Volume 14, Issue 3 (2022)
Iran J War Public Health 2022, 14(3): 331-337 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/05/31 | Accepted: 2022/08/26 | Published: 2022/10/11
Received: 2022/05/31 | Accepted: 2022/08/26 | Published: 2022/10/11
How to cite this article
Meryud Abood A, Mohammud Habash M, Mohammed M. Epidemiology of Colonic Cancer in Baghdad City, Iraq. Iran J War Public Health 2022; 14 (3) : 12
URL: http://ijwph.ir/article-1-1232-en.html
URL: http://ijwph.ir/article-1-1232-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Surgery, College of Medicine, University of Diyala, Diyala, Iraq
2- Department of Anesthesia, College of Diyala, Bilad Alrafidain University, Diyala, Iraq
2- Department of Anesthesia, College of Diyala, Bilad Alrafidain University, Diyala, Iraq
Full-Text (HTML) (1596 Views)
Introduction
Colorectal cancer (CC) begins when healthy cells in the lining of the colon or rectum change and grow out of control, forming a mass called a tumor. A tumor can be cancerous or benign [1]. A malignant tumor can grow and spread to other parts of the body [2] while, a benign tumor only grows at a specific place, but will not be able to spread in the body. These changes usually take years to develop. Both genetic and environmental factors can cause these changes [3, 4]. Based on these two factors, incidences of colonic cancer vary enormously [3, 4]. Globally, around eighty million cases of colon cancer are recorded each year, which accounts for around 10% of all incident cancers [3]. It is the most commonly diagnosed cancer in North America, Western Europe, and Australia, with a high mortality rate among males and females [5, 6, 7]. As per World Health Organization (WHO), around 87.000 cases are newly diagnosed every year [8, 9]. It is the third leading cancer after lung and prostate cancer in males, while in females; a similar trend is found in colonic cancer after breast and lung cancer [10, 11].
Various methods were used for the evaluation of colorectal cancer [12-15]. Age and gender relationship, with the incidence of CC (14) and pattern of tumor and its treatment in Iraq [15, 16] were evaluated [17]. Signs and symptoms of colonic malignancies are non-specific and usually depend on the site and the type of the tumor [18, 19]. Pre-operative staging is important to assess the penetration of colonic carcinoma, which is ultimately used to determine the excision of the tumor [20]. The standard treatment for colonic cancer is surgery with safe margins resection and anastomosis. Surgical treatment aims to remove the tumor and its lymphatic drainage and adequate clear margins ensuring the removal of an entire colonic carcinoma burden [21].
Right-sided colon cancer (RCC) occurs in the proximal colon, and consists of cancers of the cecum and ascending and transverse colon. Left-sided colorectal cancer (LCRC) occurs in the distal colorectum and consists of cancers of the descending and sigmoid colon and the rectum [21, 22]. Due to distant metastasis, approximately 15% of patients are not amenable to surgical resection with curative results [22]. The use of self-expandable stents that are introduced endoscopically either as a palliative measure or pre-operatively to allow the single-stage operation to be carried out later on in case of intestinal obstruction [23]. Adjuvant chemotherapy can be used following resection in patients with a high risk of recurrence and it can improve survival in patients [22]. After surgery, the risk of recurrence is around 20% to 45% due to incomplete tumor excision, implantation of tumor cells, or the development of new growth [18]. Hence, postoperative follow-up is important after performing a colonoscopy and barium enema [13]. Prophylactic colectomy may use to prevent the development of these cancers in patients with of polyposis coli and some cases of ulcerative colitis [16]. Earlier studies show that nonsteroidal anti-inflammatory drugs may prevent the development of cancer by inhibiting the cyclooxygenase 2 enzyme, which is over-expressed in cancer [24, 25].
Recently, very few studies were reported from Iraq [26-28]. In the period 1995-1997, the Iraqi cancer registry reported that colonic cancer was the 12th most common cancer, with an incidence of about 1.1/100000 people [29]. Colonic malignancies are slightly more common in males, they represent about 4.4% of males and 3.7 % of females of all malignant tumors registered during the period between 1995-1997 [30]. In a study done in Iraq during the period from 1989 to 1992, it was found that colonic malignancies account for 30% of all malignant tumors involving the gastrointestinal tract [31]. However, all these reports were evaluated based on the symptoms and cancer site. Hence, there is a need for evaluation of colon cancer data according to Duke’s staging system.
With this background, the present study aimed to evaluate the clinical presentation, age, sex distributions, investigations, distribution in colonic subsites, stage, grade, surgical management, and complication of colonic carcinoma in Gastroenterology and Hepatology Teaching hospital.
Materials and Methods
Patient enrollment
This prospective study was conducted at Gastroenterology and Hepatology teaching hospital, in Baghdad, Iraq, from November 2014 to February 2016. All patients with newly diagnosed colonic carcinoma during the study period were enrolled in the study through various medical procedures, e.g., imaging as ultrasound examination, computed tomography (CT), Magnetic Resonance Imaging (MRI), biochemical, colonoscopy, barium enema, confirm colonic cancer (CC). Finally, 74 patients (43 males and 31 females) were selected.
Preoperative bowel preparation oral polyethyleneglycol solution (colon clean) or antibiotics or both were given to patients when presented as an elective situation two days before surgery and fluid diet 48 hours before surgery. Prophylactic antibiotics (1mg ceftriaxone and 500 mg metronidazole) were given intravenously at induction of anesthesia and continued for two days if no clinical features of sepsis were present. The surgical technique depends on the general condition of the patient (medical status and co-morbid disease) and site of the tumor, including right and left hemicolectomy; segmental resection with anastomosis; anterior resection (high); total proctocolectomy with or without stoma; colostomy or ileostomy only; stenting and no surgery.
