Volume 17, Issue 1 (2025)
Iran J War Public Health 2025, 17(1): 51-61 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2025/02/1 | Accepted: 2025/03/5 | Published: 2025/03/8
Received: 2025/02/1 | Accepted: 2025/03/5 | Published: 2025/03/8
How to cite this article
Sedehi S, Norouzi Palangani F, Maleki Z, Banihashemi S. Effect of Conventional and Climb Milling on the Mechanical Properties and Biocompatibility of Pure Titanium; Application of the Williamson-Hall Method. Iran J War Public Health 2025; 17 (1) :51-61
URL: http://ijwph.ir/article-1-1566-en.html
URL: http://ijwph.ir/article-1-1566-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Mechanical Engineering, Faculty of Engineering, University of Tehran, Tehran, Iran
2- Department of Materials Engineering, Faculty of Materials Engineering, Amir Kabir University of Technology, Tehran, Iran
3- Department of Industrial Engineering, Faculty of Engineering, Gonabad University, Gonabad, Iran
2- Department of Materials Engineering, Faculty of Materials Engineering, Amir Kabir University of Technology, Tehran, Iran
3- Department of Industrial Engineering, Faculty of Engineering, Gonabad University, Gonabad, Iran
Full-Text (HTML) (493 Views)
Introduction
Titanium alloys are widely used across various industries, including aerospace, medical, and military, due to their unique characteristics, such as a high strength-to-weight ratio, corrosion resistance, and biocompatibility [1, 2]. Pure Ti exhibits two allotropic structures: Hexagonal close-packed (HCP, α-phase) at room temperature and body-centered cubic (BCC, β-phase) at temperatures above 882°C [3]. In addition to these excellent properties, titanium alloys can suppress metal ion release due to the formation of a strong, thin oxide layer (passive layer) that results from the material's natural activity. This makes them a highly suitable material for medical implants, where biocompatibility and safety are paramount [4]. However, machining titanium alloys have always been a significant challenge, as their specific properties hinder machinability [5]. Titanium alloys are prone to workpiece surface burning due to their low thermal conductivity, high chemical reactivity, and relatively low modulus of elasticity. This leads to severe tool wear and deformation during machining [6]. The increasing use of titanium alloys, particularly Ti-6Al-4V, in various industries, along with the challenges in machining, has led to extensive research in recent years on machining titanium alloys and pure Ti [7]. In 2018, Narita [8] studied the machining of sloped surfaces. It showed that contact patterns were critical, requiring simultaneous contact of the lower edges and upper corners to achieve a good surface finish. Yujiang & Tao [9] investigated the high-speed milling of TC11 titanium alloy and concluded that increased speed reduced tool wear resistance and significantly higher milling forces and surface roughness. Jiang et al. [6] examined the relationship between different machining paths and surface integrity, finding that machining direction significantly impacted surface integrity, and a change in direction could reduce surface roughness by almost 40%. Kumar et al. [10] studied surface roughness during the miniature milling of grade 2 and 5 titanium under dry machining conditions, demonstrating that depth of cut had the least impact on surface roughness. Danesh & Rahimi [11] investigated the effect of tool vibration and wear on workpiece surface topography during the machining of Ti-6Al-4V alloy, observing that increased free surface vibrations led to surface irregularities and reduced workpiece quality. Festas et al. [12] analyzed the challenges of machining titanium for medical applications, noting that the main issue was the extraction or dissipation of the excessive heat generated during cutting, which is inherent to the material's properties. Additionally, the high wear rate resulted in shorter tool life. Brown et al. [13] studied the deformation characteristics of machined surfaces of titanium alloys, finding that lower-speed milling resulted in greater depth of deformation. Daniyan et al. [14] conducted a numerical and experimental analysis of surface roughness during the milling of Ti6Al4V alloy, revealing that the machining parameter Ra=0.035 µm resulted in the lowest surface roughness. Zhu & Beaucamp [15] compared the surface morphology of Ti6Al4V alloys machined with uncoated and TiAlN-coated tools under various lubrication conditions, concluding that the TiAlN coating improved surface roughness. In contrast, uncoated alloy wear became more severe at a 75m/min cutting speed. Various machining processes are capable of processing almost all engineering materials. These technologies play a critical role in the properties of vital components in advanced equipment [16-18]. During machining, the tool edges contact the workpiece surface, combining compressing, scratching, plowing, and cutting to remove material or induce plastic deformation on the machined surface [19-21].
In contrast to previous studies that primarily focused on the effect of a single milling method (such as conventional or climb milling) on the mechanical properties or corrosion behavior of titanium, this research simultaneously aimed to investigate the impact of two milling methods (conventional and climb milling) on the mechanical properties, corrosion resistance, and microstructure of pure titanium. Additionally, using the Williamson-Hall method to analyze microstructural changes (such as dislocation density and crystallite size) and their correlation with improvements in mechanical properties and corrosion resistance represents an innovative approach in this study.
Materials and Methods
This experimental study applied the milling process using a three-axis Fp4md machine.
The machining was done in conventional and climb milling modes without using any coolant on the workpiece surface (Figure 1). A thickness of 4mm was selected for the titanium samples (Ti: 99.34%; Fe: 0.2%; O: 0.18%; H: 0.15%; C: 0.08%; N:0.03%) as it is closer to those used in medical applications, particularly for dental and orthopedic implants, and avoids the assumptions associated with thin sheets. The mechanical and experimental tests (such as hardness testing, wear resistance, and corrosion analysis) and microstructural analysis (using XRD and the Williamson-Hall method) were conducted. Each test was repeated multiple times, and the data were reported with high precision to ensure the reliability of the results.
The sample phases were analyzed using X-ray diffraction (XRD) with the explorer machine (GNR; Italy) under 40kV voltage and 30mA current. The hardness of the samples was measured using the Vickers method with an INNOVATEST NEXUS XL8000 hardness tester. A tungsten indenter with a 2.5mm diameter and a load of 612.9N was applied for a holding time of 15 seconds, following the ASTM A370 standards of 2020. Each sample was tested at least three times to obtain an average value.
Wear testing was conducted using the pin-on-disk method in a specialized laboratory following ASTM G99 standards. The test was performed in dry conditions at room temperature under a load of 5N for 3600 seconds, at 60rpm, with a steel pin as the abrasive material. Electrochemical corrosion tests were conducted in -250 to +250mV, with a scan rate of 1mV and an open circuit potential (OCP) test duration of 900 seconds. Surface roughness tests were conducted according to ISO 21920-3-2021 standards at 25°C and 19% humidity.
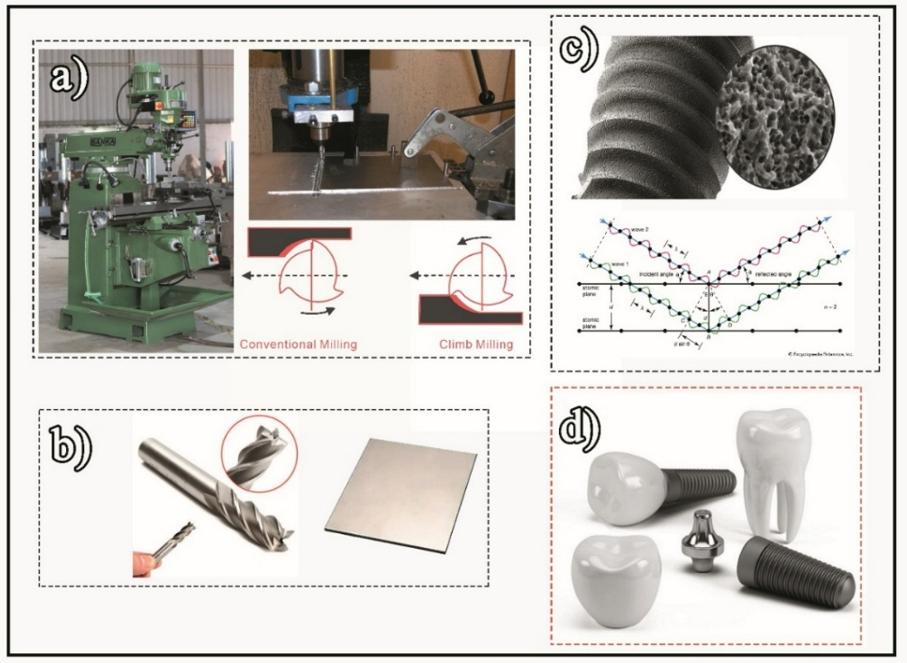
Figure 1. a: Milling machine (TiAln) and schematic of tool movement (advance speed: 34mm/s; shear speed: 160m/min; sequence length: 45mm; length of cutter: 35mm; tool diameter; table speed: 35m/h); b: Machining tool and titanium sheet (milling depth: 0.4mm; rotational speed: 1250rpm); c: Stages of the experiment; d: Intended applications of the project
The Scherrer equation is used to calculate crystallite size.
Where Dc (nm) is the crystallite size, k is the shape factor (k=1), β is the wavelength of the X-ray beam, θ is the Bragg angle at the peak maximum (radians), and (β) is the full-width at half maximum (FWHM) of the peak (in radians), referring to the deviation from peak intensity in the XRD pattern. X-ray diffraction operates based on Bragg's law:

This formula becomes important for examining small crystallites, where the broadening of peaks in the diffraction pattern is significant. The Bragg angle (θ) is where the diffraction intensity is at its maximum. The relationship between the peak width (β) and the crystallite size Dc is derived from the wave distribution theory [22, 23]. The diffraction peaks broaden when the crystallite size decreases [24, 25]. The full width at half maximum (β) is related to the crystallite size, and the distribution of these waves expresses the angular width of the peak as described by the following equation.
