Volume 16, Issue 1 (2024)
Iran J War Public Health 2024, 16(1): 1-8 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/12/18 | Accepted: 2024/02/23 | Published: 2024/04/1
Received: 2023/12/18 | Accepted: 2024/02/23 | Published: 2024/04/1
How to cite this article
Barzegari Marvast H, Akbarnejad A, Norouzi J. Effect of 12 Weeks Incremental Resistance Training on Serum Levels of Myostatin, Follistatin, and IGF-I in Sedentary Elderly Men. Iran J War Public Health 2024; 16 (1) :1-8
URL: http://ijwph.ir/article-1-1424-en.html
URL: http://ijwph.ir/article-1-1424-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Physical Education and Sports Sciences, Faculty of Psychology and Educational Sciences, Yazd University, Yazd, Iran
2- Department of Exercise Physiology, Faculty of Physical Education and Sports Sciences, University of Tehran, Tehran, Iran
2- Department of Exercise Physiology, Faculty of Physical Education and Sports Sciences, University of Tehran, Tehran, Iran
Full-Text (HTML) (1221 Views)
Introduction
Globally, the population of elderly individuals has significantly increased, rising from 382 million in 1980 to 962 million by 2017. Moreover, estimates suggest that this number will continue to grow, reaching 1.4 billion by 2030 [1]. Starting in their fifties, individuals begin to experience a gradual decline in lean mass, losing an average of approximately 1% per year [2, 3]. Longitudinal studies have shown that by around the age of 75, there is a noticeable reduction in muscle mass, with women experiencing a loss of about 0.64% to 0.7% per year, and men losing between 0.8% and 0.98% per year [4]. Consequently, by the age of 80, there is typically a substantial decrease of 30% to 50% in muscle mass [4]. The reduction in muscle mass and the accompanying weakness observed as individuals age is commonly referred to as "sarcopenia", a term initially coined by Rosenberg [5]. The onset of sarcopenia results in several changes within the muscles and nerves, including the loss of muscle mass, particularly in Type II muscle fibers, a decline in strength, an increase in fat mass, impaired coordination, elevated protein degradation (notably of contractile protein), and reduced satellite cell activity [6, 7]. The consequences of muscle atrophy and weakness lead to a variety of physiological and psychosocial effects, such as the inability to perform daily living tasks independently, increased frailty and fall risk, loss of independent living and associated depression/social isolation, a sedentary lifestyle due to physical inactivity, a heightened risk of chronic diseases, and an increased risk of all-cause mortality [8].
In recent years, extensive research has aimed to elucidate the cellular and molecular mechanisms underlying muscle hypertrophy and atrophy. The onset of age-related sarcopenia is believed to be due to one or more of the following factors: an increase in the basal-fasted rates of muscle protein breakdown, a reduction in basal muscle protein synthesis, or a combination of these factors [9].
Within the realm of signaling molecules, several myokines are recognized for their role in inhibiting the hypertrophic response in muscles [10]. A notable myokine in this context is myostatin (MSTN), identified as a key regulator of skeletal muscle mass. MSTN binds to Activin Type II receptors located in skeletal muscles, triggering the SMAD 2/3 intracellular pathway, which is responsible for inhibiting muscle growth [11]. In studies involving transgenic mice with a disrupted MSTN gene, a significant increase in muscle mass (2- to 3-fold) was observed without a corresponding rise in adipose tissue [12].
Follistatin (FLST) is another myokine that plays a critical role in muscle hypertrophy and atrophy. It functions as an antagonist to the MSTN receptor, exerting paracrine and autocrine effects [13]. By preventing MSTN from binding to its receptor, FLST decreases MSTN activity and facilitates an increase in muscle mass [14]. As a member of the TGF-β protein family, FLST is a glycoprotein that blocks MSTN's action by occupying its role [15].
Conversely, age-related changes in anabolic hormones and growth factors, such as IGF-1, are among the primary mechanisms influencing and exacerbating sarcopenia [16]. Consequently, levels of testosterone and insulin-like growth factor 1 (IGF-1) tend to decline, while catabolic factors, including cortisol and MSTN, often increase. Additionally, IGF-1 is a potent marker of satellite cell activity, leading to an increase in satellite cell numbers. It has been demonstrated that IGF-1 promotes the proliferation and differentiation of satellite cells by upregulating MSTN and downregulating P21 under various conditions [17]. Therefore, enhancing the expression of IGF-1 is considered crucial in the muscle hypertrophy process, particularly under mechanical loading [18].
Engaging in exercise training offers substantial benefits for older adults, including improved mobility and a lower risk of falls [19]. The considerable advantages of aerobic training in enhancing health, rehabilitation, well-being, and reducing cardiovascular disease risks among the elderly are well-documented [19]. Although aerobic exercise greatly benefits overall health, it does not directly contribute to an increase in muscle mass or musculoskeletal strength, both of which naturally decrease as part of the aging process. In contrast, resistance training (RT) uniquely addresses the age-related decline in muscle mass and strength, showing potential to reverse sarcopenia and, in some instances, prevent its onset [19]. Following a 2-week period of reduced physical activity, older adults exhibited anabolic resistance, characterized by diminished postprandial protein synthesis, decreased insulin sensitivity, and a reduction in leg muscle mass [20].
Despite the aging process, muscles maintain the ability to respond to increased activity levels, especially when subjected to RT. A meta-analysis in older adults has clearly shown the positive effects of incremental RT on muscle function. The improvements in muscle strength from RT are partly facilitated by proteins/hormones produced by skeletal muscle or associated tissues during exercise [21].
Therefore, while MSTN, FLST, and IGF-1 play essential roles in regulating skeletal muscle mass, the response of these growth factors to RT, particularly in the elderly, remains uncertain [22]. Previous studies have shown that three months of high-intensity RT leads to increased serum levels of FLST-like related gene [23]. In recent research, the FLST to MSTN (F:M) ratio has been identified as an important marker in assessing body composition and strength outcomes following exercise training [11]. However, research on the response of myokines to exercise training is limited. For instance, a study on older males who underwent six weeks of high-intensity interval training reported no significant change in serum FLST concentrations [10].
Given the positive physiological outcomes observed in older adults through RT, it is clear that integrating such physical activities can help maintain or even increase muscle mass, improve muscle strength, and potentially reduce or prevent the onset of sarcopenia in this demographic. Nonetheless, the response of key factors in muscle hypertrophy and atrophy, such as IGF-1, MSTN, and FLST to resistance exercise, especially among the elderly, remains both sparse and inconsistent. Therefore, this study aimed to investigate the impact of incremental RT on the serum levels of IGF-I, MSTN, and FLST in sedentary elderly men.