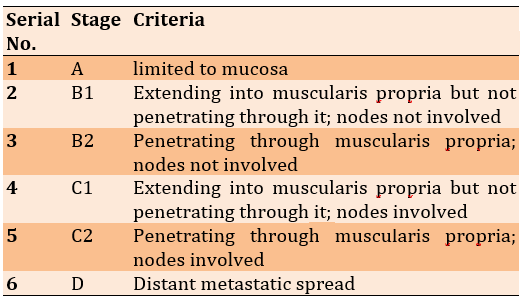
All specimens were sent for a histopathology examination. The data from physical examination, investigations, and operative findings were used in the Dukes staging system [32, 33] (Table 1).
Table 1) Dukes staging system
The collected data were analyzed in SPSS 19 software. Descriptive statistics in the form of mean, standard deviations, and frequency with percentages were calculated for interval and categorical variables, respectively. The Chi-square test between categorical variables and Student’s t-test for interval variables was used as appropriate.
Findings
The average age of male and female participants was 53.5±8.4 and 58.5±7.9 years, respectively. There were 10 patients (13.5%) below 40 years. The peak age group was affected between 60-69 years. Male to female ratio was 1.38:1. There were 8 (25.8%), female patients, under 65 years and there were 23 (74.2%) female patients above 65 years. There were 25 (58.2%) male patients below 65 years and female were 18 (41.8%) above 65 years. The average period between the onset of the disease and the final diagnosis was around 7 months. Around 51 (68.9%) patients were diagnosed within 9 months periods. 12 (16.2%) and 11 (14.8) patients were diagnosed at 10-19 months and >20 months, respectively.
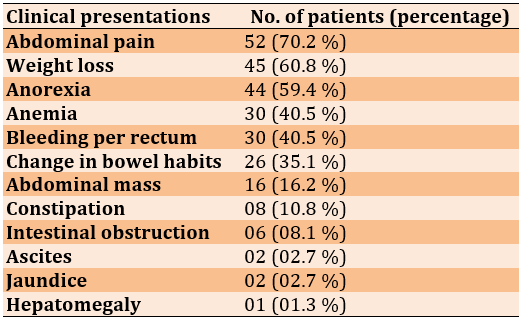
In colonic cancer, abdominal pain and weight loss were the common symptoms. In the present study, around 70.2% and 60.8% of patients showed these two symptoms, respectively. Many patients reported more than symptoms (Table 2).
Table 2) The Important signs and symptoms
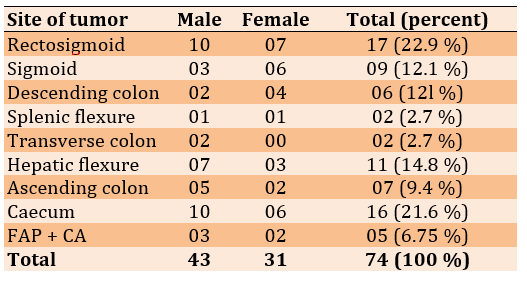
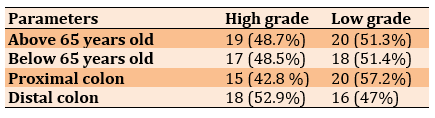
The proximal colon was from the cecum to the end of the transverse colon while the distal colon was from the splenic flexure to the rectosigmoid. There was a significant difference (p<0.05) between the number of proximal colon tumors in females (11 cases) and males (24 cases), but no significant difference was observed between females (18 cases) and males (16 cases) in distal tumors (Table 3).
Table 3) Distribution of colonic carcinoma according to site
Twelve patients (16.2%) were found to have predisposing factors; 6 (8.1%) of them had adenomatous polyps, 3 (4%) had a history of malignancy outside the bowel, 2 (2.7%) had a family history of colorectal carcinoma, 1 (1.3%) had ulcerative colitis and 62 patients (83.8%) had no predisposing factors.
The most common operation was right hemicolectomy (29.7%). Extended right hemicolectomy was 17.5% and anterior resection was 13.5 % of patients. 22, 13, 10, 8, and 5 patients underwent right hemicolectomy, extended right hemicolectomy, anterior resection, left hemicolectomy, and procto-colectomy, respectively. 9, 1, and 4 patients were torn by segmental excision with anastomosis, laparoscopic resection, and colectomy, respectively. Two patients did not undergo surgery.
The gross pathology of the right hemicolectomy of the cecal tumor and sigmoid tumor with segmental resection, anastomosis, the splenic flexure of the colon tumor with left hemicolectomy, and Double Barrel colostomy was observed (Figure 1).
The pathological stages according to the Dukes staging system were B2 (40.5%; 17 males and 13 females), C (27.0%; 11 males and 8 females), B1 (13.0%; 6 males and 4 females), D1 (11%; 5 males and 3 females), A (4.0%; 1 males and 2 females), and D2 (4.0%; 2 males and 1 females).
No significant differences were observed in each stage of tumor between proximal and distal colon tumors in males and females and different age groups (below and above 65 years).
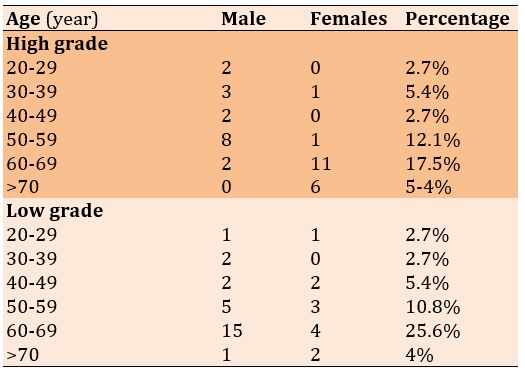
There was no significant difference between males (17 cases) and females (19 cases) in high-grade tumors, but the number of male low-graded patients (16 cases) was significantly higher than females (12 cases; p<0.05; Table 4)
Figure 1) Treatment regime

Gross pathology of right hemicolectomy of cecal tumor

Gross pathology of the sigmoid tumor with segmental resection and anastomosis
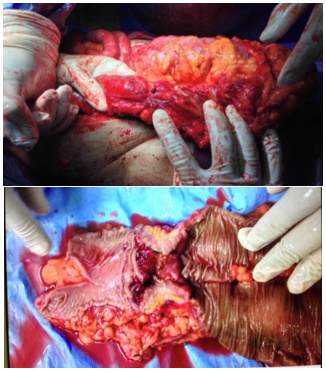
Splenic flexure of colon tumor with left hemicolectomy and Double Barrel colostomy
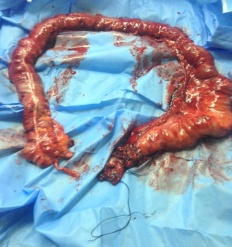
Pan proctocolectomy with end ileostomy of FAP patient with high-grade dysplasia
Table 4) Distribution of the degree of differentiation according to age in both genders
There were no significant differences between high- and low-grade patients according to age (below and above 65 years) and site of the colon (Table 5).
