Volume 17, Issue 1 (2025)
Iran J War Public Health 2025, 17(1): 1-7 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/12/20 | Accepted: 2025/02/1 | Published: 2025/02/10
Received: 2024/12/20 | Accepted: 2025/02/1 | Published: 2025/02/10
How to cite this article
Sedehi S, Sharifi S, Dastmard A, Darroudi N, Dehghan Niri M. Enhanced Titanium Structures via Spark Plasma Sintering and Shear Extrusion. Iran J War Public Health 2025; 17 (1) :1-7
URL: http://ijwph.ir/article-1-1535-en.html
URL: http://ijwph.ir/article-1-1535-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Mechanical Engineering, Faculty of Engineering, University of Tehran, Tehran, Iran
2- Department of Mechanical Engineering, Khomeinishahr Branch, Islamic Azad University, Khomeinishahr, Iran
3- Department of Biomedical Engineering, Faculty of Engineering, Mashhad Branch, Islamic Azad University, Mashhad, Iran
4- Department of Industrial Engineering, Faculty of Engineering, Gonabad University, Gonabad, Iran
2- Department of Mechanical Engineering, Khomeinishahr Branch, Islamic Azad University, Khomeinishahr, Iran
3- Department of Biomedical Engineering, Faculty of Engineering, Mashhad Branch, Islamic Azad University, Mashhad, Iran
4- Department of Industrial Engineering, Faculty of Engineering, Gonabad University, Gonabad, Iran
Full-Text (HTML) (379 Views)
Introduction
Titanium is widely recognized as the preferred material for orthopedic applications, particularly in components that endure substantial cyclic mechanical loads. Examples include stems and cups in shoulder, hip, knee, and ankle joints, where polymeric materials fail to meet the required mechanical strength [1]. Beyond strength, titanium implants exhibit excellent osseointegration properties over time, which can be further enhanced by incorporating cellular solid morphologies [2, 3]. However, despite extensive research and various proposed solutions [4], titanium is unsuitable for wear-intensive components, such as femoral heads in hip arthroplasty or femoral components in knee arthroplasty [5, 6]. The native titanium oxide layer on titanium and its alloys, although acting as a barrier, is not entirely inert.
Bone tissue can adhere to titanium surfaces, enabling complete osseointegration of devices over time [7-9]. Dental implants, in particular, demand high strength, superior corrosion resistance, and excellent biocompatibility to meet rigorous biological and mechanical criteria. Among these, titanium and its alloys are now the leading choice due to their high strength, low density, reduced elastic modulus, and superior corrosion resistance [10]. While commercially pure titanium (cp-Ti) is commonly employed, its relatively low strength (~300 MPa) restricts its reliability in load-bearing applications, such as dental screws, despite its outstanding corrosion resistance and biocompatibility [11]. Bone diseases and fractures have become increasingly prevalent worldwide, with over 50% of women and 20% of men above 50 years of age experiencing fractures in their lifetime. Such conditions often necessitate surgical interventions like complete knee or hip replacements or the implantation of temporary or permanent components. Jin & Chu [12] emphasized the role of medical materials in reconstructing alveolar bone, noting that metallic implants can degrade over time due to corrosion or wear, releasing ions into the biological environment. This degradation can trigger cytotoxicity, inflammatory responses, and other adverse reactions, ultimately compromising the biocompatibility of the material [13].
Despite the extensive use of the Ti-6Al-4V alloy, concerns over ion release and long-term biological safety persist [14]. An emerging solution involves using commercially pure titanium enhanced through severe plastic deformation (SPD) techniques, such as equal channel angular pressing (ECAP), simple shear extrusion (SSE), accumulative roll bonding (ARB), and high-pressure torsion (HPT) [13]. Among SPD techniques, SSE has proven especially effective when combined with spark plasma sintering (SPS). This synergistic approach enables rapid densification, initial grain refinement, and ultrafine grain structures, significantly improving mechanical properties. The combination’s cost-effectiveness, reduced processing time, and superior outcomes make it a promising strategy for producing advanced titanium implants [15, 16]. Specifically, ultrafine-grained structures (grain sizes below one micrometer) have been pivotal in enhancing mechanical properties, although their tribological performance requires further investigation [17]. Suwas et al. [18] demonstrated the dynamic restoration processes in commercially pure titanium during ECAE, achieving good alignment between experimental textures and simulations using the VPSC model.
Sedehi et al. [19] successfully improved the properties of commercially pure titanium through spark plasma sintering and SPD methods. Despite these advancements, more research is needed to explore the interplay between mechanical properties and biocompatibility in medical implants. The present study aimed to address this gap by examining titanium samples processed via SPS and optimized using SSE. Advanced mechanical testing and XRD peak analysis were employed to understand the microstructural changes and their impact on the material’s performance. The findings underscore the potential of these methods to enhance implant longevity and improve patient outcomes.
Materials and Methods
In this experimental study conducted in Gonabad, Iran in 2024, the primary focus was on optimizing the conditions of the two key processes, SPS and SSE, to enhance both mechanical and biomedical properties while minimizing production costs. The SPS process was conducted based on previously established procedures, with a sintering temperature of 900°C, a maximum pressure of 45 MPa, and a duration of 4 minutes. The prepared powders (Table 1) were loaded into graphite molds with an inner diameter of 25 mm and a height of 50 mm. The samples were then subjected to spark plasma sintering under the specified conditions to consolidate the powder while maintaining the structural integrity of the material. The SSE process was performed at room temperature to refine the microstructure and improve the mechanical properties of the titanium samples sintered via the SPS method. This process was carried out with a maximum of two passes, which have been shown to effectively enhance properties, such as hardness and tensile strength through SPD. The SSE mold used was specifically designed and optimized for titanium extrusion. The two-pass SSE method was selected for its proven ability to achieve grain refinement and improve mechanical strength without excessively altering the material’s original microstructure. In addition, a lubricant was used during the extrusion process to minimize friction and improve material flow efficiency. A commonly used lubricant for this purpose is stearic acid, which helps reduce wear and enhance processing stability during titanium extrusion. By optimizing both processes—SPS for consolidation and SSE for microstructure refinement—the study aimed to achieve an ideal balance of mechanical properties and biocompatibility, making the titanium components suitable for demanding applications such as biomedical implants (Figures 1 and 2).
