Volume 17, Issue 1 (2025)
Iran J War Public Health 2025, 17(1): 43-49 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/12/3 | Accepted: 2025/02/20 | Published: 2025/02/25
Received: 2024/12/3 | Accepted: 2025/02/20 | Published: 2025/02/25
How to cite this article
Dhiya A, Haji Ghasem Kashani M, Osamah N, Salehi M, Manouchehri B, Elaheh A. Effect of Osteogenic Genes Expression on Bone Tissue Treatment; an Engineered Animal Model. Iran J War Public Health 2025; 17 (1) :43-49
URL: http://ijwph.ir/article-1-1524-en.html
URL: http://ijwph.ir/article-1-1524-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Pharmaceutics, College of Pharmacy, Al-Zahraa University for Women, Karbala, Iraq, dhiya@alzahraa.edu.iq
2- Department of Cellular and Molecular Biology, Faculty of Biology and Institute of Biological Sciences, Damghan University, Damghan, Iran
3- Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Al-Zahraa University for Women, Karbala, Iraq
4- Tissue Engineering and Stem Cells Research Center, Shahroud University of Medical Sciences, Shahroud, Iran
5- Department of Biology, Faculty of Optometry, Indiana University of Bloomington, Indiana, United States of America
6- Department of Cellular and Molecular Biology, Faculty of Biology, Damghan University, Damghan, Iran
2- Department of Cellular and Molecular Biology, Faculty of Biology and Institute of Biological Sciences, Damghan University, Damghan, Iran
3- Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Al-Zahraa University for Women, Karbala, Iraq
4- Tissue Engineering and Stem Cells Research Center, Shahroud University of Medical Sciences, Shahroud, Iran
5- Department of Biology, Faculty of Optometry, Indiana University of Bloomington, Indiana, United States of America
6- Department of Cellular and Molecular Biology, Faculty of Biology, Damghan University, Damghan, Iran
Full-Text (HTML) (435 Views)
Introduction
Tissue engineering is recognized as a significant approach in bone reconstruction. Functional scaffolds must initially meet the necessary mechanical strength to replace the mechanical function lost in damaged tissues in tissue engineering. The scaffold's strength is crucial for providing adequate structural support and transferring reinforcing forces to the host tissue site. Tissue reconstruction and cell function are important for achieving angiogenesis and stable biomechanical conditions at the host site. Thus, the three-dimensional structure of the scaffold must maintain sufficient structural integrity under both in Vitro and Vivo conditions and preserve the process of growth and reconstruction [1]. An effective alternative to conventional grafting strategies is bone tissue engineering. The utilization of chitosan-based biocomposite scaffolds to accelerate the development of new bone tissue has been extensively studied among polymers [2]. Chitosan is a natural biopolymer acting as a scaffold in bone tissue engineering [3]. It is the deacetylated form of chitin, a structural element found in the exoskeleton of crustaceans like shrimp and crabs, insect cuticles, and the cell walls of fungi [4-6]. Chitosan is a natural linear polymer composed of repetitive units of N-acetyl-D-glucosamine and D-glucosamine. These units are connected by β (1-4) glycosidic bonds [7-9]. Due to its cationic nature, Chitosan is extensively employed to accelerate wound healing and exhibit antimicrobial properties. This polymer is used in tissue engineering to differentiate or repair tissues such as cartilage, bone, skin, nerve, liver, and muscles [6, 8, 9]. Chitosan is also referred to as a wound-healing accelerator because it activates and regulates inflammatory cells, thereby promoting the growth of granular tissue. It also plays a protective role in cell proliferation, osteoblast differentiation, and mineralization [2]. Numerous studies have shown that consuming many vegetables and fruits daily (240-400 g) is associated with higher Bone Mineral Density (BMD) and reduced risk of bone fractures. Recent research indicates that the risk of hip fractures and osteoporosis in postmenopausal women is inversely correlated with the consumption of soy isoflavonoids. The risk of degenerative diseases, including cardiovascular diseases, cancer, diabetes, and osteoporosis, is reduced by polyphenols' antioxidant potential, which protects cells from oxidative damage. Polyphenols influence bone metabolism by suppressing osteoclastogenesis, activating osteoblastogenesis, and suppressing osteoclastogenesis, in addition to their antioxidant properties. One of the beneficial subgroups of polyphenols, flavonoids, are found in various fruits, vegetables, and plants [10, 11]. Flavonoids are predominantly found in citrus fruits. The predominant flavonoid in lemons, oranges, and sweet oranges is hesperidin rutinoside [10]. Among natural citrus flavonoids, hesperidin is a potent anticoagulant due to its pharmacological determination. Hesperidin is effective on both cortical and trabecular bone by reducing osteoclasts and preventing the absorption of trabecular bone. Hesperidin enhances the inhibition of bone absorption and increases the concentration of minerals like calcium in the bone. Hesperidin's inhibitory effects on bone absorption are associated with an unclear molecular mechanism. Known for its antioxidant activity, hesperidin is recognized as an anticancer drug. Since osteoclast inhibition reduces bone absorption, hesperidin may inhibit bone absorption through its antioxidant activity.
In contrast to other flavonoids, hesperidin's antioxidant capacity is not as high. Another hypothesis is that hesperidin affects bone cells by activating estrogen receptors (ER). ERs have been found in bone marrow stromal cells and osteoblasts. Although there is no information on hesperidin's binding affinity for ERs. Statins increase bone formation by inducing bone morphogenic proteins. Hesperidin's mechanism on bone seems to be like that of statins. Additional research is required to determine the mechanism of action of hesperidin on bone. Hesperidin reduces serum and liver cholesterol and inhibits bone absorption by inhibiting osteoclasts [12]. Hesperidin enhances the expression of Runx2 and Osx by stimulating the BMP pathway and downregulating Noggin signaling, thereby regulating the expression of mineralization genes such as OCN and Opn [13, 14].
Therefore, considering the mentioned aspects, this research aimed to investigate the effects of expressing Runx2 and OCN genes after treating femur fractures with chitosan scaffolds enriched with various concentrations of Hesperadin flavonoids.
