Volume 17, Issue 2 (2025)
Iran J War Public Health 2025, 17(2): 113-121 |
Back to browse issues page
Article Type:
Subject:
Ethics code: IR.UM.REC.1402.276
History
Received: 2025/04/13 | Accepted: 2025/05/15 | Published: 2025/05/29
Received: 2025/04/13 | Accepted: 2025/05/15 | Published: 2025/05/29
How to cite this article
Allami M, Al-Shammari A, Neshati Z. Generation and Characterization of Purkinje-Like Cells from Differentiated Mouse Bone Marrow Mesenchymal Stem Cells. Iran J War Public Health 2025; 17 (2) :113-121
URL: http://ijwph.ir/article-1-1594-en.html
URL: http://ijwph.ir/article-1-1594-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Biology, Faculty of Science, Ferdowsi University of Mashhad, Mashhad, Iran
2- Experimental Therapy Department, Iraqi Center for Cancer and Medical Genetic Research, Mustansiriyah University, Baghdad, Iraq
3- “Department of Biology, Faculty of Science” and “Novel Diagnostics and Therapeutics Research Group, Institute of Biotechnology,” Ferdowsi University of Mashhad, Mashhad, Iran
2- Experimental Therapy Department, Iraqi Center for Cancer and Medical Genetic Research, Mustansiriyah University, Baghdad, Iraq
3- “Department of Biology, Faculty of Science” and “Novel Diagnostics and Therapeutics Research Group, Institute of Biotechnology,” Ferdowsi University of Mashhad, Mashhad, Iran
Full-Text (HTML) (257 Views)
Introduction
The cerebellum is a crucial structure responsible for ensuring that motor activities are executed with precision and well-coordinated timing. Much of the computation in the cerebellum occurs in the cerebellar cortex, which is a layered, repetitive, and meticulously structured arrangement. Information from the cerebellar cortex ultimately converges on Purkinje neurons, the central computational integrators of the cerebellar system [1]. Research has shown that the cerebellum supports multiple functions, including emotion regulation, inhibition of impulsive decision-making, attention, and working memory. Additionally, several experimental and computational studies have indicated that the cerebellum plays a role in error-free (unsupervised) learning. Damage to parts of the cerebellum that project to the motor areas, prefrontal cortex, and limbic system can result in motor, cognitive, and emotional abnormalities, respectively. Some further suggest that the role of the cerebellum in cognitive function is similar to its role in controlling purposive motor skills during motor function [2]. There is also evidence that the cerebellum may be related to a variety of cognitive abnormalities and psychopathological manifestations. Many recent studies have reported strong associations between structural and functional abnormalities of the cerebellum and psychiatric disorders, especially schizophrenia, bipolar disorder, depression, anxiety disorders, attention deficit hyperactivity disorder (ADHD), and autism [3]. Given the cerebellum’s extensive involvement in various psychological and cognitive functions, conditions that affect mental health may directly or indirectly influence cerebellar function. One such condition is exposure to armed conflict during wartime. War not only results in direct physical injuries, such as traumatic brain damage, but is also associated with an increased prevalence of psychiatric disorders, including post-traumatic stress disorder (PTSD), depression, and anxiety. Recent studies have demonstrated that many of these psychiatric conditions are linked to functional and structural alterations in several brain regions, including the cerebellum [4, 5].
Purkinje neurons exhibit repetitive firing in a tonic pattern, with their firing frequency changing during controlled movement. They are influenced by two major types of excitatory inputs: parallel fibers originating from granule cells (through the mossy fiber pathway) and climbing fibers that come from the inferior olive. Furthermore, Purkinje neurons receive feed-forward inhibitory signals from molecular layer interneurons, which are regulated by inputs from parallel fibers [6]. Purkinje neurons integrate this incoming information, and the processed signals are transmitted through inhibitory GABAergic synapses to the neurons of the deep cerebellar nuclei (DCN), which consist of paired nuclei (dentate nucleus, interposed nuclei, and fastigial nucleus) located in the deep white matter of the cerebellum. Along with direct projections from the cerebellar flocculonodular lobe to the vestibular nuclei, projections from Purkinje neurons in other parts of the cerebellum are relayed through the neurons of the deep cerebellar nuclei, serving as the exclusive output of the cerebellar cortex. The deep cerebellar nuclei, in turn, send projections to various targets within the central nervous system (CNS), such as the premotor and primary motor cortical areas (via the thalamus), the reticular nucleus, and the red nucleus. Through these outputs from the deep cerebellar nuclei, the cerebellum communicates with the rest of the nervous system to regulate movement control [1]. Therefore, the presence of any disorder in Purkinje neurons can cause a wide range of neurological disorders; for this reason, treating or replacing damaged cells is highly important for preventing these disorders [5, 6].
The pathological mechanisms of neurological diseases are primarily characterized by the progressive degeneration of neuronal structure and function, leading to impairments in motor, sensory, and cognitive functions, which can result in varying degrees of paralysis and, ultimately, permanent disability. Furthermore, the drug development process is both intricate and costly. Consequently, there is an urgent demand for the identification of effective therapeutic approaches. In recent years, with ongoing advancements in nerve injury repair, cell transplantation and cell-based therapies have gained widespread application in neurological disorders, paving the way for the emergence of novel treatment strategies. The therapeutic potential of cell-based therapy in neurological disorders lies in its ability to mimic the natural processes of cellular repair and development within the nervous system, address the underlying causes of the disease, enhance dysfunctional pathways, and promote the regeneration of damaged tissues [7, 8]. Significantly, the potential for tissue repair and regeneration in neurological disorders is limited, and there is currently no definitive treatment available to halt or reverse their progression. The clinical application of pharmacological treatments to alleviate or manage symptoms in patients is constrained, as these drugs offer limited efficacy and are incapable of repairing damaged neurons or reconstructing neural networks [9, 10].
An era of research into neural regeneration began with the discovery of neural stem cells (NSCs), and significant advancements have been made in transplantation therapies for disorders of the CNS. However, because most NSCs are derived from aborted fetuses, their use is severely limited, and they also present ethical issues [11]. Thus, the search for better stem cell sources is crucial. Mesenchymal stem cells (MSCs) have been widely studied since their discovery in the late 1960s. Due to their capacity for multiple types of differentiation, immunomodulatory roles, and ability to support tissue and organ repair, adult stem cells hold great promise for use in regenerative medicine and disease treatment [12]. Additionally, there are no ethical concerns associated with the acquisition, use, or dissemination of MSCs. MSCs can be readily isolated from several human tissues, including adipose tissue, bone marrow, and the umbilical cord [13]. MSCs constitute a promising therapeutic approach because of their ability to promote nerve regeneration through a variety of mechanisms. To support neural regeneration, MSCs can differentiate into astrocytes, neurons, and Schwann cell-like cells [12, 13].
Since access to a larger number of MSCs compared to NSCs can be achieved through a relatively simple process, this study was conducted to produce Purkinje-like neurons from defined NSCs differentiated from MSCs. The findings support the utilization of bone marrow-derived MSCs as a viable and straightforward alternative source for Purkinje-like neurons, instead of NSCs, embryonic stem cells (ESCs), or induced pluripotent stem cells, providing a suitable model for investigating cerebellar development and degeneration.
Materials and Methods
Animals
Healthy albino Swiss male mice aged 3-6 weeks and weighing 10-15 grams were obtained from the animal care facility at ICCMGR, Mustansiriyah University, as a source for obtaining MSCs. All mice involved were kept in a controlled environment at 23-25°C, with unrestricted access to food and water, and were maintained under a 12-hour light/dark cycle.
Isolation and maintenance of bone marrow MSCs
Mice were euthanized in a clean environment according to standard procedures. In an airflow chamber (under sterile conditions), the mice were cleaned using a 70% alcohol solution, and the femurs and tibias were carefully separated using sterile surgical tools. After the femurs and tibias were cut from their ends, the bone marrow was extracted via a syringe filled with phosphate-buffered saline (PBS). The cell suspensions were maintained in 25 cm² tissue culture flasks in minimum essential medium (MEM; Capricorn Scientific, Germany), which contained 2% fetal bovine serum (FBS; Capricorn Scientific, Germany), streptomycin, and penicillin (100 μg/ml; Capricorn Scientific GmbH, Ebsdorfergrund, Germany). Finally, the flasks were placed in a 5% CO₂ incubator at 37°C, with an atmosphere of 95% humidity. Since MSCs make up a small portion of the nucleated cells in mouse bone marrow, they are considered an impure population in primary culture and are purified in subcultured passages. To ensure the purity of the MSCs, we examined the phenotype, the ability to attach to the flask surface, and the expression and non-expression of specific markers. The majority of the isolated cells were adherent after overnight culture; the non-adherent cells were removed by replacing the medium with MEM supplemented with 20% FBS. After that, the cultures were maintained by replacing the culture medium every two to three days until cell colonies formed, which took three to seven days. After reaching confluence, the cells were subcultured using a 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) solution (United States Biological, USA). Cells at the third passage were used for the next steps.