Table 5) Distribution of the degree of differentiation according to age and position of colon
During the period of hospitalization, complications were recorded in 47 participants (63.5%). The commonest complication was wound infection in 18 (24.3%) followed by a chest infection in 15 (20%) patients. Two (2.7%) patients had anastomosis dehiscence that needed re-exploration, 1 (1.3%) patient developed recurrence after two years and presented with Liver metastasis (Lt lobe) and mass in the mesentery and there was observed elevation in CEA antigen above 40ng/ml. Then surgery was performed for the excision of both masses (liver and Mesentery) and the patient was sent to the oncology center 1 (1.3%) patient died during the period of hospitalization due to pulmonary embolism.
Discussion
Colonic carcinoma accounts for approximately 10% of all incident cancers [3]. The male-to-female ratio was about equal [17]. In this study the male-to-female ratio was 1.38:1. The colonic carcinoma developed in older age patients above 55 years [9, 34, 35]. A study conducted in Uzma Nabi reported only 0.8% of patients were below 40 years. In the present study, around 13.5% of patients were less than 40 years. The peak incidence of colonic cancer was between the 60-69 years age group, which was similar to the result of the previous study performed in Iraq between January 1996 and Jun 1997 [35]. In the present study, female patients showed CC at an older age of 65 years (74%). Whereas, male participants (58.8%) were below 65 years. Our results are in accordance with previous reports [34-36]. This may be due to long-time exposure to estrogens before menopause, or to hormone replacement therapy thereafter in females [37-40]. Cessation of estrogens may lead to sporadic colon cancer progressing stepwise from adenoma to carcinoma, with a latency period that may last decades [41].
Grades were based on the degree of tubule formation and cellular array in tumor tissue [42, 43]. The most common symptoms were abdominal pain, weight loss, anemia, and bleeding per rectum change of bowel habitus reported by several authors [44, 45]. Other symptoms included such as abdominal mass, constipation, intestinal obstruction, ascites, jaundice, and hepatomegaly. The present study also got similar findings regarding symptoms. Multiple symptoms were present in 82 % of patients while 18% of patients reported any one symptom.
Halder et al. [46], found that the right colon was commonly affected (30%) more than the left colon. There is a relative decrease in frequency on the left side of the colon with a relatively increased on the right side [15-17, 47]. In the present study, around 70% of individuals showed right colon cancer. The proximal colon of males 24 (32.5%) was more affected than females (14.8). This result agreed with previous reports [36, 37].
Brozeket et al. [36] found that the proximal colon cancer in males and females was 40.5% and 31.7%, respectively. Cora et al. [47] reported proximal colon incidence was 62.2% in males and 37.8% in females. Jarvinen et al. [48] found that, ulcerative colitis represents 1.7% and FAP 0.6 % [48]. Similar findings were reported by another study done in Iraq in 2001 [49]. The measures required for faster early detection of cases through proper methods for diagnosis are very vital. All patients enrolled in our study underwent abdominal ultrasonic examination and 92% of them underwent endoscopic examination.
When colonic cancer is suspected, colonoscopy is the diagnostic study of choice, it provides access to examine the entire colon, and allows a biopsy of the colonic lesion. The pitfalls of colonoscopy are an occasional incomplete examination of the right colon 5-10% of cases, so completion of barium enema time is necessary [45]. CT scans underwent 94% of our patients due to the availability of CT in our center, while in other studies done in Iraq 15% had CT [15, 50, 51]. CT scan of the abdomen and pelvis is useful in identifying metastatic disease. In our study, the most common procedure is right hemicolectomy in 22 (29.7%) of patients followed by extended right hemicolectomy in 13 (17.5%). This is due to the increase in the number of tumors involve proximal colon. Bakeri et al. [45] reported 16% of right hemicolectomies. The relatively high percentage of palliative stomas may be contributed to the delayed presentation and advanced disease. Two patients in this study did not undergo any surgical procedure either because of their advanced disease or because of their refusal to take the risk of surgery. Elzouki et al. [44] found that the Duke C 29.6 %, D1 29%%, D2 10.5%, B1 6.6%, B2 24.3% and Duke A was 0%. In the present study, there's no significant difference between males and females in staging and no difference between proximal and distal colon in staging.
The most advanced disease i.e., C, D1, and D2 presented with patients more than 65 years C (30%), D1 (8%), and D2 (4%), but still, there are 3(4%) of young patients (below the age of 40 years) involved by D1. While the results of Kim et al. [52] showed 5 % below the age of 40 years, all of them presented with an advanced disease stage and aggressive histologic types The advanced stage of the disease may be attributed to the late presentation of patients and maybe the change in the natural history of the disease from the pathological point of view [52]. Most females had a high grade (61%) and while low grade (39%). Most males had a low grade (61%) while a high grade (39%). Most of the females above age 65 years had a high grade (75%). Most of the males above age 65 years had a Low grade (88%). These results agree with Brozek et al. [36] who found about 70% of females above age 65 years had a high grade and all females (100%) above 80 years are high grades while 67% of males below age 65 years had a high grade. The explanation for such involvement is that sporadic colon cancer progresses stepwise from adenoma to carcinoma, with a latency period that may last decades [38] and with the highest incidence during advancing age and tumors start developing slowly before menopause, but rapidly progress with cessation of ovarian estrogen production.
