Volume 16, Issue 1 (2024)
Iran J War Public Health 2024, 16(1): 81-89 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/01/20 | Accepted: 2024/03/14 | Published: 2024/03/25
Received: 2024/01/20 | Accepted: 2024/03/14 | Published: 2024/03/25
How to cite this article
Awadh M, Gany S, Ghaleb R, Ameen A. Phytochemical Screening, in Vitro Antileishmanial Activity of Conyza Canadensis Extract by Neopterin. Iran J War Public Health 2024; 16 (1) :81-89
URL: http://ijwph.ir/article-1-1432-en.html
URL: http://ijwph.ir/article-1-1432-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Phytochemical Screening, in Vitro Antileishmanial Activity of Conyza Canadensis Extract by Neopterin
1- Department of Pharmacology and Therapeutics, College of Medicine, University of Kufa, Kufa, Iraq
2- Department of Anatomy and Histology, College of Medicine, University of Babylon, Iraq
3- Department of Histology, AL-Imam Al-Sadiq Hospetal, Babylon, Iraq
2- Department of Anatomy and Histology, College of Medicine, University of Babylon, Iraq
3- Department of Histology, AL-Imam Al-Sadiq Hospetal, Babylon, Iraq
Full-Text (HTML) (410 Views)
Introduction
Leishmaniasis is a parasitic disease caused by the Leishmania parasite, transmitted to humans through the bite of an infected sandfly [1]. In humans, leishmaniasis can manifest in various forms, ranging from superficial, inflammatory lesions on the skin to more severe and sometimes lethal infections of the internal organs. There are four primary clinical manifestations of the disease: visceral (LV or kala-azar), cutaneous (LCM), diffuse cutaneous (DCL), and post-kala-azar dermal (PKDL), which depend on the Leishmania spp. [2]. The Leishmania spp. has two life stages: promastigote (PRO) and amastigote (AMS). The Phlebotomus sandfly serves as both an intermediate host and a vector. Different Leishmania types elicit various immune responses [3]. During the life cycle of Leishmania, the AMS undergoes binary fission in the sandfly's midgut and eventually transforms into a PRO. The PRO then migrates to the pharynx of the sandfly and prepares for transmission to a new host. When the sandfly feeds on the blood of a vertebrate host, the PRO is injected into the host's skin [4, 5]. It is believed that a sandfly's entire life cycle lasts for approximately ten days [6].
The sandfly injects metacyclic PRO and saliva into the host's bloodstream during blood-feeding. The saliva contains biologically active components that modify the immune response and influence parasite infection. Neutrophils and monocytes/macrophages then infiltrate the bite site [7].
When PRO comes into contact with host cells, macrophages engulf them. Once inside, PRO undergoes a process of differentiation into AMS and proliferates within phagolysosomes. Leishmania must adapt metabolically and resist host immune system assaults [8]. Eventual macrophage lysis and the subsequent release of infectious stages that can invade other cells. The parasite's life cycle within the human host persists until another sandfly acquires a blood meal from the afflicted individual, completing the cycle [9].
Activation of macrophages and monocytes triggers the biosynthesis of NEO, a pteridine derived from guanosine triphosphate, reflecting an overdriven cellular immune response. The pro-inflammatory IFN-γ produced after T-lymphocyte activation is the primary factor that triggers NEO formation [10]. When the parasite infection is active, NEO release increases in visceral leishmaniasis due to activated macrophages and an increase in macrophage load. However, as the infection wanes, NEO release decreases [11].
The quantity of artificially produced NEO is directly correlated with the quantity of IFN‐γ and indirectly suggests an elevation in TNF‐α. There is a strong connection between the excessive synthesis of NEO and the stimulating effects of these cytokines on the metabolic activity of immune cells. Additionally, NEO plays a role in the mechanism by which activated macrophages exert their cytotoxic actions. NEO levels are indicative of the interaction among various cytokines in the monocyte/macrophage population [12].
Current antileishmanial drugs include amphotericin B, antimonials, sitamaquine, pentamidine, paromomycin, and miltefosine. Immunomodulatory antileishmanial drugs enhance the innate immune system [13]. The treatment regimen, however, has significant drawbacks. Pentavalent antimonials are the primary treatment for visceral leishmaniasis. Commercially available as sodium stibogluconate (SSG) and meglumine antimoniate (MA), these pentavalent antimonials (SbV) have seen reduced efficacy due to resistance [14]. Despite its effectiveness in treating visceral leishmaniasis resistant to pentavalent antimony, the injection and adverse side effects of these pharmaceuticals render them toxic and sometimes fatal [15]. The leishmanicidal efficacy of amphotericin B (AmB) reduces treatment failures and relapses. However, this drug is not recommended as a first-line treatment due to its nephrotoxicity and the need for parenteral administration [9, 17].
The World Health Organization (WHO) asserts that using plants represents the only viable path toward developing a therapeutic agent that is safe, effective, and affordable to address various health issues. Clinical trials have provided empirical evidence demonstrating the efficacy of specific indigenous flora in combating cutaneous leishmaniasis and their potential utility in facilitating wound healing through the application of herbal ointments. When native plants are abundant, they may offer a more cost-effective alternative to synthetic medications. This advantage is particularly significant in developing countries where the disease is prevalent [16].
The herb known as Conyza Canadensis grows naturally in various regions including north of Baghdad, Baquba, Kut, Rustam, Mosul, Abu Ghraib, Rowanduz, Za'franiya, Pushtashan, Qerna Qaw valley, northeast of Zakho, and 50 km from Basra to Nassiriya [18]. It is a biennial plant [18]. HPLC analysis of its extract confirms the presence of quercitrin, quercetin, apigenin, p-coumaric acid, and caffeic acid [19].
C. canadensis has been used for managing wounds, swellings, arthritis-related pain, inflammation, diarrhea, and microbial infections such as urinary and respiratory tract infections [19, 3]. Various studies have demonstrated the efficacy of the plant's ethanolic, methanolic, chloroform, and ethyl acetate fractions against both gram-positive and gram-negative bacteria. Additionally, the plant exhibits notable pharmacological activities, including anti-inflammatory, anticoagulant, anti-gastric ulcer, anti-diabetic, antioxidant, anti-cancer, and anti-mutagenic properties [19].
The study aimed to determine the antileishmanial properties of extracts from the Iraqi C. canadensis against Leishmania tropica.
Materials and Methods
