Volume 17, Issue 2 (2025)
Iran J War Public Health 2025, 17(2): 103-112 |
Back to browse issues page
Article Type:
Subject:
Ethics code: INTI-FHLS-03-01-2021
History
Received: 2025/02/26 | Accepted: 2025/04/5 | Published: 2025/04/12
Received: 2025/02/26 | Accepted: 2025/04/5 | Published: 2025/04/12
How to cite this article
Allami R, Mutlag A. Correlation between HMGB1 Expression and Cholecystokinin Levels in Patients with Gallbladder Disorders. Iran J War Public Health 2025; 17 (2) :103-112
URL: http://ijwph.ir/article-1-1558-en.html
URL: http://ijwph.ir/article-1-1558-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
R.A. Allami *1, A.M. Mutlag1
1- Department of Pathological Analyses, College of Sciences, University of Wasit, Al-Kut, Iraq
Full-Text (HTML) (237 Views)
Introduction
Gallbladder disease encompasses a range of disorders, including gallstones (cholelithiasis), inflammation (cholecystitis), polyps, and cancer. These conditions can lead to significant morbidity and may require various treatment approaches, from dietary modifications and medication to surgical interventions such as cholecystectomy. Additionally, the prevalence of gallbladder disease is influenced by several factors, including age, gender, obesity, and dietary habits, making it a complex health issue that requires careful consideration of individual risk factors. Among these, cholelithiasis is the most common condition, characterized by the formation of stones that may obstruct the bile ducts and cause inflammation, pain, or infection [1]. The development of gallbladder disease is influenced by multiple factors, including genetic predisposition, metabolic imbalances, biliary stasis, and chronic inflammation [2]. Epidemiological studies indicate that certain populations, particularly those with obesity and diabetes, are at a higher risk for developing gallstones [3]. The prevalence of gallstones is also significantly higher in women than in men, a disparity attributed to hormonal factors such as estrogen, which may influence cholesterol metabolism in the bile [4]. Additionally, dietary factors, including high-fat and low-fiber diets, have been implicated in the pathogenesis of gallbladder disease, suggesting that lifestyle modifications could play a crucial role in prevention [5].
Understanding the underlying mechanisms and risk factors associated with gallbladder disease is essential for developing effective prevention and treatment strategies, thereby reducing morbidity and healthcare costs associated with this condition [6]. Early diagnosis and intervention are crucial, as untreated gallbladder disease can result in serious complications, such as acute pancreatitis or biliary obstruction. Understanding the pathophysiology and risk factors associated with gallbladder disease is essential for developing effective prevention strategies and optimizing patient management.
High-mobility group box 1 (HMGB1) is a nuclear protein that plays a crucial role in inflammation and the immune response. It functions as a damage-associated molecular pattern (DAMP) molecule, acting as an alarm signal when it is passively released from necrotic cells or actively secreted by immune cells such as macrophages, monocytes, and dendritic cells [7]. Once extracellular, HMGB1 triggers a robust inflammatory response by signaling to adjacent immune cells [8]. It has been implicated in several pathological conditions, including sepsis, tumor metastasis, atherosclerosis, and liver injury [9, 10]. However, its precise role in the pathogenesis of gallbladder disease, particularly cholecystitis, remains unclear [11]. Recent studies suggest that elevated levels of HMGB1 correlate with the severity of inflammation in various conditions, indicating its potential as a biomarker for disease severity [12]. Furthermore, HMGB1’s interaction with the receptor for advanced glycation end products (RAGE) and toll-like receptors (TLRs) highlights its multifaceted role in modulating immune responses. These interactions not only activate pro-inflammatory signaling pathways but also promote the recruitment and activation of immune cells, amplifying the inflammatory response in various pathological conditions. Understanding these intricate signaling mechanisms could provide crucial insights into how HMGB1 contributes to chronic inflammation and tissue damage, opening avenues for the development of targeted therapies aimed at modulating HMGB1 activity in diseases characterized by excessive inflammation. Additionally, this knowledge may assist in identifying patient subgroups that could benefit from HMGB1-targeted interventions, thereby personalizing treatment strategies for conditions such as gallbladder disease and other inflammation-driven disorders [13].
Cholecystokinin (CCK) is a neuro-intestinal peptide hormone predominantly produced by enteroendocrine I-cells in the upper small intestine, with the highest density in the duodenum and proximal jejunum [14]. Its release is stimulated by the ingestion of fat- and protein-rich foods, leading to gallbladder contraction, bile secretion, and pancreatic enzyme release [15]. In addition to its gastrointestinal functions, CCK also plays a significant role in regulating appetite and satiety, as it can signal to the brain to reduce food intake after meals. This dual role highlights CCK as an important mediator of both digestive processes and metabolic control, indicating that disturbances in CCK signaling may contribute to conditions such as obesity and gallbladder disease [16]. Furthermore, research suggests that impaired CCK responses could affect the digestion and absorption of nutrients, potentially leading to malnutrition or further exacerbating gallbladder pathologies.
Understanding the full spectrum of CCK’s actions, including its interactions with other hormones and signaling pathways, is essential for developing comprehensive therapeutic strategies for digestive disorders and related metabolic diseases. Ultimately, exploring the complex relationship between CCK and gallbladder function may pave the way for innovative treatments that enhance digestive health and improve outcomes for patients suffering from gallbladder disease. CCK exerts its effects via two receptor subtypes, including CCK-A, which primarily regulates gallbladder motility and pancreatic secretion, and CCK-B, which influences gastrointestinal and central nervous system functions [17]. Defective gallbladder motility is a key contributor to cholesterol gallstone disease, and patients with cholesterol gallstones often exhibit impaired gallbladder contractility [18]. Research has also demonstrated that abnormal CCK release is associated with metabolic disorders such as obesity and gallstone formation [19].
This study is the first to investigate the association between HMGB1 gene expression and CCK levels in patients with gallbladder disease, both before and after cholecystectomy. By exploring these molecular interactions, this study aimed to improve our understanding of the pathophysiology of gallbladder disease and contribute to better diagnostic and therapeutic strategies. Given that gallbladder diseases, particularly cholecystitis, can lead to significant morbidity and healthcare costs, identifying specific biomarkers such as HMGB1 could facilitate early diagnosis and targeted treatment options. Additionally, understanding the relationship between HMGB1 and CCK levels may reveal novel insights into the regulatory mechanisms underlying gallbladder function and inflammation, potentially guiding the development of new pharmacological interventions. Moreover, this research could lay the groundwork for future studies aimed at validating HMGB1 as a therapeutic target, thereby enhancing the management of gallbladder disease and improving patient outcomes. Ultimately, the findings from this study may not only advance our scientific understanding but also translate into clinical applications that could alleviate the burden of gallbladder disease on patients and healthcare systems alike.
Materials and Methods
Subjects
This study included 60 patients with gallbladder diseases (40 patients with cholecystitis and cholelithiasis (20 pre-surgery patients and 20 post-surgery patients) and 20 healthy controls), aged 20-79 years, and was conducted at the College of Science, Wasit University, Iraq. Patient samples were collected from Al Karama Teaching Hospital and Al Zahraa Hospital between October 2023 and July 2024. Exclusion Criteria were patients with Helicobacter pylori infection and those with obesity were excluded to prevent confounding effects on HMGB1 expression and cholecystokinin levels.
EIESA kit assay
Human cholecystokinin ELISA kit
The procedure for this kit was conducted according to the guidelines provided by the manufacturer in China (SunLong Biotech; catalog number: QS2102Hu).
Reagent preparation
Before using any reagent or standard for reconstitution, we ensured that the temperature was between 20 and 25°C. A total of 300µL of the standard (12.8ng/mL) was added to 130µL of standard diluent to generate a 180.0pg/mL standard stock solution. The standard was allowed to sit for 15 minutes with gentle agitation before dilution. Standard points were prepared by serial diluting the standard stock solution (180.0pg/mL) 1:2 with standard diluent to produce 120.0pg/mL, 60.0pg/mL, 30.0pg/mL, and 15.0pg/mL solutions. Any remaining solution was frozen at -20°C and used within one month. For wash buffer preparation, we diluted 20mL of wash buffer concentrate 25x into deionized or distilled water to yield 500mL of 1x wash buffer.
Molecular assay
Primer design
The primer design was conducted using Primer 3 web version 4.1.0 (available at http://primer3.ut.ee) for HMGB1 and GAPDH genes, and the design was checked by the University Code of Student Conduct (UCSC) programs (Table 1). The primers were synthesized and lyophilized by Alpha DNA Ltd. (Canada).
Table 1. Primer sequences used in the study
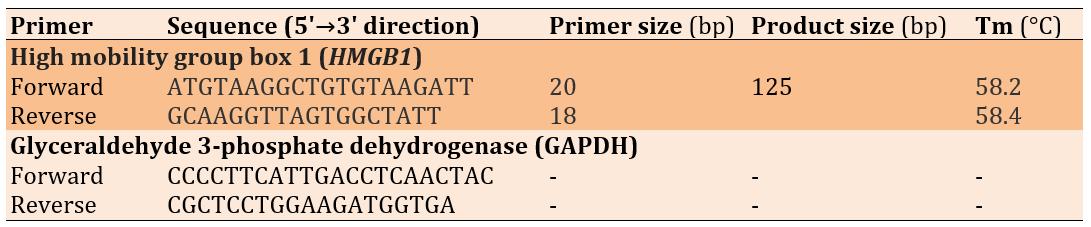
Primer Preparation
For each assay, the required primers were prepared by dissolving the lyophilized sample in nuclease-free water according to the manufacturer’s instructions. A stock solution with a concentration of 100µM was prepared and stored at -20°C. Diluting 10µL of each primer stock solution in 90µL of nuclease-free water yielded a working solution with a concentration of 10µM, which was maintained at -20°C until use.