Materials and Methods
This experimental study examined 36 twelve-week-old male Wistar rats weighing between 220 and 260g. The samples (Pasteur Institute; Iran) were maintained at 20-24°C, with a 12-hour light/dark cycle and unrestricted access to food and water. The animals underwent intramuscular ketamine/xylazine injection for anesthesia, and a 2x6mm fracture groove was made on the right femur. The rats were assigned to 6 groups (each n=6). The experiments were conducted following the Helsinki guidelines and regulations.
To construct the chitosan scaffold, 1200mg of chitosan powder is dissolved in 60cc of 1% acetic acid solution. Subsequently, hesperidin powder was added to the chitosan solution in 0.01%, 0.1%, 1%, and 10% concentrations relative to the polymer weight. A sterile scaffold was grafted onto the fracture site using chitosan (0%), chitosan+0.01% hesperidin, chitosan+0.1% hesperidin, chitosan+1% hesperidin, and chitosan+10% hesperidin. One group has not received any treatment as the control.
Following surgery, each rat received intraperitoneal injections of 200μL cefazolin (an antibiotic) and intramuscular injections of 100μl ketoprofen (an analgesic) for a maximum of five days. Following a 4-month course of treatment, the animals were slaughtered, and the femurs from the wounded legs were removed for histology and Real-time PCR analyses [15, 16].
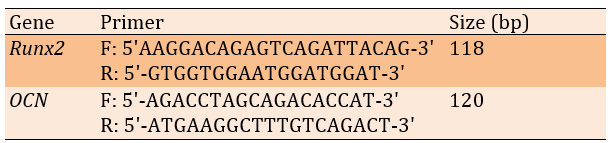
The osteogenic Runx2 (Accession number: NM_001278483) and OCN (Accession number: NM_013414) gene expression levels were quantitatively analyzed using Real-Time PCR to assess the treatment's biological impact on bone healing (Table 1).
The bone samples were embedded in paraffin after being fixed for 24 hours in 4% paraformaldehyde and then decalcified for 2-4 days using 10% EDTA. Following sectioning, Trichromasson stain (MTC) was used.
Table 1. The primers of the PCR process
Findings
The group treated with chitosan+Hesperadin scaffolds at concentrations of 1% (Hesp 1%) exhibited a significant increase in gene expression compared to the control group. Additionally, the group with 0% and 10% hesperidin showed a significant decrease in expression compared to the 1% group (p<0.05).
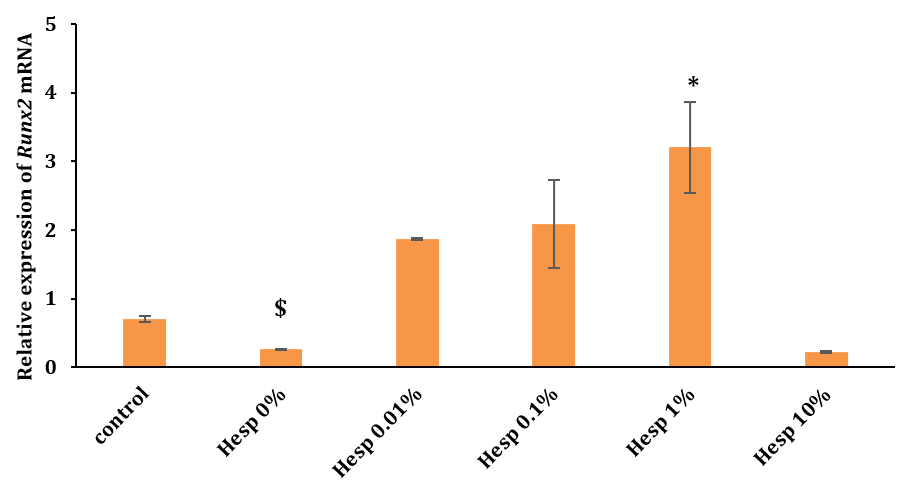
Figure 2. The levels of Runx2 gene expression across different treatment groups measured after 2 months (*: a significant difference versus the control group at <0.05; $: a significant difference versus the Hesp 10% group at <0.05)
No significant differences existed between the groups (p>0.05). This suggested that while hesperidin concentrations influenced Runx2 expression, they did not significantly affect OCN gene expression under the conditions studied. This outcome highlighted the variable impact of Hesperadin on different osteogenic markers in the context of bone healing and regeneration.
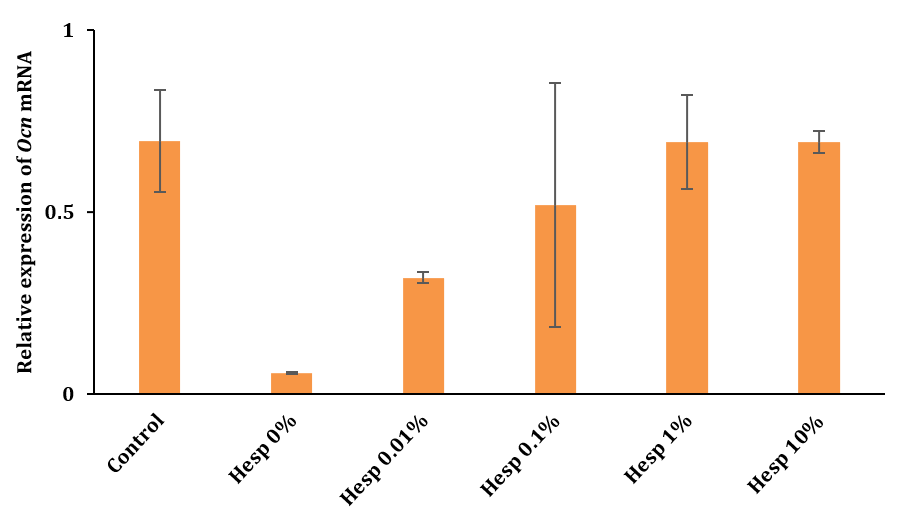
Figure 3. OCN gene expression analysis after 2 months using Real-Time PCR Technique
Histopathological assessment using MTC staining in Figure 1 (A-J) revealed that the chitosan/1% Hes-treated group (Figures A-D) displayed new bone and connective tissue replacing the defect. The Hes-treated group showed significant angiogenesis in the periphery of newly formed bone and connective tissues. The defect site in Figure 1 (E & F) displayed an organized tissue structure consisting of new bone and a cartilaginous region. In the regenerated area, MTC staining in Figure 1 (G & H) showed signs of collagen synthesis and a substantial amount of new cartilage tissue. The MTC-stained control sample in Figure 1 (I & J) did not exhibit bone fusion, which resulted in the fracture site remaining open.