This experimental study was conducted in the Pharmacology and College of Medicine of University of Babylon from September 2022 to September 2023 in which, the U937 monocyte was supplied by the National Cell Bank of Iran. The cells were grown in Roswell Park Memorial Institute (RPMI-1640) liquid medium (Gibco, UK) containing 10% fetal bovine serum (Gibco, UK) and phorbol myristate acetate (PMA) (Invevo Genes, USA). Stibogluconate (Pentostam) (GSK, UK) and C. canadensis, harvested from the mountains of Sulaymaniyah at the end of winter, were used. Neopterin (NEO) levels were measured using an ELISA Kit (Elabscience/USA). The Iraqi strain of L. tropica was obtained from the parasitology laboratory, graduate studies, Department of Biology, College of Science, University of Baghdad. The strain was then cultured in a biphasic medium (Nove-MacNeal-Nicolle) (NNN) [20].
1) Phytochemical compounds in the C. canadensis extract
A. Total alkaloid content
A total of 20g of plant material was subjected to extraction using methanol for 24hours, utilizing Soxhlet equipment. The extract underwent filtration, and the methanol was removed by evaporation using a rotary evaporator set at a temperature of 45°C.
1. Qualitative estimation (test for alkaloids)
Alkaloids were confirmed using Dragendorff's method. The extract was dissolved in dilute HCL, and Dragendorff's reagent was added, producing a crystalline precipitate indicating the presence of alkaloids. Positive samples were then quantitatively evaluated [21].
2. Quantitative estimation of alkaloid
The residue was dissolved in 2N HCl, filtered, and combined with a Bromocresol Green (BCG) solution and phosphate buffer. The resulting mixture was washed with chloroform, and its pH level was adjusted using 0.1N NaOH. To create the standard curve, an atropine standard solution was mixed with phosphate buffer and BCG solution, then shaken with the extract and chloroform. The resulting solution was collected, diluted with chloroform, and its absorbance was measured at 470nm in a UV-Spectrophotometer against the blank [22].
B. Determination of total phenolic compounds
Phenolic compounds were detected in an ethanolic extract using the Folin-Ciocalteu reagent. The mixture consisted of the extract, the reagent, and sodium carbonate. After 2 hours, the phenolic content was estimated by measuring the absorbance at 765nm against a calibration curve made with gallic acid (GA) [23].
C. Analysis of total flavonoid content
The flavonoid content was assessed using the aluminum chloride colorimetric method. A sample of the crude extract was mixed with NaNO2 and AlCl3 solutions, and NaOH was added to bring the final volume to 10ml. After 15minutes, the absorbance was measured at 510nm, and the flavonoid content was calculated as mg rutin equivalent per gram of dry weight [24].
D. Analysis of amino acids
Solid samples weighing approximately 5mg with a precision of 0.01mg, and liquid samples weighing approximately 100mg with an accuracy of 0.01mg, were hydrolyzed with 1ml of 6M hydrochloric acid solution at 100°C±20°C for 24hours. After hydrolysis, the amino acid residues were dissolved in 100µl of acetonitrile and derivatized with 100µl of OPA. The sample was then injected ten times (100µl per injection) into a gas chromatograph with a C18-ODS column and a fluorescence detector (Ex=445nm, Em=465nm) using an isocratic flow of 50/50 (v/v) water (pH=7.0) and acetonitrile at a flow rate of 1.0mL/min [25].
E. Analysis of total glycosides
To ascertain the presence of glycosides, the extracted substance was mixed with Baljet's reagent and allowed to sit for an hour. Following this, it was mixed with water and the absorbance was measured at a wavelength of 495 nm [26].
2) The preparation of the stock solution
The preparation of the C. canadensis aqueous extract stock solution (AqCC) involved dissolving 1μg of extract in 10ml of pyrogen-free Distel water. Subsequently, six serial dilutions were created with concentrations of 62.5, 125, 250, 500, 1000, and 2000μg/ml.
To create the alcoholic extract of C. canadensis stock solution (AqCC), 1mg of extract was dissolved in three milliliters of methanol. This was followed by the creation of six serial dilutions, each with concentrations of 62.5, 125, 250, 500, 1000, and 2000μg/ml.
A stock solution of stibogluconate (Pentostam) SSG was prepared at a concentration of 100mg/ml, followed by six serial dilutions at concentrations of 62.5, 125, 250, 500, 1000, and 2000μg/ml.
3) Cell line preparing and sub-culturing
Monocyte cell lines were sub-cultured for use as an in vitro model to evaluate the antileishmanial activity of AlCC and AqCC. The media used was RPMI-1640, supplemented with antimicrobial drugs such as gentamicin or penicillin (50μg/ml) and 5% fetal bovine serum. Afterwards, the cells were incubated at 37 degrees Celsius for twenty-four hours [27].
4) The development of a "macrophage-like" state in U937 monocyte
To activate NF-κB, we dissolved 5mg of PMA powder in 1.5 ml of endotoxin-free water, followed by storing at -20°C, protecting from light, avoiding repeated freeze-thaw cycles, adding 1 μl of PMA solution to the U937 monocyte cell lines, and incubating at 37°C for 24 hours [28]. PRO was used to infect macrophages from U937 in a stationary growth phase at a ratio of 20:1. The mixture was incubated in tissue culture flasks at 34°C with 5% CO2 and 95% relative humidity. After 12 hours, non-internalized PRO was removed by washing the cells five times with plain RPMI. The cells were further incubated for 96 hours in RPMI supplemented with 10% FCS. After treating the cell line with different concentrations of SSG and aqueous/alcoholic extracts, it was incubated at 37°C for 24 hours [29].
5) Detection of NEO by Enzyme-Linked Immunosorbent Assay (ELISA)
The study assessed the antileishmanial activity of extracts by introducing U973 macrophage cells into individual wells of a 96-well microplate. Following overnight treatment, promastigotes of L. tropica were administered to the macrophages and cultured at a temperature of 37°C for 96 hours. Subsequently, the cell line was exposed to varying doses of SSG, as well as aqueous and alcoholic extracts, and then incubated at 37°C for another 24 hours. After this incubation period, the liquid portion containing the treated macrophage cells was collected from each well and transferred into separate Eppendorf tubes designated for each group. The levels of nitric oxide and NEO were then measured using the sandwich enzyme-linked immunosorbent assay (ELISA) method [30, 31].
Statistical analysis
The data were analyzed using SPSS Version 26 and the student's t-test. The threshold for statistical significance was set at a p-value of ≤0.05.
Findings
HPLC analysis of the C. canadensis extract
Table 1 shows the active compounds in the extract. The analysis revealed six distinct peaks in the chloroform fraction of the Iraqi C. canadensis plant, as shown in Figures 1 and 2 for the aqueous and alcoholic extractions, respectively. Each peak represents a different type of active compound present in the plant. To further validate these findings, the retention times of each peak in the chloroform fraction were compared with those of a standard, as illustrated in Figure 3. Table 2 presents the retention time of the active compounds in comparison with the standard. Additionally, samples exhibited three peaks as depicted in Figures 4 and 5 at retention times ranging from 3.5 to 8 minutes, and when compared with the standard curve of glutathione (GSH), as shown in Figure 3G, the presence of GSH was confirmed.
Table 1. Active compounds present in the aqueous the aqueous (AqCC) and alcoholic (AlCC) extracts of C. canadensis

