Volume 15, Issue 2 (2023)
Iran J War Public Health 2023, 15(2): 191-198 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/02/14 | Accepted: 2023/04/16 | Published: 2023/06/5
Received: 2023/02/14 | Accepted: 2023/04/16 | Published: 2023/06/5
How to cite this article
Al-Saadi Z, Al-Aswad F, Sheaheed N. Oral Opportunistic Bacteria in Multiple Sclerosis with Different Treatment Modalities. Iran J War Public Health 2023; 15 (2) :191-198
URL: http://ijwph.ir/article-1-1320-en.html
URL: http://ijwph.ir/article-1-1320-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Ministry of Health and Environment, Baghdad, Iraq
2- Department of Oral Medicine, College of Dentistry, Baghdad University, Baghdad, Iraq
3- Neurology Division Baghdad Teaching Hospital Medical City, Baghdad, Iraq
2- Department of Oral Medicine, College of Dentistry, Baghdad University, Baghdad, Iraq
3- Neurology Division Baghdad Teaching Hospital Medical City, Baghdad, Iraq
Full-Text (HTML) (774 Views)
Introduction
Multiple Sclerosis (MS) is a complex chronic autoimmune disorder. It is a multifactorial disease that attacks the brain and spinal cord. MS can affect any part of the body and cause a wide range of symptoms. Moreover, MS symptoms occur due to a zone of demyelination and inflammation, which can cause motor, sensory, and visual disorders and may last for days or weeks [1, 2].
The causes of MS have not been clearly identified, but it is known that the immune system plays a vital role in the progression of MS. There are many predisposing factors, such as environmental factors, vitamin D deficiency, teenage obesity, smoking, genetic factors, and Epstein–Barr Virus (EBV) [1, 3]. In histopathology, myelin damage has been detected with mononuclear phagocytes, T-lymphocytes, dendritic cell infiltration, B-lymphocytes, and plasma cells. The T helper 1 (Th1) and Th17 pathways were involved in MS pathogenesis and demyelination [4]. No single test can positively diagnose MS, i.e., the diagnostic criteria for MS is a combination of clinical, MRI imaging, and laboratory evidence that evolves over time. The diagnosis of each case is confirmed according to the 2017 MC Donald criteria [5]. The different types of MS are as follow: 1) Relapsing–Remitting MS (RRMS), which is a common type and represents about 85% of MS cases; 2) Secondary Progressive MS (SPMS); 3) Primary Progressive MS (PPMS); which represents approximately 15% of MS cases [6].
Treating MS is a challenge, and a complete cure is not possible, but it is likely to help control the disease by reducing inflammation caused by the immune system. Moreover, it was found that the polymorphonuclear cells in MS patients, whether treated with immunomodulatory or immunosuppressive medications, had low phagocytic activity against pathogens. MS treatment interferes with the immunity of patients and may raise questions about the risk of infection [7]. The various MS therapies, each with a different mechanism of action, are as follows: Interferon beta-1b, Interferon beta-1a, Glatiramer acetate, Ocrevus, Alemtuzumab, Ocrelizumab, Fingolimod, and Natalizumab [8, 9]. The microorganisms that exist in the human body play an important role in preserving human health. The relationship between microorganisms and the host is essential in pathogenesis of certain diseases, such as Alzheimer’s, autism, Parkinson’s, and multiple sclerosis [10]. Approximately 800 different bacterial species exist in the oral cavity, and many studies indicate the role of oral microorganisms in the prevention and pathogenesis of diseases such as diabetes, dental, respiratory, and cardiovascular diseases [11]. In addition, bacteria induce inflammation and alter several signaling pathways that lead to the release of cytokines and contribute to the initiation of neuroinflammation in neurodegenerative diseases [10].
Microorganisms can enter the blood, and it is assumed that some of them can cross the blood-brain barrier and reach the brain and cause neurological disorders [12, 13]. Moreover, bacterial Lipopolysaccharides (LPS) in anaerobic bacteria such as Porphyromonas gingivalis and Bacteroides fragilis activate Toll-Like Receptors (TLRs), leading to an inflammatory response and overproduction of pro-inflammatory cytokines such as Interferon gamma (IFN-γ), Tumor Necrosis Factor Alpha (TNF-α), Interleukin-1 (IL-1), and IL-6. Furthermore, other bacteria, such as Staphylococcus aureus, can produce enterotoxins that may be implicated in various autoimmune diseases [14, 15]. There are few studies that consider the relationship between oral bacteria and MS. In 2021, a study by Zangeneh et al. [11] confirmed the relation between oral bacteria and MS, whereby oral bacteria were increased in MS patients compared to healthy individuals. However, previous studies do not focus on the relationship between bacteria and different types of MS treatment. Furthermore, there are no studies that focus on MS patients without medication (naive MS). Moreover, there is an information gap regarding microorganism detection, i.e., it is unclear whether the microorganisms occurred at the outset of the disease or later after using MS treatment.
