Volume 16, Issue 1 (2024)
Iran J War Public Health 2024, 16(1): 27-33 |
Back to browse issues page
Article Type:
Subject:
Ethics code: Extracted from master thesis of sport physiology in Islamic Azad University of Torbat –e Heydarieh
History
Received: 2023/11/20 | Accepted: 2024/02/4 | Published: 2024/02/15
Received: 2023/11/20 | Accepted: 2024/02/4 | Published: 2024/02/15
How to cite this article
Azarkamand M, Soltani H. Pulmonary Adaptation Response of Chemical Veterans Following 24 Sessions of Selected Aerobic Exercises. Iran J War Public Health 2024; 16 (1) :27-33
URL: http://ijwph.ir/article-1-1416-en.html
URL: http://ijwph.ir/article-1-1416-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
M. Azarkamand1, H. Soltani *2
1- Department of Physical Education and Sport Sciences, Torbat Heydarieh Branch, Islamic Azad University, Torbat Heydarieh, Iran
2- “Department of Physical Education and Sport Sciences” and “Sports Medicine Research Center”, Torbat Heydarieh Branch, Islamic Azad University, Torbat Heydarieh, Iran
2- “Department of Physical Education and Sport Sciences” and “Sports Medicine Research Center”, Torbat Heydarieh Branch, Islamic Azad University, Torbat Heydarieh, Iran
Full-Text (HTML) (486 Views)
Introduction
During the Iran-Iraq war, over 400,000 people suffered injuries from chemical agents, particularly mustard gas. Pulmonary complications are the main issues resulting from exposure to mustard gas, affecting approximately 42.5% of chemical veterans [1-3]. The impact of mustard gas on the respiratory system includes symptoms such as shortness of breath, irritating cough, and frequent respiratory infections, which are linked to conditions like obstructive lung disease, chronic bronchitis, asthma, bronchiectasis, COPD, and narrowing of the large airways. These lung problems frequently result in hospitalization at treatment centers [4], imposing significant treatment costs that affect their economic well-being and quality of life [5].
Chemical warfare veterans often experience persistent cough and shortness of breath, which hinder their daily activities [6, 7]. Due to diminished lung function and weakened respiratory muscles, a large amount of their energy is devoted to producing respiratory muscle energy, leading to early fatigue and reduced performance [8]. This often discourages them from engaging in sports activities [1, 9], making them more susceptible to diseases like hypertension, diabetes, and osteoporosis [10]. The long-term nature of their conditions can also reduce self-confidence and life expectancy [11]. Stahl has noted that physical health and quality of life are generally low among pulmonary patients [1]. Decreased physical activity leads to loss of muscle mass and further diminished performance [12].
Regular exercise strengthens the chest muscles, thereby enhancing inhalation and exhalation [13]. Optimally functioning muscles allow for more efficient movement activities, reducing energy consumption and shortening the duration of exercise. Strengthening and endurance training of respiratory muscles is an effective method to improve lung function, facilitate easier breathing [14], improve breathing patterns, ensure adequate ventilation exchange [4], and increase tolerance to fatigue [15]. Aerobic exercise helps reduce dynamic hyperinflation and shortness of breath, enhances exercise tolerance, and improves quality of life [18]. The respiratory system plays a crucial role in supplying oxygen to cells and regulating the body's internal environment [17]. Any inefficiency in this system affects overall body performance. Inefficiencies may alter the ratios of ventilation to blood flow and ventilation to oxygen absorption, causing oxygen removal to fall below the volume of ventilated air. This imbalance increases the energy expenditure of the respiratory muscles, leading to premature fatigue [17]. Given the proven effectiveness of exercise training in improving lung function, it is essential to incorporate exercise as a complementary approach to pharmacological treatment. This integration can enhance treatment outcomes, reduce symptoms, and decrease both treatment costs and the side effects of medications [18]. Exercising and strengthening the inspiratory muscles increases tidal volume, reduces the number of breaths taken during exercise, and improves the optimal energy consumption of lung muscles, thereby enhancing athletic performance. In Iran, these techniques are often overlooked in clinical treatment programs aimed at improving patient health. Additionally, educating patients about the benefits of exercise on health is a crucial aspect of this research [6]. Studies on the effects of physical activities on veterans have shown reductions in breath shortness and hospitalization rates, along with improvements in quality of life [1, 15]. Ghasemi et al. [16] found that the mean scores of forced vital capacity were significantly higher in the posttest compared to the pretest (p=0.001). After adjusting for the pretest scores, the forced vital capacity in the training group was significantly greater than that in the control group [16]. Abolhasani noted that while there were no significant increases in tidal volume, inspiratory reserve volume, minute ventilation, and vital capacity, a significant increase in forced vital capacity in the first second was observed [17].
Tari et al. [29] demonstrated that selected aerobic exercise using a treadmill did not significantly increase the forced expiratory volume in the first second or the forced vital capacity of chemical veterans. Given the contradictions in research outcomes, the limited number of studies focused on chemical veterans, and the scant interest among researchers in this group, it is imperative to address these gaps. Chemical veterans typically engage in less physical activity than their non-veteran counterparts due to limitations such as pulmonary and physical impairments. Therefore, this research aimed to investigate the impact of a selected aerobic exercise program consisting of 24 sessions on the lung volumes and capacities of chemical veterans, given that aerobic fitness is a key indicator of respiratory and cardiovascular system health and coordination.
Materials and Methods
This quasi-experimental study was conducted in 2023 using a pre-test and post-test design with both experimental and control groups. The participants were chemical warfare veterans from Torbat-e Heydarieh city who met the inclusion criteria for the study. The statistical sample included 17 veterans aged 50-65 years with a veteran status of 25-35%. After obtaining consent to participate in the study, participants were selected through purposive and convenience sampling methods and randomly assigned to two groups: experimental (10 participants) and control (7 participants).
The sample size was calculated using G*Power software, assuming a significance level of 0.05 and a statistical power of 0.8, employing repeated measures analysis of variance (ANOVA) for two groups. Initially, 12 participants were required; however, to accommodate potential attrition, the total was increased to 17 (10 in the experimental group and 7 in the control group).