Figure 1. HPLC chromatogram of the aqueous C. canadensis extract.

Figure 2. HPLC chromatogram of alcoholic C. canadensis extract.
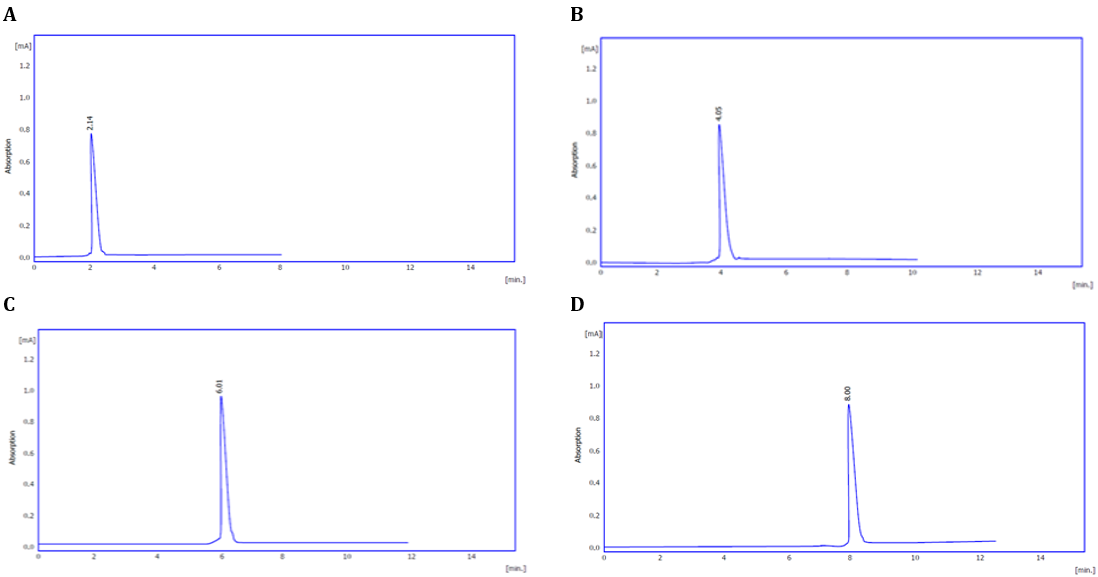

Figure 3. HPLC chromatogram of the Iraqi C. canadensis compounds compared to the standard (A: gallic acid; B: chlorogenic acid; C: caffeic acid; D: qurecetin; E: P-cumaric acid; F: apigenin; G: GSH).
Table 2. Retention time of the active compounds in comparison with the standard


Figure 4. HPLC chromatogram of alcoholic C. canadensis extract for amino acid.

Figure 5. HPLC chromatogram of aqueous C. canadensis extract for amino acid.
Effect of the extract compounds on NEO
Effect of SSG on NEO in U937 macrophage cell line
The administration of SSG led to a significant decrease in NEO levels in the U937 macrophage cell line compared to the control group (p<0.05; Figure 6).

Figure 6. Effect of SSG on U937 macrophage cell line.
Effect of SSG on NEO in U937 macrophage cell line infected with L. tropica.
The experiment revealed a substantial decrease in NEO production at high concentrations (250, 500, 1000, and 2000µg/ml) compared to the control group after 24hours of incubation (Figure 7).

Figure 7. Effect of SSG on U937 macrophage cell line infected with Leishmania tropica.
Effect of AqCC on NEO in U937 macrophage cell line
AqCC significantly reduced NEO levels in macrophages compared to the control group. The decline in NEO was statistically significant at elevated concentrations (125, 250, 500, 1000, and 2000µg/ml; p-value<0.05; Figure 8).

Figure 8. Effect of aqueous C. canadensis extract on U937 macrophage cell line.
Effect of AqCC on NEO in U937 macrophage cell line infected with L. tropica
AqCC at 1000 and 2000µg/ml caused a substantial drop in NEO levels compared to the control group (p-value≤0.05; Figure 9).

Figure 9. Effect of aqueous C. canadensis extract on U937 macrophage cell line infected with L. tropica.
Effect of AlCC on NEO in U937 macrophage cell line
AlCC markedly decreased the levels of NEO in the U937 macrophage cell line compared to the control group (Figure 10).

Figure 10. Effect of the aqueous C. canadensis extract on the U937 macrophage cell line.
Effect of AlCC on NEO in U937 macrophage cell line infected with L. tropica
In the U937 macrophage cell line, AlCC significantly reduced NEO levels compared to the control group, demonstrating its role in the immune response against L. tropica (p<0.05; Figure 11).