The novelty of this study was assessing the presence of pathogenic microorganisms in the normal oral flora of MS patients that were not taking any medication (the naive MS group) and comparing them with healthy individuals. The aim of this study was to determine the oral bacterial status in MS patients without any medication (naive group) and MS patients during different modalities of treatment (Betaferon and Natalizumab) and compare them with each other and with the healthy group.
Materials and Methods
This study is a prospective cohort study conducted at Baghdad teaching hospital. Samples were collected from January 20, 2022 to July 17, 2022. The identified microorganisms were Staphylococcus aureus, Porphyromonas gingivalis, and Bacteroides fragilis. A Real-Time PCR (RT-PCR) was used to detect the presence or absence of microorganisms. RT-PCR was used as it is one of the most sensitive and accurate methods. It was sensitive enough to detect as few as 0.001 parasites per reaction. The qPCR (quantitative PCR) was performed using the Sa Cycler-96 instrument (Sacace Company; Italy).
Groups and treatment
The total number of patients was 120, all of whom were volunteers and divided into four groups. The first group comprised 30 patients with multiple sclerosis who had been taking Natalizmab (Tysabri) for at least one year (300mg IV infusion). The second group comprised 30 patients with multiple sclerosis who had been taking Betaferon for at least one year (250mg subcutaneously every other day). The third group included 30 patients newly diagnosed with multiple sclerosis in different progressive stages of the disease (naive MS patients) who were not taking any medication yet. The fourth group consisted of 30 healthy volunteers (control group).
The inclusion criteria in the study were age between 18 and 55 years and diagnosis of multiple sclerosis based on MC Donald 2017 criteria. The exclusion criteria were severe gingivitis and severe periodontitis (good oral hygiene), pregnancy, HIV, receiving chemotherapy and/or radiotherapy, diabetes, asthma, and any other endocrine disease. Figure 1 shows the flow chart of the experiment (the study design).
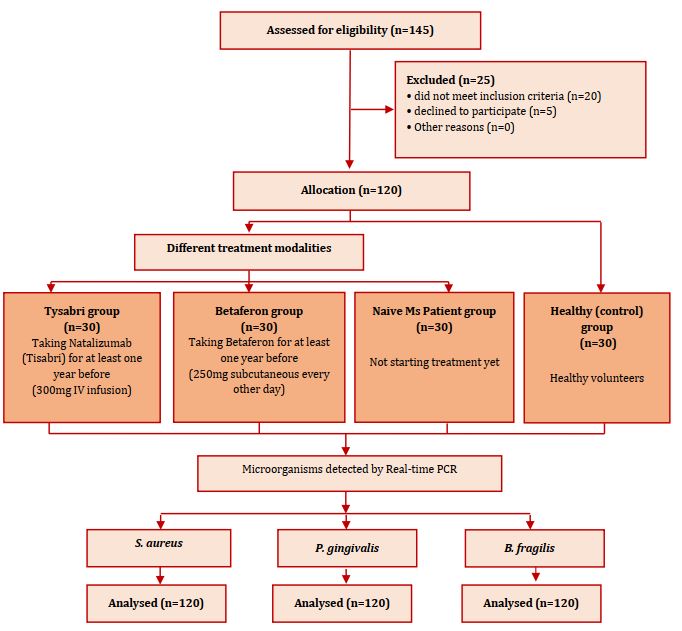
Figure 1) Flow chart of study design
Samples and DNA extraction
Participants were asked not drink, eat, brush their teeth, or use mouthwash for at least 30 minutes before swab collection. Firstly, the samples were taken from the oral cavity using a sterile swab, which was rotated and rubbed vigorously over the mucosa. Pressure was put on the swabs to pick up deeply seated microorganisms. The swab was taken from the inner surface of the cheeks, the inner surface of the upper and lower lips, the hard palate, and the dorsum of the tongue. The swab was rotated for 15-20 seconds for sample collection. The swab was kept in an Eppendorf tube with 200μL in DNA and RNA shield until DNA extraction. Thereafter, DNA extraction was performed using a special kit (iNtRON Biotechnology Company; South Korea) and according to the extraction protocol.
Real-time PCR
The real-time-PCR proceeded as follows: after preparing the SYBR FAST qPCR master mix, the required volume of each component was calculated to form a 20μL final volume (Table 1).
The target region within the genome of the bacteria was targeted by specific forward and reverse primers. The nucleotide sequences of the primers are shown in Table 2.
