Volume 15, Issue 1 (2023)
Iran J War Public Health 2023, 15(1): 1-9 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/11/11 | Accepted: 2023/01/15 | Published: 2023/01/30
Received: 2021/11/11 | Accepted: 2023/01/15 | Published: 2023/01/30
How to cite this article
Hadi H, Enayah S. RETN Gene Polymorphisms as a Risk Factor in Diabetic Patients with Covid-19 Infection. Iran J War Public Health 2023; 15 (1) :1-9
URL: http://ijwph.ir/article-1-1060-en.html
URL: http://ijwph.ir/article-1-1060-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
H.S. Hadi *1, S.H. Enayah1
1- Department of Medical Laboratory, Thi-Qar Health Directorate, Thi-Qar, Iraq
Full-Text (HTML) (896 Views)
Introduction
A pandemic is a contagious infectious disease that has spread across numerous geographic areas or continents. According to the most basic definition, the term "contagious" refers to an infection that can be passed from one person to another, either directly or indirectly [1]. According to the World Health Organization (WHO), when designating a new disease as a pandemic, there is a wide range of disagreement among members of the medical and scientific professions. “A pandemic is the worldwide spread of a new illness” [2]. In 2002, SARS came from China. It is considered that only small mammals can mutate a strain of the coronavirus that infect people. SARS spread rapidly in other Asian countries from China. Also, in several countries, including 4 in the U.K. and an important outbreak in Toronto, Canada, there were a small number of cases [3].
In 1964, June Almeida was contacted by Dr. David Arthur John Tyrrell, a British virologist, the Director of the Common Cold Unit in Salisbury, as one nasal swab sample collected from a boarding schoolboy from Surrey in 1961 and designated as “B 814”. With no other option being available, Tyrrell sent the sample to June Almeida, hoping that her electron microscope could identify the virus. His comment in his book, “Cold Wars: The Fight against the Common Cold”, was “We were not too hopeful but felt it was worth a try”. On putting the mysterious pathogen samples under the microscope, Almeida noticed that B814 had a structure similar to influenza viral particles. She knew that she was on the verge of discovering a new family of viruses with short spikey projections on the outer surface, appearing as a solar corona which led June Almeida and Tyrrell to call the new group ”coronavirus” – derived from the Latin word “corona” meaning “crown” or “halo”. When the research on the new virus was sent for publication, the journal refused to accept the finding, as the reviewer found Almeida’s micrographs of the B814 virus as “just bad pictures of influenza viruses”. June Almeida and Tyrrell had to struggle to get the work finally published in the Journal of General Virology in 1967 [4].
The first human coronavirus was described by June Dalziel Almeida in 1966. She had observed a viral structure under electron microscopy while being involved in a study investigating the causes of the common cold. A paper submitted by Almeida and her team described a crown-shaped structure supposed to be a new type of virus causing common colds. This paper was rejected as the editors claimed that “these microscopic observations resulted from distorted influenza viruses” [2].
Covid-19 symptoms vary, ranging between mild and severe diseases. Headache, loss of taste and smell, nasal congestion and rhinorrhea, cough, pain in the muscle, sore throat, fever, and breathing problems are common symptoms [3]. Nongastrointestinal symptoms of Covid-19 include fever, cough, shortness of breath, chills, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell. Gastrointestinal (GI) symptoms, including anorexia, nausea, vomiting, abdominal pain, and diarrhea, have been reported in patients with Covid-19. Additionally, abnormal liver enzymes are also observed [5]. Flu-like symptoms are present in SARS, usually starting 2-7 days after infections. The time between the virus and symptoms (incubation period) may sometimes be up to 10 days [3]. Coronaviruses infecting humans and associated with severe atypical pneumonia, including Severe Acute Respiratory Syndrome Coronaviruses 1 (SARS-CoV-1) and 2 (SARS-CoV-2), and Middle East Respiratory Syndrome Coronavirus (MERS-CoV-2), have caused three epidemics in the 21st century [6].
Viruses and the diseases they cause often have different names. For example, HIV is the virus that causes AIDS. People often know the name of a disease but not the name of the virus that causes it. There are different processes and purposes for naming viruses and diseases. Viruses are named based on their genetic structure to facilitate the development of diagnostic tests, vaccines, and medicines. Virologists and the wider scientific community do this work, so viruses are named by the International Committee on Taxonomy of Viruses (ICTV). Diseases are named to enable discussion on disease prevention, spread, transmissibility, severity and treatment. Diseases are officially named by WHO in the International Classification of Diseases (ICD). ICTV announced “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)” as the name of the new virus on 11 February 2020. This name was chosen because the virus is genetically related to the coronavirus responsible for the SARS outbreak of 2003. While related, the two viruses are different .WHO announced “Covid-19” as the name of this new disease on 11 February 2020, following guidelines previously developed with the World Organization for Animal Health (OIE) and the Food and Agriculture Organization of the United Nations (FAO). From a risk communications perspective, using the name SARS can have unintended consequences in creating unnecessary fear for some populations, especially in Asia, which was worst affected by the SARS outbreak in 2003. For that reason and others, WHO has begun referring to the virus as “the virus responsible for Covid-19” or “the Covid-19 virus” when communicating with the public. Neither of these designations are intended as replacements for the official name of the virus as agreed by the ICTV [7].
Most infected patients have mild symptoms, including fever, fatigue, and cough, but in severe cases, the disease can progress quickly to acute respiratory distress syndrome, septic shock, metabolic acidosis, and coagulopathy [8]. Outcomes of Covid-19 range from asymptomatic infection to death. Older age, male gender, and comorbid conditions, such as hypertension and diabetes, have been identified as risk factors for severe outcomes. While Covid-19 is typically characterized by symptoms of viral pneumonia, SARS-CoV-2 causes a systemic disease with possible involvement of the heart, liver, pancreas, and kidneys, as well as alterations in circulating lymphocytes and the immune system, because of the ubiquitous distribution of the main viral entry receptor, namely Angiotensin Converting Enzyme 2 (ACE2) [9]. Growing evidence indicates that environmental conditions might influence the current outbreak of Covid-19 [10]. Covid-19 is caused by the severe acute respiratory syndrome coronavirus-2 and curiously displays a propensity for thrombosis in multiple vascular beds. Covid-19-related thrombosis may contribute to severe organ injury and death. The incidence of thrombotic events was as high as 31% in one cohort [11]. Based on the epidemiologic findings of Covid-19 incidence, the severity of illness and mortality seem to be associated with multiple comorbidities such as diabetes, hypertension, and cardiovascular disease. Some studies revealed that severe Covid-19 patients have a higher incidence of diabetes and hypertension than milder forms [12]. As a direct effect, the Covid-19 infection has resulted in striking changes in patients' metabolism with significant elevations in blood glucose. It is attributed to the increased release of cytokines and inflammatory mediators, which led to increased insulin resistance and the associated hyperglycemia. In addition, it has been suggested that Covid-19 might be involved in developing acute diabetes mellitus in certain patients by targeting ACE2 receptors located in pancreatic islets resulting in pancreatic injury [13]. Insulin resistance is a pathophysiological state where cells display reduced responsiveness to the glucose-lowering activity of insulin. While there are rare cases where mutations in genes associated with insulin signaling or lipodystrophy cause insulin resistance, for the most part, insulin resistance is associated with obesity and, thus, a state of positive energy balance. This form of insulin resistance is frequently associated with hyperinsulinemia, increased waist circumference or visceral adiposity, metabolic dyslipidemia with high triglycerides and low HDL, and hepatic steatosis features collectively referred to as the metabolic syndrome. We refer to this as “common insulin resistance” [14].