Figure 11. Effect of the aqueous C. canadensis extract on the U937 macrophage cell line infected with L. tropica.
Discussion
NEO levels in individuals infected with L. tropica decreased significantly in response to treatment with SSG, AlCC, and AqCC at all concentrations, according to this study. However, a significant reduction in NEO concentrations was observed exclusively at elevated concentrations of 1000 and 2000μg/ml of AqCC. Serum NEO levels that exceed the upper limit of the established normal range indicate activation of cell-mediated immunity. It is important to recognize that serum NEO does not exhibit disease specificity. Monitoring and evaluating its concentrations in the blood serum throughout the course of an infectious disease can provide valuable insights into the disease's severity and the effectiveness of the treatment [31]. Visceral leishmaniasis (VL) is characterized by an increase in the number and activation of macrophages, which would lead to a rise in NEO concentrations during the disease's active phase. Subsequently, as the parasite infection decreases, these levels would also decrease [11-13]. Recent studies have observed antileishmanial activity in AlCC and AqCC. This is confirmed by the presence of quercitrin, quercetin, apigenin, p-coumaric acid, and caffeic acid in the extracts, as determined by HPLC analysis [20]. The results of this research are consistent with those of Monzote et al., who similarly documented the antileishmanial properties of p-coumaric acid [32]. This compound has been found to suppress the activity of three crucial enzymes involved in the progression of Leishmania braziliensis: Aldehyde dehydrogenase (ALDH), mitogen-activated protein kinase (MPK4), and DNA topoisomerase 2 (TOP2). This discovery aligns with prior investigations suggesting that SSG functions by impeding DNA topoisomerase. Conversely, measuring NEO levels in the blood serum throughout the course of treatment for VL can help determine the efficacy of the therapy [10]. Elevated levels of reactive oxygen species (ROS) are associated with these measurements. Determining NEO concentrations enables an evaluation of the extent of oxidative stress and immunological activation [33]. Flavonoids, abundant in C. canadensis, exhibit various biological effects, such as regulating enzymes responsible for the elimination of ROS [34]. These chemical compounds have the capacity to stimulate cellular apoptosis and autophagy and can inhibit the growth and penetration of cancerous cells. In healthy cells, flavonoids act as antioxidants, helping to control ROS concentrations. In cancer cells, however, they function as potent pro-oxidants. By inhibiting pro-inflammatory signaling pathways and stimulating apoptotic pathways, flavonoids regulate the balance of ROS [35]. GA has demonstrated significant immunomodulatory characteristics, including an increase in macrophage capacity to ingest and eliminate foreign particles, enlargement of lysosomes, release of nitrite, and elevated levels of calcium ions within macrophages [36]. Chlorogenic acid (CGA) shows antileishmanial properties through its ability to eradicate parasites and disrupt their cell cycle, resulting in detrimental and inhibitory effects on the PRO. In vitro, CGA eradicates intracellular AMO completely, proving its efficacy in removing parasites from host cells. The enhanced functionality of macrophages facilitates the clearance process through the concurrent elevation of IL-12, TNF, and NO levels, and the reduction of IL-10 synthesis. Majumder et al. propose that CGA may function as an innovative and non-toxic chemical agent to treat visceral leishmaniasis, presenting a viable alternative to chemotherapy [37]. Anke et al., however, found that serum NEO concentrations did not increase in patients with CL [38]; their research contradicts this finding, suggesting that the two sources are in conflict.
Our findings support the use of natural plant extracts as alternative therapies for leishmaniasis. C. canadensis shows promise due to its bioactive components and various pharmacological effects. Further research is needed to understand the mechanisms of action and assess the treatment's effectiveness in animal models and clinical trials. Developing cost-effective and accessible therapies is crucial, especially in regions with limited resources. Continued exploration of C. canadensis as a therapeutic candidate could lead to effective and sustainable solutions for leishmaniasis.
Conclusion
This study demonstrates significant antileishmanial activity of C. canadensis extracts and highlights the correlation between NEO levels and Leishmania infection.
Acknowledgments: We would like to express my deepest gratitude to everyone who has helped me throughout my time at Kufa University's College of Medicine, but especially to everyone in the Department of Pharmacology and Therapeutics.
Ethical Permission: On the first of May in the year 2021, a local ethics commission at the College of Medicine at Kufa University gave their approval to both the study protocol and the informed consent statement.
Conflicts of Interests: Considering the presentation of research, there is a complete absence of any potential conflicts of interest.
Authors’ Contribution: Awadh MAA (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (60%); Gany SN (Second Author), Introduction Writer/Methodologist/Assistant Researcher (10%); Ghaleb RA (Third Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (20%); Ameen AA (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (10%)
Funding/Support: The research was not funded.
Leishmaniasis is a parasitic disease caused by the Leishmania parasite, transmitted to humans through the bite of an infected sandfly [1]. In humans, leishmaniasis can manifest in various forms, ranging from superficial, inflammatory lesions on the skin to more severe and sometimes lethal infections of the internal organs. There are four primary clinical manifestations of the disease: visceral (LV or kala-azar), cutaneous (LCM), diffuse cutaneous (DCL), and post-kala-azar dermal (PKDL), which depend on the Leishmania spp. [2]. The Leishmania spp. has two life stages: promastigote (PRO) and amastigote (AMS). The Phlebotomus sandfly serves as both an intermediate host and a vector. Different Leishmania types elicit various immune responses [3]. During the life cycle of Leishmania, the AMS undergoes binary fission in the sandfly's midgut and eventually transforms into a PRO. The PRO then migrates to the pharynx of the sandfly and prepares for transmission to a new host. When the sandfly feeds on the blood of a vertebrate host, the PRO is injected into the host's skin [4, 5]. It is believed that a sandfly's entire life cycle lasts for approximately ten days [6].
The sandfly injects metacyclic PRO and saliva into the host's bloodstream during blood-feeding. The saliva contains biologically active components that modify the immune response and influence parasite infection. Neutrophils and monocytes/macrophages then infiltrate the bite site [7].
When PRO comes into contact with host cells, macrophages engulf them. Once inside, PRO undergoes a process of differentiation into AMS and proliferates within phagolysosomes. Leishmania must adapt metabolically and resist host immune system assaults [8]. Eventual macrophage lysis and the subsequent release of infectious stages that can invade other cells. The parasite's life cycle within the human host persists until another sandfly acquires a blood meal from the afflicted individual, completing the cycle [9].
Activation of macrophages and monocytes triggers the biosynthesis of NEO, a pteridine derived from guanosine triphosphate, reflecting an overdriven cellular immune response. The pro-inflammatory IFN-γ produced after T-lymphocyte activation is the primary factor that triggers NEO formation [10]. When the parasite infection is active, NEO release increases in visceral leishmaniasis due to activated macrophages and an increase in macrophage load. However, as the infection wanes, NEO release decreases [11].
The quantity of artificially produced NEO is directly correlated with the quantity of IFN‐γ and indirectly suggests an elevation in TNF‐α. There is a strong connection between the excessive synthesis of NEO and the stimulating effects of these cytokines on the metabolic activity of immune cells. Additionally, NEO plays a role in the mechanism by which activated macrophages exert their cytotoxic actions. NEO levels are indicative of the interaction among various cytokines in the monocyte/macrophage population [12].
Current antileishmanial drugs include amphotericin B, antimonials, sitamaquine, pentamidine, paromomycin, and miltefosine. Immunomodulatory antileishmanial drugs enhance the innate immune system [13]. The treatment regimen, however, has significant drawbacks. Pentavalent antimonials are the primary treatment for visceral leishmaniasis. Commercially available as sodium stibogluconate (SSG) and meglumine antimoniate (MA), these pentavalent antimonials (SbV) have seen reduced efficacy due to resistance [14]. Despite its effectiveness in treating visceral leishmaniasis resistant to pentavalent antimony, the injection and adverse side effects of these pharmaceuticals render them toxic and sometimes fatal [15]. The leishmanicidal efficacy of amphotericin B (AmB) reduces treatment failures and relapses. However, this drug is not recommended as a first-line treatment due to its nephrotoxicity and the need for parenteral administration [9, 17].