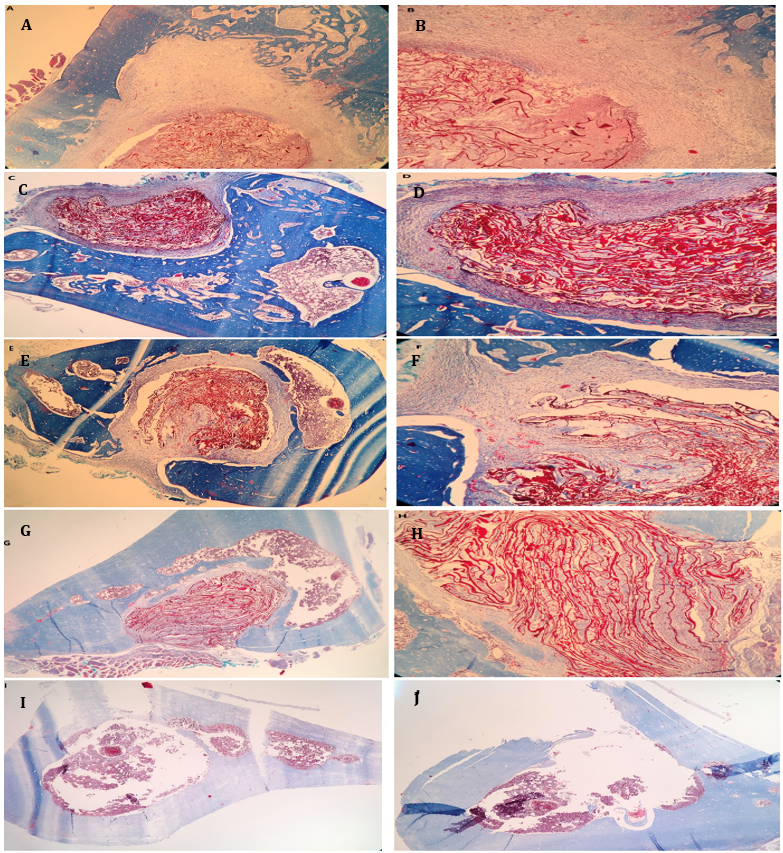
Figure 1. Histological images of bone defect regions (Masson's Trichrome staining)
Discussion
Bone transfers or grafts, such as xenografts, autografts, and allografts, are the only clinical therapeutic approaches for long-term bone reconstruction. Although these bone grafts are stimulatory and osteoconductive, they are either limited in availability or associated with complications such as bleeding, risk of infection, inadequate integration, and graft deformation, leading to reduced mechanical stability. Consequently, recent research has focused on developing alternative treatments [1, 17]. Tissue engineering is a significant therapeutic strategy for current and future medicine, intending to restore, regenerate, maintain, or enhance the function of damaged or lost tissues due to various disease conditions [6].
Tissue engineering has emerged as a crucial approach in bone reconstruction. Initially, functional scaffolds in tissue engineering must provide sufficient mechanical strength to replace the lost mechanical function in diseased or injured tissues. The structural support provided by the scaffold strength allows the transfer of reinforcing forces to the host tissue site. Cellular function and tissue reconstruction are essential for achieving stable biomechanical conditions and angiogenesis at the host site. Therefore, the three-dimensional structure of the scaffold must maintain sufficient structural integrity under both in vivo and in vitro conditions to preserve the growth and reconstruction process [1]. An ideal three-dimensional scaffold requires appropriate mechanical properties for cell adhesion, a porosity of 80-90%, and interconnected pore sizes of 50-250µm to facilitate the adequate transfer of nutrients, gases, and regulatory factors necessary for cell survival, differentiation, and proliferation [18]. Hydroxyapatite scaffolds, a material used in bone tissue engineering, are limited by their brittleness, which prevents their use in bone repair and replacement. In contrast, chitosan is an excellent candidate for bone repair due to its biodegradability, biocompatibility, antimicrobial activity, non-toxic nature, excellent pore structure, and minimal foreign body reaction [5].
Moreover, chitosan's role in enhancing cell adhesion, proliferation, differentiation of osteoblasts, and mineralization has been reported [2]. Experiments by He et al. on rat femur fractures with nano-hydroxyapatite-chitosan scaffolds loaded with BMSCs showed significant increases in bone and collagen formation and degradation compared to scaffolds without BMSCs [5]. In this study, the implanted scaffold did not elicit inflammatory responses or foreign body reactions. Both control and chitosan/1% Hes-treated groups facilitated new bone growth, but the Chitosan/1% Hes-treated group showed superior outcomes at the defect site compared to the control group. Histomorphometric analysis pointed to a higher count of osteoblasts, fibroblasts, and osteons in the Chitosan/1% Hes-treated group, whereas the control group had more chondrocytes and osteoclasts. An in vivo study in a mouse model evaluated a chitosan hydrogel infused with a natural compound with naringin, angiogenic, and anti-inflammatory properties. In the contraction phase, they illustrated the intriguing properties of chitosan by injecting it at the lesion site with a combination of active substances, which exhibited an anti-inflammatory effect on periodontal tissues [19]. Levengood & Zhang [20] used a chitosan/alginate scaffold in a rat cranial defect model, showing significant improvements in the experimental groups compared to the control after 16 weeks. Therefore, chitosan scaffolds were used in this research.