Resistin is a recently discovered adipocyte-secreted polypeptide that has been implicated in the development of insulin resistance. Resistin was first described in 2001 during a search for genes that induced adipocyte differentiation, and it was down-regulated in mature adipocytes during exposure to Thiazolidinedione (TZD). This led to the discovery of a protein that the investigators named resistin (for insulin resistance) [15]. Resistin is a 12.5-kDa polypeptide that belongs to the resistin-like molecule family of cysteine-rich proteins. Some population studies have shown that resistin levels are indeed associated with metabolic risk factors and insulin resistance, suggesting that resistin may play an important role in the pathophysiology of diabetes [16]. Resistin is expressed in pre-adipocytes in addition to adipocytes, which may contribute to the elevation of resistin content in the adipose tissue of obese humans [17]. One of the most controversial adipokines is Resistin (RETN), which is a macrophage-derived signaling polypeptide hormone that belongs to the cysteine-rich proteins family [18]. Resistin increases blood glucose levels and impairs glucose transport to the target cells [19]. Resistin is a protein hormone released by adipocytes, which contributes to IR and purportedly acts as a link between obesity and diabetes [20]. Administration of exogenous resistin or transgenic overexpression leads to decreased insulin sensitivity and altered glucose handling, and conversely, blocking resistin activity or genetically decreasing its levels improves insulin sensitivity and restores glucose homeostasis. Thus there is strongly genetic and pharmacological evidence that resistin is a mediator of insulin resistance in rodents [21]. White adipose tissue fulfills an important endocrine role in controlling the whole body's metabolism. Resistin is supposed to represent a novel link between obesity and the development of type 2 diabetes. Further investigations changed that view, however, revealed numerous species-specific properties and physiological differences of resistin actions [19]. Human resistin has been shown to induce the expression of proinflammatory cytokines and adhesion molecules in the settings of inflammation and endothelial dysfunction. Given the strong relationship between inflammation and metabolism, there is mounting evidence suggesting a role for human resistin in the pathological processes of metabolic diseases, including obesity, diabetes, and cardiovascular diseases [22]. Resistin has been implicated in the insulin resistance observed in normal pregnancy, as the level of resistin increases with gestational age and decreases after delivery. Resistin is detectable in human serum, but unlike rodents, human resistin is predominantly expressed in macrophages, and its expression in human adipose tissue is predominantly due to non-adipocyte resident inflammatory cells. Human and rodent resistin gene and protein sequences share about 60% identity, less than most hormones conserved across species. Yet, the genes are syntenic, with the gene coding resistin (Retn) on mouse chromosome 8 located a similar distance from the Insulin Receptor gene as is the Retn gene on human chromosome 19. Also, as in rodents, resistin levels are decreased with thiazolidinedione treatment in humans [21].
The present study aimed to determine RETN gene polymorphisms as a risk factor in diabetic patients with Covid-19 infection.
Instruments and Methods
Sample collection
This study was performed from January 2022 to March 2022. A total of 100 diabetic patients with Covid-19, aged between 37-69 years (46 males and 54 females), in the isolation center for people infected with Coronavirus at Al Hussein Teaching Hospital in Thi-Qar and Shatrah General Hospital were included in the study. Also, 50 diabetic patients without Covid-19 (28 males and 22 females) participated in the study as a control group.
After obtaining written consent, 5 ml of venous blood was collected from patients and controls, which was added to gel tubes and EDTA (Ethylenediaminetetraacetic acid) tubes. 50 samples were chosen randomly from Covid-19 infected patients and 19 samples from diabetic only for molecular and sequencing study. 2 ml of blood with EDTA was used to examine HbA1c and stored at -20°C until used for quantification of molecular study, and another 3 ml of blood was put into a gel tube and then the tube centrifuged at 4000 rpm for 10 min to extract serum for glucose estimation test.
All patient and control data, including age, gender, clinical presentation features, patient history, height, and weight, were recorded.
Statistical analysis
The data were statistically analyzed by SPSS 26 software using independent t-test, Chi-square test, and odds ratio expressed in mean LSD.
Findings
Diabetes parameters
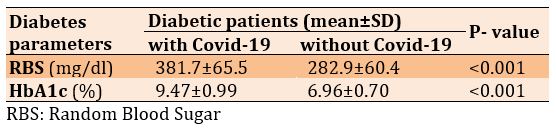
There was a significant increase in Random Blood Sugar (RBS) and HbA1c in diabetic patients with Covid-19 compared to the diabetic patients only (p<0.001; Table 1).
Table 1) Comparison of diabetes parameters in diabetic patients with (n=100) and without (n=50) Covid-19
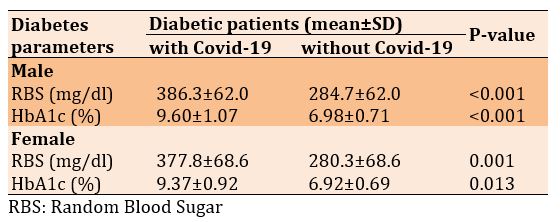
The increase in diabetes parameters in diabetic patients with Covid-19 compared to the diabetic patients without Covid-19 was observed in both men and women (Table 2).
Table 2) Comparison of diabetes parameters in diabetic patients with and without Covid-19 based on the gender
Molecular study
The results showed 10 Single Nucleotide Polymorphisms (SNPs) as follows:
rs1233732258
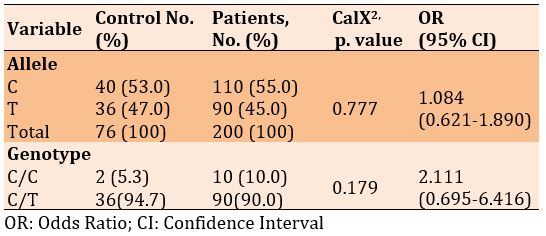
There was no significant difference in frequencies of the C and T alleles and genotypes of C/C and C/T in diabetic patients with and without Covid-19 (p>0.05). Also, the frequency of alleles and genotypes increased in patients with Covid-19 than diabetics only, 1.084 and 2.111, respectively (p<0.05; Table 3).
rs1451147074
Table 3) Allele and genotype frequency associated with rs1233732258 SNP
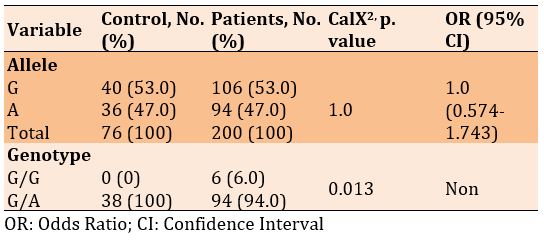
There was no significant difference in frequencies of the G and A alleles, but a significant difference was seen in the genotype of G/G and G/A in diabetic patients with and without Covid-19 (p=0.013). Also, the frequency of alleles increased in patients with Covid-19 than in diabetics only, 1.128, while there was no significant odds ratio for genotype (Table 4). X227G.A
Table 4) Allele and genotype frequency associated with rs1451147074 SNP
There was no significant difference in frequencies of the G and A alleles, but a significant difference was seen in the genotype of G/G and G/A in diabetic patients with and without Covid-19 (p=0.013). Also, the frequency of alleles increased in patients with Covid-19 than in diabetics only, 1.0, while there was no significant odds ratio for genotype (Table 5).
rs772392077
Table 5) Allele and genotype frequency associated with X227G.A SNP
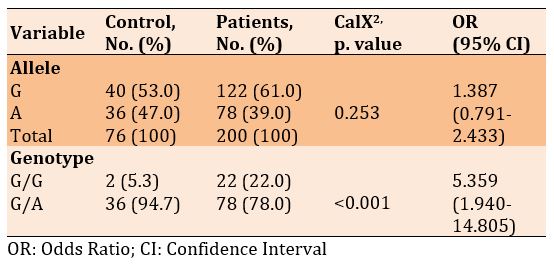
There was no significant difference in frequencies of the G and A alleles, but a significant difference was seen in the genotype of G/G and G/A in diabetic patients with and without Covid-19 (p<0.001). Also, the frequency of alleles and genotypes increased in patients with Covid-19 than diabetics only, 1.387 and 5.359, respectively (Table 6).