Total RNA extraction
Total RNA was extracted from all samples using TRIzol® LS Reagent according to the manufacturer’s protocol. Briefly, 250µL of blood was added to 750µL of TRIzol® LS Reagent in an Eppendorf tube and homogenized by vortexing. Then, 200µL of chloroform was added, and the mixture was shaken vigorously for 15 seconds before incubation on ice for 5 minutes. Following centrifugation at 12,000rpm for 10 minutes at 4°C, the mixture separated into three phases, with RNA retained in the aqueous phase. The aqueous phase was carefully transferred to a new 1.5mL tube, and an equal volume of isopropyl alcohol was added. After gentle inversion, the mixture was incubated at -20°C for 10 minutes and then centrifuged at 12,000rpm for 10 minutes at 4°C. The supernatant was removed, and the pellet was washed with 80% ethanol, vortexed, and centrifuged again at 12,000rpm for 5 minutes at 4°C. The supernatant was discarded, and the RNA pellet was dried using hot air. Finally, the RNA was dissolved in RNase-free water and incubated at 60°C for 10 minutes before being stored at freezing temperatures until further use.
Estimation of RNA purity and concentration
The NanoVue Nanodrop spectrophotometer (England) was used to evaluate the concentration and purity of the extracted RNA in order to determine the quality of samples for subsequent analysis in Reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The samples ranged in RNA concentration from 73 to 147ng/μL, while the absorbance of the samples was measured at two distinct wavelengths to determine RNA purity (260 and 280nm). The presence of an A260/A280 ratio of approximately 2.0 suggested that the RNA sample was pure.
cDNA synthesis of mRNA
Total RNA was reverse transcribed to complementary DNA (cDNA) using a cDNA kit from Addbio Company, Korea. The procedure was performed in a reaction volume of 25μL according to the manufacturer’s instructions. Three main steps were required for the preparation of the conversion reaction. Reverse transcription reactions should be assembled in an RNase-free environment. The use of clean pipettes and filter tips is recommended. The RNA templates and all reagents were thawed on ice, and each solution was gently mixed. The reaction components were then mixed.
Quantitative real-time PCR
The expression levels of HMGB1 genes were estimated by qRT-PCR. To confirm the expression of the target gene, TransStart® Top Green qPCR Super Mix (SYBR Green) was used. Primer sequences for these genes were synthesized by Alpha DNA Ltd. (Canada) and stored lyophilized at -20°C. The mRNA levels of the endogenous control Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene were used as an internal control to normalize the mRNA levels of the target genes [20].
Statistical analysis
Data were analyzed using SPSS 26 and Microsoft Excel 2010. Normality was assessed using the Kolmogorov-Smirnov test, and appropriate statistical tests (t-test, ANOVA, chi-square, receiver operating characteristic (ROC) analysis, Pearson’s correlation) were used based on the data type. The significance level was set at p<0.05, with p<0.01 indicating high significance. Results were reported with relevant statistics, including area under the curve (AUC), sensitivity, specificity, and correlation coefficients.
Findings
Cholecystokinin levels in gallbladder disease and their association with disease severity
Serum CCK analysis using one-way ANOVA revealed significantly elevated pre-surgery levels in pre-surgery patients (74.53±7.63; range: 6.69-123.60) compared to both post-surgery patients (48.37±11.61; range: 6.73-116.46) and healthy controls (37.81±10.89; range: 6.68-115.79; p=0.007). However, CCK levels in the post-surgery group were not significantly different from those of the controls (p>0.05).
Regarding disease severity, no significant difference was found in mean CCK levels across severity groups (acute cholecystitis: 91.20±14.10, chronic cholecystitis: 66.29±12.10, hyperplasia: 62.71±16.20), although levels remained elevated in gallbladder disease cases (0.374).
Diagnostic accuracy of Cholecystokinin
ROC analysis was performed to reveal the diagnostic accuracy of using CCK concentrations to distinguish patients before surgery from healthy control subjects. An optimal CCK cut-off value greater than 16.75 resulted in an AUC value of 0.717 (95% confidence interval [CI], 0.574-0.859, p=0.002), with a sensitivity of 72.5%, specificity of 70.0%, positive predictive value (PPV) of 82.9%, and negative predictive value (NPV) of 56.0%. The present results indicate that CCK is considered an acceptable diagnostic marker to distinguish patients before surgery from healthy controls (Table 2 and Figure 1).
Table 2. Receiver operating characteristic curve for cholecystokinin (CCK) levels
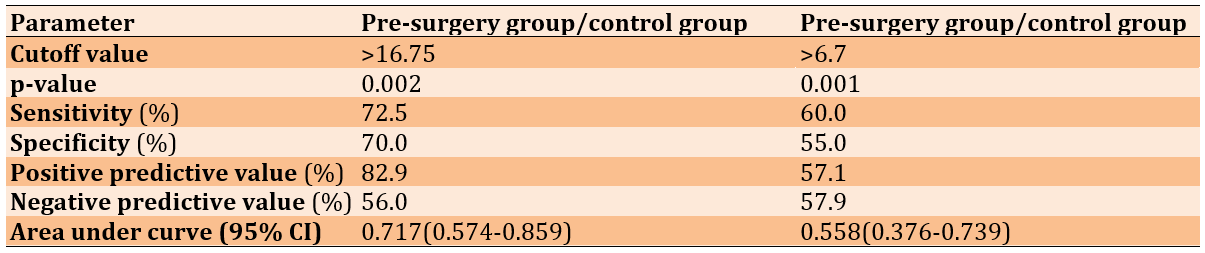
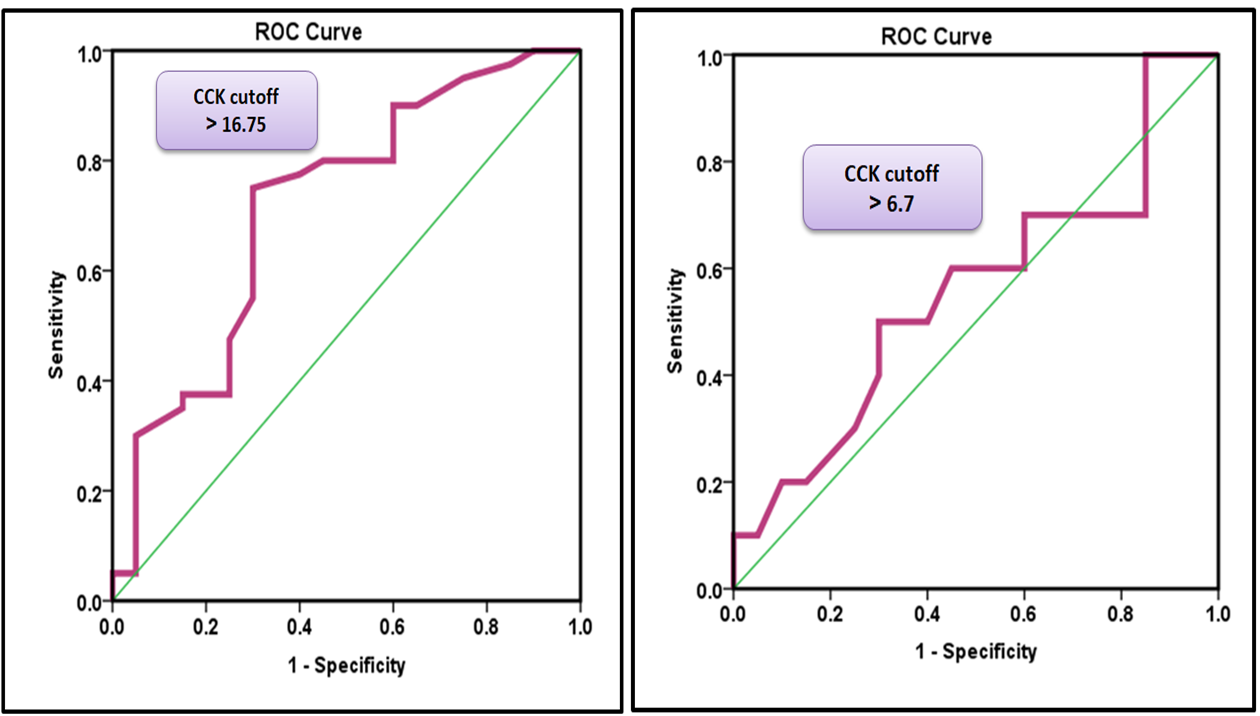
Figure 1. A) Receiver operating characteristic curve for CCK levels to distinguish patients before surgery from healthy control subjects. B) Receiver operating characteristic curve for CCK levels to distinguish patients after surgery from healthy control subjects.