On the first day, the subjects were thoroughly briefed on the test procedures. Their weight and standing height were measured using a Seka scale accurate to 0.1 kg and a measuring device accurate to 0.1 cm, respectively.
On the second day, their weight and height data were input into the spirometry machine to calculate the body mass index in kilograms per square meter and the body surface area in square meters. Subsequently, static and dynamic pulmonary volumes and capacities were measured in the laboratory using a spirometry device, Lung Test 1000 from Poland, with a reliability of 0.982. The process involved practical training for the subjects, emphasizing the need for concentration and maximum effort during the pulmonary tests. Three tests—spirometry, flow-volume, and maximum voluntary ventilation—were conducted with a five-minute rest interval between each. The best results were recorded and stored.
After completing the preliminary tests, the training program was introduced, explaining the method of work, how to use a Polar heart rate monitor to determine the heart rate and control the intensity of the training, the duration of the training sets, and guidelines for better performance during the activity and rest phases.
All subjects participated in an eight-week exercise program, consisting of three sessions per week (24 sessions in total), each lasting 45 minutes. The sessions involved intermittent aerobic exercise at an intensity of 45 to 60 percent of maximum heart rate reserve (HRR) under the supervision of a trainer and sports expert in an indoor sports hall.
The intensity of exercise was controlled by heart rate using the pulsometer display that was installed on the subjects' wrists, based on Karvonen's formula, which is a widely used equation to estimate a target heart rate (THR) training zone during exercise. It takes into account an individual's resting heart rate (RHR) and maximum heart rate (MHR) to personalize the intensity of exercise:
THR = (MHR - RHR) × %Intensity + RHR
THR is target heart rate (bpm). MHR presents maximum heart rate (bpm) that can be estimated by subtracting your age from 220. However, it is important to note that this is a general estimate and individual MHR may vary. RHR indicates resting heart rate (bpm), which can be measured by taking your pulse first thing in the morning before getting out of bed. %Intensity presents the desired percentage of your HRR to train at. This value will vary depending on your fitness goals and the specific type of exercise you're performing.
At the conclusion of the eight-week training period, the same three pulmonary tests—spirometry, flow-volume, and maximum voluntary ventilation—were administered to the subjects under the same conditions as the initial tests.
Data were recorded in the SPSS 16 software for calculating central tendency measures, dispersion, and graphing descriptive statistics. The normality of the data was confirmed using the Shapiro-Wilk test, and Levene's test was utilized for the homogeneity of variances. Additionally, repeated measures ANOVA was used to compare pre-test and post-test means and to assess between- and within-group changes. A significance level (p<0.05) was established for hypothesis testing.
Findings
The Shapiro-Wilk test results confirmed that the raw data for each variable were normally distributed. According to the results of Levene's test, the assumption of variance equality for all variables was met in both the control and experimental groups for comparing group means.
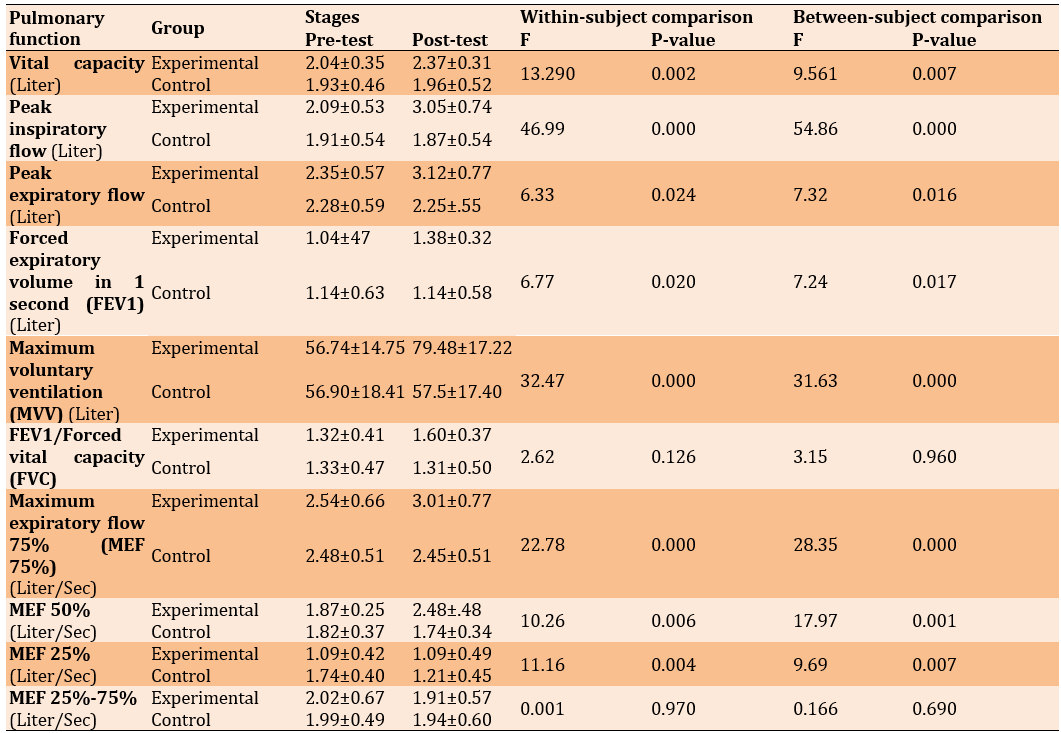
The repeated measures analysis indicated significant increases in vital capacity (p=0.007), peak inspiratory flow (PIF) (p=0.000), peak expiratory flow (PEF) (p=0.016), forced expiratory volume in 1 second (p=0.017), maximum voluntary ventilation (p=0.000), PEF 75% (p = 0.000), PEF 50% (p=0.001), and PEF 25% (p=0.007) due to aerobic exercises. However, aerobic exercises did not significantly affect the ratio of forced vital capacity to forced expiratory volume in 1 second (p=0.960) and forced expiratory flow from 25 to 75% (p=0.690) (Table 1).
