opioids. Stimulation of the mu subtype opioid receptor stimulates the exchange of GTP for GDP on the G-protein complex and subsequently inhibits adenylate cyclase. This decreases intracellular cAMP and reduces the release of neurotransmitters such as P, GABA, dopamine, acetylcholine, and noradrenaline. The analgesic effect of fentanyl is likely to be due to its metabolite morphine, which causes the opening of G-protein coupled inwardly rectifying potassium (GIRK) channels and blocks the opening of N-type voltage-gated calcium channels, thereby resulting in hyperpolarization and decreased neuronal excitability [43]. Fentanyl may decrease the incidence of EA under sevoflurane anesthesia. Fentanyl is a potent opioid receptor agonist with sedative and analgesic properties. It is routinely used in the practice of pediatric perioperative medicine. Some clinical trials have shown that fentanyl can prevent EA under sevoflurane anesthesia in children [44].
The goal of this study was to determine the efficacy of giving ketamine or fentanyl in reducing the incidence of emergence agitation in children after general anesthesia maintained with sevoflurane.
Materials and Methods
This prospective clinical trial was conducted at Al Sader Medical City from January to December 2020. Sixty children were subjected to elective ENT surgery (tonsillectomy with or without adenoidectomy) in a single-blind clinical trial. Inclusion criteria included, 1. Children aged 3-10 years; 2. American Association of Anesthesia (ASA) class I to II; 3. Planned to undergo tonsillectomy with or without adenoidectomy; 4. Total anesthesia time 30-60 minutes; and 5. Both genders have average weight and age. Children with congenital anomalies (uncorrected CHD), with delayed mental development (e.g., CP, autism, down syndrome), with respiratory disease (e.g., untreated chest infection), with known allergy to the drugs used, and with prolonged surgery (more than 60 minutes) were not entered the study.
Assessment of emergence delirium and diagnosis
The incidence of agitation was assessed using the PAED scale [22] (Table 1).
Table 1. Pediatric anesthesia emergence delirium scale [23]

The score in the first and third articles explains the disturbance in the child’s consciousness. The second article, where the child's actions are purposeful, reveals an abnormality in the child’s cognition. However, all these do not explain pain. The fourth and fifth articles, “Restlessness and Inconsolability,” may reveal pain. PAED above 12 indicates the presence of emergence agitation.
Study Protocol and procedures
After the agreement of the Scientific Council of Anesthesia and Intensive Care in Iraqi board for medical specializations in the Anesthesia and intensive care department, and after informed written signed consent was obtained from parents or patients’ relatives of participant children, Children were divided into three groups according to every other case manner; the patient’s age, gender, weight, and ASA state with complete medical history was recorded.
Twenty children were involved in the ketamine group, twenty children were involved in the fentanyl group, and twenty children were involved in the control group.
- All children were fasting for eight hours for solid food, four hours for milk, and two hours for water.
- All patients in three groups were prepared to undergo elective surgery
- Upon arrival at the operating room, patients were assessed by history, physical examination, chest auscultation, IV cannula was established, and patients were monitored by pulse oximetry.
- All patients given the same premedication (Atropine 0.01mg/kg, dexamethasone 1m/kg).
- Patients were received preoxygenation with 100% oxygen (6L/min) through a face mask.
- Anesthesia was induced with propofol (2mg/kg), [anesthetizing dose], neuromuscular blocking with traction or rocuronium (0.5mg/kg) to facilitate tracheal intubation.
- Maintenance of Anesthesia done with 2% sevoflurane.
In all patients, a 10mg/kg paracetamol infusion was administered prior to the start of the operation.
Patients received mechanical ventilation, and the following timings were recorded: time of induction, time of surgery, time of studied agent, and duration of anesthesia, defined as “time from the start of induction until the time of turning off inhalational agent.”
For patients in the ketamine group, 0.5mg/kg of IV ketamine was given 10 minutes before the end of the surgery.
- Patients with fentanyl group 1µg/kg of IV fentanyl were given 10 minutes before the end of the surgery.
In patients with the control group, nothing was given.
- At the end of the surgery, when hemostasis was achieved, sevoflurane was discontinued, the patient was placed in the lateral decubitus position, and spontaneous ventilation was performed with 100% oxygen.
Ventilation continued until the child’s spontaneous respiration resumed, and neuromuscular blocking was reversed with neostigmine (0.05mg/kg) and atropine (0.01mg/kg). Then, the patient was extubated.