Recently, Lin et al. [53] and Berube et al. [54] reported that in premenopausal women, adequate calcium and vitamin D intake was particularly protective against more aggressive cancer. Kumar et al [55] reported postoperative complications in (39.6%), i.e., wound infection and anastomosis leak was 25% and 14.6, respectively. Our results are in accordance with this report. In the present study, the postoperative complication was 47 (60.0%), i.e., wound infection, chest infection, renal failure, DVT, fistula, and anastomosis leak were 24.3%, 20.2%, 5.4%, 4%, 4%, and 2.7%, respectively. Follow-up was generally poor because most patients were referred to the oncology center for chemotherapy and most patients de-faulted from follow-up after completion of adjuvant therapy [56]. Bakeri et al. [45] found only 27% of patients were seen after 6 months of discharge. The four patients were seen at 24 months and only one at 5 years. However, in our study, all the recruited patients completed the study.
Conclusion
The peak incidence of colonic cancer was between 60-69 years. There is a relative decrease in the frequency of left-side colon cancer to right-side colon cancer. The male’s proximal colon is more affected than the female’s. The advanced disease stages i.e., C1, D2, and D are observed in the later stages of life (more than 65 years). The high-grade CC is in the earlier life of males as compared to females.
Acknowledgments: None declared by the authors.
Ethical Permissions: The research was ethically approved by the Ethical Committee of Baghdad Medical City.
Conflicts of Interests: No conflicts of interests were reported.
Authors’ Contributions: Meryud Abood A (First Author), Introduction Writer/Main Researcher (40%); Mohammud Habash M (Second Author), Methodologist/Discussion Writer (35%); Mohammed MJ (Third Author), Assistant Researcher/Data Analyst (25%)
Funding/Support: None declared by the authors.
Colorectal cancer (CC) begins when healthy cells in the lining of the colon or rectum change and grow out of control, forming a mass called a tumor. A tumor can be cancerous or benign [1]. A malignant tumor can grow and spread to other parts of the body [2] while, a benign tumor only grows at a specific place, but will not be able to spread in the body. These changes usually take years to develop. Both genetic and environmental factors can cause these changes [3, 4]. Based on these two factors, incidences of colonic cancer vary enormously [3, 4]. Globally, around eighty million cases of colon cancer are recorded each year, which accounts for around 10% of all incident cancers [3]. It is the most commonly diagnosed cancer in North America, Western Europe, and Australia, with a high mortality rate among males and females [5, 6, 7]. As per World Health Organization (WHO), around 87.000 cases are newly diagnosed every year [8, 9]. It is the third leading cancer after lung and prostate cancer in males, while in females; a similar trend is found in colonic cancer after breast and lung cancer [10, 11].
Various methods were used for the evaluation of colorectal cancer [12-15]. Age and gender relationship, with the incidence of CC (14) and pattern of tumor and its treatment in Iraq [15, 16] were evaluated [17]. Signs and symptoms of colonic malignancies are non-specific and usually depend on the site and the type of the tumor [18, 19]. Pre-operative staging is important to assess the penetration of colonic carcinoma, which is ultimately used to determine the excision of the tumor [20]. The standard treatment for colonic cancer is surgery with safe margins resection and anastomosis. Surgical treatment aims to remove the tumor and its lymphatic drainage and adequate clear margins ensuring the removal of an entire colonic carcinoma burden [21].
Right-sided colon cancer (RCC) occurs in the proximal colon, and consists of cancers of the cecum and ascending and transverse colon. Left-sided colorectal cancer (LCRC) occurs in the distal colorectum and consists of cancers of the descending and sigmoid colon and the rectum [21, 22]. Due to distant metastasis, approximately 15% of patients are not amenable to surgical resection with curative results [22]. The use of self-expandable stents that are introduced endoscopically either as a palliative measure or pre-operatively to allow the single-stage operation to be carried out later on in case of intestinal obstruction [23]. Adjuvant chemotherapy can be used following resection in patients with a high risk of recurrence and it can improve survival in patients [22]. After surgery, the risk of recurrence is around 20% to 45% due to incomplete tumor excision, implantation of tumor cells, or the development of new growth [18]. Hence, postoperative follow-up is important after performing a colonoscopy and barium enema [13]. Prophylactic colectomy may use to prevent the development of these cancers in patients with of polyposis coli and some cases of ulcerative colitis [16]. Earlier studies show that nonsteroidal anti-inflammatory drugs may prevent the development of cancer by inhibiting the cyclooxygenase 2 enzyme, which is over-expressed in cancer [24, 25].
Recently, very few studies were reported from Iraq [26-28]. In the period 1995-1997, the Iraqi cancer registry reported that colonic cancer was the 12th most common cancer, with an incidence of about 1.1/100000 people [29]. Colonic malignancies are slightly more common in males, they represent about 4.4% of males and 3.7 % of females of all malignant tumors registered during the period between 1995-1997 [30]. In a study done in Iraq during the period from 1989 to 1992, it was found that colonic malignancies account for 30% of all malignant tumors involving the gastrointestinal tract [31]. However, all these reports were evaluated based on the symptoms and cancer site. Hence, there is a need for evaluation of colon cancer data according to Duke’s staging system.
With this background, the present study aimed to evaluate the clinical presentation, age, sex distributions, investigations, distribution in colonic subsites, stage, grade, surgical management, and complication of colonic carcinoma in Gastroenterology and Hepatology Teaching hospital.
Materials and Methods
Patient enrollment
This prospective study was conducted at Gastroenterology and Hepatology teaching hospital, in Baghdad, Iraq, from November 2014 to February 2016. All patients with newly diagnosed colonic carcinoma during the study period were enrolled in the study through various medical procedures, e.g., imaging as ultrasound examination, computed tomography (CT), Magnetic Resonance Imaging (MRI), biochemical, colonoscopy, barium enema, confirm colonic cancer (CC). Finally, 74 patients (43 males and 31 females) were selected.