Table 1. The results of the repeated measures ANOVA (within- and between-group changes) regarding the mean volume and lung capacity of chemical veterans in the control (n=7) and experimental (n=10) groups
Discussion
One of the primary objectives of this research was to examine pulmonary responses to exercise using non-invasive methods to evaluate the effectiveness of exercise programs aimed at improving health, therapy, and regeneration. The findings of this study demonstrated that eight weeks of selected aerobic exercises significantly enhanced vital capacity, aligning with the findings of Shamlou et al. [19] and Attarzadeh et al. [15]. This research highlighted that strengthening the chest muscles, which increases chest volume, significantly enhances pulmonary function and spirometry indicators. Essentially, the increase in chest volume results from changes in the total volume of the pulmonary alveoli [20].
According to the results of this study, the selected aerobic exercises significantly increased the average PIF and PEF. These flows, like other volumes, reflect the static properties of the respiratory system [21], and enhancing the elasticity of the main and auxiliary intercostal muscle fibers increases effective inspiratory force [21]. Consistent with this research, Sharifian et al. [22] found that eight weeks of aerobic exercise significantly improved respiratory indices of PEF and PIF. The inspiratory muscles expand the chest; this expansion decreases the pressure difference between the alveoli and the pleural cavity, allowing the force of expansion to overcome elasticity and quickly fill the intrapulmonary volume with air. Maximally filling the lungs with air is crucial for stimulating the secretion of surfactant, which increases the level of prostaglandins in the alveolar space, reducing the tone of the bronchial smooth muscles and enhancing lung efficiency. Surfactant improves lung function by increasing the size of lung cells, facilitating cell-to-cell communication, and reducing the surface tension of air sacs. Additionally, surfactant acts as a bronchodilator by increasing the diameter of airways and reducing air resistance, thereby enhancing lung volumes and capacities. This mechanism may explain the observed increase in PIF [23].
In the study by Rupam Bassi, a significant decrease in BMI and a significant increase in PEF and PFI were observed over a 10-week period. They suggested that the improvement in PEF values could be attributed to aerobic exercises, which enhance breathing efficiency, reduce pulmonary resistance, and decrease body fat percentage [20].
Similarly, the present study found a significant increase in the FEV1 index, aligning with the findings of Azad et al. [24], Qanbarzadeh et al. [25], and Moodi et al. [12]. Azad et al. investigated the effect of aerobic exercise on lung function in overweight and obese students and concluded that aerobic exercise improves FEV1. These studies collectively demonstrate that aerobic exercises increase the endurance and tolerance of respiratory muscles, potentially leading to expanded chest volume and increased lung capacities. Aerobic capacity is often enhanced through activities such as walking, which is likely why an increase in FEV1 was observed following exercise intervention, due to reduced bronchial compression or the lessened occurrence of airway bronchospasm, consistent with the results of the current study.
However, the results from Attarzadeh et al. [15] and Tari et al. [23] showed that aerobic exercise programs had an effect on subjects' FEV1 and FVC, showing a slight increase in the post-test, though these changes were not statistically significant. Differences between these studies and the current research may be attributed to factors such as the type of subjects, intensity and duration of training, type of exercise, age of participants, and their physical fitness levels. For instance, in Tari et al.’s study, the training period was 4 weeks and involved sedentary female education students, whereas Attarzadeh worked with inactive girls.
The ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC) is an indicator of airway obstruction. The development of respiratory and trunk muscles helps improve chest mobility and positively affects FEV1/FVC [32]. In this study, aerobic exercises did not cause significant changes in FEV1/FVC. Although FEV1 increased due to aerobic exercise, the FEV1/FVC ratio did not change significantly, suggesting that both FEV1 and FVC increased proportionally. This finding is consistent with the results of Towfiqi et al. [26].
Physical inactivity and obesity can impair FEV1 and FVC, while appropriate aerobic exercise training can partially improve these indices due to enhanced respiratory muscle function. However, participation in long-term physical activity and achieving a normal BMI are crucial for significant improvement in FVC and FEV1 in overweight and sedentary patients [26].
Among respiratory maneuvers, maximum voluntary ventilation (MVV) is particularly important as a dynamic test of ventilatory capacity, where reductions in MVV can indicate airway obstruction or stenosis. Thus, MVV values depend on an individual's exercise capacity and ability, as well as limitations from shortness of breath [17]. In this study, MVV increased significantly by 42.36%. This result aligns with the findings of Azad et al. [24], Hojati et al. [30], and Plankeel et al. [37]. Babaei Bonab [28], who studied the effect of aerobic exercise on 30 obese women with mild asthma over eight weeks, also found a significant effect of aerobic exercise on MVV, FEF, FEV, and PEF indices. Nazem et al. [29] reported a significant increase in MVV after three months of aerobic exercise in middle-aged men.
O'Donnell et al. [35] have identified four main physiological mechanisms that reduce shortness of breath due to aerobic exercise in chronic obstructive pulmonary diseases: reducing ventilatory requirements or increasing ventilatory reserve, reducing apparent ventilatory resistance, improving ventilatory muscle performance, and psychological factors.
However, the results of Casaburi et al. [31] regarding the effect of aerobic exercise on MVV do not align with the findings of this study. The discrepancies can be attributed to differences in the number of subjects, their gender and age, the type of exercise, and the duration of the exercise.
In the present study, the maximum expiratory flow of 75% (MEF 75%), maximum expiratory flow of 50% (MEF 50%), and maximum expiratory flow of 25% (MEF 25%) increased significantly. This indicates that exercise has increased the speed of muscle shortening and consequently muscle strength. In line with the results of this study, Hojati et al. [30] investigated the effect of intermittent aerobic exercises on the lung volumes of 100 inactive female students who participated in a protocol of aerobic intermittent running at 65-80% of the reserve heart rate. They concluded that intermittent aerobic training had a significant effect on MEF 75% and MEF 50%, but it had no significant effect on MEF 25%. Park et al. [32] investigated the effects of high-intensity aerobic exercise on the pulmonary function of elderly women after eight weeks of exercise. According to their findings, high-intensity aerobic exercise significantly increased MEF 75%, MEF 50% remained unchanged, and MEF 25% decreased, which was not significant.