- Patients were transported to the post-anesthesia care unit, and the incidence of agitation was assessed five minutes after arrival.
Emergence time was defined as the time from the first moment a child responds to a command by opening their eyes or making any other purposeful movement.
- Follow patients through recovery using the Pediatric Anesthesia Emergence Delirium Scale.
Statistical analysis
The data of the 60 patients were entered, processed, and analyzed with the statistical package for social sciences; all data were tested for error or inconsistency. Descriptive statistics reported as mean, standard deviation, range, median, interquartile range (IQR), and 95% confidence interval of the mean were calculated for the PAED score. Frequency and percentage were used to present the gender variable and the ED incidence. Scale (continuous) variables, including age, weight, total anesthesia time, operation time, and PAED score, were tested for statistical normal distribution; all variables did not follow the normal statistical distribution. Therefore, comparisons of these variables were performed using the non-parametric analysis. Gender was compared using the Chi-square test. All other variables were compared using the Kruskal–Wallis one-way analysis of variance test for multiple comparisons(pairwise) with the least significant difference (LSD) post hoc tests. Bivariate correlation analysis: Pearson’s and Spearman’s tests assessed the correlation between the PAED score and another variable.
Findings
60 child patients were enrolled in this study, and 60 surgeries were performed, including 47 adenoidectomies + tonsillectomy and 13 tonsillectomies.
Patients were equally assigned into three groups (each n=20); Ketamine (11 male+9 female), Fentanyl (13 male+7 female), and Control (10 male+10 female). Patients in all groups were almost matched for age and gender (p=0.622). The mean age and weight were not significantly different among the three groups (Table 2).
Table 2. Baseline pre-operative characteristics of the studied groups (each n=20)

The mean total anesthesia time was significantly (p<0.001) longer in Ketamine (51.5±8.6) than in Fentanyl (37.0±9.5) and also significantly (p=0.002) longer than in the Control (40.7±12.2) group. There was no significant difference between Fentanyl and Control groups (p=0.25).
The operation time was significantly (p<0.001) longer in Ketamine (39.0±8.2min) than in Fentanyl (26.0±9.4min) group and also significantly (p=0.008) longer than in the Control (30.7±10.8min) group. There was no significant difference between Fentanyl and Control groups.
The mean PAED score was much higher in the Control group (13.5±1.5) compared to that in the Ketamine group (9.3±2.0) and (8.0±1.1) in the Fentanyl group (p<0.001). Furthermore, multiple pairwise comparisons (LSD) post hoc analysis for the mean PAED score across the three groups revealed a significant difference in all possible pairs of comparisons. The ketamine group had a higher PAED score than the fentanyl group, which had the lowest mean score compared to the other two groups in all comparisons (p<0.05; Figure 1).

Figure 1. Graphical comparison of mean PAED score between the studied groups
From other points of view, according to the standard cutoff point of the PAED score of 12 used to diagnose ED, it had been found that only three patients with ketamine (15%) had ED. In contrast, all patients in the control group developed ED, and none of the patients in the Fentanyl group had (p<0.001).
The PAED score was not significantly affected by the studied parameters, but a used agent significantly affected the lower PAED score and incidence of ED (Table 3).
Table 3. Correlation of PAED score and incidence of Emergence Delirium with other parameters

Discussion
Emergence agitation is a common problem in pediatric anesthesia and has increased in incidence with the use of inhalational anesthesia [8]. Many different factors have been associated with the emergence of agitation, including age, postoperative pain, psychological, social, and environmental factors related to the operation, type of surgical procedure, inhaled and intravenous anesthetics, anesthetic premedication, and adjuvant drugs [45]. Surgery is usually performed in the preschool period, and it can be an additional important factor associated with the highest incidence of EA after sevoflurane anesthesia, so it is important to provide effective prevention of this clinical dilemma, allowing early ambulation [8, 46].
It is often difficult to distinguish between post-operative pain and EA in younger children, as symptoms of both might be similar. Although postoperative pain is regarded as a contributing factor in the etiology of EA [8], there are more supporting reports of increased EA after sevoflurane in pain-free children, even if adequate analgesia was given intra-operatively or even if the regional block was applied [8, 46, 47]. Therefore, pain cannot be considered the sole contributing factor to EA.