rs764296340
Table 6) Allele and genotype frequency associated with rs772392077 SNP
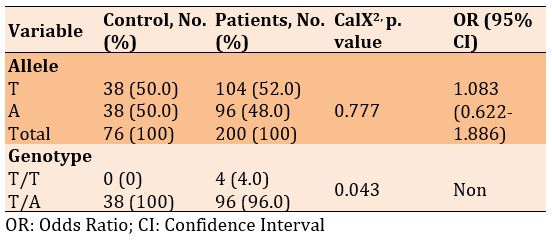
There was no significant difference in frequencies of the T and A alleles, but a significant difference was seen in the genotype of T/T and T/A in diabetic patients with and without Covid-19 (p=0.043). Also, the frequency of alleles increased in patients with Covid-19 than in diabetics only, 1.083, while there was no significant odds ratio for genotype (Table 7).
rs3219177
Table 7) Allele and genotype frequency associated with rs764296340 SNP
X663A.C
There was a significant difference in frequencies of the C and A alleles, as well as in the genotypes of codominant and recessive. However, there was no significant difference
There was a significant difference in frequencies of the C and T alleles, and the genotypes of codominant, dominant, recessive, and over dominant in diabetic patients with and without Covid-19 (p<0.05; Table 8). X494T.C
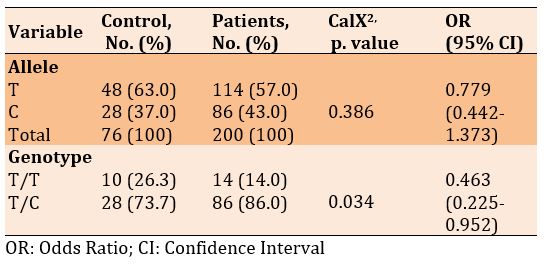
There was no significant difference in frequencies of the T and C alleles, but a significant difference was seen in the genotype of T/T and T/C in diabetic patients with and without Covid-19 (p=0.043). Also, the frequency of alleles increased in patients with Covid-19 than in diabetics only, 1.083, while there was no significant odds ratio for genotype (Table 9).
X542A.C
There was no significant difference in frequencies of the T and C alleles, but a significant difference was seen in the genotype of T/T and T/C in diabetic patients with and without Covid-19 (p=0.034; Table 10).
rs3745367
There was no significant difference in frequencies of the G and A alleles, as well as in the genotypes of codominant and recessive. Hewer, there was a significant difference in the genotypes of dominant and over dominant in diabetic patients with and without Covid-19 (p<0.05). Also, the odds ratio of recessive genotype increased in patients with Covid-19, by 1.490, compared to the diabetics only (Table 11).
Table 8) Allele and genotype frequency associated with rs3219177 SNP
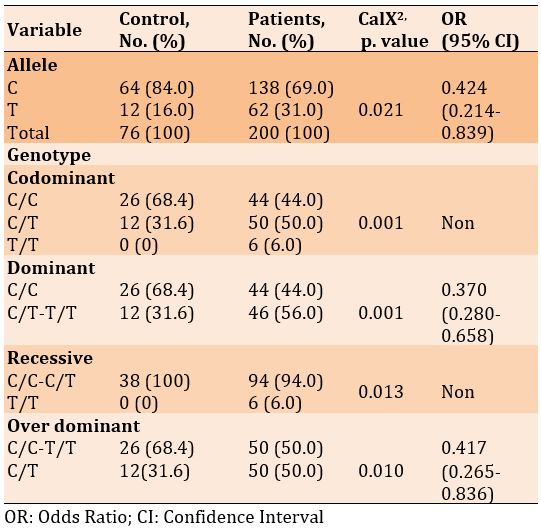
Table 9) Allele and genotype frequency associated with X494T.C SNP
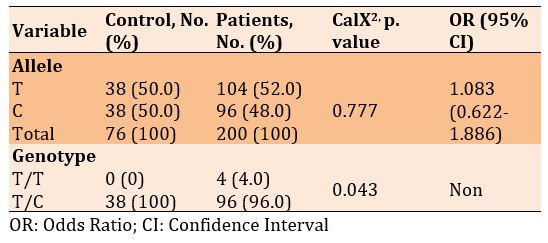
Table 10) Allele and genotype frequency associated with X542A.C SNP
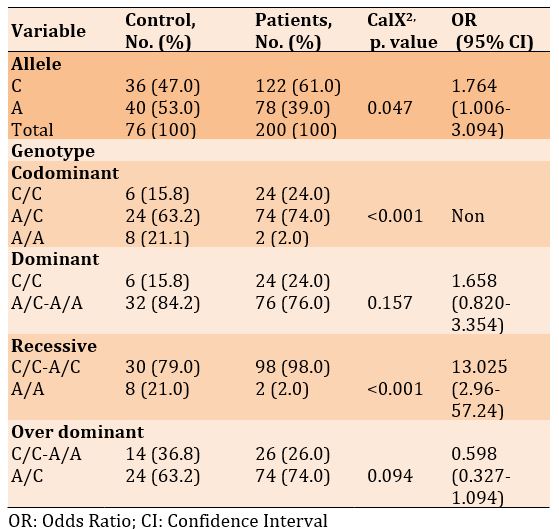
X663A.C
There was a significant difference in frequencies of the C and A alleles, as well as in the genotypes of codominant and recessive. However, there was no significant difference in the genotypes of dominant and over dominant in diabetic patients with and without Covid-19 (p<0.05). Also, the odds ratio of alleles and dominant and recessive genotypes increased in patients with Covid-19 compared to the diabetics only, 1.764, 1.658, and 13.025, respectively (Table 12).
Table 11) Allele and genotype frequency associated with rs3745367 SNP
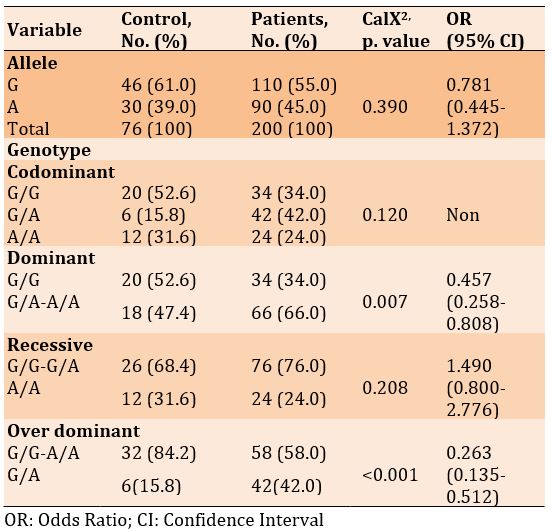
Table 12) Allele and genotype frequency associated with X663A.C SNP
Linkage disequilibrium of the studied variants is represented by haplo plot diagram of D’ (Figure 1).
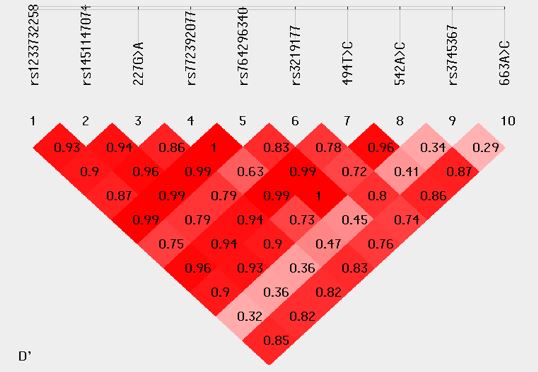
Figure 1) Linkage disequilibrium of the studied variants represented by haplo plot diagram of D’
Discussion
The current study indicated a significant increase in the concentration of RBS and HbA1c in Covid-19 infected diabetic patients than in diabetic patients only.
The current study agreed with the study of Kumar et al. [23]. Their study investigated inflammatory markers in diabetic and non-diabetic Covid-19 and concluded a Covid-19 infection induce a high level of RBS and HbA1c in diabetic patients compared with patients without Covid-19 infection. Hyperglycemia promotes SARS-CoV-2 replication in human monocytes, resulting in increased viral proliferation. Thus, hyperglycemia is an independent risk factor for Covid-19 infection [24].