Role of HMGB1 in gallbladder disease and its association with disease severity
HMGB1 gene expression was markedly elevated in patients with gallbladder disease before surgery compared to postoperative patients and healthy individuals, indicating an active inflammatory response. Following cholecystectomy, HMGB1 expression significantly declined, suggesting an attenuation of inflammation and the restoration of normal physiological levels. The mean HMGB1 gene expression levels were 4.91±0.51 in preoperative patients, 1.95±0.24 in postoperative patients, and 1.00±0.12 in the healthy control group, with significant differences observed between all groups (one-way ANOVA; p=0.001). Additionally, a significant difference (p<0.05) was observed between preoperative and postoperative patients (Figure 2).
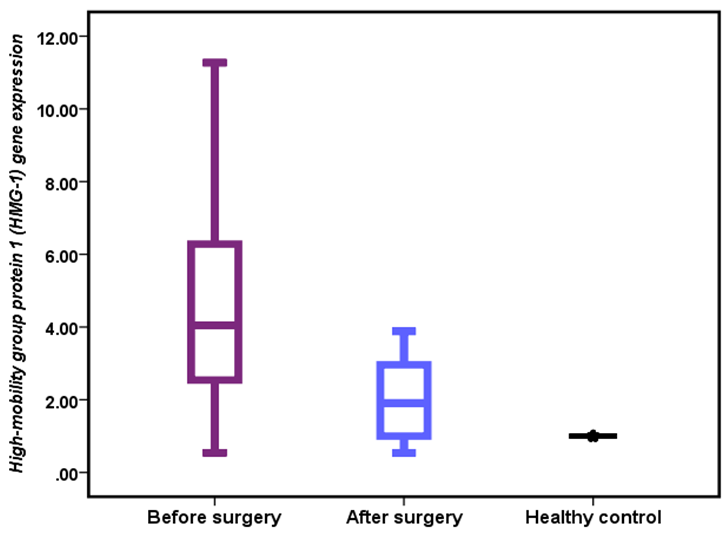
Figure 2. HMGB1 gene expression in patients and healthy controls.
There was a significant increase in HMGB1 expression in patients with hyperplasia compared to other groups (6.87±0.96 vs. 4.91±0.71 and 3.77±0.67, p=0.035).
Diagnostic accuracy of HMGB1 gene expression
ROC analysis was performed to reveal the diagnostic accuracy of using HMGB1 gene expression to distinguish patients before surgery from healthy control subjects. An optimal HMGB1 gene cut-off value greater than 1.20 resulted in an AUC value of 0.950 (95% CI, 0.882-1.000, p=0.001), with a sensitivity of 95.0%, specificity of 100.0%, PPV of 100.0%, and NPV of 90.9% (Table 3 and Figure 3).
Table 3. Receiver operating characteristic curve for HMGB1 gene expression
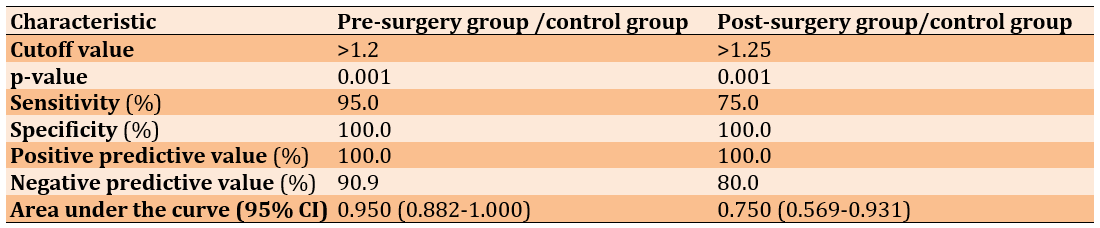
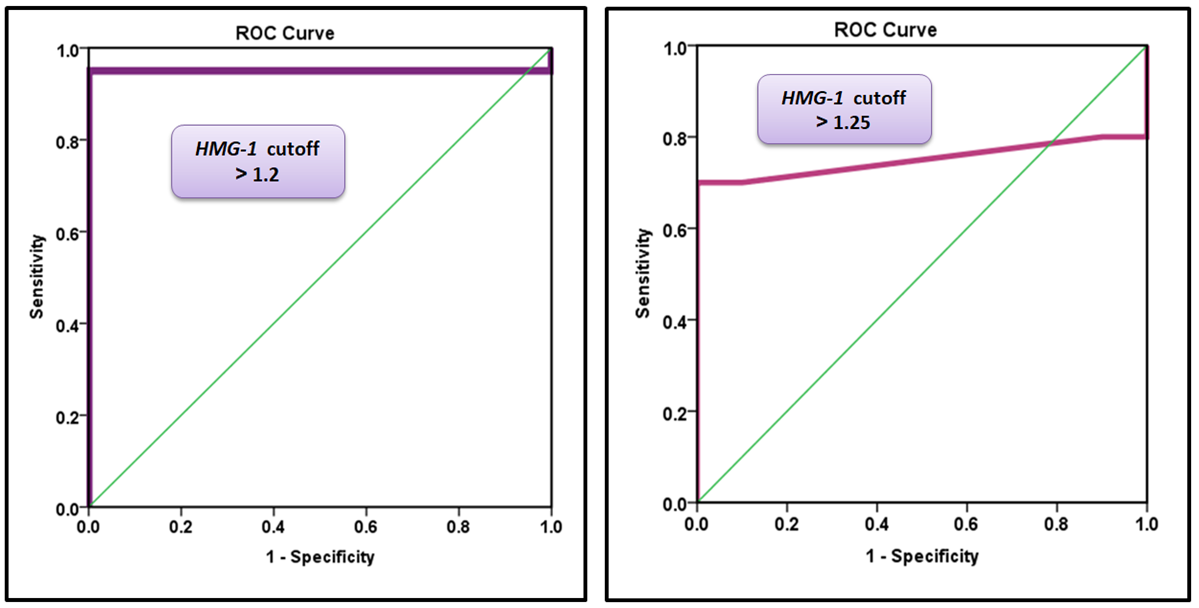
Figure 3. A) Receiver operating characteristic curve for HMGB1 gene expression to distinguish patients before surgery from healthy control subjects. B) Receiver operating characteristic curve for HMGB1 gene expression to distinguish patients after surgery from healthy control subjects.
Correlations between gene expression parameters
According to the logistic regression model, HMGB1 was directly correlated with CCK among patients before and after treatment (Figures 4 and 5). Logistic regression analysis demonstrated a significant positive correlation between HMGB1 and CCK expression in patients with chronic cholecystitis and gallstone disease. Postoperatively, a significant decrease in HMGB1 and CCK expression was observed, suggesting that cholecystectomy effectively reduces inflammation.
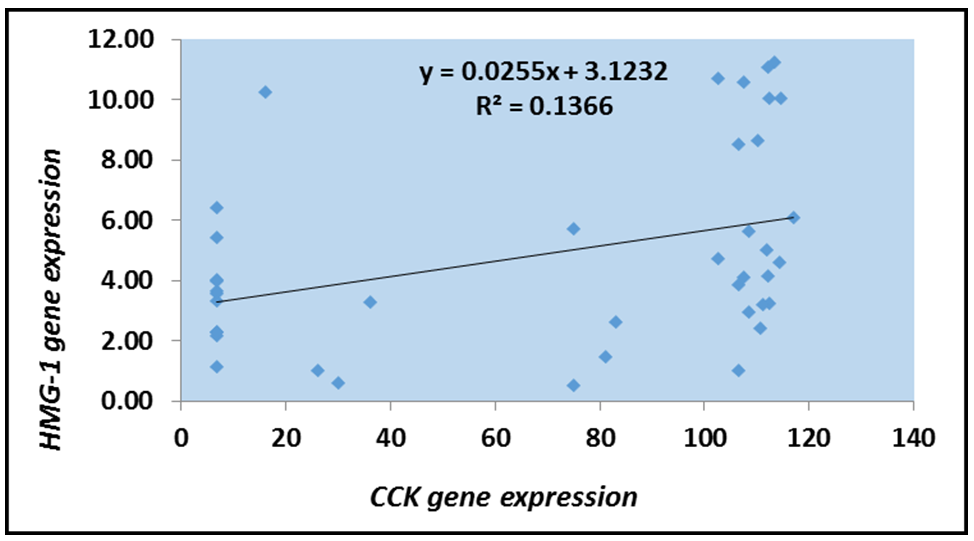
Figure 4. Logistic scatter plot of HMGB1 gene expression and CCK levels among patients before treatment.
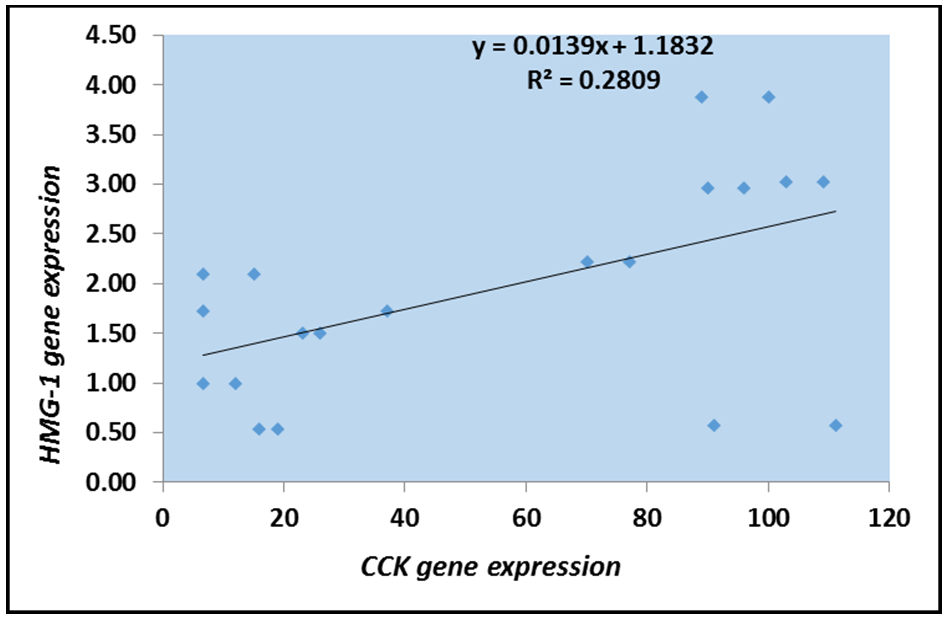
Figure 5. Logistic scatter plot of HMGB1 gene expression and CCK levels among patients after treatment.