Many different studies and meta-analyses have studied the effectiveness of ketamine and fentanyl on decreasing emergence agitation after sevoflurane. Still, the incidence of postoperative emergence agitation was significantly less in groups using intraoperative fentanyl compared to groups using ketamine, and this is consistent with Batarseh et al. [48], which studied the impact of intravenous administration of ketamine, fentanyl, and propofol in decreasing Pediatric post-tonsillectomy emergence agitation.
The PAED was used in this study because it is valid and reliable. According to Sikich and Lerman, the PAED scale is a reliable and valid tool based on its reliability, content, and initial construct validity profile determined in their study [22]. Aouad and Nasr recommended using the PAED scale as a reliable and valid tool to minimize measurement error in the clinical evaluation of EA [49].
Patients in all three groups were matched for age, gender, and ASA status, and mean weight was not significantly different among the three groups. The mean total anesthesia time was relatively longer in Ketamine than in fentanyl and the control group, which was a statistically significant difference due to prolonged operative time with ketamine. Regarding the operation time, it was significantly longer in the ketamine group than in the fentanyl group and in controls, which was related to surgeon experience; however, no significant difference was found between the fentanyl and control groups in mean operative time.
The mean PAED score was much higher in the control group, compared to that in the ketamine group and in the fentanyl group, which was significant; the ketamine group appears to have a higher PAED score than the fentanyl group; this might be due to many factors; which include, longer anesthesia time in ketamine group and this may be associated with increased incidence of emergence agitation, may be due to circumstances of the research done. It is shown that ketamine increases overall activity in the prefrontal cortex, which may be due to preferential inhibition of NMDA receptors. This study appears to be inconsistent with Ashraf Arafat Abdelhalim et al. [50] in that the incidence of emergence is 15% with the ketamine group, 17.5% with the fentanyl group, and 42.5% with the control group because this study took a larger sample size (120 patients). Anesthesia was induced with sevoflurane, fentanyl, and rocuronium; in this study, the Children's Hospital of Eastern Ontario Pain Scale (CHEOPS) was used, which included the pain score, and the score was taken at different time intervals.
Also, the results are comparable with Kim et al. [52], comparing the effect of propofol and fentanyl on PAED scores in children undergoing inguinal hernia repair. In this study, anesthesia was induced and maintained by inhaling sevoflurane in oxygen via a face mask, and Spontaneous ventilation was maintained in all subjects. Furthermore, multiple pairwise comparisons (LSD) post hoc analysis for the mean PAED score across the three groups revealed a significant difference in all possible pairs of comparison and that the ketamine group had a higher PAED score than the fentanyl group, had the lowest mean score compared to the other two groups, in all comparisons.
According to the standard cutoff point of the PAED score of 12, used to diagnose ED, it had been found that only three patients with ketamine (15%) had ED. In contrast, all patients in the control group developed ED, and none of the patients in the Fentanyl group did have a highly significant difference.
Further analysis was performed to assess the effect of other variables on the PAED score among the studied groups. Whether the lower PAED score and lower incidence of ED could be attributed to the administration of ketamine or fentanyl or due to other factors, the results of these analyses demonstrated that where the PAED score was not significantly affected by these variables, indicating that ketamine and fentanyl had the good ameliorating effect that reduces the incidence of EA which is consistent with David Costi et al. 2014 [52], which compare the effect of sevoflurane versus other intravenous anesthetic agents (ketamine, opioids; especially fentanyl, alpha two agonist, propofol, and midazolam) and halothane in the incidence of ED, and found that the effect of IV anesthetic and halothane are superior to sevoflurane.
These are the recommendations:
1- Administration of ketamine in a dose of (0.5mg/kg), or fentanyl at a dose of (1μg/kg) at the end of inhalational anesthesia for children undergoing surgeries under general anesthesia to reduce ED.
2- Further studies with a larger sample size are highly suggested, considering the use of multiple centers and evaluating the effect of sedation as it is necessary when using the PAED scale.
3- By incorporating the use of ketamine and fentanyl in the anesthetic plan for pediatric patients, anesthesia providers will be able to decrease the incidence of EA and its associated adverse outcomes.
4- Take scoring at different time points.
5- Including pain scales in the analysis.
Conclusion
The intravenous administration of fentanyl before the end of surgery in sevoflurane anesthetized children undergoing tonsillectomy with or without adenoidectomy reduces the incidence of postoperative agitation more effectively than ketamine.