Also, the current study agreed with recent study performed by Smith et al. [25]. They studied impaired glucose metabolism in patients with diabetes with severe Covid‐19. Their study showed a significant increase in diabetic parameters in Covid-19 infected patients than diabetic only. In addition, it was found a positive correlation between body mass index and diabetic parameters. A possible explanation for a link between hyperglycemia and ACE2 levels in the severity of Covid‐19 could be explained by several clinical observations in SARS and preclinical observations in the non-diabetic. Potential changes in glycosylation of the ACE2, as well as glycosylation of the viral spike protein, both possibly induced by uncontrolled hyperglycemia, may alter both the binding of the viral spike protein to ACE2 and the degree of the immune response to the virus [25]. Several studies showed that Diabetes Mellitus (DM) as an associated distinctive comorbidity increased the morbidity and mortality of Covid-19 patients [26].
The present study disagreed with previous studies that reported higher glucose levels than the current study. Reddy et al. [27] recorded a concentration of glucose equal to 555 mg/dl, while the HbA1c was 14.2 mg/dl. Also, in the study of Chee et al. [28], the glucose level was 714, and HbA1c was 14.2, and in the study of Kim et al. [29], the hyperglycemia in Covid-19 patients was 655 mg/dl, and HbA1c was 11.4. In contrast, the study of Li et al. [30] reported a level of glucose equal to 298 mg/dl and HbA1c equal to 6.8, which was lower than those reported in the present study.
Diabetes is linked to the severity of infection by a number of factors. Hyperglycemia can initiate, intensify, or extend the acute inflammatory response. It also causes a fibrinolysis and coagulation mismatch, leading to increased factors of coagulation and relative inhibition of the system of fibrinolysis, promoting a state of pro-coagulation. Furthermore, SARS- CoV-2 is thought to use ACE2 as an entrance receptor on the islets of Langerhans.
The current study showed, according to males and females, that the Hba1c level and Random blood sugar increased in diabetic patients infected with the Covid19 compared to diabetic patients only, and there was a significant difference between the two groups. Several observational studies have provided clinical evidence that uncontrolled hyperglycemia may lead to a longer LOS and significantly higher mortality in Covid-19 patients. The current study agreed with study of Lei et al. [31]. Their study included that high blood sugar in the body leads to a doubling of the symptoms of Covid and leads to death due to the relationship between Covid and high sugar in the body. As for age and body mass, a significant increase was observed in the level of normal sugar and cumulative sugar for diabetic patients with Covid and diabetic patients only. The current study agreed with study of AbdElaleem et al. [32]. Their study included to study Covid-19 related diabetes and try to find predictors of mortality in these patients. Hyperglycemia is commonly seen in critically unwell patients and can be correlated to disease severity [33].
Our data showed that higher HbA1c levels were a factor in disease severity in patients with Covid-19 and diabetes. These results are consistent with those of previous studies. A retrospective cohort study on the association between pre-infection glycemic control and disease severity in patients with type 2 diabetes and Covid-19 in Israel found a gradual dose-response relationship between HbA1c level and the risk of severe Covid-19 [34]. A population-based cohort study in England also suggested a higher risk of mortality from Covid-19 in patients with either type 1 or type 2 diabetes with HbA1c levels >10.0% than in those with HbA1c levels of less than 6.5% [35]. In addition, a Japanese study showed that HbA1c levels might be a risk factor for severe disease requiring oxygenation in patients with Covid-19 [36].
The newly discovered hormone resistin has been suggested to link obesity in patients with diabetes [37, 38]. In animal models, resistin is specifically secreted by adipocytes and significantly increased in both genetic and diet-induced obesity. Resistin overexpression was associated with impaired glucose tolerance and insulin action in mice, whereas thiazolidinedione treatment significantly reduced resistin gene polymorphism, and neutralization of the resistin protein enhanced blood glucose uptake and insulin sensitivity. It has been suggested that resistin modulates one or more steps in the insulin signaling pathway and may be involved in the pathogenesis of insulin resistance. Thus, the resistin gene represents a potential candidate for the etiology of insulin resistance and type 2 diabetes, although the absence of the resistin gene has been reported in fat cells and adipose tissue from nearly overweight subjects [39].
The current study investigated the Covid-19 infected diabetic patients have a high frequency of single nucleotide polymorphism than patients with diabetes only in rs1233732258, rs1451147074, X227G.A, rs772392077, rs764296340, X494T.C, X663A.C, While no significant difference was recorded in the frequency of SNPs rs3219177, X542A.C, and rs3745367.
The study of Meizlish et al. [40] described the elevated levels of resistin in Covid-19 patients with a fatal progression. They report that the discovery of resistin, along with lipocalin 2 and matrix metallopeptidase [41], at high concentrations, in the circulation of critically ill Covid-19 patients strongly suggests neutrophil activation and granulocyte clearance. They hypothesized that the components of the described neutrophil activation have effector functions that may be harmful to a patient with Covid-19. In addition, it was shown that resistin is a biomarker of disease severity and possibly a mediator of the prolonged inflammatory state in septic shock/acute sepsis [42]. Perpiñan et al. [43] concluded that resistin is the best early indicator of invasive ventilation in Covid-19 pneumonia. Therefore, this cytokine profile should be included in the personalized treatment decision algorithm for patients with Covid-19. On the other hand, Covid-19 produces such an intense cellular storm in the acute phase of the disease that it overwhelms the underlying chronic inflammation found in obesity and metabolic syndrome. Several mechanisms for resistin secretion have been reported in inflammation and sepsis [44], but the mechanism in Covid-19 has remained unclear. In humans, resistin is delivered from peripheral blood mononuclear cells, macrophages, and bone marrow rather than from adipocytes [45].
In future studies, it is suggested to investigate other genes that are related to the severity of diabetes expressed during the covid-19 infection.
Conclusion
Diabetic parameters increase in diabetic patients with covid-19 compared to diabetic patients without Covid-19. In addition, diabetic patients with Covid-19 have a high frequency of single nucleotide polymorphisms of the RETN gene.
Acknowledgements: We hereby express our gratitude to the Deanship of the College of Science/Thi-Qar University for their valuable assistance and the assistance of the head and faculty members of the Department of Biology - College of Science/Thi-Qar University. We also thank all the participants in this study and the laboratory staff of Shatrah General Hospital and the isolation center for people infected with the corona virus.
Ethical Permission: This study subjected to the qualifications of ethical considerations and according to the form prepared for this purpose by the Iraqi Ministry of Health. Also, the research got the agreement by the committee of ethical standards at the College of Science, Thi-Qar University, one of the colleges belonging to the Ministry of Higher Education and Scientific Research, Iraq. In addition, informed consent was obtained from all patients and healthy persons before taking samples.
Conflict of Interests: There is no conflict of interests.
Authors’ Contribution: Hadi HS (First Author), Introduction Writer/Main Researcher/Statistical Analyst/Discussion Writer (60%); Enayah SH (Second Author), Methodologist/Statistical Analyst/Discussion Writer (40%)
Funding: This research was sponsored by Thi-Qar University.
A pandemic is a contagious infectious disease that has spread across numerous geographic areas or continents. According to the most basic definition, the term "contagious" refers to an infection that can be passed from one person to another, either directly or indirectly [1]. According to the World Health Organization (WHO), when designating a new disease as a pandemic, there is a wide range of disagreement among members of the medical and scientific professions. “A pandemic is the worldwide spread of a new illness” [2]. In 2002, SARS came from China. It is considered that only small mammals can mutate a strain of the coronavirus that infect people. SARS spread rapidly in other Asian countries from China. Also, in several countries, including 4 in the U.K. and an important outbreak in Toronto, Canada, there were a small number of cases [3].
In 1964, June Almeida was contacted by Dr. David Arthur John Tyrrell, a British virologist, the Director of the Common Cold Unit in Salisbury, as one nasal swab sample collected from a boarding schoolboy from Surrey in 1961 and designated as “B 814”. With no other option being available, Tyrrell sent the sample to June Almeida, hoping that her electron microscope could identify the virus. His comment in his book, “Cold Wars: The Fight against the Common Cold”, was “We were not too hopeful but felt it was worth a try”. On putting the mysterious pathogen samples under the microscope, Almeida noticed that B814 had a structure similar to influenza viral particles. She knew that she was on the verge of discovering a new family of viruses with short spikey projections on the outer surface, appearing as a solar corona which led June Almeida and Tyrrell to call the new group ”coronavirus” – derived from the Latin word “corona” meaning “crown” or “halo”. When the research on the new virus was sent for publication, the journal refused to accept the finding, as the reviewer found Almeida’s micrographs of the B814 virus as “just bad pictures of influenza viruses”. June Almeida and Tyrrell had to struggle to get the work finally published in the Journal of General Virology in 1967 [4].