Table 1) Each component of the required volume to form a 20μL final volume
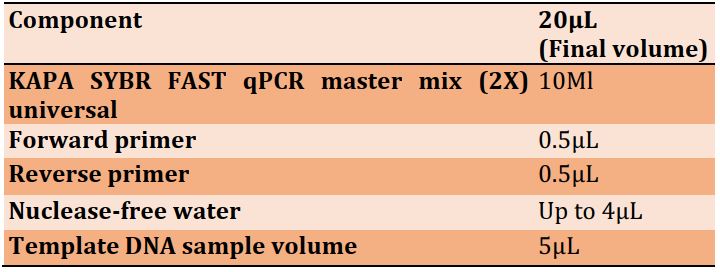
Table 2) The forward and reverse primer sequences for each microorganism
Real-time PCR program
The tubes were sealed and placed in different temperatures, which were programmed in the following steps: The first step was enzyme activation at 95°C for 5 minutes. Then, the denaturation step was performed at 95°C for 30sec. Thereafter, annealing was performed for 30sec at different temperatures: 60°C for S. aureus and P. gingivalis, and 66°C for B. fragilis. Lastly, the extension was run for 30 and 15sec and at 72°C and 90°C, respectively. RT-PCR cycling curves are shown in Figure 2.
Statistical analysis
Data analysis was done using SPSS 22.0 software. A p-value of less than 0.05 was considered statistically significant and was automatically calculated by the linear trapezoidal method. The data were analyzed using the Chi-Square test to compare the four groups with the different treatment modalities. Moreover, Fisher’s exact test was used to compare both groups. The difference between the mean ages in the four groups was measured by a one-way Analysis of Variance (ANOVA).
Figure 2) RT-PCR cycling
Findings
Demographic variables such as age, disease duration and gender were randomly distributed in the studied groups and did not affect the results. There was no significant difference between the four groups in terms of mean age (p>0.05; Figure 3a). In addition, the mean age of onset of the disease did not show a significant difference between the three groups of Taysabri group, Betaferon group and the naive MS patient group (p>0.05; Figure 3b).
The bacteria detected by real-time PCR in all groups were S. aureus, P. gingivalis, and B. fragilis. There was a significant difference in the prevalence of S. aureus (p=0.0001), P. gingivalis (p=0.001), and B. fragilis (p=0.030) in four studied groups (Table 3).
For S. aureus, a significant difference was observed between the Naive MS group (p=0.016), Betaferon group (p=0.001), and Tysabri group (p=0.0001) with the healthy group, as well as between Naive MS group and Tysabri group (p=0.005). However, no significant difference was found between the Betaferon group and the Naive MS group (p=0.215), also between the Tysabri group and the Betaferon group (p=0.064; Table 4).
For P. gingivalis, a significant difference was observed between the Betaferon group (p=0.002) and Tysabri group (p=0.0001) with the healthy group, as well as between Naive MS group and Tysabri group (p=0.020). However, no significant difference was found between other groups (p>0.05; Table 4).
For B. fragilis, a significant difference was observed between the Naive MS group (p=0.008), and Betaferon group (p=0.015), and Tysabri group (p=0.008) with the healthy group, but there was no significant difference between other groups (p>0.05; Table 4).
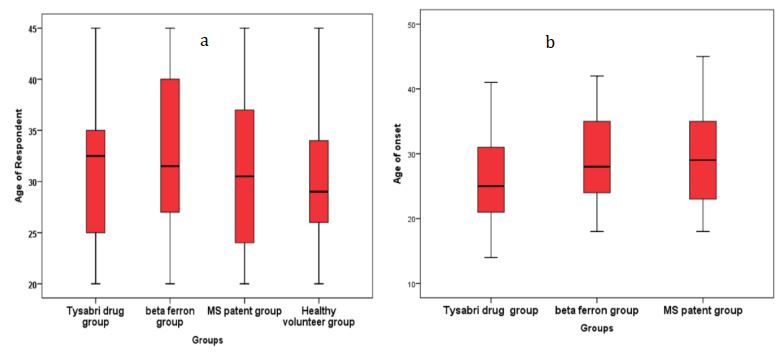
Figure 3) Comparison of demographic characteristics in the studied groups (each group = 30 people)
a) Difference in mean age in the four studied groups; b) Difference in mean age of disease onset between Tysabri group, Betaferon group,
and MS patients group
Table 3) Comparison of the prevalence of oral bacteria in different groups (each group = 30 people; Numbers in parentheses are percentages)
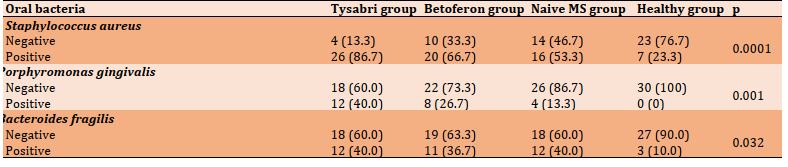
Table 4) p-values of paired comparison of the studied groups in terms of oral bacteria prevalence

Discussion
The relationship between host and microorganisms plays an important role in the regression or progression of several autoimmune disorders, such as multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease [11]. Most studies regarding the interactions between MS and microorganisms are limited to gut microorganisms. These studies suggest that gut microorganisms have an impact on the pathogenesis of MS [19-22]. However, previous studies that have focused on the interaction between oral bacteria and MS have been limited and have not examined the details of volunteer patients and whether these patients are receiving treatment. Therefore, our data cannot be compared with previous results [21, 22]. Moreover, other study indicated that oral bacteria may contribute to other autoimmune disorders, such as rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, Crohn’s disease, and Behcet’s disease. There is a potential association between oral bacteria and neurodegenerative disorders such as Alzheimer's [10, 23, 24]. The current unique study compared the naive MS patient group with other groups taking different treatment modalities. Bacteria comprise the majority of microorganisms in the oral cavity. Therefore, this study investigated Staphylococcus aureus, Porphyromonas gingivalis, and Bacteroides fragilis.
