Volume 16, Issue 1 (2024)
Iran J War Public Health 2024, 16(1): 35-42 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/01/11 | Accepted: 2024/03/14 | Published: 2024/03/22
Received: 2024/01/11 | Accepted: 2024/03/14 | Published: 2024/03/22
How to cite this article
Jafari E, Bagheriyeh Yazdi K, Hendi A, Koochaki M. Low-Level Laser Therapy in Management of Recurrent Aphthous Stomatitis; A Comprehensive Review. Iran J War Public Health 2024; 16 (1) :35-42
URL: http://ijwph.ir/article-1-1430-en.html
URL: http://ijwph.ir/article-1-1430-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- “Student Research Committee” and “School of Dentistry”, Guilan University of Medical Sciences, Rasht, Iran
2- “Dental Sciences Research Center” and “Department of Prosthodontics, School of Dentistry”, Guilan University of Medical Sciences, Rasht, Iran
3- Department of Oral and Maxillofacial Medicine, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran
2- “Dental Sciences Research Center” and “Department of Prosthodontics, School of Dentistry”, Guilan University of Medical Sciences, Rasht, Iran
3- Department of Oral and Maxillofacial Medicine, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran
Full-Text (HTML) (2108 Views)
Introduction
Low-level laser therapy (LLLT), a subtype of photobiomodulation therapy (PBMT), utilizes photons at a lower intensity compared to other forms of laser therapy. This method does not rely on ablative or thermal mechanisms but instead operates through a photochemical effect, where light is absorbed and induces a chemical change. It can have either a stimulating or inhibitory effect on target tissues. Its medical applications include the reduction of pain and inflammation, enhancement of tissue repair, promotion of tissue and nerve regeneration, and prevention of tissue damage in various situations [1-4].
Studies have shown that far-red and near-infrared light (NIR), used in LLLT, improve wounds by stimulating collagen metabolism and reducing pain, although our current understanding of these mechanisms is still incomplete. LLLT is known to exert beneficial biostimulatory effects on both soft and hard tissues. In soft tissue, it promotes healing, reduces inflammation, and stimulates collagen synthesis. In hard tissue, LLLT has demonstrated potential in accelerating tissue repair [3, 5-8].
Oral ulcers, arising from traumatic, immunological, or other pathological processes, are a common complaint involving the oral mucosa and can cause varying levels of pain. The primary goal of treatment is to alleviate symptoms and accelerate the healing process. Despite various treatment attempts to enhance wound healing, the challenge of managing and treating these conditions persists. Orofacial ulcers and pain hinder essential functions such as eating, swallowing, maintaining oral hygiene, and speaking, especially when severe pain accompanies acute or chronic lesions. Ulcerative lesions, particularly recurrent aphthous stomatitis (RAS), are a leading cause of pain in the oral mucosa, significantly impacting quality of life [9-13].
The oral mucosa is frequently afflicted by RAS, which stands as the prevailing clinical condition in this particular domain. Its prevalence in the general population ranges from 5% to 25%, with the highest occurrence during the second decade of life. T-lymphocytes are believed to play a vital role in the emergence of this type of immunological dysfunction. Numerous factors contribute to its development, including genetics, immunology, hypersensitivity to food and drugs, hormonal fluctuations, physical trauma, deficiencies in essential nutrients like serum iron, folate, and vitamin B12, environmental and psychological stress, as well as viral infections. However, the exact cause remains uncharted.
Oral aphthous stomatitis represents a painful inflammatory condition in the oral mucosa, which can occur individually or as a manifestation of an underlying disease. Due to its frequent recurrence, it is referred to as RAS. In the early stages, individuals usually experience a tingling sensation, along with redness and subtle swelling in the specific non-keratinized mucosal area that is affected. Within a few hours, distinct ulcers with identifiable features become visible. The ulcers associated with RAS are round or oval-shaped, with well-defined erythematous haloes and a shallow center covered by a gray or yellowish fibrinous pseudomembrane. These ulcers may appear intermittently, with intervals spanning from a few days to several months [4-12].
When aphthous-like symptoms are found in the oral mucosa, it is crucial to collect a thorough medical history and establish an accurate differential diagnosis, eliminate the related systemic condition, and identify remediable factors prior to diagnosing aphthous stomatitis. Based on the pain, number, size, and duration of ulcers, RAS can be classified into three types: minor, major, and herpetiform ulcers. Minor RAS is the most common form, accounting for approximately 70-85% of all RAS ulcers. These ulcers typically occur in the non-keratinized oral mucosa and can persist for days or weeks. The pathophysiology of aphthous ulcers is still not fully understood, although various bacteria are implicated in its microbiological culture. The effective management of aphthous ulcers poses a significant challenge. There are various types of therapies available for the treatment of this condition, which can be broadly classified as either topical or systemic. Topical treatments encompass a range of options such as mouthwashes, gels, pastes, sprays, injections, and even laser treatments. Among these, there is a laser therapy technique known as PBMT that has shown promise in effectively targeting and treating localized instances of this condition. For oral aphthous derived from an underlying disease, topical medications are preferred due to their minimal side effects [14-21].
LLLT has been widely studied for its clinical efficacy in tissue healing as a topical method. According to in-vitro research, LLLT can significantly enhance a wide range of cellular processes. It increases the motility of keratinocyte cells, stimulates the release of growth factors, and induces the transformation of fibroblasts into myofibroblasts. In studies where LLLT was applied to one of two randomly chosen wounds spaced apart, both the treated and untreated wounds showed improved healing compared to a placebo. These findings were reported by Braverman et al., who observed increased wound contraction in rabbit wounds treated with LLLT compared to control wounds on the opposite side. They suggested that LLLT may have induced the production of tissue growth factors that affected surrounding tissues or entire systems. Indirect repair could be a valuable effect of LLLT in treating large-sized or multiple-location tissue damage. It also implies that deeper tissues could be influenced by laser therapy [22, 23].
Studies provide supporting evidence for the potential use of laser therapy as a treatment for oral ulcers. This treatment has been found to impact macrophage polarization and promote wound healing in the oral cavity. The proteins found in the body, particularly those within the mitochondrial-cytochrome system, are thought to be the primary receptors for laser light. These receptors are likely involved in mediating the effects of LLLT, although the exact mechanism of immunomodulation is not yet fully comprehended. One of the key benefits of LLLT is its analgesic effect, which is achieved through the inhibition of various nociceptive stimuli. These stimuli can be related to changes in temperature and chemical irritations. By applying LLLT, the pain threshold can be increased. This effect is attributed to the stabilization of cell membranes and the regulation of resting cell potential. LLLT helps to modulate and reduce pain sensations, thereby yielding favorable outcomes in terms of alleviating pain [5, 24-30].
LLLT limits the production of proinflammatory mediators within damaged nerve cells, while simultaneously promoting their maturation and post-traumatic regeneration. Furthermore, LLLT stimulates the production of adenosine triphosphate (ATP) and reduces cellular oxygen demand by targeting and stimulating the mitochondria, which are the powerhouses of the cells. This metabolic enhancement aids in tissue repair and regeneration. The application of LLLT has been found to affect various biochemical factors. It results in increased levels of serotonin and endorphins, which are neurotransmitters associated with mood and pain relief. Conversely, it reduces the levels of prostaglandin E2 and interleukin-1 beta, which are pro-inflammatory substances. The overall outcome of these biochemical changes is a reduction in pain perception. Additionally, LLLT inhibits the activity of plasminogen activator, an enzyme responsible for collagen breakdown. By doing so, it helps to mitigate the inflammatory response and facilitate the healing process [31].