In this study, the forced expiratory flow of 25-75% (FEF 25-75%) was not statistically significant. Wu et al. [36] investigated the effects of continuous aerobic exercise on lung function in a meta-analysis and concluded that aerobic exercise improved forced expiratory volume in one second, PEF, and forced vital capacity, but changes in FEF 25-75% were not significant.
Other research on the effects of combined exercises on lung function in smokers showed that 16 weeks of physical exercises in 50 sedentary male smokers, divided into groups of aerobic, resistance, combined, and control, did not yield significant changes in FEF 75-25% [37]. Similarly, in the study by Barari et al. [33], which examined the effects of resistance training and ivy extract consumption on a selection of spirometric indicators in 48 men with respiratory diseases, the FEF 25-75% value was not significant after training. Womack et al. [34] conducted a study on the effects of aerobic training (eight weeks of training at an intensity of 50-75% HRmax) on the lung function of obese men. Their research did not show significant changes in respiratory indices after eight weeks of aerobic exercise, which is consistent with the present research.
Overall, multiple lines of evidence support the enhancement of pulmonary function through exercise training. This includes the compensatory effects of exercise on muscle imbalances in the chest, strengthening of accessory respiratory muscles, increased residual airflow, and reduced ventilation in asthmatic patients by enhancing bronchial dilation during exercise. Other benefits are reducing airway resistance, enlarging airway diameter, strengthening respiratory muscles, increasing chest elasticity through exercise, reducing pulmonary reversibility, and increasing pulmonary vascular dilation due to heightened activation of the adrenaline system during exercise. Additionally, decreased airway resistance and improved FEV1 and FVC from increased airflow (following pulmonary vasodilation), as well as the relationship between serum cortisol, bronchial dilation, and pulmonary surfactant production, all contribute to improved lung function [35]. Since the training program significantly impacted most pulmonary indicators, it can be concluded that it has enhanced the ability and coordination of the respiratory muscles.
Incorporating exercise into the management of these patients is essential for symptom improvement, lung function enhancement, and reducing dependence on inhalation sprays and oral corticosteroids. This approach not only advances treatment but also reduces costs and minimizes side effects from medications. Overall, the study highlights the effectiveness of aerobic exercise as a viable and appropriate method for improving pulmonary function in individuals with normal physical fitness levels, including chemical veterans.
Conclusion
Exercise, especially aerobic activities such as running and jogging, enhances respiratory muscle endurance, which in turn improves ventilation and increases expiratory flow in patients with respiratory diseases.
Acknowledgments: We hereby express our thanks and appreciation for the sincere cooperation of the Martyr Foundation and the affairs of Torbat-e Heydarieh city and the chemical veterans.
Ethical Permissions: Extracted from master thesis of sport physiology in Islamic Azad University of Torbat-e Heydarieh Branch.
Conflicts of Interests: The authors declare no conflict of interest.
Authors’ Contribution: Azarkamand M (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (50%); Soltani H (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (50%)
Funding/Support: The present research has not received financial support from any institution or organization.
During the Iran-Iraq war, over 400,000 people suffered injuries from chemical agents, particularly mustard gas. Pulmonary complications are the main issues resulting from exposure to mustard gas, affecting approximately 42.5% of chemical veterans [1-3]. The impact of mustard gas on the respiratory system includes symptoms such as shortness of breath, irritating cough, and frequent respiratory infections, which are linked to conditions like obstructive lung disease, chronic bronchitis, asthma, bronchiectasis, COPD, and narrowing of the large airways. These lung problems frequently result in hospitalization at treatment centers [4], imposing significant treatment costs that affect their economic well-being and quality of life [5].
Chemical warfare veterans often experience persistent cough and shortness of breath, which hinder their daily activities [6, 7]. Due to diminished lung function and weakened respiratory muscles, a large amount of their energy is devoted to producing respiratory muscle energy, leading to early fatigue and reduced performance [8]. This often discourages them from engaging in sports activities [1, 9], making them more susceptible to diseases like hypertension, diabetes, and osteoporosis [10]. The long-term nature of their conditions can also reduce self-confidence and life expectancy [11]. Stahl has noted that physical health and quality of life are generally low among pulmonary patients [1]. Decreased physical activity leads to loss of muscle mass and further diminished performance [12].
Regular exercise strengthens the chest muscles, thereby enhancing inhalation and exhalation [13]. Optimally functioning muscles allow for more efficient movement activities, reducing energy consumption and shortening the duration of exercise. Strengthening and endurance training of respiratory muscles is an effective method to improve lung function, facilitate easier breathing [14], improve breathing patterns, ensure adequate ventilation exchange [4], and increase tolerance to fatigue [15]. Aerobic exercise helps reduce dynamic hyperinflation and shortness of breath, enhances exercise tolerance, and improves quality of life [18]. The respiratory system plays a crucial role in supplying oxygen to cells and regulating the body's internal environment [17]. Any inefficiency in this system affects overall body performance. Inefficiencies may alter the ratios of ventilation to blood flow and ventilation to oxygen absorption, causing oxygen removal to fall below the volume of ventilated air. This imbalance increases the energy expenditure of the respiratory muscles, leading to premature fatigue [17]. Given the proven effectiveness of exercise training in improving lung function, it is essential to incorporate exercise as a complementary approach to pharmacological treatment. This integration can enhance treatment outcomes, reduce symptoms, and decrease both treatment costs and the side effects of medications [18]. Exercising and strengthening the inspiratory muscles increases tidal volume, reduces the number of breaths taken during exercise, and improves the optimal energy consumption of lung muscles, thereby enhancing athletic performance. In Iran, these techniques are often overlooked in clinical treatment programs aimed at improving patient health. Additionally, educating patients about the benefits of exercise on health is a crucial aspect of this research [6]. Studies on the effects of physical activities on veterans have shown reductions in breath shortness and hospitalization rates, along with improvements in quality of life [1, 15]. Ghasemi et al. [16] found that the mean scores of forced vital capacity were significantly higher in the posttest compared to the pretest (p=0.001). After adjusting for the pretest scores, the forced vital capacity in the training group was significantly greater than that in the control group [16]. Abolhasani noted that while there were no significant increases in tidal volume, inspiratory reserve volume, minute ventilation, and vital capacity, a significant increase in forced vital capacity in the first second was observed [17].