Table 1. Specifications of the Ti powder used

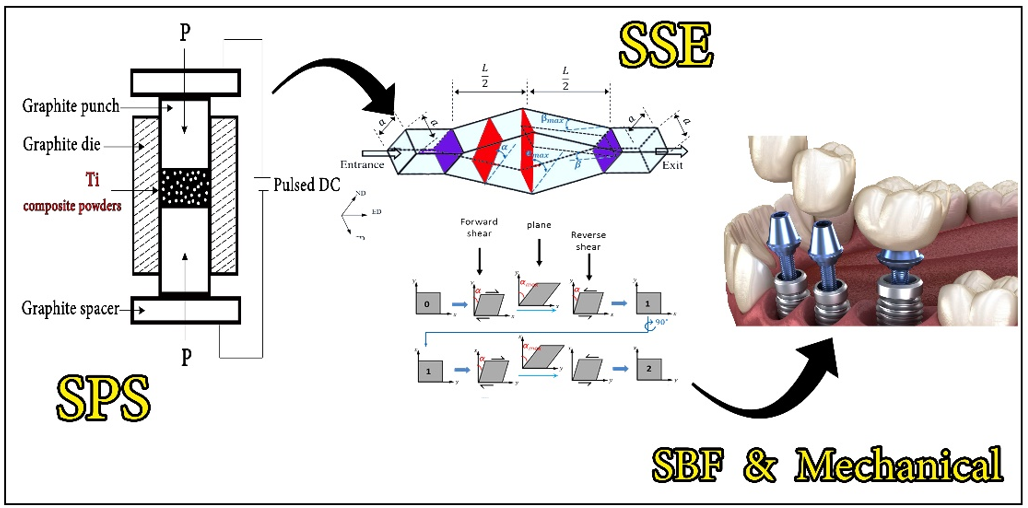
Figure 1. Schematic of the fabrication cycle.
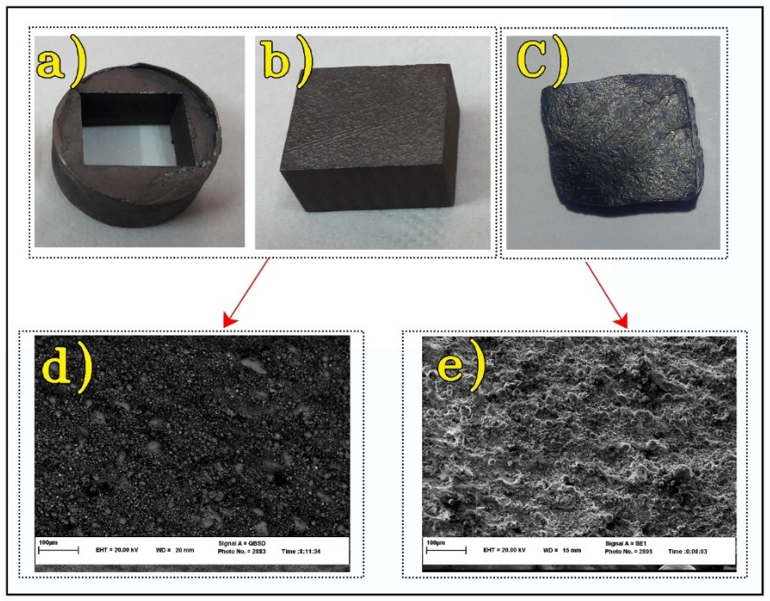
Figure 2. Fabricated parts: a) Sintered initial sample, b) Cut sample for spark plasma sintering (SPS) die, c) Post-simple shear extrusion (SSE) sample, d) SEM image of the sintered initial sample, e) SEM image of the post-SSE sample.
Characterization
To comprehensively analyze the existing phases and evaluate the impact of different stages, X-ray diffraction (XRD) analysis was performed using an explorer instrument manufactured by GNR (Italy). Morphological changes in the fabricated samples were examined utilizing a field emission scanning electron microscope (FESEM) model MIRA3 from TESCAN (Czech Republic), equipped with a resolution of up to 1.5 nm at an operating voltage of 15 kV. The hardness of the samples was determined using a Vickers hardness tester (model KOOPA-UV1) under a 30 kg load and a dwell time of 10 seconds. To ensure accuracy, at least three measurements were taken for each sample. This comprehensive methodology ensures precise characterization and reliability of the mechanical and structural properties of the samples.
Findings
XRD and Williamson-Hall Analysis
After performing SPS at 900°C, the XRD pattern of titanium revealed significant changes in its phase structure, which notably affected its mechanical properties and biocompatibility. The pronounced reduction in the intensity of the peak at approximately 40°, associated with the alpha (α) phase of titanium, suggests a decrease in the amount of this phase or alterations in its crystalline structure. Such a decrease could be attributed to grain refinement or the redistribution of crystallographic orientations during the extrusion process. Additionally, the emergence of a new peak at approximately 42° raised the possibility of phase transformation or the formation of a secondary phase. This transformation may involve the stabilization of a metastable phase with distinct crystallographic characteristics. Following the synthesis of titanium samples via SPS at 900°C and subsequent exposure to SPD through SSE, significant alterations in the material’s structural and mechanical properties were observed. The primary titanium peak, initially located at 40° 2θ, shifted slightly in the positive direction and appeared at approximately 43°. This shift indicates modifications in the crystal lattice structure, potentially resulting from the stress introduced during the SSE process. Such changes in peak position may be attributed to increased lattice strain, phase transformation, or alterations in the crystallite size induced by severe deformation. Williamson-Hall introduced grain size and lattice strains as factors contributing to the broadening of XRD peaks. According to the theory proposed by Williamson-Hall, the peak width at half of the maximum intensity is a function of both particle size and lattice strains:
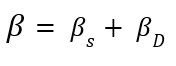
βs and βD represent the broadening of peaks, which is sequentially caused by grain size and lattice strains. By analyzing the broadening of diffraction peaks, this method provides valuable information about internal strains and crystallite sizes in the material. Moreover, an optimal and uniform strain distribution can enhance corrosion resistance, as regions with high strain concentration are usually the initiation sites for corrosion [20]. Therefore, using the Williamson-Hall method and precise analysis of XRD data can lead to improved mechanical properties and increased durability of materials under various environmental conditions:
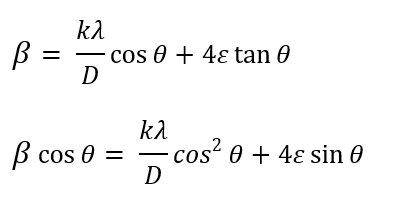
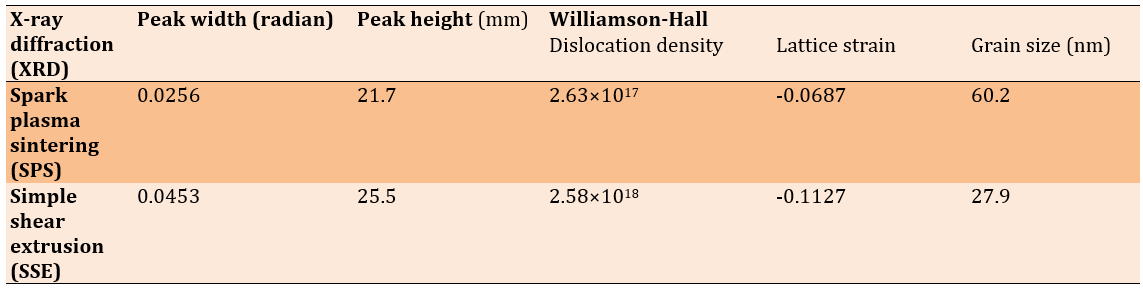
The former is obtained by multiplying both sides of the latter by cosinus θ. According to Williamson-Hall analysis, the grain size in the sintered sample was 60.6 nm, which was reduced to 27.2 nm after extrusion. This reduction in grain size, facilitated by the extrusion process, led to a considerable increase in the hardness of the sample. According to the Williamson-Hall analysis, grain size and lattice strains are factors contributing to the broadening of XRD peaks. The broadening of peaks is sequentially caused by grain size and lattice strains. By analyzing the broadening of diffraction peaks, this method provides valuable information about internal strains and crystallite sizes in the material. Moreover, an optimal and uniform strain distribution can enhance corrosion resistance, as regions with high strain concentration are usually the initiation sites for corrosion [20]. Therefore, using the Williamson-Hall method and precise analysis of XRD data can lead to improved mechanical properties and increased durability of materials under various environmental conditions. The grain size in the sintered sample was 60.6 nm, which was reduced to 27.2 nm after extrusion. This reduction in grain size, facilitated by the extrusion process, led to a considerable increase in the hardness of the sample. The fine-grained structure is correlated with a higher dislocation density and increased lattice strain in the extruded sample, indicating an improvement in mechanical properties due to SPD. The dislocation density in the extruded sample increased to 2.58×10¹⁸ m⁻², a significant rise compared to the sintered sample, which had a dislocation density of 2.63×10¹⁷ m⁻² (Figures 3 and 4 and Table 2).
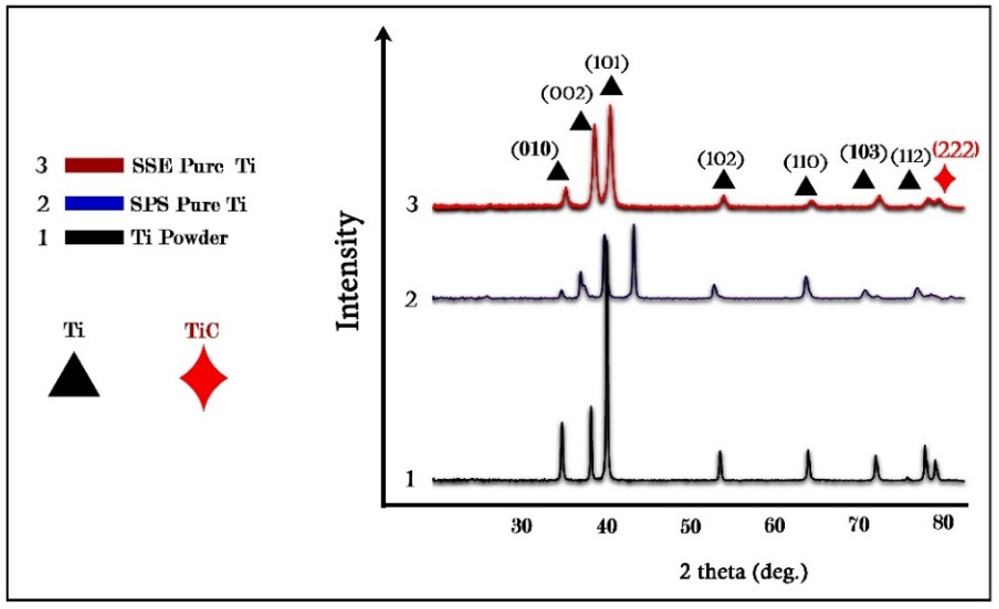
Figure 3. XRD results.
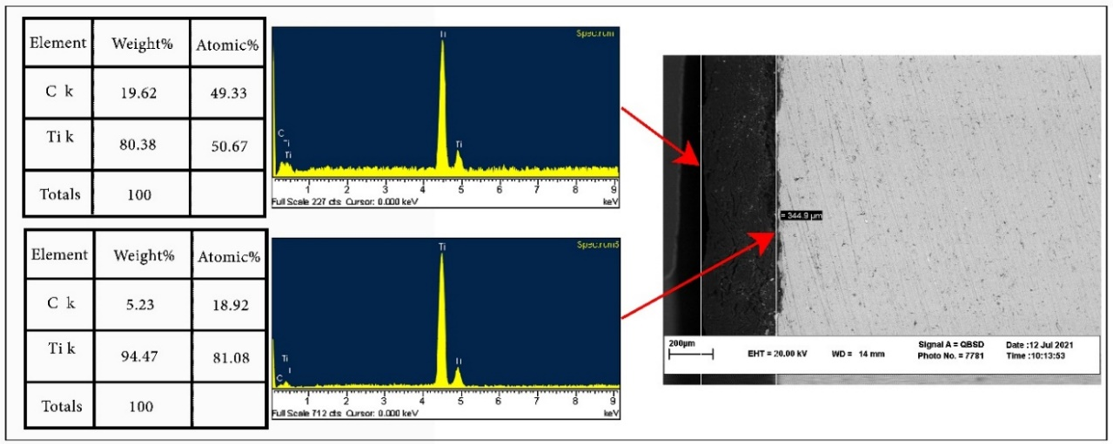
Figure 4. Carbon penetration from the spark plasma sintering (SPS) die into the workpiece.