Characterization of MSCs
Immunocytochemistry (ICC) was used to study stem cell markers. Cells (4×104) were cultured in each well of 8-well tissue culture chamber slides (SPL, Korea). After three to five days of growth in the chamber slides, the medium was removed, the cells were washed with PBS, and they were fixed for ten minutes with 4% paraformaldehyde in a humid chamber with 1% hydrogen peroxide (H₂O₂) to block endogenous peroxidase activity. Fixed cells were washed with PBS and maintained at room temperature for 30 to 40 minutes before being incubated in 1.5% blocking serum for 30 minutes. Four specific markers—CD73 and CD44 as positive markers and CD34 and CD45 as negative markers—were used for the characterization of MSCs, utilizing CD73 (E-AB-14725-120), CD44 (E-AB-66948), CD34 (E-AB-70337), and CD45 (E-AB-70024) polyclonal primary antibodies (Elabscience Biotechnology, USA). The primary antibodies against each of the CD markers were added, and the cells were incubated in a humid chamber overnight at 4°C. The staining protocol was followed according to a 2-step plus Poly-HRP Anti-Mouse/Rabbit IgG Detection System (with DAB Solution; E-IR-R217; Elabscience Biotechnology, USA). After washing with PBS, the cells were incubated with anti-mouse/rabbit IgG secondary antibody for 30 minutes and were washed with PBS. Horseradish peroxidase (HRP) conjugated with avidin was added to each slide and incubated for 30 minutes, after which the slides were washed with PBS. A liquid DAB chromogen mixture was introduced and allowed to react for 2-5 minutes, after which the samples were washed with PBS and stained for 30-60 seconds with hematoxylin and eosin (H & E). Washing with PBS was repeated at least three times after each step. Negative control cells were stained with secondary antibody only, without any primary antibody. Finally, all slides were mounted using DPX, analyzed under an optical microscope, and captured using a digital camera.
Differentiation of MSCs into NSCs
Subconfluent cultures of P3 MSCs (2.50×106) in a 25cm² plastic flask (SPL, Korea) were used to induce neurogenesis. This was accomplished by using β-mercaptoethanol (BME; Santa Cruz Biotechnology Inc.) as a differentiation factor. The induction process followed these steps: A). The cells were exposed to MEM supplemented with 20% FBS and 1mM BME as preinduction media for 24 hours [14, 15]. B) The cells were subjected to neural differentiation induction (as postinduction media) using MEM without FBS (serum-free media) and 5mM BME for 10 hours [16, 17]. The ICC assay for Nestin and SOX-2 markers was carried out to characterize NSCs using an anti-mouse Nestin IgG monoclonal antibody (E-AB-70343) and anti-mouse SOX2 IgG polyclonal antibody (E-AB-70108) (Elabscience Biotechnology, USA) as primary antibodies.
Neurosphere formation
After the induction of NSCs, the induced cells were maintained in culture to form neurospheres (the typical morphology of NSCs in culture) in MEM supplemented with 5% FBS, 100µg/ml streptomycin and penicillin, 100µg/ml Amphotericin B, and 50ng/mL of both epidermal growth factor (EGF; Elabscience Biotechnology, USA) and basic fibroblast growth factor (b-FGF; Elabscience Biotechnology, USA) [18]. The induced cells were cultured in small tissue culture Petri dishes, 96-well flat-bottom microtitration plates (NEST Biotechnology, China), or tissue culture flasks (25cm²) for three days, one week, or two weeks to facilitate neurosphere formation. Neurosphere formation was confirmed through morphology and the expression of Nestin and SOX2 markers.
Differentiation of NSCs into Purkinje-like cells
NSCs were washed with PBS (pH 7.4) and then cultured in the Purkinje cell induction medium DMEM (Capricorn Scientific, Germany), which contained 3.15g/L (w/v) glucose, 3.57g/L (w/v) HEPES (pH 8.0), 1mM sodium pyruvate, and 2.5mM glutamine (Elabscience Biotechnology, USA), supplemented with 20μg/mL transferrin (Santa Cruz Biotechnology, USA), 50μg/mL bovine serum albumin (BSA; Elabscience Biotechnology, USA), and 20μg/mL insulin (Elabscience Biotechnology, USA). After 24 hours, 20ng/mL FGF2 was added to the aforementioned medium, and the cells were cultured for an additional nine days, with the medium changed every other day. From days seven to ten, the cells were treated with 20 μM cyclopamine (Santa Cruz Biotechnology, USA) [19].
Characterization of Purkinje-like cells
The morphology of the Purkinje cells was examined via microscopy. To characterize the Purkinje cells, the ICC assay for Calbindin D28K and IP3R-specific positive markers was conducted using CALB1 (E-AB-11020) and IP3R (E-AB-93302; Elabscience Biotechnology, USA) polyclonal primary antibodies.
Statistical analysis
Statistical analysis was conducted using one-way ANOVA followed by the least significant difference (LSD) test in SPSS Software (version 20) to calculate the average percentage mean values. Significant values were considered as p<0.05 (*), p<0.01 (**), and p<0.001 (***).
Findings
Characterization of MSCs
CD34 and CD45 expression levels were negative in cells stained blue in H & E staining. The cells were positive for both CD73 and CD44 markers, which were stained brown (Figure 1). MSCs stained with HRP secondary antibody only served as a negative control.
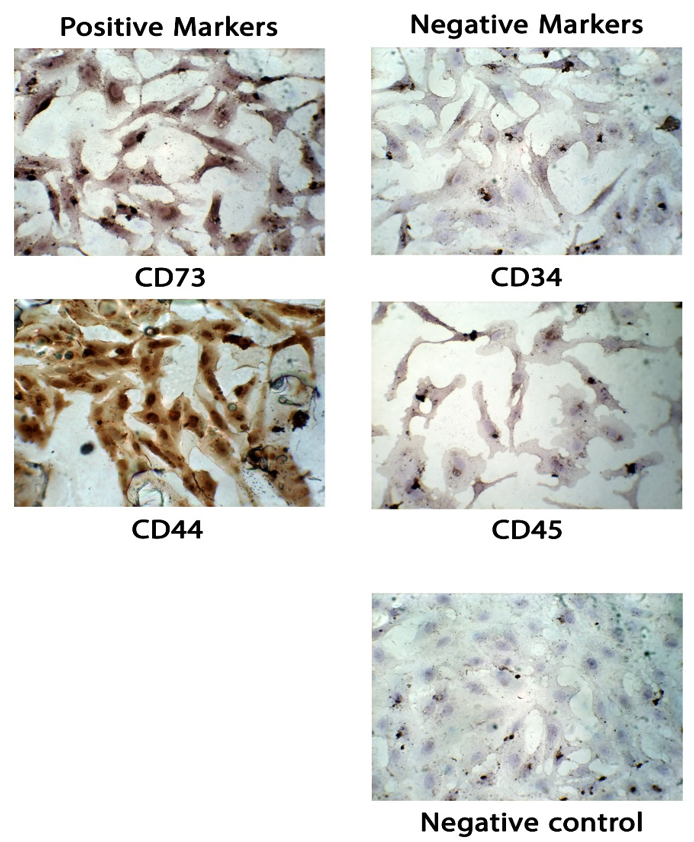
Figure 1. Immunophenotypic analysis of bone marrow mesenchymal stem cells (MSCs) under a light microscope. The cells exhibited the typical fibroblastic morphology of MSCs, which were maintained at the third passage. Positive MSCs (CD73 and CD44) were stained brown, while negative cells (CD34 and CD45) were stained blue. Negative control: MSCs stained with HRP secondary antibody only. All figures are shown at 10x magnification.
MSC culture and induction into neural stem cells
MSCs were successfully isolated from mouse bone marrow and cultured. The cells exhibited a spindle-shaped, fibroblast-like morphology and demonstrated rapid proliferation in vitro (Figure 2). The cultured cells underwent a proliferative phase lasting 7 days, after which they transitioned into the plateau phase by day ten. After 24 hours of exposure to the induction media, MSCs displayed a spindle-shaped, fibroblast-like morphology. After 34 hours in the post-induction media, MSCs became more spherical and polygonal, with an increase in size. Eventually, they developed elongated cellular branches resembling neural cells.
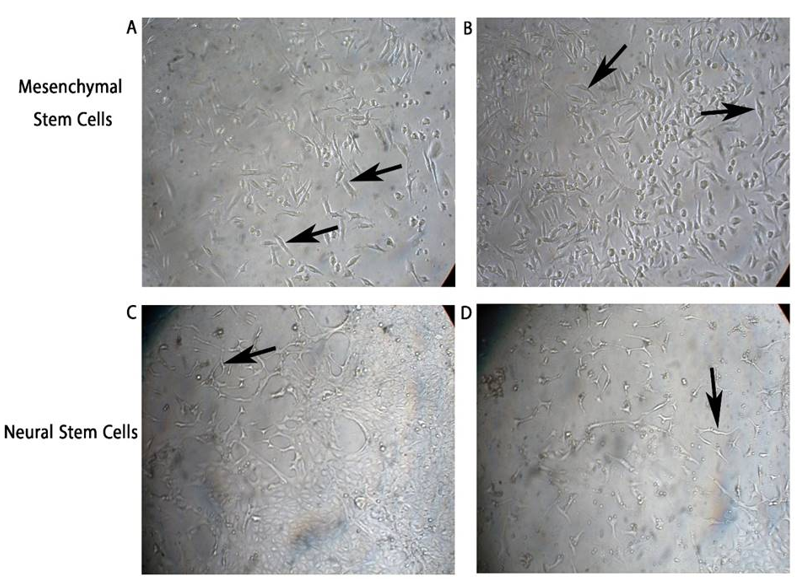
Figure 2. Morphology of mesenchymal stem cells (MSCs) and induced neural stem cells in vitro. (A and B) MSCs isolated from mouse bone marrow exhibited a spindle-shaped, fibroblast-like morphology (black arrows) and proliferated rapidly. After exposure to the induction media (34 hours), they became more elongated with branches, as shown in (C and D). Neural stem cells (NSCs) displayed their characteristic morphology with elongated projections (black arrows).