Acknowledgments: None declared.
Ethical Permissions: After agreement of the scientific council of anaesthesia and intensive care in Iraqi boardfor medical specializations Anesthesia and intensivecare department, and after informed written signedconsent was obtained from parents or patients’ relatives of participant children.
Conflicts of Interests: No conflict of interest.
Authors’ Contribution: M.Saeed ZM (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer (50%); Jaafar JH (Second Author), Assistant Researcher/Discussion Writer (25%); Najim RF (Third Author), Assistant Researcher/Statistical Analyst (25%)
Funding/Support: None declared.
References
1. Munk L, Andersen G, Møller AM. Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anesthesia Scans 2016;60:1059-66. [Link] [DOI:10.1111/aas.12717]
2. Dahmani S, Delivet H, Hilly J. Emergence delirium in children: an update. Curr Opin Anaesthesiol 2014;27(3):309-15. [Link] [DOI:10.1097/ACO.0000000000000076]
3. Lee SJ, Choi SJ, In CB, Sung TY. Effects of tramadol on emergence agitation after general anesthesia for nasal surgery: a retrospective cohort study. Medicine (Baltimore) 2019;98(10):e14763. [Link] [DOI:10.1097/MD.0000000000014763]
4. Jee YS, You HJ, Sung TY, Cho CK. Effects of nefopam on emergence agitation after general anesthesia for nasal surgery: a prospective, randomized, and controlled trial. Medicine (Baltimore) 2017;96(47):e8843. [Link] [DOI:10.1097/MD.0000000000008843]
5. Kuratani N, Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: A meta-analysis of randomized controlled trials. Anesthesiology. 2008;109(2):225-32. [Link] [DOI:10.1097/ALN.0b013e31817f5c18]
6. Uezono S, Goto T, Terui K, Ichinose F, Ishguro Y, Nakata Y, et al. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anesth Analg. 2000;91(3):563-6. [Link] [DOI:10.1213/00000539-200009000-00012]
7. Kim JH. Mechanism of emergence agitation induced by sevoflurane anesthesia. Korean J Anesthesiol 2011;60(2):73-4. [Link] [DOI:10.4097/kjae.2011.60.2.73]
8. Vlajkovic GP, Sindjelic RP. Emergence delirium in children: Many questions, few answers. Anesth Analg. 2007;104(1):84-91. [Link] [DOI:10.1213/01.ane.0000250914.91881.a8]
9. Dahmani S, Stany I, Brasher C, Lejeune C, Bruneau B, Wood C, et al. Pharmacological prevention of sevofluraneand desflurane-related emergence agitation in children: A meta-analysis of published studies. Br J Anaesth. 2010;104(2):216-23. [Link] [DOI:10.1093/bja/aep376]
10. Lerman J. Emergence delirium and agitation in children. Anesthesia Anal. 2017;126(1):365. [Link] [DOI:10.1213/ANE.0000000000002587]
11. Butterworth J, Mackey D, Wasnick J. Morgan & Mikhail's Clinical Anesthesiology. 5th ed. New York: McGraw-Hill Education; 2015. [Link]
12. Koner O, Ture H, Mercan A, Menda F, Sozubir S. Effects of hydroxyzine-midazolam premedication on sevoflurane-induced paediatric emergence agitation: A prospective randomised clinical trial. Eur J Anaesthesiol. 2011;28(9):640-5. [Link] [DOI:10.1097/EJA.0b013e328344db1a]
13. Aouad MT, Yazbeck-Karam VG, Nasr VG, El-Khatib MF, Kanazi GE, Bleik JH. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevofluraneanesthesia. Anesthesiology. 2007;107(5):733-8. [Link] [DOI:10.1097/01.anes.0000287009.46896.a7]
14. Kim JY, Chang YJ, Lee JY, Park HY, Kwak HJ. Post-induction alfentanil reduces sevoflurane-associated emergence agitation in children undergoing an adenotonsillectomy. Acta Anaesthesiol Scand. 2009;53(5):678-81. [Link] [DOI:10.1111/j.1399-6576.2009.01943.x]
15. Abu-Shahwan I, Chowdary K. Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr Anaesth. 2007;17(9):846-50. [Link] [DOI:10.1111/j.1460-9592.2007.02298.x]