Additionally, we employed the flavonoid hesperidin as an osteogenic factor during the in vivo bone repair process [17, 20]. Hesperidin is an affordable option with excellent performance in inhibiting bone resorption, making it suitable for osteogenesis. In Horcajada et al., three-month-old rodents consumed a diet that contained hesperidin supplements, increasing bone mass. It also prevented bone degradation caused by ovariectomy in six-month-old rodents [21, 22].
Furthermore, in experiments by Habauzit, Hesperidin supplementation of the diet of 20-month-old male rodents resulted in a substantial increase in trabecular bone volume and femur bone density over three months [23]. Xue et al. observed increased fracture healing in rats with human mesenchymal stem cells loaded in a gelatin/hesperidin scaffold, concluding that hesperidin could be used as a growth factor for fracture repair surgeries or bone tissue engineering [24]. Studies indicate that Runx2 is a transcription factor specific to osteogenesis that can affect the expression of genes related to osteogenesis and regulate the cell cycle, impacting the function of chondrocytes and osteoclasts. Runx2 is crucial for maintaining function, differentiation, and maturation of osteoblasts. It stimulates angiogenesis and induces bone formation and growth. Mutations and deficiencies in the Runx2 gene may cause abnormal bone growth and development [25]. The mechanism of Runx2 action involves weak expression in undifferentiated mesenchymal cells (with high differentiation potential), increased expression in osteoblast precursors, maximum expression in immature osteoblasts, and decreased expression in mature osteoblasts [26]. Runx2 also regulates chondrocyte and osteoclast generation, stimulating angiogenesis and inducing bone formation [25].
Significant increases in Runx2 gene expression in the 1% Hesp group compared to the control group demonstrate the stimulatory effect of this treatment on osteoblast differentiation. No significant difference was observed in the hesperidin groups (0.01, 0.1, 10, and 0%) compared to the control. Given that the 1% Hesp group showed a significant increase in Runx2 expression compared to the 10% Hesp group, this treatment method's effective role in osteoblast differentiation at the fracture site can be discerned. The absence of significant differences in the expression of the OCN gene among treatment groups suggests that the treatment stimulates osteoblast differentiation up to the point of bone formation without playing a role in osteoblast mineralization activity. OCN is significantly increased in human peripheral blood during maturity and in adult patients with fractures. Its primary role in mineral deposition in both in vivo and in vitro conditions has been established and is expected to play an important role in the healing process of fractures [27]. The findings are consistent with those of Liu et al., who discovered that overexpression of the Runx2 gene in the later stages of osteoblast differentiation prevents the maturation of osteoblasts and reduces bone mass [28]. Additionally, Galindo et al. found that Runx2 expression significantly increases during slow cellular proliferation (such as the G0 phase) but decreases during rapid cell proliferation [29]. Furthermore, a study by Zhao et al. showed that scaffolds carrying mesenchymal stem cells induced with the Runx2 gene in vivo could form more bone cells than uninduced cells [30].
Yang et al. observed that injection of the flavonoid Kaempferol into the upper periosteum of rat parietal bones over 12 days increased calcification in new bone formation and raised the expression of the Runx2 antibody compared to the control group [31]. According to the experiment by Zhong et al., the expression of genes OCN, ALP, and type I collagen in an animal model with fractures, treated with tri-calcium phosphate (TCP) scaffolds loaded with PRP, was significantly increased compared to the scaffold without PRP group, 8 and 12 weeks after scaffold implantation [32].
Hesperidin can regulate the expression of mineralization genes, such as OCN and OPN, by activating the BMP pathway and inhibiting Noggin signaling. Consequently, the expression of Runx-2 is increased [13]. Various research groups have reported on the function of Runx-2 in in vivo conditions. Otto et al. illustrated the critical role of Runx2 in bone formation and osteoblasts' differentiation. Additionally, they observed that mice with a homozygous mutation in Runx-2 died shortly after birth and exhibited incomplete bone formation, while cartilage development was nearly normal. Other studies also presented the essential role of Runx-2 in intramembranous and endochondral ossification. The expression of numerous genes associated with osteoblast differentiation is regulated by Runx-2, which is essential for osteoblast differentiation.
Additionally, it was found that Runx2, apart from OCN, regulates the expression of several osteoblast marker genes [33]. Understanding the complete process of fracture healing, especially the molecular events, can accelerate recovery rates. The clinical evidence increasingly indicates that artificial scaffolds may be a new gold standard for treating specific fractures. Moreover, studies suggest that implants from artificial scaffolds offer superior functional potential in the future compared to autografts. Thus, the ultimate objective is to develop an artificial bone graft that provides compressive resistance superior to natural bone grafts.
Conclusion
Increasing the expression of the Runx2 gene following the application of chitosan scaffolds containing 1% Hesperadin improves bone fracture treatment.
Acknowledgments: The authors would like to thank all members of the Faculty of Biology at Damghan University.
Ethical Permissions: The Ethical Committee of the Faculty of Biology at Damghan University, Iran (IR.DU.REC.1400.006) approved the research project.
Conflicts of Interests: The authors deny any financial or other conflicts of interest.
Authors' Contribution: Dhiya A (First Author), Introduction Writer/Main Researcher (25%); Haji Ghasem Kashani M (Second Author), Methodologist (25%); Osamah NW (Third Author), Assistant Researcher/Statistical Analyst (15%); Salehi M (Fourth Author), Assistant Researcher/Discussion Writer (15%); Manouchehri B (Fifth Author), Assistant Researcher (10%); Elaheh A (Sixth Author), Assistant Researcher (10%)
Funding/Support: There was no external financial support. It is a self-funded study.