Materials and Methods
Participants
This semi-experimental study, employing a pretest/posttest control group design, was carried out in the summer of 2022. The statistical research population included all employees (58 men aged 60 to 70 years) of the Razavi Khorasan Gas Company, Iran. From this population, 30 individuals were purposefully selected through available and voluntary sampling and were randomly divided into two groups, including experimental (15 men) and control (15 men). The sample size determination was estimated using G-Power software, setting the size of the intervention effect with 95% power at a significance level of 0.05 for 15 participants per group. Individuals with a history of cardiovascular and respiratory diseases (acute myocardial infarction, asthma), neurological disorders (stroke, paralysis, Parkinson’s disease), spinal deformities, severe lower limb disabilities, skin diseases, smokers, those who were unmarried, and those regularly engaged in sports activities were excluded from the selection. Inclusion criteria included being at least 60 years old and sedentary, defined as not engaging in at least 30 minutes of moderate physical activity per day for three days a week.
Test Procedures
Body Composition Measurements
To evaluate body composition, the non-invasive and user-friendly electrical impedance analysis (BIA) method was utilized, employing the Olympia 3.3 Jawon device from Korea. This device provides an effective way to measure body composition with minimal intrusion. For the assessments, subjects in a fasting state and wearing minimal clothing had four electrodes attached to their body by the device. These electrodes were positioned under the feet and in the hands. After entering individual details, various parameters, such as total body water, intracellular water, extracellular water, fat percentage, and fat-free mass were measured. Like blood sampling, this test was carried out twice: once before starting the training protocol and once after its completion.
Determine One-Repetition Maximum (1-RM) at Each Station
Participants were asked to participate in a resistance exercise session to determine their 1-RM at each station. The measurement of one repetition maximum (1-RM) followed the protocol established by Brzycki et al. [24]. The procedure for determining 1-RM was as follows:
To minimize the risk of injury, participants underwent both general and specific warm-up exercises before assessing 1-RM. The warm-up began with a general activity that involved 5 minutes of moderate-intensity jogging on a treadmill. This was followed by a specific warm-up, where participants performed 5 to 10 repetitions with a weight approximately 50% of their estimated maximum strength. After a one-minute rest with stretching exercises, they then completed 3-5 repetitions with 60% to 80% of their estimated maximum strength. Following another 3 to 5 minutes of rest, the weight was incrementally increased until the final attempt to determine their 1-RM. If a participant successfully lifted the weight, it was increased for the next attempt. After a rest period of 3 to 5 minutes, the participant attempted the lift again. To avoid excessive fatigue, participants were advised to find their 1-RM within a maximum of five attempts. Adhering to the principle of progressive overload, 1-RM was reevaluated at each station during the 6th and 9th weeks to ensure gradual progression.
Training Intervention
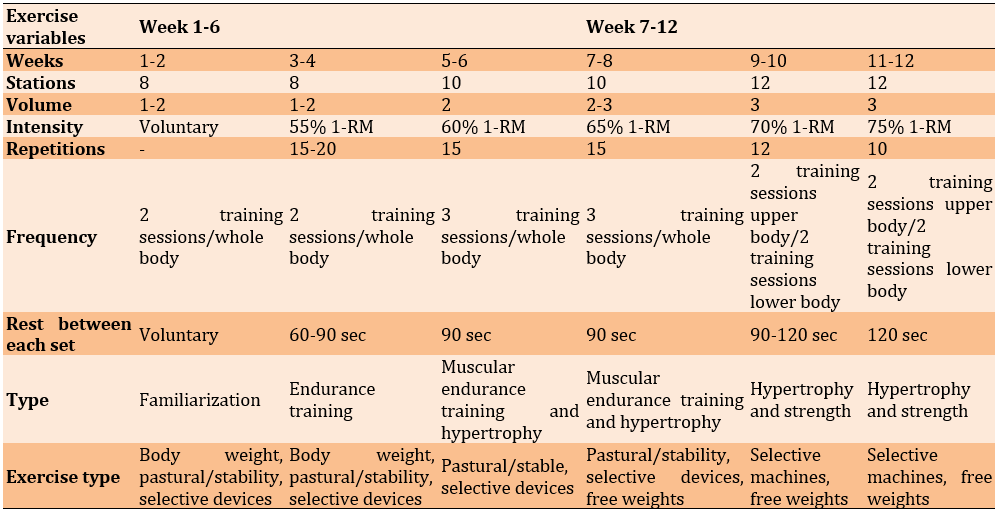
The training protocol, which included training volume, intensity, frequency, and type per week, is detailed in Table 1. This protocol adheres to the recommendations provided by the American College of Sports Medicine for RT in the elderly population [25].
Table 1. Resistance training protocol
Blood Sampling and Analysis
The subjects attended an introductory session to become acquainted with the correct blood sampling procedure. To minimize potential interferences and confounding factors that could affect the research outcomes, and to limit the influence of food types on hormonal indicators, subjects were advised to avoid consuming fast food and beverages for at least 24 hours before blood sampling.
Blood sampling occurred in two stages: one day before the first training session (pre-test) and 24 hours after the final training session in the twelfth week. Both control and experimental groups were tested after fasting for 12-14 hours, specifically between 8-9 am. Before the initial blood sampling, subjects were instructed to avoid any strenuous physical activity for two days. Subsequently, 10 ml of blood was drawn from the brachial vein of the right arm while the subjects were seated and at rest. In the second stage, following the training period and a 48-hour break from the last session, blood collection was repeated under the same conditions as the first stage. After collection, the blood samples were centrifuged at 1500 rpm for 15 minutes. The separated serum was then stored at -20°C.
To measure concentrations of FLST and MSTN, enzyme immunoassay methods were used with ELISA kits from R and D Systems (Minneapolis, MN, USA). IGF-1 levels were determined using ELISA kits from Mediagnost (Reutlingen, BW, Germany). To ensure accuracy and consistency across the tests, coefficients of variance for both intra- and inter-assay measurements were calculated for all variables, consistently showing values below 10%.
Statistical Analysis
The data obtained were analyzed using SPSS version 20. After verifying the normality of data distribution with the Kolmogorov-Smirnov test and the homogeneity of variances with Levene’s test, comparisons of means within and between groups were conducted using the paired sample t-test and ANCOVA test, respectively. A significance level of p<0.05 was established for all test results.
Findings
The Kolmogorov-Smirnov test results confirmed that the data for each variable were normally distributed. Table 2 lists the demographic characteristics, body composition, and biochemical variables of the subjects before and after the intervention.
Table 2. Characteristics and physiological variables measured pre-test and post-test in the experimental and control groups
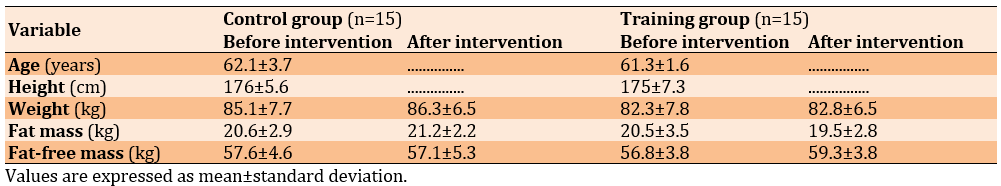
The independent t-test revealed no significant differences between the average values of age, weight, height, fat mass, and fat-free mass of the training and control groups prior to the intervention, indicating homogeneity in these characteristics between both groups, as depicted in Table 2. At the start of the intervention, no differences in the assessed variables were noted between the groups.