Neural stem cell characterization
NSCs were positive for Nestin and SOX2 markers. NSCs stained only with the secondary antibody served as a negative control (NC). Moreover, non-induced stem cells were negative for Nestin (Figure 3 A-D).
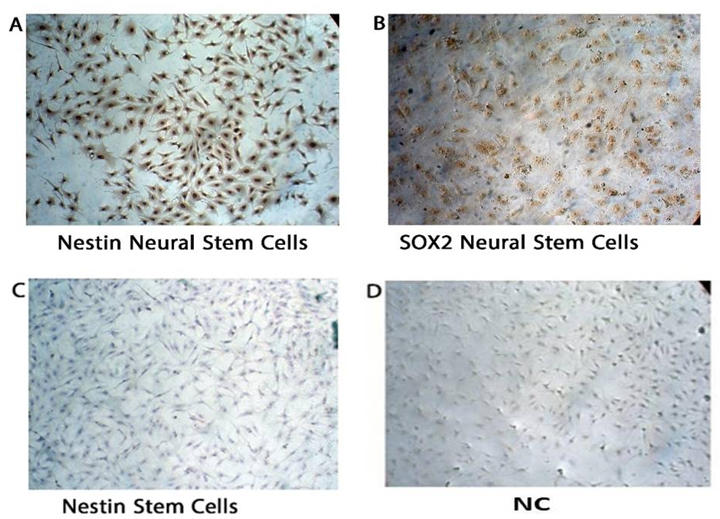
Figure 3. Mesenchymal stem cells (MSCs) after treatment with β-mercaptoethanol revealed that newly formed neural stem cells are positive for Nestin (A) and SOX2 (B), while the original stem cells are negative for Nestin (C). (D) Negative control (NC): neural stem cells stained with HRP secondary antibody only, which showed no nonspecific staining. All figures are illustrated at 10x magnification. NC: Negative control.
To evaluate the expression levels of NSC markers Nestin and SOX2, their protein levels were analyzed and compared to the NC group. NSCs showed higher expression of these markers than the NC group. Thus, both Nestin and SOX2 are expressed in NSCs, providing additional evidence for their roles as important markers in the maintenance and identity of NSCs. The lower expression observed in the NC group further supports the specificity of Nestin and SOX2 in NSCs (Figure 4A).
To assess Nestin protein expression in different cell groups, a quantitative analysis was performed. NSCs exhibited the highest expression levels of Nestin compared to both the NC and stem cell groups. Statistical analysis using one-way ANOVA followed by multiple comparisons indicated a significant difference (p<0.05) between NSCs and both NC and stem cells, while no significant difference was observed between the NC and stem cell groups. These results confirm Nestin as a neural stem cell marker, highlighting its role in distinguishing NSCs from other cell types (Figure 4B).
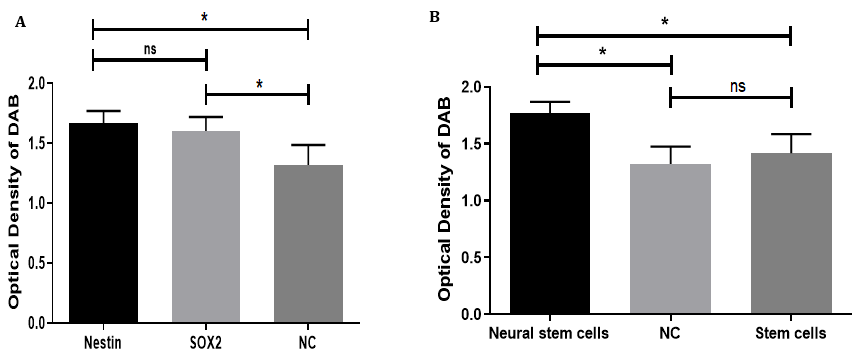
Figure 4. Statistical analysis of neural stem cell marker expression: (A) The bar graph illustrates the expression levels of the neural stem cell markers Nestin and SOX2 compared to the negative control (NC). Nestin exhibited the highest expression, followed by SOX2, while the NC group showed the lowest levels. Statistical analysis revealed a significant difference (p<0.05) between both markers and the NC group; however, there was no significant difference (ns) between the expression levels of Nestin and SOX2. (B) The expression of Nestin was further compared among neural stem cells (NSCs), negative control (NC), and untreated mesenchymal stem cells. NSCs demonstrated a significantly higher expression of Nestin compared to both the NC and stem cell groups (p<0.05), whereas no significant difference was observed between the NC group and stem cells. This indicates that Nestin expression is specifically upregulated in NSCs.
Neurosphere formation
NSCs formed floating, spherical structures one hour after being placed in the neural induction culture medium. Within the next 24 hours, the floating cells aggregated and formed 3D spheroid clusters, referred to as “neurospheres.” The ICC results on day 7 revealed that these cell clusters expressed Nestin and SOX2 markers (Figure 5).
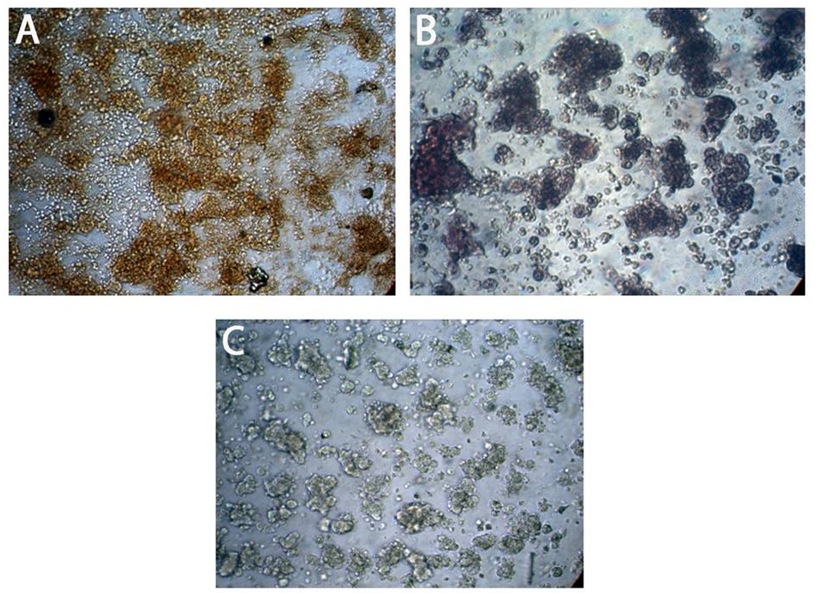
Figure 5. Expression of neural stem cell proteins Nestin and SOX2 markers in neurospheres by immunocytochemistry: (A) shows positive expression of Nestin in neurospheres. (B) shows a positive expression of SOX2 in neurospheres. (C) The negative control (NC) shows fully suspended neurospheres with no positive staining, indicating the absence of nonspecific staining. All figures are shown at 10x magnification.
The negative control showed unstained suspended neurospheres, indicating no nonspecific staining. Statistical analysis revealed a highly significant expression of Nestin (Figure 6).
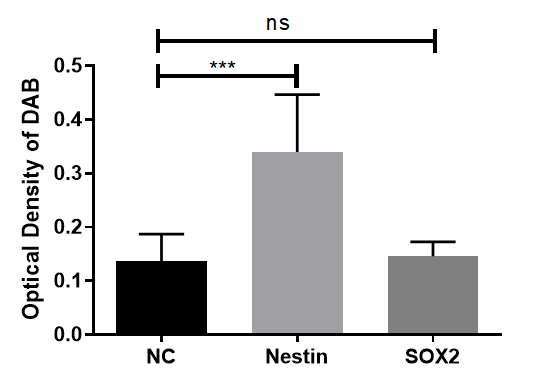
Figure 6. Statistical analysis of neural stem cell marker expression in neurospheres: Expression of Nestin showed a statistically significant increase compared to the negative control (NC) group (p<0.05), while SOX2 expression was not significantly different (ns).
Purkinje-like cells
Calbindin D28K- and IP3R1-positive cells were readily detected ten days after culture in specific media (Figure 7).