Tissue engineering is recognized as a significant approach in bone reconstruction. Functional scaffolds must initially meet the necessary mechanical strength to replace the mechanical function lost in damaged tissues in tissue engineering. The scaffold's strength is crucial for providing adequate structural support and transferring reinforcing forces to the host tissue site. Tissue reconstruction and cell function are important for achieving angiogenesis and stable biomechanical conditions at the host site. Thus, the three-dimensional structure of the scaffold must maintain sufficient structural integrity under both in Vitro and Vivo conditions and preserve the process of growth and reconstruction [1]. An effective alternative to conventional grafting strategies is bone tissue engineering. The utilization of chitosan-based biocomposite scaffolds to accelerate the development of new bone tissue has been extensively studied among polymers [2]. Chitosan is a natural biopolymer acting as a scaffold in bone tissue engineering [3]. It is the deacetylated form of chitin, a structural element found in the exoskeleton of crustaceans like shrimp and crabs, insect cuticles, and the cell walls of fungi [4-6]. Chitosan is a natural linear polymer composed of repetitive units of N-acetyl-D-glucosamine and D-glucosamine. These units are connected by β (1-4) glycosidic bonds [7-9]. Due to its cationic nature, Chitosan is extensively employed to accelerate wound healing and exhibit antimicrobial properties. This polymer is used in tissue engineering to differentiate or repair tissues such as cartilage, bone, skin, nerve, liver, and muscles [6, 8, 9]. Chitosan is also referred to as a wound-healing accelerator because it activates and regulates inflammatory cells, thereby promoting the growth of granular tissue. It also plays a protective role in cell proliferation, osteoblast differentiation, and mineralization [2]. Numerous studies have shown that consuming many vegetables and fruits daily (240-400 g) is associated with higher Bone Mineral Density (BMD) and reduced risk of bone fractures. Recent research indicates that the risk of hip fractures and osteoporosis in postmenopausal women is inversely correlated with the consumption of soy isoflavonoids. The risk of degenerative diseases, including cardiovascular diseases, cancer, diabetes, and osteoporosis, is reduced by polyphenols' antioxidant potential, which protects cells from oxidative damage. Polyphenols influence bone metabolism by suppressing osteoclastogenesis, activating osteoblastogenesis, and suppressing osteoclastogenesis, in addition to their antioxidant properties. One of the beneficial subgroups of polyphenols, flavonoids, are found in various fruits, vegetables, and plants [10, 11]. Flavonoids are predominantly found in citrus fruits. The predominant flavonoid in lemons, oranges, and sweet oranges is hesperidin rutinoside [10]. Among natural citrus flavonoids, hesperidin is a potent anticoagulant due to its pharmacological determination. Hesperidin is effective on both cortical and trabecular bone by reducing osteoclasts and preventing the absorption of trabecular bone. Hesperidin enhances the inhibition of bone absorption and increases the concentration of minerals like calcium in the bone. Hesperidin's inhibitory effects on bone absorption are associated with an unclear molecular mechanism. Known for its antioxidant activity, hesperidin is recognized as an anticancer drug. Since osteoclast inhibition reduces bone absorption, hesperidin may inhibit bone absorption through its antioxidant activity.
In contrast to other flavonoids, hesperidin's antioxidant capacity is not as high. Another hypothesis is that hesperidin affects bone cells by activating estrogen receptors (ER). ERs have been found in bone marrow stromal cells and osteoblasts. Although there is no information on hesperidin's binding affinity for ERs. Statins increase bone formation by inducing bone morphogenic proteins. Hesperidin's mechanism on bone seems to be like that of statins. Additional research is required to determine the mechanism of action of hesperidin on bone. Hesperidin reduces serum and liver cholesterol and inhibits bone absorption by inhibiting osteoclasts [12]. Hesperidin enhances the expression of Runx2 and Osx by stimulating the BMP pathway and downregulating Noggin signaling, thereby regulating the expression of mineralization genes such as OCN and Opn [13, 14].
Therefore, considering the mentioned aspects, this research aimed to investigate the effects of expressing Runx2 and OCN genes after treating femur fractures with chitosan scaffolds enriched with various concentrations of Hesperadin flavonoids.
Materials and Methods
This experimental study examined 36 twelve-week-old male Wistar rats weighing between 220 and 260g. The samples (Pasteur Institute; Iran) were maintained at 20-24°C, with a 12-hour light/dark cycle and unrestricted access to food and water. The animals underwent intramuscular ketamine/xylazine injection for anesthesia, and a 2x6mm fracture groove was made on the right femur. The rats were assigned to 6 groups (each n=6). The experiments were conducted following the Helsinki guidelines and regulations.
To construct the chitosan scaffold, 1200mg of chitosan powder is dissolved in 60cc of 1% acetic acid solution. Subsequently, hesperidin powder was added to the chitosan solution in 0.01%, 0.1%, 1%, and 10% concentrations relative to the polymer weight. A sterile scaffold was grafted onto the fracture site using chitosan (0%), chitosan+0.01% hesperidin, chitosan+0.1% hesperidin, chitosan+1% hesperidin, and chitosan+10% hesperidin. One group has not received any treatment as the control.
Following surgery, each rat received intraperitoneal injections of 200μL cefazolin (an antibiotic) and intramuscular injections of 100μl ketoprofen (an analgesic) for a maximum of five days. Following a 4-month course of treatment, the animals were slaughtered, and the femurs from the wounded legs were removed for histology and Real-time PCR analyses [15, 16].
The osteogenic Runx2 (Accession number: NM_001278483) and OCN (Accession number: NM_013414) gene expression levels were quantitatively analyzed using Real-Time PCR to assess the treatment's biological impact on bone healing (Table 1).
The bone samples were embedded in paraffin after being fixed for 24 hours in 4% paraformaldehyde and then decalcified for 2-4 days using 10% EDTA. Following sectioning, Trichromasson stain (MTC) was used.
Table 1. The primers of the PCR process

Findings
The group treated with chitosan+Hesperadin scaffolds at concentrations of 1% (Hesp 1%) exhibited a significant increase in gene expression compared to the control group. Additionally, the group with 0% and 10% hesperidin showed a significant decrease in expression compared to the 1% group (p<0.05).

Figure 2. The levels of Runx2 gene expression across different treatment groups measured after 2 months (*: a significant difference versus the control group at <0.05; $: a significant difference versus the Hesp 10% group at <0.05)
No significant differences existed between the groups (p>0.05). This suggested that while hesperidin concentrations influenced Runx2 expression, they did not significantly affect OCN gene expression under the conditions studied. This outcome highlighted the variable impact of Hesperadin on different osteogenic markers in the context of bone healing and regeneration.