Table 2. Extracted results from the XRD test and Williamson-Hall analysis
Hardness analysis
Hardness measurements revealed a significant increase in the extruded sample compared to the sintered one. The hardness of the sintered sample was 320 HV, whereas, after extrusion, the hardness increased to 415 HV. This improvement is well documented in the literature, where decreased grain size increases the number of grain boundaries, serving as effective barriers to dislocation motion [21]. This increase can be attributed to the reduction in grain size, which follows the Hall-Petch relationship, where smaller grains enhance the material’s strength and hardness due to the increased grain boundary area, restricting dislocation motion. Additionally, the high dislocation density and increased lattice strain in the extruded sample further contributed to its improved mechanical performance.
Simulated body fluid (SBF) immersion test
Grain refinement of titanium has a positive impact on its biocompatibility, especially in biomedical fields [22]. Therefore, to evaluate the biocompatibility of the processed titanium samples, they were immersed in SBF for 14 days. The surface morphology analysis after immersion indicated enhanced bioactivity in the extruded sample compared to the sintered one. The presence of hydroxyapatite-like deposits was observed on the surface of the extruded sample, suggesting superior biointegration potential. The increased surface roughness due to SPD promotes better nucleation sites for calcium phosphate precipitation, which is essential for osseointegration in biomedical implants. Additionally, the formation of TiO₂ on the surface of the extruded sample contributes to its improved corrosion resistance and biocompatibility, making it a promising candidate for medical applications. Furthermore, the SBF solution was periodically replaced (every two to three days) to prevent any changes in its ionic composition [23] (Table 3 and Figure 5).
Table 3. Comparison of soluble compounds of simulated body fluid (SBF) and human blood plasma
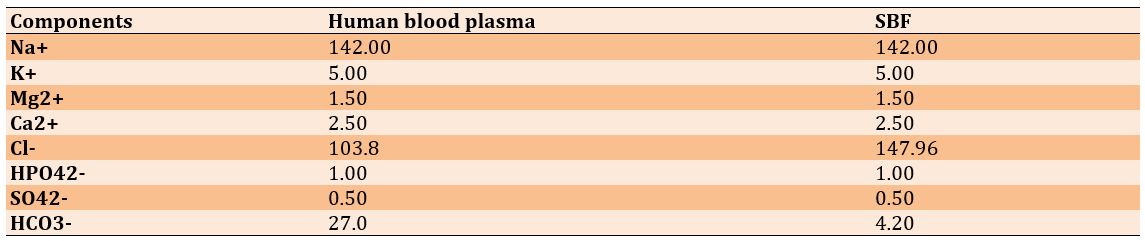
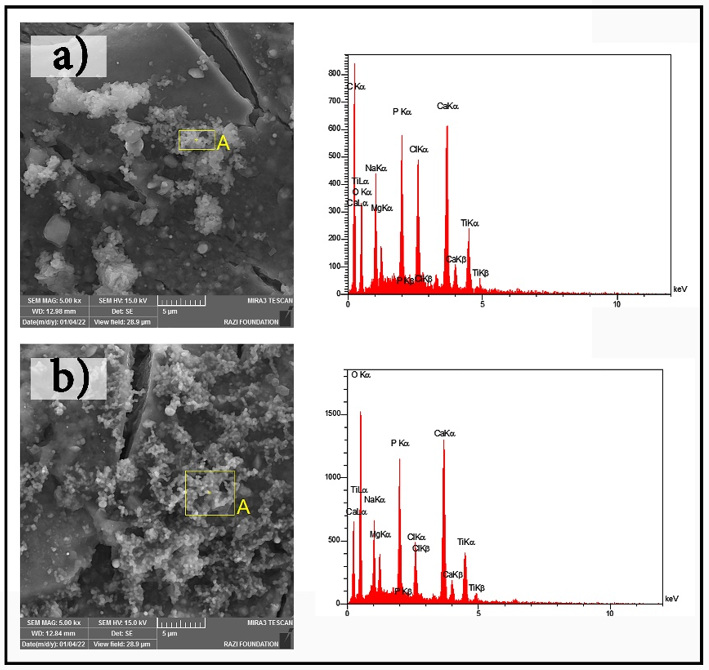
Figure 5. SEM images of bone-like growth: a) spark plasma sintering (SPS) and b) simple shear extrusion (SSE).
Discussion
The present study aimed to address this gap by examining titanium samples processed via SPS and optimized using SSE. The observed changes in the XRD pattern and the increased lattice strain resulting from the SSE process are consistent with results from previous studies on the effects of SPD on the structural and mechanical properties of materials. Previous research has shown that reducing grain size and altering the crystal structure are correlated with improvements in mechanical properties, such as hardness and tensile strength [21]. In this study, the reduction in grain size from 60.6 nm to 27.2 nm and the increase in dislocation density to 2.58×10¹⁸ m⁻² in the extruded samples are consistent with findings from other studies, indicating that increased dislocation density enhances resistance to plastic deformation and improves tensile strength. The increase in hardness from 315 to 411 HV is particularly noteworthy, as previous studies have also demonstrated that a decrease in grain size below 30 nm significantly enhances hardness and strength. This increase in hardness is highly beneficial for biomedical applications, particularly in implantable devices where mechanical stability and wear resistance are critical factors.
The emergence of a new peak at approximately 62.5°, attributed to titanium oxide, was a significant observation. The formation of this oxide phase can enhance biocompatibility, as titanium oxide is known to interact favorably with biological tissues and promote better tissue growth [20]. Furthermore, the formation of the TiC phase in titanium composites could also play a crucial role in improving the mechanical properties of titanium, especially in terms of hardness and wear resistance, which are critical for medical implant applications. Topolski et al. [24] have obtained a maximum hardness of 279 HV for commercially pure titanium after SSE under optimal experimental conditions. However, in this research, the hardness of commercially pure titanium reached 411 HV after a single pass of SSE. TiC is a highly stable and wear-resistant phase that can increase the longevity and performance of implants, especially when subjected to severe mechanical forces and wear within the body. The presence of TiC has not been fully validated for medical applications but has gained attention in research on in vivo implants. Its potential to improve the mechanical and biological performance of titanium implants is promising. The harder and more wear-resistant surface of TiC can also reduce the release of wear particles from the implant surface, which could otherwise lead to inflammatory responses and long-term complications. Thus, the formation of the TiC phase in titanium-based implants could significantly enhance their mechanical and biological performance, improving their lifespan and efficacy in medical applications. One of the most prominent shortcomings in previous studies that have refined commercially pure titanium through SSE is the lack of attention to and discussion of the biocompatibility aspects of the processed material [25, 24].