LLLT has been investigated for the treatment of oral mucositis and RAS. Given recent reports on the successful application of LLLT as an advanced treatment modality for RAS, the objective of this review is to comprehensively assess and summarize clinical studies to determine the efficacy of laser therapy as a viable option for treating aphthous ulcers. To accomplish this, we have conducted a review of relevant studies published between the years 2013 and 2023 [32-35].
Information and Methods
Search strategy
Three online databases have been searched from 2013 to 2023. The following keywords "Low-level laser therapy", "LLLT", "aphthous", "recurrent aphthous", "aphthous stomatitis", "pain", and "laser therapy" have been used to search among titles and abstracts in Science Direct and in titles in Google Scholar. "Low-Level Light Therapy"[Mesh]) OR "Lasers"[Mesh] AND "Stomatitis, Aphthous"[Mesh] with/without "Wound Healing"[Mesh] OR "Pain "[Mesh] as MeSH terms were utilized to search in Medline. The article selection was performed in 5 phases:
In phase I, duplicated articles were removed. In phase II, titles and abstracts were studied, and unrelated articles were excluded. In phase III, by studying the materials and methods of the articles, some were eliminated. In phase IV, full-text articles were analyzed, and some were excluded. In phase V, the eligibility criteria consisted of randomized controlled trials (RCTs), and all found RCTs were included.
Exclusion Criteria
Exclusion criteria were studies involving other interventions with RAS or systemic diseases affecting treatment outcomes, methodological issues, including lack of a control group, unclear group sizes, inconsistent post-intervention observation protocols, absence of statistical analysis, or papers unavailable in full-text, non-English articles, reviews outside the selected timeline, in vitro studies, animal studies, and case reports, and studies focusing on the laser treatment of non-aphthous oral or extra-oral lesions.
Inclusion Criteria
Exclusion criteria were English language papers, randomized controlled trials (RCTs) and case series, papers available in full text, retrospective studies, and studies specifically addressing laser treatment of oral aphthous ulcerations.
Laser Type
Among the lasers used in dental practice are high-power lasers such as the Carbon Dioxide Laser (CO2), Neodymium-Doped Yttrium Aluminium Garnet (Nd:YAG), diode lasers, and Erbium: Yttrium Aluminium Garnet (Er:YAG) and Erbium, Chromium-doped Yttrium Scandium Gallium Garnet (Er,Cr:YSGG). These can be applied using focused/defocused and contact/non-contact methods to achieve various effects on the target tissues.
Pain Measurement
The Visual Analogue Scale (VAS) is employed to measure pain levels, ranging from 1 (no pain) to 10 (severe pain). In some studies, pain relief is categorized as complete, partial, or no relief, rather than comparing VAS scores over time.
Ulcer Size
Ulcer dimensions are measured using a periodontal probe in millimeters across different days. Some studies evaluate the effectiveness of size reduction using a scoring system: 1 (healed), 2 (marked improvement), 3 (moderate improvement), and 4 (no improvement).
Patient Satisfaction and Functional Complications
Patient satisfaction and reductions in functional complications are assessed using the VAS. Difficulties in eating, drinking, and brushing are evaluated from day 0 to day 3 with a questionnaire offering options of none, slight, moderate, and severe for each activity.
Healing Time
Healing of Recurrent Aphthous Stomatitis (HRAS) is compared between the study and placebo groups in terms of days until re-epithelialization is observed clinically. Some studies assess the stages of epithelialization as absent, initiating, or complete.
Recurrence of Lesions
Recurrence is documented in terms of months if the patient reports new lesions.
Gender Differences
Some studies assess the differential response to treatment between men and women.
Findings
A total of 88 articles were initially found during the search. After conducting data selection phases, 8 original papers met the criteria and were selected for this study [5, 24-30, 38]. Figure 1 shows the study selection process.
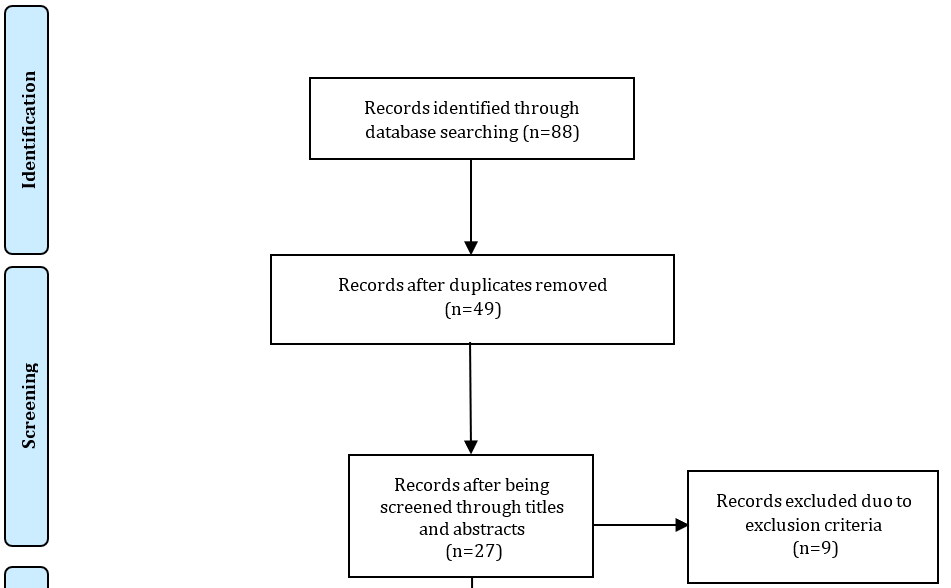
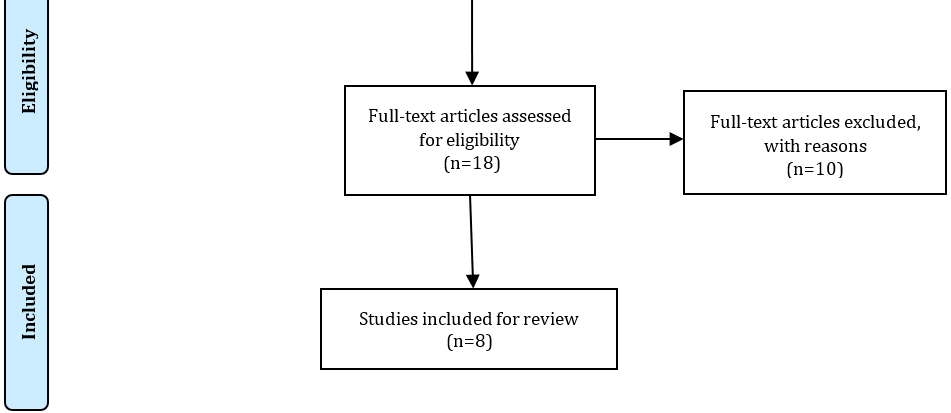
Figure 1. The study selection process.