The World Health Organization (WHO) asserts that using plants represents the only viable path toward developing a therapeutic agent that is safe, effective, and affordable to address various health issues. Clinical trials have provided empirical evidence demonstrating the efficacy of specific indigenous flora in combating cutaneous leishmaniasis and their potential utility in facilitating wound healing through the application of herbal ointments. When native plants are abundant, they may offer a more cost-effective alternative to synthetic medications. This advantage is particularly significant in developing countries where the disease is prevalent [16].
The herb known as Conyza Canadensis grows naturally in various regions including north of Baghdad, Baquba, Kut, Rustam, Mosul, Abu Ghraib, Rowanduz, Za'franiya, Pushtashan, Qerna Qaw valley, northeast of Zakho, and 50 km from Basra to Nassiriya [18]. It is a biennial plant [18]. HPLC analysis of its extract confirms the presence of quercitrin, quercetin, apigenin, p-coumaric acid, and caffeic acid [19].
C. canadensis has been used for managing wounds, swellings, arthritis-related pain, inflammation, diarrhea, and microbial infections such as urinary and respiratory tract infections [19, 3]. Various studies have demonstrated the efficacy of the plant's ethanolic, methanolic, chloroform, and ethyl acetate fractions against both gram-positive and gram-negative bacteria. Additionally, the plant exhibits notable pharmacological activities, including anti-inflammatory, anticoagulant, anti-gastric ulcer, anti-diabetic, antioxidant, anti-cancer, and anti-mutagenic properties [19].
The study aimed to determine the antileishmanial properties of extracts from the Iraqi C. canadensis against Leishmania tropica.
Materials and Methods
This experimental study was conducted in the Pharmacology and College of Medicine of University of Babylon from September 2022 to September 2023 in which, the U937 monocyte was supplied by the National Cell Bank of Iran. The cells were grown in Roswell Park Memorial Institute (RPMI-1640) liquid medium (Gibco, UK) containing 10% fetal bovine serum (Gibco, UK) and phorbol myristate acetate (PMA) (Invevo Genes, USA). Stibogluconate (Pentostam) (GSK, UK) and C. canadensis, harvested from the mountains of Sulaymaniyah at the end of winter, were used. Neopterin (NEO) levels were measured using an ELISA Kit (Elabscience/USA). The Iraqi strain of L. tropica was obtained from the parasitology laboratory, graduate studies, Department of Biology, College of Science, University of Baghdad. The strain was then cultured in a biphasic medium (Nove-MacNeal-Nicolle) (NNN) [20].
1) Phytochemical compounds in the C. canadensis extract
A. Total alkaloid content
A total of 20g of plant material was subjected to extraction using methanol for 24hours, utilizing Soxhlet equipment. The extract underwent filtration, and the methanol was removed by evaporation using a rotary evaporator set at a temperature of 45°C.
1. Qualitative estimation (test for alkaloids)
Alkaloids were confirmed using Dragendorff's method. The extract was dissolved in dilute HCL, and Dragendorff's reagent was added, producing a crystalline precipitate indicating the presence of alkaloids. Positive samples were then quantitatively evaluated [21].
2. Quantitative estimation of alkaloid
The residue was dissolved in 2N HCl, filtered, and combined with a Bromocresol Green (BCG) solution and phosphate buffer. The resulting mixture was washed with chloroform, and its pH level was adjusted using 0.1N NaOH. To create the standard curve, an atropine standard solution was mixed with phosphate buffer and BCG solution, then shaken with the extract and chloroform. The resulting solution was collected, diluted with chloroform, and its absorbance was measured at 470nm in a UV-Spectrophotometer against the blank [22].
B. Determination of total phenolic compounds
Phenolic compounds were detected in an ethanolic extract using the Folin-Ciocalteu reagent. The mixture consisted of the extract, the reagent, and sodium carbonate. After 2 hours, the phenolic content was estimated by measuring the absorbance at 765nm against a calibration curve made with gallic acid (GA) [23].
C. Analysis of total flavonoid content
The flavonoid content was assessed using the aluminum chloride colorimetric method. A sample of the crude extract was mixed with NaNO2 and AlCl3 solutions, and NaOH was added to bring the final volume to 10ml. After 15minutes, the absorbance was measured at 510nm, and the flavonoid content was calculated as mg rutin equivalent per gram of dry weight [24].
D. Analysis of amino acids
Solid samples weighing approximately 5mg with a precision of 0.01mg, and liquid samples weighing approximately 100mg with an accuracy of 0.01mg, were hydrolyzed with 1ml of 6M hydrochloric acid solution at 100°C±20°C for 24hours. After hydrolysis, the amino acid residues were dissolved in 100µl of acetonitrile and derivatized with 100µl of OPA. The sample was then injected ten times (100µl per injection) into a gas chromatograph with a C18-ODS column and a fluorescence detector (Ex=445nm, Em=465nm) using an isocratic flow of 50/50 (v/v) water (pH=7.0) and acetonitrile at a flow rate of 1.0mL/min [25].
E. Analysis of total glycosides
To ascertain the presence of glycosides, the extracted substance was mixed with Baljet's reagent and allowed to sit for an hour. Following this, it was mixed with water and the absorbance was measured at a wavelength of 495 nm [26].
2) The preparation of the stock solution
The preparation of the C. canadensis aqueous extract stock solution (AqCC) involved dissolving 1μg of extract in 10ml of pyrogen-free Distel water. Subsequently, six serial dilutions were created with concentrations of 62.5, 125, 250, 500, 1000, and 2000μg/ml.
To create the alcoholic extract of C. canadensis stock solution (AqCC), 1mg of extract was dissolved in three milliliters of methanol. This was followed by the creation of six serial dilutions, each with concentrations of 62.5, 125, 250, 500, 1000, and 2000μg/ml.
A stock solution of stibogluconate (Pentostam) SSG was prepared at a concentration of 100mg/ml, followed by six serial dilutions at concentrations of 62.5, 125, 250, 500, 1000, and 2000μg/ml.
3) Cell line preparing and sub-culturing
Monocyte cell lines were sub-cultured for use as an in vitro model to evaluate the antileishmanial activity of AlCC and AqCC. The media used was RPMI-1640, supplemented with antimicrobial drugs such as gentamicin or penicillin (50μg/ml) and 5% fetal bovine serum. Afterwards, the cells were incubated at 37 degrees Celsius for twenty-four hours [27].
4) The development of a "macrophage-like" state in U937 monocyte
To activate NF-κB, we dissolved 5mg of PMA powder in 1.5 ml of endotoxin-free water, followed by storing at -20°C, protecting from light, avoiding repeated freeze-thaw cycles, adding 1 μl of PMA solution to the U937 monocyte cell lines, and incubating at 37°C for 24 hours [28]. PRO was used to infect macrophages from U937 in a stationary growth phase at a ratio of 20:1. The mixture was incubated in tissue culture flasks at 34°C with 5% CO2 and 95% relative humidity. After 12 hours, non-internalized PRO was removed by washing the cells five times with plain RPMI. The cells were further incubated for 96 hours in RPMI supplemented with 10% FCS. After treating the cell line with different concentrations of SSG and aqueous/alcoholic extracts, it was incubated at 37°C for 24 hours [29].
5) Detection of NEO by Enzyme-Linked Immunosorbent Assay (ELISA)
The study assessed the antileishmanial activity of extracts by introducing U973 macrophage cells into individual wells of a 96-well microplate. Following overnight treatment, promastigotes of L. tropica were administered to the macrophages and cultured at a temperature of 37°C for 96 hours. Subsequently, the cell line was exposed to varying doses of SSG, as well as aqueous and alcoholic extracts, and then incubated at 37°C for another 24 hours. After this incubation period, the liquid portion containing the treated macrophage cells was collected from each well and transferred into separate Eppendorf tubes designated for each group. The levels of nitric oxide and NEO were then measured using the sandwich enzyme-linked immunosorbent assay (ELISA) method [30, 31].
Statistical analysis
The data were analyzed using SPSS Version 26 and the student's t-test. The threshold for statistical significance was set at a p-value of ≤0.05.
Findings
HPLC analysis of the C. canadensis extract
Table 1 shows the active compounds in the extract. The analysis revealed six distinct peaks in the chloroform fraction of the Iraqi C. canadensis plant, as shown in Figures 1 and 2 for the aqueous and alcoholic extractions, respectively. Each peak represents a different type of active compound present in the plant. To further validate these findings, the retention times of each peak in the chloroform fraction were compared with those of a standard, as illustrated in Figure 3. Table 2 presents the retention time of the active compounds in comparison with the standard. Additionally, samples exhibited three peaks as depicted in Figures 4 and 5 at retention times ranging from 3.5 to 8 minutes, and when compared with the standard curve of glutathione (GSH), as shown in Figure 3G, the presence of GSH was confirmed.
Table 1. Active compounds present in the aqueous the aqueous (AqCC) and alcoholic (AlCC) extracts of C. canadensis