Preoperative bowel preparation oral polyethyleneglycol solution (colon clean) or antibiotics or both were given to patients when presented as an elective situation two days before surgery and fluid diet 48 hours before surgery. Prophylactic antibiotics (1mg ceftriaxone and 500 mg metronidazole) were given intravenously at induction of anesthesia and continued for two days if no clinical features of sepsis were present. The surgical technique depends on the general condition of the patient (medical status and co-morbid disease) and site of the tumor, including right and left hemicolectomy; segmental resection with anastomosis; anterior resection (high); total proctocolectomy with or without stoma; colostomy or ileostomy only; stenting and no surgery.
All specimens were sent for a histopathology examination. The data from physical examination, investigations, and operative findings were used in the Dukes staging system [32, 33] (Table 1).
Table 1) Dukes staging system

The collected data were analyzed in SPSS 19 software. Descriptive statistics in the form of mean, standard deviations, and frequency with percentages were calculated for interval and categorical variables, respectively. The Chi-square test between categorical variables and Student’s t-test for interval variables was used as appropriate.
Findings
The average age of male and female participants was 53.5±8.4 and 58.5±7.9 years, respectively. There were 10 patients (13.5%) below 40 years. The peak age group was affected between 60-69 years. Male to female ratio was 1.38:1. There were 8 (25.8%), female patients, under 65 years and there were 23 (74.2%) female patients above 65 years. There were 25 (58.2%) male patients below 65 years and female were 18 (41.8%) above 65 years. The average period between the onset of the disease and the final diagnosis was around 7 months. Around 51 (68.9%) patients were diagnosed within 9 months periods. 12 (16.2%) and 11 (14.8) patients were diagnosed at 10-19 months and >20 months, respectively.
In colonic cancer, abdominal pain and weight loss were the common symptoms. In the present study, around 70.2% and 60.8% of patients showed these two symptoms, respectively. Many patients reported more than symptoms (Table 2).
Table 2) The Important signs and symptoms

The proximal colon was from the cecum to the end of the transverse colon while the distal colon was from the splenic flexure to the rectosigmoid. There was a significant difference (p<0.05) between the number of proximal colon tumors in females (11 cases) and males (24 cases), but no significant difference was observed between females (18 cases) and males (16 cases) in distal tumors (Table 3).
Table 3) Distribution of colonic carcinoma according to site

Twelve patients (16.2%) were found to have predisposing factors; 6 (8.1%) of them had adenomatous polyps, 3 (4%) had a history of malignancy outside the bowel, 2 (2.7%) had a family history of colorectal carcinoma, 1 (1.3%) had ulcerative colitis and 62 patients (83.8%) had no predisposing factors.
The most common operation was right hemicolectomy (29.7%). Extended right hemicolectomy was 17.5% and anterior resection was 13.5 % of patients. 22, 13, 10, 8, and 5 patients underwent right hemicolectomy, extended right hemicolectomy, anterior resection, left hemicolectomy, and procto-colectomy, respectively. 9, 1, and 4 patients were torn by segmental excision with anastomosis, laparoscopic resection, and colectomy, respectively. Two patients did not undergo surgery.
The gross pathology of the right hemicolectomy of the cecal tumor and sigmoid tumor with segmental resection, anastomosis, the splenic flexure of the colon tumor with left hemicolectomy, and Double Barrel colostomy was observed (Figure 1).
The pathological stages according to the Dukes staging system were B2 (40.5%; 17 males and 13 females), C (27.0%; 11 males and 8 females), B1 (13.0%; 6 males and 4 females), D1 (11%; 5 males and 3 females), A (4.0%; 1 males and 2 females), and D2 (4.0%; 2 males and 1 females).
No significant differences were observed in each stage of tumor between proximal and distal colon tumors in males and females and different age groups (below and above 65 years).
There was no significant difference between males (17 cases) and females (19 cases) in high-grade tumors, but the number of male low-graded patients (16 cases) was significantly higher than females (12 cases; p<0.05; Table 4)
Figure 1) Treatment regime

Gross pathology of right hemicolectomy of cecal tumor

Gross pathology of the sigmoid tumor with segmental resection and anastomosis

Splenic flexure of colon tumor with left hemicolectomy and Double Barrel colostomy

Pan proctocolectomy with end ileostomy of FAP patient with high-grade dysplasia
Table 4) Distribution of the degree of differentiation according to age in both genders

There were no significant differences between high- and low-grade patients according to age (below and above 65 years) and site of the colon (Table 5).
Table 5) Distribution of the degree of differentiation according to age and position of colon

During the period of hospitalization, complications were recorded in 47 participants (63.5%). The commonest complication was wound infection in 18 (24.3%) followed by a chest infection in 15 (20%) patients. Two (2.7%) patients had anastomosis dehiscence that needed re-exploration, 1 (1.3%) patient developed recurrence after two years and presented with Liver metastasis (Lt lobe) and mass in the mesentery and there was observed elevation in CEA antigen above 40ng/ml. Then surgery was performed for the excision of both masses (liver and Mesentery) and the patient was sent to the oncology center 1 (1.3%) patient died during the period of hospitalization due to pulmonary embolism.
Discussion
Colonic carcinoma accounts for approximately 10% of all incident cancers [3]. The male-to-female ratio was about equal [17]. In this study the male-to-female ratio was 1.38:1. The colonic carcinoma developed in older age patients above 55 years [9, 34, 35]. A study conducted in Uzma Nabi reported only 0.8% of patients were below 40 years. In the present study, around 13.5% of patients were less than 40 years. The peak incidence of colonic cancer was between the 60-69 years age group, which was similar to the result of the previous study performed in Iraq between January 1996 and Jun 1997 [35]. In the present study, female patients showed CC at an older age of 65 years (74%). Whereas, male participants (58.8%) were below 65 years. Our results are in accordance with previous reports [34-36]. This may be due to long-time exposure to estrogens before menopause, or to hormone replacement therapy thereafter in females [37-40]. Cessation of estrogens may lead to sporadic colon cancer progressing stepwise from adenoma to carcinoma, with a latency period that may last decades [41].