Laser biostimulation of tissue employs a laser beam within specific wavelengths, typically between 630 and 1000 nm, and with power outputs ranging from 2 to 200 mW. The effectiveness of this technique is influenced by factors such as tissue vascularization and the dispersion of energy by erythrocytes, which leads to reduced antioxidants and oxidation in the area, resulting in collagen deposition and epithelialization, thereby improving wound healing [26, 31].
The type of laser and its therapeutic application method can induce different levels of biological changes [1].
Fekrazad et al. compared the efficacy of two methods of ND:YAG laser treatment with a sham laser (Time=60s; Frequency=30Hz; Power=3W). Group A was treated with focused laser with the following specifications: Energy=100mJ, Energy density=48 [J/cm2], Power density=0.797 [W/cm2], Spot size=0.1256 [cm2], while Group B received defocused laser treatment, with the laser tip positioned 6 mm away from the lesion to achieve defocused irradiation, Energy density=21 [J/cm2], Power density=0.354 [W/cm2], Spot size=0.2826 [cm2]. The control group received a similar treatment using a He-Ne red light laser. The defocused technique demonstrated better results in all assessed parameters [38]. These results were supported by findings from the study conducted by Chen et al., which demonstrated that a defocused laser shows better macroscopic properties, while having lower temperature and mechanical performance, and less collagen distortion and deformation, all of which vary by adjusting the focus [39].
A total of four papers utilized diode lasers with varying wavelengths for PBMT. Bardellini et al. employed a laser with a wavelength of 645 nm, power of 100 mW, a spot size of 1 cm², an energy density of 10 J/cm² in the study group, and a switched-off mode in the control group. Similarly, another study by Soliman et al. used a diode laser at a wavelength of 660 nm, with an intensity of 100-130 mW/cm, and an energy of 4 J/cm2, while employing a placebo in the study and control groups, respectively [24, 25].
lbrektson et al. reported the use of a GaAlAs diode laser with a wavelength of 809 nm, power of 60 mW, frequency of 1800 Hz, duration of 80 seconds per treatment, and a dose of 6.3 J/cm², compared to a no-power laser in a sham control group. In a similar vein, Aggarwal utilized an output power of 500 mW and a wavelength of 810 nm, maintaining a distance of 2-3 mm between the laser tip and the ulcer surface in his evaluations [29, 30]. All studies found these methods beneficial for managing RAS. A clinical assessment to evaluate the effects of different wavelengths and output powers on tissue showed that increasing both can enhance the thermal effect of lasers. The increase in thermal changes with higher wavelengths is due to greater water and hemoglobin absorption with the 980 nm diode laser compared to the 810 nm and 940 nm diode lasers. At higher wavelengths, good absorption by water and hemoglobin in tissues results in a further increase in temperature, which is more suitable for ablation and biopsy procedures.
A comparison between diode lasers and CO2 lasers was conducted by Zeini Jahromi et al., who analyzed a CO2 laser (DEKA, 10600 nm) for approximately 5-10 seconds at a power output of 2W and a distance of 12.5 mm from the tissue as Group A. An InGaAlP Diode laser (AZOR-2K, 660 nm), at a frequency of 80 Hz, power output of 25 mW, power density of 3 J/cm², and a focal spot size of 9.04 mm was used in contact with the lesion surface for 4 minutes in Group B, while Group C utilized a sham laser. Results indicated pain reduction immediately after treatment with the CO2 laser, although the pain persisted longer after this type of treatment compared to the InGaAlP Diode laser. CO2 laser treatment also resulted in fewer functional complications compared to the placebo group. However, laser treatment did not show significant improvements in healing time in this study. Zand et al. evaluated the effects of a non-thermal non-ablative 1 W CO2 laser on the wound healing of minor RAS in a single session. Their study revealed that laser treatment can improve the healing of ulcers, which contrasts with these results. The use of a non-ablative method in this study may explain the different outcomes compared to the current article [26, 42].
CO2 lasers have also been utilized at 0.7 W power, using a defocused handpiece in continuous mode for 5-8 seconds, at a distance of 5-7mm from the mucosal surface in a spiral motion, as investigated by Prasad et al. This method, which was compared to a placebo, confirms the efficacy of this therapy in a single session. It employs a non-contact, non-ablative, and non-invasive method without anesthesia and offers pain relief (sustained for up to 24 hours) and accelerated healing. These studies reveal that even when high-power lasers are positioned further from the tissue, reducing laser power relative to the distance, they can achieve similar effects to low-level lasers [5]. An Er,Cr:YSGG laser was applied in one of the studies, with a 600 μm diameter, 6 mm length tip at an energy level of 0.25W with a repetition rate of 20kHz, pulse duration of 140μs without water and 10% air at 5 J/cm2 energy density in non-contact mode. According to Yilmaz's findings, using an Er,Cr:YSGG laser at 0.25W without water may be suitable for reducing pain and accelerating the healing of RAS compared to a sham control [27].
Several studies have investigated the efficacy of different interventions for managing pain levels over time. These studies employed the VAS to assign numerical values to pain intensity levels reported by individuals. Bardellini et al. observed a decrease in pain levels from Day 0 to Day 7, with the study group exhibiting reductions from 4 to 1 by Day 4, and 1 by Day 7, while the control group showed reductions from 4 to 3 by Day 4 and 1 by Day 7. Soliman et al. demonstrated pain reductions in the study group with values decreasing from 2 on Day 1 to 0.82 on Day 3, in contrast to the control group, which maintained constant values around 10 throughout the study period. Zeini Jahromi et al. highlighted reductions in idiopathic and contact pain with different lasers; the CO2 laser significantly reduced pain from 1.71 to 0.7, whereas the Diode laser reduced pain from 0.58 to 2.08. Additionally, pain reduction after treatment was immediate with the CO2 laser but lasted longer with the Diode laser. Yilmaz et al. showed reductions in pain levels from a baseline of 8.3 to 0 by Day 10 in the study group, compared to minimal reductions in the control group, with statistically significant differences at various time points post-treatment. Albrektson et al. reported decreasing VAS scores from Day 0 to Day 2 in both study and control groups, with statistically significant differences observed due to the treatment. Aggarwal et al. demonstrated varying pain reduction scores between the treatment and control groups, with the laser group showing significantly greater reductions compared to the control group. Prasad et al. emphasized the effectiveness of non-contact CO2 laser treatment in providing immediate and lasting pain relief for up to 24 hours after a single session, further supporting the positive impact of this modality on pain reduction. Overall, these studies showcase the numerical impact of various treatments on pain reduction, providing valuable insights into the efficacy of different interventions in managing pain levels over time [5, 24-30, 38].