By inserting the shape factor (k=1), which corrects for particle shape and sample geometry, the equation takes the general form of the Scherrer formula:
The Williamson-Hall equation is as follows:
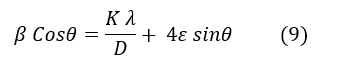
Where k is the shape factor (k=1), β is the wavelength of the Xλ, D is the crystalline size (nm), the average crystal size, ϵ is the microstrain, which indicates structural deformation in the crystals, and θ is the Bragg angle at the maximum peak (radians), with the peak width at half maximum (radians) indicating the deviation from the intensity peak in the XRD pattern. This equation demonstrates the relationship between two phenomena: β represents the fluctuations and variations in peak width due to ϵ and D, and ϵ is also related to crystal deformation under the influence of stresses. A line can be analyzed by plotting the data points using the Bragg relationship (2), where n is an integer representing the number of crystal planes. If the Williamson-Hall relationship is divided by λ:
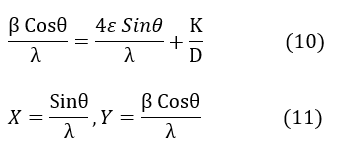
The slope of the line represents 4ε, indicating the crystallite size. The Williamson-Hall formula helps analyze the effect of ϵ (strain) and crystallite size on materials' structural quality and properties. Micro strain refers to very small deformations within the crystal [26], and peak broadening in diffraction corresponds to the deviation in atomic positions [27]. Dislocation density, or defect density [28], refers to the number of defects within a material unit. These defects can exist as dislocations in the crystal structure, influencing the mechanical and physical properties of the material. As crystallite size Dc decreases, the contact surface of crystal edges increases, leading to the creation and rise in defect numbers. There is an inverse relationship between the square of crystallite size and dislocation density. Overall, these concepts are particularly important in analyzing the mechanical properties of materials and X-ray diffraction patterns. The XRD results and Williamson-Hall analysis show that the milling process, especially in conventional milling, has significant effects on the crystalline structure of titanium. These changes can be applied in designing and producing medical implants, particularly for dental and knee applications, to provide patients with longer-lasting and safer performance.
Findings
XRD and Williamson-Hall Analysis
The XRD results indicated that the peak intensities at 35° and 40° had significantly decreased in the titanium samples machined using conventional and climb milling methods (Table 1; Figure 2).
Table 1. Results extracted from the XRD test and Williamson-Hall analysis
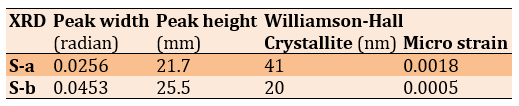
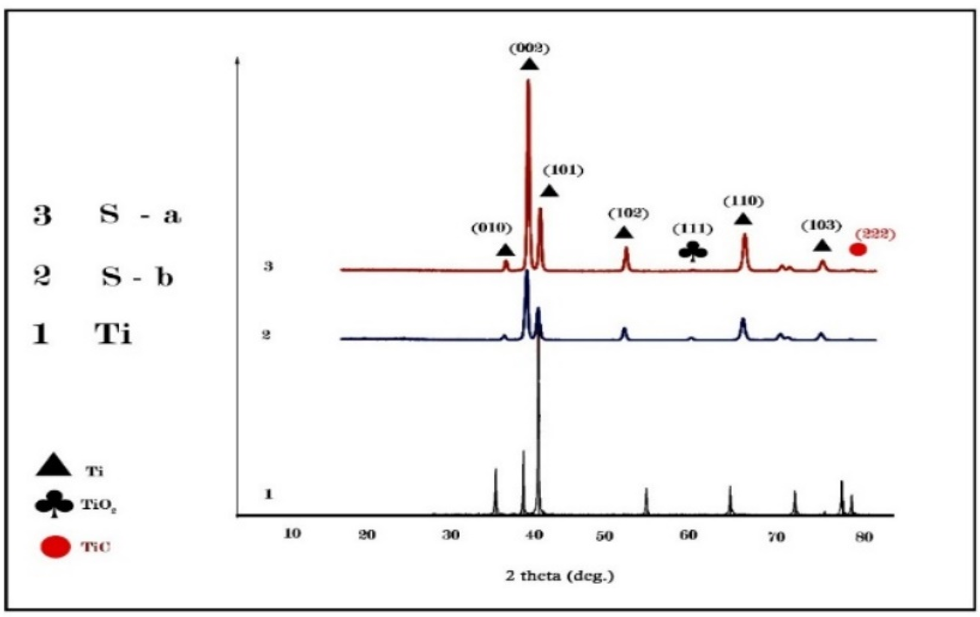
Figure 2. XRD chart of the samples at different stages
Surface roughness
The sample machined with conventional milling (S-a: 2.709μm) had a smoother surface than the one machined with climb milling (S-b: 7.129μm).
Hardness testing
The Vickers hardness results, which were recognized as an indicator of a material's resistance to deformation, indicated that the hardness of titanium increased to 315HV during conventional milling and 334HV during climb milling. In comparison, the hardness of pure titanium was measured at 292HV.
Weight loss after wear testing
Pure Ti exhibited a weight loss of 18mg after wear testing, with 11 and 10mg reductions observed for the conventional and climb milling methods, respectively (Figure 3).
Corrosion rate
The corrosion rate of titanium samples machined using the climb milling method (0.0003mm/year) was significantly lower than that of samples machined using the conventional milling method (0.02mm/year). Climb milling resulted in a smoother surface with fewer cracks, which can substantially increase corrosion resistance. Pure Ti, with a corrosion rate of 0.07mm/year, achieved significantly lower rates after milling by both methods, especially in the case of conventional milling, where corrosion was reduced to 0.0003mm/year (Table 2; Figures 4 & 5).
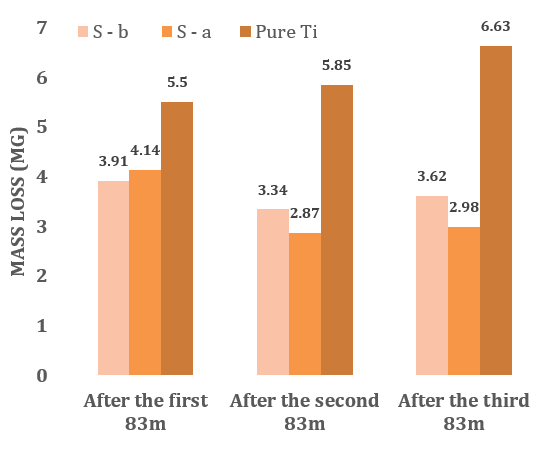
Figure 3. Weight loss due to wear
Table 2. Results of the corrosion test
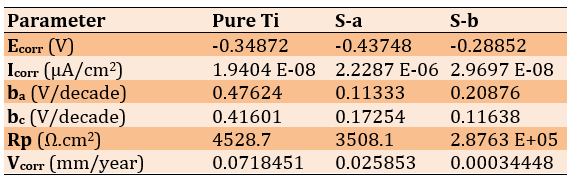
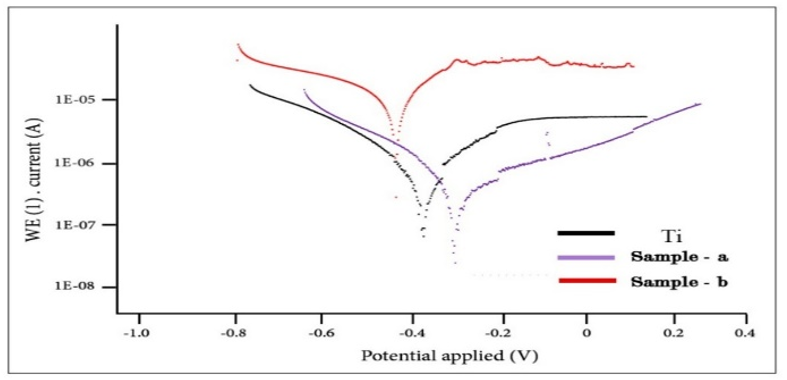
Figure 4. The corrosion test results
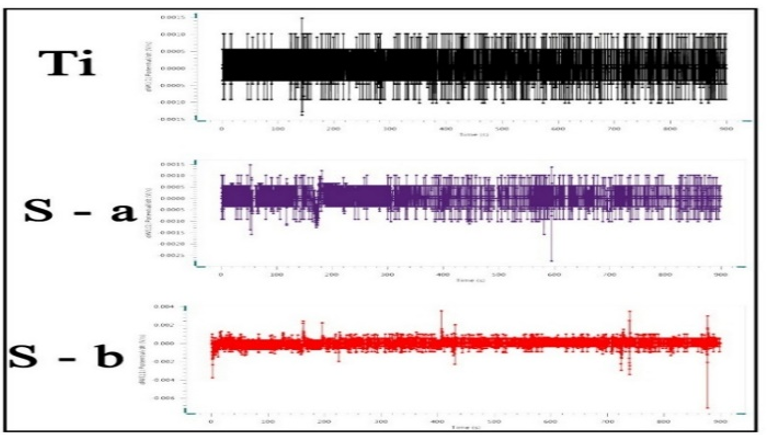
Figure 5. The OCP corrosion test results
Discussion
The reduction in peak intensity is attributed to structural changes in the titanium. The 40° peak, known as the indicator of pure titanium's crystalline structure, is reduced in conventional and climb milling. This may be due to changes in internal stresses and crystallite size reduction after milling. Compared to climb milling, the more pronounced reduction in peak intensity observed in conventional milling is likely due to deeper structural changes, cutting force, and more severe plastic deformation in this method. This phenomenon is particularly important in medical industries, such as the production of dental and knee implants, because the reduction in crystallinity can directly impact the mechanical properties and durability of implants. Changes in the peak positions, which result from accumulated stresses and atomic displacements in the structure, can affect titanium's mechanical properties and biocompatibility. These alterations are significant for dental and knee implant applications, as they can influence the mechanical compatibility of the implant with the surrounding bone and improve its ability to withstand applied forces over time. This peak, which is absent in the control sample, indicates the formation of a titanium oxide layer after milling. The formation of this oxide, particularly in implants, can enhance biocompatibility, as titanium oxide is known to interact well with the body and promote better growth of biological tissues. The difference in the titanium oxide peak height between conventional and climb milling also indicates greater oxide formation in conventional milling, which may be due to more intense thermal and mechanical changes. The crystallite size in climb milling is 41nm, while in conventional milling, it is 20nm, indicating a significant reduction in grain size. Reducing crystallite size can enhance mechanical properties such as hardness and wear resistance but may also affect biocompatibility. On the other hand, the recorded micro strains show that climb milling can lead to greater internal strains, which may improve implants' mechanical properties and fracture resistance.