Tari et al. [29] demonstrated that selected aerobic exercise using a treadmill did not significantly increase the forced expiratory volume in the first second or the forced vital capacity of chemical veterans. Given the contradictions in research outcomes, the limited number of studies focused on chemical veterans, and the scant interest among researchers in this group, it is imperative to address these gaps. Chemical veterans typically engage in less physical activity than their non-veteran counterparts due to limitations such as pulmonary and physical impairments. Therefore, this research aimed to investigate the impact of a selected aerobic exercise program consisting of 24 sessions on the lung volumes and capacities of chemical veterans, given that aerobic fitness is a key indicator of respiratory and cardiovascular system health and coordination.
Materials and Methods
This quasi-experimental study was conducted in 2023 using a pre-test and post-test design with both experimental and control groups. The participants were chemical warfare veterans from Torbat-e Heydarieh city who met the inclusion criteria for the study. The statistical sample included 17 veterans aged 50-65 years with a veteran status of 25-35%. After obtaining consent to participate in the study, participants were selected through purposive and convenience sampling methods and randomly assigned to two groups: experimental (10 participants) and control (7 participants).
The sample size was calculated using G*Power software, assuming a significance level of 0.05 and a statistical power of 0.8, employing repeated measures analysis of variance (ANOVA) for two groups. Initially, 12 participants were required; however, to accommodate potential attrition, the total was increased to 17 (10 in the experimental group and 7 in the control group).
On the first day, the subjects were thoroughly briefed on the test procedures. Their weight and standing height were measured using a Seka scale accurate to 0.1 kg and a measuring device accurate to 0.1 cm, respectively.
On the second day, their weight and height data were input into the spirometry machine to calculate the body mass index in kilograms per square meter and the body surface area in square meters. Subsequently, static and dynamic pulmonary volumes and capacities were measured in the laboratory using a spirometry device, Lung Test 1000 from Poland, with a reliability of 0.982. The process involved practical training for the subjects, emphasizing the need for concentration and maximum effort during the pulmonary tests. Three tests—spirometry, flow-volume, and maximum voluntary ventilation—were conducted with a five-minute rest interval between each. The best results were recorded and stored.
After completing the preliminary tests, the training program was introduced, explaining the method of work, how to use a Polar heart rate monitor to determine the heart rate and control the intensity of the training, the duration of the training sets, and guidelines for better performance during the activity and rest phases.
All subjects participated in an eight-week exercise program, consisting of three sessions per week (24 sessions in total), each lasting 45 minutes. The sessions involved intermittent aerobic exercise at an intensity of 45 to 60 percent of maximum heart rate reserve (HRR) under the supervision of a trainer and sports expert in an indoor sports hall.
The intensity of exercise was controlled by heart rate using the pulsometer display that was installed on the subjects' wrists, based on Karvonen's formula, which is a widely used equation to estimate a target heart rate (THR) training zone during exercise. It takes into account an individual's resting heart rate (RHR) and maximum heart rate (MHR) to personalize the intensity of exercise:
THR = (MHR - RHR) × %Intensity + RHR
THR is target heart rate (bpm). MHR presents maximum heart rate (bpm) that can be estimated by subtracting your age from 220. However, it is important to note that this is a general estimate and individual MHR may vary. RHR indicates resting heart rate (bpm), which can be measured by taking your pulse first thing in the morning before getting out of bed. %Intensity presents the desired percentage of your HRR to train at. This value will vary depending on your fitness goals and the specific type of exercise you're performing.
At the conclusion of the eight-week training period, the same three pulmonary tests—spirometry, flow-volume, and maximum voluntary ventilation—were administered to the subjects under the same conditions as the initial tests.
Data were recorded in the SPSS 16 software for calculating central tendency measures, dispersion, and graphing descriptive statistics. The normality of the data was confirmed using the Shapiro-Wilk test, and Levene's test was utilized for the homogeneity of variances. Additionally, repeated measures ANOVA was used to compare pre-test and post-test means and to assess between- and within-group changes. A significance level (p<0.05) was established for hypothesis testing.
Findings
The Shapiro-Wilk test results confirmed that the raw data for each variable were normally distributed. According to the results of Levene's test, the assumption of variance equality for all variables was met in both the control and experimental groups for comparing group means.
The repeated measures analysis indicated significant increases in vital capacity (p=0.007), peak inspiratory flow (PIF) (p=0.000), peak expiratory flow (PEF) (p=0.016), forced expiratory volume in 1 second (p=0.017), maximum voluntary ventilation (p=0.000), PEF 75% (p = 0.000), PEF 50% (p=0.001), and PEF 25% (p=0.007) due to aerobic exercises. However, aerobic exercises did not significantly affect the ratio of forced vital capacity to forced expiratory volume in 1 second (p=0.960) and forced expiratory flow from 25 to 75% (p=0.690) (Table 1).
Table 1. The results of the repeated measures ANOVA (within- and between-group changes) regarding the mean volume and lung capacity of chemical veterans in the control (n=7) and experimental (n=10) groups

Discussion
One of the primary objectives of this research was to examine pulmonary responses to exercise using non-invasive methods to evaluate the effectiveness of exercise programs aimed at improving health, therapy, and regeneration. The findings of this study demonstrated that eight weeks of selected aerobic exercises significantly enhanced vital capacity, aligning with the findings of Shamlou et al. [19] and Attarzadeh et al. [15]. This research highlighted that strengthening the chest muscles, which increases chest volume, significantly enhances pulmonary function and spirometry indicators. Essentially, the increase in chest volume results from changes in the total volume of the pulmonary alveoli [20].