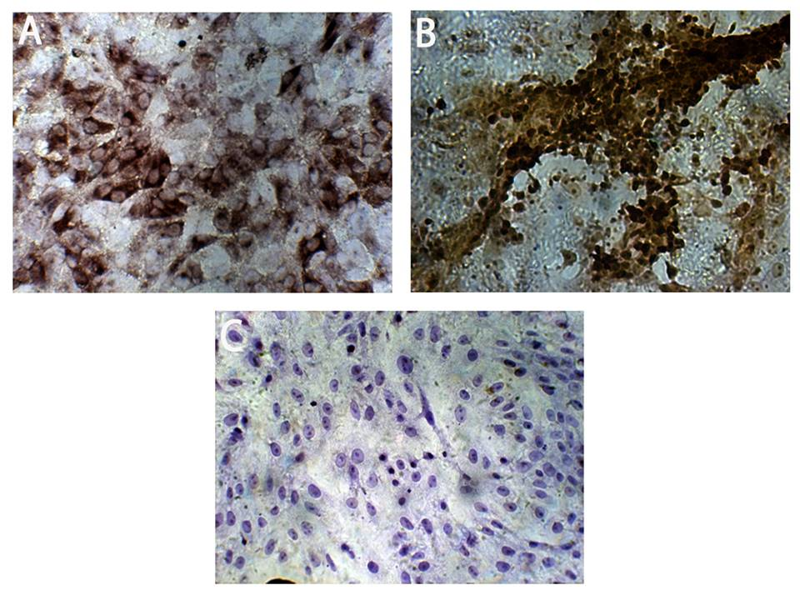
Figure 7. Differentiation of Purkinje-like cells from MSCs: (A) Purkinje-like cells expressing the marker calbindin D28K. (B) Purkinje-like cells expressing the marker IP3R1. (C) Purkinje-like cells stained with secondary antibody only, which shows no nonspecific staining, serve as a negative control. All figures are shown at 10x magnification. NC: Negative control
Statistical analysis of marker expression in these cells indicated a highly significant increase in calbindin D28K (p<0.001) and a significant increase in IP3R1 (p<0.01) compared to the negative control group (Figure 8).
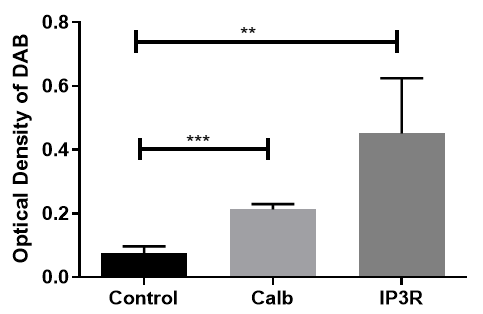
Figure 8. Statistical analysis of Purkinje-like cell marker expression: Statistical analysis revealed a highly significant difference between calbindin D28K (p<0.001), IP3R1 (p<0.01), and the NC group.
Discussion
Repair of neuronal damage to the CNS and neuronal regeneration are among the most important concerns for neurologists worldwide. Recent research has shown that ESCs, NSCs, and MSCs have the potential to differentiate into neurons, and in animal models, they can exhibit neuronal function after transplantation [13, 17-19]. Adult stem cells, particularly MSCs, have received much attention because they do not raise ethical concerns associated with ESCs. MSCs are considered multipotent stem cells that can differentiate into a variety of cell types, such as adipocytes, chondrocytes, osteoblasts, and neurons [10, 20]. We presented a protocol to obtain a population of Purkinje-like cells from mouse bone marrow MSCs. Our process begins with a streamlined protocol for generating NSCs from MSCs, which eliminates the need for embryoid body formation and rosette selection. This method results in monolayers of NSCs and can be completed in roughly half the time compared to traditional embryoid body-based techniques. Next, we separately differentiated these NSCs into Purkinje-like cells using a specific set of extrinsic morphogens and growth factors that mimic in vivo cerebellar development. Our improved approach also reduces animal costs by eliminating the need for postnatal feeder layers, which are typically used in other protocols for differentiating mouse ESCs into Purkinje cells. This research was conducted to highlight the potential of MSCs, as they allow for the production of neurons (especially Purkinje cells) and their use in the treatment of neurological diseases in both civilian and military individuals, particularly those who have suffered from brain injuries caused by war or other incidents [21, 22].
The International Society for Cell Therapy (ISCT) [23] defines the minimum criteria that must be met to address the issue of varying characteristics of MSCs due to their isolation from different tissue types. First, MSCs must adhere to plastic surfaces during in vitro culture, and second, they should express certain surface antigens, such as CD73 and CD44, while not expressing CD34 and CD45. The MSCs isolated in this study fulfilled these two conditions.
NSCs can proliferate and subsequently differentiate into all major neuronal cell lineages in the brain, including neurons, astrocytes, and oligodendrocytes [24]. This study utilized NSCs differentiated from MSCs instead of directly using NSCs isolated from the CNS. Although NSCs can be generated directly from human brain tissue or human ESCs [25], their availability is limited by the number of brain donors and available ESC lines. In a study using mouse MSCs, Suzuki et al. reported that a sizable portion (20-60%) of mouse MSCs differentiate into NSCs [26]. In another study, Hermann et al. reported that more than 60% of human bone marrow MSCs can become clonogenic NSCs [27]. In both studies, MSC-derived NSCs differentiated in vitro into cells with morphological and functional characteristics of neurons, astrocytes, and oligodendrocytes [24, 25].
In this study, BME was successfully employed as a key compound to facilitate the sustained production of NSCs in culture. BME has been widely utilized as a pre-induction or induction reagent in various experimental protocols [15, 16, 26, 27, 28]. The findings of this study align with those of Mareschi et al. [29] and Mohammad et al. [18], utilizing BME as an inducer and reporting the expression of Nestin, MAP-2, NF genes, and various other neural markers throughout the differentiation process [15, 27]. The ICC results in this study confirmed the differentiation of MSCs into NSCs, as the differentiated cells showed positive expression of the neuronal markers Nestin and SOX2, consistent with the results reported in previous studies [15, 22, 25, 27, 28].
NES, a gene whose expression differentiates NSCs from more differentiated cells (neurons), is a commonly used marker for NSCs. Due to its specific expression in neuroepithelial stem cells, this gene was named NES. A distinct sixth class of intermediate filament proteins is characterized by NES, based on the predicted amino acid sequence of the NES gene product. These findings support the theory that major stages in the neural differentiation pathway are reflected in changes in intermediate filament gene expression [17]. In the mammalian CNS, NES expression occurs at key steps of cell type differentiation. Nearly all mature CNS cells do not express NES, but stem cells do; when they transition from proliferating stem cells to postmitotic neurons, they drastically downregulate NES [30, 31].
Since SOX2 is expressed in neural stem and progenitor cells, its deletion during mouse embryogenesis results in the loss of hippocampal NSCs during the prenatal or early postnatal period, and its loss after birth reduces the proliferation of hippocampal neural stem/progenitor cells [32]. The molecular mechanisms by which SOX2 supports the maintenance of NSCs have been extensively studied. In vitro studies on NSCs revealed that the loss of SOX2 causes progressive exhaustion of NSCs, in contrast to the long-term proliferation of control wild-type cells [32].
Recent strategies for differentiating cells into neurons include the use of small molecules, psychotropic drugs, epigenetic modifications, and enriched medium formulations combined with chemical inducers. However, placing MSCs in a neuronal induction medium containing chemical inducers leads to a faster neuronal differentiation rate compared to other approaches. The formation of neurospheres serves as a promising in vitro model for investigating CNS disorders [31, 32, 33]. This study investigated the neuronal differentiation of characterized MSCs into NSCs through a 3D neurosphere induction process conducted in a neural induction medium containing EGF and bFGF, within a low-adherence culture system. Fu et al. reported that NSCs can be generated from human bone marrow-derived MSCs and that 8% of NSCs can generate neurospheres. These MSC-derived neurospheres expressed NSC-characteristic antigens, such as Nestin and Musashi-1 [7]. Songsaad et al. [34] reported that neurospheres induced from human stem cells from the apical papilla (hSCAPs) exhibit a three-dimensional floating spherical shape, and their investigation revealed that these cells within the neurospheres expressed Nestin and SOX2. NSCs created from differentiated MSCs were able to form neurospheres. The neurosphere formation test confirmed the identity of the NSCs produced in this study. The neurospheres generated here expressed the Nestin and SOX2 markers, which was consistent with the findings of other studies [16, 25, 32].
In this study, we used a modified method from Alexander & Hammer to differentiate NSCs into Purkinje-like cells [19]. This is the first study to utilize NSCs differentiated from MSCs to generate Purkinje-like cells. However, in some studies, MSCs have been differentiated into neuron-like cells through co-culture [16]. Valencia-Salgado differentiated human adipose tissue-derived mesenchymal stem cells (hADSCs) directly into oligodendrocyte-like cells and neuron-like cells [20]. In other studies, the differentiation of ESCs or induced pluripotent stem cells has been used to produce Purkinje cells [17, 19].
The resulting cells in this study were morphologically similar to cerebellar Purkinje cells. To confirm the identity of the cells, we examined the expression of Calbindin D28K and IP3R1 markers, which are specific to Purkinje cells or are strongly expressed in them. These two markers were detected in the differentiated Purkinje cells. Alexander & Hammer attempted the differentiation of mouse ESCs to produce Purkinje neurons and demonstrated the expression of calbindin D28K, IP3R1, PSD93, IRBIT, PLCβ4, and myosin IIB-B2 markers in these cells [19]. Watson et al. developed a protocol to regenerate Purkinje cells from human-induced pluripotent stem cells in vitro, demonstrating the presence of human calbindin-positive Purkinje cells after 15 days of co-culture with human cerebellar progenitors [21].
