Volume 16, Issue 1 (2024)
Iran J War Public Health 2024, 16(1): 67-74 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/11/7 | Accepted: 2024/03/4 | Published: 2024/03/28
Received: 2023/11/7 | Accepted: 2024/03/4 | Published: 2024/03/28
How to cite this article
Saeed Z, Jaafar H, Alburkiebh S. Effect of Nebulized Lidocaine and Intravenous Lidocaine on Hemodynamic Responses to Laryngoscopy and Endotracheal Intubation. Iran J War Public Health 2024; 16 (1) :67-74
URL: http://ijwph.ir/article-1-1414-en.html
URL: http://ijwph.ir/article-1-1414-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Clinical Laboratory, Faculty of Pharmacy, Kufa University Jaafar, Kufa, Iraq
2- Department of Surgery, Medical College, Kufa University, Kufa, Iraq
3- Department of Anesthesia, Faculty of Surgery, Kufa University, Kufa, Iraq
2- Department of Surgery, Medical College, Kufa University, Kufa, Iraq
3- Department of Anesthesia, Faculty of Surgery, Kufa University, Kufa, Iraq
Full-Text (HTML) (1239 Views)
Introduction
Direct laryngoscopy is the most commonly used technique for endotracheal intubation, as it allows direct visualization of the vocal cords [1]. This technique was pioneered by Kirstein, Killian, and Jackson in the late 1800s and early 1900s and is now the predominant method for endotracheal intubation [2]. Preparation for direct laryngoscopy includes proper patient positioning, preoxygenation, and ensuring the equipment functions correctly. The presence of a skilled assistant is crucial for a successful outcome. Thorough and precise preparation is vital, as the initial attempt at securing the airway should be optimal. Successful direct laryngoscopy requires a straight line of sight from the mouth to the larynx, aligning the oral, oropharyngeal, hypopharyngeal, and laryngeal axes. Positioning the patient in the sniffing position helps achieve this alignment, and optimal head elevation is achieved by aligning a straight horizontal line from the external auditory meatus to the sternal notch. The laryngoscope is a handheld instrument with a blade attached to a handle that includes a light source. There are two types of laryngoscope blades: the curved blade (with Macintosh being the most popular) and the straight blade (with Miller being the most popular). Each type has specific techniques, advantages, and disadvantages [1].
Laryngoscopy involves inserting the blade through the mouth, lifting the tongue, positioning the blade tip with a lifting force to expose the glottis area, and then inserting the endotracheal tube through the vocal cords into the trachea. Endotracheal intubation is the gold standard procedure for airway management. A laryngoscope typically facilitates it, which helps establish a definitive airway, offers maximum protection against gastric acid aspiration, and facilitates positive pressure ventilation with higher airway pressures than a supraglottic airway or face mask [1]. The endotracheal tube is inserted through the vocal cords into the trachea under continuous visualization [1].
Tracheal intubation was initially described by Kite in 1788 for resuscitation and for laryngeal obstruction. Macewen first advocated using tracheal intubation instead of tracheostomy for anesthesia during head and neck surgery in 1880. O'Dwyer described an intubating tube in 1885. Modern endotracheal anesthesia was further developed by Magill and Rowbotham after World War I [2].
The modern, standard endotracheal tube is a disposable, single-use, cuffed, plastic tube designed to be inserted through the nose or mouth, positioned with its distal end in the mid-trachea to maintain a patent airway for lung ventilation. Various endotracheal tubes are available for specific situations. Common features across these models include a universal 15mm adapter for attaching the proximal end to different ventilating circuits and devices, a high-volume, low-pressure cuff, a beveled tip to ease passage through the vocal cords, and a Murphy eye—a distal opening on the tube's side wall that provides an additional ventilation port if the tube's end becomes obstructed by secretions or soft tissue. In most patients, a cuffed endotracheal tube is used for routine intubation; however, a cuffless tube may be used in neonates and infants. This low-pressure, high-volume cuff is inflated with air to ensure that tidal volume ventilates the lungs without escaping upward into the upper airways and to prevent aspiration of gastric contents into the lungs [1]. Securing the airway with intubation induces hemodynamic responses such as tachycardia, hypertension, and dysrhythmias due to sympatho-adrenal stimulation. These responses are more severe and pronounced in hypertensive patients than in normotensive ones [3].
The force applied by the laryngoscope to lift the epiglottis during endotracheal intubation, coupled with the irritation caused by the tube entering the trachea and subsequent cuff expansion, can lead to cardiovascular responses. This may result in aneurysm rupture or intracranial hemorrhage in patients with a history of cerebrovascular diseases [4, 5]. The force exerted by the laryngoscope is measured in units of force, approximately 40N, which causes significant irritation, although not as much as the tube's entry into the trachea, which has the most substantial effect on hemodynamic changes [6]. Shribman et al. found that a 10-second laryngoscopy produced hemodynamic stress responses and plasma catecholamine levels similar to those seen with laryngoscopy followed by tracheal intubation [5]. Conversely, Bucx et al. reported that a laryngoscopy lasting only 3 seconds resulted in fewer hemodynamic changes than one followed by endotracheal intubation [7].
These responses begin within 5 seconds, peak within 1-2 minutes, and return to baseline within 5 minutes [8]. Heart rate (HR) and blood pressure increases are usually transient, unpredictable, and variable. The average increase in HR has been reported to be 23bpm, and the average increase in blood pressure has been reported as 53-54mmHg [9, 10].
To prevent excessive hyperdynamic responses to endotracheal intubation, many anesthesiologists concur that it is advisable to apply the lowest possible force to the patient’s larynx when using a laryngoscope [5, 11, 12]. The mechanisms underlying these hemodynamic responses are not fully understood, but they are thought to be due to a reflex sympathetic discharge triggered by stimulation of the upper respiratory tract. This theory is supported by previous observations that hemodynamic responses to endotracheal intubation are associated with increased plasma catecholamine levels [5, 13]. The sympathetic innervation of the heart, originating from T1 to T4 in the spinal cord, contrasts with the vascular system's innervation from T1 to L2 and the adrenal medulla's from T3 to L3 [14, 15]. The cardiovascular response to intubation is eliminated in patients undergoing total thoracolumbar anesthesia. In contrast, blocking sympathetic outflow with epidural anesthesia, either cervicothoracic without affecting the adrenal gland or lumbar without affecting the heart, does not alter the cardiovascular response to tracheal intubation [16, 17].
A confirmed correlation exists between the increase in mean arterial pressure following intubation and elevated plasma norepinephrine levels [13]. The rise in plasma norepinephrine likely indicates the extent of its release from the adrenal gland and adrenergic nerve endings, particularly the latter, in response to sympathetic stimulation [18]. In hypertensive patients, elevated catecholamine levels and increased peripheral vascular sensitivity to these catecholamines result in an exaggerated hemodynamic response compared to normotensive patients [19-22].