Figure 1. HPLC chromatogram of the aqueous C. canadensis extract.

Figure 2. HPLC chromatogram of alcoholic C. canadensis extract.


Figure 3. HPLC chromatogram of the Iraqi C. canadensis compounds compared to the standard (A: gallic acid; B: chlorogenic acid; C: caffeic acid; D: qurecetin; E: P-cumaric acid; F: apigenin; G: GSH).
Table 2. Retention time of the active compounds in comparison with the standard


Figure 4. HPLC chromatogram of alcoholic C. canadensis extract for amino acid.

Figure 5. HPLC chromatogram of aqueous C. canadensis extract for amino acid.
Effect of the extract compounds on NEO
Effect of SSG on NEO in U937 macrophage cell line
The administration of SSG led to a significant decrease in NEO levels in the U937 macrophage cell line compared to the control group (p<0.05; Figure 6).

Figure 6. Effect of SSG on U937 macrophage cell line.
Effect of SSG on NEO in U937 macrophage cell line infected with L. tropica.
The experiment revealed a substantial decrease in NEO production at high concentrations (250, 500, 1000, and 2000µg/ml) compared to the control group after 24hours of incubation (Figure 7).

Figure 7. Effect of SSG on U937 macrophage cell line infected with Leishmania tropica.
Effect of AqCC on NEO in U937 macrophage cell line
AqCC significantly reduced NEO levels in macrophages compared to the control group. The decline in NEO was statistically significant at elevated concentrations (125, 250, 500, 1000, and 2000µg/ml; p-value<0.05; Figure 8).

Figure 8. Effect of aqueous C. canadensis extract on U937 macrophage cell line.
Effect of AqCC on NEO in U937 macrophage cell line infected with L. tropica
AqCC at 1000 and 2000µg/ml caused a substantial drop in NEO levels compared to the control group (p-value≤0.05; Figure 9).

Figure 9. Effect of aqueous C. canadensis extract on U937 macrophage cell line infected with L. tropica.
Effect of AlCC on NEO in U937 macrophage cell line
AlCC markedly decreased the levels of NEO in the U937 macrophage cell line compared to the control group (Figure 10).

Figure 10. Effect of the aqueous C. canadensis extract on the U937 macrophage cell line.
Effect of AlCC on NEO in U937 macrophage cell line infected with L. tropica
In the U937 macrophage cell line, AlCC significantly reduced NEO levels compared to the control group, demonstrating its role in the immune response against L. tropica (p<0.05; Figure 11).