The successful fabrication of fine-grained titanium components was achieved through the synergistic combination of SPS and SSE, leading to significant improvements in both mechanical properties and biocompatibility, making them highly relevant for medical applications. The incorporation of the TiC phase into the titanium matrix notably enhanced its mechanical properties, including a remarkable increase in hardness (up to 411 HV) and chemical stability. These improvements are essential for extending the service life of medical implants, particularly under physiological conditions. Additionally, the SSE process further refined the microstructure, contributing to better integration with bone-like tissues, as evidenced by data from SBF studies. These findings highlight the promising potential of combining SPS and SSE methods to advance the production of high-performance medical implants. The ability to tailor the material properties precisely for clinical needs, such as improved durability and lower failure rates, positions these techniques as viable alternatives to conventional titanium manufacturing methods, especially in critical biomedical applications. These implants promise extended longevity and increased reliability, addressing the growing demand for more durable medical solutions. Future research should focus on optimizing these techniques for large-scale production and exploring their potential for complex implant geometries.
Conclusion
The fusion of SPS and SSE not only enhances the mechanical integrity and biocompatibility of titanium components but also establishes a solid foundation for developing next-generation medical implants.
Acknowledgments: The authors would like to express their appreciation to Bargaz (https://bargaz.ir/-Iran, Gonabad) and Ms. Maryam Mohammadzadeh (Iran, Gonabad) for their constructive support, which has greatly benefited the authors.
Ethical Permissions: This research was conducted on a laboratory scale, and obtaining an ethics code was not required.
Conflicts of Interests: The authors confirm that there are no financial interests or personal relationships that could have influenced the outcomes of the study presented in this paper.
Authors' Contribution: Sedehi SM (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer (40%); Sharifi SN (Second Author), Introduction Writer/Methodologist/Assistant Researcher (15%); Dastmard AM (Third Author), Assistant Researcher/Discussion Writer/Statistical Analyst (15%); Darroudi N (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (15%); Dehghan Niri MS (Fifth Author), Methodologist/Assistant Researcher/Statistical Analyst (15%)
Funding/Support: This research did not receive any financial support.
Titanium is widely recognized as the preferred material for orthopedic applications, particularly in components that endure substantial cyclic mechanical loads. Examples include stems and cups in shoulder, hip, knee, and ankle joints, where polymeric materials fail to meet the required mechanical strength [1]. Beyond strength, titanium implants exhibit excellent osseointegration properties over time, which can be further enhanced by incorporating cellular solid morphologies [2, 3]. However, despite extensive research and various proposed solutions [4], titanium is unsuitable for wear-intensive components, such as femoral heads in hip arthroplasty or femoral components in knee arthroplasty [5, 6]. The native titanium oxide layer on titanium and its alloys, although acting as a barrier, is not entirely inert.
Bone tissue can adhere to titanium surfaces, enabling complete osseointegration of devices over time [7-9]. Dental implants, in particular, demand high strength, superior corrosion resistance, and excellent biocompatibility to meet rigorous biological and mechanical criteria. Among these, titanium and its alloys are now the leading choice due to their high strength, low density, reduced elastic modulus, and superior corrosion resistance [10]. While commercially pure titanium (cp-Ti) is commonly employed, its relatively low strength (~300 MPa) restricts its reliability in load-bearing applications, such as dental screws, despite its outstanding corrosion resistance and biocompatibility [11]. Bone diseases and fractures have become increasingly prevalent worldwide, with over 50% of women and 20% of men above 50 years of age experiencing fractures in their lifetime. Such conditions often necessitate surgical interventions like complete knee or hip replacements or the implantation of temporary or permanent components. Jin & Chu [12] emphasized the role of medical materials in reconstructing alveolar bone, noting that metallic implants can degrade over time due to corrosion or wear, releasing ions into the biological environment. This degradation can trigger cytotoxicity, inflammatory responses, and other adverse reactions, ultimately compromising the biocompatibility of the material [13].
Despite the extensive use of the Ti-6Al-4V alloy, concerns over ion release and long-term biological safety persist [14]. An emerging solution involves using commercially pure titanium enhanced through severe plastic deformation (SPD) techniques, such as equal channel angular pressing (ECAP), simple shear extrusion (SSE), accumulative roll bonding (ARB), and high-pressure torsion (HPT) [13]. Among SPD techniques, SSE has proven especially effective when combined with spark plasma sintering (SPS). This synergistic approach enables rapid densification, initial grain refinement, and ultrafine grain structures, significantly improving mechanical properties. The combination’s cost-effectiveness, reduced processing time, and superior outcomes make it a promising strategy for producing advanced titanium implants [15, 16]. Specifically, ultrafine-grained structures (grain sizes below one micrometer) have been pivotal in enhancing mechanical properties, although their tribological performance requires further investigation [17]. Suwas et al. [18] demonstrated the dynamic restoration processes in commercially pure titanium during ECAE, achieving good alignment between experimental textures and simulations using the VPSC model.
Sedehi et al. [19] successfully improved the properties of commercially pure titanium through spark plasma sintering and SPD methods. Despite these advancements, more research is needed to explore the interplay between mechanical properties and biocompatibility in medical implants. The present study aimed to address this gap by examining titanium samples processed via SPS and optimized using SSE. Advanced mechanical testing and XRD peak analysis were employed to understand the microstructural changes and their impact on the material’s performance. The findings underscore the potential of these methods to enhance implant longevity and improve patient outcomes.