Grades were based on the degree of tubule formation and cellular array in tumor tissue [42, 43]. The most common symptoms were abdominal pain, weight loss, anemia, and bleeding per rectum change of bowel habitus reported by several authors [44, 45]. Other symptoms included such as abdominal mass, constipation, intestinal obstruction, ascites, jaundice, and hepatomegaly. The present study also got similar findings regarding symptoms. Multiple symptoms were present in 82 % of patients while 18% of patients reported any one symptom.
Halder et al. [46], found that the right colon was commonly affected (30%) more than the left colon. There is a relative decrease in frequency on the left side of the colon with a relatively increased on the right side [15-17, 47]. In the present study, around 70% of individuals showed right colon cancer. The proximal colon of males 24 (32.5%) was more affected than females (14.8). This result agreed with previous reports [36, 37].
Brozeket et al. [36] found that the proximal colon cancer in males and females was 40.5% and 31.7%, respectively. Cora et al. [47] reported proximal colon incidence was 62.2% in males and 37.8% in females. Jarvinen et al. [48] found that, ulcerative colitis represents 1.7% and FAP 0.6 % [48]. Similar findings were reported by another study done in Iraq in 2001 [49]. The measures required for faster early detection of cases through proper methods for diagnosis are very vital. All patients enrolled in our study underwent abdominal ultrasonic examination and 92% of them underwent endoscopic examination.
When colonic cancer is suspected, colonoscopy is the diagnostic study of choice, it provides access to examine the entire colon, and allows a biopsy of the colonic lesion. The pitfalls of colonoscopy are an occasional incomplete examination of the right colon 5-10% of cases, so completion of barium enema time is necessary [45]. CT scans underwent 94% of our patients due to the availability of CT in our center, while in other studies done in Iraq 15% had CT [15, 50, 51]. CT scan of the abdomen and pelvis is useful in identifying metastatic disease. In our study, the most common procedure is right hemicolectomy in 22 (29.7%) of patients followed by extended right hemicolectomy in 13 (17.5%). This is due to the increase in the number of tumors involve proximal colon. Bakeri et al. [45] reported 16% of right hemicolectomies. The relatively high percentage of palliative stomas may be contributed to the delayed presentation and advanced disease. Two patients in this study did not undergo any surgical procedure either because of their advanced disease or because of their refusal to take the risk of surgery. Elzouki et al. [44] found that the Duke C 29.6 %, D1 29%%, D2 10.5%, B1 6.6%, B2 24.3% and Duke A was 0%. In the present study, there's no significant difference between males and females in staging and no difference between proximal and distal colon in staging.
The most advanced disease i.e., C, D1, and D2 presented with patients more than 65 years C (30%), D1 (8%), and D2 (4%), but still, there are 3(4%) of young patients (below the age of 40 years) involved by D1. While the results of Kim et al. [52] showed 5 % below the age of 40 years, all of them presented with an advanced disease stage and aggressive histologic types The advanced stage of the disease may be attributed to the late presentation of patients and maybe the change in the natural history of the disease from the pathological point of view [52]. Most females had a high grade (61%) and while low grade (39%). Most males had a low grade (61%) while a high grade (39%). Most of the females above age 65 years had a high grade (75%). Most of the males above age 65 years had a Low grade (88%). These results agree with Brozek et al. [36] who found about 70% of females above age 65 years had a high grade and all females (100%) above 80 years are high grades while 67% of males below age 65 years had a high grade. The explanation for such involvement is that sporadic colon cancer progresses stepwise from adenoma to carcinoma, with a latency period that may last decades [38] and with the highest incidence during advancing age and tumors start developing slowly before menopause, but rapidly progress with cessation of ovarian estrogen production.
Recently, Lin et al. [53] and Berube et al. [54] reported that in premenopausal women, adequate calcium and vitamin D intake was particularly protective against more aggressive cancer. Kumar et al [55] reported postoperative complications in (39.6%), i.e., wound infection and anastomosis leak was 25% and 14.6, respectively. Our results are in accordance with this report. In the present study, the postoperative complication was 47 (60.0%), i.e., wound infection, chest infection, renal failure, DVT, fistula, and anastomosis leak were 24.3%, 20.2%, 5.4%, 4%, 4%, and 2.7%, respectively. Follow-up was generally poor because most patients were referred to the oncology center for chemotherapy and most patients de-faulted from follow-up after completion of adjuvant therapy [56]. Bakeri et al. [45] found only 27% of patients were seen after 6 months of discharge. The four patients were seen at 24 months and only one at 5 years. However, in our study, all the recruited patients completed the study.
Conclusion
The peak incidence of colonic cancer was between 60-69 years. There is a relative decrease in the frequency of left-side colon cancer to right-side colon cancer. The male’s proximal colon is more affected than the female’s. The advanced disease stages i.e., C1, D2, and D are observed in the later stages of life (more than 65 years). The high-grade CC is in the earlier life of males as compared to females.
Acknowledgments: None declared by the authors.
Ethical Permissions: The research was ethically approved by the Ethical Committee of Baghdad Medical City.
Conflicts of Interests: No conflicts of interests were reported.
Authors’ Contributions: Meryud Abood A (First Author), Introduction Writer/Main Researcher (40%); Mohammud Habash M (Second Author), Methodologist/Discussion Writer (35%); Mohammed MJ (Third Author), Assistant Researcher/Data Analyst (25%)
Funding/Support: None declared by the authors.