Staphylococcus aureus is the most common opportunistic pathogen that colonizes humans. It causes different infections, ranging from uncomplicated skin and soft tissue infections to more serious and life-threatening infections. Moreover, S. aureus may produce toxins that act as super-antigens. These activate a large numbers of T cells and CD4 cells, which are implicated in different autoimmune diseases, such as Wegener’s granulomatosis, MS, and rheumatoid arthritis [16, 17]. As shown in the results, six comparisons were made between groups for S. aureus. Firstly, oral S. aureus in the naive MS patient group was significantly higher compared to the healthy group. These results are in agreement with the results of Zangeneh et al. [11], even though their study did not consider whether MS individuals were taking treatment or not. Moreover, another study suggested that the ability to quickly screen patients for the existence of S. aureus may provide a marker of potential MS exacerbation [15]. Furthermore, Marrodan et al. [25] propose that S. aureus isolated from the upper respiratory tract and gut may be associated with developing or exacerbating MS [26, 27]. The above can be explained because Super-Antigens (SAgs) produced by S. aureus are considered as specific triggers, activating T cells and CD4 cells, which may play an essential role in the development of MS and several diseases [11, 14, 28].
The prevalence of S. aureus in the Betaferon group was significantly higher compared to the healthy group, because the Betaferon medication causes immune modulation, which inhibits T cells proliferation and alters the immune response [9, 29]. S. aureus in the oral cavity is considered an opportunistic pathogen and increases the risk of infection when the immune system is affected. Thus, it may be implicated in several diseases [30, 31]. This is in agreement with another study, suggesting that immunomodulation is usually linked with an increased risk of infection [29]. Although the prevalence of S. aureus was not significantly different between the Betaferon group and naive group, it still slightly increased due to Betaferon-related immune modulation. Scientists have found evidence that this substance has an immunosuppressive effect, which may be the reason [29].
The prevalence of S. aureus was significantly higher in the Tysabri group compared to the healthy group and naive group. Natalizumab (Tysabri) is a monoclonal antibody, which prevents the migration of autoreactive lymphocytes from blood vessels into the target organs by binding to alpha 4-integrin. Tysabri affects the immune response and causes suppression in the immune system, which increases the risk of infection [4, 29-32]. S. aureus is considered opportunistic pathogen, and the risk of S. aureus infection increases when the immune system is affected, which may contribute to several diseases [33]. The data of the present study are consistent with Winkelmann et al. [29], suggesting that Tysabri increases the risk of opportunistic infections. Although the prevalence of S. aureus was not significantly different between the Betaferon and Tysabri groups, it increased in the Tysabri group compared to the Betaferon group. These results are in agreement with a previous study that found that Tysabri is associated with a higher risk of infections [7].
Porphyromonas gingivalis is well-known as a key pathogen for periodontal disease and is considered a major opportunistic pathogen for periodontitis [34]. The prevalence of P. gingivalis was not significantly different between the naive MS and healthy groups, although it was higher in the naive group compared to the healthy group. This result is in agreement with the results of Zangeneh et al. [11], but they found the significant result. It may be because they did not specify the number of patients who received treatment compared to those who did not. In addition, the results of the present study showed that the prevalence of P. gingivalis in patients under treatment (Tysabri or Betaferon) was significantly higher compared to the healthy group, which confirms that the treatments cause an increase in bacterial infections. Moreover, as previously mentioned, Betaferon and Tysabri have a suppressive effect on the immune system and increase the risk of infection [29]. An abnormal immune response is evident in periodontal conditions and may cause periodontal disease [35, 36]. The results of this study can be explained by the virulence products of P. gingivalis, such as gingipain, fimbrins, and lipopolysaccharide. These virulence products may enter the bloodstream and promote the production of cytokines. Moreover, the lipid structure of P. gingivalis lipopolysaccharides affects the immune system by activating TLRs, leading to an inflammatory response associated with the overproduction of pro-inflammatory cytokines, such as like IFN-γ, TNF-α, IL-1, and IL-6. In addition, the spread of P. gingivalis from the oral cavity to other sites is possible because of the formation of circulating Outer Membrane Vesicles (OMVs), which lead to secondary non-oral diseases [12]. Moreover, P. gingivalis has been shown to increase other diseases such as COVID-19 and is associated with numerous systemic diseases, such as diabetes mellitus and neurological disease like Alzheimer’s disease [12, 34, 37, 38]. Additionally, P. gingivalis is related to the progression of other autoimmune disease, such as rheumatoid arthritis [39- 43].