Figure 3. OCN gene expression analysis after 2 months using Real-Time PCR Technique
Histopathological assessment using MTC staining in Figure 1 (A-J) revealed that the chitosan/1% Hes-treated group (Figures A-D) displayed new bone and connective tissue replacing the defect. The Hes-treated group showed significant angiogenesis in the periphery of newly formed bone and connective tissues. The defect site in Figure 1 (E & F) displayed an organized tissue structure consisting of new bone and a cartilaginous region. In the regenerated area, MTC staining in Figure 1 (G & H) showed signs of collagen synthesis and a substantial amount of new cartilage tissue. The MTC-stained control sample in Figure 1 (I & J) did not exhibit bone fusion, which resulted in the fracture site remaining open.

Figure 1. Histological images of bone defect regions (Masson's Trichrome staining)
Discussion
Bone transfers or grafts, such as xenografts, autografts, and allografts, are the only clinical therapeutic approaches for long-term bone reconstruction. Although these bone grafts are stimulatory and osteoconductive, they are either limited in availability or associated with complications such as bleeding, risk of infection, inadequate integration, and graft deformation, leading to reduced mechanical stability. Consequently, recent research has focused on developing alternative treatments [1, 17]. Tissue engineering is a significant therapeutic strategy for current and future medicine, intending to restore, regenerate, maintain, or enhance the function of damaged or lost tissues due to various disease conditions [6].
Tissue engineering has emerged as a crucial approach in bone reconstruction. Initially, functional scaffolds in tissue engineering must provide sufficient mechanical strength to replace the lost mechanical function in diseased or injured tissues. The structural support provided by the scaffold strength allows the transfer of reinforcing forces to the host tissue site. Cellular function and tissue reconstruction are essential for achieving stable biomechanical conditions and angiogenesis at the host site. Therefore, the three-dimensional structure of the scaffold must maintain sufficient structural integrity under both in vivo and in vitro conditions to preserve the growth and reconstruction process [1]. An ideal three-dimensional scaffold requires appropriate mechanical properties for cell adhesion, a porosity of 80-90%, and interconnected pore sizes of 50-250µm to facilitate the adequate transfer of nutrients, gases, and regulatory factors necessary for cell survival, differentiation, and proliferation [18]. Hydroxyapatite scaffolds, a material used in bone tissue engineering, are limited by their brittleness, which prevents their use in bone repair and replacement. In contrast, chitosan is an excellent candidate for bone repair due to its biodegradability, biocompatibility, antimicrobial activity, non-toxic nature, excellent pore structure, and minimal foreign body reaction [5].
Moreover, chitosan's role in enhancing cell adhesion, proliferation, differentiation of osteoblasts, and mineralization has been reported [2]. Experiments by He et al. on rat femur fractures with nano-hydroxyapatite-chitosan scaffolds loaded with BMSCs showed significant increases in bone and collagen formation and degradation compared to scaffolds without BMSCs [5]. In this study, the implanted scaffold did not elicit inflammatory responses or foreign body reactions. Both control and chitosan/1% Hes-treated groups facilitated new bone growth, but the Chitosan/1% Hes-treated group showed superior outcomes at the defect site compared to the control group. Histomorphometric analysis pointed to a higher count of osteoblasts, fibroblasts, and osteons in the Chitosan/1% Hes-treated group, whereas the control group had more chondrocytes and osteoclasts. An in vivo study in a mouse model evaluated a chitosan hydrogel infused with a natural compound with naringin, angiogenic, and anti-inflammatory properties. In the contraction phase, they illustrated the intriguing properties of chitosan by injecting it at the lesion site with a combination of active substances, which exhibited an anti-inflammatory effect on periodontal tissues [19]. Levengood & Zhang [20] used a chitosan/alginate scaffold in a rat cranial defect model, showing significant improvements in the experimental groups compared to the control after 16 weeks. Therefore, chitosan scaffolds were used in this research.
Additionally, we employed the flavonoid hesperidin as an osteogenic factor during the in vivo bone repair process [17, 20]. Hesperidin is an affordable option with excellent performance in inhibiting bone resorption, making it suitable for osteogenesis. In Horcajada et al., three-month-old rodents consumed a diet that contained hesperidin supplements, increasing bone mass. It also prevented bone degradation caused by ovariectomy in six-month-old rodents [21, 22].
Furthermore, in experiments by Habauzit, Hesperidin supplementation of the diet of 20-month-old male rodents resulted in a substantial increase in trabecular bone volume and femur bone density over three months [23]. Xue et al. observed increased fracture healing in rats with human mesenchymal stem cells loaded in a gelatin/hesperidin scaffold, concluding that hesperidin could be used as a growth factor for fracture repair surgeries or bone tissue engineering [24]. Studies indicate that Runx2 is a transcription factor specific to osteogenesis that can affect the expression of genes related to osteogenesis and regulate the cell cycle, impacting the function of chondrocytes and osteoclasts. Runx2 is crucial for maintaining function, differentiation, and maturation of osteoblasts. It stimulates angiogenesis and induces bone formation and growth. Mutations and deficiencies in the Runx2 gene may cause abnormal bone growth and development [25]. The mechanism of Runx2 action involves weak expression in undifferentiated mesenchymal cells (with high differentiation potential), increased expression in osteoblast precursors, maximum expression in immature osteoblasts, and decreased expression in mature osteoblasts [26]. Runx2 also regulates chondrocyte and osteoclast generation, stimulating angiogenesis and inducing bone formation [25].