Over the years, researchers have developed various methods to attenuate the pressure response caused by laryngoscopy and tracheal intubation [23]. These methods include antihypertensive drugs such as β-blockers, hydralazine, nitroglycerine, and sodium nitroprusside. While some may be effective when administered orally or preoperatively (e.g., β-blockers), they can cause bradycardia. Other options include benzodiazepines, deep inhalational anesthesia, IV lidocaine at 1-2mg/kg, and opioids such as Fentanyl (6-8µg/kg), Alfentanil (30-50µg/kg), or Sufentanil (0.5-1.0µg/kg) administered 1-2 minutes before intubation.
Inserting a laryngeal mask airway typically results in fewer hemodynamic changes [24]. If not blunted or attenuated, the hemodynamic response in patients with hypertension, ischemic heart disease, aneurysmal vascular disease, and elevated intracranial pressure may lead to disastrous outcomes such as myocardial ischemia and infarction, stroke, or ruptured aneurysm [25, 26]. Cardiac arrhythmias, especially ventricular bigeminy, sometimes occur and may indicate light anesthesia [2].
Lidocaine hydrochloride, an amide-type local anesthetic introduced in 1947, revolutionized regional anesthesia due to its higher safety profile compared to earlier agents. It quickly became a standard against which other local anesthetics are compared. Approximately 95% of the injected dose undergoes hepatic metabolism and is excreted by the kidneys. The onset of action for a 1% solution is typically about 1 hour, which increases to about 1.5-2 hours when the solution is mixed with adrenaline [2].
Lidocaine's site of action is on the sodium ion channels, specifically on the internal surface of nerve cell membranes. It diffuses in its uncharged form through neural sheaths into the axoplasm, where it ionizes by binding with hydrogen ions. The resulting cation binds reversibly to the sodium channels from the inside, locking them in the open state and preventing nerve depolarization. As lidocaine is a weak base with a dissociation constant (pKa) of 7.7, approximately 25% of the molecules are un-ionized at a physiological pH of 7.35-7.45, allowing them to cross into the nerve cells. This property means lidocaine with a higher pKa has a more rapid onset of action than other local anesthetics [27]. As a local anesthetic, lidocaine is often mixed with adrenaline to counteract the local vasodilation it induces. Its intravenous administration is used to diminish increases in intracranial pressure associated with laryngoscopy, reduce potassium rise and muscular pain after succinylcholine administration, provide analgesia (despite its low therapeutic ratio), depress laryngeal and tracheal reflexes during tracheal intubation or extubation, treat ventricular tachyarrhythmias as a class I antiarrhythmic drug, and manage neuropathic pain [28]. Lidocaine is available in various formulations: 0.25-0.5% solutions for intravenous regional and infiltration anesthesia, 1-2% solutions for epidural anesthesia and nerve blocks, 4% solution for topical anesthesia (used on the mucosa of the respiratory tract, pharynx, and mouth), 1-2% gel for urethral instillation, and 5% ointment for use on the skin, rectum, and other mucous membranes [28].
Lidocaine is administered at 1-2mg/kg IV 2-5 minutes before intubation or extubation, or initially at 1mg/kg IV for ventricular arrhythmias followed by 4mg/kg/min for 30 minutes, 2mg/kg/min for 2 hours, and then 1mg/kg/min thereafter. The maximum recommended dose is 3mg/kg without adrenaline and 7mg/kg with adrenaline. Its toxic plasma level is >10µg/ml [28]. Lidocaine is considered more directly neurotoxic than other local anesthetics, as it has a higher incidence of transient radicular irritation syndrome following spinal injection compared to other drugs. Lidocaine is contraindicated in patients with known severe adverse reactions. Although rare, an anaphylactic reaction to lidocaine is possible, and methemoglobinemia can occur due to its metabolism of O-toluidine [6].
Lidocaine has been used both topically and intravenously to attenuate the hemodynamic response to laryngoscopy and endotracheal intubation. Intravenous lidocaine, known for its anti-arrhythmic and centrally depressant effects, has been found effective in minimizing hemodynamic responses; however, the effectiveness of topical lidocaine remains controversial [29-31]. The pharyngeal nerves can be anesthetized by inhaling nebulized lidocaine. The glossopharyngeal nerve innervates the oropharynx, soft palate, posterior part of the tongue, and the pharyngeal surface of the epiglottis [32]. Lidocaine, when applied topically to the larynx and trachea, remains a popular method used either alone or in combination with other techniques [31]. However, its rate and extent of absorption following topical application depend on the concentration, dose administered, site of action, and exposure time [29].
Inhalation of lidocaine leads to a peak mean plasma concentration of only 1.1±0.5μg/ml, far below the presumed toxic threshold of 5μg/ml [33-36]. Using a nebulizer for lidocaine administration appears to be a safe method as it produces low serum levels and a reduced occurrence of adverse effects compared to spray or gel formulations [37]. However, convulsions after nebulization of lidocaine have been reported as a symptom of local anesthetic toxicity [38, 39]. Systemic absorption may be rapid, and maximum safe doses should not be exceeded [2]. At plasma levels greater than 5μg/mL, signs and symptoms of mild toxicity, such as slurred speech, tinnitus, circumoral paresthesia, and feeling faint, become apparent. Above 10μg/mL, seizures or loss of consciousness may occur. At 15μg/mL, depression of the myocardium and central nervous system intensifies. At levels above 20μg/mL, cardiac arrhythmias, respiratory arrest, and cardiac arrest can occur [40].
The primary aim of this study was to evaluate and compare the effects of nebulized and intravenous lidocaine on the hemodynamic changes that typically accompany laryngoscopy and endotracheal intubation to determine the most effective method for preventing potential complications.
Materials and Methods
This randomized clinical trial was conducted on 78 patients from both sexes aged 18 to 50 who were admitted for emergency open appendectomy requiring general anesthesia with endotracheal intubation in the emergency department of Al-Sadder Teaching Hospital in Al-Najaf Province, Iraq, from April to September 2022. According to the American Society of Anesthesiologists, the patients were classified under physical status I and II. Following approval from the local ethics committee, informed consent was obtained from all participants before the operation. The inclusion criteria were age between 18 and 50 years, patients of both genders classified as ASA physical status I and II, and undergoing operations under general anesthesia with endotracheal intubation. Exclusion criteria were patient refusal, known allergy to the study drug or any administered drugs, anticipated difficult airway, history of difficult intubation or intubation time of 15 seconds or longer, preexisting hypertension, and patients on beta blockers.
Collected data included name, age, gender, weight, medical history, history of previous surgery, smoking history, drug allergy history, ASA physical status, blood pressure, pulse rate, and SpO2%. All patients underwent a detailed pre-anesthesia check, including history taking, clinical examination, and measurement of vital signs. All patients fasted for at least 8 hours. The anesthetic and surgical team prepared the operating room, equipment, and medications. Patients were seated on the operating table, and an intravenous line, pulse oximetry probe, and non-invasive blood pressure cuff were placed and secured. Baseline HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were measured.
After applying inclusion and exclusion criteria, 78 patients were included and divided into three groups of 26 each:
- The control group received 5ml of normal saline IV and the induction drugs.
- The IVL group received 1.5mg/kg of 2% IV lidocaine two minutes before intubation.
- The NL group received 4ml of nebulized lidocaine administered through a cirrus nebulizer with a face mask at an O2 flow rate of 5L/min. Nebulization continued until the drug was fully utilized (approximately 10-15 minutes), after which anesthesia induction was immediately initiated.