Bacteroidetes represent the largest phylum of bacteria in the Gastrointestinal (GI) tract. While commonly useful to the host when limited to the GI tract, they have the ability to secrete pro-inflammatory neurotoxins, which include toxic proteolytic peptides and surface lipopolysaccharides [42]. Gut bacteria may trigger the onset of autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, and ulcerative colitis [43]. The prevalence of B. fragilis was significantly higher in naive MS patient group compared to the healthy group. B. fragilis has been shown to be involved in the pathogenesis of many diseases, such as Alzheimer's and autism. The pathogenesis is linked to the pro-inflammatory effects of lipopolysaccharide, agglutinin, capsule, and fimbriae [11, 42]. In addition to endotoxins, B. fragilis can cause various diseases by expressing pro-inflammatory cytokines and zinc metalloprotease, metalloproteinase and B. fragilis Toxin (BFT) fragilysin. Finally, the production of pro-inflammatory cytokines, such as Th17, IL-17, IL-21, and IL22, is the primary mechanism involved in developing MS, as T cells are activated by bacteria [11]. These results are in agreement with those of Zangeneh et al. [11], although their study did not ascertain whether the patients were receiving treatment or not. Furthermore, the prevalence of B. fragilis was significantly higher in the Betaferon and Tysabri groups compared to the healthy group. However, when comparing both the Betaferon and Tysabri groups with the naive MS group, the results were not significant. Thus, future studies are needed to resolve this matter.
The difficulty of finding a laboratory to determine the quantity of bacteria and the short study period were the limitations of this research. It is suggested that more studies be done in the future to explain why the Bacteroides fragilis does not affect MS.
Conclusion
The prevalence of oral bacteria (Staphylococcus aureus, Prophyromonos gingivalis, and Bacteroides fragilis) increases in naive MS patients compared to healthy individuals. Therefore, oral bacteria are involved in MS development. Furthermore, MS patients are more susceptible to periodontal disease due to high P. gingivalis presence compared to healthy individuals, and these patients need to receive extra care to prevent periodontal disease. Contrarily, Bacteroides fragilis is not affected in any of the MS treatments. However, other bacterial species (S. aureus and P. gingivalis) increase in the MS treatment groups, and more bacterial infections occurs in the Tysabri treatment.
Acknowledgements: Special thanks to Dr. Haider Jaafar Chilabi for his help and guidance in doing the work. We are also very grateful to Dr. Hiba Murtadha Al-Saadi, Dr. Mohammed Murtadha Al-Saadi, and Dr. Ahmed Mustafa Sadiq for their help and guidance.
Ethical Permission: The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (Research Ethics Committee) of College of Dentistry, Baghdad University (Protocol code: 461722 19/01/2022).
Informed consent was obtained from all subjects involved in the study. Informed written consent was obtained from the patient(s) for publication of this article.
Conflict of Interests: The authors declare no conflict of interests.
Authors’ Contribution: Al-Saadi ZM (First Author), Introduction Writer/Methodologist/Main Researcher/Statistical Analyst (40%); Al-Aswad FD (Second Author), Introduction Writer/Assistant Researcher/Statistical Analyst/Discussion Writer (30%); Sheaheed NM (Third Author), Methodologist/Assistant Researcher/Statistical Analyst/Discussion Writer (20%)
Funding: This research did not receive any external funding (self-funded).
Multiple Sclerosis (MS) is a complex chronic autoimmune disorder. It is a multifactorial disease that attacks the brain and spinal cord. MS can affect any part of the body and cause a wide range of symptoms. Moreover, MS symptoms occur due to a zone of demyelination and inflammation, which can cause motor, sensory, and visual disorders and may last for days or weeks [1, 2].
The causes of MS have not been clearly identified, but it is known that the immune system plays a vital role in the progression of MS. There are many predisposing factors, such as environmental factors, vitamin D deficiency, teenage obesity, smoking, genetic factors, and Epstein–Barr Virus (EBV) [1, 3]. In histopathology, myelin damage has been detected with mononuclear phagocytes, T-lymphocytes, dendritic cell infiltration, B-lymphocytes, and plasma cells. The T helper 1 (Th1) and Th17 pathways were involved in MS pathogenesis and demyelination [4]. No single test can positively diagnose MS, i.e., the diagnostic criteria for MS is a combination of clinical, MRI imaging, and laboratory evidence that evolves over time. The diagnosis of each case is confirmed according to the 2017 MC Donald criteria [5]. The different types of MS are as follow: 1) Relapsing–Remitting MS (RRMS), which is a common type and represents about 85% of MS cases; 2) Secondary Progressive MS (SPMS); 3) Primary Progressive MS (PPMS); which represents approximately 15% of MS cases [6].