16. von Ungern-Sternberg BS, Boda K, Chambers NA, Rebmann C, Johnson C, Sly PD, Habre W. Risk assessment for respiratory complications in paediatric anesthesia: a prospective cohort study. Lancet. 2010;376(9743):773-83. [Link] [DOI:10.1016/S0140-6736(10)61193-2]
17. Brouilette R, Hanson D, David R, Klemka L, Szatkowski A, Fernbach S, et al. A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr. 1984;105(1):10-4. [Link] [DOI:10.1016/S0022-3476(84)80348-0]
18. Leach JOlson JHermann JManning S Polysomnographic and clinical findings in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118(7):741-744. [Link] [DOI:10.1001/archotol.1992.01880070071013]
19. Carroll JL. Sleep-related upper-airway obstruction in children and adolescents. Child Adolesc Psychiatr Clin North Am. 1996;5(3):617-47. [Link] [DOI:10.1016/S1056-4993(18)30352-3]
20. Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung. 1981;159(5):275-87. [Link] [DOI:10.1007/BF02713925]
21. Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100(1):31-40. [Link] [DOI:10.1016/S0022-3476(82)80231-X]
22. Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138-45. [Link] [DOI:10.1097/00000542-200405000-00015]
23. Bajwa SA, Costi D, Cyna AM. A comparison of emergence delirium scales following general anesthesia in children. Paediatr Anaesth. 2010;20(8):704-11. [Link] [DOI:10.1111/j.1460-9592.2010.03328.x]
24. Bryce-Smith R, O'Brien HD. Fluothane: A non-explosive volatile anaesthetic agent. BMJ. 1956;2(4999):969-72. [Link] [DOI:10.1136/bmj.2.4999.969]
25. Johnstone M. The human cardiovascular response to fluothane anesthesia. Br J Anaesth. 1956;28(9):392-410 [Link] [DOI:10.1093/bja/28.9.392]
26. Suckling CW. Some chemical and physical factors in the development of fluothane. Br J Anaesth. 1957;29(10):466-72. [Link] [DOI:10.1093/bja/29.10.466]
27. Morray JP, Geiduschek JM, Ramamoorthy C, Haberkern CM, Hackel A, Caplan RA, et al. Anesthesia-related cardiac arrest in childreninitial findings of the pediatric perioperative cardiac arrest (POCA) registry. Anesthesiol J Am Soc Anesthesiol. 2000;93(1):6-14. [Link] [DOI:10.1097/00000542-200007000-00007]
28. Sarner JB, Levine M, Davis PJ, Lerman J, Cook DR, Motoyama EK. Clinical characteristics of sevoflurane in children. A comparison with halothane. Anesthesiology. 1995;82(1):38-46. [Link] [DOI:10.1097/00000542-199501000-00006]
29. Komatu H. Electrical seizures during sevoflurane anesthesia in two pediatric patients with epilepsy. Anesthesiology. 1994;81(6):1535-7. [Link] [DOI:10.1097/00000542-199412000-00030]
30. Woodforth IJ, Hicks RG, Crawford MR, Stephen JPH, Burke DJ. Electroencephalographic evidence of seizure activity under deep sevoflurane anesthesia in a nonepileptic patient. Anesthesiol J Am Soc Anesthesiol. 1997;87(6):1579-82. [Link] [DOI:10.1097/00000542-199712000-00041]
31. Yasuda N, Lockhart SH, Weiskopf RB, Liu J, Laster M, Taheri S, et al. Comparison of kinetics of sevoflurane and isoflurane in humans. Anesth Analg. 1991;72(3):316-24. [Link] [DOI:10.1213/00000539-199103000-00007]
32. Shiraishi Y, Ikeda K. Uptake and biotransformation of sevof lurane in humans: A comparative study of sevof lurane with halothane, enflurane, and isoflurane. J Clin Anesth. 1990;2(6):381-6. [Link] [DOI:10.1016/0952-8180(90)90024-W]
33. Kharasch ED. Biotransformation of sevoflurane. Anesth Analg. 1995;81(6S):27S-38. [Link] [DOI:10.1097/00000539-199512001-00005]
34. Barash PG, Cullen BF, Stoelting RK, Cahalan MK. Clinical Anesthesia. Philadelphia:Wolters Kluwer; 2017. [Link]
35. Meller ST. Ketamine: Relief from chronic pain through actions at the NMDA receptor?. Pain. 1996;68(2-3):435-6. [Link]