Rapid sequence induction of anesthesia was performed using IV ketamine (1mg/kg), IV propofol (2mg/kg), and rocuronium (0.9mg/kg). Volume-controlled ventilation with an O2 flow rate of 6L/min was initiated after apnea was observed. Intubation was then performed using direct laryngoscopy with an appropriately sized cuffed endotracheal tube. The entire airway instrumentation procedure was designed to take less than 15 seconds. SBP, DBP, MAP, and HR were measured before and immediately after induction, as well as at 1, 3, 5, and 7 minutes post-intubation. Maintenance of anesthesia was achieved with 1.2% MAC isoflurane in O2. The ventilator settings were a tidal volume of 8-10ml/kg, a frequency of 12 breaths per minute, a PEEP of 5 cm H2O, and an I:E ratio of 1:2.
Data were analyzed using SPSS 26 software. by analysis of variance (ANOVA), followed by the post hoc LSD test for multiple comparisons. A linear graph was used to compare means across study times. A p-value of 0.05 or less was considered significant.
Findings
The mean age of the Control (27.6±7.8 years), IV Lidocaine (25.0±7.6 years), and Nebulized Lidocaine (27.1±8.5 years) groups was not significantly different (p=0.4). There were no significant differences among the three groups in demographic data (Table 1).
Table 1. Comparison of the frequency of the Control, IV Lidocaine (IVL), and Nebulized Lidocaine (NL) groups (each n=26) according to demographic data

The maximum increase in heart rate was observed in the C group, followed by the IVL group, with the NL group showing a minimal increase post-intubation. The first comparison between the C and IVL groups shows a significant difference (p=0.04) only at 1 minute post-intubation. Comparing the C and NL groups, significant differences were noted at 1- and 3 minutes post-intubation (Table 2).
Table 2. Comparison of mean heart rate between the Control (C), Intravenous Lidocaine (IVL), and Nebulized Lidocaine (NL) groups at different times

There was a significant difference between the C and IVL systolic blood pressure at 1- and 3 minutes post-intubation. At the same time, there were significant differences between the C and NL groups at 1-, 3-, 5-, and 7 minutes after intubation, with the lidocaine nebulizer offering an advantage in attenuating systolic blood pressure. However, when comparing the IVL and NL groups, no significant differences were found in any of the readings (Table 3).
Table 3. Comparison of mean systolic blood pressure between the Control (C), Intravenous Lidocaine (IVL), and Nebulized Lidocaine (NL) groups at different times

There was no significant difference between the C and IVL diastolic blood pressure, as both showed an increase in diastolic blood pressure post-intubation. However, significant differences were found between the C and NL groups at 1- and 3 minutes post-intubation, with NL reducing diastolic blood pressure in response to laryngoscopy and intubation. No notable differences were observed between the NL and IVL groups (Table 4).
Table 4. Comparison of mean diastolic blood pressure between the Control (C), Intravenous Lidocaine (IVL), and Nebulized Lidocaine (NL) groups at different times

There was a significant difference between the C and IVL mean arterial pressure 1 minute post-intubation, but significant differences between the C and NL groups were noted at 1- and 3 minutes post-intubation (Table 5).
Table 5. Comparison of mean arterial pressure between the Control (C), Intravenous Lidocaine (IVL), and Nebulized Lidocaine (NL) groups at different times
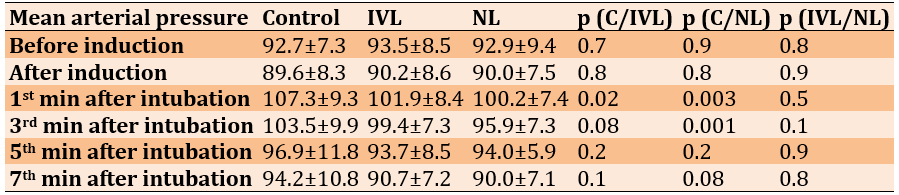
Overall, the attenuation of HR, SBP, DBP, and MAP was greater in the NL group compared to the Control group at the 1st and 3rd minutes post-intubation. No arrhythmias of any type were noted in the studied cases.
Discussion
This study evaluated the effectiveness of two methods of administering lidocaine in attenuating the hemodynamic response to laryngoscopy and endotracheal intubation. Airway instrumentation using a laryngoscope and endotracheal tube insertion can elicit a significant sympatho-adrenal response. While this transient response may have limited consequences for healthy individuals, it could pose risks to patients with hypertension due to their labile cardiovascular systems [20, 41]. However, Forbes and Dally reported a case of acute ischemia in a previously healthy, normotensive male patient whose blood pressure reached 190/130mmHg [42], indicating the importance of suppressing hypertensive responses to airway manipulation as a crucial prerequisite for proper general anesthesia.
Lidocaine has been used both topically and intravenously to blunt the pressure response. The effectiveness of topical lidocaine in reducing this response to laryngoscopy and endotracheal intubation has been controversial. In this study, the effect of nebulized lidocaine was significantly beneficial compared to the control group in attenuating tachycardia and hypertension. Several studies support the findings of this research. Venus et al. reported that the pressor response and tachycardia associated with airway instrumentation were successfully abolished by using 240mg of nebulized lidocaine, resulting in greater cardiovascular stability. This technique of anesthetizing the upper airways before intubation may be considered [43]. Additionally, Pelton et al. mentioned that the topical application of lidocaine on the tracheobronchial mucosa, as an adjunct to general anesthesia, effectively abolishes laryngeal reflexes [44]. Both Shehata et al. and Sklar et al. found that nebulized lidocaine was effective in attenuating the hemodynamic response to intubation, suggesting that the attenuating effects of a lidocaine nebulizer could be due to its direct local anesthetic action [45, 46].
However, Kumar et al. reported that nebulization with lidocaine did not show any additional benefits in attenuating the hemodynamic response. Thus, nebulized lidocaine should not be used alone for this purpose. This discrepancy could be attributed to the duration of laryngoscopy with endotracheal intubation included in his study, which allowed a maximum time of less than 30 seconds [47]. However, it is ideally recommended that the procedure take less than 15 seconds to alleviate hypertension and tachycardia, as advised by Stoelting et al. in their study [48].
The systemic absorption of lidocaine after nebulization is unpredictable. Consequently, when topical administration involves continuous nebulization, the estimated loss of nebulized lidocaine will likely exceed 50%. This loss is due to mist escaping around the patient's mouth during exhaling and breath-holding, resulting in more than 50% of nebulized lidocaine being lost [49].
When comparing the efficacy of lidocaine nebulization to intravenous administration for attenuating hemodynamic responses, nebulization might be more effective. This difference could be attributed to intravenous administration increasing the depth of anesthesia. According to Himes et al. [50], while intravenous use deepens anesthesia, local application through nebulization serves as a local anesthetic. It enhances anesthesia depth due to systemic absorption through the airway mucosa [31]. The absorption of lidocaine depends on the dose concentration, site of action, and exposure time [29]. Plasma lidocaine concentration reaches approximately 2.7μg/ml after topical aerosol application at a dosage of 3mg/kg [44].