This research highlighted the potential for differentiating MSCs into Purkinje-like cells via a neural induction medium. The Purkinje-like cells created in this study can serve as an in vitro model to develop appropriate treatments for diseases caused by the dysfunction of Purkinje cells. This inexpensive model is available in large volumes and presents fewer ethical/legal issues compared to other models created from ESCs, induced pluripotent stem cells, and NSCs, each of which typically has its own limitations. Future studies should also focus on the transplantation of Purkinje cells into damaged cerebellar regions to investigate factors, such as host integration and cell survival in in vivo models.
Conclusion
The Purkinje-like cells created in this study can serve as an in vitro model to develop appropriate treatments for diseases caused by the dysfunction of Purkinje cells.
Acknowledgments: The authors of this project sincerely thank the Experimental Therapy Department at the Iraqi Center for Cancer and Medical Genetic Research, Mustansiriyah University, Baghdad, Iraq, for their support in conducting the experimental part of this work.
Ethical Permissions: All experiments were conducted under the FUM-approved codes for the care and use of laboratory animals (IR.UM.REC.1402.276).
Conflicts of Interests: No conflicts of interest are declared.
Authors' Contribution: Allami M (First Author), Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (40%); Al-Shammari AM (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (30%); Neshati Z (Third Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (30%)
Funding/Support: This work was supported by Ferdowsi University of Mashhad [grant numbers 3/61701].
The cerebellum is a crucial structure responsible for ensuring that motor activities are executed with precision and well-coordinated timing. Much of the computation in the cerebellum occurs in the cerebellar cortex, which is a layered, repetitive, and meticulously structured arrangement. Information from the cerebellar cortex ultimately converges on Purkinje neurons, the central computational integrators of the cerebellar system [1]. Research has shown that the cerebellum supports multiple functions, including emotion regulation, inhibition of impulsive decision-making, attention, and working memory. Additionally, several experimental and computational studies have indicated that the cerebellum plays a role in error-free (unsupervised) learning. Damage to parts of the cerebellum that project to the motor areas, prefrontal cortex, and limbic system can result in motor, cognitive, and emotional abnormalities, respectively. Some further suggest that the role of the cerebellum in cognitive function is similar to its role in controlling purposive motor skills during motor function [2]. There is also evidence that the cerebellum may be related to a variety of cognitive abnormalities and psychopathological manifestations. Many recent studies have reported strong associations between structural and functional abnormalities of the cerebellum and psychiatric disorders, especially schizophrenia, bipolar disorder, depression, anxiety disorders, attention deficit hyperactivity disorder (ADHD), and autism [3]. Given the cerebellum’s extensive involvement in various psychological and cognitive functions, conditions that affect mental health may directly or indirectly influence cerebellar function. One such condition is exposure to armed conflict during wartime. War not only results in direct physical injuries, such as traumatic brain damage, but is also associated with an increased prevalence of psychiatric disorders, including post-traumatic stress disorder (PTSD), depression, and anxiety. Recent studies have demonstrated that many of these psychiatric conditions are linked to functional and structural alterations in several brain regions, including the cerebellum [4, 5].
Purkinje neurons exhibit repetitive firing in a tonic pattern, with their firing frequency changing during controlled movement. They are influenced by two major types of excitatory inputs: parallel fibers originating from granule cells (through the mossy fiber pathway) and climbing fibers that come from the inferior olive. Furthermore, Purkinje neurons receive feed-forward inhibitory signals from molecular layer interneurons, which are regulated by inputs from parallel fibers [6]. Purkinje neurons integrate this incoming information, and the processed signals are transmitted through inhibitory GABAergic synapses to the neurons of the deep cerebellar nuclei (DCN), which consist of paired nuclei (dentate nucleus, interposed nuclei, and fastigial nucleus) located in the deep white matter of the cerebellum. Along with direct projections from the cerebellar flocculonodular lobe to the vestibular nuclei, projections from Purkinje neurons in other parts of the cerebellum are relayed through the neurons of the deep cerebellar nuclei, serving as the exclusive output of the cerebellar cortex. The deep cerebellar nuclei, in turn, send projections to various targets within the central nervous system (CNS), such as the premotor and primary motor cortical areas (via the thalamus), the reticular nucleus, and the red nucleus. Through these outputs from the deep cerebellar nuclei, the cerebellum communicates with the rest of the nervous system to regulate movement control [1]. Therefore, the presence of any disorder in Purkinje neurons can cause a wide range of neurological disorders; for this reason, treating or replacing damaged cells is highly important for preventing these disorders [5, 6].
The pathological mechanisms of neurological diseases are primarily characterized by the progressive degeneration of neuronal structure and function, leading to impairments in motor, sensory, and cognitive functions, which can result in varying degrees of paralysis and, ultimately, permanent disability. Furthermore, the drug development process is both intricate and costly. Consequently, there is an urgent demand for the identification of effective therapeutic approaches. In recent years, with ongoing advancements in nerve injury repair, cell transplantation and cell-based therapies have gained widespread application in neurological disorders, paving the way for the emergence of novel treatment strategies. The therapeutic potential of cell-based therapy in neurological disorders lies in its ability to mimic the natural processes of cellular repair and development within the nervous system, address the underlying causes of the disease, enhance dysfunctional pathways, and promote the regeneration of damaged tissues [7, 8]. Significantly, the potential for tissue repair and regeneration in neurological disorders is limited, and there is currently no definitive treatment available to halt or reverse their progression. The clinical application of pharmacological treatments to alleviate or manage symptoms in patients is constrained, as these drugs offer limited efficacy and are incapable of repairing damaged neurons or reconstructing neural networks [9, 10].
An era of research into neural regeneration began with the discovery of neural stem cells (NSCs), and significant advancements have been made in transplantation therapies for disorders of the CNS. However, because most NSCs are derived from aborted fetuses, their use is severely limited, and they also present ethical issues [11]. Thus, the search for better stem cell sources is crucial. Mesenchymal stem cells (MSCs) have been widely studied since their discovery in the late 1960s. Due to their capacity for multiple types of differentiation, immunomodulatory roles, and ability to support tissue and organ repair, adult stem cells hold great promise for use in regenerative medicine and disease treatment [12]. Additionally, there are no ethical concerns associated with the acquisition, use, or dissemination of MSCs. MSCs can be readily isolated from several human tissues, including adipose tissue, bone marrow, and the umbilical cord [13]. MSCs constitute a promising therapeutic approach because of their ability to promote nerve regeneration through a variety of mechanisms. To support neural regeneration, MSCs can differentiate into astrocytes, neurons, and Schwann cell-like cells [12, 13].
Since access to a larger number of MSCs compared to NSCs can be achieved through a relatively simple process, this study was conducted to produce Purkinje-like neurons from defined NSCs differentiated from MSCs. The findings support the utilization of bone marrow-derived MSCs as a viable and straightforward alternative source for Purkinje-like neurons, instead of NSCs, embryonic stem cells (ESCs), or induced pluripotent stem cells, providing a suitable model for investigating cerebellar development and degeneration.
Materials and Methods
Animals
Healthy albino Swiss male mice aged 3-6 weeks and weighing 10-15 grams were obtained from the animal care facility at ICCMGR, Mustansiriyah University, as a source for obtaining MSCs. All mice involved were kept in a controlled environment at 23-25°C, with unrestricted access to food and water, and were maintained under a 12-hour light/dark cycle.
Isolation and maintenance of bone marrow MSCs
Mice were euthanized in a clean environment according to standard procedures. In an airflow chamber (under sterile conditions), the mice were cleaned using a 70% alcohol solution, and the femurs and tibias were carefully separated using sterile surgical tools. After the femurs and tibias were cut from their ends, the bone marrow was extracted via a syringe filled with phosphate-buffered saline (PBS). The cell suspensions were maintained in 25 cm² tissue culture flasks in minimum essential medium (MEM; Capricorn Scientific, Germany), which contained 2% fetal bovine serum (FBS; Capricorn Scientific, Germany), streptomycin, and penicillin (100 μg/ml; Capricorn Scientific GmbH, Ebsdorfergrund, Germany). Finally, the flasks were placed in a 5% CO₂ incubator at 37°C, with an atmosphere of 95% humidity. Since MSCs make up a small portion of the nucleated cells in mouse bone marrow, they are considered an impure population in primary culture and are purified in subcultured passages. To ensure the purity of the MSCs, we examined the phenotype, the ability to attach to the flask surface, and the expression and non-expression of specific markers. The majority of the isolated cells were adherent after overnight culture; the non-adherent cells were removed by replacing the medium with MEM supplemented with 20% FBS. After that, the cultures were maintained by replacing the culture medium every two to three days until cell colonies formed, which took three to seven days. After reaching confluence, the cells were subcultured using a 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) solution (United States Biological, USA). Cells at the third passage were used for the next steps.