Table 3 showcases the outcomes of the independent t-test, highlighting the differences in the mean serum indices of the training and control groups before and after RT. Serum levels of IGF-1 and FLST experienced a significant increase of 17% (p=0.004) and 20% (p=0.001), respectively, after 12 weeks of RT with progressively increasing intensity. In contrast, serum levels of MSTN saw a significant decrease of 14% (p=0.001). Notably, these changes were significant in comparison to the control group.
Table 3. Mean MSTN, FLST and IGF-I levels in the pre-test and post-test
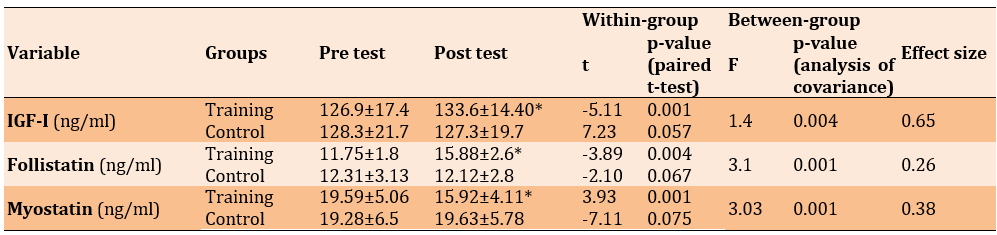
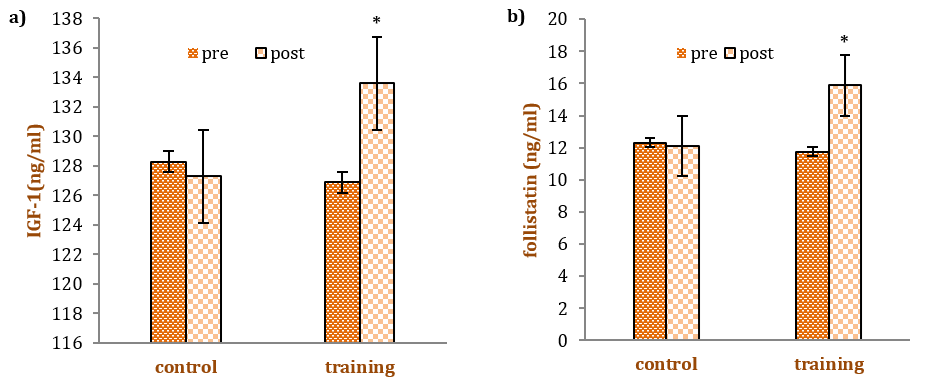
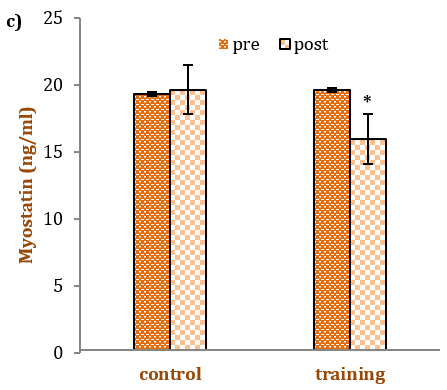
Figure 1. Mean IGF-I, a) Follistatin, b) Myostatin, c) levels in the pre-test and post-test in each group. The * denotes statistical significance (p<0.05) in respective pre- to post-comparisons
Discussion
The main goal of this study was to assess the effects of a 12-week incremental RT program on the serum levels of MSTN, FLST, and IGF-1 in sedentary elderly men.
IGF-1, known for enhancing muscle development, acts as a positive regulator. Activation of the muscle IGF-1 receptor initiates a cascade of signaling pathways that lead to mitogenic and myogenic responses [26]. Increased levels of IGF-1 have been associated with numerous health benefits, including improved muscle, bone, tendon, body composition, and cognitive functions [16]. In the context of sarcopenia, IGF-1 plays a vital role in maintaining lean body mass through its effects on skeletal muscles [17]. As individuals age, they experience not only a decline in musculoskeletal system integrity, resulting in reduced muscle and bone mass but also a decrease in circulating IGF-1 levels [16]. The level of circulating IGF-1 can potentially increase the vulnerability of older adults to a higher risk of sarcopenia, functional decline, and loss of lean body mass [27].
The findings of this research demonstrated that 12 weeks of incremental RT significantly increased IGF-1 levels in sedentary elderly individuals (p=0.004). While some studies have observed chronic increases in circulating IGF-1 and muscle hypertrophy following resistance exercise training [28, 29], other studies have reported no change or even decreases in serum IGF-1 levels resulting from RT [30, 31]. For instance, the study by Rashidi et al. [31] involved an 8-week training protocol, whereas, in the current study, the participants engaged in RT for 12 weeks. This suggests that the length of the training period is crucial in determining the degree of increase in IGF-1 expression.
In some studies, the response of IGF-I to RT is characterized by two distinct phases. The first phase, which lasts about 5 to 6 weeks, is often termed the catabolic phase, during which serum IGF-I levels may remain unchanged or even decrease in response to training. This phase is followed by an anabolic phase, beginning after 7 weeks or more, where serum IGF-I levels start to rise [32]. Therefore, the lack of changes in IGF-I observed in the study by Rashidi et al. underscores the two-stage adaptation process of IGF-I in response to exercise [31]. In essence, during the initial period, IGF-I is in a catabolic phase, playing a limited role in muscle adaptations during RT. Additionally, the study by Haddad et al. found that the expression of Mechano growth factor (MGF) precedes that of IGF-IEa in response to exercise [33], indicating that following mechanical strain and/or muscle damage, the IGF-I gene first shifts towards MGF and later towards IGF-IEa in rodent muscles. Jiang et al. further showed that an extended duration of RT is required to increase the expression of hepatic IGF-I [34].
Conversely, the lack of change in serum IGF-I concentration after RT, as noted in the study by Walker et al., might relate to the initial IGF-I levels of the subjects in the current study [35]. Interestingly, our study's subjects had significantly lower initial IGF-I levels than those in Walker's study. Therefore, according to the research findings, numerous confounding factors could influence the observed results. These factors include inadequate dietary control, differences in subject characteristics, changes in plasma volume, dynamics of synthesis, release and receptor uptake, variations in exercise modes and intensities, limitations regarding the timing of post-exercise sampling, types of exercise protocols, study durations, training frequencies, sample sizes, and the age, sex, and baseline IGF-1 levels of participants.
MSTN acts as an inhibitor of growth and differentiation, specifically expressed in both developing and mature skeletal muscle tissues [11]. Disruption or mutation of the gene responsible for MSTN leads to a significant increase in skeletal muscle mass during development [12]. Additionally, serum MSTN levels are inversely related to total body muscle mass relative to height in older compared to younger adults, both males and females. Higher MSTN levels in the bloodstream are associated with muscle atrophy resulting from prolonged bed rest and thyroxine administration, underscoring its role in muscle changes during immobilization and hormonal interventions [11].