36. Herd DW, Anderson BJ, Keene NA, Holford NHG. Investigating the pharmacodynamics of ketamine in children. Paediatr Anaesth. 2008;18(1):36-42. [Link] [DOI:10.1111/j.1460-9592.2007.02384.x]
37. Dallimore D, Anderson BJ, Short TG, Herd DW. Ketamine anesthesia in children-exploring infusion regimens. Paediatr Anaesth. 2008;18(8):708-14. [Link] [DOI:10.1111/j.1460-9592.2008.02665.x]
38. Anderson BJ, McKee AD, Holford NH. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin Pharmacokinet. 1997;33(5):313-27. [Link] [DOI:10.2165/00003088-199733050-00001]
39. Dalens BJ, Pinard AM, Létourneau DR, Albert NT, Truchon RJ. Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesth Analg. 2006;102(4):1056-61. [Link] [DOI:10.1213/01.ane.0000200282.38041.1f]
40. Lee YS, Kim WY, Choi JH, Son JH, Kim JH, Park YC. The effect of ketamine on the incidence of emergence agitation in children undergoing tonsillectomy and adenoidectomy under sevoflurane general anesthesia. Korean J Anaesthesiol. 2010;58(5):440-5. [Link] [DOI:10.4097/kjae.2010.58.5.440]
41. Kararmaz A, Kaya S, Turhanoglu S, Ozyilmaz MA. Oral ketamine premedication can prevent emergence agitation in children after desflurane anesthesia. Pediatr Anesth. 2004;14(6):477-82. [Link] [DOI:10.1111/j.1460-9592.2004.01224.x]
42. Khattab AM, El-Seify ZA. Sevoflurane-emergence agitation: Effect of supplementary low-dose oral ketamine premedication in preschool children undergoing dental surgery. Saudi J Anesth. 2009;3(2):61-6. [Link] [DOI:10.4103/1658-354X.57878]
43. Finkel JC, Cohen IT, Hannallah RS, Patel KM, Kim MS, Hummer KA, et al. The effect of intranasal fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing surgery for bilateral myringotomy tube placement. Anesth Analg. 2001;92(5):1164-8. [Link] [DOI:10.1097/00000539-200105000-00016]
44. Cohen IT, Finkel JC, Hannallah RS, Hummer KA, Patel KM. The effect of fentanyl on the emergence characteristics after desflurane or sevoflurane anesthesia in children. Anesth Analg. 2002;94(5):1178-81. [Link] [DOI:10.1097/00000539-200205000-00023]
45. Silva LMD, Braz LG, Módolo NSP. Emergence agitation in pediatric anesthesia: current features. J Pediatr. 2008;84(2):107-13. [Link] [DOI:10.2223/JPED.1763]
46. Aono J, Ueda W, Mamiya K, Takimoto E, Manabe M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology. 1997;87:1298-300. [Link] [DOI:10.1097/00000542-199712000-00006]
47. Cravero JP, Beach M, Thyr B, Whalen K. The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesth Analg. 2003;97(2):364-7. [Link] [DOI:10.1213/01.ANE.0000070227.78670.43]
48. Alkhaldi RN. Impact of intraoperative intravenous administration of ketamine, fentanyl or propofol in decreasing pediatric post-tonsillectomy emergence agitation. IntJ Med Investigat. 2013;2(3). [Link]
49. Aouad MT, Nasr VG. Emergence agitation in children: An update. Curr Opinion Anesthesiol. 2005;18(6):614-9. [Link] [DOI:10.1097/01.aco.0000188420.84763.35]
50. Sarner JB, Levine M, Davis PJ, Lerman J, Cook DR, Motoyama EK. Clinical characteristics of sevoflurane in children. A comparison with halothane. Anesthesiology. 1995;82:38-46. [Link] [DOI:10.1097/00000542-199501000-00006]
51. Cravero J, Surgenor S, Whalen K. Emergence agitation in pediatric patients after sevoflurane anesthesia and no surgery: A comparison with halothane. Paediatr Anaesth. 2000;10(4):419-24. [Link] [DOI:10.1046/j.1460-9592.2000.00560.x]
52. Costi D, Cyna AM, Ahmed S, Stephens K, Strickland P, Ellwood J, et al. Effects of sevoflurane versus other general anesthesia on emergence agitation in children. Cochrane Database Syst Rev. 2014;12(9):CD007084. [Link] [DOI:10.1002/14651858.CD007084.pub2]