The first human coronavirus was described by June Dalziel Almeida in 1966. She had observed a viral structure under electron microscopy while being involved in a study investigating the causes of the common cold. A paper submitted by Almeida and her team described a crown-shaped structure supposed to be a new type of virus causing common colds. This paper was rejected as the editors claimed that “these microscopic observations resulted from distorted influenza viruses” [2].
Covid-19 symptoms vary, ranging between mild and severe diseases. Headache, loss of taste and smell, nasal congestion and rhinorrhea, cough, pain in the muscle, sore throat, fever, and breathing problems are common symptoms [3]. Nongastrointestinal symptoms of Covid-19 include fever, cough, shortness of breath, chills, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell. Gastrointestinal (GI) symptoms, including anorexia, nausea, vomiting, abdominal pain, and diarrhea, have been reported in patients with Covid-19. Additionally, abnormal liver enzymes are also observed [5]. Flu-like symptoms are present in SARS, usually starting 2-7 days after infections. The time between the virus and symptoms (incubation period) may sometimes be up to 10 days [3]. Coronaviruses infecting humans and associated with severe atypical pneumonia, including Severe Acute Respiratory Syndrome Coronaviruses 1 (SARS-CoV-1) and 2 (SARS-CoV-2), and Middle East Respiratory Syndrome Coronavirus (MERS-CoV-2), have caused three epidemics in the 21st century [6].
Viruses and the diseases they cause often have different names. For example, HIV is the virus that causes AIDS. People often know the name of a disease but not the name of the virus that causes it. There are different processes and purposes for naming viruses and diseases. Viruses are named based on their genetic structure to facilitate the development of diagnostic tests, vaccines, and medicines. Virologists and the wider scientific community do this work, so viruses are named by the International Committee on Taxonomy of Viruses (ICTV). Diseases are named to enable discussion on disease prevention, spread, transmissibility, severity and treatment. Diseases are officially named by WHO in the International Classification of Diseases (ICD). ICTV announced “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)” as the name of the new virus on 11 February 2020. This name was chosen because the virus is genetically related to the coronavirus responsible for the SARS outbreak of 2003. While related, the two viruses are different .WHO announced “Covid-19” as the name of this new disease on 11 February 2020, following guidelines previously developed with the World Organization for Animal Health (OIE) and the Food and Agriculture Organization of the United Nations (FAO). From a risk communications perspective, using the name SARS can have unintended consequences in creating unnecessary fear for some populations, especially in Asia, which was worst affected by the SARS outbreak in 2003. For that reason and others, WHO has begun referring to the virus as “the virus responsible for Covid-19” or “the Covid-19 virus” when communicating with the public. Neither of these designations are intended as replacements for the official name of the virus as agreed by the ICTV [7].
Most infected patients have mild symptoms, including fever, fatigue, and cough, but in severe cases, the disease can progress quickly to acute respiratory distress syndrome, septic shock, metabolic acidosis, and coagulopathy [8]. Outcomes of Covid-19 range from asymptomatic infection to death. Older age, male gender, and comorbid conditions, such as hypertension and diabetes, have been identified as risk factors for severe outcomes. While Covid-19 is typically characterized by symptoms of viral pneumonia, SARS-CoV-2 causes a systemic disease with possible involvement of the heart, liver, pancreas, and kidneys, as well as alterations in circulating lymphocytes and the immune system, because of the ubiquitous distribution of the main viral entry receptor, namely Angiotensin Converting Enzyme 2 (ACE2) [9]. Growing evidence indicates that environmental conditions might influence the current outbreak of Covid-19 [10]. Covid-19 is caused by the severe acute respiratory syndrome coronavirus-2 and curiously displays a propensity for thrombosis in multiple vascular beds. Covid-19-related thrombosis may contribute to severe organ injury and death. The incidence of thrombotic events was as high as 31% in one cohort [11]. Based on the epidemiologic findings of Covid-19 incidence, the severity of illness and mortality seem to be associated with multiple comorbidities such as diabetes, hypertension, and cardiovascular disease. Some studies revealed that severe Covid-19 patients have a higher incidence of diabetes and hypertension than milder forms [12]. As a direct effect, the Covid-19 infection has resulted in striking changes in patients' metabolism with significant elevations in blood glucose. It is attributed to the increased release of cytokines and inflammatory mediators, which led to increased insulin resistance and the associated hyperglycemia. In addition, it has been suggested that Covid-19 might be involved in developing acute diabetes mellitus in certain patients by targeting ACE2 receptors located in pancreatic islets resulting in pancreatic injury [13]. Insulin resistance is a pathophysiological state where cells display reduced responsiveness to the glucose-lowering activity of insulin. While there are rare cases where mutations in genes associated with insulin signaling or lipodystrophy cause insulin resistance, for the most part, insulin resistance is associated with obesity and, thus, a state of positive energy balance. This form of insulin resistance is frequently associated with hyperinsulinemia, increased waist circumference or visceral adiposity, metabolic dyslipidemia with high triglycerides and low HDL, and hepatic steatosis features collectively referred to as the metabolic syndrome. We refer to this as “common insulin resistance” [14].
Resistin is a recently discovered adipocyte-secreted polypeptide that has been implicated in the development of insulin resistance. Resistin was first described in 2001 during a search for genes that induced adipocyte differentiation, and it was down-regulated in mature adipocytes during exposure to Thiazolidinedione (TZD). This led to the discovery of a protein that the investigators named resistin (for insulin resistance) [15]. Resistin is a 12.5-kDa polypeptide that belongs to the resistin-like molecule family of cysteine-rich proteins. Some population studies have shown that resistin levels are indeed associated with metabolic risk factors and insulin resistance, suggesting that resistin may play an important role in the pathophysiology of diabetes [16]. Resistin is expressed in pre-adipocytes in addition to adipocytes, which may contribute to the elevation of resistin content in the adipose tissue of obese humans [17]. One of the most controversial adipokines is Resistin (RETN), which is a macrophage-derived signaling polypeptide hormone that belongs to the cysteine-rich proteins family [18]. Resistin increases blood glucose levels and impairs glucose transport to the target cells [19]. Resistin is a protein hormone released by adipocytes, which contributes to IR and purportedly acts as a link between obesity and diabetes [20]. Administration of exogenous resistin or transgenic overexpression leads to decreased insulin sensitivity and altered glucose handling, and conversely, blocking resistin activity or genetically decreasing its levels improves insulin sensitivity and restores glucose homeostasis. Thus there is strongly genetic and pharmacological evidence that resistin is a mediator of insulin resistance in rodents [21]. White adipose tissue fulfills an important endocrine role in controlling the whole body's metabolism. Resistin is supposed to represent a novel link between obesity and the development of type 2 diabetes. Further investigations changed that view, however, revealed numerous species-specific properties and physiological differences of resistin actions [19]. Human resistin has been shown to induce the expression of proinflammatory cytokines and adhesion molecules in the settings of inflammation and endothelial dysfunction. Given the strong relationship between inflammation and metabolism, there is mounting evidence suggesting a role for human resistin in the pathological processes of metabolic diseases, including obesity, diabetes, and cardiovascular diseases [22]. Resistin has been implicated in the insulin resistance observed in normal pregnancy, as the level of resistin increases with gestational age and decreases after delivery. Resistin is detectable in human serum, but unlike rodents, human resistin is predominantly expressed in macrophages, and its expression in human adipose tissue is predominantly due to non-adipocyte resident inflammatory cells. Human and rodent resistin gene and protein sequences share about 60% identity, less than most hormones conserved across species. Yet, the genes are syntenic, with the gene coding resistin (Retn) on mouse chromosome 8 located a similar distance from the Insulin Receptor gene as is the Retn gene on human chromosome 19. Also, as in rodents, resistin levels are decreased with thiazolidinedione treatment in humans [21].