Discussion
This study aimed to evaluate the association between HMGB1 gene expression and CCK levels in patients with gallbladder disease before and after cholecystectomy.
Serum CCK analysis revealed significantly elevated pre-surgery levels in gallbladder disease patients compared to both post-surgery patients and healthy controls. This finding aligns with previous studies indicating that gallbladder dysfunction (such as cholelithiasis and chronic cholecystitis) leads to compensatory CCK elevation due to impaired bile release [21]. Gallbladder dysfunction is a significant health concern that can manifest in various forms, including conditions such as cholelithiasis (gallstones) and chronic cholecystitis (inflammation of the gallbladder). These disorders disrupt the normal functioning of the gallbladder, which plays a critical role in the digestion and absorption of fats by storing and releasing bile. When the gallbladder is unable to effectively release bile due to these dysfunctions, the body responds with compensatory mechanisms to maintain digestive processes. One such mechanism involves the elevation of CCK, a hormone that stimulates gallbladder contraction and bile secretion. Elevated levels of CCK can indicate the body’s attempt to compensate for impaired bile release, but this response may also contribute to further complications, such as increased gallbladder pressure and exacerbation of symptoms. Understanding the relationship between gallbladder dysfunction and CCK elevation is essential for developing effective treatment strategies and improving patient outcomes.
The surgical removal of the gallbladder, known as cholecystectomy, is a common procedure performed to alleviate conditions, such as cholelithiasis and chronic cholecystitis. Following this surgery, the body undergoes significant physiological changes, particularly in the mechanisms responsible for bile storage and release. Post-cholecystectomy, enteroendocrine cells remain functional, but bile storage and release mechanisms are altered. The continuous bile flow reduces baseline CCK levels, while post-meal CCK secretion may increase due to the absence of a bile reservoir [22, 23]. This shift in CCK dynamics can impact digestion and metabolic regulation. Animal studies suggest that CCK-OP administration prevents gallbladder stasis and reduces the risk of gallstone formation [24]. However, post-meal, the secretion of CCK may increase due to the absence of a bile reservoir, which can significantly impact digestive processes and metabolic regulation. These shifts in CCK dynamics may have important implications for post-operative patients, affecting both digestion and the risk of developing complications such as gallstone formation.
Regarding disease severity, no significant difference was found in mean CCK levels across severity groups, although levels remained elevated in gallbladder disease cases. Consistent with Northfield et al. [25], gallstone patients may exhibit altered gallbladder sensitivity to CCK, contributing to disease progression. Similarly, studies by Zhu et al. [18], Masclee et al. [26], and Otsuki [21] highlight increased CCK levels, particularly in gallstone-induced pancreatitis. While CCK plays a key regulatory role, impaired motility and receptor sensitivity may limit its protective effects against gallstone formation.
An optimal CCK cut-off value greater than 16.75 caused an AUC value of 0.717, with a sensitivity of 72.5%, specificity of 70.0%, PPV of 82.9%, and NPV of 56.0%. These findings are consistent with those of Sonne et al. [23], indicating that CCK can potentially be used as a diagnostic marker for gallbladder dysfunction. Monitoring CCK levels can improve diagnostic accuracy and aid in clinical decision-making. However, its diagnostic utility remains limited. Post-surgery, an optimal CCK cut-off value of >6.7 can differentiate post-cholecystectomy patients from healthy controls, with a sensitivity of 60.0%, specificity of 55.0%, PPV of 57.1%, and NPV of 57.9%, with an AUC of 0.558 (0.376-0.739). These results indicate that CCK is a weak marker for distinguishing postsurgical patients from healthy individuals. Due to its low sensitivity and specificity, CCK alone is insufficient as a reliable diagnostic marker after cholecystectomy. To enhance the accuracy of clinical assessments, researchers should explore alternative biomarkers or a multifaceted diagnostic approach in future investigations.
HMGB1 functions as a DAMP, interacting with TLR4 and the receptor for advanced glycation end-products (RAGE), which leads to immune activation and cytokine release [27]. Elevated HMGB1 levels have been documented in both acute and chronic cholecystitis, with significant reductions observed post-surgery, emphasizing its role in disease progression [11]. Furthermore, Mohammed et al. [28] confirm higher preoperative HMGB1 levels in gallstone patients, which significantly decline postoperatively, reinforcing its association with inflammation. These findings suggest that HMGB1 is a key mediator of gallbladder disease and that its reduction post-surgery highlights the resolution of inflammation following gallbladder removal.
As a DAMP, HMGB1 is released during cellular injury, activating immune responses and influencing pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6 [29, 30]. Previous studies have confirmed its association with advanced inflammatory changes in cholecystitis [31]. HMGB1’s function as a key regulator of gallbladder inflammation highlights its potential as a therapeutic target for managing gallbladder disease. By monitoring HMGB1 expression, clinicians may be able to assess disease severity and predict inflammatory resolution post-surgery, ultimately improving patient outcomes.
An optimal HMGB1 gene cut-off value greater than 1.20 resulted in an AUC value of 0.950, with a sensitivity of 95.0%, specificity of 100.0%, PPV of 100.0%, and NPV of 90.9%. These results indicate that the HMGB1 gene is considered an excellent diagnostic marker to distinguish patients before surgery from healthy controls. The HMGB1 gene is considered an acceptable diagnostic marker to distinguish patients after surgery from healthy controls. These results demonstrate the potential of HMGB1 as an effective biomarker to enhance the diagnostic accuracy of acute and chronic cholecystitis when used in combination with standard diagnostic tests. This finding is recently corroborated by Amini et al. [11], who report that HMGB serves as a valuable biomarker for diagnosing acute cholecystitis and differentiating affected patients from healthy individuals. Their study reveals significantly elevated serum HMGB1 levels in patients with both mild and severe acute cholecystitis compared with healthy controls, with a reported sensitivity of 79.41% and specificity of 54.3% for distinguishing between these groups. Mohammed et al. [28] observed that HMGB1 serum levels are significantly higher in patients with gallstones than in healthy individuals. Notably, HMGB1 concentration decreased following surgical intervention, suggesting its potential utility in monitoring patient recovery post-surgery. These findings collectively support the use of HMGB1 as a diagnostic marker to differentiate patients with cholecystitis from healthy individuals and track changes in patient status following surgical treatment.
HMGB1 was directly correlated with CCK among patients before and after treatment. This result may suggest that the disease condition enhances the production of HMGB1 in relation to the expression of CCK. Logistic regression analysis demonstrated a significant positive correlation between HMGB1 and CCK expression in patients with chronic cholecystitis and gallstone disease. This suggests that disease progression enhances HMGB1 production in response to CCK expression. A key mechanistic pathway involves CCK-stimulated HMGB1 production via CCK1 receptor (CCK1R)-mediated activation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling, both of which regulate HMGB1 transcription [32]. Supporting evidence indicates that CCK receptor activation in epithelial and immune cells enhances inflammatory gene expression and cytokine release [33].
Gallbladder motility impairment and mechanical stress contribute to HMGB1 release, as CCK plays a crucial role in promoting bile flow. Chronic obstruction due to gallstones disrupts CCK responses, leading to tissue injury and inflammation [28]. Cellular damage from necrosis or pyroptosis results in passive HMGB1 release, further amplifying the inflammatory response [34]. Extracellular HMGB1 activates inflammatory pathways by binding to receptors for advanced glycation end products (RAGE) and TLR4, which may further influence CCK secretion. Additionally, HMGB1-mediated macrophage activation enhances interleukin-1 (IL-1) and chole IL-6 release, both of which regulate CCK [35, 36].
Postoperatively, a significant decrease in HMGB1 and CCK expression was observed, suggesting that cholecystectomy effectively reduces inflammation. This aligns with studies showing that HMGB1 levels decline post-surgery, correlating with a reduction in systemic inflammatory burden [11]. The absence of the gallbladder also affects bile storage and secretion, reducing the need for CCK stimulation due to continuous bile flow into the duodenum [21, 37].
From a metabolic perspective, HMGB1 promotes chronic inflammation and insulin resistance through dysregulated autophagy [38, 39]. In contrast, CCK exhibits anti-inflammatory properties through p38 MAPK signaling [40, 41]. The opposing roles of HMGB1 and CCK suggest a potential counter-regulatory interaction influencing inflammation and metabolic function.