Treating MS is a challenge, and a complete cure is not possible, but it is likely to help control the disease by reducing inflammation caused by the immune system. Moreover, it was found that the polymorphonuclear cells in MS patients, whether treated with immunomodulatory or immunosuppressive medications, had low phagocytic activity against pathogens. MS treatment interferes with the immunity of patients and may raise questions about the risk of infection [7]. The various MS therapies, each with a different mechanism of action, are as follows: Interferon beta-1b, Interferon beta-1a, Glatiramer acetate, Ocrevus, Alemtuzumab, Ocrelizumab, Fingolimod, and Natalizumab [8, 9]. The microorganisms that exist in the human body play an important role in preserving human health. The relationship between microorganisms and the host is essential in pathogenesis of certain diseases, such as Alzheimer’s, autism, Parkinson’s, and multiple sclerosis [10]. Approximately 800 different bacterial species exist in the oral cavity, and many studies indicate the role of oral microorganisms in the prevention and pathogenesis of diseases such as diabetes, dental, respiratory, and cardiovascular diseases [11]. In addition, bacteria induce inflammation and alter several signaling pathways that lead to the release of cytokines and contribute to the initiation of neuroinflammation in neurodegenerative diseases [10].
Microorganisms can enter the blood, and it is assumed that some of them can cross the blood-brain barrier and reach the brain and cause neurological disorders [12, 13]. Moreover, bacterial Lipopolysaccharides (LPS) in anaerobic bacteria such as Porphyromonas gingivalis and Bacteroides fragilis activate Toll-Like Receptors (TLRs), leading to an inflammatory response and overproduction of pro-inflammatory cytokines such as Interferon gamma (IFN-γ), Tumor Necrosis Factor Alpha (TNF-α), Interleukin-1 (IL-1), and IL-6. Furthermore, other bacteria, such as Staphylococcus aureus, can produce enterotoxins that may be implicated in various autoimmune diseases [14, 15]. There are few studies that consider the relationship between oral bacteria and MS. In 2021, a study by Zangeneh et al. [11] confirmed the relation between oral bacteria and MS, whereby oral bacteria were increased in MS patients compared to healthy individuals. However, previous studies do not focus on the relationship between bacteria and different types of MS treatment. Furthermore, there are no studies that focus on MS patients without medication (naive MS). Moreover, there is an information gap regarding microorganism detection, i.e., it is unclear whether the microorganisms occurred at the outset of the disease or later after using MS treatment.
The novelty of this study was assessing the presence of pathogenic microorganisms in the normal oral flora of MS patients that were not taking any medication (the naive MS group) and comparing them with healthy individuals. The aim of this study was to determine the oral bacterial status in MS patients without any medication (naive group) and MS patients during different modalities of treatment (Betaferon and Natalizumab) and compare them with each other and with the healthy group.
Materials and Methods
This study is a prospective cohort study conducted at Baghdad teaching hospital. Samples were collected from January 20, 2022 to July 17, 2022. The identified microorganisms were Staphylococcus aureus, Porphyromonas gingivalis, and Bacteroides fragilis. A Real-Time PCR (RT-PCR) was used to detect the presence or absence of microorganisms. RT-PCR was used as it is one of the most sensitive and accurate methods. It was sensitive enough to detect as few as 0.001 parasites per reaction. The qPCR (quantitative PCR) was performed using the Sa Cycler-96 instrument (Sacace Company; Italy).
Groups and treatment
The total number of patients was 120, all of whom were volunteers and divided into four groups. The first group comprised 30 patients with multiple sclerosis who had been taking Natalizmab (Tysabri) for at least one year (300mg IV infusion). The second group comprised 30 patients with multiple sclerosis who had been taking Betaferon for at least one year (250mg subcutaneously every other day). The third group included 30 patients newly diagnosed with multiple sclerosis in different progressive stages of the disease (naive MS patients) who were not taking any medication yet. The fourth group consisted of 30 healthy volunteers (control group).
The inclusion criteria in the study were age between 18 and 55 years and diagnosis of multiple sclerosis based on MC Donald 2017 criteria. The exclusion criteria were severe gingivitis and severe periodontitis (good oral hygiene), pregnancy, HIV, receiving chemotherapy and/or radiotherapy, diabetes, asthma, and any other endocrine disease. Figure 1 shows the flow chart of the experiment (the study design).

Figure 1) Flow chart of study design
Samples and DNA extraction
Participants were asked not drink, eat, brush their teeth, or use mouthwash for at least 30 minutes before swab collection. Firstly, the samples were taken from the oral cavity using a sterile swab, which was rotated and rubbed vigorously over the mucosa. Pressure was put on the swabs to pick up deeply seated microorganisms. The swab was taken from the inner surface of the cheeks, the inner surface of the upper and lower lips, the hard palate, and the dorsum of the tongue. The swab was rotated for 15-20 seconds for sample collection. The swab was kept in an Eppendorf tube with 200μL in DNA and RNA shield until DNA extraction. Thereafter, DNA extraction was performed using a special kit (iNtRON Biotechnology Company; South Korea) and according to the extraction protocol.
Real-time PCR
The real-time-PCR proceeded as follows: after preparing the SYBR FAST qPCR master mix, the required volume of each component was calculated to form a 20μL final volume (Table 1).