The difference in surface roughness is primarily due to the direction of tool movement in both milling methods. In conventional milling, the tool moves in alignment with the cutting direction of the workpiece, resulting in a more uniform surface and reduced roughness. This approach minimizes shear forces and enhances surface quality. In conventional milling (also known as down milling), the direction of the tool's movement is aligned with the workpiece's movement. This alignment reduces cutting forces and vibrations, leading to a smoother surface with lower roughness. The reduction in surface roughness is due to more uniform and stable cutting in this method. In contrast, in climb milling (or up milling), the direction of the tool's movement is opposite to that of the workpiece, which increases cutting forces and vibrations. This condition results in a rougher surface with higher roughness. The increase in surface roughness is caused by instability in the cutting process and the formation of deeper scratches. When assessing the surface behavior of pure titanium using both milling methods, the surface roughness results are significantly influenced by the machining techniques. The conventional milling method yielded a surface roughness of 2.7 microns, indicating a smoother and more uniform surface. This characteristic is particularly important in dental applications, as dental implants require a low roughness surface to facilitate better interaction with the mouth's soft tissues and prevent bacterial plaque accumulation. A smoother surface increases the success of implant integration with the jawbone. It reduces the risk of bacterial infections, a crucial factor in extending the lifespan of dental implants. On the other hand, the surface roughness of the samples produced by climb milling was measured at 7.12 microns, indicating a rougher and more uneven surface. This surface type is advantageous for knee implants subjected to heavy and continuous loads. Higher roughness can improve the mechanical bonding of the implant to the bone tissue, which is particularly important in the working conditions of knee joints that require greater strength and wear resistance.
The increase in hardness is attributed to the effects of work hardening and the formation of crystalline discontinuities on the material's surface, which occur due to the shear forces and heat generated during the milling process. In this study, the hardness properties of pure titanium sheets were investigated after machining with both milling methods, and the results demonstrated a significant improvement in surface hardness for both approaches. This increase in hardness has significant implications for biomedical implants, particularly in dental and knee applications. In dental implants, a harder surface enhances wear resistance and reduces deformation, thereby improving the longevity of the implant under chewing forces. The hardness enhancement in machined samples, particularly in the conventional milling method, can improve knee implant performance and reduce the risk of wear or premature failure. This improvement in mechanical properties guarantees the durability and performance of knee implants and enhances patients' confidence in the efficacy and longevity of internal implants in complex physical conditions. Whenever the strength and hardness of an alloy increase, its ductility tends to decrease. Low hardness can transfer uncoordinated stresses during mechanical movements, resulting in localized stress shielding from osteoporosis. Furthermore, the increase in hardness indicates enhanced wear resistance. Higher hardness can extend the lifespan of components and equipment while reducing maintenance costs, which is crucial for improving efficiency and safety in these industries.
The significant reduction in wear, especially with climb milling, indicates an increase in the surface resistance of titanium to wear. The difference in performance may be related to the distinct surface structures generated by the two milling methods. The surface of titanium machined using the conventional method is likely more resilient to wear and erosion due to the formation of protective layers and a rougher structure, whereas the surface of titanium machined with the climbing method may experience wear sooner due to reduced protective layers and a less porous surface. As is evident, bones are the most crucial and abundant structures, serving as the primary load-bearing components of the human body, making bone health paramount. When positioned as joints within the body, implants are subject to wear and potential failure. As load-bearing biomaterials, wear is an inevitable consequence of bodily movement. Moreover, prolonged wear of implants in various joint regions can release numerous metallic particles from the implant surfaces, triggering foreign body reactions with surrounding tissues. Broken surfaces of implants may interact with their environment, leading to serious complications for the body. Due to increased wear, these released metallic particles can deposit in organs distant from the implants, potentially causing various adverse reactions and damage, including toxic effects, inflammatory responses, tissue damage, and disruption of organ function. Particles released as a result of wear hinder bone regeneration processes, including survival, proliferation, and adhesion, while also making the damaged surface susceptible to cracking. This susceptibility allows bodily fluids, including blood, serum, interstitial fluids, and lymph, to invade easily, increasing the likelihood of corrosion and infection. In summary, the high wear resistance of implants ensures their safe and stable use in the human body. Improved wear resistance is particularly crucial for knee implants, which endure heavy and continuous physical pressures. In such conditions, any reduction in wear can greatly enhance the durability and stability of the implant. The results showed that conventional milling, with a weight loss of 11mg during wear testing, demonstrated excellent performance for knee working conditions, as high wear resistance minimizes the likelihood of surface degradation or loosening of the implant.
Various factors influence corrosion, including alloy composition, microstructure, and grain size. In biomedical titanium alloys, corrosion occurs in contact with body fluids such as blood, serum, interstitial fluids, and lymph, primarily due to corrosive environments like chloride ions and proteins [29]. Although titanium alloys can naturally form a stable passive layer on their surfaces, providing excellent corrosion resistance, the complexity of the human body environment can lead to the detachment and dissolution of this surface passive layer. This process is exacerbated by the combined effects of external forces and human body fluids, including blood, serum, interstitial fluids, and lymph, which can potentially result in the release of harmful substances or waste materials that may be toxic and cause inflammation or lead to blood clotting in the body. Non-harmful waste materials are suspended in blood, posing no harm to human health and being naturally excreted [30]. Considering the prolonged implantation time in the human body, implantable materials must demonstrate sufficient corrosion resistance to ensure long-term biocompatibility while avoiding any potential interference that may arise from accumulated corrosion products or the release of metal ions. Corrosion resistance is a fundamental property of biomedical materials. Unfortunately, the human body constitutes an electrolyte environment containing plasma, blood serum, chloride ions, lymphatic fluid, amino acids, and proteins. Research findings indicate that metal implants exhibit gradual electrochemical reactions in the human body. Compared to non-passivated metals with high corrosion resistance, biodegradable metals have attracted considerable attention due to their gradual absorption and consumption in biological tissues [30]. Reduced corrosion is critical as implants are continuously exposed to an acidic and moist oral environment. A lower corrosion rate enhances the resistance of dental implants to wear from saliva and food, thereby increasing their longevity and reducing the risk of infection or adverse reactions. Conversely, knee implants require high corrosion resistance due to their exposure to joints' complex and high-pressure environments. The significant improvement in corrosion resistance observed in conventional milling samples suggests that this method can play a key role in increasing the durability of knee implants. This improvement not only ensures resistance against the corrosive environments of the body but also prevents premature degradation or fracture of the implant, thereby reducing the likelihood of replacement surgeries. Combining these results with enhancements in mechanical properties under various working conditions guarantees the long-term efficacy of both dental and knee implants. In analyzing titanium's corrosion behavior and the milling process's impact on its properties, the corrosion potential plays a key role in determining the material's susceptibility to corrosion. A negative corrosion potential indicates a greater tendency for oxidation and electron loss. Here, the corrosion potential of the conventional milling sample is lower than that of pure titanium, signifying enhanced corrosion resistance. In contrast, the corrosion potential in climb milling is lower and more negative, indicating an increased susceptibility to corrosion. This aspect is particularly relevant in the medical design of dental and knee implants, as higher corrosion resistance leads to longer implant life and reduced release of harmful ions into the body. Corrosion current density is another crucial parameter that indicates the amount of electric current generated due to the corrosion process and is directly related to the rate of material degradation in a corrosive environment. The higher the current density, the greater the corrosion. In this case, the corrosion current density in the conventional milling sample is approximately similar to that of pure titanium. Still, the current density in climb milling is significantly higher, indicating more severe corrosion. This discrepancy suggests that climb milling may produce a surface that is more prone to corrosion, which is undesirable for implants, as higher degradation rates result in greater titanium ion release and negatively affect biocompatibility. The cathodic and anodic slopes are important parameters in determining the electrochemical behavior of materials during the corrosion process. Notably, the reduction in the anodic slope suggests that the formation of the oxide layer on the surface of conventional milled titanium occurs more extensively and rapidly, which can enhance biocompatibility and corrosion resistance in dental and knee implants. From a mechanical perspective, the reduction in the cathodic and anodic slopes indicates a greater tendency to form protective oxide layers, improving surface hardness and wear resistance. This could directly impact the lifespan of metallic implants, as a harder and more corrosion-resistant surface implies better resistance to both mechanical and chemical wear. The open circuit potential (OCP) diagram is crucial in studying the corrosion behavior of metallic materials, especially in biological environments where implants come into contact with body fluids. This diagram reflects the metal surface's ability to maintain its electrochemical potential without external current. The results indicate that pure titanium exhibits greater stability than milled samples. This suggests that pure titanium demonstrates higher resistance to environmental fluctuations and prevents corrosion by forming a protective layer on its surface. In a medical context, this greater stability translates to better biocompatibility, as implants with higher OCP stability are less prone to releasing metal ions into the body, thus posing a lower risk to patient health. Mechanically, the broader range recorded for the conventional milling sample indicates that this sample has lower electrochemical stability due to structural changes and stresses caused by the milling process. This could lead to a higher corrosion rate and reduced mechanical longevity of the implant. Moreover, reduced stability in milled samples may result from creating surface defects and an increased surface area exposed to the corrosive environment. Consequently, selecting the appropriate milling method and controlling process conditions can play a crucial role in enhancing both mechanical resistance and the longevity of dental and knee implants.