Materials and Methods
In this experimental study conducted in Gonabad, Iran in 2024, the primary focus was on optimizing the conditions of the two key processes, SPS and SSE, to enhance both mechanical and biomedical properties while minimizing production costs. The SPS process was conducted based on previously established procedures, with a sintering temperature of 900°C, a maximum pressure of 45 MPa, and a duration of 4 minutes. The prepared powders (Table 1) were loaded into graphite molds with an inner diameter of 25 mm and a height of 50 mm. The samples were then subjected to spark plasma sintering under the specified conditions to consolidate the powder while maintaining the structural integrity of the material. The SSE process was performed at room temperature to refine the microstructure and improve the mechanical properties of the titanium samples sintered via the SPS method. This process was carried out with a maximum of two passes, which have been shown to effectively enhance properties, such as hardness and tensile strength through SPD. The SSE mold used was specifically designed and optimized for titanium extrusion. The two-pass SSE method was selected for its proven ability to achieve grain refinement and improve mechanical strength without excessively altering the material’s original microstructure. In addition, a lubricant was used during the extrusion process to minimize friction and improve material flow efficiency. A commonly used lubricant for this purpose is stearic acid, which helps reduce wear and enhance processing stability during titanium extrusion. By optimizing both processes—SPS for consolidation and SSE for microstructure refinement—the study aimed to achieve an ideal balance of mechanical properties and biocompatibility, making the titanium components suitable for demanding applications such as biomedical implants (Figures 1 and 2).
Table 1. Specifications of the Ti powder used


Figure 1. Schematic of the fabrication cycle.

Figure 2. Fabricated parts: a) Sintered initial sample, b) Cut sample for spark plasma sintering (SPS) die, c) Post-simple shear extrusion (SSE) sample, d) SEM image of the sintered initial sample, e) SEM image of the post-SSE sample.
Characterization
To comprehensively analyze the existing phases and evaluate the impact of different stages, X-ray diffraction (XRD) analysis was performed using an explorer instrument manufactured by GNR (Italy). Morphological changes in the fabricated samples were examined utilizing a field emission scanning electron microscope (FESEM) model MIRA3 from TESCAN (Czech Republic), equipped with a resolution of up to 1.5 nm at an operating voltage of 15 kV. The hardness of the samples was determined using a Vickers hardness tester (model KOOPA-UV1) under a 30 kg load and a dwell time of 10 seconds. To ensure accuracy, at least three measurements were taken for each sample. This comprehensive methodology ensures precise characterization and reliability of the mechanical and structural properties of the samples.
Findings
XRD and Williamson-Hall Analysis
After performing SPS at 900°C, the XRD pattern of titanium revealed significant changes in its phase structure, which notably affected its mechanical properties and biocompatibility. The pronounced reduction in the intensity of the peak at approximately 40°, associated with the alpha (α) phase of titanium, suggests a decrease in the amount of this phase or alterations in its crystalline structure. Such a decrease could be attributed to grain refinement or the redistribution of crystallographic orientations during the extrusion process. Additionally, the emergence of a new peak at approximately 42° raised the possibility of phase transformation or the formation of a secondary phase. This transformation may involve the stabilization of a metastable phase with distinct crystallographic characteristics. Following the synthesis of titanium samples via SPS at 900°C and subsequent exposure to SPD through SSE, significant alterations in the material’s structural and mechanical properties were observed. The primary titanium peak, initially located at 40° 2θ, shifted slightly in the positive direction and appeared at approximately 43°. This shift indicates modifications in the crystal lattice structure, potentially resulting from the stress introduced during the SSE process. Such changes in peak position may be attributed to increased lattice strain, phase transformation, or alterations in the crystallite size induced by severe deformation. Williamson-Hall introduced grain size and lattice strains as factors contributing to the broadening of XRD peaks. According to the theory proposed by Williamson-Hall, the peak width at half of the maximum intensity is a function of both particle size and lattice strains:
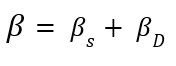
βs and βD represent the broadening of peaks, which is sequentially caused by grain size and lattice strains. By analyzing the broadening of diffraction peaks, this method provides valuable information about internal strains and crystallite sizes in the material. Moreover, an optimal and uniform strain distribution can enhance corrosion resistance, as regions with high strain concentration are usually the initiation sites for corrosion [20]. Therefore, using the Williamson-Hall method and precise analysis of XRD data can lead to improved mechanical properties and increased durability of materials under various environmental conditions:
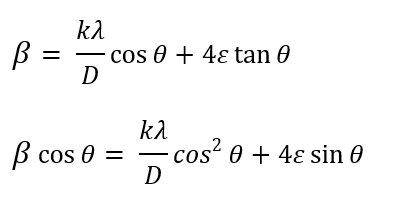
The former is obtained by multiplying both sides of the latter by cosinus θ. According to Williamson-Hall analysis, the grain size in the sintered sample was 60.6 nm, which was reduced to 27.2 nm after extrusion. This reduction in grain size, facilitated by the extrusion process, led to a considerable increase in the hardness of the sample. According to the Williamson-Hall analysis, grain size and lattice strains are factors contributing to the broadening of XRD peaks. The broadening of peaks is sequentially caused by grain size and lattice strains. By analyzing the broadening of diffraction peaks, this method provides valuable information about internal strains and crystallite sizes in the material. Moreover, an optimal and uniform strain distribution can enhance corrosion resistance, as regions with high strain concentration are usually the initiation sites for corrosion [20]. Therefore, using the Williamson-Hall method and precise analysis of XRD data can lead to improved mechanical properties and increased durability of materials under various environmental conditions. The grain size in the sintered sample was 60.6 nm, which was reduced to 27.2 nm after extrusion. This reduction in grain size, facilitated by the extrusion process, led to a considerable increase in the hardness of the sample. The fine-grained structure is correlated with a higher dislocation density and increased lattice strain in the extruded sample, indicating an improvement in mechanical properties due to SPD. The dislocation density in the extruded sample increased to 2.58×10¹⁸ m⁻², a significant rise compared to the sintered sample, which had a dislocation density of 2.63×10¹⁷ m⁻² (Figures 3 and 4 and Table 2).

Figure 3. XRD results.

Figure 4. Carbon penetration from the spark plasma sintering (SPS) die into the workpiece.