In this study, it was observed that sedentary elderly men exhibited a significant decrease in serum MSTN levels after participating in 12 weeks of incremental RT. Roth et al. were among the first to report a decrease in MSTN mRNA expression in both young and older individuals, including women and men, following 9 weeks of RT [36]. Conversely, Willoughby et al. noted an increase in MSTN mRNA expression, despite a rise in muscle mass, after 12 weeks of RT [37].
The inconsistency in findings could be due to differences in sampling times, methods, exercise intensity and duration, or methods of measuring MSTN. Bagheri et al. reported a significant decrease in serum MSTN concentrations in middle-aged men after 8 weeks of combined upper and lower body RT at 50 to 80% of 1-RM. However, serum MSTN concentration did not significantly decrease after 12 weeks of non-periodized upper and lower body RT at 85 to 90% of 1-RM in untrained males [11]. Additionally, Binns et al. observed a trend toward reduced serum MSTN concentration in community-dwelling older adults after 20 weeks of high-velocity whole-body RT at 70% of 1-RM [38].
The evidence suggests that exercise leads to decreased serum MSTN concentrations in middle-aged and elderly individuals, but not in young healthy men [11]. Although our data does not clarify the precise mechanism behind the reduction in plasma MSTN, the existing literature indicates that the decreased levels might be due to diminished production, processing, and/or secretion of the protein into the circulation. Alternatively, the observed lower MSTN levels could result from reduced stability, increased distribution in the circulatory system, enhanced disposal or reuptake of the protein, or a combination of these factors, potentially influenced by exercise training [11].
FLST is another myokine that plays a crucial role in muscle hypertrophy and atrophy, particularly in the elderly [13]. As a single-chain polypeptide, FLST belongs to the transforming growth factor (TGF) superfamily [14] and is ubiquitously expressed in various human tissues, including skeletal muscle, exerting both paracrine and autocrine effects [13]. Previous research has shown that FLST has the capacity to promote both anabolic and catabolic effects on skeletal muscle by binding to and neutralizing MSTN [14].
The results of this study reveal a significant increase in serum FLST levels among sedentary elderly men following 12 weeks of incremental RT. These findings are in line with those from other studies, which reported that serum FLST concentrations increased in sedentary females and middle-aged men after 8 weeks of high-intensity RT [11, 39]. An elevated FLST:MSTN ratio, indicative of a more favorable anabolic environment, resulted from decreased MSTN concentrations and increased FLST levels [11]. However, these results contrast with those of Diel et al., which found no effect of 12 weeks of RT on serum FLST levels in participants [40]. It's crucial to consider the limitations and various factors that could account for these differing outcomes. The inconsistency may arise from differences in the age of the subjects and the training protocol intensities across studies, likely leading to the varied results reported in the literature. The consistent studies involved middle-aged and elderly participants, whereas Diel et al.'s study focused on young, healthy males. Additionally, it should be noted that the exercise protocol intensity in Diel et al.'s study was lower compared to that used in the present study.
To summarize, a 12-week resistance exercise training program in elderly men led to significant changes: a 14% reduction in plasma MSTN, a 20% increase in plasma FLST, and a 17% increase in IGF-1.
Normally, the maintenance of muscle fiber size is dependent on a delicate balance between positive regulators (such as FLST and IGF-1) and negative regulators (such as MSTN) of muscle growth. However, in situations of muscle atrophy, such as that experienced by older individuals, this equilibrium is disrupted, leading to a dominance of negative influences. Consequently, the introduction of RT, which involves muscle loading, can potentially tip the balance in favor of positive regulators. While the exact mechanism of interaction between these regulators is still not fully understood, it seems to involve a complex negative feedback loop.
Conclusion
From the results of this study, it can be cautiously inferred that RT, through its dual role of enhancing muscle growth factors and reducing atrophy factors, has the potential to alleviate the effects of sarcopenia in elderly individuals. This, in turn, could address various sarcopenia-associated conditions, including metabolic syndrome, inflammation, glucose intolerance, reduced arterial elasticity, and obesity.
Acknowledgments: Thank all the employees and workers of Khorasan Razavi Gas Company for helping us in conducting this research.
Ethical Permissions: The permission of this document was approved by the Research Ethics Committee of Khorasan Razavi Gas Company, which included the research protocol, subject information and consent form.
Conflicts of Interests: There is no conflict of interest.
Authors' Contribution: Barzegari Marvast H (First Author), Introduction Writer/Main Researcher/Statistical Analyst (40%); Akbarnejad A (Second Author), Assistant Researcher/Statistical Analyst (30%); Norouzi J (Third Author), Methodologist/Statistical Analyst/Assistant Researcher/Discussion Writer (30%)
Funding/Support: The present study was not financially supported.
Globally, the population of elderly individuals has significantly increased, rising from 382 million in 1980 to 962 million by 2017. Moreover, estimates suggest that this number will continue to grow, reaching 1.4 billion by 2030 [1]. Starting in their fifties, individuals begin to experience a gradual decline in lean mass, losing an average of approximately 1% per year [2, 3]. Longitudinal studies have shown that by around the age of 75, there is a noticeable reduction in muscle mass, with women experiencing a loss of about 0.64% to 0.7% per year, and men losing between 0.8% and 0.98% per year [4]. Consequently, by the age of 80, there is typically a substantial decrease of 30% to 50% in muscle mass [4]. The reduction in muscle mass and the accompanying weakness observed as individuals age is commonly referred to as "sarcopenia", a term initially coined by Rosenberg [5]. The onset of sarcopenia results in several changes within the muscles and nerves, including the loss of muscle mass, particularly in Type II muscle fibers, a decline in strength, an increase in fat mass, impaired coordination, elevated protein degradation (notably of contractile protein), and reduced satellite cell activity [6, 7]. The consequences of muscle atrophy and weakness lead to a variety of physiological and psychosocial effects, such as the inability to perform daily living tasks independently, increased frailty and fall risk, loss of independent living and associated depression/social isolation, a sedentary lifestyle due to physical inactivity, a heightened risk of chronic diseases, and an increased risk of all-cause mortality [8].
In recent years, extensive research has aimed to elucidate the cellular and molecular mechanisms underlying muscle hypertrophy and atrophy. The onset of age-related sarcopenia is believed to be due to one or more of the following factors: an increase in the basal-fasted rates of muscle protein breakdown, a reduction in basal muscle protein synthesis, or a combination of these factors [9].
Within the realm of signaling molecules, several myokines are recognized for their role in inhibiting the hypertrophic response in muscles [10]. A notable myokine in this context is myostatin (MSTN), identified as a key regulator of skeletal muscle mass. MSTN binds to Activin Type II receptors located in skeletal muscles, triggering the SMAD 2/3 intracellular pathway, which is responsible for inhibiting muscle growth [11]. In studies involving transgenic mice with a disrupted MSTN gene, a significant increase in muscle mass (2- to 3-fold) was observed without a corresponding rise in adipose tissue [12].