To gain a comprehensive understanding of the effects of different interventions on ulcer healing, a review of relevant studies provides valuable insights into changes in ulcer size and healing time. These factors are crucial in identifying effective treatment approaches and optimizing patient outcomes. In this analysis, we examine the findings of several studies conducted by Fekrazad et al., Bardellini et al., Soliman et al., Zeini Jahromi et al., Yilmaz et al., Aggarwal et al., and Prasad et al., which illuminate the impact of various interventions on ulcer size reduction and healing time. The data presented by Fekrazad et al. indicated a notable decrease in ulcer size over three consecutive days, demonstrating that a focused treatment technique led to a shorter healing time. Bardellini et al. reported statistically significant differences in ulcer diameter reduction between two groups on days 4 and 7, with both groups showing progressive reduction and complete healing by day 10. Soliman et al. observed statistically significant differences in ulcer size between the study group on days 1, 4, and 6 compared to the control group, particularly highlighting the efficacy of laser treatment in reducing ulcer size. Zeini Jahromi et al. found no significant differences in ulcer repair time among different groups and highlighted a lack of satisfaction with laser treatment in their study. Yilmaz et al. showcased the significant healing effect of laser treatment, with HRAS scores differing from the control group, indicating maintained effectiveness throughout the study. Aggarwal et al. reported a statistically significant reduction in lesion size and a highly significant shorter healing time in the LLLT group compared to the sham-controlled group. Prasad et al. also found faster healing times in laser-treated ulcers, further supporting the efficacy of laser treatments in promoting ulcer healing based on scientific assessments and statistical analyses [5, 34-30, 38].
Discussion
Lasers are considered an alternative treatment option due to their promising properties, such as excellent water absorption and effective penetration of biological tissues [43]. RAS is characterized by painful mouth ulcers causing dysfunction without an underlying disease explanation. Standardized treatments for this condition are lacking, and none are curative. The primary goal of any treatment should be to alleviate pain, shorten ulcer duration, and prevent recurrence [44]. Assessing laser efficacy in pain reduction is crucial.
LLLT also positively affects microcirculation by enhancing arteriolar dilation, improving blood flow, and contributing to tissue healing and regeneration. LLLT stimulates fibroblast proliferation, which are crucial cells in wound healing, and their maturation into myofibroblasts, thereby accelerating wound healing. Additionally, LLLT enhances fibroblastic growth factor secretion, further aiding tissue repair and reducing overall healing time [31].
To assess the impact of laser treatment on patient satisfaction and functional complications, researchers used the Visual Analog Scale (VAS) to record satisfaction ratings and reduction in functional complications for different treatment groups. Patient satisfaction was measured using the VAS, a tool allowing individuals to rate satisfaction or discomfort on a scale, with ratings recorded for different groups.
Analyzing the data provided, several conclusions can be drawn regarding patient satisfaction and reduction in functional complications post-laser treatment for painful mouth ulcers. The average VAS rating for reduction in functional complications in the study group was 8.57±1.50, indicating a significant improvement after laser treatment.
The average reduction in functional complications for the control group was 7.50±2.19 on the VAS, indicating a relatively lower improvement level compared to the study group [26]. Both groups experienced reduced functional complications after laser treatment for painful mouth ulcers. However, the study group showed a higher improvement level in functional complications than the control group. Regarding patient satisfaction, the control group reported relatively higher satisfaction compared to the study group. It's essential to note that satisfaction ratings may vary based on individual experiences and expectations. Further analysis and evaluation may be necessary to fully assess the effectiveness and outcomes of laser treatment for painful mouth ulcers [25, 26].
Upon thorough review of the studies, no discernible disparity was found in lesion localization or between genders.
However, more extensive comparisons between high-power and low-power lasers are needed to determine the ultimate effectiveness of lesion ablation. Further research is warranted to elucidate the underlying mechanisms of LLLT, laser focus effects, and optimize treatment protocols for favorable clinical outcomes.
Conclusion
Among various laser types, the diode laser is most commonly used, providing immediate pain relief. Albrektson's study demonstrates the fastest pain reduction with the diode laser at low power settings. Functional complications decreased as healing accelerated with LLLT in most cases.
Acknowledgments: None declared.
Ethical Permissions: This study was approved by the Ethics Committee of Guilan University of Medical Sciences.
Conflicts of Interests: The authors declared no conflicts of interests.
Authors’ Contribution: Jafari E (First Author), Introduction Writer/Main Researcher (40%); Bagheriyeh Yazdi K (Second Author), Assistant Researcher/Discussion Writer (20%); Hendi (Third Author), Methodologist/Statistical Analyst (20%); Koochaki M (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher (20%)
Funding/Support: None declared.
Low-level laser therapy (LLLT), a subtype of photobiomodulation therapy (PBMT), utilizes photons at a lower intensity compared to other forms of laser therapy. This method does not rely on ablative or thermal mechanisms but instead operates through a photochemical effect, where light is absorbed and induces a chemical change. It can have either a stimulating or inhibitory effect on target tissues. Its medical applications include the reduction of pain and inflammation, enhancement of tissue repair, promotion of tissue and nerve regeneration, and prevention of tissue damage in various situations [1-4].
Studies have shown that far-red and near-infrared light (NIR), used in LLLT, improve wounds by stimulating collagen metabolism and reducing pain, although our current understanding of these mechanisms is still incomplete. LLLT is known to exert beneficial biostimulatory effects on both soft and hard tissues. In soft tissue, it promotes healing, reduces inflammation, and stimulates collagen synthesis. In hard tissue, LLLT has demonstrated potential in accelerating tissue repair [3, 5-8].
Oral ulcers, arising from traumatic, immunological, or other pathological processes, are a common complaint involving the oral mucosa and can cause varying levels of pain. The primary goal of treatment is to alleviate symptoms and accelerate the healing process. Despite various treatment attempts to enhance wound healing, the challenge of managing and treating these conditions persists. Orofacial ulcers and pain hinder essential functions such as eating, swallowing, maintaining oral hygiene, and speaking, especially when severe pain accompanies acute or chronic lesions. Ulcerative lesions, particularly recurrent aphthous stomatitis (RAS), are a leading cause of pain in the oral mucosa, significantly impacting quality of life [9-13].
The oral mucosa is frequently afflicted by RAS, which stands as the prevailing clinical condition in this particular domain. Its prevalence in the general population ranges from 5% to 25%, with the highest occurrence during the second decade of life. T-lymphocytes are believed to play a vital role in the emergence of this type of immunological dysfunction. Numerous factors contribute to its development, including genetics, immunology, hypersensitivity to food and drugs, hormonal fluctuations, physical trauma, deficiencies in essential nutrients like serum iron, folate, and vitamin B12, environmental and psychological stress, as well as viral infections. However, the exact cause remains uncharted.
Oral aphthous stomatitis represents a painful inflammatory condition in the oral mucosa, which can occur individually or as a manifestation of an underlying disease. Due to its frequent recurrence, it is referred to as RAS. In the early stages, individuals usually experience a tingling sensation, along with redness and subtle swelling in the specific non-keratinized mucosal area that is affected. Within a few hours, distinct ulcers with identifiable features become visible. The ulcers associated with RAS are round or oval-shaped, with well-defined erythematous haloes and a shallow center covered by a gray or yellowish fibrinous pseudomembrane. These ulcers may appear intermittently, with intervals spanning from a few days to several months [4-12].