The target region within the genome of the bacteria was targeted by specific forward and reverse primers. The nucleotide sequences of the primers are shown in Table 2.
Table 1) Each component of the required volume to form a 20μL final volume

Table 2) The forward and reverse primer sequences for each microorganism

Real-time PCR program
The tubes were sealed and placed in different temperatures, which were programmed in the following steps: The first step was enzyme activation at 95°C for 5 minutes. Then, the denaturation step was performed at 95°C for 30sec. Thereafter, annealing was performed for 30sec at different temperatures: 60°C for S. aureus and P. gingivalis, and 66°C for B. fragilis. Lastly, the extension was run for 30 and 15sec and at 72°C and 90°C, respectively. RT-PCR cycling curves are shown in Figure 2.
Statistical analysis
Data analysis was done using SPSS 22.0 software. A p-value of less than 0.05 was considered statistically significant and was automatically calculated by the linear trapezoidal method. The data were analyzed using the Chi-Square test to compare the four groups with the different treatment modalities. Moreover, Fisher’s exact test was used to compare both groups. The difference between the mean ages in the four groups was measured by a one-way Analysis of Variance (ANOVA).

Figure 2) RT-PCR cycling
Findings
Demographic variables such as age, disease duration and gender were randomly distributed in the studied groups and did not affect the results. There was no significant difference between the four groups in terms of mean age (p>0.05; Figure 3a). In addition, the mean age of onset of the disease did not show a significant difference between the three groups of Taysabri group, Betaferon group and the naive MS patient group (p>0.05; Figure 3b).
The bacteria detected by real-time PCR in all groups were S. aureus, P. gingivalis, and B. fragilis. There was a significant difference in the prevalence of S. aureus (p=0.0001), P. gingivalis (p=0.001), and B. fragilis (p=0.030) in four studied groups (Table 3).
For S. aureus, a significant difference was observed between the Naive MS group (p=0.016), Betaferon group (p=0.001), and Tysabri group (p=0.0001) with the healthy group, as well as between Naive MS group and Tysabri group (p=0.005). However, no significant difference was found between the Betaferon group and the Naive MS group (p=0.215), also between the Tysabri group and the Betaferon group (p=0.064; Table 4).
For P. gingivalis, a significant difference was observed between the Betaferon group (p=0.002) and Tysabri group (p=0.0001) with the healthy group, as well as between Naive MS group and Tysabri group (p=0.020). However, no significant difference was found between other groups (p>0.05; Table 4).
For B. fragilis, a significant difference was observed between the Naive MS group (p=0.008), and Betaferon group (p=0.015), and Tysabri group (p=0.008) with the healthy group, but there was no significant difference between other groups (p>0.05; Table 4).

Figure 3) Comparison of demographic characteristics in the studied groups (each group = 30 people)
a) Difference in mean age in the four studied groups; b) Difference in mean age of disease onset between Tysabri group, Betaferon group,
and MS patients group
Table 3) Comparison of the prevalence of oral bacteria in different groups (each group = 30 people; Numbers in parentheses are percentages)

Table 4) p-values of paired comparison of the studied groups in terms of oral bacteria prevalence

Discussion
The relationship between host and microorganisms plays an important role in the regression or progression of several autoimmune disorders, such as multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease [11]. Most studies regarding the interactions between MS and microorganisms are limited to gut microorganisms. These studies suggest that gut microorganisms have an impact on the pathogenesis of MS [19-22]. However, previous studies that have focused on the interaction between oral bacteria and MS have been limited and have not examined the details of volunteer patients and whether these patients are receiving treatment. Therefore, our data cannot be compared with previous results [21, 22]. Moreover, other study indicated that oral bacteria may contribute to other autoimmune disorders, such as rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, Crohn’s disease, and Behcet’s disease. There is a potential association between oral bacteria and neurodegenerative disorders such as Alzheimer's [10, 23, 24]. The current unique study compared the naive MS patient group with other groups taking different treatment modalities. Bacteria comprise the majority of microorganisms in the oral cavity. Therefore, this study investigated Staphylococcus aureus, Porphyromonas gingivalis, and Bacteroides fragilis.
Staphylococcus aureus is the most common opportunistic pathogen that colonizes humans. It causes different infections, ranging from uncomplicated skin and soft tissue infections to more serious and life-threatening infections. Moreover, S. aureus may produce toxins that act as super-antigens. These activate a large numbers of T cells and CD4 cells, which are implicated in different autoimmune diseases, such as Wegener’s granulomatosis, MS, and rheumatoid arthritis [16, 17]. As shown in the results, six comparisons were made between groups for S. aureus. Firstly, oral S. aureus in the naive MS patient group was significantly higher compared to the healthy group. These results are in agreement with the results of Zangeneh et al. [11], even though their study did not consider whether MS individuals were taking treatment or not. Moreover, another study suggested that the ability to quickly screen patients for the existence of S. aureus may provide a marker of potential MS exacerbation [15]. Furthermore, Marrodan et al. [25] propose that S. aureus isolated from the upper respiratory tract and gut may be associated with developing or exacerbating MS [26, 27]. The above can be explained because Super-Antigens (SAgs) produced by S. aureus are considered as specific triggers, activating T cells and CD4 cells, which may play an essential role in the development of MS and several diseases [11, 14, 28].