Follistatin (FLST) is another myokine that plays a critical role in muscle hypertrophy and atrophy. It functions as an antagonist to the MSTN receptor, exerting paracrine and autocrine effects [13]. By preventing MSTN from binding to its receptor, FLST decreases MSTN activity and facilitates an increase in muscle mass [14]. As a member of the TGF-β protein family, FLST is a glycoprotein that blocks MSTN's action by occupying its role [15].
Conversely, age-related changes in anabolic hormones and growth factors, such as IGF-1, are among the primary mechanisms influencing and exacerbating sarcopenia [16]. Consequently, levels of testosterone and insulin-like growth factor 1 (IGF-1) tend to decline, while catabolic factors, including cortisol and MSTN, often increase. Additionally, IGF-1 is a potent marker of satellite cell activity, leading to an increase in satellite cell numbers. It has been demonstrated that IGF-1 promotes the proliferation and differentiation of satellite cells by upregulating MSTN and downregulating P21 under various conditions [17]. Therefore, enhancing the expression of IGF-1 is considered crucial in the muscle hypertrophy process, particularly under mechanical loading [18].
Engaging in exercise training offers substantial benefits for older adults, including improved mobility and a lower risk of falls [19]. The considerable advantages of aerobic training in enhancing health, rehabilitation, well-being, and reducing cardiovascular disease risks among the elderly are well-documented [19]. Although aerobic exercise greatly benefits overall health, it does not directly contribute to an increase in muscle mass or musculoskeletal strength, both of which naturally decrease as part of the aging process. In contrast, resistance training (RT) uniquely addresses the age-related decline in muscle mass and strength, showing potential to reverse sarcopenia and, in some instances, prevent its onset [19]. Following a 2-week period of reduced physical activity, older adults exhibited anabolic resistance, characterized by diminished postprandial protein synthesis, decreased insulin sensitivity, and a reduction in leg muscle mass [20].
Despite the aging process, muscles maintain the ability to respond to increased activity levels, especially when subjected to RT. A meta-analysis in older adults has clearly shown the positive effects of incremental RT on muscle function. The improvements in muscle strength from RT are partly facilitated by proteins/hormones produced by skeletal muscle or associated tissues during exercise [21].
Therefore, while MSTN, FLST, and IGF-1 play essential roles in regulating skeletal muscle mass, the response of these growth factors to RT, particularly in the elderly, remains uncertain [22]. Previous studies have shown that three months of high-intensity RT leads to increased serum levels of FLST-like related gene [23]. In recent research, the FLST to MSTN (F:M) ratio has been identified as an important marker in assessing body composition and strength outcomes following exercise training [11]. However, research on the response of myokines to exercise training is limited. For instance, a study on older males who underwent six weeks of high-intensity interval training reported no significant change in serum FLST concentrations [10].
Given the positive physiological outcomes observed in older adults through RT, it is clear that integrating such physical activities can help maintain or even increase muscle mass, improve muscle strength, and potentially reduce or prevent the onset of sarcopenia in this demographic. Nonetheless, the response of key factors in muscle hypertrophy and atrophy, such as IGF-1, MSTN, and FLST to resistance exercise, especially among the elderly, remains both sparse and inconsistent. Therefore, this study aimed to investigate the impact of incremental RT on the serum levels of IGF-I, MSTN, and FLST in sedentary elderly men.
Materials and Methods
Participants
This semi-experimental study, employing a pretest/posttest control group design, was carried out in the summer of 2022. The statistical research population included all employees (58 men aged 60 to 70 years) of the Razavi Khorasan Gas Company, Iran. From this population, 30 individuals were purposefully selected through available and voluntary sampling and were randomly divided into two groups, including experimental (15 men) and control (15 men). The sample size determination was estimated using G-Power software, setting the size of the intervention effect with 95% power at a significance level of 0.05 for 15 participants per group. Individuals with a history of cardiovascular and respiratory diseases (acute myocardial infarction, asthma), neurological disorders (stroke, paralysis, Parkinson’s disease), spinal deformities, severe lower limb disabilities, skin diseases, smokers, those who were unmarried, and those regularly engaged in sports activities were excluded from the selection. Inclusion criteria included being at least 60 years old and sedentary, defined as not engaging in at least 30 minutes of moderate physical activity per day for three days a week.
Test Procedures
Body Composition Measurements
To evaluate body composition, the non-invasive and user-friendly electrical impedance analysis (BIA) method was utilized, employing the Olympia 3.3 Jawon device from Korea. This device provides an effective way to measure body composition with minimal intrusion. For the assessments, subjects in a fasting state and wearing minimal clothing had four electrodes attached to their body by the device. These electrodes were positioned under the feet and in the hands. After entering individual details, various parameters, such as total body water, intracellular water, extracellular water, fat percentage, and fat-free mass were measured. Like blood sampling, this test was carried out twice: once before starting the training protocol and once after its completion.
Determine One-Repetition Maximum (1-RM) at Each Station
Participants were asked to participate in a resistance exercise session to determine their 1-RM at each station. The measurement of one repetition maximum (1-RM) followed the protocol established by Brzycki et al. [24]. The procedure for determining 1-RM was as follows:
To minimize the risk of injury, participants underwent both general and specific warm-up exercises before assessing 1-RM. The warm-up began with a general activity that involved 5 minutes of moderate-intensity jogging on a treadmill. This was followed by a specific warm-up, where participants performed 5 to 10 repetitions with a weight approximately 50% of their estimated maximum strength. After a one-minute rest with stretching exercises, they then completed 3-5 repetitions with 60% to 80% of their estimated maximum strength. Following another 3 to 5 minutes of rest, the weight was incrementally increased until the final attempt to determine their 1-RM. If a participant successfully lifted the weight, it was increased for the next attempt. After a rest period of 3 to 5 minutes, the participant attempted the lift again. To avoid excessive fatigue, participants were advised to find their 1-RM within a maximum of five attempts. Adhering to the principle of progressive overload, 1-RM was reevaluated at each station during the 6th and 9th weeks to ensure gradual progression.
Training Intervention
The training protocol, which included training volume, intensity, frequency, and type per week, is detailed in Table 1. This protocol adheres to the recommendations provided by the American College of Sports Medicine for RT in the elderly population [25].
Table 1. Resistance training protocol

Blood Sampling and Analysis
The subjects attended an introductory session to become acquainted with the correct blood sampling procedure. To minimize potential interferences and confounding factors that could affect the research outcomes, and to limit the influence of food types on hormonal indicators, subjects were advised to avoid consuming fast food and beverages for at least 24 hours before blood sampling.