Pure titanium is widely used in the production of medical implants due to its excellent mechanical properties, superior biocompatibility, and high corrosion resistance. However, machining this material presents significant challenges. The heat generated in the cutting zone rapidly increases at higher machining speeds or greater cutting depths. This rise in temperature can lead to undesirable changes in the material's microstructure, such as localized annealing and a reduction in surface hardness. Additionally, titanium tends to adhere to cutting tools, a phenomenon known as "built-up edge", which can cause deep scratches and increase surface roughness. These factors collectively limit the use of higher machining speeds and depths, as they can negatively impact the implant's surface quality and mechanical properties. The results of this study demonstrate that improving surface hardness and reducing surface roughness in titanium machining directly influence the performance of medical implants. Increased surface hardness enhances wear resistance and prevents implant deformation under mechanical loads, such as those experienced by artificial joints. On the other hand, reduced surface roughness minimizes friction between the implant and surrounding tissues, promoting better bone cell adhesion and accelerating the osseointegration process. Furthermore, the higher corrosion resistance observed in this study is particularly significant, as medical implants are exposed to corrosive biological environments. These combined properties extend the implant's lifespan and reduce the risk of inflammation or adverse reactions. For instance, conventional milling at moderate speeds and controlled cutting depths induces beneficial compressive stresses on the surface and prevents excessive heating and structural degradation. These conditions help maintain surface hardness and reduce roughness, both of which are crucial for the performance of medical implants. Additionally, precise control of machining parameters can minimize the generation of abrasive particles, preventing the introduction of metallic debris into the body and subsequent inflammation. As a result, optimizing these parameters enhances implant quality and ensures their safety and efficacy in medical applications.
The importance of selecting the appropriate machining method for titanium in medical applications, particularly for dental and knee implants, is increasing due to its impact on the material's mechanical properties, corrosion resistance, and biocompatibility. Changes induced by different machining processes can significantly affect the performance of these implants in the human body. This study examined the effects of conventional and climb milling on pure titanium sheets' mechanical and corrosion properties to draw effective conclusions for selecting the best method for specific applications, especially in the medical field.
The Williamson-Hall analysis provides crucial insights into structural changes induced by the milling process, particularly through crystallite size and microstrain measurements [31]. According to the results of this study, the shift of the highest peak from 40° to 38° in both milling methods may indicate changes in the titanium crystal lattice structure, possibly due to stress variations or defect formation caused by milling [32]. Additionally, the emergence of a new peak at 62.5°, attributed to titanium oxide, is a significant observation [33]. In XRD patterns, each peak corresponds to a specific set of crystal planes, which can broaden due to stresses and defects (microstrain), meaning that increased stresses lead to peak broadening [34, 35]. Dislocation density, which represents the number of dislocations per unit volume of material [26], significantly influences the mechanical properties of metals, as dislocations act as weak points within the crystal structure [27]. According to the findings of this study, variations in crystallite size, internal stresses, and titanium oxide formation collectively enhance this material's mechanical properties and biocompatibility [36, 37]. Furthermore, the smoother surface achieved in conventional milling reduces friction and improves bone cell adhesion, which is critical for the success of medical implants [38]. In contrast, rougher surfaces promote new bone growth at the implant site, ensuring long-term integrity and durability [39]. This study's results show that dental implants, which experience small but continuous stresses, require hard and biocompatible surfaces to ensure mechanical durability and optimal interaction with surrounding tissues [40]. Conversely, knee implants, subjected to more demanding working conditions and heavier loads, require greater hardness to withstand continuous compressive forces and friction [41-43]. Additionally, corrosion analysis demonstrated that smoother and more homogeneous surfaces resist corrosive agents due to fewer weak points and microscopic cracks [44, 45]. According to this study, the cathodic slope, which describes the rate of reduction reactions at the cathode, and the anodic slope, which represents the oxidation kinetics of the material, are lower in conventionally milled titanium than in pure titanium. This indicates reduced electrochemical reaction rates, suggesting improved corrosion resistance and enhanced protective oxide layer formation [46, 47]. Finally, due to titanium’s sensitivity to high temperatures, optimizing machining parameters such as cutting speed, depth of cut, and machining strategy plays a crucial role in achieving the desired properties for medical implants [48-50].
These findings suggest that selecting the appropriate milling method can optimize the performance of titanium implants according to their specific application.
Conclusion
Conventional and climb milling methods impact pure titanium sheets' mechanical and corrosion properties, influencing their performance in dental and knee implants.
Acknowledgments: We would like to express our sincere gratitude to the esteemed nurses, Ms. Salimeh Sadat Sedehi and Ms. Sahar Sadat Sedehi, for their invaluable contributions during this research, particularly in patient care. Their unwavering support and presence during many challenging moments have been our research team's immense motivation and hope. Undoubtedly, their role in the advancement of this project and the enhancement of its results is undeniable.
Ethical Permissions: Not needed.
Conflicts of Interests: The authors confirm that no financial interests or personal relationships could have influenced the outcomes of the study presented in this paper.
Authors' Contribution: Sedehi SMR (First Author), Introduction Writer/Methodologist/Original Researcher/Discussion Writer (55%); Norouzi Palangani F (Second Author), Introduction Writer/Methodologist/Assistant Researcher (15%); Maleki Z (Third Author), Assistant Researcher/Discussion Writer/Statistical Analyst (15%); Banihashemi SF (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (15%)
Funding/Support: This research did not receive any financial support.
Titanium alloys are widely used across various industries, including aerospace, medical, and military, due to their unique characteristics, such as a high strength-to-weight ratio, corrosion resistance, and biocompatibility [1, 2]. Pure Ti exhibits two allotropic structures: Hexagonal close-packed (HCP, α-phase) at room temperature and body-centered cubic (BCC, β-phase) at temperatures above 882°C [3]. In addition to these excellent properties, titanium alloys can suppress metal ion release due to the formation of a strong, thin oxide layer (passive layer) that results from the material's natural activity. This makes them a highly suitable material for medical implants, where biocompatibility and safety are paramount [4]. However, machining titanium alloys have always been a significant challenge, as their specific properties hinder machinability [5]. Titanium alloys are prone to workpiece surface burning due to their low thermal conductivity, high chemical reactivity, and relatively low modulus of elasticity. This leads to severe tool wear and deformation during machining [6]. The increasing use of titanium alloys, particularly Ti-6Al-4V, in various industries, along with the challenges in machining, has led to extensive research in recent years on machining titanium alloys and pure Ti [7]. In 2018, Narita [8] studied the machining of sloped surfaces. It showed that contact patterns were critical, requiring simultaneous contact of the lower edges and upper corners to achieve a good surface finish. Yujiang & Tao [9] investigated the high-speed milling of TC11 titanium alloy and concluded that increased speed reduced tool wear resistance and significantly higher milling forces and surface roughness. Jiang et al. [6] examined the relationship between different machining paths and surface integrity, finding that machining direction significantly impacted surface integrity, and a change in direction could reduce surface roughness by almost 40%. Kumar et al. [10] studied surface roughness during the miniature milling of grade 2 and 5 titanium under dry machining conditions, demonstrating that depth of cut had the least impact on surface roughness. Danesh & Rahimi [11] investigated the effect of tool vibration and wear on workpiece surface topography during the machining of Ti-6Al-4V alloy, observing that increased free surface vibrations led to surface irregularities and reduced workpiece quality. Festas et al. [12] analyzed the challenges of machining titanium for medical applications, noting that the main issue was the extraction or dissipation of the excessive heat generated during cutting, which is inherent to the material's properties. Additionally, the high wear rate resulted in shorter tool life. Brown et al. [13] studied the deformation characteristics of machined surfaces of titanium alloys, finding that lower-speed milling resulted in greater depth of deformation. Daniyan et al. [14] conducted a numerical and experimental analysis of surface roughness during the milling of Ti6Al4V alloy, revealing that the machining parameter Ra=0.035 µm resulted in the lowest surface roughness. Zhu & Beaucamp [15] compared the surface morphology of Ti6Al4V alloys machined with uncoated and TiAlN-coated tools under various lubrication conditions, concluding that the TiAlN coating improved surface roughness. In contrast, uncoated alloy wear became more severe at a 75m/min cutting speed. Various machining processes are capable of processing almost all engineering materials. These technologies play a critical role in the properties of vital components in advanced equipment [16-18]. During machining, the tool edges contact the workpiece surface, combining compressing, scratching, plowing, and cutting to remove material or induce plastic deformation on the machined surface [19-21].