When aphthous-like symptoms are found in the oral mucosa, it is crucial to collect a thorough medical history and establish an accurate differential diagnosis, eliminate the related systemic condition, and identify remediable factors prior to diagnosing aphthous stomatitis. Based on the pain, number, size, and duration of ulcers, RAS can be classified into three types: minor, major, and herpetiform ulcers. Minor RAS is the most common form, accounting for approximately 70-85% of all RAS ulcers. These ulcers typically occur in the non-keratinized oral mucosa and can persist for days or weeks. The pathophysiology of aphthous ulcers is still not fully understood, although various bacteria are implicated in its microbiological culture. The effective management of aphthous ulcers poses a significant challenge. There are various types of therapies available for the treatment of this condition, which can be broadly classified as either topical or systemic. Topical treatments encompass a range of options such as mouthwashes, gels, pastes, sprays, injections, and even laser treatments. Among these, there is a laser therapy technique known as PBMT that has shown promise in effectively targeting and treating localized instances of this condition. For oral aphthous derived from an underlying disease, topical medications are preferred due to their minimal side effects [14-21].
LLLT has been widely studied for its clinical efficacy in tissue healing as a topical method. According to in-vitro research, LLLT can significantly enhance a wide range of cellular processes. It increases the motility of keratinocyte cells, stimulates the release of growth factors, and induces the transformation of fibroblasts into myofibroblasts. In studies where LLLT was applied to one of two randomly chosen wounds spaced apart, both the treated and untreated wounds showed improved healing compared to a placebo. These findings were reported by Braverman et al., who observed increased wound contraction in rabbit wounds treated with LLLT compared to control wounds on the opposite side. They suggested that LLLT may have induced the production of tissue growth factors that affected surrounding tissues or entire systems. Indirect repair could be a valuable effect of LLLT in treating large-sized or multiple-location tissue damage. It also implies that deeper tissues could be influenced by laser therapy [22, 23].
Studies provide supporting evidence for the potential use of laser therapy as a treatment for oral ulcers. This treatment has been found to impact macrophage polarization and promote wound healing in the oral cavity. The proteins found in the body, particularly those within the mitochondrial-cytochrome system, are thought to be the primary receptors for laser light. These receptors are likely involved in mediating the effects of LLLT, although the exact mechanism of immunomodulation is not yet fully comprehended. One of the key benefits of LLLT is its analgesic effect, which is achieved through the inhibition of various nociceptive stimuli. These stimuli can be related to changes in temperature and chemical irritations. By applying LLLT, the pain threshold can be increased. This effect is attributed to the stabilization of cell membranes and the regulation of resting cell potential. LLLT helps to modulate and reduce pain sensations, thereby yielding favorable outcomes in terms of alleviating pain [5, 24-30].
LLLT limits the production of proinflammatory mediators within damaged nerve cells, while simultaneously promoting their maturation and post-traumatic regeneration. Furthermore, LLLT stimulates the production of adenosine triphosphate (ATP) and reduces cellular oxygen demand by targeting and stimulating the mitochondria, which are the powerhouses of the cells. This metabolic enhancement aids in tissue repair and regeneration. The application of LLLT has been found to affect various biochemical factors. It results in increased levels of serotonin and endorphins, which are neurotransmitters associated with mood and pain relief. Conversely, it reduces the levels of prostaglandin E2 and interleukin-1 beta, which are pro-inflammatory substances. The overall outcome of these biochemical changes is a reduction in pain perception. Additionally, LLLT inhibits the activity of plasminogen activator, an enzyme responsible for collagen breakdown. By doing so, it helps to mitigate the inflammatory response and facilitate the healing process [31].
LLLT has been investigated for the treatment of oral mucositis and RAS. Given recent reports on the successful application of LLLT as an advanced treatment modality for RAS, the objective of this review is to comprehensively assess and summarize clinical studies to determine the efficacy of laser therapy as a viable option for treating aphthous ulcers. To accomplish this, we have conducted a review of relevant studies published between the years 2013 and 2023 [32-35].
Information and Methods
Search strategy
Three online databases have been searched from 2013 to 2023. The following keywords "Low-level laser therapy", "LLLT", "aphthous", "recurrent aphthous", "aphthous stomatitis", "pain", and "laser therapy" have been used to search among titles and abstracts in Science Direct and in titles in Google Scholar. "Low-Level Light Therapy"[Mesh]) OR "Lasers"[Mesh] AND "Stomatitis, Aphthous"[Mesh] with/without "Wound Healing"[Mesh] OR "Pain "[Mesh] as MeSH terms were utilized to search in Medline. The article selection was performed in 5 phases:
In phase I, duplicated articles were removed. In phase II, titles and abstracts were studied, and unrelated articles were excluded. In phase III, by studying the materials and methods of the articles, some were eliminated. In phase IV, full-text articles were analyzed, and some were excluded. In phase V, the eligibility criteria consisted of randomized controlled trials (RCTs), and all found RCTs were included.
Exclusion Criteria
Exclusion criteria were studies involving other interventions with RAS or systemic diseases affecting treatment outcomes, methodological issues, including lack of a control group, unclear group sizes, inconsistent post-intervention observation protocols, absence of statistical analysis, or papers unavailable in full-text, non-English articles, reviews outside the selected timeline, in vitro studies, animal studies, and case reports, and studies focusing on the laser treatment of non-aphthous oral or extra-oral lesions.
Inclusion Criteria
Exclusion criteria were English language papers, randomized controlled trials (RCTs) and case series, papers available in full text, retrospective studies, and studies specifically addressing laser treatment of oral aphthous ulcerations.
Laser Type
Among the lasers used in dental practice are high-power lasers such as the Carbon Dioxide Laser (CO2), Neodymium-Doped Yttrium Aluminium Garnet (Nd:YAG), diode lasers, and Erbium: Yttrium Aluminium Garnet (Er:YAG) and Erbium, Chromium-doped Yttrium Scandium Gallium Garnet (Er,Cr:YSGG). These can be applied using focused/defocused and contact/non-contact methods to achieve various effects on the target tissues.
Pain Measurement
The Visual Analogue Scale (VAS) is employed to measure pain levels, ranging from 1 (no pain) to 10 (severe pain). In some studies, pain relief is categorized as complete, partial, or no relief, rather than comparing VAS scores over time.
Ulcer Size
Ulcer dimensions are measured using a periodontal probe in millimeters across different days. Some studies evaluate the effectiveness of size reduction using a scoring system: 1 (healed), 2 (marked improvement), 3 (moderate improvement), and 4 (no improvement).
Patient Satisfaction and Functional Complications
Patient satisfaction and reductions in functional complications are assessed using the VAS. Difficulties in eating, drinking, and brushing are evaluated from day 0 to day 3 with a questionnaire offering options of none, slight, moderate, and severe for each activity.
Healing Time
Healing of Recurrent Aphthous Stomatitis (HRAS) is compared between the study and placebo groups in terms of days until re-epithelialization is observed clinically. Some studies assess the stages of epithelialization as absent, initiating, or complete.
Recurrence of Lesions
Recurrence is documented in terms of months if the patient reports new lesions.
Gender Differences
Some studies assess the differential response to treatment between men and women.
Findings
A total of 88 articles were initially found during the search. After conducting data selection phases, 8 original papers met the criteria and were selected for this study [5, 24-30, 38]. Figure 1 shows the study selection process.


Figure 1. The study selection process.
Laser biostimulation of tissue employs a laser beam within specific wavelengths, typically between 630 and 1000 nm, and with power outputs ranging from 2 to 200 mW. The effectiveness of this technique is influenced by factors such as tissue vascularization and the dispersion of energy by erythrocytes, which leads to reduced antioxidants and oxidation in the area, resulting in collagen deposition and epithelialization, thereby improving wound healing [26, 31].