Blood sampling occurred in two stages: one day before the first training session (pre-test) and 24 hours after the final training session in the twelfth week. Both control and experimental groups were tested after fasting for 12-14 hours, specifically between 8-9 am. Before the initial blood sampling, subjects were instructed to avoid any strenuous physical activity for two days. Subsequently, 10 ml of blood was drawn from the brachial vein of the right arm while the subjects were seated and at rest. In the second stage, following the training period and a 48-hour break from the last session, blood collection was repeated under the same conditions as the first stage. After collection, the blood samples were centrifuged at 1500 rpm for 15 minutes. The separated serum was then stored at -20°C.
To measure concentrations of FLST and MSTN, enzyme immunoassay methods were used with ELISA kits from R and D Systems (Minneapolis, MN, USA). IGF-1 levels were determined using ELISA kits from Mediagnost (Reutlingen, BW, Germany). To ensure accuracy and consistency across the tests, coefficients of variance for both intra- and inter-assay measurements were calculated for all variables, consistently showing values below 10%.
Statistical Analysis
The data obtained were analyzed using SPSS version 20. After verifying the normality of data distribution with the Kolmogorov-Smirnov test and the homogeneity of variances with Levene’s test, comparisons of means within and between groups were conducted using the paired sample t-test and ANCOVA test, respectively. A significance level of p<0.05 was established for all test results.
Findings
The Kolmogorov-Smirnov test results confirmed that the data for each variable were normally distributed. Table 2 lists the demographic characteristics, body composition, and biochemical variables of the subjects before and after the intervention.
Table 2. Characteristics and physiological variables measured pre-test and post-test in the experimental and control groups

The independent t-test revealed no significant differences between the average values of age, weight, height, fat mass, and fat-free mass of the training and control groups prior to the intervention, indicating homogeneity in these characteristics between both groups, as depicted in Table 2. At the start of the intervention, no differences in the assessed variables were noted between the groups.
Table 3 showcases the outcomes of the independent t-test, highlighting the differences in the mean serum indices of the training and control groups before and after RT. Serum levels of IGF-1 and FLST experienced a significant increase of 17% (p=0.004) and 20% (p=0.001), respectively, after 12 weeks of RT with progressively increasing intensity. In contrast, serum levels of MSTN saw a significant decrease of 14% (p=0.001). Notably, these changes were significant in comparison to the control group.
Table 3. Mean MSTN, FLST and IGF-I levels in the pre-test and post-test



Figure 1. Mean IGF-I, a) Follistatin, b) Myostatin, c) levels in the pre-test and post-test in each group. The * denotes statistical significance (p<0.05) in respective pre- to post-comparisons
Discussion
The main goal of this study was to assess the effects of a 12-week incremental RT program on the serum levels of MSTN, FLST, and IGF-1 in sedentary elderly men.
IGF-1, known for enhancing muscle development, acts as a positive regulator. Activation of the muscle IGF-1 receptor initiates a cascade of signaling pathways that lead to mitogenic and myogenic responses [26]. Increased levels of IGF-1 have been associated with numerous health benefits, including improved muscle, bone, tendon, body composition, and cognitive functions [16]. In the context of sarcopenia, IGF-1 plays a vital role in maintaining lean body mass through its effects on skeletal muscles [17]. As individuals age, they experience not only a decline in musculoskeletal system integrity, resulting in reduced muscle and bone mass but also a decrease in circulating IGF-1 levels [16]. The level of circulating IGF-1 can potentially increase the vulnerability of older adults to a higher risk of sarcopenia, functional decline, and loss of lean body mass [27].
The findings of this research demonstrated that 12 weeks of incremental RT significantly increased IGF-1 levels in sedentary elderly individuals (p=0.004). While some studies have observed chronic increases in circulating IGF-1 and muscle hypertrophy following resistance exercise training [28, 29], other studies have reported no change or even decreases in serum IGF-1 levels resulting from RT [30, 31]. For instance, the study by Rashidi et al. [31] involved an 8-week training protocol, whereas, in the current study, the participants engaged in RT for 12 weeks. This suggests that the length of the training period is crucial in determining the degree of increase in IGF-1 expression.
In some studies, the response of IGF-I to RT is characterized by two distinct phases. The first phase, which lasts about 5 to 6 weeks, is often termed the catabolic phase, during which serum IGF-I levels may remain unchanged or even decrease in response to training. This phase is followed by an anabolic phase, beginning after 7 weeks or more, where serum IGF-I levels start to rise [32]. Therefore, the lack of changes in IGF-I observed in the study by Rashidi et al. underscores the two-stage adaptation process of IGF-I in response to exercise [31]. In essence, during the initial period, IGF-I is in a catabolic phase, playing a limited role in muscle adaptations during RT. Additionally, the study by Haddad et al. found that the expression of Mechano growth factor (MGF) precedes that of IGF-IEa in response to exercise [33], indicating that following mechanical strain and/or muscle damage, the IGF-I gene first shifts towards MGF and later towards IGF-IEa in rodent muscles. Jiang et al. further showed that an extended duration of RT is required to increase the expression of hepatic IGF-I [34].
Conversely, the lack of change in serum IGF-I concentration after RT, as noted in the study by Walker et al., might relate to the initial IGF-I levels of the subjects in the current study [35]. Interestingly, our study's subjects had significantly lower initial IGF-I levels than those in Walker's study. Therefore, according to the research findings, numerous confounding factors could influence the observed results. These factors include inadequate dietary control, differences in subject characteristics, changes in plasma volume, dynamics of synthesis, release and receptor uptake, variations in exercise modes and intensities, limitations regarding the timing of post-exercise sampling, types of exercise protocols, study durations, training frequencies, sample sizes, and the age, sex, and baseline IGF-1 levels of participants.
MSTN acts as an inhibitor of growth and differentiation, specifically expressed in both developing and mature skeletal muscle tissues [11]. Disruption or mutation of the gene responsible for MSTN leads to a significant increase in skeletal muscle mass during development [12]. Additionally, serum MSTN levels are inversely related to total body muscle mass relative to height in older compared to younger adults, both males and females. Higher MSTN levels in the bloodstream are associated with muscle atrophy resulting from prolonged bed rest and thyroxine administration, underscoring its role in muscle changes during immobilization and hormonal interventions [11].
In this study, it was observed that sedentary elderly men exhibited a significant decrease in serum MSTN levels after participating in 12 weeks of incremental RT. Roth et al. were among the first to report a decrease in MSTN mRNA expression in both young and older individuals, including women and men, following 9 weeks of RT [36]. Conversely, Willoughby et al. noted an increase in MSTN mRNA expression, despite a rise in muscle mass, after 12 weeks of RT [37].
The inconsistency in findings could be due to differences in sampling times, methods, exercise intensity and duration, or methods of measuring MSTN. Bagheri et al. reported a significant decrease in serum MSTN concentrations in middle-aged men after 8 weeks of combined upper and lower body RT at 50 to 80% of 1-RM. However, serum MSTN concentration did not significantly decrease after 12 weeks of non-periodized upper and lower body RT at 85 to 90% of 1-RM in untrained males [11]. Additionally, Binns et al. observed a trend toward reduced serum MSTN concentration in community-dwelling older adults after 20 weeks of high-velocity whole-body RT at 70% of 1-RM [38].