Significant increases in Runx2 gene expression in the 1% Hesp group compared to the control group demonstrate the stimulatory effect of this treatment on osteoblast differentiation. No significant difference was observed in the hesperidin groups (0.01, 0.1, 10, and 0%) compared to the control. Given that the 1% Hesp group showed a significant increase in Runx2 expression compared to the 10% Hesp group, this treatment method's effective role in osteoblast differentiation at the fracture site can be discerned. The absence of significant differences in the expression of the OCN gene among treatment groups suggests that the treatment stimulates osteoblast differentiation up to the point of bone formation without playing a role in osteoblast mineralization activity. OCN is significantly increased in human peripheral blood during maturity and in adult patients with fractures. Its primary role in mineral deposition in both in vivo and in vitro conditions has been established and is expected to play an important role in the healing process of fractures [27]. The findings are consistent with those of Liu et al., who discovered that overexpression of the Runx2 gene in the later stages of osteoblast differentiation prevents the maturation of osteoblasts and reduces bone mass [28]. Additionally, Galindo et al. found that Runx2 expression significantly increases during slow cellular proliferation (such as the G0 phase) but decreases during rapid cell proliferation [29]. Furthermore, a study by Zhao et al. showed that scaffolds carrying mesenchymal stem cells induced with the Runx2 gene in vivo could form more bone cells than uninduced cells [30].
Yang et al. observed that injection of the flavonoid Kaempferol into the upper periosteum of rat parietal bones over 12 days increased calcification in new bone formation and raised the expression of the Runx2 antibody compared to the control group [31]. According to the experiment by Zhong et al., the expression of genes OCN, ALP, and type I collagen in an animal model with fractures, treated with tri-calcium phosphate (TCP) scaffolds loaded with PRP, was significantly increased compared to the scaffold without PRP group, 8 and 12 weeks after scaffold implantation [32].
Hesperidin can regulate the expression of mineralization genes, such as OCN and OPN, by activating the BMP pathway and inhibiting Noggin signaling. Consequently, the expression of Runx-2 is increased [13]. Various research groups have reported on the function of Runx-2 in in vivo conditions. Otto et al. illustrated the critical role of Runx2 in bone formation and osteoblasts' differentiation. Additionally, they observed that mice with a homozygous mutation in Runx-2 died shortly after birth and exhibited incomplete bone formation, while cartilage development was nearly normal. Other studies also presented the essential role of Runx-2 in intramembranous and endochondral ossification. The expression of numerous genes associated with osteoblast differentiation is regulated by Runx-2, which is essential for osteoblast differentiation.
Additionally, it was found that Runx2, apart from OCN, regulates the expression of several osteoblast marker genes [33]. Understanding the complete process of fracture healing, especially the molecular events, can accelerate recovery rates. The clinical evidence increasingly indicates that artificial scaffolds may be a new gold standard for treating specific fractures. Moreover, studies suggest that implants from artificial scaffolds offer superior functional potential in the future compared to autografts. Thus, the ultimate objective is to develop an artificial bone graft that provides compressive resistance superior to natural bone grafts.
Conclusion
Increasing the expression of the Runx2 gene following the application of chitosan scaffolds containing 1% Hesperadin improves bone fracture treatment.
Acknowledgments: The authors would like to thank all members of the Faculty of Biology at Damghan University.
Ethical Permissions: The Ethical Committee of the Faculty of Biology at Damghan University, Iran (IR.DU.REC.1400.006) approved the research project.
Conflicts of Interests: The authors deny any financial or other conflicts of interest.
Authors' Contribution: Dhiya A (First Author), Introduction Writer/Main Researcher (25%); Haji Ghasem Kashani M (Second Author), Methodologist (25%); Osamah NW (Third Author), Assistant Researcher/Statistical Analyst (15%); Salehi M (Fourth Author), Assistant Researcher/Discussion Writer (15%); Manouchehri B (Fifth Author), Assistant Researcher (10%); Elaheh A (Sixth Author), Assistant Researcher (10%)
Funding/Support: There was no external financial support. It is a self-funded study.
Keywords:
Chitosan [MeSH], Hesperidin [MeSH], Bone Fracture [MeSH], Tissue Engineering [MeSH], Gene [MeSH], Rat [MeSH]
References
1. Reichert JC, Wullschleger ME, Cipitria A, Lienau J, Cheng TK, Schütz MA, et al. Custom-made composite scaffolds for segmental defect repair in long bones. Int Orthop. 2011;35(8):1229-36. [Link] [DOI:10.1007/s00264-010-1146-x]
2. Saravanan S, Leena R, Selvamurugan N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int J Biol Macromol. 2016;93(Pt B):1354-65. [Link] [DOI:10.1016/j.ijbiomac.2016.01.112]
3. Dhivya S, Keshav Narayan A, Logith Kumar R, Viji Chandran S, Vairamani M, Selvamurugan N. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2018;51(1):e12408. [Link] [DOI:10.1111/cpr.12408]
4. Bianchera A, Salomi E, Pezzanera M, Ruwet E, Bettini R, Elviri L. Chitosan hydrogels for chondroitin sulphate controlled release: An analytical characterization. J Anal Methods Chem. 2014;2014(1):808703. [Link] [DOI:10.1155/2014/808703]