According to the results of this study, the selected aerobic exercises significantly increased the average PIF and PEF. These flows, like other volumes, reflect the static properties of the respiratory system [21], and enhancing the elasticity of the main and auxiliary intercostal muscle fibers increases effective inspiratory force [21]. Consistent with this research, Sharifian et al. [22] found that eight weeks of aerobic exercise significantly improved respiratory indices of PEF and PIF. The inspiratory muscles expand the chest; this expansion decreases the pressure difference between the alveoli and the pleural cavity, allowing the force of expansion to overcome elasticity and quickly fill the intrapulmonary volume with air. Maximally filling the lungs with air is crucial for stimulating the secretion of surfactant, which increases the level of prostaglandins in the alveolar space, reducing the tone of the bronchial smooth muscles and enhancing lung efficiency. Surfactant improves lung function by increasing the size of lung cells, facilitating cell-to-cell communication, and reducing the surface tension of air sacs. Additionally, surfactant acts as a bronchodilator by increasing the diameter of airways and reducing air resistance, thereby enhancing lung volumes and capacities. This mechanism may explain the observed increase in PIF [23].
In the study by Rupam Bassi, a significant decrease in BMI and a significant increase in PEF and PFI were observed over a 10-week period. They suggested that the improvement in PEF values could be attributed to aerobic exercises, which enhance breathing efficiency, reduce pulmonary resistance, and decrease body fat percentage [20].
Similarly, the present study found a significant increase in the FEV1 index, aligning with the findings of Azad et al. [24], Qanbarzadeh et al. [25], and Moodi et al. [12]. Azad et al. investigated the effect of aerobic exercise on lung function in overweight and obese students and concluded that aerobic exercise improves FEV1. These studies collectively demonstrate that aerobic exercises increase the endurance and tolerance of respiratory muscles, potentially leading to expanded chest volume and increased lung capacities. Aerobic capacity is often enhanced through activities such as walking, which is likely why an increase in FEV1 was observed following exercise intervention, due to reduced bronchial compression or the lessened occurrence of airway bronchospasm, consistent with the results of the current study.
However, the results from Attarzadeh et al. [15] and Tari et al. [23] showed that aerobic exercise programs had an effect on subjects' FEV1 and FVC, showing a slight increase in the post-test, though these changes were not statistically significant. Differences between these studies and the current research may be attributed to factors such as the type of subjects, intensity and duration of training, type of exercise, age of participants, and their physical fitness levels. For instance, in Tari et al.’s study, the training period was 4 weeks and involved sedentary female education students, whereas Attarzadeh worked with inactive girls.
The ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC) is an indicator of airway obstruction. The development of respiratory and trunk muscles helps improve chest mobility and positively affects FEV1/FVC [32]. In this study, aerobic exercises did not cause significant changes in FEV1/FVC. Although FEV1 increased due to aerobic exercise, the FEV1/FVC ratio did not change significantly, suggesting that both FEV1 and FVC increased proportionally. This finding is consistent with the results of Towfiqi et al. [26].
Physical inactivity and obesity can impair FEV1 and FVC, while appropriate aerobic exercise training can partially improve these indices due to enhanced respiratory muscle function. However, participation in long-term physical activity and achieving a normal BMI are crucial for significant improvement in FVC and FEV1 in overweight and sedentary patients [26].
Among respiratory maneuvers, maximum voluntary ventilation (MVV) is particularly important as a dynamic test of ventilatory capacity, where reductions in MVV can indicate airway obstruction or stenosis. Thus, MVV values depend on an individual's exercise capacity and ability, as well as limitations from shortness of breath [17]. In this study, MVV increased significantly by 42.36%. This result aligns with the findings of Azad et al. [24], Hojati et al. [30], and Plankeel et al. [37]. Babaei Bonab [28], who studied the effect of aerobic exercise on 30 obese women with mild asthma over eight weeks, also found a significant effect of aerobic exercise on MVV, FEF, FEV, and PEF indices. Nazem et al. [29] reported a significant increase in MVV after three months of aerobic exercise in middle-aged men.
O'Donnell et al. [35] have identified four main physiological mechanisms that reduce shortness of breath due to aerobic exercise in chronic obstructive pulmonary diseases: reducing ventilatory requirements or increasing ventilatory reserve, reducing apparent ventilatory resistance, improving ventilatory muscle performance, and psychological factors.
However, the results of Casaburi et al. [31] regarding the effect of aerobic exercise on MVV do not align with the findings of this study. The discrepancies can be attributed to differences in the number of subjects, their gender and age, the type of exercise, and the duration of the exercise.
In the present study, the maximum expiratory flow of 75% (MEF 75%), maximum expiratory flow of 50% (MEF 50%), and maximum expiratory flow of 25% (MEF 25%) increased significantly. This indicates that exercise has increased the speed of muscle shortening and consequently muscle strength. In line with the results of this study, Hojati et al. [30] investigated the effect of intermittent aerobic exercises on the lung volumes of 100 inactive female students who participated in a protocol of aerobic intermittent running at 65-80% of the reserve heart rate. They concluded that intermittent aerobic training had a significant effect on MEF 75% and MEF 50%, but it had no significant effect on MEF 25%. Park et al. [32] investigated the effects of high-intensity aerobic exercise on the pulmonary function of elderly women after eight weeks of exercise. According to their findings, high-intensity aerobic exercise significantly increased MEF 75%, MEF 50% remained unchanged, and MEF 25% decreased, which was not significant.
In this study, the forced expiratory flow of 25-75% (FEF 25-75%) was not statistically significant. Wu et al. [36] investigated the effects of continuous aerobic exercise on lung function in a meta-analysis and concluded that aerobic exercise improved forced expiratory volume in one second, PEF, and forced vital capacity, but changes in FEF 25-75% were not significant.