The present study aimed to determine RETN gene polymorphisms as a risk factor in diabetic patients with Covid-19 infection.
Instruments and Methods
Sample collection
This study was performed from January 2022 to March 2022. A total of 100 diabetic patients with Covid-19, aged between 37-69 years (46 males and 54 females), in the isolation center for people infected with Coronavirus at Al Hussein Teaching Hospital in Thi-Qar and Shatrah General Hospital were included in the study. Also, 50 diabetic patients without Covid-19 (28 males and 22 females) participated in the study as a control group.
After obtaining written consent, 5 ml of venous blood was collected from patients and controls, which was added to gel tubes and EDTA (Ethylenediaminetetraacetic acid) tubes. 50 samples were chosen randomly from Covid-19 infected patients and 19 samples from diabetic only for molecular and sequencing study. 2 ml of blood with EDTA was used to examine HbA1c and stored at -20°C until used for quantification of molecular study, and another 3 ml of blood was put into a gel tube and then the tube centrifuged at 4000 rpm for 10 min to extract serum for glucose estimation test.
All patient and control data, including age, gender, clinical presentation features, patient history, height, and weight, were recorded.
Statistical analysis
The data were statistically analyzed by SPSS 26 software using independent t-test, Chi-square test, and odds ratio expressed in mean LSD.
Findings
Diabetes parameters
There was a significant increase in Random Blood Sugar (RBS) and HbA1c in diabetic patients with Covid-19 compared to the diabetic patients only (p<0.001; Table 1).
Table 1) Comparison of diabetes parameters in diabetic patients with (n=100) and without (n=50) Covid-19

The increase in diabetes parameters in diabetic patients with Covid-19 compared to the diabetic patients without Covid-19 was observed in both men and women (Table 2).
Table 2) Comparison of diabetes parameters in diabetic patients with and without Covid-19 based on the gender

Molecular study
The results showed 10 Single Nucleotide Polymorphisms (SNPs) as follows:
rs1233732258
There was no significant difference in frequencies of the C and T alleles and genotypes of C/C and C/T in diabetic patients with and without Covid-19 (p>0.05). Also, the frequency of alleles and genotypes increased in patients with Covid-19 than diabetics only, 1.084 and 2.111, respectively (p<0.05; Table 3).
rs1451147074
Table 3) Allele and genotype frequency associated with rs1233732258 SNP

There was no significant difference in frequencies of the G and A alleles, but a significant difference was seen in the genotype of G/G and G/A in diabetic patients with and without Covid-19 (p=0.013). Also, the frequency of alleles increased in patients with Covid-19 than in diabetics only, 1.128, while there was no significant odds ratio for genotype (Table 4). X227G.A
Table 4) Allele and genotype frequency associated with rs1451147074 SNP

There was no significant difference in frequencies of the G and A alleles, but a significant difference was seen in the genotype of G/G and G/A in diabetic patients with and without Covid-19 (p=0.013). Also, the frequency of alleles increased in patients with Covid-19 than in diabetics only, 1.0, while there was no significant odds ratio for genotype (Table 5).
rs772392077
Table 5) Allele and genotype frequency associated with X227G.A SNP
There was no significant difference in frequencies of the G and A alleles, but a significant difference was seen in the genotype of G/G and G/A in diabetic patients with and without Covid-19 (p<0.001). Also, the frequency of alleles and genotypes increased in patients with Covid-19 than diabetics only, 1.387 and 5.359, respectively (Table 6).
rs764296340
Table 6) Allele and genotype frequency associated with rs772392077 SNP

There was no significant difference in frequencies of the T and A alleles, but a significant difference was seen in the genotype of T/T and T/A in diabetic patients with and without Covid-19 (p=0.043). Also, the frequency of alleles increased in patients with Covid-19 than in diabetics only, 1.083, while there was no significant odds ratio for genotype (Table 7).
rs3219177
Table 7) Allele and genotype frequency associated with rs764296340 SNP

X663A.C
There was a significant difference in frequencies of the C and A alleles, as well as in the genotypes of codominant and recessive. However, there was no significant difference
There was a significant difference in frequencies of the C and T alleles, and the genotypes of codominant, dominant, recessive, and over dominant in diabetic patients with and without Covid-19 (p<0.05; Table 8). X494T.C
There was no significant difference in frequencies of the T and C alleles, but a significant difference was seen in the genotype of T/T and T/C in diabetic patients with and without Covid-19 (p=0.043). Also, the frequency of alleles increased in patients with Covid-19 than in diabetics only, 1.083, while there was no significant odds ratio for genotype (Table 9).
X542A.C
There was no significant difference in frequencies of the T and C alleles, but a significant difference was seen in the genotype of T/T and T/C in diabetic patients with and without Covid-19 (p=0.034; Table 10).
rs3745367
There was no significant difference in frequencies of the G and A alleles, as well as in the genotypes of codominant and recessive. Hewer, there was a significant difference in the genotypes of dominant and over dominant in diabetic patients with and without Covid-19 (p<0.05). Also, the odds ratio of recessive genotype increased in patients with Covid-19, by 1.490, compared to the diabetics only (Table 11).
Table 8) Allele and genotype frequency associated with rs3219177 SNP

Table 9) Allele and genotype frequency associated with X494T.C SNP

Table 10) Allele and genotype frequency associated with X542A.C SNP

X663A.C
There was a significant difference in frequencies of the C and A alleles, as well as in the genotypes of codominant and recessive. However, there was no significant difference in the genotypes of dominant and over dominant in diabetic patients with and without Covid-19 (p<0.05). Also, the odds ratio of alleles and dominant and recessive genotypes increased in patients with Covid-19 compared to the diabetics only, 1.764, 1.658, and 13.025, respectively (Table 12).
Table 11) Allele and genotype frequency associated with rs3745367 SNP

Table 12) Allele and genotype frequency associated with X663A.C SNP

Linkage disequilibrium of the studied variants is represented by haplo plot diagram of D’ (Figure 1).

Figure 1) Linkage disequilibrium of the studied variants represented by haplo plot diagram of D’
Discussion
The current study indicated a significant increase in the concentration of RBS and HbA1c in Covid-19 infected diabetic patients than in diabetic patients only.
The current study agreed with the study of Kumar et al. [23]. Their study investigated inflammatory markers in diabetic and non-diabetic Covid-19 and concluded a Covid-19 infection induce a high level of RBS and HbA1c in diabetic patients compared with patients without Covid-19 infection. Hyperglycemia promotes SARS-CoV-2 replication in human monocytes, resulting in increased viral proliferation. Thus, hyperglycemia is an independent risk factor for Covid-19 infection [24].
Also, the current study agreed with recent study performed by Smith et al. [25]. They studied impaired glucose metabolism in patients with diabetes with severe Covid‐19. Their study showed a significant increase in diabetic parameters in Covid-19 infected patients than diabetic only. In addition, it was found a positive correlation between body mass index and diabetic parameters. A possible explanation for a link between hyperglycemia and ACE2 levels in the severity of Covid‐19 could be explained by several clinical observations in SARS and preclinical observations in the non-diabetic. Potential changes in glycosylation of the ACE2, as well as glycosylation of the viral spike protein, both possibly induced by uncontrolled hyperglycemia, may alter both the binding of the viral spike protein to ACE2 and the degree of the immune response to the virus [25]. Several studies showed that Diabetes Mellitus (DM) as an associated distinctive comorbidity increased the morbidity and mortality of Covid-19 patients [26].
The present study disagreed with previous studies that reported higher glucose levels than the current study. Reddy et al. [27] recorded a concentration of glucose equal to 555 mg/dl, while the HbA1c was 14.2 mg/dl. Also, in the study of Chee et al. [28], the glucose level was 714, and HbA1c was 14.2, and in the study of Kim et al. [29], the hyperglycemia in Covid-19 patients was 655 mg/dl, and HbA1c was 11.4. In contrast, the study of Li et al. [30] reported a level of glucose equal to 298 mg/dl and HbA1c equal to 6.8, which was lower than those reported in the present study.