5. He Y, Dong Y, Cui F, Chen X, Lin R. Ectopic osteogenesis and scaffold biodegradation of nano-hydroxyapatite-chitosan in a rat model. PLoS One. 2015;10(8):e0135366. [Link] [DOI:10.1371/journal.pone.0135366]
6. Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, Saldaña-Koppel DA, Quiñones-Olvera LF. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed Res Int. 2015;2015(1):821279. [Link] [DOI:10.1155/2015/821279]
7. Muxika A, Etxabide A, Uranga J, Guerrero P, De La Caba K. Chitosan as a bioactive polymer: Processing, properties and applications. Int J Biol Macromol. 2017;105(Pt 2):1358-68. [Link] [DOI:10.1016/j.ijbiomac.2017.07.087]
8. Costa-Pinto AR, Reis RL, Neves NM. Scaffolds based bone tissue engineering: The role of chitosan. Tissue Eng Part B Rev. 2011;17(5):331-47. [Link] [DOI:10.1089/ten.teb.2010.0704]
9. Furuike T, Komoto D, Hashimoto H, Tamura H. Preparation of chitosan hydrogel and its solubility in organic acids. Int J Biol Macromol. 2017;104(Pt B):1620-5. [Link] [DOI:10.1016/j.ijbiomac.2017.02.099]
10. Austermann K, Baecker N, Stehle P, Heer M. Putative effects of nutritive polyphenols on bone metabolism in vivo-evidence from human studies. Nutrients. 2019;11(4):871. [Link] [DOI:10.3390/nu11040871]
11. Martin BR, McCabe GP, McCabe L, Jackson GS, Horcajada MN, Offord-Cavin E, et al. Effect of hesperidin with and without a calcium (Calcilock) supplement on bone health in postmenopausal women. J Clin Endocrinol Metab. 2016;101(3):923-7. [Link] [DOI:10.1210/jc.2015-3767]
12. Chiba H, Uehara M, Wu J, Wang X, Masuyama R, Suzuki K, et al. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr. 2003;133(6):1892-7. [Link] [DOI:10.1093/jn/133.6.1892]
13. Torre E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem Rev. 2017;16(6):1183-226. [Link] [DOI:10.1007/s11101-017-9529-x]
14. Altememy D, Kashani MHG, Fateme A, Khosravian P. New method to induce neurotrophin gene expression in human adipose-derived stem cells in vitro. J Adv Pharm Technol Res. 2024;15(3):214-9. [Link] [DOI:10.4103/JAPTR.JAPTR_390_23]
15. Akman AC, Seda Tığlı R, Gümüşderelioğlu M, Nohutcu RM. Bone morphogenetic protein‐6‐loaded chitosan scaffolds enhance the osteoblastic characteristics of MC3T3‐E1 cells. Artif Organs. 2010;34(1):65-74. [Link] [DOI:10.1111/j.1525-1594.2009.00798.x]
16. Maji K, Dasgupta S, Pramanik K, Bissoyi A. Preparation and evaluation of gelatin‐chitosan‐nanobioglass 3D porous scaffold for bone tissue engineering. Int J Biomater. 2016;2016(1):9825659. [Link] [DOI:10.1155/2016/9825659]
17. Altememy D, Ghasem Kashani MH, Wenas O. Sinusoidal electromagnetic field decreases osteogenic differentiation of rat bone marrow mesenchymal stem cells. Int J Appl Pharm. 2024;16(3). [Link] [DOI:10.22159/ijap.2024v16i3.50382]
18. Wang F, Pang Y, Chen G, Wang W, Chen Z. Enhanced physical and biological properties of chitosan scaffold by silk proteins cross-linking. Carbohydr Polym. 2020;229:115529. [Link] [DOI:10.1016/j.carbpol.2019.115529]
19. Aguilar A, Zein N, Harmouch E, Hafdi B, Bornert F, Offner D, et al. Application of chitosan in bone and dental engineering. Molecules. 2019;24(16):3009. [Link] [DOI:10.3390/molecules24163009]
20. Levengood SKL, Zhang M. Chitosan-based scaffolds for bone tissue engineering. J Mater Chem B. 2014;2(21):3161-84. [Link] [DOI:10.1039/c4tb00027g]
21. Horcajada MN, Habauzit V, Trzeciakiewicz A, Morand C, Gil-Izquierdo A, Mardon J, et al. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J Appl Physiol. 2008;104(3):648-54. [Link] [DOI:10.1152/japplphysiol.00441.2007]
22. Altememy D, Kashani MHG, Khosravian P. Selegiline induced differentiation of rat bone marrow mesenchymal stem cells to dopaminergic neurons in vitro. Pharmacia. 2023;70(4):959-65. [Link] [DOI:10.3897/pharmacia.70.e107909]
23. Habauzit V, Sacco SM, Gil-Izquierdo A, Trzeciakiewicz A, Morand C, Barron D, et al. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone. 2011;49(5):1108-16. [Link] [DOI:10.1016/j.bone.2011.07.030]
24. Xue D, Chen E, Zhang W, Gao X, Wang S, Zheng Q, et al. The role of hesperetin on osteogenesis of human mesenchymal stem cells and its function in bone regeneration. Oncotarget. 2017;8(13):21031-43. [Link] [DOI:10.18632/oncotarget.15473]
25. Xu J, Li Z, Hou Y, Fang W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am J Transl Res. 2015;7(12):2527-35. [Link]
26. Komori T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int J Mol Sci. 2019;20(7):1694. [Link] [DOI:10.3390/ijms20071694]
27. Abe Y, Chiba M, Yaklai S, Pechayco RS, Suzuki H, Takahashi T. Increase in bone metabolic markers and circulating osteoblast-lineage cells after orthognathic surgery. Sci Rep. 2019;9(1):20106. [Link] [DOI:10.1038/s41598-019-56484-x]
28. Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, et al. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001;155(1):157-66. [Link] [DOI:10.1083/jcb.200105052]
29. Galindo M, Kahler RA, Teplyuk NM, Stein JL, Lian JB, Stein GS, et al. Cell cycle related modulations in Runx2 protein levels are independent of lymphocyte enhancer-binding factor 1 (Lef1) in proliferating osteoblasts. J Mol Histol. 2007;38(5):501-6. [Link] [DOI:10.1007/s10735-007-9143-0]
30. Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12(2):247-53. [Link] [DOI:10.1016/j.ymthe.2005.03.009]
31. Yang L, Takai H, Utsunomiya T, Li X, Li Z, Wang Z, et al. Kaempferol stimulates bone sialoprotein gene transcription and new bone formation. J Cell Biochem. 2010;110(6):1342-55. [Link] [DOI:10.1002/jcb.22649]
32. Zhong D, Wang CG, Yin K, Liao Q, Zhou X, Liu AS, et al. In vivo ossification of a scaffold combining β‑tricalcium phosphate and platelet‑rich plasma. Exp Ther Med. 2014;8(5):1381-8. [Link] [DOI:10.3892/etm.2014.1969]
33. Bruderer M, Richards R, Alini M, Stoddart MJ. Role and regulation of RUNX2 in osteogenesis. Eur Cell Mater. 2014;28(1):269-86. [Link] [DOI:10.22203/eCM.v028a19]