Table 2. Extracted results from the XRD test and Williamson-Hall analysis

Hardness analysis
Hardness measurements revealed a significant increase in the extruded sample compared to the sintered one. The hardness of the sintered sample was 320 HV, whereas, after extrusion, the hardness increased to 415 HV. This improvement is well documented in the literature, where decreased grain size increases the number of grain boundaries, serving as effective barriers to dislocation motion [21]. This increase can be attributed to the reduction in grain size, which follows the Hall-Petch relationship, where smaller grains enhance the material’s strength and hardness due to the increased grain boundary area, restricting dislocation motion. Additionally, the high dislocation density and increased lattice strain in the extruded sample further contributed to its improved mechanical performance.
Simulated body fluid (SBF) immersion test
Grain refinement of titanium has a positive impact on its biocompatibility, especially in biomedical fields [22]. Therefore, to evaluate the biocompatibility of the processed titanium samples, they were immersed in SBF for 14 days. The surface morphology analysis after immersion indicated enhanced bioactivity in the extruded sample compared to the sintered one. The presence of hydroxyapatite-like deposits was observed on the surface of the extruded sample, suggesting superior biointegration potential. The increased surface roughness due to SPD promotes better nucleation sites for calcium phosphate precipitation, which is essential for osseointegration in biomedical implants. Additionally, the formation of TiO₂ on the surface of the extruded sample contributes to its improved corrosion resistance and biocompatibility, making it a promising candidate for medical applications. Furthermore, the SBF solution was periodically replaced (every two to three days) to prevent any changes in its ionic composition [23] (Table 3 and Figure 5).
Table 3. Comparison of soluble compounds of simulated body fluid (SBF) and human blood plasma


Figure 5. SEM images of bone-like growth: a) spark plasma sintering (SPS) and b) simple shear extrusion (SSE).
Discussion
The present study aimed to address this gap by examining titanium samples processed via SPS and optimized using SSE. The observed changes in the XRD pattern and the increased lattice strain resulting from the SSE process are consistent with results from previous studies on the effects of SPD on the structural and mechanical properties of materials. Previous research has shown that reducing grain size and altering the crystal structure are correlated with improvements in mechanical properties, such as hardness and tensile strength [21]. In this study, the reduction in grain size from 60.6 nm to 27.2 nm and the increase in dislocation density to 2.58×10¹⁸ m⁻² in the extruded samples are consistent with findings from other studies, indicating that increased dislocation density enhances resistance to plastic deformation and improves tensile strength. The increase in hardness from 315 to 411 HV is particularly noteworthy, as previous studies have also demonstrated that a decrease in grain size below 30 nm significantly enhances hardness and strength. This increase in hardness is highly beneficial for biomedical applications, particularly in implantable devices where mechanical stability and wear resistance are critical factors.
The emergence of a new peak at approximately 62.5°, attributed to titanium oxide, was a significant observation. The formation of this oxide phase can enhance biocompatibility, as titanium oxide is known to interact favorably with biological tissues and promote better tissue growth [20]. Furthermore, the formation of the TiC phase in titanium composites could also play a crucial role in improving the mechanical properties of titanium, especially in terms of hardness and wear resistance, which are critical for medical implant applications. Topolski et al. [24] have obtained a maximum hardness of 279 HV for commercially pure titanium after SSE under optimal experimental conditions. However, in this research, the hardness of commercially pure titanium reached 411 HV after a single pass of SSE. TiC is a highly stable and wear-resistant phase that can increase the longevity and performance of implants, especially when subjected to severe mechanical forces and wear within the body. The presence of TiC has not been fully validated for medical applications but has gained attention in research on in vivo implants. Its potential to improve the mechanical and biological performance of titanium implants is promising. The harder and more wear-resistant surface of TiC can also reduce the release of wear particles from the implant surface, which could otherwise lead to inflammatory responses and long-term complications. Thus, the formation of the TiC phase in titanium-based implants could significantly enhance their mechanical and biological performance, improving their lifespan and efficacy in medical applications. One of the most prominent shortcomings in previous studies that have refined commercially pure titanium through SSE is the lack of attention to and discussion of the biocompatibility aspects of the processed material [25, 24].
The successful fabrication of fine-grained titanium components was achieved through the synergistic combination of SPS and SSE, leading to significant improvements in both mechanical properties and biocompatibility, making them highly relevant for medical applications. The incorporation of the TiC phase into the titanium matrix notably enhanced its mechanical properties, including a remarkable increase in hardness (up to 411 HV) and chemical stability. These improvements are essential for extending the service life of medical implants, particularly under physiological conditions. Additionally, the SSE process further refined the microstructure, contributing to better integration with bone-like tissues, as evidenced by data from SBF studies. These findings highlight the promising potential of combining SPS and SSE methods to advance the production of high-performance medical implants. The ability to tailor the material properties precisely for clinical needs, such as improved durability and lower failure rates, positions these techniques as viable alternatives to conventional titanium manufacturing methods, especially in critical biomedical applications. These implants promise extended longevity and increased reliability, addressing the growing demand for more durable medical solutions. Future research should focus on optimizing these techniques for large-scale production and exploring their potential for complex implant geometries.
Conclusion
The fusion of SPS and SSE not only enhances the mechanical integrity and biocompatibility of titanium components but also establishes a solid foundation for developing next-generation medical implants.
Acknowledgments: The authors would like to express their appreciation to Bargaz (https://bargaz.ir/-Iran, Gonabad) and Ms. Maryam Mohammadzadeh (Iran, Gonabad) for their constructive support, which has greatly benefited the authors.
Ethical Permissions: This research was conducted on a laboratory scale, and obtaining an ethics code was not required.
Conflicts of Interests: The authors confirm that there are no financial interests or personal relationships that could have influenced the outcomes of the study presented in this paper.
Authors' Contribution: Sedehi SM (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer (40%); Sharifi SN (Second Author), Introduction Writer/Methodologist/Assistant Researcher (15%); Dastmard AM (Third Author), Assistant Researcher/Discussion Writer/Statistical Analyst (15%); Darroudi N (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (15%); Dehghan Niri MS (Fifth Author), Methodologist/Assistant Researcher/Statistical Analyst (15%)
Funding/Support: This research did not receive any financial support.