Characterization of MSCs
Immunocytochemistry (ICC) was used to study stem cell markers. Cells (4×104) were cultured in each well of 8-well tissue culture chamber slides (SPL, Korea). After three to five days of growth in the chamber slides, the medium was removed, the cells were washed with PBS, and they were fixed for ten minutes with 4% paraformaldehyde in a humid chamber with 1% hydrogen peroxide (H₂O₂) to block endogenous peroxidase activity. Fixed cells were washed with PBS and maintained at room temperature for 30 to 40 minutes before being incubated in 1.5% blocking serum for 30 minutes. Four specific markers—CD73 and CD44 as positive markers and CD34 and CD45 as negative markers—were used for the characterization of MSCs, utilizing CD73 (E-AB-14725-120), CD44 (E-AB-66948), CD34 (E-AB-70337), and CD45 (E-AB-70024) polyclonal primary antibodies (Elabscience Biotechnology, USA). The primary antibodies against each of the CD markers were added, and the cells were incubated in a humid chamber overnight at 4°C. The staining protocol was followed according to a 2-step plus Poly-HRP Anti-Mouse/Rabbit IgG Detection System (with DAB Solution; E-IR-R217; Elabscience Biotechnology, USA). After washing with PBS, the cells were incubated with anti-mouse/rabbit IgG secondary antibody for 30 minutes and were washed with PBS. Horseradish peroxidase (HRP) conjugated with avidin was added to each slide and incubated for 30 minutes, after which the slides were washed with PBS. A liquid DAB chromogen mixture was introduced and allowed to react for 2-5 minutes, after which the samples were washed with PBS and stained for 30-60 seconds with hematoxylin and eosin (H & E). Washing with PBS was repeated at least three times after each step. Negative control cells were stained with secondary antibody only, without any primary antibody. Finally, all slides were mounted using DPX, analyzed under an optical microscope, and captured using a digital camera.
Differentiation of MSCs into NSCs
Subconfluent cultures of P3 MSCs (2.50×106) in a 25cm² plastic flask (SPL, Korea) were used to induce neurogenesis. This was accomplished by using β-mercaptoethanol (BME; Santa Cruz Biotechnology Inc.) as a differentiation factor. The induction process followed these steps: A). The cells were exposed to MEM supplemented with 20% FBS and 1mM BME as preinduction media for 24 hours [14, 15]. B) The cells were subjected to neural differentiation induction (as postinduction media) using MEM without FBS (serum-free media) and 5mM BME for 10 hours [16, 17]. The ICC assay for Nestin and SOX-2 markers was carried out to characterize NSCs using an anti-mouse Nestin IgG monoclonal antibody (E-AB-70343) and anti-mouse SOX2 IgG polyclonal antibody (E-AB-70108) (Elabscience Biotechnology, USA) as primary antibodies.
Neurosphere formation
After the induction of NSCs, the induced cells were maintained in culture to form neurospheres (the typical morphology of NSCs in culture) in MEM supplemented with 5% FBS, 100µg/ml streptomycin and penicillin, 100µg/ml Amphotericin B, and 50ng/mL of both epidermal growth factor (EGF; Elabscience Biotechnology, USA) and basic fibroblast growth factor (b-FGF; Elabscience Biotechnology, USA) [18]. The induced cells were cultured in small tissue culture Petri dishes, 96-well flat-bottom microtitration plates (NEST Biotechnology, China), or tissue culture flasks (25cm²) for three days, one week, or two weeks to facilitate neurosphere formation. Neurosphere formation was confirmed through morphology and the expression of Nestin and SOX2 markers.
Differentiation of NSCs into Purkinje-like cells
NSCs were washed with PBS (pH 7.4) and then cultured in the Purkinje cell induction medium DMEM (Capricorn Scientific, Germany), which contained 3.15g/L (w/v) glucose, 3.57g/L (w/v) HEPES (pH 8.0), 1mM sodium pyruvate, and 2.5mM glutamine (Elabscience Biotechnology, USA), supplemented with 20μg/mL transferrin (Santa Cruz Biotechnology, USA), 50μg/mL bovine serum albumin (BSA; Elabscience Biotechnology, USA), and 20μg/mL insulin (Elabscience Biotechnology, USA). After 24 hours, 20ng/mL FGF2 was added to the aforementioned medium, and the cells were cultured for an additional nine days, with the medium changed every other day. From days seven to ten, the cells were treated with 20 μM cyclopamine (Santa Cruz Biotechnology, USA) [19].
Characterization of Purkinje-like cells
The morphology of the Purkinje cells was examined via microscopy. To characterize the Purkinje cells, the ICC assay for Calbindin D28K and IP3R-specific positive markers was conducted using CALB1 (E-AB-11020) and IP3R (E-AB-93302; Elabscience Biotechnology, USA) polyclonal primary antibodies.
Statistical analysis
Statistical analysis was conducted using one-way ANOVA followed by the least significant difference (LSD) test in SPSS Software (version 20) to calculate the average percentage mean values. Significant values were considered as p<0.05 (*), p<0.01 (**), and p<0.001 (***).
Findings
Characterization of MSCs
CD34 and CD45 expression levels were negative in cells stained blue in H & E staining. The cells were positive for both CD73 and CD44 markers, which were stained brown (Figure 1). MSCs stained with HRP secondary antibody only served as a negative control.

Figure 1. Immunophenotypic analysis of bone marrow mesenchymal stem cells (MSCs) under a light microscope. The cells exhibited the typical fibroblastic morphology of MSCs, which were maintained at the third passage. Positive MSCs (CD73 and CD44) were stained brown, while negative cells (CD34 and CD45) were stained blue. Negative control: MSCs stained with HRP secondary antibody only. All figures are shown at 10x magnification.
MSC culture and induction into neural stem cells
MSCs were successfully isolated from mouse bone marrow and cultured. The cells exhibited a spindle-shaped, fibroblast-like morphology and demonstrated rapid proliferation in vitro (Figure 2). The cultured cells underwent a proliferative phase lasting 7 days, after which they transitioned into the plateau phase by day ten. After 24 hours of exposure to the induction media, MSCs displayed a spindle-shaped, fibroblast-like morphology. After 34 hours in the post-induction media, MSCs became more spherical and polygonal, with an increase in size. Eventually, they developed elongated cellular branches resembling neural cells.

Figure 2. Morphology of mesenchymal stem cells (MSCs) and induced neural stem cells in vitro. (A and B) MSCs isolated from mouse bone marrow exhibited a spindle-shaped, fibroblast-like morphology (black arrows) and proliferated rapidly. After exposure to the induction media (34 hours), they became more elongated with branches, as shown in (C and D). Neural stem cells (NSCs) displayed their characteristic morphology with elongated projections (black arrows).
Neural stem cell characterization
NSCs were positive for Nestin and SOX2 markers. NSCs stained only with the secondary antibody served as a negative control (NC). Moreover, non-induced stem cells were negative for Nestin (Figure 3 A-D).

Figure 3. Mesenchymal stem cells (MSCs) after treatment with β-mercaptoethanol revealed that newly formed neural stem cells are positive for Nestin (A) and SOX2 (B), while the original stem cells are negative for Nestin (C). (D) Negative control (NC): neural stem cells stained with HRP secondary antibody only, which showed no nonspecific staining. All figures are illustrated at 10x magnification. NC: Negative control.
To evaluate the expression levels of NSC markers Nestin and SOX2, their protein levels were analyzed and compared to the NC group. NSCs showed higher expression of these markers than the NC group. Thus, both Nestin and SOX2 are expressed in NSCs, providing additional evidence for their roles as important markers in the maintenance and identity of NSCs. The lower expression observed in the NC group further supports the specificity of Nestin and SOX2 in NSCs (Figure 4A).
To assess Nestin protein expression in different cell groups, a quantitative analysis was performed. NSCs exhibited the highest expression levels of Nestin compared to both the NC and stem cell groups. Statistical analysis using one-way ANOVA followed by multiple comparisons indicated a significant difference (p<0.05) between NSCs and both NC and stem cells, while no significant difference was observed between the NC and stem cell groups. These results confirm Nestin as a neural stem cell marker, highlighting its role in distinguishing NSCs from other cell types (Figure 4B).

Figure 4. Statistical analysis of neural stem cell marker expression: (A) The bar graph illustrates the expression levels of the neural stem cell markers Nestin and SOX2 compared to the negative control (NC). Nestin exhibited the highest expression, followed by SOX2, while the NC group showed the lowest levels. Statistical analysis revealed a significant difference (p<0.05) between both markers and the NC group; however, there was no significant difference (ns) between the expression levels of Nestin and SOX2. (B) The expression of Nestin was further compared among neural stem cells (NSCs), negative control (NC), and untreated mesenchymal stem cells. NSCs demonstrated a significantly higher expression of Nestin compared to both the NC and stem cell groups (p<0.05), whereas no significant difference was observed between the NC group and stem cells. This indicates that Nestin expression is specifically upregulated in NSCs.
Neurosphere formation
NSCs formed floating, spherical structures one hour after being placed in the neural induction culture medium. Within the next 24 hours, the floating cells aggregated and formed 3D spheroid clusters, referred to as “neurospheres.” The ICC results on day 7 revealed that these cell clusters expressed Nestin and SOX2 markers (Figure 5).