The evidence suggests that exercise leads to decreased serum MSTN concentrations in middle-aged and elderly individuals, but not in young healthy men [11]. Although our data does not clarify the precise mechanism behind the reduction in plasma MSTN, the existing literature indicates that the decreased levels might be due to diminished production, processing, and/or secretion of the protein into the circulation. Alternatively, the observed lower MSTN levels could result from reduced stability, increased distribution in the circulatory system, enhanced disposal or reuptake of the protein, or a combination of these factors, potentially influenced by exercise training [11].
FLST is another myokine that plays a crucial role in muscle hypertrophy and atrophy, particularly in the elderly [13]. As a single-chain polypeptide, FLST belongs to the transforming growth factor (TGF) superfamily [14] and is ubiquitously expressed in various human tissues, including skeletal muscle, exerting both paracrine and autocrine effects [13]. Previous research has shown that FLST has the capacity to promote both anabolic and catabolic effects on skeletal muscle by binding to and neutralizing MSTN [14].
The results of this study reveal a significant increase in serum FLST levels among sedentary elderly men following 12 weeks of incremental RT. These findings are in line with those from other studies, which reported that serum FLST concentrations increased in sedentary females and middle-aged men after 8 weeks of high-intensity RT [11, 39]. An elevated FLST:MSTN ratio, indicative of a more favorable anabolic environment, resulted from decreased MSTN concentrations and increased FLST levels [11]. However, these results contrast with those of Diel et al., which found no effect of 12 weeks of RT on serum FLST levels in participants [40]. It's crucial to consider the limitations and various factors that could account for these differing outcomes. The inconsistency may arise from differences in the age of the subjects and the training protocol intensities across studies, likely leading to the varied results reported in the literature. The consistent studies involved middle-aged and elderly participants, whereas Diel et al.'s study focused on young, healthy males. Additionally, it should be noted that the exercise protocol intensity in Diel et al.'s study was lower compared to that used in the present study.
To summarize, a 12-week resistance exercise training program in elderly men led to significant changes: a 14% reduction in plasma MSTN, a 20% increase in plasma FLST, and a 17% increase in IGF-1.
Normally, the maintenance of muscle fiber size is dependent on a delicate balance between positive regulators (such as FLST and IGF-1) and negative regulators (such as MSTN) of muscle growth. However, in situations of muscle atrophy, such as that experienced by older individuals, this equilibrium is disrupted, leading to a dominance of negative influences. Consequently, the introduction of RT, which involves muscle loading, can potentially tip the balance in favor of positive regulators. While the exact mechanism of interaction between these regulators is still not fully understood, it seems to involve a complex negative feedback loop.
Conclusion
From the results of this study, it can be cautiously inferred that RT, through its dual role of enhancing muscle growth factors and reducing atrophy factors, has the potential to alleviate the effects of sarcopenia in elderly individuals. This, in turn, could address various sarcopenia-associated conditions, including metabolic syndrome, inflammation, glucose intolerance, reduced arterial elasticity, and obesity.
Acknowledgments: Thank all the employees and workers of Khorasan Razavi Gas Company for helping us in conducting this research.
Ethical Permissions: The permission of this document was approved by the Research Ethics Committee of Khorasan Razavi Gas Company, which included the research protocol, subject information and consent form.
Conflicts of Interests: There is no conflict of interest.
Authors' Contribution: Barzegari Marvast H (First Author), Introduction Writer/Main Researcher/Statistical Analyst (40%); Akbarnejad A (Second Author), Assistant Researcher/Statistical Analyst (30%); Norouzi J (Third Author), Methodologist/Statistical Analyst/Assistant Researcher/Discussion Writer (30%)
Funding/Support: The present study was not financially supported.
Keywords:
References
1. Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, Almas K. Global prevalence of periodontal disease and lack of its surveillance. Sci World J. 2020;2020:2146160. [Link] [DOI:10.1155/2020/2146160]
2. Bell KE, Von Allmen MT, Devries MC, Phillips SM. Muscle disuse as a pivotal problem in sarcopenia-related muscle loss and dysfunction. J Frailty Aging. 2016;5(1):33-41. [Link] [DOI:10.14283/jfa.2016.78]
3. Visser M. Epidemiology of muscle mass loss with age. In: Cruz-Jentoft AJ, Morley JE, editors. Sarcopenia. Hoboken: John Wiley & Sons; 2012. p. 1-7. [Link] [DOI:10.1002/9781118338032.ch1]
4. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; A quantitative review. Front Physiol. 2012;3:260. [Link] [DOI:10.3389/fphys.2012.00260]
5. Rosenberg IH. Sarcopenia: Origins and clinical relevance. J Nutr. 1997;127(5):990S-1S. [Link] [DOI:10.1093/jn/127.5.990S]
6. Delbono O. Excitation-contraction coupling regulation in aging skeletal muscle. In: Lynch G, editor. Sarcopenia-Age-Related Muscle Wasting and Weakness. Dordrecht: Springer; 2011. p. 113-34. [Link] [DOI:10.1007/978-90-481-9713-2_6]
7. Mero AA, Hulmi JJ, Salmijärvi H, Katajavuori M, Haverinen M, Holviala J, et al. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol. 2013;113(3):641-50. [Link] [DOI:10.1007/s00421-012-2466-x]
8. Escriche-Escuder A, Fuentes-Abolafio IJ, Roldan-Jimenez C, Cuesta-Vargas AI. Effects of exercise on muscle mass, strength, and physical performance in older adults with sarcopenia: A systematic review and meta-analysis according to the EWGSOP criteria. Exp Gerontol. 2021;151:111420. [Link] [DOI:10.1016/j.exger.2021.111420]
9. Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123-32. [Link] [DOI:10.1016/j.arr.2018.07.005]
10. Elliott B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol. 2012;205(3):324-40. [Link] [DOI:10.1111/j.1748-1716.2012.02423.x]
11. Bagheri R, Rashidlamir A, Motevalli MS, Elliott BT, Mehrabani J, Wong A. Effects of upper-body, lower-body, or combined resistance training on the ratio of follistatin and myostatin in middle-aged men. Eur J Appl Physiol. 2019;119(9):1921-31. [Link] [DOI:10.1007/s00421-019-04180-z]
12. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature. 1997;387(6628):83-90. [Link] [DOI:10.1038/387083a0]