The type of laser and its therapeutic application method can induce different levels of biological changes [1].
Fekrazad et al. compared the efficacy of two methods of ND:YAG laser treatment with a sham laser (Time=60s; Frequency=30Hz; Power=3W). Group A was treated with focused laser with the following specifications: Energy=100mJ, Energy density=48 [J/cm2], Power density=0.797 [W/cm2], Spot size=0.1256 [cm2], while Group B received defocused laser treatment, with the laser tip positioned 6 mm away from the lesion to achieve defocused irradiation, Energy density=21 [J/cm2], Power density=0.354 [W/cm2], Spot size=0.2826 [cm2]. The control group received a similar treatment using a He-Ne red light laser. The defocused technique demonstrated better results in all assessed parameters [38]. These results were supported by findings from the study conducted by Chen et al., which demonstrated that a defocused laser shows better macroscopic properties, while having lower temperature and mechanical performance, and less collagen distortion and deformation, all of which vary by adjusting the focus [39].
A total of four papers utilized diode lasers with varying wavelengths for PBMT. Bardellini et al. employed a laser with a wavelength of 645 nm, power of 100 mW, a spot size of 1 cm², an energy density of 10 J/cm² in the study group, and a switched-off mode in the control group. Similarly, another study by Soliman et al. used a diode laser at a wavelength of 660 nm, with an intensity of 100-130 mW/cm, and an energy of 4 J/cm2, while employing a placebo in the study and control groups, respectively [24, 25].
lbrektson et al. reported the use of a GaAlAs diode laser with a wavelength of 809 nm, power of 60 mW, frequency of 1800 Hz, duration of 80 seconds per treatment, and a dose of 6.3 J/cm², compared to a no-power laser in a sham control group. In a similar vein, Aggarwal utilized an output power of 500 mW and a wavelength of 810 nm, maintaining a distance of 2-3 mm between the laser tip and the ulcer surface in his evaluations [29, 30]. All studies found these methods beneficial for managing RAS. A clinical assessment to evaluate the effects of different wavelengths and output powers on tissue showed that increasing both can enhance the thermal effect of lasers. The increase in thermal changes with higher wavelengths is due to greater water and hemoglobin absorption with the 980 nm diode laser compared to the 810 nm and 940 nm diode lasers. At higher wavelengths, good absorption by water and hemoglobin in tissues results in a further increase in temperature, which is more suitable for ablation and biopsy procedures.
A comparison between diode lasers and CO2 lasers was conducted by Zeini Jahromi et al., who analyzed a CO2 laser (DEKA, 10600 nm) for approximately 5-10 seconds at a power output of 2W and a distance of 12.5 mm from the tissue as Group A. An InGaAlP Diode laser (AZOR-2K, 660 nm), at a frequency of 80 Hz, power output of 25 mW, power density of 3 J/cm², and a focal spot size of 9.04 mm was used in contact with the lesion surface for 4 minutes in Group B, while Group C utilized a sham laser. Results indicated pain reduction immediately after treatment with the CO2 laser, although the pain persisted longer after this type of treatment compared to the InGaAlP Diode laser. CO2 laser treatment also resulted in fewer functional complications compared to the placebo group. However, laser treatment did not show significant improvements in healing time in this study. Zand et al. evaluated the effects of a non-thermal non-ablative 1 W CO2 laser on the wound healing of minor RAS in a single session. Their study revealed that laser treatment can improve the healing of ulcers, which contrasts with these results. The use of a non-ablative method in this study may explain the different outcomes compared to the current article [26, 42].
CO2 lasers have also been utilized at 0.7 W power, using a defocused handpiece in continuous mode for 5-8 seconds, at a distance of 5-7mm from the mucosal surface in a spiral motion, as investigated by Prasad et al. This method, which was compared to a placebo, confirms the efficacy of this therapy in a single session. It employs a non-contact, non-ablative, and non-invasive method without anesthesia and offers pain relief (sustained for up to 24 hours) and accelerated healing. These studies reveal that even when high-power lasers are positioned further from the tissue, reducing laser power relative to the distance, they can achieve similar effects to low-level lasers [5]. An Er,Cr:YSGG laser was applied in one of the studies, with a 600 μm diameter, 6 mm length tip at an energy level of 0.25W with a repetition rate of 20kHz, pulse duration of 140μs without water and 10% air at 5 J/cm2 energy density in non-contact mode. According to Yilmaz's findings, using an Er,Cr:YSGG laser at 0.25W without water may be suitable for reducing pain and accelerating the healing of RAS compared to a sham control [27].
Several studies have investigated the efficacy of different interventions for managing pain levels over time. These studies employed the VAS to assign numerical values to pain intensity levels reported by individuals. Bardellini et al. observed a decrease in pain levels from Day 0 to Day 7, with the study group exhibiting reductions from 4 to 1 by Day 4, and 1 by Day 7, while the control group showed reductions from 4 to 3 by Day 4 and 1 by Day 7. Soliman et al. demonstrated pain reductions in the study group with values decreasing from 2 on Day 1 to 0.82 on Day 3, in contrast to the control group, which maintained constant values around 10 throughout the study period. Zeini Jahromi et al. highlighted reductions in idiopathic and contact pain with different lasers; the CO2 laser significantly reduced pain from 1.71 to 0.7, whereas the Diode laser reduced pain from 0.58 to 2.08. Additionally, pain reduction after treatment was immediate with the CO2 laser but lasted longer with the Diode laser. Yilmaz et al. showed reductions in pain levels from a baseline of 8.3 to 0 by Day 10 in the study group, compared to minimal reductions in the control group, with statistically significant differences at various time points post-treatment. Albrektson et al. reported decreasing VAS scores from Day 0 to Day 2 in both study and control groups, with statistically significant differences observed due to the treatment. Aggarwal et al. demonstrated varying pain reduction scores between the treatment and control groups, with the laser group showing significantly greater reductions compared to the control group. Prasad et al. emphasized the effectiveness of non-contact CO2 laser treatment in providing immediate and lasting pain relief for up to 24 hours after a single session, further supporting the positive impact of this modality on pain reduction. Overall, these studies showcase the numerical impact of various treatments on pain reduction, providing valuable insights into the efficacy of different interventions in managing pain levels over time [5, 24-30, 38].
To gain a comprehensive understanding of the effects of different interventions on ulcer healing, a review of relevant studies provides valuable insights into changes in ulcer size and healing time. These factors are crucial in identifying effective treatment approaches and optimizing patient outcomes. In this analysis, we examine the findings of several studies conducted by Fekrazad et al., Bardellini et al., Soliman et al., Zeini Jahromi et al., Yilmaz et al., Aggarwal et al., and Prasad et al., which illuminate the impact of various interventions on ulcer size reduction and healing time. The data presented by Fekrazad et al. indicated a notable decrease in ulcer size over three consecutive days, demonstrating that a focused treatment technique led to a shorter healing time. Bardellini et al. reported statistically significant differences in ulcer diameter reduction between two groups on days 4 and 7, with both groups showing progressive reduction and complete healing by day 10. Soliman et al. observed statistically significant differences in ulcer size between the study group on days 1, 4, and 6 compared to the control group, particularly highlighting the efficacy of laser treatment in reducing ulcer size. Zeini Jahromi et al. found no significant differences in ulcer repair time among different groups and highlighted a lack of satisfaction with laser treatment in their study. Yilmaz et al. showcased the significant healing effect of laser treatment, with HRAS scores differing from the control group, indicating maintained effectiveness throughout the study. Aggarwal et al. reported a statistically significant reduction in lesion size and a highly significant shorter healing time in the LLLT group compared to the sham-controlled group. Prasad et al. also found faster healing times in laser-treated ulcers, further supporting the efficacy of laser treatments in promoting ulcer healing based on scientific assessments and statistical analyses [5, 34-30, 38].