This study is the first to establish a strong link between CCK signaling and HMGB1 expression in gallbladder disease. The observed postoperative decline in HMGB1 and CCK levels highlights their roles in sustaining inflammation and mechanical stress. Targeting CCK pathways or HMGB1-mediated signaling may offer novel therapeutic strategies for managing chronic gallbladder inflammation. Further research should explore non-surgical interventions for HMGB1 modulation, particularly in patients who are unsuitable for surgery or those experiencing persistent post-cholecystectomy symptoms.
Targeting the CCK and HMGB1 pathways could offer promising new therapeutic strategies for managing gallbladder inflammation. The interplay between these two pathways suggests that they may play pivotal roles in the pathophysiology of gallbladder disorders, including cholecystitis and other inflammatory conditions. By modulating CCK levels, it may be possible to enhance bile flow and reduce inflammation, thereby alleviating symptoms and preventing disease progression. Similarly, targeting HMGB1, a protein known to act as a pro-inflammatory mediator, could help mitigate the inflammatory response associated with gallbladder dysfunction. There is a pressing need for further research to elucidate the specific mechanisms by which CCK and HMGB1 influence gallbladder pathology. Future studies should aim to explore the therapeutic potential of agents that can selectively modulate these pathways, potentially leading to more effective management strategies for patients suffering from gallbladder-related conditions.
Conclusion
There is a strong link between elevated HMGB1 and CCK in chronic cholecystitis, with postoperative declines indicating reduced inflammation and altered bile regulation.
Acknowledgments: We are profoundly grateful to Allah for granting me the strength and patience to complete this research. I sincerely thank my supervisor, Assistant Professor Dr. Ali Mahdi Mutlag, for his guidance and continuous support, as well as the College of Science, Wasit University, for providing the academic environment necessary for this work. We also acknowledge my own dedication and perseverance throughout this journey.
Ethical Permissions: This study was approved by the Ethics Committee of the Department of Biology, College of Science, University of Wasit, as well as the Iraqi Ministry of Health, prior to including participants in the study tests. A signed written consent was obtained from each individual participating in the study.
Conflicts of Interests: The authors declared no conflicts of interests.
Authors' Contribution: Allami RA (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (85%); Mutlag AM (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (15%)
Funding/Support: This research was self-funded by the first author without any external financial support.
Gallbladder disease encompasses a range of disorders, including gallstones (cholelithiasis), inflammation (cholecystitis), polyps, and cancer. These conditions can lead to significant morbidity and may require various treatment approaches, from dietary modifications and medication to surgical interventions such as cholecystectomy. Additionally, the prevalence of gallbladder disease is influenced by several factors, including age, gender, obesity, and dietary habits, making it a complex health issue that requires careful consideration of individual risk factors. Among these, cholelithiasis is the most common condition, characterized by the formation of stones that may obstruct the bile ducts and cause inflammation, pain, or infection [1]. The development of gallbladder disease is influenced by multiple factors, including genetic predisposition, metabolic imbalances, biliary stasis, and chronic inflammation [2]. Epidemiological studies indicate that certain populations, particularly those with obesity and diabetes, are at a higher risk for developing gallstones [3]. The prevalence of gallstones is also significantly higher in women than in men, a disparity attributed to hormonal factors such as estrogen, which may influence cholesterol metabolism in the bile [4]. Additionally, dietary factors, including high-fat and low-fiber diets, have been implicated in the pathogenesis of gallbladder disease, suggesting that lifestyle modifications could play a crucial role in prevention [5].
Understanding the underlying mechanisms and risk factors associated with gallbladder disease is essential for developing effective prevention and treatment strategies, thereby reducing morbidity and healthcare costs associated with this condition [6]. Early diagnosis and intervention are crucial, as untreated gallbladder disease can result in serious complications, such as acute pancreatitis or biliary obstruction. Understanding the pathophysiology and risk factors associated with gallbladder disease is essential for developing effective prevention strategies and optimizing patient management.
High-mobility group box 1 (HMGB1) is a nuclear protein that plays a crucial role in inflammation and the immune response. It functions as a damage-associated molecular pattern (DAMP) molecule, acting as an alarm signal when it is passively released from necrotic cells or actively secreted by immune cells such as macrophages, monocytes, and dendritic cells [7]. Once extracellular, HMGB1 triggers a robust inflammatory response by signaling to adjacent immune cells [8]. It has been implicated in several pathological conditions, including sepsis, tumor metastasis, atherosclerosis, and liver injury [9, 10]. However, its precise role in the pathogenesis of gallbladder disease, particularly cholecystitis, remains unclear [11]. Recent studies suggest that elevated levels of HMGB1 correlate with the severity of inflammation in various conditions, indicating its potential as a biomarker for disease severity [12]. Furthermore, HMGB1’s interaction with the receptor for advanced glycation end products (RAGE) and toll-like receptors (TLRs) highlights its multifaceted role in modulating immune responses. These interactions not only activate pro-inflammatory signaling pathways but also promote the recruitment and activation of immune cells, amplifying the inflammatory response in various pathological conditions. Understanding these intricate signaling mechanisms could provide crucial insights into how HMGB1 contributes to chronic inflammation and tissue damage, opening avenues for the development of targeted therapies aimed at modulating HMGB1 activity in diseases characterized by excessive inflammation. Additionally, this knowledge may assist in identifying patient subgroups that could benefit from HMGB1-targeted interventions, thereby personalizing treatment strategies for conditions such as gallbladder disease and other inflammation-driven disorders [13].
Cholecystokinin (CCK) is a neuro-intestinal peptide hormone predominantly produced by enteroendocrine I-cells in the upper small intestine, with the highest density in the duodenum and proximal jejunum [14]. Its release is stimulated by the ingestion of fat- and protein-rich foods, leading to gallbladder contraction, bile secretion, and pancreatic enzyme release [15]. In addition to its gastrointestinal functions, CCK also plays a significant role in regulating appetite and satiety, as it can signal to the brain to reduce food intake after meals. This dual role highlights CCK as an important mediator of both digestive processes and metabolic control, indicating that disturbances in CCK signaling may contribute to conditions such as obesity and gallbladder disease [16]. Furthermore, research suggests that impaired CCK responses could affect the digestion and absorption of nutrients, potentially leading to malnutrition or further exacerbating gallbladder pathologies.
Understanding the full spectrum of CCK’s actions, including its interactions with other hormones and signaling pathways, is essential for developing comprehensive therapeutic strategies for digestive disorders and related metabolic diseases. Ultimately, exploring the complex relationship between CCK and gallbladder function may pave the way for innovative treatments that enhance digestive health and improve outcomes for patients suffering from gallbladder disease. CCK exerts its effects via two receptor subtypes, including CCK-A, which primarily regulates gallbladder motility and pancreatic secretion, and CCK-B, which influences gastrointestinal and central nervous system functions [17]. Defective gallbladder motility is a key contributor to cholesterol gallstone disease, and patients with cholesterol gallstones often exhibit impaired gallbladder contractility [18]. Research has also demonstrated that abnormal CCK release is associated with metabolic disorders such as obesity and gallstone formation [19].
This study is the first to investigate the association between HMGB1 gene expression and CCK levels in patients with gallbladder disease, both before and after cholecystectomy. By exploring these molecular interactions, this study aimed to improve our understanding of the pathophysiology of gallbladder disease and contribute to better diagnostic and therapeutic strategies. Given that gallbladder diseases, particularly cholecystitis, can lead to significant morbidity and healthcare costs, identifying specific biomarkers such as HMGB1 could facilitate early diagnosis and targeted treatment options. Additionally, understanding the relationship between HMGB1 and CCK levels may reveal novel insights into the regulatory mechanisms underlying gallbladder function and inflammation, potentially guiding the development of new pharmacological interventions. Moreover, this research could lay the groundwork for future studies aimed at validating HMGB1 as a therapeutic target, thereby enhancing the management of gallbladder disease and improving patient outcomes. Ultimately, the findings from this study may not only advance our scientific understanding but also translate into clinical applications that could alleviate the burden of gallbladder disease on patients and healthcare systems alike.
Materials and Methods
Subjects
This study included 60 patients with gallbladder diseases (40 patients with cholecystitis and cholelithiasis (20 pre-surgery patients and 20 post-surgery patients) and 20 healthy controls), aged 20-79 years, and was conducted at the College of Science, Wasit University, Iraq. Patient samples were collected from Al Karama Teaching Hospital and Al Zahraa Hospital between October 2023 and July 2024. Exclusion Criteria were patients with Helicobacter pylori infection and those with obesity were excluded to prevent confounding effects on HMGB1 expression and cholecystokinin levels.
EIESA kit assay
Human cholecystokinin ELISA kit
The procedure for this kit was conducted according to the guidelines provided by the manufacturer in China (SunLong Biotech; catalog number: QS2102Hu).
Reagent preparation
Before using any reagent or standard for reconstitution, we ensured that the temperature was between 20 and 25°C. A total of 300µL of the standard (12.8ng/mL) was added to 130µL of standard diluent to generate a 180.0pg/mL standard stock solution. The standard was allowed to sit for 15 minutes with gentle agitation before dilution. Standard points were prepared by serial diluting the standard stock solution (180.0pg/mL) 1:2 with standard diluent to produce 120.0pg/mL, 60.0pg/mL, 30.0pg/mL, and 15.0pg/mL solutions. Any remaining solution was frozen at -20°C and used within one month. For wash buffer preparation, we diluted 20mL of wash buffer concentrate 25x into deionized or distilled water to yield 500mL of 1x wash buffer.