Keywords:
References
1. Marin E, Lanzutti A. Biomedical applications of titanium alloys: A comprehensive review. Materials. 2024;17(1):114. [Link] [DOI:10.3390/ma17010114]
2. Zaid MB, O'Donnell RJ, Potter BK, Forsberg JA. Orthopaedic osseointegration: State of the art. J Am Acad Orthop Surg. 2019;27(22):e977-85. [Link] [DOI:10.5435/JAAOS-D-19-00016]
3. Marin E, Fedrizzi L, Zagra L. Porous metallic structures for orthopaedic applications: A short review of materials and technologies. Eur Orthop Traumatol. 2010;1:103-9. [Link] [DOI:10.1007/s12570-010-0020-z]
4. Raimondi MT, Pietrabissa R. The in-vivo wear performance of prosthetic femoral heads with titanium nitride coating. Biomaterials. 2000;21(9):907-13. [Link] [DOI:10.1016/S0142-9612(99)00246-X]
5. Lalor PA, Revell PA, Gray AB, Wright S, Railton GT, Freeman MA. Sensitivity to titanium. A cause of implant failure?. J Bone Joint Surg Br. 1991;73(1):25-8. [Link] [DOI:10.1302/0301-620X.73B1.1991768]
6. Jacobs JJ, Silverton C, Hallab NJ, Skipor AK, Patterson L, Black J, et al. Metal release and excretion from cementless titanium alloy total knee replacements. Clin Orthop Relat Res. 1999;(358):173-80. [Link] [DOI:10.1097/00003086-199901000-00021]
7. Jacobs Z, Schipani R, Pastrama M, Ahmadi SM, Sajadi B. Evaluation of biocompatibility and osseointegration of multi‐component TiAl6V4 titanium alloy implants. J Orthop Res. 2025;43(1):139-52. [Link] [DOI:10.1002/jor.25974]
8. Branemark R, Branemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: A review. J Rehabil Res Dev. 2001;38(2):175-82. [Link]
9. Yang S, Jiang W, Ma X, Wang Z, Sah RL, Wang J, et al. Nanoscale morphologies on the surface of 3D-printed titanium implants for improved osseointegration: A systematic review of the literature. Int J Nanomed. 2023;18:4171-91. [Link] [DOI:10.2147/IJN.S409033]
10. Pałka K, Pokrowiecki R. Porous titanium implants: A review. Adv Eng Mater. 2018;20(5):1700648. [Link] [DOI:10.1002/adem.201700648]
11. Niu J, Guo Y, Li K, Liu W, Dan Z, Sun Z, et al. Improved mechanical, bio-corrosion properties and in vitro cell responses of Ti-Fe alloys as candidate dental implants. Mater Sci Eng C. 2021;122:111917. [Link] [DOI:10.1016/j.msec.2021.111917]
12. Jin W, Chu PK. Orthopedic implants. Encycl Biomed Eng. 2019;1(3):425-39. [Link] [DOI:10.1016/B978-0-12-801238-3.10999-7]
13. Wang K. The biological effects of corrosion products from titanium implants. BioMetals; 2019. [Link]
14. Rominiyi AL, Mashinini PM, Rominiyi OL. Microstructure, phase evolution and mechanical properties of nickel-silicon carbide reinforced Ti6Al4V alloy processed by pulsed electric current sintering. Ceram Int. 2024;50(18):33926-36. [Link] [DOI:10.1016/j.ceramint.2024.06.212]
15. Valiev RZ, Estrin Y, Horita Z, Langdon TG, Zechetbauer MJ, Zhu YT. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM. 2006;58:33-9. [Link] [DOI:10.1007/s11837-006-0213-7]
16. Estrin Y, Vinogradov A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013;61(3):782-817. [Link] [DOI:10.1016/j.actamat.2012.10.038]
17. Shi X, Wang X, Zhang J, Du H. High-temperature tribological behavior of the Al/Mg/Cu multilayered composite produced by the severe plastic deformation. Tribol Int. 2024;199:110037. [Link] [DOI:10.1016/j.triboint.2024.110037]
18. Suwas S, Beausir B, Tóth LS, Fundenberger JJ, Gottstein G. Texture evolution in commercially pure titanium after warm equal channel angular extrusion. Acta Mater. 2011;59(3):1121-33. [Link] [DOI:10.1016/j.actamat.2010.10.045]
19. Sedehi SM, Khosravi M, Yaghoubinezhad Y. Mechanical properties and microstructures of reduced graphene oxide reinforced titanium matrix composites produced by spark plasma sintering and simple shear extrusion. Ceram Int. 2021;47(23):33180-90. [Link] [DOI:10.1016/j.ceramint.2021.08.219]
20. Alam MK, Hossain MS, Bahadur NM, Ahmed S. A comparative study in estimating of crystallite sizes of synthesized and natural hydroxyapatites using Scherrer Method, Williamson-Hall model, Size-Strain Plot and Halder-Wagner Method. J Mol Struct. 2024;1306:137820. [Link] [DOI:10.1016/j.molstruc.2024.137820]
21. Izi A, Honarpisheh M, Ahmadi F. Investigation of mechanical properties and residual stress in the combined simple shear extrusion-forward extrusion (CSSE-FE) process of 1050 aluminum alloy. Proceedings of the Institution of Mechanical Engineers, Part L: Journal of Materials: Design and Applications; 2025. [Link] [DOI:10.1177/14644207241308975]
22. Sotniczuk A, Chromiński W, Adamczyk-Cieślak B, Pisarek M, Garbacz H. Corrosion behaviour of biomedical Ti under simulated inflammation: Exploring the relevance of grain refinement and crystallographic texture. Corros Sci. 2022;200:110238. [Link] [DOI:10.1016/j.corsci.2022.110238]
23. Salas L, Chávez J, Jimenez O, Flores-Jimenez M, Alvarado-Hernandez F, Olmos L, et al. Tribocorrosion and corrosion behavior of quaternary Ti-24Nb-xZr-ySn alloys in SBF. Mater Lett. 2021;283:128903. [Link] [DOI:10.1016/j.matlet.2020.128903]
24. Topolski K, Pachla W, Garbacz H. Progress in hydrostatic extrusion of titanium. J Mater Sci. 2013;48:4543-8. [Link] [DOI:10.1007/s10853-012-7086-7]
25. Thomas BM, Derguti F, Jackson M. Continuous extrusion of a commercially pure titanium powder via the Conform process. Mater Sci Technol. 2017;33(7):899-903. [Link] [DOI:10.1080/02670836.2016.1245256]