Discussion
Lasers are considered an alternative treatment option due to their promising properties, such as excellent water absorption and effective penetration of biological tissues [43]. RAS is characterized by painful mouth ulcers causing dysfunction without an underlying disease explanation. Standardized treatments for this condition are lacking, and none are curative. The primary goal of any treatment should be to alleviate pain, shorten ulcer duration, and prevent recurrence [44]. Assessing laser efficacy in pain reduction is crucial.
LLLT also positively affects microcirculation by enhancing arteriolar dilation, improving blood flow, and contributing to tissue healing and regeneration. LLLT stimulates fibroblast proliferation, which are crucial cells in wound healing, and their maturation into myofibroblasts, thereby accelerating wound healing. Additionally, LLLT enhances fibroblastic growth factor secretion, further aiding tissue repair and reducing overall healing time [31].
To assess the impact of laser treatment on patient satisfaction and functional complications, researchers used the Visual Analog Scale (VAS) to record satisfaction ratings and reduction in functional complications for different treatment groups. Patient satisfaction was measured using the VAS, a tool allowing individuals to rate satisfaction or discomfort on a scale, with ratings recorded for different groups.
Analyzing the data provided, several conclusions can be drawn regarding patient satisfaction and reduction in functional complications post-laser treatment for painful mouth ulcers. The average VAS rating for reduction in functional complications in the study group was 8.57±1.50, indicating a significant improvement after laser treatment.
The average reduction in functional complications for the control group was 7.50±2.19 on the VAS, indicating a relatively lower improvement level compared to the study group [26]. Both groups experienced reduced functional complications after laser treatment for painful mouth ulcers. However, the study group showed a higher improvement level in functional complications than the control group. Regarding patient satisfaction, the control group reported relatively higher satisfaction compared to the study group. It's essential to note that satisfaction ratings may vary based on individual experiences and expectations. Further analysis and evaluation may be necessary to fully assess the effectiveness and outcomes of laser treatment for painful mouth ulcers [25, 26].
Upon thorough review of the studies, no discernible disparity was found in lesion localization or between genders.
However, more extensive comparisons between high-power and low-power lasers are needed to determine the ultimate effectiveness of lesion ablation. Further research is warranted to elucidate the underlying mechanisms of LLLT, laser focus effects, and optimize treatment protocols for favorable clinical outcomes.
Conclusion
Among various laser types, the diode laser is most commonly used, providing immediate pain relief. Albrektson's study demonstrates the fastest pain reduction with the diode laser at low power settings. Functional complications decreased as healing accelerated with LLLT in most cases.
Acknowledgments: None declared.
Ethical Permissions: This study was approved by the Ethics Committee of Guilan University of Medical Sciences.
Conflicts of Interests: The authors declared no conflicts of interests.
Authors’ Contribution: Jafari E (First Author), Introduction Writer/Main Researcher (40%); Bagheriyeh Yazdi K (Second Author), Assistant Researcher/Discussion Writer (20%); Hendi (Third Author), Methodologist/Statistical Analyst (20%); Koochaki M (Fourth Author), Introduction Writer/Methodologist/Assistant Researcher (20%)
Funding/Support: None declared.
Keywords:
References
1. Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41-52. [Link]
2. Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516-33. [Link] [DOI:10.1007/s10439-011-0454-7]
3. Wu CH. 17-Physical agent modalities. In: Cifu DX, Lew HL, editors. Braddom's Rehabilitation Care: A Clinical Handbook. Amsterdam: Elsevier; 2018. pp. 119-25. [Link] [DOI:10.1016/B978-0-323-47904-2.00017-9]
4. Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358-83. [Link] [DOI:10.2203/dose-response.09-027.Hamblin]
5. Prasad RS, Pai A. Assessment of immediate pain relief with laser treatment in recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):189-93. [Link] [DOI:10.1016/j.oooo.2013.02.011]
6. Chiari S. Photobiomodulation and lasers. Front Oral Biol. 2016;18:118-23. [Link] [DOI:10.1159/000351906]
7. Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761-71. [Link] [DOI:10.1074/jbc.M409650200]
8. Curtis D, Habegger A, Kendrick A, James D, Traywick L, Varela A, et al. Pilot study on the effects of low level laser therapy treatment for acute and persistent discomfort. Arch Physical Med Rehabil. 2022;103(3):e10. [Link] [DOI:10.1016/j.apmr.2022.01.026]
9. Pedersen AML, Forssell H, Grinde B. Orofacial pain conditions-Pain and oral mucosa. Nor Tannlegeforen Tid. 2016;126:96-102. [Link] [DOI:10.56373/2016-2-3]
10. Radwan-Oczko M, Sokół I, Babuśka K, Owczarek-Drabińska JE. Prevalence and characteristic of oral mucosa lesions. Symmetry. 2022;14(2):307. [Link] [DOI:10.3390/sym14020307]
11. Chung MK, Wang S, Oh SL, Kim YS. Acute and chronic pain from facial skin and oral mucosa: unique neurobiology and challenging treatment. Int J Mol Sci. 2021;22(11):5810. [Link] [DOI:10.3390/ijms22115810]
12. Kuffler DP. Photobiomodulation in promoting wound healing: A review. Regen Med. 2016;11(1):107-22. [Link] [DOI:10.2217/rme.15.82]
13. Cheng KK, Leung SF, Liang RH, Tai JW, Yeung RM, Thompson DR. Severe oral mucositis associated with cancer therapy: impact on oral functional status and quality of life. Support Care Cancer. 2010;18(11):1477-85. [Link] [DOI:10.1007/s00520-009-0771-7]
14. Skučas K, Balčiūnaitė A, Basevičienė N. Systemic and topical treatment methods of recurrent aphthous stomatitis: A systematic review. Stomatologija. 2023;25(1):11-20. [Link]
15. Gasmi Benahmed A, Noor S, Menzel A, Gasmi A. Oral aphthous: Pathophysiology, clinical aspects and medical treatment. Arch Razi Inst. 2021;76(5):1155-63. [Link]