Other research on the effects of combined exercises on lung function in smokers showed that 16 weeks of physical exercises in 50 sedentary male smokers, divided into groups of aerobic, resistance, combined, and control, did not yield significant changes in FEF 75-25% [37]. Similarly, in the study by Barari et al. [33], which examined the effects of resistance training and ivy extract consumption on a selection of spirometric indicators in 48 men with respiratory diseases, the FEF 25-75% value was not significant after training. Womack et al. [34] conducted a study on the effects of aerobic training (eight weeks of training at an intensity of 50-75% HRmax) on the lung function of obese men. Their research did not show significant changes in respiratory indices after eight weeks of aerobic exercise, which is consistent with the present research.
Overall, multiple lines of evidence support the enhancement of pulmonary function through exercise training. This includes the compensatory effects of exercise on muscle imbalances in the chest, strengthening of accessory respiratory muscles, increased residual airflow, and reduced ventilation in asthmatic patients by enhancing bronchial dilation during exercise. Other benefits are reducing airway resistance, enlarging airway diameter, strengthening respiratory muscles, increasing chest elasticity through exercise, reducing pulmonary reversibility, and increasing pulmonary vascular dilation due to heightened activation of the adrenaline system during exercise. Additionally, decreased airway resistance and improved FEV1 and FVC from increased airflow (following pulmonary vasodilation), as well as the relationship between serum cortisol, bronchial dilation, and pulmonary surfactant production, all contribute to improved lung function [35]. Since the training program significantly impacted most pulmonary indicators, it can be concluded that it has enhanced the ability and coordination of the respiratory muscles.
Incorporating exercise into the management of these patients is essential for symptom improvement, lung function enhancement, and reducing dependence on inhalation sprays and oral corticosteroids. This approach not only advances treatment but also reduces costs and minimizes side effects from medications. Overall, the study highlights the effectiveness of aerobic exercise as a viable and appropriate method for improving pulmonary function in individuals with normal physical fitness levels, including chemical veterans.
Conclusion
Exercise, especially aerobic activities such as running and jogging, enhances respiratory muscle endurance, which in turn improves ventilation and increases expiratory flow in patients with respiratory diseases.
Acknowledgments: We hereby express our thanks and appreciation for the sincere cooperation of the Martyr Foundation and the affairs of Torbat-e Heydarieh city and the chemical veterans.
Ethical Permissions: Extracted from master thesis of sport physiology in Islamic Azad University of Torbat-e Heydarieh Branch.
Conflicts of Interests: The authors declare no conflict of interest.
Authors’ Contribution: Azarkamand M (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (50%); Soltani H (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (50%)
Funding/Support: The present research has not received financial support from any institution or organization.
Keywords:
References
1. Jafari F, Guitynavard F, Soroush M, Mousavi B. Quality of life in chemical war victims with sever pulmonary damage. Iran J War Public Health. 2012;4(1):46-52. [Link]
2. Amini H, Solaymani-Dodaran M, Mousavi B, Alam Beladi SN, Soroush MR, Abolghasemi J, et al. Long-term health outcomes among survivors exposed to sulfur mustard in Iran. JAMA Netw Open. 2020;3(12):e2028894. [Link] [DOI:10.1001/jamanetworkopen.2020.28894]
3. Mousavi ZB, Ebrahimi A, Mirian M. Long-term effects of Sulfur Mustard gas exposure on the skin of Iranian combaters. Iran J Dermatol. 2001;5(1):9-19. [Persian] [Link]
4. Ghahri Sarabi AR, Heydarizadeh K, Barahimi N, Alavi Majd H, Yaghmai F. Effects of respiratory exercises on pulmonary function of chemically afflicted soldiers in Khoramabad. Adv Nurs Midwifery. 2007;16(58):13-20. [Persian] [Link]
5. Qolipour S, Parhizgar Kalat SZ, Qolipour M. Type of Injury and quality of life among veterans with disabilities of Kermanshah province. J Soc Work Res. 2017;2(4):66-99. [Persian] [Link]
6. Amini M, Gholami M, Aabed Natanzi H, Shakeri N, Haddad H. Effect of diaphragmatic respiratory training on some pulmonary indexes in older people with chronic obstructive pulmonary disease. Salmand: Iran J Ageing. 2019;14(3):332-41. [Persian] [Link]
7. Salehi M, Shahrabi J, Aliannejad R, Khiabani I. Estimation of VO2php in chemical veterans based on the values of spirometry test parameters using decision tree and SVR. Proceedings of the 4th Iran Data Mining Conference; 2010. Tehran. [Persian] [Link]
8. Azad A, Gharakhanlou R, Niknam A, Ghanbari A. Effects of aerobic exercise on lung function in overweight and obese students. Tanaffos. 2011;10(3):24-31. [Link]
9. Boskabady M, Boskabady MH, Zabihi NA, Boskabady M. The effect of chemical warfare on respiratory symptoms, pulmonary function tests and their reversibility 23-25 years after exposure. Toxicol Ind Health. 2012;31(1):79-84. [Link] [DOI:10.1177/0748233712468025]
10. Ahmadi Z, Moradi M, Abedi B. Effect of breathing exercises on lung volumes and fatigue in chemical victims. Iran J War Public Health. 2016;8(3):127-33. [Link]
11. Sedighi Moghadam MR, Ghanei M, Kenn K, Hopkinson NS. Pulmonary rehabilitation in patients with mustard gas lung disease: A study protocol for a randomized controlled trial. Trials. 2019;20:132. [Link] [DOI:10.1186/s13063-019-3180-3]