Figure 11. Effect of the aqueous C. canadensis extract on the U937 macrophage cell line infected with L. tropica.
Discussion
NEO levels in individuals infected with L. tropica decreased significantly in response to treatment with SSG, AlCC, and AqCC at all concentrations, according to this study. However, a significant reduction in NEO concentrations was observed exclusively at elevated concentrations of 1000 and 2000μg/ml of AqCC. Serum NEO levels that exceed the upper limit of the established normal range indicate activation of cell-mediated immunity. It is important to recognize that serum NEO does not exhibit disease specificity. Monitoring and evaluating its concentrations in the blood serum throughout the course of an infectious disease can provide valuable insights into the disease's severity and the effectiveness of the treatment [31]. Visceral leishmaniasis (VL) is characterized by an increase in the number and activation of macrophages, which would lead to a rise in NEO concentrations during the disease's active phase. Subsequently, as the parasite infection decreases, these levels would also decrease [11-13]. Recent studies have observed antileishmanial activity in AlCC and AqCC. This is confirmed by the presence of quercitrin, quercetin, apigenin, p-coumaric acid, and caffeic acid in the extracts, as determined by HPLC analysis [20]. The results of this research are consistent with those of Monzote et al., who similarly documented the antileishmanial properties of p-coumaric acid [32]. This compound has been found to suppress the activity of three crucial enzymes involved in the progression of Leishmania braziliensis: Aldehyde dehydrogenase (ALDH), mitogen-activated protein kinase (MPK4), and DNA topoisomerase 2 (TOP2). This discovery aligns with prior investigations suggesting that SSG functions by impeding DNA topoisomerase. Conversely, measuring NEO levels in the blood serum throughout the course of treatment for VL can help determine the efficacy of the therapy [10]. Elevated levels of reactive oxygen species (ROS) are associated with these measurements. Determining NEO concentrations enables an evaluation of the extent of oxidative stress and immunological activation [33]. Flavonoids, abundant in C. canadensis, exhibit various biological effects, such as regulating enzymes responsible for the elimination of ROS [34]. These chemical compounds have the capacity to stimulate cellular apoptosis and autophagy and can inhibit the growth and penetration of cancerous cells. In healthy cells, flavonoids act as antioxidants, helping to control ROS concentrations. In cancer cells, however, they function as potent pro-oxidants. By inhibiting pro-inflammatory signaling pathways and stimulating apoptotic pathways, flavonoids regulate the balance of ROS [35]. GA has demonstrated significant immunomodulatory characteristics, including an increase in macrophage capacity to ingest and eliminate foreign particles, enlargement of lysosomes, release of nitrite, and elevated levels of calcium ions within macrophages [36]. Chlorogenic acid (CGA) shows antileishmanial properties through its ability to eradicate parasites and disrupt their cell cycle, resulting in detrimental and inhibitory effects on the PRO. In vitro, CGA eradicates intracellular AMO completely, proving its efficacy in removing parasites from host cells. The enhanced functionality of macrophages facilitates the clearance process through the concurrent elevation of IL-12, TNF, and NO levels, and the reduction of IL-10 synthesis. Majumder et al. propose that CGA may function as an innovative and non-toxic chemical agent to treat visceral leishmaniasis, presenting a viable alternative to chemotherapy [37]. Anke et al., however, found that serum NEO concentrations did not increase in patients with CL [38]; their research contradicts this finding, suggesting that the two sources are in conflict.
Our findings support the use of natural plant extracts as alternative therapies for leishmaniasis. C. canadensis shows promise due to its bioactive components and various pharmacological effects. Further research is needed to understand the mechanisms of action and assess the treatment's effectiveness in animal models and clinical trials. Developing cost-effective and accessible therapies is crucial, especially in regions with limited resources. Continued exploration of C. canadensis as a therapeutic candidate could lead to effective and sustainable solutions for leishmaniasis.
Conclusion
This study demonstrates significant antileishmanial activity of C. canadensis extracts and highlights the correlation between NEO levels and Leishmania infection.
Acknowledgments: We would like to express my deepest gratitude to everyone who has helped me throughout my time at Kufa University's College of Medicine, but especially to everyone in the Department of Pharmacology and Therapeutics.
Ethical Permission: On the first of May in the year 2021, a local ethics commission at the College of Medicine at Kufa University gave their approval to both the study protocol and the informed consent statement.
Conflicts of Interests: Considering the presentation of research, there is a complete absence of any potential conflicts of interest.
Authors’ Contribution: Awadh MAA (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (60%); Gany SN (Second Author), Introduction Writer/Methodologist/Assistant Researcher (10%); Ghaleb RA (Third Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (20%); Ameen AA (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (10%)
Funding/Support: The research was not funded.
Keywords:
References
1. Irshad H, Khalid MZ. Types and treatments of leishmaniasis. Biomed J Sci Tech Res. 2022;42(5):34037-42. [Link] [DOI:10.26717/BJSTR.2022.42.006815]
2. De Vries HJC, Schallig HD. Cutaneous leishmaniasis: A 2022 updated narrative review into diagnosis and management developments. Am J Clin Dermatol. 2022;23(6):823-40. [Link] [DOI:10.1007/s40257-022-00726-8]
3. Ferreira C, Estaquier J, Silvestre R. Immune-metabolic interactions between Leishmania and macrophage host. Curr Opin Microbiol. 2021;63:231-7. [Link] [DOI:10.1016/j.mib.2021.07.012]
4. Martínez-López M, Soto M, Iborra S, Sancho D. Leishmania hijacks myeloid cells for immune escape. Front Microbiol. 2018;9:883. [Link] [DOI:10.3389/fmicb.2018.00883]
5. Saunders EC, McConville MJ. Immunometabolism of Leishmania granulomas. Immunol Cell Biol. 2020;98(10):832-44. [Link] [DOI:10.1111/imcb.12394]
6. Lazar LTY, Abass KS. Morphology, life cycle, pathogenesis and virulence factors of genus Leishmania: A review. Plant Arch. 2020;20(2):4057-60. [Link]
7. Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18(6):1296. [Link] [DOI:10.3390/ijms18061296]
8. Sloan MA, Brooks K, Otto TD, Sanders MJ, Cotton JA, Ligoxygakis P. Transcriptional and genomic parallels between the monoxenous parasite Herpetomonas muscarum and Leishmania. PLoS Genet. 2019;15(11):e1008452. [Link] [DOI:10.1371/journal.pgen.1008452]
9. Majeed RK, Muhammed HF, Rahim HM. Acute toxicity study of indomethacin and oxytetracycline in rabbits. Med J Babylon. 2018;15(3):218-21. [Link] [DOI:10.4103/MJBL.MJBL_60_18]
10. Hamerlinck FF, Van Gool T, Faber WR, Kager PA. Serum neopterin concentrations during treatment of leishmaniasis: Useful as test of cure?. FEMS Immunol Med Microbiol. 2000;27(1):31-4. [Link] [DOI:10.1111/j.1574-695X.2000.tb01408.x]
11. Palić S, Beijnen JH, Dorlo TPC. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int J Antimicrob Agents. 2022;59(1):106459. [Link] [DOI:10.1016/j.ijantimicag.2021.106459]
12. Kuryltsiv NB, Zborovska OV. Neopterin, a promising biomarker for the diagnosis of intraocular inflammation. J Ophthalmol. 2021;(3):55-60. [Link] [DOI:10.31288/oftalmolzh202135560]
13. Campos BLS, Silva TN, Ribeiro SP, Carvalho KI, Kallás EG, Laurenti MD, et al. Analysis of iron superoxide dismutase-encoding DNA vaccine on the evolution of the Leishmania amazonensis experimental infection. Parasite Immunol. 2015;37(8):407-16. [Link] [DOI:10.1111/pim.12206]
14. Kristanc L, Božič B, Jokhadar ŠZ, Dolenc MS, Gomišček G. The pore-forming action of polyenes: From model membranes to living organisms. Biochim Biophys Acta Biomembr. 2019;1861(2):418-30. [Link] [DOI:10.1016/j.bbamem.2018.11.006]
15. Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019;20(10):1251-65. [Link] [DOI:10.1080/14656566.2019.1609940]
16. Bahmani M, Saki K, Ezatpour B, Shahsavari S, Eftekhari Z, Jelodari M, et al. Leishmaniosis phytotherapy: Review of plants used in Iranian traditional medicine on leishmaniasis. Asian Pac J Trop Biomed. 2015;5(9):695-701. [Link] [DOI:10.1016/j.apjtb.2015.05.018]
17. Al-Douri NA. A survey of medicinal plants and their traditional uses in Iraq. Pharm Biol. 2000;38(1):74-9. [Link] [DOI:10.1076/1388-0209(200001)3811-BFT074]
18. Kadereit JW, Bittrich V. Flowering plants. Eudicots. Cham: Springer; 2018. [Link] [DOI:10.1007/978-3-319-93605-5]
19. Abood MA, Kadhim EJ. Phytochemical investigation of some active components in Iraqi Conyza Canadensis (Syn. Erigeron canadensis). Int J Drug Deliv Technol. 2021;11(3):669-75. [Link]
20. Hai N, Akhter RP, Ali I. Isolation, maintenance and detection of Leishmania parasite by microscopy and culture technique. Proceedings of the 2017 14th International Bhurban Conference on Applied Sciences and Technology (IBCAST). Islamabad; 2017. p. 179-82. [Link] [DOI:10.1109/IBCAST.2017.7868052]
21. Evans WC. Trease and Evans Pharmacognosy. 15th edition. Paris: Bailliere Tindall; 2002. [Link]
22. Ajanal M, Gundkalle MB, Nayak SU. Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer. Anc Sci Life. 2012;31(4):198-201. [Link] [DOI:10.4103/0257-7941.107361]
23. Laouini SE, Ouahrani MR. Phytochemical screening, in vitro antioxidant and antibacterial activity of Rumex vesicarius L. Extract. Sci Study Res Chem Chem Eng Biotechnol Food Ind. 2017;18(4):367-76. [Link] [DOI:10.26832/24566632.2018.030406]
24. Habibatni S, Abdessalam FZ, Hani K, Anwar S, Mansi I, Ali N. In vitro antioxidant, Xanthine oxidase-inhibitory and in vivo Anti-inflammatory, analgesic, antipyretic activity of Onopordum acanthium. Int J Phytomed. 2017;9(1):92-100. [Link] [DOI:10.5138/09750185.2030]
25. Sutariya V, Wehrung D, Geldenhuys WJ. Development and validation of a novel RP-HPLC method for the analysis of reduced glutathione. J Chromatogr Sci. 2012;50(3):271-6. [Link] [DOI:10.1093/chromsci/bmr055]
26. Tofighi Z, Ghazi SN, Hadjiakhoondi A, Yassa N. Determination of cardiac glycosides and total phenols in different generations of Securigera securidaca suspension culture. Res J Pharmacognosy. 2016;3(2):23-31. [Link]
27. Kuo CF, Chen CC, Lin CF, Jan MS, Huang RY, Luo YH, et al. Abrogation of streptococcal pyrogenic exotoxin B-mediated suppression of phagocytosis in U937 cells by Cordyceps sinensis mycelium via production of cytokines. Food Chem Toxicol. 2007; 45(2):278-85. [Link] [DOI:10.1016/j.fct.2006.08.017]
28. Sharp BM. Conversion of the U937 Monocyte into" Macrophage-Like" Populations Exhibiting M1 or M2 Characteristics. Ohio: Wright State University; 2013. [Link]
29. Puentes F, Diaz D, Hoya RD, Gutíerrez JA, Lozano JM, Patarroyo ME, et al. Cultivation and characterization of stable Leishmania guyanensis complex axenic amastigotes derived from infected U937 cells. Am J Trop Med Hyg. 2000;63(1):102-10. [Link] [DOI:10.4269/ajtmh.2000.63.102]
30. Kip AE, Wasunna M, Alves F, Schellens JHM, Beijnen JH, Musa AH, et al. Macrophage activation marker neopterin: A candidate biomarker for treatment response and relapse in visceral leishmaniasis. Front Cell Infect Microbiol. 2018;8:181. [Link] [DOI:10.3389/fcimb.2018.00181]
31. Bührer-Sekula S, Hamerlinck FFV, Out TA, Bordewijk LG, Klatser PR. Simple dipstick assay for semi-quantitative detection of neopterin in sera. J Immunol Methods. 2000;238(1-2):55-8. [Link] [DOI:10.1016/S0022-1759(00)00148-4]
32. Monzote L, Córdova WHP, García M, Piñón A, Setzer WN. In-vitro and In-vivo Activities of Phenolic Compounds Against Cutaneous Leishmaniasis. Rec Nat Prod. 2016;10(3):269. [Link]
33. Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3(2):175-87. [Link] [DOI:10.2174/1389200024605082]
34. Shareef RH, Sharba ZF, Hameed EN. The positive role of antioxidants on body immunity: An overview. Med J Babylon. 2021;18(3):169-71. [Link] [DOI:10.4103/MJBL.MJBL_18_21]
35. Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2):457. [Link] [DOI:10.3390/nu12020457]
36. Alves MM, Brito LM, Souza AC, Queiroz BCSH, de Carvalho TP, Batista JF, et al. Gallic and ellagic acids: Two natural immunomodulator compounds solve infection of macrophages by Leishmania major. Naunyn. Schmiedebergs Arch Pharmacol. 2017;390:893-903. [Link] [DOI:10.1007/s00210-017-1387-y]
37. Majumder N, Ganguly S, Ghosh AK, Kundu S, Banerjee A, Saha S. Chlorogenic acid acts upon Leishmania donovani arresting cell cycle and modulating cytokines and nitric oxide in vitro. Parasite Immunol. 2020; 42(6):e12719. [Link] [DOI:10.1111/pim.12719]
38. Kip AE, Balasegaram M, Beijnen JH, Schellens JHM, de Peter J, Vries TPC. Systematic review of biomarkers to monitor therapeutic response in leishmaniasis. Antimicrob Agents Chemother. 2015;59(1):1-14. [Link] [DOI:10.1128/AAC.04298-14]