In contrast to previous studies that primarily focused on the effect of a single milling method (such as conventional or climb milling) on the mechanical properties or corrosion behavior of titanium, this research simultaneously aimed to investigate the impact of two milling methods (conventional and climb milling) on the mechanical properties, corrosion resistance, and microstructure of pure titanium. Additionally, using the Williamson-Hall method to analyze microstructural changes (such as dislocation density and crystallite size) and their correlation with improvements in mechanical properties and corrosion resistance represents an innovative approach in this study.
Materials and Methods
This experimental study applied the milling process using a three-axis Fp4md machine.
The machining was done in conventional and climb milling modes without using any coolant on the workpiece surface (Figure 1). A thickness of 4mm was selected for the titanium samples (Ti: 99.34%; Fe: 0.2%; O: 0.18%; H: 0.15%; C: 0.08%; N:0.03%) as it is closer to those used in medical applications, particularly for dental and orthopedic implants, and avoids the assumptions associated with thin sheets. The mechanical and experimental tests (such as hardness testing, wear resistance, and corrosion analysis) and microstructural analysis (using XRD and the Williamson-Hall method) were conducted. Each test was repeated multiple times, and the data were reported with high precision to ensure the reliability of the results.
The sample phases were analyzed using X-ray diffraction (XRD) with the explorer machine (GNR; Italy) under 40kV voltage and 30mA current. The hardness of the samples was measured using the Vickers method with an INNOVATEST NEXUS XL8000 hardness tester. A tungsten indenter with a 2.5mm diameter and a load of 612.9N was applied for a holding time of 15 seconds, following the ASTM A370 standards of 2020. Each sample was tested at least three times to obtain an average value.
Wear testing was conducted using the pin-on-disk method in a specialized laboratory following ASTM G99 standards. The test was performed in dry conditions at room temperature under a load of 5N for 3600 seconds, at 60rpm, with a steel pin as the abrasive material. Electrochemical corrosion tests were conducted in -250 to +250mV, with a scan rate of 1mV and an open circuit potential (OCP) test duration of 900 seconds. Surface roughness tests were conducted according to ISO 21920-3-2021 standards at 25°C and 19% humidity.

Figure 1. a: Milling machine (TiAln) and schematic of tool movement (advance speed: 34mm/s; shear speed: 160m/min; sequence length: 45mm; length of cutter: 35mm; tool diameter; table speed: 35m/h); b: Machining tool and titanium sheet (milling depth: 0.4mm; rotational speed: 1250rpm); c: Stages of the experiment; d: Intended applications of the project
The Scherrer equation is used to calculate crystallite size.
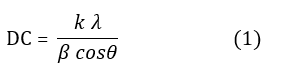
Where Dc (nm) is the crystallite size, k is the shape factor (k=1), β is the wavelength of the X-ray beam, θ is the Bragg angle at the peak maximum (radians), and (β) is the full-width at half maximum (FWHM) of the peak (in radians), referring to the deviation from peak intensity in the XRD pattern. X-ray diffraction operates based on Bragg's law:

This formula becomes important for examining small crystallites, where the broadening of peaks in the diffraction pattern is significant. The Bragg angle (θ) is where the diffraction intensity is at its maximum. The relationship between the peak width (β) and the crystallite size Dc is derived from the wave distribution theory [22, 23]. The diffraction peaks broaden when the crystallite size decreases [24, 25]. The full width at half maximum (β) is related to the crystallite size, and the distribution of these waves expresses the angular width of the peak as described by the following equation.
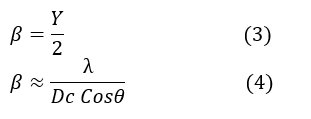
By inserting the shape factor (k=1), which corrects for particle shape and sample geometry, the equation takes the general form of the Scherrer formula:
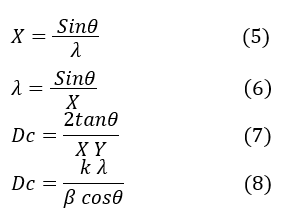
The Williamson-Hall equation is as follows:
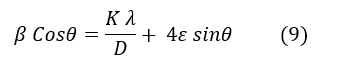
Where k is the shape factor (k=1), β is the wavelength of the Xλ, D is the crystalline size (nm), the average crystal size, ϵ is the microstrain, which indicates structural deformation in the crystals, and θ is the Bragg angle at the maximum peak (radians), with the peak width at half maximum (radians) indicating the deviation from the intensity peak in the XRD pattern. This equation demonstrates the relationship between two phenomena: β represents the fluctuations and variations in peak width due to ϵ and D, and ϵ is also related to crystal deformation under the influence of stresses. A line can be analyzed by plotting the data points using the Bragg relationship (2), where n is an integer representing the number of crystal planes. If the Williamson-Hall relationship is divided by λ:
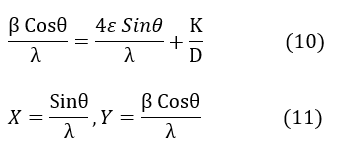
The slope of the line represents 4ε, indicating the crystallite size. The Williamson-Hall formula helps analyze the effect of ϵ (strain) and crystallite size on materials' structural quality and properties. Micro strain refers to very small deformations within the crystal [26], and peak broadening in diffraction corresponds to the deviation in atomic positions [27]. Dislocation density, or defect density [28], refers to the number of defects within a material unit. These defects can exist as dislocations in the crystal structure, influencing the mechanical and physical properties of the material. As crystallite size Dc decreases, the contact surface of crystal edges increases, leading to the creation and rise in defect numbers. There is an inverse relationship between the square of crystallite size and dislocation density. Overall, these concepts are particularly important in analyzing the mechanical properties of materials and X-ray diffraction patterns. The XRD results and Williamson-Hall analysis show that the milling process, especially in conventional milling, has significant effects on the crystalline structure of titanium. These changes can be applied in designing and producing medical implants, particularly for dental and knee applications, to provide patients with longer-lasting and safer performance.
Findings
XRD and Williamson-Hall Analysis
The XRD results indicated that the peak intensities at 35° and 40° had significantly decreased in the titanium samples machined using conventional and climb milling methods (Table 1; Figure 2).
Table 1. Results extracted from the XRD test and Williamson-Hall analysis


Figure 2. XRD chart of the samples at different stages
Surface roughness
The sample machined with conventional milling (S-a: 2.709μm) had a smoother surface than the one machined with climb milling (S-b: 7.129μm).
Hardness testing
The Vickers hardness results, which were recognized as an indicator of a material's resistance to deformation, indicated that the hardness of titanium increased to 315HV during conventional milling and 334HV during climb milling. In comparison, the hardness of pure titanium was measured at 292HV.
Weight loss after wear testing
Pure Ti exhibited a weight loss of 18mg after wear testing, with 11 and 10mg reductions observed for the conventional and climb milling methods, respectively (Figure 3).
Corrosion rate
The corrosion rate of titanium samples machined using the climb milling method (0.0003mm/year) was significantly lower than that of samples machined using the conventional milling method (0.02mm/year). Climb milling resulted in a smoother surface with fewer cracks, which can substantially increase corrosion resistance. Pure Ti, with a corrosion rate of 0.07mm/year, achieved significantly lower rates after milling by both methods, especially in the case of conventional milling, where corrosion was reduced to 0.0003mm/year (Table 2; Figures 4 & 5).

Figure 3. Weight loss due to wear
Table 2. Results of the corrosion test


Figure 4. The corrosion test results

Figure 5. The OCP corrosion test results
Discussion
The reduction in peak intensity is attributed to structural changes in the titanium. The 40° peak, known as the indicator of pure titanium's crystalline structure, is reduced in conventional and climb milling. This may be due to changes in internal stresses and crystallite size reduction after milling. Compared to climb milling, the more pronounced reduction in peak intensity observed in conventional milling is likely due to deeper structural changes, cutting force, and more severe plastic deformation in this method. This phenomenon is particularly important in medical industries, such as the production of dental and knee implants, because the reduction in crystallinity can directly impact the mechanical properties and durability of implants. Changes in the peak positions, which result from accumulated stresses and atomic displacements in the structure, can affect titanium's mechanical properties and biocompatibility. These alterations are significant for dental and knee implant applications, as they can influence the mechanical compatibility of the implant with the surrounding bone and improve its ability to withstand applied forces over time. This peak, which is absent in the control sample, indicates the formation of a titanium oxide layer after milling. The formation of this oxide, particularly in implants, can enhance biocompatibility, as titanium oxide is known to interact well with the body and promote better growth of biological tissues. The difference in the titanium oxide peak height between conventional and climb milling also indicates greater oxide formation in conventional milling, which may be due to more intense thermal and mechanical changes. The crystallite size in climb milling is 41nm, while in conventional milling, it is 20nm, indicating a significant reduction in grain size. Reducing crystallite size can enhance mechanical properties such as hardness and wear resistance but may also affect biocompatibility. On the other hand, the recorded micro strains show that climb milling can lead to greater internal strains, which may improve implants' mechanical properties and fracture resistance.
The difference in surface roughness is primarily due to the direction of tool movement in both milling methods. In conventional milling, the tool moves in alignment with the cutting direction of the workpiece, resulting in a more uniform surface and reduced roughness. This approach minimizes shear forces and enhances surface quality. In conventional milling (also known as down milling), the direction of the tool's movement is aligned with the workpiece's movement. This alignment reduces cutting forces and vibrations, leading to a smoother surface with lower roughness. The reduction in surface roughness is due to more uniform and stable cutting in this method. In contrast, in climb milling (or up milling), the direction of the tool's movement is opposite to that of the workpiece, which increases cutting forces and vibrations. This condition results in a rougher surface with higher roughness. The increase in surface roughness is caused by instability in the cutting process and the formation of deeper scratches. When assessing the surface behavior of pure titanium using both milling methods, the surface roughness results are significantly influenced by the machining techniques. The conventional milling method yielded a surface roughness of 2.7 microns, indicating a smoother and more uniform surface. This characteristic is particularly important in dental applications, as dental implants require a low roughness surface to facilitate better interaction with the mouth's soft tissues and prevent bacterial plaque accumulation. A smoother surface increases the success of implant integration with the jawbone. It reduces the risk of bacterial infections, a crucial factor in extending the lifespan of dental implants. On the other hand, the surface roughness of the samples produced by climb milling was measured at 7.12 microns, indicating a rougher and more uneven surface. This surface type is advantageous for knee implants subjected to heavy and continuous loads. Higher roughness can improve the mechanical bonding of the implant to the bone tissue, which is particularly important in the working conditions of knee joints that require greater strength and wear resistance.