In addition, Ahmed and Haider compared sprayed and inhaled nebulized lidocaine for suppression of hemodynamic response to laryngoscopy and oral endotracheal intubation and reported a statistically significant difference (p<0.05) between nebulized and sprayed lidocaine in HR, SBP, DBP, and MAP at different time points after tracheal intubation, with nebulized lidocaine being most effective and better toleration [51].
Furthermore, the timing of intravenous lidocaine administration before intubation also affects cardiovascular stability. Tarn et al. reported that 1.5 mg/kg of intravenous lidocaine administered 3 minutes before intubation significantly attenuates hemodynamic changes. However, when administered 2 minutes before intubation—the same timing and dosage used in our study—it does not offer significant protection against hypertension and tachycardia [30]. Thus, timing can significantly influence the differences between intravenous lidocaine and nebulized lidocaine.
Further research should evaluate the effects of lidocaine nebulization and correlate them with plasma lidocaine levels. It would be beneficial to target a hypertensive group of patients requiring general anesthesia using the same or different doses.
Conclusion
Nebulized lidocaine is more effective than intravenous lidocaine in attenuating hypertension and tachycardia associated with laryngoscopy and endotracheal intubation.
Acknowledgments: None declared by the authors.
Ethical Permissions: This study is approve by Iraqi board for medical specialization with the ethical code of 452.
Conflicts of Interests: There were no conflicts.
Authors’ Contribution: Saeed ZM (First Author), Introduction Writer/Methodologist/Main Researcher (40%); Jaafar H (Second Author), Main Researcher/Discussion Writer (30%); Alburkiebh SHA (Third Author), Main Researcher/Statistical Analyst (30%)
Funding/Support: None declared by the authors.
Direct laryngoscopy is the most commonly used technique for endotracheal intubation, as it allows direct visualization of the vocal cords [1]. This technique was pioneered by Kirstein, Killian, and Jackson in the late 1800s and early 1900s and is now the predominant method for endotracheal intubation [2]. Preparation for direct laryngoscopy includes proper patient positioning, preoxygenation, and ensuring the equipment functions correctly. The presence of a skilled assistant is crucial for a successful outcome. Thorough and precise preparation is vital, as the initial attempt at securing the airway should be optimal. Successful direct laryngoscopy requires a straight line of sight from the mouth to the larynx, aligning the oral, oropharyngeal, hypopharyngeal, and laryngeal axes. Positioning the patient in the sniffing position helps achieve this alignment, and optimal head elevation is achieved by aligning a straight horizontal line from the external auditory meatus to the sternal notch. The laryngoscope is a handheld instrument with a blade attached to a handle that includes a light source. There are two types of laryngoscope blades: the curved blade (with Macintosh being the most popular) and the straight blade (with Miller being the most popular). Each type has specific techniques, advantages, and disadvantages [1].
Laryngoscopy involves inserting the blade through the mouth, lifting the tongue, positioning the blade tip with a lifting force to expose the glottis area, and then inserting the endotracheal tube through the vocal cords into the trachea. Endotracheal intubation is the gold standard procedure for airway management. A laryngoscope typically facilitates it, which helps establish a definitive airway, offers maximum protection against gastric acid aspiration, and facilitates positive pressure ventilation with higher airway pressures than a supraglottic airway or face mask [1]. The endotracheal tube is inserted through the vocal cords into the trachea under continuous visualization [1].
Tracheal intubation was initially described by Kite in 1788 for resuscitation and for laryngeal obstruction. Macewen first advocated using tracheal intubation instead of tracheostomy for anesthesia during head and neck surgery in 1880. O'Dwyer described an intubating tube in 1885. Modern endotracheal anesthesia was further developed by Magill and Rowbotham after World War I [2].
The modern, standard endotracheal tube is a disposable, single-use, cuffed, plastic tube designed to be inserted through the nose or mouth, positioned with its distal end in the mid-trachea to maintain a patent airway for lung ventilation. Various endotracheal tubes are available for specific situations. Common features across these models include a universal 15mm adapter for attaching the proximal end to different ventilating circuits and devices, a high-volume, low-pressure cuff, a beveled tip to ease passage through the vocal cords, and a Murphy eye—a distal opening on the tube's side wall that provides an additional ventilation port if the tube's end becomes obstructed by secretions or soft tissue. In most patients, a cuffed endotracheal tube is used for routine intubation; however, a cuffless tube may be used in neonates and infants. This low-pressure, high-volume cuff is inflated with air to ensure that tidal volume ventilates the lungs without escaping upward into the upper airways and to prevent aspiration of gastric contents into the lungs [1]. Securing the airway with intubation induces hemodynamic responses such as tachycardia, hypertension, and dysrhythmias due to sympatho-adrenal stimulation. These responses are more severe and pronounced in hypertensive patients than in normotensive ones [3].
The force applied by the laryngoscope to lift the epiglottis during endotracheal intubation, coupled with the irritation caused by the tube entering the trachea and subsequent cuff expansion, can lead to cardiovascular responses. This may result in aneurysm rupture or intracranial hemorrhage in patients with a history of cerebrovascular diseases [4, 5]. The force exerted by the laryngoscope is measured in units of force, approximately 40N, which causes significant irritation, although not as much as the tube's entry into the trachea, which has the most substantial effect on hemodynamic changes [6]. Shribman et al. found that a 10-second laryngoscopy produced hemodynamic stress responses and plasma catecholamine levels similar to those seen with laryngoscopy followed by tracheal intubation [5]. Conversely, Bucx et al. reported that a laryngoscopy lasting only 3 seconds resulted in fewer hemodynamic changes than one followed by endotracheal intubation [7].
These responses begin within 5 seconds, peak within 1-2 minutes, and return to baseline within 5 minutes [8]. Heart rate (HR) and blood pressure increases are usually transient, unpredictable, and variable. The average increase in HR has been reported to be 23bpm, and the average increase in blood pressure has been reported as 53-54mmHg [9, 10].
To prevent excessive hyperdynamic responses to endotracheal intubation, many anesthesiologists concur that it is advisable to apply the lowest possible force to the patient’s larynx when using a laryngoscope [5, 11, 12]. The mechanisms underlying these hemodynamic responses are not fully understood, but they are thought to be due to a reflex sympathetic discharge triggered by stimulation of the upper respiratory tract. This theory is supported by previous observations that hemodynamic responses to endotracheal intubation are associated with increased plasma catecholamine levels [5, 13]. The sympathetic innervation of the heart, originating from T1 to T4 in the spinal cord, contrasts with the vascular system's innervation from T1 to L2 and the adrenal medulla's from T3 to L3 [14, 15]. The cardiovascular response to intubation is eliminated in patients undergoing total thoracolumbar anesthesia. In contrast, blocking sympathetic outflow with epidural anesthesia, either cervicothoracic without affecting the adrenal gland or lumbar without affecting the heart, does not alter the cardiovascular response to tracheal intubation [16, 17].