Diabetes is linked to the severity of infection by a number of factors. Hyperglycemia can initiate, intensify, or extend the acute inflammatory response. It also causes a fibrinolysis and coagulation mismatch, leading to increased factors of coagulation and relative inhibition of the system of fibrinolysis, promoting a state of pro-coagulation. Furthermore, SARS- CoV-2 is thought to use ACE2 as an entrance receptor on the islets of Langerhans.
The current study showed, according to males and females, that the Hba1c level and Random blood sugar increased in diabetic patients infected with the Covid19 compared to diabetic patients only, and there was a significant difference between the two groups. Several observational studies have provided clinical evidence that uncontrolled hyperglycemia may lead to a longer LOS and significantly higher mortality in Covid-19 patients. The current study agreed with study of Lei et al. [31]. Their study included that high blood sugar in the body leads to a doubling of the symptoms of Covid and leads to death due to the relationship between Covid and high sugar in the body. As for age and body mass, a significant increase was observed in the level of normal sugar and cumulative sugar for diabetic patients with Covid and diabetic patients only. The current study agreed with study of AbdElaleem et al. [32]. Their study included to study Covid-19 related diabetes and try to find predictors of mortality in these patients. Hyperglycemia is commonly seen in critically unwell patients and can be correlated to disease severity [33].
Our data showed that higher HbA1c levels were a factor in disease severity in patients with Covid-19 and diabetes. These results are consistent with those of previous studies. A retrospective cohort study on the association between pre-infection glycemic control and disease severity in patients with type 2 diabetes and Covid-19 in Israel found a gradual dose-response relationship between HbA1c level and the risk of severe Covid-19 [34]. A population-based cohort study in England also suggested a higher risk of mortality from Covid-19 in patients with either type 1 or type 2 diabetes with HbA1c levels >10.0% than in those with HbA1c levels of less than 6.5% [35]. In addition, a Japanese study showed that HbA1c levels might be a risk factor for severe disease requiring oxygenation in patients with Covid-19 [36].
The newly discovered hormone resistin has been suggested to link obesity in patients with diabetes [37, 38]. In animal models, resistin is specifically secreted by adipocytes and significantly increased in both genetic and diet-induced obesity. Resistin overexpression was associated with impaired glucose tolerance and insulin action in mice, whereas thiazolidinedione treatment significantly reduced resistin gene polymorphism, and neutralization of the resistin protein enhanced blood glucose uptake and insulin sensitivity. It has been suggested that resistin modulates one or more steps in the insulin signaling pathway and may be involved in the pathogenesis of insulin resistance. Thus, the resistin gene represents a potential candidate for the etiology of insulin resistance and type 2 diabetes, although the absence of the resistin gene has been reported in fat cells and adipose tissue from nearly overweight subjects [39].
The current study investigated the Covid-19 infected diabetic patients have a high frequency of single nucleotide polymorphism than patients with diabetes only in rs1233732258, rs1451147074, X227G.A, rs772392077, rs764296340, X494T.C, X663A.C, While no significant difference was recorded in the frequency of SNPs rs3219177, X542A.C, and rs3745367.
The study of Meizlish et al. [40] described the elevated levels of resistin in Covid-19 patients with a fatal progression. They report that the discovery of resistin, along with lipocalin 2 and matrix metallopeptidase [41], at high concentrations, in the circulation of critically ill Covid-19 patients strongly suggests neutrophil activation and granulocyte clearance. They hypothesized that the components of the described neutrophil activation have effector functions that may be harmful to a patient with Covid-19. In addition, it was shown that resistin is a biomarker of disease severity and possibly a mediator of the prolonged inflammatory state in septic shock/acute sepsis [42]. Perpiñan et al. [43] concluded that resistin is the best early indicator of invasive ventilation in Covid-19 pneumonia. Therefore, this cytokine profile should be included in the personalized treatment decision algorithm for patients with Covid-19. On the other hand, Covid-19 produces such an intense cellular storm in the acute phase of the disease that it overwhelms the underlying chronic inflammation found in obesity and metabolic syndrome. Several mechanisms for resistin secretion have been reported in inflammation and sepsis [44], but the mechanism in Covid-19 has remained unclear. In humans, resistin is delivered from peripheral blood mononuclear cells, macrophages, and bone marrow rather than from adipocytes [45].
In future studies, it is suggested to investigate other genes that are related to the severity of diabetes expressed during the covid-19 infection.
Conclusion
Diabetic parameters increase in diabetic patients with covid-19 compared to diabetic patients without Covid-19. In addition, diabetic patients with Covid-19 have a high frequency of single nucleotide polymorphisms of the RETN gene.
Acknowledgements: We hereby express our gratitude to the Deanship of the College of Science/Thi-Qar University for their valuable assistance and the assistance of the head and faculty members of the Department of Biology - College of Science/Thi-Qar University. We also thank all the participants in this study and the laboratory staff of Shatrah General Hospital and the isolation center for people infected with the corona virus.
Ethical Permission: This study subjected to the qualifications of ethical considerations and according to the form prepared for this purpose by the Iraqi Ministry of Health. Also, the research got the agreement by the committee of ethical standards at the College of Science, Thi-Qar University, one of the colleges belonging to the Ministry of Higher Education and Scientific Research, Iraq. In addition, informed consent was obtained from all patients and healthy persons before taking samples.
Conflict of Interests: There is no conflict of interests.
Authors’ Contribution: Hadi HS (First Author), Introduction Writer/Main Researcher/Statistical Analyst/Discussion Writer (60%); Enayah SH (Second Author), Methodologist/Statistical Analyst/Discussion Writer (40%)
Funding: This research was sponsored by Thi-Qar University.
References
1. Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, et al. COVID-19: A global challenge with old history, epidemiology and progress so far. Molecules. 2021;26(1):39. [Link] [DOI:10.3390/molecules26010039]
2. Pitlik SD. COVID-19 compared to other pandemic diseases. Rambam Maimonides Med J. 2020;11(3):e0027. [Link] [DOI:10.5041/RMMJ.10418]
3. Habeeb,NJ, Abbas YA, Abass KS, Hussein KR. Detection of Covid-19 (SARS-COV-2) and their virulence factor orf8 gene among patients in Thi-Qar Province, Iraq. Biochem Cell Arch. 2021;21(2):5365-70. [Link]
4. Bhattacharya K, Bhattacharya N, Bhattacharya AS. The discovery of coronavirus-an interesting journey. Galician Med J. 2021;28(3):e202131. [Link] [DOI:10.21802/gmj.2021.3.1]
5. Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, et al. aga institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159(1):320-34. [Link] [DOI:10.1053/j.gastro.2020.05.001]
6. Zhu Z, Lian X, Su X, Wu W, Marraro GA, Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21(1):224. [Link] [DOI:10.1186/s12931-020-01479-w]
7. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Brazil J Implantol Health Sci. 2020;2(3):173. [Link]
8. 8- Melo-Oliveira ME, Sá-Caputo D, Bachur JA, Paineiras-Domingos LL, Sonza A, Lacerda AC, et al. Reported quality of life in countries with cases of COVID19: a systematic review. Expert Rev Respir Med. 2021;15(2):213-20. [Link] [DOI:10.1080/17476348.2021.1826315]
9. Lv Y, Zhao X, Wang Y, Zhu J, Ma C, Feng X, et al. Abnormal liver function tests were associated with adverse clinical outcomes: an observational cohort study of 2,912 patients with covid-19. Front Med. 2021;8:639855. [Link] [DOI:10.3389/fmed.2021.639855]
10. Akter R, Rahman M, Bhattacharya T, Kaushik D, Mittal V, Parashar J, et al. Novel coronavirus pathogen in humans and animals: an overview on its social impact, economic impact, and potential treatments. Environ Sci Pollut Res Int. 2021;28(48):68071-89. [Link] [DOI:10.1007/s11356-021-16809-8]
11. Sahai A, Bhandari R, Koupenova M, Freedman JE, Godwin M, McIntyre T, et al. SARS-CoV-2 receptors are expressed on human platelets and the effect of aspirin on clinical outcomes in COVID-19 patients. Res Sq. 2020;rs.3.rs-119031. [Link] [DOI:10.21203/rs.3.rs-119031/v1]
12. Moazzami B, Chaichian S, Kasaeian A, Djalalinia S, Akhlaghdoust M, Eslami M, et al. Metabolic risk factors and risk of Covid-19: A systematic review and meta-analysis. PLoS One. 2020;15(12):e0243600. [Link] [DOI:10.1371/journal.pone.0243600]
13. Nassar M, Daoud A, Nso N, Medina L, Ghernautan V, Bhangoo H, et al. Diabetes mellitus and COVID-19. Diabetes Metabo Syndr. 2021;15(6):102268. [Link] [DOI:10.1016/j.dsx.2021.102268]
14. Fazakerley DJ, Krycer JR, Kearney AL, Hocking SL, James DE. Muscle and adipose tissue insulin resistance: malady without mechanism? J Lipid Res. 2019;60(10):1720-32. [Link] [DOI:10.1194/jlr.R087510]
15. Devanoorkar A, Kathariya R, Guttiganur N, Gopalakrishnan D, Bagchi P. Resistin: a potential biomarker for periodontitis influenced diabetes mellitus and diabetes induced periodontitis. Dis Markers. 2014;2014:930206. [Link] [DOI:10.1155/2014/930206]
16. Hivert MF, Manning AK, McAteer JB, Dupuis J, Fox CS, Cupples LA, et al. Association of variants in RETN with plasma resistin levels and diabetes-related traits in the Framingham Offspring Study. Diabetes. 2009;58(3):750-6. [Link] [DOI:10.2337/db08-1339]
17. Al-Harithy RN, Al-Ghamdi S. Serum resistin, adiposity and insulin resistance in Saudi women with type 2 diabetes mellitus. Ann Saudi Med. 2005;25(4):283-7. [Link] [DOI:10.5144/0256-4947.2005.283]
18. Zayani N, Hamdouni H, Boumaiza I, Achour O, Neffati F, Omezzine A, et al. Resistin polymorphims, plasma resistin levels and obesity in Tunisian volunteers. J Clin Lab Anal. 2018;32(2):e22227. [Link] [DOI:10.1002/jcla.22227]
19. Sassek M, Pruszynska-Oszmalek E, Kołodziejski PA, Szczepankiewicz D, Kaczmarek P, Wieloch M, et al. Resistin is produced by rat pancreatic islets and regulates insulin and glucagon in vitro secretion. Islets. 2016;8(6):177-85. [Link] [DOI:10.1080/19382014.2016.1251538]
20. Liu SX, Zheng F, Xie KL, Xie MR, Jiang LJ, Cai Y. Exercise reduces insulin resistance in type 2 diabetes mellitus via mediating the lncRNA MALAT1/microRNA-382-3p/resistin axis. Mol Ther Nucleic Acids. 2019;18:34-44. [Link] [DOI:10.1016/j.omtn.2019.08.002]
21. Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrinol Metab. 2011;22(7):259-65. [Link] [DOI:10.1016/j.tem.2011.03.005]
22. Jiang S, Teague AM, Tryggestad JB, Lyons TJ, Chernausek SD. Fetal circulating human resistin increases in diabetes during pregnancy and impairs placental mitochondrial biogenesis. Mol Med. 2020;26(1):76. [Link] [DOI:10.1186/s10020-020-00205-y]
23. Kumar M, Bindu CM, Shyam AC, Reena R. Ferritin-The key model inflammatory marker in diabetic and non-diabetic COVID-19. Asian J Med Sci. 2021;12(12):23-31. [Link] [DOI:10.3126/ajms.v12i12.39717]
24. Codo AC, Davanzo GG, de Brito Monteiro L, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32(3):437-46. [Link] [DOI:10.2139/ssrn.3606770]
25. Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, Chao K, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID‐19. J Med Virol. 2021;93(1):409-15. [Link] [DOI:10.1002/jmv.26227]
26. Malik SUF, Chowdhury PA, Hakim A, Islam MS, Alam MJ, Azad AK. Blood biochemical parameters for assessment of COVID-19 in diabetic and non-diabetic subjects: a cross-sectional study. Int J Environ Health Res. 2022;32(6):1344-58. [Link] [DOI:10.1080/09603123.2021.1879741]
27. Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab Syndr. 2020;14(5):1459-62. [Link] [DOI:10.1016/j.dsx.2020.07.050]
28. Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. [Link] [DOI:10.1016/j.diabres.2020.108166]
29. Kim NY, Ha E, Moon JS, Lee YH, Choi EY. Acute hyperglycemic crises with coronavirus disease-19. Diabetes Metab J. 2020;44(2):349-53. [Link] [DOI:10.4093/dmj.2020.0091]
30. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID‐19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935-41. [Link] [DOI:10.1111/dom.14057]
31. Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology. 2020;72(2):389-98. [Link] [DOI:10.1002/hep.31301]
32. AbdElaleem AAM, Rasheed MM, Hamid HA, Alsayed E, Zanaty MM, Ibrahim AA. Diabetes incidence and impact on mortality with COVID-19 patients. Diabetes. 2022;29(05):1-2. [Link]
33. Chandrashekhar Joshi S, Pozzilli P. COVID-19 induced Diabetes: A novel presentation. Diabetes Res Clin Pract. 2022;191:110034. [Link] [DOI:10.1016/j.diabres.2022.110034]
34. Hayek S, Ben-Shlomo Y, Balicer R, Byrne K, Katz M, Kepten E, et al. Preinfection glycaemic control and disease severity among patients with type 2 diabetes and COVID-19: A retrospective, cohort study. Diabetes Obes Metab. 2021;23(8):1995-2000. [Link] [DOI:10.1111/dom.14393]
35. Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-33. [Link] [DOI:10.1016/S2213-8587(20)30271-0]
36. Yanagisawa S, Oikawa Y, Takagi S, Horikoshi Y, Satomura A, Imai K, et al. HbA1c level may be a risk factor for oxygen therapy requirement in patients with coronavirus disease 2019. J Diabetes Investig. 2022;13(5):909-17. [Link] [DOI:10.1111/jdi.13743]
37. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307-12. [Link] [DOI:10.1038/35053000]
38. Sentinelli F, Romeo S, Arca M, Filippi E, Leonetti F, Banchieri M, et al. Human resistin gene, obesity, and type 2 diabetes: mutation analysis and population study. Diabetes. 2002;51(3):860-2. [Link] [DOI:10.2337/diabetes.51.3.860]
39. Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001;285(2):561-4. [Link] [DOI:10.1006/bbrc.2001.5173]
40. Meizlish ML, Pine AB, Bishai JD, Goshua G, Nadelmann ER, Simonov M, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5(5):1164-77. [Link] [DOI:10.1182/bloodadvances.2020003568]
41. Mahase E. Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. [Link] [DOI:10.1136/bmj.m641]
42. Sundén-Cullberg J, Nyström T, Lee ML, Mullins GE, Tokics L, Andersson J, et al. Pronounced elevation of resistin correlates with severity of disease in severe sepsis and septic shock. Crit Care Med. 2007;35(6):1536-42. [Link] [DOI:10.1097/01.CCM.0000266536.14736.03]
43. Perpiñan C, Bertran L, Terra X, Aguilar C, Binetti J, Lopez-Dupla M, et al. Resistin and IL-15 as predictors of invasive mechanical ventilation in covid-19 pneumonia irrespective of the presence of obesity and metabolic syndrome. J Pers Med. 2022;12(3):391. [Link] [DOI:10.3390/jpm12030391]
44. Nikonovas T, Spessa A, Doerr SH, Clay GD, Mezbahuddin S. Near-complete loss of fire-resistant primary tropical forest cover in Sumatra and Kalimantan. Commun Earth Environ. 2020;1(1):1234567890. [Link] [DOI:10.1038/s43247-020-00069-4]
45. Lin C-Y. Social reaction toward the 2019 novel coronavirus (COVID-19). Soc Health Behav. 2020;3(1):1-2. [Link] [DOI:10.4103/SHB.SHB_11_20]