The increase in hardness is attributed to the effects of work hardening and the formation of crystalline discontinuities on the material's surface, which occur due to the shear forces and heat generated during the milling process. In this study, the hardness properties of pure titanium sheets were investigated after machining with both milling methods, and the results demonstrated a significant improvement in surface hardness for both approaches. This increase in hardness has significant implications for biomedical implants, particularly in dental and knee applications. In dental implants, a harder surface enhances wear resistance and reduces deformation, thereby improving the longevity of the implant under chewing forces. The hardness enhancement in machined samples, particularly in the conventional milling method, can improve knee implant performance and reduce the risk of wear or premature failure. This improvement in mechanical properties guarantees the durability and performance of knee implants and enhances patients' confidence in the efficacy and longevity of internal implants in complex physical conditions. Whenever the strength and hardness of an alloy increase, its ductility tends to decrease. Low hardness can transfer uncoordinated stresses during mechanical movements, resulting in localized stress shielding from osteoporosis. Furthermore, the increase in hardness indicates enhanced wear resistance. Higher hardness can extend the lifespan of components and equipment while reducing maintenance costs, which is crucial for improving efficiency and safety in these industries.
The significant reduction in wear, especially with climb milling, indicates an increase in the surface resistance of titanium to wear. The difference in performance may be related to the distinct surface structures generated by the two milling methods. The surface of titanium machined using the conventional method is likely more resilient to wear and erosion due to the formation of protective layers and a rougher structure, whereas the surface of titanium machined with the climbing method may experience wear sooner due to reduced protective layers and a less porous surface. As is evident, bones are the most crucial and abundant structures, serving as the primary load-bearing components of the human body, making bone health paramount. When positioned as joints within the body, implants are subject to wear and potential failure. As load-bearing biomaterials, wear is an inevitable consequence of bodily movement. Moreover, prolonged wear of implants in various joint regions can release numerous metallic particles from the implant surfaces, triggering foreign body reactions with surrounding tissues. Broken surfaces of implants may interact with their environment, leading to serious complications for the body. Due to increased wear, these released metallic particles can deposit in organs distant from the implants, potentially causing various adverse reactions and damage, including toxic effects, inflammatory responses, tissue damage, and disruption of organ function. Particles released as a result of wear hinder bone regeneration processes, including survival, proliferation, and adhesion, while also making the damaged surface susceptible to cracking. This susceptibility allows bodily fluids, including blood, serum, interstitial fluids, and lymph, to invade easily, increasing the likelihood of corrosion and infection. In summary, the high wear resistance of implants ensures their safe and stable use in the human body. Improved wear resistance is particularly crucial for knee implants, which endure heavy and continuous physical pressures. In such conditions, any reduction in wear can greatly enhance the durability and stability of the implant. The results showed that conventional milling, with a weight loss of 11mg during wear testing, demonstrated excellent performance for knee working conditions, as high wear resistance minimizes the likelihood of surface degradation or loosening of the implant.
Various factors influence corrosion, including alloy composition, microstructure, and grain size. In biomedical titanium alloys, corrosion occurs in contact with body fluids such as blood, serum, interstitial fluids, and lymph, primarily due to corrosive environments like chloride ions and proteins [29]. Although titanium alloys can naturally form a stable passive layer on their surfaces, providing excellent corrosion resistance, the complexity of the human body environment can lead to the detachment and dissolution of this surface passive layer. This process is exacerbated by the combined effects of external forces and human body fluids, including blood, serum, interstitial fluids, and lymph, which can potentially result in the release of harmful substances or waste materials that may be toxic and cause inflammation or lead to blood clotting in the body. Non-harmful waste materials are suspended in blood, posing no harm to human health and being naturally excreted [30]. Considering the prolonged implantation time in the human body, implantable materials must demonstrate sufficient corrosion resistance to ensure long-term biocompatibility while avoiding any potential interference that may arise from accumulated corrosion products or the release of metal ions. Corrosion resistance is a fundamental property of biomedical materials. Unfortunately, the human body constitutes an electrolyte environment containing plasma, blood serum, chloride ions, lymphatic fluid, amino acids, and proteins. Research findings indicate that metal implants exhibit gradual electrochemical reactions in the human body. Compared to non-passivated metals with high corrosion resistance, biodegradable metals have attracted considerable attention due to their gradual absorption and consumption in biological tissues [30]. Reduced corrosion is critical as implants are continuously exposed to an acidic and moist oral environment. A lower corrosion rate enhances the resistance of dental implants to wear from saliva and food, thereby increasing their longevity and reducing the risk of infection or adverse reactions. Conversely, knee implants require high corrosion resistance due to their exposure to joints' complex and high-pressure environments. The significant improvement in corrosion resistance observed in conventional milling samples suggests that this method can play a key role in increasing the durability of knee implants. This improvement not only ensures resistance against the corrosive environments of the body but also prevents premature degradation or fracture of the implant, thereby reducing the likelihood of replacement surgeries. Combining these results with enhancements in mechanical properties under various working conditions guarantees the long-term efficacy of both dental and knee implants. In analyzing titanium's corrosion behavior and the milling process's impact on its properties, the corrosion potential plays a key role in determining the material's susceptibility to corrosion. A negative corrosion potential indicates a greater tendency for oxidation and electron loss. Here, the corrosion potential of the conventional milling sample is lower than that of pure titanium, signifying enhanced corrosion resistance. In contrast, the corrosion potential in climb milling is lower and more negative, indicating an increased susceptibility to corrosion. This aspect is particularly relevant in the medical design of dental and knee implants, as higher corrosion resistance leads to longer implant life and reduced release of harmful ions into the body. Corrosion current density is another crucial parameter that indicates the amount of electric current generated due to the corrosion process and is directly related to the rate of material degradation in a corrosive environment. The higher the current density, the greater the corrosion. In this case, the corrosion current density in the conventional milling sample is approximately similar to that of pure titanium. Still, the current density in climb milling is significantly higher, indicating more severe corrosion. This discrepancy suggests that climb milling may produce a surface that is more prone to corrosion, which is undesirable for implants, as higher degradation rates result in greater titanium ion release and negatively affect biocompatibility. The cathodic and anodic slopes are important parameters in determining the electrochemical behavior of materials during the corrosion process. Notably, the reduction in the anodic slope suggests that the formation of the oxide layer on the surface of conventional milled titanium occurs more extensively and rapidly, which can enhance biocompatibility and corrosion resistance in dental and knee implants. From a mechanical perspective, the reduction in the cathodic and anodic slopes indicates a greater tendency to form protective oxide layers, improving surface hardness and wear resistance. This could directly impact the lifespan of metallic implants, as a harder and more corrosion-resistant surface implies better resistance to both mechanical and chemical wear. The open circuit potential (OCP) diagram is crucial in studying the corrosion behavior of metallic materials, especially in biological environments where implants come into contact with body fluids. This diagram reflects the metal surface's ability to maintain its electrochemical potential without external current. The results indicate that pure titanium exhibits greater stability than milled samples. This suggests that pure titanium demonstrates higher resistance to environmental fluctuations and prevents corrosion by forming a protective layer on its surface. In a medical context, this greater stability translates to better biocompatibility, as implants with higher OCP stability are less prone to releasing metal ions into the body, thus posing a lower risk to patient health. Mechanically, the broader range recorded for the conventional milling sample indicates that this sample has lower electrochemical stability due to structural changes and stresses caused by the milling process. This could lead to a higher corrosion rate and reduced mechanical longevity of the implant. Moreover, reduced stability in milled samples may result from creating surface defects and an increased surface area exposed to the corrosive environment. Consequently, selecting the appropriate milling method and controlling process conditions can play a crucial role in enhancing both mechanical resistance and the longevity of dental and knee implants.
Pure titanium is widely used in the production of medical implants due to its excellent mechanical properties, superior biocompatibility, and high corrosion resistance. However, machining this material presents significant challenges. The heat generated in the cutting zone rapidly increases at higher machining speeds or greater cutting depths. This rise in temperature can lead to undesirable changes in the material's microstructure, such as localized annealing and a reduction in surface hardness. Additionally, titanium tends to adhere to cutting tools, a phenomenon known as "built-up edge", which can cause deep scratches and increase surface roughness. These factors collectively limit the use of higher machining speeds and depths, as they can negatively impact the implant's surface quality and mechanical properties. The results of this study demonstrate that improving surface hardness and reducing surface roughness in titanium machining directly influence the performance of medical implants. Increased surface hardness enhances wear resistance and prevents implant deformation under mechanical loads, such as those experienced by artificial joints. On the other hand, reduced surface roughness minimizes friction between the implant and surrounding tissues, promoting better bone cell adhesion and accelerating the osseointegration process. Furthermore, the higher corrosion resistance observed in this study is particularly significant, as medical implants are exposed to corrosive biological environments. These combined properties extend the implant's lifespan and reduce the risk of inflammation or adverse reactions. For instance, conventional milling at moderate speeds and controlled cutting depths induces beneficial compressive stresses on the surface and prevents excessive heating and structural degradation. These conditions help maintain surface hardness and reduce roughness, both of which are crucial for the performance of medical implants. Additionally, precise control of machining parameters can minimize the generation of abrasive particles, preventing the introduction of metallic debris into the body and subsequent inflammation. As a result, optimizing these parameters enhances implant quality and ensures their safety and efficacy in medical applications.