Figure 5. Expression of neural stem cell proteins Nestin and SOX2 markers in neurospheres by immunocytochemistry: (A) shows positive expression of Nestin in neurospheres. (B) shows a positive expression of SOX2 in neurospheres. (C) The negative control (NC) shows fully suspended neurospheres with no positive staining, indicating the absence of nonspecific staining. All figures are shown at 10x magnification.
The negative control showed unstained suspended neurospheres, indicating no nonspecific staining. Statistical analysis revealed a highly significant expression of Nestin (Figure 6).

Figure 6. Statistical analysis of neural stem cell marker expression in neurospheres: Expression of Nestin showed a statistically significant increase compared to the negative control (NC) group (p<0.05), while SOX2 expression was not significantly different (ns).
Purkinje-like cells
Calbindin D28K- and IP3R1-positive cells were readily detected ten days after culture in specific media (Figure 7).

Figure 7. Differentiation of Purkinje-like cells from MSCs: (A) Purkinje-like cells expressing the marker calbindin D28K. (B) Purkinje-like cells expressing the marker IP3R1. (C) Purkinje-like cells stained with secondary antibody only, which shows no nonspecific staining, serve as a negative control. All figures are shown at 10x magnification. NC: Negative control
Statistical analysis of marker expression in these cells indicated a highly significant increase in calbindin D28K (p<0.001) and a significant increase in IP3R1 (p<0.01) compared to the negative control group (Figure 8).

Figure 8. Statistical analysis of Purkinje-like cell marker expression: Statistical analysis revealed a highly significant difference between calbindin D28K (p<0.001), IP3R1 (p<0.01), and the NC group.
Discussion
Repair of neuronal damage to the CNS and neuronal regeneration are among the most important concerns for neurologists worldwide. Recent research has shown that ESCs, NSCs, and MSCs have the potential to differentiate into neurons, and in animal models, they can exhibit neuronal function after transplantation [13, 17-19]. Adult stem cells, particularly MSCs, have received much attention because they do not raise ethical concerns associated with ESCs. MSCs are considered multipotent stem cells that can differentiate into a variety of cell types, such as adipocytes, chondrocytes, osteoblasts, and neurons [10, 20]. We presented a protocol to obtain a population of Purkinje-like cells from mouse bone marrow MSCs. Our process begins with a streamlined protocol for generating NSCs from MSCs, which eliminates the need for embryoid body formation and rosette selection. This method results in monolayers of NSCs and can be completed in roughly half the time compared to traditional embryoid body-based techniques. Next, we separately differentiated these NSCs into Purkinje-like cells using a specific set of extrinsic morphogens and growth factors that mimic in vivo cerebellar development. Our improved approach also reduces animal costs by eliminating the need for postnatal feeder layers, which are typically used in other protocols for differentiating mouse ESCs into Purkinje cells. This research was conducted to highlight the potential of MSCs, as they allow for the production of neurons (especially Purkinje cells) and their use in the treatment of neurological diseases in both civilian and military individuals, particularly those who have suffered from brain injuries caused by war or other incidents [21, 22].
The International Society for Cell Therapy (ISCT) [23] defines the minimum criteria that must be met to address the issue of varying characteristics of MSCs due to their isolation from different tissue types. First, MSCs must adhere to plastic surfaces during in vitro culture, and second, they should express certain surface antigens, such as CD73 and CD44, while not expressing CD34 and CD45. The MSCs isolated in this study fulfilled these two conditions.
NSCs can proliferate and subsequently differentiate into all major neuronal cell lineages in the brain, including neurons, astrocytes, and oligodendrocytes [24]. This study utilized NSCs differentiated from MSCs instead of directly using NSCs isolated from the CNS. Although NSCs can be generated directly from human brain tissue or human ESCs [25], their availability is limited by the number of brain donors and available ESC lines. In a study using mouse MSCs, Suzuki et al. reported that a sizable portion (20-60%) of mouse MSCs differentiate into NSCs [26]. In another study, Hermann et al. reported that more than 60% of human bone marrow MSCs can become clonogenic NSCs [27]. In both studies, MSC-derived NSCs differentiated in vitro into cells with morphological and functional characteristics of neurons, astrocytes, and oligodendrocytes [24, 25].
In this study, BME was successfully employed as a key compound to facilitate the sustained production of NSCs in culture. BME has been widely utilized as a pre-induction or induction reagent in various experimental protocols [15, 16, 26, 27, 28]. The findings of this study align with those of Mareschi et al. [29] and Mohammad et al. [18], utilizing BME as an inducer and reporting the expression of Nestin, MAP-2, NF genes, and various other neural markers throughout the differentiation process [15, 27]. The ICC results in this study confirmed the differentiation of MSCs into NSCs, as the differentiated cells showed positive expression of the neuronal markers Nestin and SOX2, consistent with the results reported in previous studies [15, 22, 25, 27, 28].
NES, a gene whose expression differentiates NSCs from more differentiated cells (neurons), is a commonly used marker for NSCs. Due to its specific expression in neuroepithelial stem cells, this gene was named NES. A distinct sixth class of intermediate filament proteins is characterized by NES, based on the predicted amino acid sequence of the NES gene product. These findings support the theory that major stages in the neural differentiation pathway are reflected in changes in intermediate filament gene expression [17]. In the mammalian CNS, NES expression occurs at key steps of cell type differentiation. Nearly all mature CNS cells do not express NES, but stem cells do; when they transition from proliferating stem cells to postmitotic neurons, they drastically downregulate NES [30, 31].
Since SOX2 is expressed in neural stem and progenitor cells, its deletion during mouse embryogenesis results in the loss of hippocampal NSCs during the prenatal or early postnatal period, and its loss after birth reduces the proliferation of hippocampal neural stem/progenitor cells [32]. The molecular mechanisms by which SOX2 supports the maintenance of NSCs have been extensively studied. In vitro studies on NSCs revealed that the loss of SOX2 causes progressive exhaustion of NSCs, in contrast to the long-term proliferation of control wild-type cells [32].
Recent strategies for differentiating cells into neurons include the use of small molecules, psychotropic drugs, epigenetic modifications, and enriched medium formulations combined with chemical inducers. However, placing MSCs in a neuronal induction medium containing chemical inducers leads to a faster neuronal differentiation rate compared to other approaches. The formation of neurospheres serves as a promising in vitro model for investigating CNS disorders [31, 32, 33]. This study investigated the neuronal differentiation of characterized MSCs into NSCs through a 3D neurosphere induction process conducted in a neural induction medium containing EGF and bFGF, within a low-adherence culture system. Fu et al. reported that NSCs can be generated from human bone marrow-derived MSCs and that 8% of NSCs can generate neurospheres. These MSC-derived neurospheres expressed NSC-characteristic antigens, such as Nestin and Musashi-1 [7]. Songsaad et al. [34] reported that neurospheres induced from human stem cells from the apical papilla (hSCAPs) exhibit a three-dimensional floating spherical shape, and their investigation revealed that these cells within the neurospheres expressed Nestin and SOX2. NSCs created from differentiated MSCs were able to form neurospheres. The neurosphere formation test confirmed the identity of the NSCs produced in this study. The neurospheres generated here expressed the Nestin and SOX2 markers, which was consistent with the findings of other studies [16, 25, 32].
In this study, we used a modified method from Alexander & Hammer to differentiate NSCs into Purkinje-like cells [19]. This is the first study to utilize NSCs differentiated from MSCs to generate Purkinje-like cells. However, in some studies, MSCs have been differentiated into neuron-like cells through co-culture [16]. Valencia-Salgado differentiated human adipose tissue-derived mesenchymal stem cells (hADSCs) directly into oligodendrocyte-like cells and neuron-like cells [20]. In other studies, the differentiation of ESCs or induced pluripotent stem cells has been used to produce Purkinje cells [17, 19].
The resulting cells in this study were morphologically similar to cerebellar Purkinje cells. To confirm the identity of the cells, we examined the expression of Calbindin D28K and IP3R1 markers, which are specific to Purkinje cells or are strongly expressed in them. These two markers were detected in the differentiated Purkinje cells. Alexander & Hammer attempted the differentiation of mouse ESCs to produce Purkinje neurons and demonstrated the expression of calbindin D28K, IP3R1, PSD93, IRBIT, PLCβ4, and myosin IIB-B2 markers in these cells [19]. Watson et al. developed a protocol to regenerate Purkinje cells from human-induced pluripotent stem cells in vitro, demonstrating the presence of human calbindin-positive Purkinje cells after 15 days of co-culture with human cerebellar progenitors [21].
This research highlighted the potential for differentiating MSCs into Purkinje-like cells via a neural induction medium. The Purkinje-like cells created in this study can serve as an in vitro model to develop appropriate treatments for diseases caused by the dysfunction of Purkinje cells. This inexpensive model is available in large volumes and presents fewer ethical/legal issues compared to other models created from ESCs, induced pluripotent stem cells, and NSCs, each of which typically has its own limitations. Future studies should also focus on the transplantation of Purkinje cells into damaged cerebellar regions to investigate factors, such as host integration and cell survival in in vivo models.
Conclusion
The Purkinje-like cells created in this study can serve as an in vitro model to develop appropriate treatments for diseases caused by the dysfunction of Purkinje cells.