Molecular assay
Primer design
The primer design was conducted using Primer 3 web version 4.1.0 (available at http://primer3.ut.ee) for HMGB1 and GAPDH genes, and the design was checked by the University Code of Student Conduct (UCSC) programs (Table 1). The primers were synthesized and lyophilized by Alpha DNA Ltd. (Canada).
Table 1. Primer sequences used in the study

Primer Preparation
For each assay, the required primers were prepared by dissolving the lyophilized sample in nuclease-free water according to the manufacturer’s instructions. A stock solution with a concentration of 100µM was prepared and stored at -20°C. Diluting 10µL of each primer stock solution in 90µL of nuclease-free water yielded a working solution with a concentration of 10µM, which was maintained at -20°C until use.
Total RNA extraction
Total RNA was extracted from all samples using TRIzol® LS Reagent according to the manufacturer’s protocol. Briefly, 250µL of blood was added to 750µL of TRIzol® LS Reagent in an Eppendorf tube and homogenized by vortexing. Then, 200µL of chloroform was added, and the mixture was shaken vigorously for 15 seconds before incubation on ice for 5 minutes. Following centrifugation at 12,000rpm for 10 minutes at 4°C, the mixture separated into three phases, with RNA retained in the aqueous phase. The aqueous phase was carefully transferred to a new 1.5mL tube, and an equal volume of isopropyl alcohol was added. After gentle inversion, the mixture was incubated at -20°C for 10 minutes and then centrifuged at 12,000rpm for 10 minutes at 4°C. The supernatant was removed, and the pellet was washed with 80% ethanol, vortexed, and centrifuged again at 12,000rpm for 5 minutes at 4°C. The supernatant was discarded, and the RNA pellet was dried using hot air. Finally, the RNA was dissolved in RNase-free water and incubated at 60°C for 10 minutes before being stored at freezing temperatures until further use.
Estimation of RNA purity and concentration
The NanoVue Nanodrop spectrophotometer (England) was used to evaluate the concentration and purity of the extracted RNA in order to determine the quality of samples for subsequent analysis in Reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The samples ranged in RNA concentration from 73 to 147ng/μL, while the absorbance of the samples was measured at two distinct wavelengths to determine RNA purity (260 and 280nm). The presence of an A260/A280 ratio of approximately 2.0 suggested that the RNA sample was pure.
cDNA synthesis of mRNA
Total RNA was reverse transcribed to complementary DNA (cDNA) using a cDNA kit from Addbio Company, Korea. The procedure was performed in a reaction volume of 25μL according to the manufacturer’s instructions. Three main steps were required for the preparation of the conversion reaction. Reverse transcription reactions should be assembled in an RNase-free environment. The use of clean pipettes and filter tips is recommended. The RNA templates and all reagents were thawed on ice, and each solution was gently mixed. The reaction components were then mixed.
Quantitative real-time PCR
The expression levels of HMGB1 genes were estimated by qRT-PCR. To confirm the expression of the target gene, TransStart® Top Green qPCR Super Mix (SYBR Green) was used. Primer sequences for these genes were synthesized by Alpha DNA Ltd. (Canada) and stored lyophilized at -20°C. The mRNA levels of the endogenous control Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene were used as an internal control to normalize the mRNA levels of the target genes [20].
Statistical analysis
Data were analyzed using SPSS 26 and Microsoft Excel 2010. Normality was assessed using the Kolmogorov-Smirnov test, and appropriate statistical tests (t-test, ANOVA, chi-square, receiver operating characteristic (ROC) analysis, Pearson’s correlation) were used based on the data type. The significance level was set at p<0.05, with p<0.01 indicating high significance. Results were reported with relevant statistics, including area under the curve (AUC), sensitivity, specificity, and correlation coefficients.
Findings
Cholecystokinin levels in gallbladder disease and their association with disease severity
Serum CCK analysis using one-way ANOVA revealed significantly elevated pre-surgery levels in pre-surgery patients (74.53±7.63; range: 6.69-123.60) compared to both post-surgery patients (48.37±11.61; range: 6.73-116.46) and healthy controls (37.81±10.89; range: 6.68-115.79; p=0.007). However, CCK levels in the post-surgery group were not significantly different from those of the controls (p>0.05).
Regarding disease severity, no significant difference was found in mean CCK levels across severity groups (acute cholecystitis: 91.20±14.10, chronic cholecystitis: 66.29±12.10, hyperplasia: 62.71±16.20), although levels remained elevated in gallbladder disease cases (0.374).
Diagnostic accuracy of Cholecystokinin
ROC analysis was performed to reveal the diagnostic accuracy of using CCK concentrations to distinguish patients before surgery from healthy control subjects. An optimal CCK cut-off value greater than 16.75 resulted in an AUC value of 0.717 (95% confidence interval [CI], 0.574-0.859, p=0.002), with a sensitivity of 72.5%, specificity of 70.0%, positive predictive value (PPV) of 82.9%, and negative predictive value (NPV) of 56.0%. The present results indicate that CCK is considered an acceptable diagnostic marker to distinguish patients before surgery from healthy controls (Table 2 and Figure 1).
Table 2. Receiver operating characteristic curve for cholecystokinin (CCK) levels


Figure 1. A) Receiver operating characteristic curve for CCK levels to distinguish patients before surgery from healthy control subjects. B) Receiver operating characteristic curve for CCK levels to distinguish patients after surgery from healthy control subjects.
Role of HMGB1 in gallbladder disease and its association with disease severity
HMGB1 gene expression was markedly elevated in patients with gallbladder disease before surgery compared to postoperative patients and healthy individuals, indicating an active inflammatory response. Following cholecystectomy, HMGB1 expression significantly declined, suggesting an attenuation of inflammation and the restoration of normal physiological levels. The mean HMGB1 gene expression levels were 4.91±0.51 in preoperative patients, 1.95±0.24 in postoperative patients, and 1.00±0.12 in the healthy control group, with significant differences observed between all groups (one-way ANOVA; p=0.001). Additionally, a significant difference (p<0.05) was observed between preoperative and postoperative patients (Figure 2).

Figure 2. HMGB1 gene expression in patients and healthy controls.
There was a significant increase in HMGB1 expression in patients with hyperplasia compared to other groups (6.87±0.96 vs. 4.91±0.71 and 3.77±0.67, p=0.035).
Diagnostic accuracy of HMGB1 gene expression
ROC analysis was performed to reveal the diagnostic accuracy of using HMGB1 gene expression to distinguish patients before surgery from healthy control subjects. An optimal HMGB1 gene cut-off value greater than 1.20 resulted in an AUC value of 0.950 (95% CI, 0.882-1.000, p=0.001), with a sensitivity of 95.0%, specificity of 100.0%, PPV of 100.0%, and NPV of 90.9% (Table 3 and Figure 3).
Table 3. Receiver operating characteristic curve for HMGB1 gene expression


Figure 3. A) Receiver operating characteristic curve for HMGB1 gene expression to distinguish patients before surgery from healthy control subjects. B) Receiver operating characteristic curve for HMGB1 gene expression to distinguish patients after surgery from healthy control subjects.
Correlations between gene expression parameters
According to the logistic regression model, HMGB1 was directly correlated with CCK among patients before and after treatment (Figures 4 and 5). Logistic regression analysis demonstrated a significant positive correlation between HMGB1 and CCK expression in patients with chronic cholecystitis and gallstone disease. Postoperatively, a significant decrease in HMGB1 and CCK expression was observed, suggesting that cholecystectomy effectively reduces inflammation.

Figure 4. Logistic scatter plot of HMGB1 gene expression and CCK levels among patients before treatment.

Figure 5. Logistic scatter plot of HMGB1 gene expression and CCK levels among patients after treatment.
Discussion
This study aimed to evaluate the association between HMGB1 gene expression and CCK levels in patients with gallbladder disease before and after cholecystectomy.
Serum CCK analysis revealed significantly elevated pre-surgery levels in gallbladder disease patients compared to both post-surgery patients and healthy controls. This finding aligns with previous studies indicating that gallbladder dysfunction (such as cholelithiasis and chronic cholecystitis) leads to compensatory CCK elevation due to impaired bile release [21]. Gallbladder dysfunction is a significant health concern that can manifest in various forms, including conditions such as cholelithiasis (gallstones) and chronic cholecystitis (inflammation of the gallbladder). These disorders disrupt the normal functioning of the gallbladder, which plays a critical role in the digestion and absorption of fats by storing and releasing bile. When the gallbladder is unable to effectively release bile due to these dysfunctions, the body responds with compensatory mechanisms to maintain digestive processes. One such mechanism involves the elevation of CCK, a hormone that stimulates gallbladder contraction and bile secretion. Elevated levels of CCK can indicate the body’s attempt to compensate for impaired bile release, but this response may also contribute to further complications, such as increased gallbladder pressure and exacerbation of symptoms. Understanding the relationship between gallbladder dysfunction and CCK elevation is essential for developing effective treatment strategies and improving patient outcomes.