Keywords:
References
1. AL-Bahrany Zuhair R, Dezzay O, Alkhateeb AK, Butrous GS. Cancer of colon and rectum in Iraq. Am J Proct Gastro Colon Resct Surg. 1980;1:20-2. [Link]
2. Al Dahhan SA, Al Lami FH. Epidemiology of colorectal cancer in Iraq, 2002-2014. Gulf J Oncol. 2018;1(26):23-6. [Link]
3. Al-Saigh THT, Al-Bayati SA, Abdulmawjood SA, Ahmed FA. Descriptive study of colorectal cancer in iraq, 1999-2016. Ann Coll Med Mosul. 2019;41(1):81-5. [Link] [DOI:10.33899/mmed.2019.161330]
4. American Cancer Society. Global cancer facts and figures. 4th Edition. Atlanta: American Cancer Society Inc.; 2018. [Link]
5. Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139(6):846-52. [Linkv] [DOI:10.1097/00000658-195406000-00015]
6. Bakeri AA. Clinicopathologica pattern and challenge in the management of colorectal cancer in sub-Saharan Africa. J Chines Clin Med. 2007;21(121):20-5. [Link]
7. Berube S, Diorio C, Masse B, Hebert-Croteau N, Byrne C, Cote G, et al. Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol Biomarkers Prev, 2005;14(7):1653-9. [Link] [DOI:10.1158/1055-9965.EPI-05-0068]
8. Binkert C A, Ledermann H, Jost R, Saurenmann P, Decurtins M, Zollikofer CL. Acute colonic obstruction: Clinical aspects and cost-effectiveness of preoperative and palliative treatment with self-expanding metallic stents--a preliminary report. Radiology. 1998;206(1):199-204. [Link] [DOI:10.1148/radiology.206.1.9423673]
9. Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, et al. Cancer mortality in Europe, 2005‑ 2009, and an overview of trends since 1980. Ann Oncol. 2013;24(10):2657-71. [Linkvvv] [DOI:10.1093/annonc/mdt301]
10. Boyle P, Langman JS. Epidemiology. BMJ. 2000;321(7264):805‑8. [Link] [DOI:10.1136/bmj.321.7264.805]
11. Boyle P, Leon ME. Epidemiology of colorectal cancer. Br Med Bull. 2002;64(1):1‑25. [Link] [DOI:10.1093/bmb/64.1.1]
12. Brozek W, Kriwanek S, Bonner E, Peterlik M, Cross H. Mutual associations between malignancy, age, gender, and subsite incidence of colorectal cancer. Anticancer Res. 2009;29(9):3721-6. [Link]
13. Bruce E. Jarrel, R. National medical series for independent study-surgery. 4th Edition. Philadelphia: Lippincott William & Wilkins; 2000. pp. 234-40. [Link]
14. Cairns S, Scholfield JH. Guidelines for colorectal cancer screening in high-risk groups. Gut. 2002;51(Suppl 5):V1-2. [Link] [DOI:10.1136/gut.51.suppl_5.v1]
15. Calle EE, Miracle-McMahill HL, Thun MJ, Heath CW. Estrogen replacement therapy and risk of fatal colon cancer in a prospective cohort of postmenopausal women. J Natl Cancer Inst. 1995;87(7):517-23. [Link] [DOI:10.1093/jnci/87.7.517]
16. Chattar-Cora D, Onime GD, Coppa GF, Valentine IS, Rivera L. Anatomic, age, and sex distribution of colorectal cancer in a New York City hispanic population. J Natl Med Assoc. 1998;90(1):19-24. [Link]
17. Cho VR, Vogelstein B. Colorectal cancer-genetic alterations in the adenoma-carcinoma sequence. Cancer. 1992;70(Suppl 6):1727-31.
https://doi.org/10.1002/1097-0142(19920915)70:4+<1727::AID-CNCR2820701613>3.0.CO;2-P [Link] [DOI:10.1002/1097-0142(19920915)70:4+3.0.CO;2-P]
18. Corman MI, Allison SI, Kuehene JP. Hand book of colon and rectal surgery. Philadelphia PA: Lippincott, Williams and Wilkins; 2002. pp. 518-21. [Link]
19. Doğusoy G. Kolon Kanserinin Patolojik Özellikleri. In: Alemdaroğlu K, Akçal T, Buğra D, editors. Kolon Rektum ve Anal Bölge Hastalıkları. İstanbul: Türk Kolon ve Rektum Cerrahi Derneği; 2003. pp. 413-20. [Turkish] [Link]
20. Dukes CE. The classification of cancer of the rectum. J Pathol Bacteriol. 1932;35(3):323-32. [Link] [DOI:10.1002/path.1700350303]
21. El-Hasseni MB. The changing pattern of cancer in Iraq during the last 18 years. Baghdad: Ministry of Health Iraqi Cancer Board; 1999. [Link]
22. Elzouki AN, Habel S, Alsoaeiti S, Abosedra A, Khan F. Epidemiology and clinical findings of colorectal carcinoma in two tertiary care hospitals in Benghazi, Libya. Avicenna J Med. 2014;4(4)-94-8. [Link] [DOI:10.4103/2231-0770.140659]
23. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759-67. [Link] [DOI:10.1016/0092-8674(90)90186-I]
24. Garcia-Anguilar J, Mellgren A, Sirivangs P, Buie D, Madoff RD, Rothenberger DA. Local excision of rectal cancer without adjuvant therapy: A word of caution. Ann Surg. 2000;231(3):345-51. [Link] [DOI:10.1097/00000658-200003000-00007]
25. Gericke D. Early diagnosis saves human life on the status of colorectal tumors. Versicherungsmedizin. 1992;44(2):60-3. [German] [Linkv]
26. Grigg M, McDermott FT, Pihl EA, Hughes ES. Curative local excision in the treatment of carcinoma of the rectum. Dis Colon Rectum. 1984;27(2):81-3. [Link] [DOI:10.1007/BF02553979]
27. Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: A review and meta-analysis. Am J Med. 1999;106(5):574-82. [Link] [DOI:10.1016/S0002-9343(99)00063-7]