A confirmed correlation exists between the increase in mean arterial pressure following intubation and elevated plasma norepinephrine levels [13]. The rise in plasma norepinephrine likely indicates the extent of its release from the adrenal gland and adrenergic nerve endings, particularly the latter, in response to sympathetic stimulation [18]. In hypertensive patients, elevated catecholamine levels and increased peripheral vascular sensitivity to these catecholamines result in an exaggerated hemodynamic response compared to normotensive patients [19-22].
Over the years, researchers have developed various methods to attenuate the pressure response caused by laryngoscopy and tracheal intubation [23]. These methods include antihypertensive drugs such as β-blockers, hydralazine, nitroglycerine, and sodium nitroprusside. While some may be effective when administered orally or preoperatively (e.g., β-blockers), they can cause bradycardia. Other options include benzodiazepines, deep inhalational anesthesia, IV lidocaine at 1-2mg/kg, and opioids such as Fentanyl (6-8µg/kg), Alfentanil (30-50µg/kg), or Sufentanil (0.5-1.0µg/kg) administered 1-2 minutes before intubation.
Inserting a laryngeal mask airway typically results in fewer hemodynamic changes [24]. If not blunted or attenuated, the hemodynamic response in patients with hypertension, ischemic heart disease, aneurysmal vascular disease, and elevated intracranial pressure may lead to disastrous outcomes such as myocardial ischemia and infarction, stroke, or ruptured aneurysm [25, 26]. Cardiac arrhythmias, especially ventricular bigeminy, sometimes occur and may indicate light anesthesia [2].
Lidocaine hydrochloride, an amide-type local anesthetic introduced in 1947, revolutionized regional anesthesia due to its higher safety profile compared to earlier agents. It quickly became a standard against which other local anesthetics are compared. Approximately 95% of the injected dose undergoes hepatic metabolism and is excreted by the kidneys. The onset of action for a 1% solution is typically about 1 hour, which increases to about 1.5-2 hours when the solution is mixed with adrenaline [2].
Lidocaine's site of action is on the sodium ion channels, specifically on the internal surface of nerve cell membranes. It diffuses in its uncharged form through neural sheaths into the axoplasm, where it ionizes by binding with hydrogen ions. The resulting cation binds reversibly to the sodium channels from the inside, locking them in the open state and preventing nerve depolarization. As lidocaine is a weak base with a dissociation constant (pKa) of 7.7, approximately 25% of the molecules are un-ionized at a physiological pH of 7.35-7.45, allowing them to cross into the nerve cells. This property means lidocaine with a higher pKa has a more rapid onset of action than other local anesthetics [27]. As a local anesthetic, lidocaine is often mixed with adrenaline to counteract the local vasodilation it induces. Its intravenous administration is used to diminish increases in intracranial pressure associated with laryngoscopy, reduce potassium rise and muscular pain after succinylcholine administration, provide analgesia (despite its low therapeutic ratio), depress laryngeal and tracheal reflexes during tracheal intubation or extubation, treat ventricular tachyarrhythmias as a class I antiarrhythmic drug, and manage neuropathic pain [28]. Lidocaine is available in various formulations: 0.25-0.5% solutions for intravenous regional and infiltration anesthesia, 1-2% solutions for epidural anesthesia and nerve blocks, 4% solution for topical anesthesia (used on the mucosa of the respiratory tract, pharynx, and mouth), 1-2% gel for urethral instillation, and 5% ointment for use on the skin, rectum, and other mucous membranes [28].
Lidocaine is administered at 1-2mg/kg IV 2-5 minutes before intubation or extubation, or initially at 1mg/kg IV for ventricular arrhythmias followed by 4mg/kg/min for 30 minutes, 2mg/kg/min for 2 hours, and then 1mg/kg/min thereafter. The maximum recommended dose is 3mg/kg without adrenaline and 7mg/kg with adrenaline. Its toxic plasma level is >10µg/ml [28]. Lidocaine is considered more directly neurotoxic than other local anesthetics, as it has a higher incidence of transient radicular irritation syndrome following spinal injection compared to other drugs. Lidocaine is contraindicated in patients with known severe adverse reactions. Although rare, an anaphylactic reaction to lidocaine is possible, and methemoglobinemia can occur due to its metabolism of O-toluidine [6].
Lidocaine has been used both topically and intravenously to attenuate the hemodynamic response to laryngoscopy and endotracheal intubation. Intravenous lidocaine, known for its anti-arrhythmic and centrally depressant effects, has been found effective in minimizing hemodynamic responses; however, the effectiveness of topical lidocaine remains controversial [29-31]. The pharyngeal nerves can be anesthetized by inhaling nebulized lidocaine. The glossopharyngeal nerve innervates the oropharynx, soft palate, posterior part of the tongue, and the pharyngeal surface of the epiglottis [32]. Lidocaine, when applied topically to the larynx and trachea, remains a popular method used either alone or in combination with other techniques [31]. However, its rate and extent of absorption following topical application depend on the concentration, dose administered, site of action, and exposure time [29].
Inhalation of lidocaine leads to a peak mean plasma concentration of only 1.1±0.5μg/ml, far below the presumed toxic threshold of 5μg/ml [33-36]. Using a nebulizer for lidocaine administration appears to be a safe method as it produces low serum levels and a reduced occurrence of adverse effects compared to spray or gel formulations [37]. However, convulsions after nebulization of lidocaine have been reported as a symptom of local anesthetic toxicity [38, 39]. Systemic absorption may be rapid, and maximum safe doses should not be exceeded [2]. At plasma levels greater than 5μg/mL, signs and symptoms of mild toxicity, such as slurred speech, tinnitus, circumoral paresthesia, and feeling faint, become apparent. Above 10μg/mL, seizures or loss of consciousness may occur. At 15μg/mL, depression of the myocardium and central nervous system intensifies. At levels above 20μg/mL, cardiac arrhythmias, respiratory arrest, and cardiac arrest can occur [40].
The primary aim of this study was to evaluate and compare the effects of nebulized and intravenous lidocaine on the hemodynamic changes that typically accompany laryngoscopy and endotracheal intubation to determine the most effective method for preventing potential complications.
Materials and Methods
This randomized clinical trial was conducted on 78 patients from both sexes aged 18 to 50 who were admitted for emergency open appendectomy requiring general anesthesia with endotracheal intubation in the emergency department of Al-Sadder Teaching Hospital in Al-Najaf Province, Iraq, from April to September 2022. According to the American Society of Anesthesiologists, the patients were classified under physical status I and II. Following approval from the local ethics committee, informed consent was obtained from all participants before the operation. The inclusion criteria were age between 18 and 50 years, patients of both genders classified as ASA physical status I and II, and undergoing operations under general anesthesia with endotracheal intubation. Exclusion criteria were patient refusal, known allergy to the study drug or any administered drugs, anticipated difficult airway, history of difficult intubation or intubation time of 15 seconds or longer, preexisting hypertension, and patients on beta blockers.
Collected data included name, age, gender, weight, medical history, history of previous surgery, smoking history, drug allergy history, ASA physical status, blood pressure, pulse rate, and SpO2%. All patients underwent a detailed pre-anesthesia check, including history taking, clinical examination, and measurement of vital signs. All patients fasted for at least 8 hours. The anesthetic and surgical team prepared the operating room, equipment, and medications. Patients were seated on the operating table, and an intravenous line, pulse oximetry probe, and non-invasive blood pressure cuff were placed and secured. Baseline HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were measured.