The surgical removal of the gallbladder, known as cholecystectomy, is a common procedure performed to alleviate conditions, such as cholelithiasis and chronic cholecystitis. Following this surgery, the body undergoes significant physiological changes, particularly in the mechanisms responsible for bile storage and release. Post-cholecystectomy, enteroendocrine cells remain functional, but bile storage and release mechanisms are altered. The continuous bile flow reduces baseline CCK levels, while post-meal CCK secretion may increase due to the absence of a bile reservoir [22, 23]. This shift in CCK dynamics can impact digestion and metabolic regulation. Animal studies suggest that CCK-OP administration prevents gallbladder stasis and reduces the risk of gallstone formation [24]. However, post-meal, the secretion of CCK may increase due to the absence of a bile reservoir, which can significantly impact digestive processes and metabolic regulation. These shifts in CCK dynamics may have important implications for post-operative patients, affecting both digestion and the risk of developing complications such as gallstone formation.
Regarding disease severity, no significant difference was found in mean CCK levels across severity groups, although levels remained elevated in gallbladder disease cases. Consistent with Northfield et al. [25], gallstone patients may exhibit altered gallbladder sensitivity to CCK, contributing to disease progression. Similarly, studies by Zhu et al. [18], Masclee et al. [26], and Otsuki [21] highlight increased CCK levels, particularly in gallstone-induced pancreatitis. While CCK plays a key regulatory role, impaired motility and receptor sensitivity may limit its protective effects against gallstone formation.
An optimal CCK cut-off value greater than 16.75 caused an AUC value of 0.717, with a sensitivity of 72.5%, specificity of 70.0%, PPV of 82.9%, and NPV of 56.0%. These findings are consistent with those of Sonne et al. [23], indicating that CCK can potentially be used as a diagnostic marker for gallbladder dysfunction. Monitoring CCK levels can improve diagnostic accuracy and aid in clinical decision-making. However, its diagnostic utility remains limited. Post-surgery, an optimal CCK cut-off value of >6.7 can differentiate post-cholecystectomy patients from healthy controls, with a sensitivity of 60.0%, specificity of 55.0%, PPV of 57.1%, and NPV of 57.9%, with an AUC of 0.558 (0.376-0.739). These results indicate that CCK is a weak marker for distinguishing postsurgical patients from healthy individuals. Due to its low sensitivity and specificity, CCK alone is insufficient as a reliable diagnostic marker after cholecystectomy. To enhance the accuracy of clinical assessments, researchers should explore alternative biomarkers or a multifaceted diagnostic approach in future investigations.
HMGB1 functions as a DAMP, interacting with TLR4 and the receptor for advanced glycation end-products (RAGE), which leads to immune activation and cytokine release [27]. Elevated HMGB1 levels have been documented in both acute and chronic cholecystitis, with significant reductions observed post-surgery, emphasizing its role in disease progression [11]. Furthermore, Mohammed et al. [28] confirm higher preoperative HMGB1 levels in gallstone patients, which significantly decline postoperatively, reinforcing its association with inflammation. These findings suggest that HMGB1 is a key mediator of gallbladder disease and that its reduction post-surgery highlights the resolution of inflammation following gallbladder removal.
As a DAMP, HMGB1 is released during cellular injury, activating immune responses and influencing pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6 [29, 30]. Previous studies have confirmed its association with advanced inflammatory changes in cholecystitis [31]. HMGB1’s function as a key regulator of gallbladder inflammation highlights its potential as a therapeutic target for managing gallbladder disease. By monitoring HMGB1 expression, clinicians may be able to assess disease severity and predict inflammatory resolution post-surgery, ultimately improving patient outcomes.
An optimal HMGB1 gene cut-off value greater than 1.20 resulted in an AUC value of 0.950, with a sensitivity of 95.0%, specificity of 100.0%, PPV of 100.0%, and NPV of 90.9%. These results indicate that the HMGB1 gene is considered an excellent diagnostic marker to distinguish patients before surgery from healthy controls. The HMGB1 gene is considered an acceptable diagnostic marker to distinguish patients after surgery from healthy controls. These results demonstrate the potential of HMGB1 as an effective biomarker to enhance the diagnostic accuracy of acute and chronic cholecystitis when used in combination with standard diagnostic tests. This finding is recently corroborated by Amini et al. [11], who report that HMGB serves as a valuable biomarker for diagnosing acute cholecystitis and differentiating affected patients from healthy individuals. Their study reveals significantly elevated serum HMGB1 levels in patients with both mild and severe acute cholecystitis compared with healthy controls, with a reported sensitivity of 79.41% and specificity of 54.3% for distinguishing between these groups. Mohammed et al. [28] observed that HMGB1 serum levels are significantly higher in patients with gallstones than in healthy individuals. Notably, HMGB1 concentration decreased following surgical intervention, suggesting its potential utility in monitoring patient recovery post-surgery. These findings collectively support the use of HMGB1 as a diagnostic marker to differentiate patients with cholecystitis from healthy individuals and track changes in patient status following surgical treatment.
HMGB1 was directly correlated with CCK among patients before and after treatment. This result may suggest that the disease condition enhances the production of HMGB1 in relation to the expression of CCK. Logistic regression analysis demonstrated a significant positive correlation between HMGB1 and CCK expression in patients with chronic cholecystitis and gallstone disease. This suggests that disease progression enhances HMGB1 production in response to CCK expression. A key mechanistic pathway involves CCK-stimulated HMGB1 production via CCK1 receptor (CCK1R)-mediated activation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling, both of which regulate HMGB1 transcription [32]. Supporting evidence indicates that CCK receptor activation in epithelial and immune cells enhances inflammatory gene expression and cytokine release [33].
Gallbladder motility impairment and mechanical stress contribute to HMGB1 release, as CCK plays a crucial role in promoting bile flow. Chronic obstruction due to gallstones disrupts CCK responses, leading to tissue injury and inflammation [28]. Cellular damage from necrosis or pyroptosis results in passive HMGB1 release, further amplifying the inflammatory response [34]. Extracellular HMGB1 activates inflammatory pathways by binding to receptors for advanced glycation end products (RAGE) and TLR4, which may further influence CCK secretion. Additionally, HMGB1-mediated macrophage activation enhances interleukin-1 (IL-1) and chole IL-6 release, both of which regulate CCK [35, 36].
Postoperatively, a significant decrease in HMGB1 and CCK expression was observed, suggesting that cholecystectomy effectively reduces inflammation. This aligns with studies showing that HMGB1 levels decline post-surgery, correlating with a reduction in systemic inflammatory burden [11]. The absence of the gallbladder also affects bile storage and secretion, reducing the need for CCK stimulation due to continuous bile flow into the duodenum [21, 37].
From a metabolic perspective, HMGB1 promotes chronic inflammation and insulin resistance through dysregulated autophagy [38, 39]. In contrast, CCK exhibits anti-inflammatory properties through p38 MAPK signaling [40, 41]. The opposing roles of HMGB1 and CCK suggest a potential counter-regulatory interaction influencing inflammation and metabolic function.
This study is the first to establish a strong link between CCK signaling and HMGB1 expression in gallbladder disease. The observed postoperative decline in HMGB1 and CCK levels highlights their roles in sustaining inflammation and mechanical stress. Targeting CCK pathways or HMGB1-mediated signaling may offer novel therapeutic strategies for managing chronic gallbladder inflammation. Further research should explore non-surgical interventions for HMGB1 modulation, particularly in patients who are unsuitable for surgery or those experiencing persistent post-cholecystectomy symptoms.
Targeting the CCK and HMGB1 pathways could offer promising new therapeutic strategies for managing gallbladder inflammation. The interplay between these two pathways suggests that they may play pivotal roles in the pathophysiology of gallbladder disorders, including cholecystitis and other inflammatory conditions. By modulating CCK levels, it may be possible to enhance bile flow and reduce inflammation, thereby alleviating symptoms and preventing disease progression. Similarly, targeting HMGB1, a protein known to act as a pro-inflammatory mediator, could help mitigate the inflammatory response associated with gallbladder dysfunction. There is a pressing need for further research to elucidate the specific mechanisms by which CCK and HMGB1 influence gallbladder pathology. Future studies should aim to explore the therapeutic potential of agents that can selectively modulate these pathways, potentially leading to more effective management strategies for patients suffering from gallbladder-related conditions.
Conclusion
There is a strong link between elevated HMGB1 and CCK in chronic cholecystitis, with postoperative declines indicating reduced inflammation and altered bile regulation.
Acknowledgments: We are profoundly grateful to Allah for granting me the strength and patience to complete this research. I sincerely thank my supervisor, Assistant Professor Dr. Ali Mahdi Mutlag, for his guidance and continuous support, as well as the College of Science, Wasit University, for providing the academic environment necessary for this work. We also acknowledge my own dedication and perseverance throughout this journey.
Ethical Permissions: This study was approved by the Ethics Committee of the Department of Biology, College of Science, University of Wasit, as well as the Iraqi Ministry of Health, prior to including participants in the study tests. A signed written consent was obtained from each individual participating in the study.
Conflicts of Interests: The authors declared no conflicts of interests.
Authors' Contribution: Allami RA (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (85%); Mutlag AM (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (15%)
Funding/Support: This research was self-funded by the first author without any external financial support.