13. Tortoriello DV, Sidis Y, Holtzman DA, Holmes WE, Schneyer AL. Human follistatin-related protein: A structural homologue of follistatin with nuclear localization. Endocrinology. 2001;142(8):3426-34. [Link] [DOI:10.1210/endo.142.8.8319]
14. Wewege MA, Desai I, Honey C, Coorie B, Jones MD, Clifford BK, et al. The effect of resistance training in healthy adults on body fat percentage, fat mass and visceral fat: A systematic review and meta-analysis. Sports Med. 2022;52(2):287-300. [Link] [DOI:10.1007/s40279-021-01562-2]
15. Hansen J, Brandt C, Nielsen AR, Hojman P, Whitham M, Febbraio MA, et al. Exercise induces a marked increase in plasma follistatin: Evidence that follistatin is a contraction-induced hepatokine. Endocrinology. 2011;152(1):164-71. [Link] [DOI:10.1210/en.2010-0868]
16. Kraemer WJ, Ratamess NA, Hymer WC, Nindl BC, Fragala MS. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: Roles and integration for cellular development and growth with exercise. Front Endocrinol. 2020;11:33. [Link] [DOI:10.3389/fendo.2020.00033]
17. Huygens W, Thomis MA, Peeters MW, Aerssens J, Janssen R, Vlietinck RF, et al. Linkage of myostatin pathway genes with knee strength in humans. Physiol Genomics. 2004;17(3):264-70. [Link] [DOI:10.1152/physiolgenomics.00224.2003]
18. Moustogiannis A, Philippou A, Taso O, Zevolis E, Pappa M, Chatzigeorgiou A, et al. The effects of muscle cell aging on myogenesis. Int J Mol Sci. 2021;22(7):3721. [Link] [DOI:10.3390/ijms22073721]
19. Maltais ML, Ladouceur JP, Dionne IJ. The effect of resistance training and different sources of postexercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. J Strength Cond Res. 2016;30(6):1680-7. [Link] [DOI:10.1519/JSC.0000000000001255]
20. Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, et al. Two weeks of reduced activity decreases leg lean mass and induces "anabolic resistance" of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98(6):2604-12. [Link] [DOI:10.1210/jc.2013-1502]
21. Bosaeus I, Rothenberg E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc Nutr Soc. 2016;75(2):174-80. [Link] [DOI:10.1017/S002966511500422X]
22. Luk HY, Levitt DE, Boyett JC, Rojas S, Flader SM, McFarlin BK, et al. Resistance exercise-induced hormonal response promotes satellite cell proliferation in untrained men but not in women. Am J Physiol Endocrinol Metab. 2019;317(2):E421-32. [Link] [DOI:10.1152/ajpendo.00473.2018]
23. Willoughby DS. Effects of an alleged myostatin-binding supplement and heavy resistance training on serum myostatin, muscle strength and mass, and body composition. Int J Sport Nutr Exerc Metab. 2004;14(4):461-72. [Link] [DOI:10.1123/ijsnem.14.4.461]
24. Brzycki M. A practical approach to strength training. 2nd ed. Carrollton: Masters Press; 1991. [Link]
25. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-30. [Link] [DOI:10.1249/MSS.0b013e3181a0c95c]
26. Velloso CP. Regulation of muscle mass by growth hormone and IGF‐I. Br J Pharmacol. 2008;154(3):557-68. [Link] [DOI:10.1038/bjp.2008.153]
27. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: A 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321-6. [Link] [DOI:10.1152/jappl.2000.88.4.1321]
28. Molina-Sotomayor E, Castillo-Quezada H, Martínez-Salazar C, González-Orb M, Espinoza-Salinas A, Gonzalez-Jurado JA. Effects of progressive resistance training on cognition and IGF-1 levels in elder women who live in areas with high air pollution. Int J Environ Res Public Health. 2020;17(17):6203. [Link] [DOI:10.3390/ijerph17176203]
29. Cunha PM, Nunes JP, Tomeleri CM, Nascimento MA, Schoenfeld BJ, Antunes M, et al. Resistance training performed with single and multiple sets induces similar improvements in muscular strength, muscle mass, muscle quality, and IGF-1 in older women: A randomized controlled trial. J Strength Cond Res. 2020;34(4):1008-16. [Link] [DOI:10.1519/JSC.0000000000002847]
30. Nunes PRP, Barcelos LC, Oliveira AA, Furlanetto R Jr, Martins FM, Resende EAMR, et al. Muscular strength adaptations and hormonal responses after two different multiple-set protocols of resistance training in postmenopausal women. J Strength Cond Res. 2019;33(5):1276-85. [Link] [DOI:10.1519/JSC.0000000000001788]
31. Rashidi E, Hosseini Kakhak SAR, Askari R. The effect of 8 weeks resistance training with low load and high load on testosterone, insulin-like growth factor-1, insulin-like growth factor binding protein-3 levels, and functional adaptations in older women. Salmand: Iran J Ageing. 2019;14(3):356-67. [Persian] [Link]
32. Hatfield DL, Kraemer WJ, Volek JS, Nindl BC, Caldwell LK, Vingren JL, et al. Hormonal stress responses of growth hormone and insulin-like growth factor-I in highly resistance trained women and men. Growth Horm IGF Res. 2021;59:101407. [Link] [DOI:10.1016/j.ghir.2021.101407]
33. Haddad F, Adams GR. Selected contribution: Acute cellular and molecular responses to resistance exercise. J Appl Physiol. 2002;93(1):394-403. [Link] [DOI:10.1152/japplphysiol.01153.2001]
34. Jiang Q, Lou K, Hou L, Lu Y, Sun L, Tan SC, et al. The effect of resistance training on serum insulin-like growth factor 1 (IGF-1): A systematic review and meta-analysis. Complement Ther Med. 2020;50:102360. [Link] [DOI:10.1016/j.ctim.2020.102360]
35. Walker KS, Kambadur R, Sharma M, Smith HK. Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med Sci Sports Exerc. 2004;36(5):787-93. [Link] [DOI:10.1249/01.MSS.0000126384.04778.29]
36. Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA. Myostatin gene expression is reduced in humans with heavy-resistance strength training: A brief communication. Exp Biol Med. 2003;228(6):706-9. [Link] [DOI:10.1177/153537020322800609]
37. Willoughby DS. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc. 2004;36(4):574-82. [Link] [DOI:10.1249/01.MSS.0000121952.71533.EA]
38. Binns A, Gray M, Henson AC, Fort IL. Changes in lean mass and serum myostatin with habitual protein intake and high-velocity resistance training. J Nutr Health Aging. 2017;21(10):1111-7. [Link] [DOI:10.1007/s12603-017-0883-6]
39. Bagheri R, Moghadam BH, Church DD, Tinsley GM, Eskandari M, Moghadam BH, et al. The effects of concurrent training order on body composition and serum concentrations of follistatin, myostatin and GDF11 in sarcopenic elderly men. Exp Gerontol. 2020;133:110869. [Link] [DOI:10.1016/j.exger.2020.110869]
40. Diel P, Schiffer T, Geisler S, Hertrampf T, Mosler S, Schulz S, et al. Analysis of the effects of androgens and training on myostatin propeptide and follistatin concentrations in blood and skeletal muscle using highly sensitive Immuno PCR. Mol Cell Endocrinol. 2010;330(1-2):1-9. [Link] [DOI:10.1016/j.mce.2010.08.015]