After applying inclusion and exclusion criteria, 78 patients were included and divided into three groups of 26 each:
- The control group received 5ml of normal saline IV and the induction drugs.
- The IVL group received 1.5mg/kg of 2% IV lidocaine two minutes before intubation.
- The NL group received 4ml of nebulized lidocaine administered through a cirrus nebulizer with a face mask at an O2 flow rate of 5L/min. Nebulization continued until the drug was fully utilized (approximately 10-15 minutes), after which anesthesia induction was immediately initiated.
Rapid sequence induction of anesthesia was performed using IV ketamine (1mg/kg), IV propofol (2mg/kg), and rocuronium (0.9mg/kg). Volume-controlled ventilation with an O2 flow rate of 6L/min was initiated after apnea was observed. Intubation was then performed using direct laryngoscopy with an appropriately sized cuffed endotracheal tube. The entire airway instrumentation procedure was designed to take less than 15 seconds. SBP, DBP, MAP, and HR were measured before and immediately after induction, as well as at 1, 3, 5, and 7 minutes post-intubation. Maintenance of anesthesia was achieved with 1.2% MAC isoflurane in O2. The ventilator settings were a tidal volume of 8-10ml/kg, a frequency of 12 breaths per minute, a PEEP of 5 cm H2O, and an I:E ratio of 1:2.
Data were analyzed using SPSS 26 software. by analysis of variance (ANOVA), followed by the post hoc LSD test for multiple comparisons. A linear graph was used to compare means across study times. A p-value of 0.05 or less was considered significant.
Findings
The mean age of the Control (27.6±7.8 years), IV Lidocaine (25.0±7.6 years), and Nebulized Lidocaine (27.1±8.5 years) groups was not significantly different (p=0.4). There were no significant differences among the three groups in demographic data (Table 1).
Table 1. Comparison of the frequency of the Control, IV Lidocaine (IVL), and Nebulized Lidocaine (NL) groups (each n=26) according to demographic data

The maximum increase in heart rate was observed in the C group, followed by the IVL group, with the NL group showing a minimal increase post-intubation. The first comparison between the C and IVL groups shows a significant difference (p=0.04) only at 1 minute post-intubation. Comparing the C and NL groups, significant differences were noted at 1- and 3 minutes post-intubation (Table 2).
Table 2. Comparison of mean heart rate between the Control (C), Intravenous Lidocaine (IVL), and Nebulized Lidocaine (NL) groups at different times

There was a significant difference between the C and IVL systolic blood pressure at 1- and 3 minutes post-intubation. At the same time, there were significant differences between the C and NL groups at 1-, 3-, 5-, and 7 minutes after intubation, with the lidocaine nebulizer offering an advantage in attenuating systolic blood pressure. However, when comparing the IVL and NL groups, no significant differences were found in any of the readings (Table 3).
Table 3. Comparison of mean systolic blood pressure between the Control (C), Intravenous Lidocaine (IVL), and Nebulized Lidocaine (NL) groups at different times

There was no significant difference between the C and IVL diastolic blood pressure, as both showed an increase in diastolic blood pressure post-intubation. However, significant differences were found between the C and NL groups at 1- and 3 minutes post-intubation, with NL reducing diastolic blood pressure in response to laryngoscopy and intubation. No notable differences were observed between the NL and IVL groups (Table 4).
Table 4. Comparison of mean diastolic blood pressure between the Control (C), Intravenous Lidocaine (IVL), and Nebulized Lidocaine (NL) groups at different times

There was a significant difference between the C and IVL mean arterial pressure 1 minute post-intubation, but significant differences between the C and NL groups were noted at 1- and 3 minutes post-intubation (Table 5).
Table 5. Comparison of mean arterial pressure between the Control (C), Intravenous Lidocaine (IVL), and Nebulized Lidocaine (NL) groups at different times

Overall, the attenuation of HR, SBP, DBP, and MAP was greater in the NL group compared to the Control group at the 1st and 3rd minutes post-intubation. No arrhythmias of any type were noted in the studied cases.
Discussion
This study evaluated the effectiveness of two methods of administering lidocaine in attenuating the hemodynamic response to laryngoscopy and endotracheal intubation. Airway instrumentation using a laryngoscope and endotracheal tube insertion can elicit a significant sympatho-adrenal response. While this transient response may have limited consequences for healthy individuals, it could pose risks to patients with hypertension due to their labile cardiovascular systems [20, 41]. However, Forbes and Dally reported a case of acute ischemia in a previously healthy, normotensive male patient whose blood pressure reached 190/130mmHg [42], indicating the importance of suppressing hypertensive responses to airway manipulation as a crucial prerequisite for proper general anesthesia.
Lidocaine has been used both topically and intravenously to blunt the pressure response. The effectiveness of topical lidocaine in reducing this response to laryngoscopy and endotracheal intubation has been controversial. In this study, the effect of nebulized lidocaine was significantly beneficial compared to the control group in attenuating tachycardia and hypertension. Several studies support the findings of this research. Venus et al. reported that the pressor response and tachycardia associated with airway instrumentation were successfully abolished by using 240mg of nebulized lidocaine, resulting in greater cardiovascular stability. This technique of anesthetizing the upper airways before intubation may be considered [43]. Additionally, Pelton et al. mentioned that the topical application of lidocaine on the tracheobronchial mucosa, as an adjunct to general anesthesia, effectively abolishes laryngeal reflexes [44]. Both Shehata et al. and Sklar et al. found that nebulized lidocaine was effective in attenuating the hemodynamic response to intubation, suggesting that the attenuating effects of a lidocaine nebulizer could be due to its direct local anesthetic action [45, 46].
However, Kumar et al. reported that nebulization with lidocaine did not show any additional benefits in attenuating the hemodynamic response. Thus, nebulized lidocaine should not be used alone for this purpose. This discrepancy could be attributed to the duration of laryngoscopy with endotracheal intubation included in his study, which allowed a maximum time of less than 30 seconds [47]. However, it is ideally recommended that the procedure take less than 15 seconds to alleviate hypertension and tachycardia, as advised by Stoelting et al. in their study [48].
The systemic absorption of lidocaine after nebulization is unpredictable. Consequently, when topical administration involves continuous nebulization, the estimated loss of nebulized lidocaine will likely exceed 50%. This loss is due to mist escaping around the patient's mouth during exhaling and breath-holding, resulting in more than 50% of nebulized lidocaine being lost [49].
When comparing the efficacy of lidocaine nebulization to intravenous administration for attenuating hemodynamic responses, nebulization might be more effective. This difference could be attributed to intravenous administration increasing the depth of anesthesia. According to Himes et al. [50], while intravenous use deepens anesthesia, local application through nebulization serves as a local anesthetic. It enhances anesthesia depth due to systemic absorption through the airway mucosa [31]. The absorption of lidocaine depends on the dose concentration, site of action, and exposure time [29]. Plasma lidocaine concentration reaches approximately 2.7μg/ml after topical aerosol application at a dosage of 3mg/kg [44].