Acknowledgments: The authors of this project sincerely thank the Experimental Therapy Department at the Iraqi Center for Cancer and Medical Genetic Research, Mustansiriyah University, Baghdad, Iraq, for their support in conducting the experimental part of this work.
Ethical Permissions: All experiments were conducted under the FUM-approved codes for the care and use of laboratory animals (IR.UM.REC.1402.276).
Conflicts of Interests: No conflicts of interest are declared.
Authors' Contribution: Allami M (First Author), Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (40%); Al-Shammari AM (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (30%); Neshati Z (Third Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (30%)
Funding/Support: This work was supported by Ferdowsi University of Mashhad [grant numbers 3/61701].
Keywords:
References
1. Chopra R, Shakkottai VG. Translating cerebellar Purkinje neuron physiology to progress in dominantly inherited ataxia. Future Neurol. 2014;9(2):187-96. [Link] [DOI:10.2217/fnl.14.6]
2. Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry. 2001;49(1):20-7. [Link] [DOI:10.1016/S0006-3223(00)01081-7]
3. Phillips JR, Hewedi DH, Eissa AM, Moustafa AA. The cerebellum and psychiatric disorders. Front Public Health. 2015;3:66. [Link] [DOI:10.3389/fpubh.2015.00066]
4. Blithikioti C, Nuño L, Guell X, Pascual-Diaz S, Gual A, Balcells-Olivero Μ, et al. The cerebellum and psychological trauma: A systematic review of neuroimaging studies. Neurobiol Stress. 2022;17:100429. [Link] [DOI:10.1016/j.ynstr.2022.100429]
5. Schutter DJLG, Honk JV. The cerebellum on the rise in human emotion. Cerebellum. 2005;4(4):290-4. [Link] [DOI:10.1080/14734220500348584]
6. Van Der Heijden ME, Sillitoe RV. Interactions between Purkinje cells and granule cells coordinate the development of functional cerebellar circuits. Neuroscience. 2021;462:4-21. [Link] [DOI:10.1016/j.neuroscience.2020.06.010]
7. Fu L, Zhu L, Huang Y, Lee TD, Forman SJ, Shih CC. Derivation of neural stem cells from mesenchymal stem cells: Evidence for a bipotential stem cell population. Stem Cells Dev. 2008;17(6):1109-22. [Link] [DOI:10.1089/scd.2008.0068]
8. Zhu J, Qiu W, Wei F, Zhang J, Yuan Y, Liu L, et al. Toll-like receptor 4 deficiency in Purkinje neurons drives cerebellar ataxia by impairing the BK channel-mediated after-hyperpolarization and cytosolic calcium homeostasis. Cell Death Dis. 2024;15(8):594. [Link] [DOI:10.1038/s41419-024-06988-w]
9. Yang L, Liu SC, Liu YY, Zhu FQ, Xiong MJ, Hu DX, et al. Therapeutic role of neural stem cells in neurological diseases. Front Bioeng Biotechnol. 2024;12:1329712. [Link] [DOI:10.3389/fbioe.2024.1329712]
10. Zhang LP, Liao JX, Liu YY, Luo HL, Zhang WJ. Potential therapeutic effect of olfactory ensheathing cells in neurological diseases: Neurodegenerative diseases and peripheral nerve injuries. Front Immunol. 2023;14:1280186. [Link] [DOI:10.3389/fimmu.2023.1280186]
11. Wang Q, Zhou L, Guo Y, Liu G, Cheng J, Yu H. Differentiation of human adipose-derived stem cells into neuron-like cells by Radix Angelicae Sinensis. Neural Regen Res. 2013;8(35):3353-8. [Link]
12. Tan L, Liu X, Dou H, Hou Y. Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment-Specific factors involved in the regulation of MSC plasticity. Genes Dis. 2020;9(2):296-309. [Link] [DOI:10.1016/j.gendis.2020.10.006]
13. Han I, Kwon BS, Park HK, Kim KS. Differentiation potential of mesenchymal stem cells is related to their intrinsic mechanical properties. Int Neurourol J. 2017;21(Suppl 1):S24-31. [Link] [DOI:10.5213/inj.1734856.428]
14. Rehman A, Nigam A, Laino L, Russo D, Todisco C, Esposito G, et al. Mesenchymal stem cells in soft tissue regenerative medicine: A comprehensive review. Medicina. 2023;59(8):1449. [Link] [DOI:10.3390/medicina59081449]
15. Ramli K, Aminath Gasim I, Ahmad AA, Hassan S, Law ZK, Tan GC, et al. Human bone marrow‐derived MSCs spontaneously express specific Schwann cell markers. Cell Biol Int. 2019;43(3):233-52. [Link] [DOI:10.1002/cbin.11067]
16. Abdullah RH, Yaseen NY, Salih SM, Al-Juboory AA, Hassan A, Al-Shammari AM. Induction of mice adult bone marrow mesenchymal stem cells into functional motor neuron-like cells. J Chem Neuroanat. 2016;77:129-42. [Link] [DOI:10.1016/j.jchemneu.2016.07.003]
17. Mohammad MH, Al-Shammari AM, Al-Juboory AA, Yaseen NY. Characterization of neural stemness status through the neurogenesis process for bone marrow mesenchymal stem cells. Stem Cells Cloning. 2016;9:1-15. [Link] [DOI:10.2147/SCCAA.S94545]
18. Mohammad MH, Almzaien AK, Al-Joubory AA, Al-Shammari AM, Ahmed AA, Shaker HK, et al. In vitro isolation and expansion of neural stem cells NSCs. Baghdad Sci J. 2022;20(3):0787. [Link] [DOI:10.21123/bsj.2022.7280]
19. Alexander CJ, Hammer JA. An improved method for differentiating mouse embryonic stem cells into cerebellar Purkinje neurons. Cerebellum. 2019;18(3):406-21. [Link] [DOI:10.1007/s12311-019-1007-0]
20. Valencia-Salgado C, Jacobo-Arreola S, Said-Fernandez S, Soto-Dominguez A, Camacho-Morales A, Martinez-Rodriguez H, et al. Promoting differentiation of human adipose mesenchymal stem cells into oligodendrocyte-like cells and neuron-like cells through coculture on decellularized sciatic nerves. Sci Lett. 2022;1(1):2. [Link]
21. Watson LM, Wong MMK, Vowles J, Cowley SA, Becker EBE. A simplified method for generating Purkinje cells from human-induced pluripotent stem cells. Cerebellum. 2018;17(4):419-27. [Link] [DOI:10.1007/s12311-017-0913-2]
22. Baghaei K, Hashemi SM, Tokhanbigli S, Rad AA, Assadzadeh-Aghdaei H, Sharifian A, et al. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol Hepatol Bed Bench. 2017;10(3):208-13. [Link]
23. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315-7. [Link] [DOI:10.1080/14653240600855905]
24. Gu P, Qiu FC, Han R, Zhang ZX, Dong C, Zhang LN, et al. Efficient differentiation of neural stem cells induced by the rat bone marrow stromal cells. Int J Clin Exp Med. 2015;8(5):6713-24. [Link]
25. Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129-33. [Link] [DOI:10.1038/nbt1201-1129]
26. Suzuki H, Taguchi T, Tanaka H, Kataoka H, Li Z, Muramatsu K, et al. Neurospheres induced from bone marrow stromal cells are multipotent for differentiation into neuron, astrocyte, and oligodendrocyte phenotypes. Biochem Biophys Res Commun. 2004;322(3):918-22. [Link] [DOI:10.1016/j.bbrc.2004.07.201]
27. Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117(19):4411-22. [Link] [DOI:10.1242/jcs.01307]
28. Divya MS, Roshin GE, Divya TS, Rasheed VA, Santhoshkumar TR, Elizabeth KE, et al. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res Ther. 2012;3(6):57. [Link] [DOI:10.1186/scrt148]
29. Mareschi K, Novara M, Rustichelli D, Ferrero I, Guido D, Carbone E, et al. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Exp Hematol. 2006;34(11):1563-72. [Link] [DOI:10.1016/j.exphem.2006.06.020]
30. Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26(2-4):148-65. [Link] [DOI:10.1159/000082134]
31. Park D, Xiang AP, Mao FF, Zhang L, Di CG, Liu XM, et al. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells. 2010;28(12):2162-71. [Link] [DOI:10.1002/stem.541]
32. Pagin M, Pernebrink M, Giubbolini S, Barone C, Sambruni G, Zhu Y, et al. Sox2 controls neural stem cell self-renewal through a Fos-centered gene regulatory network. Stem Cells. 2021;39(8):1107-19. [Link] [DOI:10.1002/stem.3373]
33. Da Silva Siqueira L, Majolo F, Da Silva APB, Da Costa JC, Marinowic DR. Neurospheres: A potential in vitro model for the study of central nervous system disorders. Mol Biol Rep. 2021;48(4):3649-63. [Link] [DOI:10.1007/s11033-021-06301-4]
34. Songsaad AT, Thairat S, Seemaung P, Thongsuk A, Balit T, Ruangsawasdi N, et al. Characterization of neural stem cells derived from human stem cells from the apical papilla undergoing three-dimensional neurosphere induction. J Appl Oral Sci. 2023;31:e20230209. [Link] [DOI:10.1590/1678-7757-2023-0209]