The importance of selecting the appropriate machining method for titanium in medical applications, particularly for dental and knee implants, is increasing due to its impact on the material's mechanical properties, corrosion resistance, and biocompatibility. Changes induced by different machining processes can significantly affect the performance of these implants in the human body. This study examined the effects of conventional and climb milling on pure titanium sheets' mechanical and corrosion properties to draw effective conclusions for selecting the best method for specific applications, especially in the medical field.
The Williamson-Hall analysis provides crucial insights into structural changes induced by the milling process, particularly through crystallite size and microstrain measurements [31]. According to the results of this study, the shift of the highest peak from 40° to 38° in both milling methods may indicate changes in the titanium crystal lattice structure, possibly due to stress variations or defect formation caused by milling [32]. Additionally, the emergence of a new peak at 62.5°, attributed to titanium oxide, is a significant observation [33]. In XRD patterns, each peak corresponds to a specific set of crystal planes, which can broaden due to stresses and defects (microstrain), meaning that increased stresses lead to peak broadening [34, 35]. Dislocation density, which represents the number of dislocations per unit volume of material [26], significantly influences the mechanical properties of metals, as dislocations act as weak points within the crystal structure [27]. According to the findings of this study, variations in crystallite size, internal stresses, and titanium oxide formation collectively enhance this material's mechanical properties and biocompatibility [36, 37]. Furthermore, the smoother surface achieved in conventional milling reduces friction and improves bone cell adhesion, which is critical for the success of medical implants [38]. In contrast, rougher surfaces promote new bone growth at the implant site, ensuring long-term integrity and durability [39]. This study's results show that dental implants, which experience small but continuous stresses, require hard and biocompatible surfaces to ensure mechanical durability and optimal interaction with surrounding tissues [40]. Conversely, knee implants, subjected to more demanding working conditions and heavier loads, require greater hardness to withstand continuous compressive forces and friction [41-43]. Additionally, corrosion analysis demonstrated that smoother and more homogeneous surfaces resist corrosive agents due to fewer weak points and microscopic cracks [44, 45]. According to this study, the cathodic slope, which describes the rate of reduction reactions at the cathode, and the anodic slope, which represents the oxidation kinetics of the material, are lower in conventionally milled titanium than in pure titanium. This indicates reduced electrochemical reaction rates, suggesting improved corrosion resistance and enhanced protective oxide layer formation [46, 47]. Finally, due to titanium’s sensitivity to high temperatures, optimizing machining parameters such as cutting speed, depth of cut, and machining strategy plays a crucial role in achieving the desired properties for medical implants [48-50].
These findings suggest that selecting the appropriate milling method can optimize the performance of titanium implants according to their specific application.
Conclusion
Conventional and climb milling methods impact pure titanium sheets' mechanical and corrosion properties, influencing their performance in dental and knee implants.
Acknowledgments: We would like to express our sincere gratitude to the esteemed nurses, Ms. Salimeh Sadat Sedehi and Ms. Sahar Sadat Sedehi, for their invaluable contributions during this research, particularly in patient care. Their unwavering support and presence during many challenging moments have been our research team's immense motivation and hope. Undoubtedly, their role in the advancement of this project and the enhancement of its results is undeniable.
Ethical Permissions: Not needed.
Conflicts of Interests: The authors confirm that no financial interests or personal relationships could have influenced the outcomes of the study presented in this paper.
Authors' Contribution: Sedehi SMR (First Author), Introduction Writer/Methodologist/Original Researcher/Discussion Writer (55%); Norouzi Palangani F (Second Author), Introduction Writer/Methodologist/Assistant Researcher (15%); Maleki Z (Third Author), Assistant Researcher/Discussion Writer/Statistical Analyst (15%); Banihashemi SF (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (15%)
Funding/Support: This research did not receive any financial support.
Keywords:
Titanium [MeSH], Machining [MeSH], Milling [MeSH], Medical Applications [MeSH], Surface Modification [MeSH], Biocompatibility [MeSH]
References
1. Liu GL, Zheng JT, Huang CZ, Sun SF, Liu XF, Dai LJ, et al. Coupling effect of micro-textured tools and cooling conditions on the turning performance of aluminum alloy 6061. Adv Manuf. 2023;11(4):663-81. [Link] [DOI:10.1007/s40436-022-00432-y]
2. Zhang S, Shi H, Wang B, Ma C, Li Q. Research on the milling performance of micro-groove ball end mills for titanium alloys. Lubricants. 2024;12(6):204. [Link] [DOI:10.3390/lubricants12060204]
3. Hong SY, Markus I, Jeong WC. New cooling approach and tool life improvement in cryogenic machining of titanium alloy Ti-6Al-4V. Int J Mach Tools Manuf. 2001;41(15):2245-60. [Link] [DOI:10.1016/S0890-6955(01)00041-4]
4. Kato H. Milling of medical titanium alloy. J Jpn Soc Precis Eng. 2021;87(3):275-8. [Japanese] [Link] [DOI:10.2493/jjspe.87.275]
5. Outeiro J, Cheng W, Chinesta F, Ammar A. Modelling and optimization of machining of Ti-6Al-4V titanium alloy using machine learning and design of experiments methods. J Manuf Mater Process. 2022;6(3):58. [Link] [DOI:10.3390/jmmp6030058]
6. Jiang G, Yang H, Xiao G, Zhao Z, Wu Y. Titanium alloys surface integrity of belt grinding considering different machining trajectory direction. Front Mater. 2022;9:1052523. [Link] [DOI:10.3389/fmats.2022.1052523]
7. Shokrani A, Al-Samarrai I, Newman ST. Hybrid cryogenic MQL for improving tool life in machining of Ti-6Al-4V titanium alloy. J Manuf Process. 2019;43:229-43. [Link] [DOI:10.1016/j.jmapro.2019.05.006]
8. Narita H. Cutting features between surface roughness in feed direction and machining state of radius end mill against inclined surfaces (in case of contour machining and five-axis machining with constant tilt angle). Int J Autom Technol. 2020;14(1):46-51. [Japanese] [Link] [DOI:10.20965/ijat.2020.p0046]
9. Yujiang L, Tao C. Research on cutting performance in high-speed milling of TC11 titanium alloy using self-propelled rotary milling cutters. Int J Adv Manuf Technol. 2021;116:2125-35. [Link] [DOI:10.1007/s00170-021-07592-4]
10. Kumar MK, Gurudatt B, Reddappa HN, Suresh R. Investigations on the effect of machining parameters on machining force and roughness in micro-milling of titanium Gr5 and Gr12 alloys under dry machining conditions using carbide tool. Mater Today Proc. 2021;47(10):2598-602. [Link] [DOI:10.1016/j.matpr.2021.05.082]
11. Danesh M, Rahimi A. Effect of cutting tool vibration and tool wear on the surface topography of workpiece while machining Ti6Al4V Titanium alloy using laser profilometry. Iran J Manuf Eng. 2020;7(10):34-45. [Persian] [Link]
12. Festas A, Ramos A, Davim JP. Machining of titanium alloys for medical application-a review. Proc Inst Mech Eng Part B J Eng Manuf. 2022;236(4):309-18. [Link] [DOI:10.1177/09544054211028531]
13. Brown M, M'saoubi R, Crawforth P, Mantle A, McGourlay J, Ghadbeigi H. On deformation characterization of machined surfaces and machining-induced white layers in a milled titanium alloy. J Mater Process Technol. 2022;299:117378. [Link] [DOI:10.1016/j.jmatprotec.2021.117378]
14. Daniyan IA, Tlhabadira I, Mpofu K, Muvunzi R. Numerical and experimental analysis of surface roughness during the milling operation of titanium alloy Ti6Al4V. Int J Mech Eng Robotics Res. 2021;10(12):683-93. [Link] [DOI:10.18178/ijmerr.10.12.683-693]
15. Zhu WL, Beaucamp A. Compliant grinding and polishing: A review. Int J Mach Tools Manuf. 2020;158:103634. [Link] [DOI:10.1016/j.ijmachtools.2020.103634]
16. Li C, Hu Y, Zhang F, Geng Y, Meng B. Molecular dynamics simulation of laser-assisted grinding of GaN crystals. Int J Mech Sci. 2023;239:107856. [Link] [DOI:10.1016/j.ijmecsci.2022.107856]