In addition, Ahmed and Haider compared sprayed and inhaled nebulized lidocaine for suppression of hemodynamic response to laryngoscopy and oral endotracheal intubation and reported a statistically significant difference (p<0.05) between nebulized and sprayed lidocaine in HR, SBP, DBP, and MAP at different time points after tracheal intubation, with nebulized lidocaine being most effective and better toleration [51].
Furthermore, the timing of intravenous lidocaine administration before intubation also affects cardiovascular stability. Tarn et al. reported that 1.5 mg/kg of intravenous lidocaine administered 3 minutes before intubation significantly attenuates hemodynamic changes. However, when administered 2 minutes before intubation—the same timing and dosage used in our study—it does not offer significant protection against hypertension and tachycardia [30]. Thus, timing can significantly influence the differences between intravenous lidocaine and nebulized lidocaine.
Further research should evaluate the effects of lidocaine nebulization and correlate them with plasma lidocaine levels. It would be beneficial to target a hypertensive group of patients requiring general anesthesia using the same or different doses.
Conclusion
Nebulized lidocaine is more effective than intravenous lidocaine in attenuating hypertension and tachycardia associated with laryngoscopy and endotracheal intubation.
Acknowledgments: None declared by the authors.
Ethical Permissions: This study is approve by Iraqi board for medical specialization with the ethical code of 452.
Conflicts of Interests: There were no conflicts.
Authors’ Contribution: Saeed ZM (First Author), Introduction Writer/Methodologist/Main Researcher (40%); Jaafar H (Second Author), Main Researcher/Discussion Writer (30%); Alburkiebh SHA (Third Author), Main Researcher/Statistical Analyst (30%)
Funding/Support: None declared by the authors.
Keywords:
References
1. Gropper MA, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Cohen NA, Leslie K, editors. Miller's Anesthesia. 9th ed. Philadelphia: Elsevier; 2019. [Link]
2. Yentis S, Hirsch NP, Ip J. Anaesthesia, intensive care, and perioperative medicine A-Z: An encyclopedia of principles and practice. 6th ed. New York: Elsevier; 2018. [Link]
3. Prys-Roberts C, Foëx P, Biro GP, Roberts JG. Studies of anaesthesia in relation to hypertension V: Adrenergic beta-receptor blockade. Br J Anaesth. 1973;45(7):671-81. [Link] [DOI:10.1093/bja/45.7.671]
4. Mort TC. Complications of emergency tracheal intubation: Hemodynamic alterations-part I. J Intensive Care Med. 2007;22(3):157-65. [Link] [DOI:10.1177/0885066607299525]
5. Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59(3):295-9. [Link] [DOI:10.1093/bja/59.3.295]
6. Bucx MJL, Snijders CJ. Force, torque, and stress relaxation with direct laryngoscopy. Anesth Analg. 1996;83(5):1130. [Link] [DOI:10.1213/00000539-199611000-00048]
7. Bucx MJL, Scheck PAE, Geel RTM, Ouden AH, Niesing R. Measurement of forces during laryngoscopy. Anaesthesia. 1992;47(4):348-51. [Link] [DOI:10.1111/j.1365-2044.1992.tb02180.x]
8. Henderson J. Airway management in the adult. In: Miller RD, editor. Miller's Anesthesia. 7th ed. London: Elsevier Churchill Livingstone; 2010. p. 1573-610. [Link] [DOI:10.1016/B978-0-443-06959-8.00050-9]
9. King BD, Harris LC, Greifenstein FE, Elder JD, Dripps RD. Reflex circulatory responses to direct laryngoscopy and tracheal intubation performed during general anesthesia. Anesthesiology. 1951;12(5):556-66. [Link] [DOI:10.1097/00000542-195109000-00002]
10. Ng WS. Pathological effects of tracheal intubation. In: Latto IP, Rosen M, editors. Difficulties in tracheal intubation. London: Elsevier Health Sciences; 1985. p. 14. [Link]
11. Finfer SR, Mackenzie SI, Saddler JM, Watkins TG. Cardiovascular responses to tracheal intubation: A comparison of direct laryngoscopy and fibreoptic intubation. Anaesth Intensive Care. 1989;17(1):44-8. [Link] [DOI:10.1177/0310057X8901700110]
12. Bishop MJ, Harrington RM, Tencer AF. Force applied during tracheal intubation. Anesth Analg. 1992;74(3):411-4. [Link] [DOI:10.1213/00000539-199203000-00016]
13. Derbyshire DR, Chmielewski A, Fell D, Vater M, Achola K, Smith G. Plasma catecholamine responses to tracheal intubation. Br J Anaesth. 1983;55(9):855-60. [Link] [DOI:10.1093/bja/55.9.855]
14. Bonica JJ. Autonomic innervation of the viscera in relation to nerve block. Anesthesiology. 1968;29(4):793-813. [Link] [DOI:10.1097/00000542-196807000-00023]
15. Landsberg L, Young JB. Catecholamines and the adrenal medulla. In: Wilson JD, editor. Williams' textbook of endocrinology. Philadelphia: WB Saunders; 1998. p. 621-705. [Link]
16. Wattwil M, Sundberg A, Olsson J, Nordström S. Thoracolumbar epidural anaesthesia blocks the circulatory response to laryngoscopy and intubation. Acta Anaesthesiol Scand. 1987;31(6):529-31. [Link] [DOI:10.1111/j.1399-6576.1987.tb02616.x]
17. Dohi S, Nishikawa T, Ujike Y, Mayumi T. Circulatory responses to airway stimulation and cervical epidural blockade. Anesthesiology. 1982;57(5):359-63. [Link] [DOI:10.1097/00000542-198211000-00002]
18. Barret K, Barman S, Boitano S, Brooks H. Ganong's review of medical physiology. 25th ed. New York: McGraw-Hill; 2015. [Link]
19. Stone JG, Foëx P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Risk of myocardial ischaemia during anaesthesia in treated and untreated hypertensive patients. Br J Anaesth. 1988;61(6):675-9. [Link] [DOI:10.1093/bja/61.6.675]
20. Fujii Y, Tanaka H, Toyooka H. Circulatory responses to laryngeal mask airway insertion or tracheal intubation in normotensive and hypertensive patients. Can J Anaesth. 1995;42(1):32-6. [Link] [DOI:10.1007/BF03010568]
21. Low JM, Harvey JT, Prys-Roberts C, Dagnino J. Studies of anaesthesia in relation to hypertension. Br J Anaesth. 1986;58(5):471-7. [Link] [DOI:10.1093/bja/58.5.471]
22. Goldman L, Caldera DL. Risks of general anesthesia and elective operation in the hypertensive patient. Anesthesiology. 1979;50(4):285-92. [Link] [DOI:10.1097/00000542-197904000-00002]