12. Moodi H, Ghiasi F, Afshar M, Akbari A, Harat H, Moodi M, et al. The effect of one kind of plyometric and aerobic exercises on chest expansion and respiratory volumes in high school students. J Shahrekord Univ Med Sci. 2009;11(2):22-9. [Persian] [Link]
13. Haji Rasouli M, Mehzeb A, Soroush MR, Amini R, Ghanei M. Investigating the amount of changes in the physical parameters of chemical veterans after participating in a regular exercise program. Sport Sci Res Q. 2011;4(9):29-50. [Persian] [Link]
14. Burr JF, Davidson W, Shephard RJ, Eves N. Physical activity in chronic respiratory conditions: Assessing risks for physical activity clearance and prescription. Can Fam Physician. 2012;58(7):761-4. [Link]
15. Attarzadeh Hosseini SR, Hojati Oshtovani Z, Soltani H, Hossein Kakhk SA. Changes in pulmonary function and peak oxygen consumption in response to interval aerobic training in sedentary girls. J Sabzevar Univ Med Sci. 2012;19(1):42-51. [Persian] [Link]
16. Ghasemi GH, Barati Rokati P, Salehi OR. Effect of eight weeks of modified Pilates training on pulmonary function and quality of life of veterans exposed to chemical warfare. Iran J War Public Health. 2019;11(4):183-8. [Link] [DOI:10.29252/ijwph.11.4.183]
17. Abolhasani H. Investigating the effect of a period of submaximal exercises on the amount of changes in lung volumes and capacities in Isfahan chemically injured patients [dissertation]. Tehran: University of Tehran, Faculty of Physical Education and Sports Sciences; 1995. [Persian] [Link]
18. Moghaddasi B, Moghaddasi Z, Taheri Nasab P. Effects of physical exercise on pulmonary function and clinical manifestations by asthmatic patients. J Arak Univ Med Sci. 2010;13(2):134-40. [Persian] [Link]
19. Shamlou S, Taheri M, Nowrozi N, Sohrabi S. Investigating the effect of eight weeks of aerobic exercise in water on respiratory indices of middle-aged men. Proceedings of the 1st National Conference on Developments in Sports Science in the Field of Health, Prevention and Championship; 2016. Qazvin: Imam Khomeini International University. [Persian] [Link]
20. Bassi R, Sharma S, Sharma A, Kaur D, Kaur H. The effect of aerobic exercises on peak expiratory flow rate and physical fitness index in female subjects. Natl J Physiol, Pharm Pharmacol. 2015;5(5):376-81. [Link] [DOI:10.5455/njppp.2015.5.2107201560]
21. Cancelliero-Gaiad KM, Ike D, Pantoni CBF, Borghi-Silva A, Costa D. Respiratory pattern of diaphragmatic breathing and pilates breathing in COPD subjects. Braz J Phys Ther. 2014;18(4):291-9. [Link] [DOI:10.1590/bjpt-rbf.2014.0042]
22. Sharifian A, Sigari N, Rahimi E, Yazhdanpanah K. Survey of normal indices of pulmonary function test by use of spirometry in the people of Kurdistan province. Sci J Kurdistan Univ Med Sci. 2007;12(2). [Persian] [Link]
23. Tari M, Fallah Mohammadi Z, Dabidi Roshan VA, Aliaei M. The effect of cycle ergometer aerobic training program on FVC, FEVL, exercise tolerance and dyspnearatein lung chemically injured veterans. Olympic. 2018;17(1):9-32. [Persian] [Link]
24. Azad A, Gharakhanlou R, Niknam A, Ghanbari A. Effects of aerobic exercise on lung function in overweight and obese students. Tanaffos. 2011;10(3):24-3. [Link]
25. Qanbarzadeh M. Comparison of the effect of two types of special exercise programs on spirometry indicators and activity tolerance in the injured pulmonary effects of chemical warfare gases in Khuzestan province [dissertation]. Tehran: Tarbiat Modares University; 2002. [Persian] [Link]
26. Towfiqi A, Seyed Ameri MH, Toloei Azar J. The effect of 12 weeks of endurance, resistance and parallel training on the volume and lung capacities of inactive male students. Olympic. 2012;20(2):99-113. [Persian] [Link]
27. Eftekhari E. Pilates training on pulmonary and endurance function in female patients with multiple sclerosis. Middle East J Disabil Stud. 2020;10:92. [Persian] [Link]
28. Babaei Bonab S. Changes in spirometric parameters of obese women with mild asthma following selected aerobic exercises. Middle East J Disabil Stud. 2019;9:57. [Persian] [Link]
29. Nazem F, Izadi M, Jalili M, Keshavarz B. Impact of aerobic exercise and detraining on pulmonary function indexes in obese middle-aged patients with chronic asthma. J Arak Univ Med Sci. 2013;15(9):85-93. [Persian] [Link]
30. Hojati Z, Kumar R. The effect of interval aerobic exercises on some dynamic pulmonary volumes of non-athlete female students. Adv Environ Biol. 2013;7(7):1249-54. [Link]
31. Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(8):870-8. [Link] [DOI:10.1164/rccm.200305-617OC]
32. Park J, Han D. Effects of high intensity aerobic exercise on treadmill on maximum-expiratory lung capacity of elderly women. J Phys Ther Sci. 2017;29(8):1454-7. [Link] [DOI:10.1589/jpts.29.1454]
33. Barari A, Kazemi M, Abdi A. Resistance training and consumption of Glechoma extracts on selection of spirometric indices in men with respiratory diseases. Razi J Med Sci. 2016;23(146):17-25. [Persian] [Link]
34. Womack CJ, Harris DL, Katzel LI, Hagberg JM, Bleecker ER, Goldberg AP. Weight loss, not aerobic exercise, improves pulmonary function in older obese men. J Gerontol: Ser A. 2000;55(8):M453-7. [Link] [DOI:10.1093/gerona/55.8.M453]
35. O'Donnell DE. Breathlessness in patients with chronic airflow limitation. Chest. 1994;106(3):904-12. [Link] [DOI:10.1378/chest.106.3.904]
36. Wu X, Gao S, Lian Y. Effects of continuous aerobic exercise on lung function and quality of life with asthma: A systematic review and meta-analysis. J Thorac Dis. 2020;12(9):4781-95. [Link] [DOI:10.21037/jtd-19-2813]
37. Plankeel JF, McMullen B, MacIntyre NR. Exercise outcomes after pulmonary rehabilitation depend on the initial mechanism of exercise limitation among non-oxygen-dependent COPD patients. Chest. 2005;127(1):110-6. [Link] [DOI:10.1378/chest.127.1.110]