Keywords:
References
1. Wang HH, Portincasa P, Wang DQ. Molecular pathophysiology and physical chemistry of cholesterol gallstones. Front Biosci. 2008;13:401-23. [Link] [DOI:10.2741/2688]
2. Aerts R, Penninckx F. The burden of gallstone disease in Europe. Aliment Pharmacol Ther. 2003;18 Suppl 3:49-53. [Link] [DOI:10.1046/j.0953-0673.2003.01721.x]
3. Acalovschi M. Epidemiology of gallstone disease. InFALK SYMPOSIUM 2001 (pp. 117-130). Dordrecht; London; Kluwer Academic; 1999. [Link]
4. Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century?. Curr Gastroenterol Rep. 2005;7(2):132-40. [Link] [DOI:10.1007/s11894-005-0051-8]
5. Wang Y, Lu J, Wen N, Nie G, Peng D, Xiong X, et al. The role of diet and nutrition related indicators in biliary diseases: an umbrella review of systematic review and meta-analysis. Nutr Metabol. 2022;19(1):51. [Link] [DOI:10.1186/s12986-022-00677-1]
6. Jones C, Mawhinney A, Brown R. The true cost of gallstone disease. Ulster Med J. 2012;81(1):10. [Link] [DOI:10.53347/rID-17178]
7. Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. Bratisl Lek Listy. 2012;113(3):163-71. [Link] [DOI:10.4149/BLL_2012_039]
8. Xue J, Suarez JS, Minaai M, Li S, Gaudino G, Pass HI, et al. HMGB1 as a therapeutic target in disease. J Cell Physiol. 2021;236(5):3406-19. [Link] [DOI:10.1002/jcp.30125]
9. Vijayakumar EC, Bhatt LK, Prabhavalkar KS. High mobility group box-1 (HMGB1): A potential target in therapeutics. Curr Drug Targets. 2019;20(14):1474-85. [Link] [DOI:10.2174/1389450120666190618125100]
10. Lea JD, Clarke JI, McGuire N, Antoine DJ. Redox-dependent HMGB1 isoforms as pivotal Co-Ordinators of drug-induced liver injury: Mechanistic biomarkers and therapeutic targets. Antioxid Redox Signal. 2016;24(12):652-65. [Link] [DOI:10.1089/ars.2015.6406]
11. Amini M, Pakdaman A, Shapoori S, Mosayebi G. High mobility group box-1 (HMGB1) protein as a biomarker for acute cholecystitis. Rep Biochem Mol Biol. 2019;7(2):204-9. [Link]
12. Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, et al. HMGB1 in health and disease. Molecular aspects of medicine. 2014;40:1-16. [Link] [DOI:10.1016/j.mam.2014.05.001]
13. Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51(2):119-26. [Link] [DOI:10.1016/j.cyto.2010.02.021]
14. Wang HH, Portincasa P, Wang DQ. Update on the molecular mechanisms underlying the effect of cholecystokinin and cholecystokinin-1 receptor on the formation of cholesterol gallstones. Curr Med Chem. 2019;26(19):3407-23. [Link] [DOI:10.2174/0929867324666170619104801]
15. Schjoldager BT. Role of CCK in gallbladder function. Ann N Y Acad Sci. 1994;713:207-18. [Link] [DOI:10.1111/j.1749-6632.1994.tb44067.x]
16. Liu Y, Ting J, Zhu W. Role of cholecystokinin in appetite regulation: A review. Octa J Biosci. 2023;11(1). [Link]
17. Morisset J. The gastrointestinal cholecystokinin receptors in health and diseases. Rocz Akad Med Bialymst. 2005;50:21-36. [Link]
18. Zhu J, Han TQ, Chen S, Jiang Y, Zhang SD. Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World J Gastroenterol. 2005;11(11):1685-9. [Link] [DOI:10.3748/wjg.v11.i11.1685]
19. Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104(4):531-43. [Link] [DOI:10.1016/S0092-8674(01)00240-9]
20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-8. [Link] [DOI:10.1006/meth.2001.1262]
21. Otsuki M. Pathophysiological role of cholecystokinin in humans. J Gastroenterol Hepatol. 2000;15:D71-83. [Link] [DOI:10.1046/j.1440-1746.2000.02178.x]
22. Guimbaud R, Moreau JA, Bouisson M, Durand S, Escourrou J, Vaysse N, et al. Intraduodenal free fatty acids rather than triglycerides are responsible for the release of CCK in humans. Pancreas. 1997;14(1):76-82. [Link] [DOI:10.1097/00006676-199701000-00012]
23. Sonne DP, Hare KJ, Martens P, Rehfeld JF, Holst JJ, Vilsbøll T, et al. Postprandial gut hormone responses and glucose metabolism in cholecystectomized patients. Am J Physiol Gastrointest Liver Physiol. 2013;304(4):G413-9. [Link] [DOI:10.1152/ajpgi.00435.2012]
24. Roslyn JJ, DenBesten L, Pitt HA, Kuchenbecker S, Polarek JW. Resident research award. Effects of cholecystokinin on gallbladder stasis and cholesterol gallstone formation. J Surg Res. 1981;30(3):200-4. [Link] [DOI:10.1016/0022-4804(81)90148-7]
25. Northfield TC, Kupfer RM, Maudgal DP, Zentler-Munro PL, Meller ST, Garvie NW, et al. Gall-bladder sensitivity to cholecystokinin in patients with gall stones. Br Med J. 1980;280(6208):143-4. [Link] [DOI:10.1136/bmj.280.6208.143]
26. Masclee AA, Jansen JB, Driessen WM, Geuskens LM, Lamers CB. Plasma cholecystokinin and gallbladder responses to intraduodenal fat in gallstone patients. Dig Dis Sci. 1989;34(3):353-9. [Link] [DOI:10.1007/BF01536255]
27. Ren W, Zhao L, Sun Y, Wang X, Shi X. HMGB1 and Toll-like receptors: Potential therapeutic targets in autoimmune diseases. Mol Med. 2023;29(1):117. [Link] [DOI:10.1186/s10020-023-00717-3]
28. Mohammed SH, Al-Dujaili AN, El Katib WA. High mobility group box protein-1 (HMGB1) level in gallstones patients. AIP Conf Proc. 2022;2398(1). [Link] [DOI:10.1063/5.0093559]
29. Popovic PJ, DeMarco R, Lotze MT, Winikoff SE, Bartlett DL, Krieg AM, et al. High mobility group B1 protein suppresses the human plasmacytoid dendritic cell response to TLR9 agonists. J Immunol. 2006;177(12):8701-7. [Link] [DOI:10.4049/jimmunol.177.12.8701]
30. Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE. High-mobility group box 1 (HMGB1) in COVID-19: Extrapolation of dangerous liaisons. Inflammopharmacology. 2022;30(3):811-20. [Link] [DOI:10.1007/s10787-022-00988-y]
31. Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A. 2010;107(28):12611-6. [Link] [DOI:10.1073/pnas.1006542107]
32. Williams JA, Sans MD, Tashiro M, Schäfer C, Bragado MJ, Dabrowski A. Cholecystokinin activates a variety of intracellular signal transduction mechanisms in rodent pancreatic acinar cells. Pharmacol Toxicol. 2002;91(6):297-303. [Link] [DOI:10.1034/j.1600-0773.2002.910606.x]
33. Gilyard SN, Hamlin SL, Johnson JO, Herr KD. Imaging review of sickle cell disease for the emergency radiologist. Emerg Radiol. 2021;28(1):153-64. [Link] [DOI:10.1007/s10140-020-01828-8]
34. Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93(6):865-73. [Link] [DOI:10.1189/jlb.1212662]
35. Daun JM, McCarthy DO. The role of cholecystokinin in interleukin-1-induced anorexia. Physiol Behav. 1993;54(2):237-41. [Link] [DOI:10.1016/0031-9384(93)90105-O]
36. Zhong H, Li X, Zhou S, Jiang P, Liu X, Ouyang M, et al. Interplay between RAGE and TLR4 regulates HMGB1-induced inflammation by promoting cell surface expression of RAGE and TLR4. J Immunol. 2020;205(3):767-75. [Link] [DOI:10.4049/jimmunol.1900860]
37. Kennedy NS, Campbell FC, Cullen PT, Sutton DG, Millar BW, Cuschieri A. Gallbladder function and fasting enterogastric bile reflux. Nucl Med Commun. 1989;10(3):193-8. [Link] [DOI:10.1097/00006231-198903000-00013]
38. Moghetti P, Catellani C, Sartori C, Migazzi M, Cirillo F, Villani M, et al. Serum HMGB1 levels are independently associated with glucose clamp-derived measures of insulin resistance in women with PCOS. J Endocrinol Invest. 2023;46(12):2629-37. [Link] [DOI:10.1007/s40618-023-02119-y]
39. Yang K, Cao F, Wang W, Tian Z, Yang L. The relationship between HMGB1 and autophagy in the pathogenesis of diabetes and its complications. Front Endocrinol. 2023;14:1141516. [Link] [DOI:10.3389/fendo.2023.1141516]
40. Saia RS, Ribeiro AB, Giusti H. Cholecystokinin modulates the mucosal inflammatory response and prevents the lipopolysaccharide-induced intestinal epithelial barrier dysfunction. Shock. 2020;53(2):242-51. [Link] [DOI:10.1097/SHK.0000000000001355]
41. Meng AH, Ling YL, Zhang XP, Zhang JL. Anti-inflammatory effect of cholecystokinin and its signal transduction mechanism in endotoxic shock rat. World J Gastroenterol. 2002;8(4):712-7. [Link] [DOI:10.3748/wjg.v8.i4.712]