28. Halder SK, Bhattacharjee PK, Bahr P, Pachaury A. Epidemiological, clinico-pathological profile and management of colorectal carcinoma in a tertiary referral center of eastern India. J Krishna Ins Med Sci Univ. 2013;2(1):45-50 [Link]
29. Hassan HA, Sameen AN. Colorectal carcinoma, presentation and pattern of surgical management at the university hospital [dissertation]; 2002. [Link]
30. Huddy SP, Husband EM, Cook MG, Gibbs NM, Marks CG, Heald RJ. Lymph node metastasis in early rectal cancer. Br J Surg. 1993;80(11):1458-8. [Link] [DOI:10.1002/bjs.1800801135]
31. Ibrahem S, Ahmed H, Zangana S. Trends in colorectal cancer in Iraq over two decades: Incidence, mortality, topography and morphology. Ann Saudi Med. 2022;42(4):252-61. [Link] [DOI:10.5144/0256-4947.2022.252]
32. Iraqi Cancer Board. A report of the results of the Iraqi cancer registry from 1995 to 1997. Baghdad: Ministry of Health Publishing; 1999. [Arabic] [Link]
33. Jacobs EJ, White E, Weiss NS. Exogenous hormones, reproductive history, and colon cancer (Seattle, Washington, USA). Cancer Causes Control. 1994;5(4):359-66. [Link] [DOI:10.1007/BF01804987]
34. Janout V, Kollarova H. Epidemiology of colorectal cancer 2001. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145(1):5-10. [Link] [DOI:10.5507/bp.2001.001]
35. Jarvinen HJ, Ovaska J, Meklin JP. Improvment in treatment and prognosis of colorectal carcinoma. Br J Surg. 1988;75(1):25-7. [Link] [DOI:10.1002/bjs.1800750110]
36. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893‑907. [Link] [DOI:10.1158/1055-9965.EPI-10-0437]
37. Jensen OM. Different age and sex relationship for cancer of subsites of the large bowel. Br J Cancer. 1984;5(6):825-9. [Link] [DOI:10.1038/bjc.1984.262]
38. Kim G, Baik SH, Lee KY, Hur H, Min BS, Lyu CJ, et al. Colon carcinoma in childhood: Review of the literature with four case reports. Int J Colorectal Dis. 2013;28(2):157-64. [Link] [DOI:10.1007/s00384-012-1603-7]
39. Kuipers EJ, Rösch T, Bretthauer M. Colorectal cancer screening--optimizing current strategies and new directions. Nat Rev Clin Oncol. 2013;10(3):130-42. [Link] [DOI:10.1038/nrclinonc.2013.12]
40. Kumar A, Daga R, Vijayaragavan P, Prakash A, Singh RK, Behari A, et al. Anterior resection for rectal carcinoma risk factors for anastomotic leaks and strictures. World J Gastroenterol. 2011;17(11):1475-79. [Link] [DOI:10.3748/wjg.v17.i11.1475]
41. Kumar V, Robbins S, Cotran R, editors. Temel Patoloji. Istanbul: Nobel Tıp Kitabevleri; 2000. pp. 505-14. [Turkish] [Link]
42. Lefebure B, Tuech JJ, Bridoux V, Costaglioli B, Scotte M, Teniere P, et al. Evaluation of selective defunctioning stoma after low anterior resection for rectal cancer. Int J Colorectal Dis. 2008;23(23):283-8. [Link] [DOI:10.1007/s00384-007-0380-1]
43. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Cheifec G. Use of colonoscopy to screen asymptomatic adult for colorectal cancer. veterans affairs cooperative study group 380. N Engl J Med. 2000;343(3):162-8. [Link] [DOI:10.1056/NEJM200007203430301]
44. Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167(10):1050-9. [Link] [DOI:10.1001/archinte.167.10.1050]
45. McMichael AJ, Potter JD. Reproduction, endogenous and exogenous sex hormones, and colon cancer: A review and hypothesis. J Natl Cancer Inst. 1980;65(6):1201-7. [Link]
46. Nabi U, Nagi AH, Riaz S, Sami W. Morphological evaluation of colorectal carcinoma with grading staging and histological types. J Pak Med Assoc. 2010;60(12):998-1001. [Link]
47. Neil J. The small and large intestines. O'Connell PR, Williams NS, McCaskie AW, editors. Bailey & love's short practice of surgery. 26th Edition. Boca Raton, Florida: CRC Press; 2012. []
48. O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69(10):866‑72. []
49. Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin .1999;49:33‑64. [Link] [DOI:10.3322/canjclin.49.1.33]
50. Petrović T, Breberina M, Radovanović Z, Nikolić I, Ivković-Kapicl T. The results of the surgical treatment of rectal cancer. Arch Oncol. 2010;18(3):51-5. [Link] [DOI:10.2298/AOO1003051P]
51. Rahman MM, Al-Janabyi KA. The pattern of colorectal and anal tumor and its surgical treatment 1999. J Fac Med Baghdad. 2000;(1):38-44. [Link]
52. Sarraf S, Abdul-Jabbar A. Clinical course of colorectal cancer in young patients comparing with old patients [dissertation]; 1997. [Link]
53. Shahrudin MD, Noori SM. Cancer of the colon and rectum in the first three decades of life. HepatoGastroentrology. 1997;44(14):441-4. [Link]
54. Vasen HFA, Tomlinson I, Castells A. Clinical management of hereditary colorectal cancer syndromes. Nat Rev Gastroenterol Hepatol. 2015;12(2):88-97. [Link] [DOI:10.1038/nrgastro.2014.229]
55. Vone J R, Botting RM. Mechanism of action of asperin-like drugs. Semin Arthritis Rheum. 1997;26(6 Suppl 1):2-10. [Link] [DOI:10.1016/S0049-0172(97)80046-7]
56. Waseem T. Colorectal carcinoma presentation and manegment [dissertation]; 2009. [Link]