17. Sabarinathan P, Annamalai VE, Xavier Kennedy A. On the use of grains recovered from spent vitrified wheels in resinoid applications. J Mater Cycles Waste Manag. 2020;22(1):197-206. [Link] [DOI:10.1007/s10163-019-00927-0]
18. Li L, Ren X, Feng H, Chen H, Chen X. A novel material removal rate model based on single grain force for robotic belt grinding. J Manuf Process. 2021;68(Pt A):1-12. [Link] [DOI:10.1016/j.jmapro.2021.05.029]
19. Palaniyappan S, Veiravan A, Kumar V, Mathusoothanaperumal Sukanya N, Veeman D. Process optimization and removal of phenol formaldehyde resin coating using mechanical erosion process. Prog Rubber Plast Recycl Technol. 2022;38(2):141-54. [Link] [DOI:10.1177/14777606211066316]
20. Davis R, Singh A, Jackson MJ, Coelho RT, Prakash D, Charalambous CP, et al. A comprehensive review on metallic implant biomaterials and their subtractive manufacturing. Int J Adv Manuf Technol. 2022;120(3-4):1473-530. [Link] [DOI:10.1007/s00170-022-08770-8]
21. Nasiri S, Rabiei M, Palevicius A, Janusas G, Vilkauskas A, Nutalapati V, et al. Modified scherrer equation to calculate crystal size by XRD with high accuracy, examples Fe2O3, TiO2 and V2O5. Nano Trends. 2023;3:100015. [Link] [DOI:10.1016/j.nwnano.2023.100015]
22. Holzwarth U, Gibson N. The Scherrer equation versus the 'Debye-Scherrer equation'. Nat Nanotechnol. 2011;6(9):534. [Link] [DOI:10.1038/nnano.2011.145]
23. Chérif I, Dkhil YO, Smaoui S, Elhadef K, Ferhi M, Ammar S. X-ray diffraction analysis by modified Scherrer, Williamson-Hall and size-strain plot methods of ZnO nanocrystals synthesized by oxalate route: A potential antimicrobial candidate against foodborne pathogens. J Clust Sci. 2023;34(1):623-38. [Link] [DOI:10.1007/s10876-022-02248-z]
24. Aftab M, Aftab A, Butt MZ, Ali D, Bashir F, Iqbal SS. Surface hardness of pristine and laser-treated zinc as a function of indentation load and its correlation with crystallite size valued by Williamson-Hall analysis, size-strain plot, Halder-Wagner and Wagner-Aqua models. Mater Chem Phys. 2023;295:127117. [Link] [DOI:10.1016/j.matchemphys.2022.127117]
25. Nath D, Singh F, Das R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles-a comparative study. Mater Chem Phys. 2020;239:122021. [Link] [DOI:10.1016/j.matchemphys.2019.122021]
26. Mendez-Lozano N, Apátiga-Castro M, Soto KM, Manzano-Ramírez A, Zamora-Antuñano M, Gonzalez-Gutierrez C. Effect of temperature on crystallite size of hydroxyapatite powders obtained by wet precipitation process. J Saudi Chem Soc. 2022;26(4):101513. [Link] [DOI:10.1016/j.jscs.2022.101513]
27. Hossain MS, Hasan MM, Mahmud M, Mobarak MB, Ahmed S. Assessment of crystallite size of UV-synthesized hydroxyapatite using different model equations. Chem Pap. 2023;77(1):463-71. [Link] [DOI:10.1007/s11696-022-02501-9]
28. Kumar U, Padalia D, Kumar P, Bhandari P. Estimation of lattice strain and structural study of BaTiO3/PS polymer composite using X-ray peak profile analysis. J Nanoparticle Res. 2023;25(6):124. [Link] [DOI:10.1007/s11051-023-05779-2]
29. Bavil AY, Eghan-Acquah E, Dastgerdi AK, Diamond LE, Barrett R, Walsh HP, et al. Simulated effects of surgical corrections on bone-implant micromotion and implant stresses in paediatric proximal femoral osteotomy. Comput Biol Med. 2025;185:109544. [Link] [DOI:10.1016/j.compbiomed.2024.109544]
30. Ali A, Polepalli L, Chowdhury S, Carr MA, Janorkar AV, Marquart ME, et al. Silver-doped titanium oxide layers for improved photocatalytic activity and antibacterial properties of titanium implants. J Funct Biomater. 2024;15(6):163. [Link] [DOI:10.3390/jfb15060163]
31. Himabindu B, Latha Devi NSMP, Sandhya G, Naveen Reddy T, Saha T, Rajini Kanth B, et al. Structure-based photocatalytic efficiency and optical properties of ZnO nanoparticles modified by annealing including Williamson-Hall microstructural investigation. Mater Sci Eng B. 2023;296:116666. [Link] [DOI:10.1016/j.mseb.2023.116666]
32. Cui YW, Wang L, Zhang LC. Towards load-bearing biomedical titanium-based alloys: From essential requirements to future developments. Prog Mater Sci. 2024;144:101277. [Link] [DOI:10.1016/j.pmatsci.2024.101277]
33. Alam MK, Hossain MS, Bahadur NM, Ahmed S. A comparative study in estimating of crystallite sizes of synthesized and natural hydroxyapatites using Scherrer Method, Williamson-Hall model, Size-Strain Plot and Halder-Wagner Method. J Mol Struct. 2024;1306:137820. [Link] [DOI:10.1016/j.molstruc.2024.137820]
34. Sedehi SM, Maraki MR, Houshyar Eftekhari SD, Fazeli M, Maleki Z, Norouzi Palangani F. Experimental investigation of the effect of reduced graphene oxide addition on the mechanical properties and behavior of Ti/RGO composites in spark plasma sintering process with reference to potential applications in medical implants. Adv Ceram Prog. 2023;9(4):22-31. [Link]
35. Scherrer PJ. Estimation of the size and internal structure of colloidal particles by means of Röntgen. NACHRICHTEN VON DER GESELLSCHAFT DER WISSENSCHAFTEN ZU GÖTTINGEN. 1918;1918:98-100. [German] [Link]
36. Manh DH, Nha TTN, Phong LTH, Nam PH, Thanh TD, Phong PT. Determination of the crystalline size of hexagonal La1-xSrxMnO3 (x=0.3) nanoparticles from X-ray diffraction-a comparative study. RSC Adv. 2023;13(36):25007-17. [Link] [DOI:10.1039/D3RA04018F]
37. Madhavi J. Comparison of average crystallite size by X-ray peak broadening and Williamson-Hall and size-strain plots for VO2+ doped ZnS/CdS composite nanopowder. SN Appl Sci. 2019;1(11):1509. [Link] [DOI:10.1007/s42452-019-1291-9]
38. Ching HA, Choudhury D, Nine MJ, Osman NA. Effects of surface coating on reducing friction and wear of orthopaedic implants. Sci Technol Adv Mater. 2014;15(1):014402. [Link] [DOI:10.1088/1468-6996/15/1/014402]
39. Alla RK, Ginjupalli K, Upadhya N, Shammas M, Ravi RK, Sekhar R. Surface roughness of implants: A review. Trends Biomater Artif Organs. 2011;25(3):112-8. [Link]
40. Soares PB, Nunes SA, Franco SD, Pires RR, Zanetta-Barbosa D, Soares CJ. Measurement of elastic modulus and Vickers hardness of surround bone implant using dynamic microindentation-parameters definition. Braz Dent J. 2014;25(5):385-90. [Link] [DOI:10.1590/0103-6440201300169]
41. Silva AM, Matos JDM, Tribst JPM, Lopes GRS, Martinelli CSM, Borges ALS, et al. Effect of titanium hardness on the integrity and stress concentration of external hexagon dental implants. Int J Odontostomatol. 2021;15(4):1053-9. [Link] [DOI:10.4067/S0718-381X2021000401053]
42. Arnold JS, Bartley MH, Tont SA, Jenkins DP. Skeletal changes in aging and disease. Clin Orthop Relat Res. 1966;49:17-38. [Link] [DOI:10.1097/00003086-196611000-00002]
43. Herbster M, Garke B, Harnisch K, Michael O, Lieb A, Betke U, et al. Effects of Cr addition on Ti implant alloys (Ti-Cr/Ti-Al-V-Cr) to enhance corrosion and wear resistance. J Mech Behav Biomed Mater. 2025;164:106899. [Link] [DOI:10.1016/j.jmbbm.2025.106899]
44. Yang S, Hu Z, Xu J, Sun X, Wang Y. Surface microstructures and corrosion behavior of Zircaloy-4 induced by the composite action of micro electrical discharge machining and high current pulsed electron beam. Vacuum. 2025;234:114050. [Link] [DOI:10.1016/j.vacuum.2025.114050]
45. Yang J, Wang Y, Yang Y, Liu Y, Zhang W. Fabrication of micro holes with confined pitting corrosion by laser and electrochemical machining: Pitting corrosion formation mechanisms and protection method. J Mater Process Technol. 2025;335:118677. [Link] [DOI:10.1016/j.jmatprotec.2024.118677]
46. Goidanich S, Lazzari L, Ormellese M. AC corrosion-Part 1: Effects on overpotentials of anodic and cathodic processes. Corros Sci. 2010;52(2):491-7. [Link] [DOI:10.1016/j.corsci.2009.10.005]
47. Driver R, Meakins RJ. Tafel slopes and chemical structure of inhibitors of the acid corrosion of steel. Br Corros J. 1974;9(4):233-7. [Link] [DOI:10.1179/000705974798321224]
48. Yazar KU, Mishra S, Karmakar A, Bhattacharjee A, Suwas S. On the temperature sensitivity of dwell fatigue of a near alpha titanium alloy: Role of strain hardening and strain rate sensitivity. Metall Mater Trans A. 2020;51(10):5036-42. [Link] [DOI:10.1007/s11661-020-05914-x]
49. Antil P, Kumar Antil S, Prakash C, Krolczyk G, Pruncu C. Multi-objective optimization of drilling parameters for orthopaedic implants. Meas Control. 2020;53(9-10):1902-10. [Link] [DOI:10.1177/0020294020947126]
50. Singh B, Saxena KK, Dagwa IM, Singhal P, Malik V. Optimization of machining characteristics of titanium-based biomaterials: Approach to optimize surface integrity for implants applications. Surf Rev Lett. 2023;1:2340008. [Link] [DOI:10.1142/S0218625X23400085]