23. Mohan K, Mohana RL. Attenuation of cardiovascular responses to laryngoscopy and intubation by diltiazem and lignocaine: A comparative study. Int J Med Res Health Sci. 2013;2(3):557-63. [Link] [DOI:10.5958/j.2319-5886.2.3.098]
24. Butterworth JF, Mackey DC, Wasnick JD. Morgan & Mikhail's clinical anesthesiology. 5th ed. New York: McGraw-Hill; 2013. [Link]
25. Fox EJ, Sklar GS, Hill CH, Villanueva R, King BD. Complications related to the pressor response to endotracheal intubation. Anesthesiology. 1977;47(6):524-5. [Link] [DOI:10.1097/00000542-197712000-00013]
26. Swarnamba UN, Veena K, Shaikh SI. Comparison of the efficacy of lornoxicam and fentanyl in attenuating the hemodynamic response to laryngoscopy and intubation. Anesth Essays Res. 2016;10(3):478-82. [Link] [DOI:10.4103/0259-1162.177521]
27. Tetzlaff JE. The pharmacology of local anesthetics. Anesthesiol Clin North Am. 2000;18(2):217-33. [Link] [DOI:10.1016/S0889-8537(05)70161-9]
28. Barash M, Reich KA, Rademaker D. Lidocaine-induced methemoglobinemia: A clinical reminder. J Am Osteopath Assoc. 2015;115(2):94-8. [Link] [DOI:10.7556/jaoa.2015.020]
29. Jee D, Park SY. Lidocaine sprayed down the endotracheal tube attenuates the airway-circulatory reflexes by local anesthesia during emergence and extubation. Anesth Analg. 2003;96(1):293-7. [Link] [DOI:10.1213/00000539-200301000-00058]
30. Tarn S, Chung F, Campbell M. Intravenous lidocaine: Optimal time of injection before tracheal intubation. Anesth Analg. 1987;66(10):1036-38. [Link] [DOI:10.1213/00000539-198710000-00026]
31. Abou-Madi MN, Keszler H, Yacoub JM. Cardiovascular reactions to laryngoscopy and tracheal intubation following small and large intravenous doses of lidocaine. Can Anaesth Soc J. 1977;24(1):12-9. [Link] [DOI:10.1007/BF03006808]
32. John G. Regional anesthesia. In: Dershwitz M, Walz J, editors. Anesthesiology examination and board review. 7th ed. New York: McGraw-Hill; 2013. [Link]
33. Weiss EB, Patwardhan AV. The response to lidocaine in bronchial asthma. Chest. 1977;72(4):429-38. [Link] [DOI:10.1378/chest.72.4.429]
34. Bromage PR, Robson JG. Concentrations of lidocaine in the blood after intravenous, intramuscular, epidural, and endotracheal administration. Anesthesia. 1961;16(4):461-78. [Link] [DOI:10.1111/j.1365-2044.1961.tb13426.x]
35. Viegas O, Stoelting RK. Lidocaine in arterial blood after laryngotracheal administration. Anesthesiology. 1975;43(4):491-3. [Link] [DOI:10.1097/00000542-197510000-00019]
36. Scott DB, Littlewood DG, Covino BG, Drummond GB. Plasma lignocaine concentrations following endotracheal spraying with an aerosol. Br J Anaesth. 1976;48(9):899-902. [Link] [DOI:10.1093/bja/48.9.899]
37. Chong CF, Chen CC, Ma HP, Wu YC, Chen YC, Wang TL. Comparison of lidocaine and bronchodilator inhalation treatments for cough suppression in patients with chronic obstructive pulmonary disease. Emerg Med J. 2005;22(6):429-32. [Link] [DOI:10.1136/emj.2004.015719]
38. Groeben H, Schlicht M, Stieglitz S, Pavlakovic G, Peters J. Both local anesthetics and salbutamol pretreatment affect reflex bronchoconstriction in volunteers with asthma undergoing awake fiberoptic intubation. Anesthesiology. 2002;97(6):1445-50. [Link] [DOI:10.1097/00000542-200212000-00016]
39. Efthimiou J, Higenbottam T, Holt D, Cochrane GM. Plasma concentrations of lignocaine during fibreoptic bronchoscopy. Thorax. 1982;37(1):68-71. [Link] [DOI:10.1136/thx.37.1.68]
40. Becker DE, Reed KL. Local anesthetics: Review of pharmacological considerations. Anesth Prog. 2012;59(2):90-102. [Link] [DOI:10.2344/0003-3006-59.2.90]
41. Omote K, Kirita A, Namiki A, Iwasaki H. Effects of nicardipine on the circulatory responses to tracheal intubation in normotensive and hypertensive patients. Anaesthesia. 1992;47(1):24-7. [Link] [DOI:10.1111/j.1365-2044.1992.tb01947.x]
42. Forbes AM, Dally FG. Acute hypertension during induction of anaesthesia and endotracheal intubation in normotensive man. Br J Anaesth. 1970;42(7):618-24. [Link] [DOI:10.1093/bja/42.7.618]
43. Venus B, Polassani V, Pham CG. Effects of aerosolized lidocaine on circulatory responses to laryngoscopy and tracheal intubation. Crit Care Med. 1984;12(4):391-4. [Link] [DOI:10.1097/00003246-198404000-00011]
44. Pelton DA, Daly M, Cooper PD, Conn AW. Plasma lidocaine concentrations following topical aerosol application to the trachea and bronchi. Can Anaesth Soc J. 1970;17(3):250-5. [Link] [DOI:10.1007/BF03004603]
45. Shehata A, Abd El-Hamid AM, Hasan AM, Abd El-Fattah MH. Lidocaine nebulizer reduce response to endotracheal intubation and the need for postoperative analgesia after nasal operations. J Am Sci. 2013;9(12):287-91. [Link]
46. Sklar BZ, Lurie S, Ezri T, Krichelli D, Savir I, Soroker D. Lidocaine inhalation attenuates the circulatory response to laryngoscopy and endotracheal intubation. J Clin Anesth. 1992;4(5):382-5. [Link] [DOI:10.1016/0952-8180(92)90160-3]
47. Kumar A, Seth A, Prakash S, Deganwa M, Gogia AR. Attenuation of the hemodynamic response to laryngoscopy and tracheal intubation with fentanyl, lignocaine nebulization, and a combination of both. Anesth Essays Res. 2016;10(3):661-6. [Link] [DOI:10.4103/0259-1162.191113]
48. Stoelting RK. Blood pressure and heart rate changes during short-duration laryngoscopy for tracheal intubation. Anesth Analg. 1978;57(2):197-9. [Link] [DOI:10.1213/00000539-197803000-00009]
49. Chinn WM, Zavala DC, Ambre J. Plasma levels of lidocaine following nebulized aerosol administration. Chest. 1977;71(3):346-8. [Link] [DOI:10.1378/chest.71.3.346]
50. Himes RS, Difazio CA, Burney RG. Effects of lidocaine on the anesthetic requirements for nitrous oxide and halothane. Anesthesiology. 1977;47(5):437-40. [Link] [DOI:10.1097/00000542-197711000-00010]
51. Ahmed AA, Haider HS. Comparative study between sprayed and inhaled nebulized lidocaine for suppression of hemodynamic response to laryngoscopy and oral endotracheal intubation. J Univ Shanghai Sci Technol. 2021;23(9):772. [Link] [DOI:10.51201/JUSST/21/09599]