16. Conejero Del Mazo R, García Forcén L, Navarro Aguilar ME. Recurrent aphthous stomatitis. Med Clin (Barc). 2023;161(6):251-9. [Link] [DOI:10.1016/j.medcli.2023.05.007]
17. Chavan M, Jain H, Diwan N, Khedkar S, Shete A, Durkar S. Recurrent aphthous stomatitis: A review. J Oral Pathol Med. 2012;41(8):577-83. [Link] [DOI:10.1111/j.1600-0714.2012.01134.x]
18. Jurge S, Kuffer R, Scully C, Porter S. Number VI recurrent aphthous stomatitis. Oral Diseases. 2006;12(1):1-21. [Link] [DOI:10.1111/j.1601-0825.2005.01143.x]
19. Kalhori KAM, Vahdatinia F, Jamalpour MR, Vescovi P, Fornaini C, Merigo E, et al. Photobiomodulation in oral medicine. Photobiomodul Photomed Laser Surg. 2019;37(12):837-61. [Link] [DOI:10.1089/photob.2019.4706]
20. Walsh LJ. The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications. Aust Dent J. 1997;42(4):247-54. [Link] [DOI:10.1111/j.1834-7819.1997.tb00129.x]
21. Dhopte A, Bagde H. Comparative evaluation of low-level laser therapy and topical triamcinolone acetonide 0.1% in recurrent aphthous stomatitis subjects. Cureus. 2022;14(6):e25564. [Link] [DOI:10.7759/cureus.25564]
22. Hopkins JT, McLoda TA, Seegmiller JG, David Baxter G. Low-level laser therapy facilitates superficial wound healing in humans: A triple-blind, sham-controlled study. J Athl Train. 2004;39(3):223-9. [Link]
23. Braverman B, McCarthy RJ, Ivankovich AD, Forde DE, Overfield M, Bapna MS. Effect of helium-neon and infrared laser irradiation on wound healing in rabbits. Lasers Surg Med. 1989;9(1):50-8. [Link] [DOI:10.1002/lsm.1900090111]
24. Bardellini E, Veneri F, Amadori F, Conti G, Majorana A. Photobiomodulation therapy for the management of recurrent aphthous stomatitis in children: clinical effectiveness and parental satisfaction. Med Oral Patol Oral Cir Bucal. 2020;25(4):e549-53. [Link] [DOI:10.4317/medoral.23573]
25. Soliman HA, Mostafaa D. Clinical evaluation of 660 nm diode laser therapy on the pain, size and functional disorders of recurrent aphthous stomatitis. Open Access Maced J Med Sci. 2019;7(9):1516-22. [Link] [DOI:10.3889/oamjms.2019.268]
26. Zeini Jahromi N, Ghapanchi J, Pourshahidi S, Zahed M, Ebrahimi H. Clinical evaluation of high and low-level laser treatment (CO2vsInGaAlP Diode Laser) for recurrent aphthous stomatitis. J Dent (Shiraz). 2017;18(1):17-23. [Link]
27. Yilmaz HG, Albaba MR, Caygur A, Cengiz E, Boke-Karacaoglu F, Tumer H. Treatment of recurrent aphthous stomatitis with Er,Cr:YSGG laser irradiation: A randomized controlled split mouth clinical study. J Photochem Photobiol B. 2017;170:1-5. [Link] [DOI:10.1016/j.jphotobiol.2017.03.011]
28. Lalabonova H, Daskalov H. Clinical assessment of the therapeutic effect of low-level laser therapy on chronic recurrent aphthous stomatitis. Biotechnol Biotechnol Equip. 2014;28(5):929-33. [Link] [DOI:10.1080/13102818.2014.966526]
29. Albrektson M, Hedström L, Bergh H. Recurrent aphthous stomatitis and pain management with low-level laser therapy: a randomized controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(5):590-4. [Link] [DOI:10.1016/j.oooo.2014.01.228]
30. Aggarwal H, Singh MP, Nahar P, Mathur H, Sowmya G. Efficacy of low-level laser therapy in treatment of recurrent aphthous ulcers-a sham controlled, split mouth follow up study. J Clin Diagnostic Res 2014;8(2):218. [Link] [DOI:10.7860/JCDR/2014/7639.4064]
31. Ślebioda Z, Dorocka-Bobkowska B. Low-level laser therapy in the treatment of recurrent aphthous stomatitis and oral lichen planus: a literature review. Postępy Dermatol Alergol. 2020;37(4):475-81. [Link] [DOI:10.5114/ada.2020.98258]
32. Cruz AR, Minicucci EM, Betini M, Almeida-Lopes L, Tieghi Neto V, Cataneo AJM. Efficacy of photobiomodulation in the treatment of oral mucositis in patients undergoing antineoplastic therapy: Systematic review and meta-analysis. Support Care Cancer. 2023;31(12):645. [Link] [DOI:10.1007/s00520-023-08105-7]
33. Ryu HS, Lim NK, Padalhin AR, Abueva C, Park SY, Chung PS, et al. Improved healing and macrophage polarization in oral ulcers treated with photobiomodulation (PBM). Lasers Surg Med. 2022;54(4):600-10. [Link] [DOI:10.1002/lsm.23510]
34. Parker S. Low-level laser use in dentistry. Br Dent J. 2007;202(3):131-8. [Link] [DOI:10.1038/bdj.2007.75]
35. Basirat M. The effects of low power lasers in healing of oral ulcers. J Lasers Med Sci. 2012;3. [Link]
36. Seyyedi SA, Khashabi E, Falaki F. Laser application in periodontics. J Lasers Med Sci. 2012;3(1):26-32. [Link]
37. Heller GZ, Manuguerra M, Chow R. How to analyze the Visual Analogue Scale: Myths, truths and clinical relevance. Scand J Pain. 2016;13:67-75. [Link] [DOI:10.1016/j.sjpain.2016.06.012]
38. Fekrazad R, Gholami GA, Sadr Eshkevari P, Nokhbatolfoghahaei H, Mohaghegh S. Management of recurrent aphthous ulcers with therapeutic Nd:YAG laser, using two different methods. Dent Med Probl. 2023;60(3):467-72. [Link] [DOI:10.17219/dmp/147048]
39. Chen Y, Huang J, Xia S, Wang K, Rui Y. Evaluation of fusion performances of skin wound incisions under different defocus amounts in laser tissue welding. Optics Laser Technol. 2023;165:109570. [Link] [DOI:10.1016/j.optlastec.2023.109570]
40. Shahbazi S, Moezzi ghadim N, Mirzaei A, Azizi A. Comparison of Soft Tissue Thermal Changes Induced by Three Types of Diode Lasers at 810, 940, and 980nm Wavelengths. J Res Dental Maxillofac Sci. 2020;5(2):7-13. [Link] [DOI:10.29252/jrdms.5.2.7]
41. Tenore G, Mohsen A, Nuvoli A, Palaia G, Rocchetti F, Di Gioia CR, et al. The impact of laser thermal effect on histological evaluation of oral soft tissue biopsy: Systematic review. Dentistry J. 2023; 11(2):28. [Link] [DOI:10.3390/dj11020028]
42. Zand N. Non-Thermal, Non-Ablative CO2 Laser Therapy (NACLT): A new approach to relieve pain in some painful oral diseases. 2012. [Link] [DOI:10.5772/37828]
43. Elad S, Or R, Shapira MY, Haviv A, Galili D, Garfunkel AA, et al. CO2 laser in oral graft-versus-host disease: A pilot study. Bone Marrow Transplant. 2003;32(10):1031-4. [Link] [DOI:10.1038/sj.bmt.1704272]
44. Sánchez-Bernal J, Conejero C, Conejero R. Recurrent Aphthous Stomatitis. Actas Dermosifiliogr. 2020;111(6):471-80. [Link] [DOI:10.1016/j.adengl.2019.09.006]