The prevalence of S. aureus in the Betaferon group was significantly higher compared to the healthy group, because the Betaferon medication causes immune modulation, which inhibits T cells proliferation and alters the immune response [9, 29]. S. aureus in the oral cavity is considered an opportunistic pathogen and increases the risk of infection when the immune system is affected. Thus, it may be implicated in several diseases [30, 31]. This is in agreement with another study, suggesting that immunomodulation is usually linked with an increased risk of infection [29]. Although the prevalence of S. aureus was not significantly different between the Betaferon group and naive group, it still slightly increased due to Betaferon-related immune modulation. Scientists have found evidence that this substance has an immunosuppressive effect, which may be the reason [29].
The prevalence of S. aureus was significantly higher in the Tysabri group compared to the healthy group and naive group. Natalizumab (Tysabri) is a monoclonal antibody, which prevents the migration of autoreactive lymphocytes from blood vessels into the target organs by binding to alpha 4-integrin. Tysabri affects the immune response and causes suppression in the immune system, which increases the risk of infection [4, 29-32]. S. aureus is considered opportunistic pathogen, and the risk of S. aureus infection increases when the immune system is affected, which may contribute to several diseases [33]. The data of the present study are consistent with Winkelmann et al. [29], suggesting that Tysabri increases the risk of opportunistic infections. Although the prevalence of S. aureus was not significantly different between the Betaferon and Tysabri groups, it increased in the Tysabri group compared to the Betaferon group. These results are in agreement with a previous study that found that Tysabri is associated with a higher risk of infections [7].
Porphyromonas gingivalis is well-known as a key pathogen for periodontal disease and is considered a major opportunistic pathogen for periodontitis [34]. The prevalence of P. gingivalis was not significantly different between the naive MS and healthy groups, although it was higher in the naive group compared to the healthy group. This result is in agreement with the results of Zangeneh et al. [11], but they found the significant result. It may be because they did not specify the number of patients who received treatment compared to those who did not. In addition, the results of the present study showed that the prevalence of P. gingivalis in patients under treatment (Tysabri or Betaferon) was significantly higher compared to the healthy group, which confirms that the treatments cause an increase in bacterial infections. Moreover, as previously mentioned, Betaferon and Tysabri have a suppressive effect on the immune system and increase the risk of infection [29]. An abnormal immune response is evident in periodontal conditions and may cause periodontal disease [35, 36]. The results of this study can be explained by the virulence products of P. gingivalis, such as gingipain, fimbrins, and lipopolysaccharide. These virulence products may enter the bloodstream and promote the production of cytokines. Moreover, the lipid structure of P. gingivalis lipopolysaccharides affects the immune system by activating TLRs, leading to an inflammatory response associated with the overproduction of pro-inflammatory cytokines, such as like IFN-γ, TNF-α, IL-1, and IL-6. In addition, the spread of P. gingivalis from the oral cavity to other sites is possible because of the formation of circulating Outer Membrane Vesicles (OMVs), which lead to secondary non-oral diseases [12]. Moreover, P. gingivalis has been shown to increase other diseases such as COVID-19 and is associated with numerous systemic diseases, such as diabetes mellitus and neurological disease like Alzheimer’s disease [12, 34, 37, 38]. Additionally, P. gingivalis is related to the progression of other autoimmune disease, such as rheumatoid arthritis [39- 43].
Bacteroidetes represent the largest phylum of bacteria in the Gastrointestinal (GI) tract. While commonly useful to the host when limited to the GI tract, they have the ability to secrete pro-inflammatory neurotoxins, which include toxic proteolytic peptides and surface lipopolysaccharides [42]. Gut bacteria may trigger the onset of autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, and ulcerative colitis [43]. The prevalence of B. fragilis was significantly higher in naive MS patient group compared to the healthy group. B. fragilis has been shown to be involved in the pathogenesis of many diseases, such as Alzheimer's and autism. The pathogenesis is linked to the pro-inflammatory effects of lipopolysaccharide, agglutinin, capsule, and fimbriae [11, 42]. In addition to endotoxins, B. fragilis can cause various diseases by expressing pro-inflammatory cytokines and zinc metalloprotease, metalloproteinase and B. fragilis Toxin (BFT) fragilysin. Finally, the production of pro-inflammatory cytokines, such as Th17, IL-17, IL-21, and IL22, is the primary mechanism involved in developing MS, as T cells are activated by bacteria [11]. These results are in agreement with those of Zangeneh et al. [11], although their study did not ascertain whether the patients were receiving treatment or not. Furthermore, the prevalence of B. fragilis was significantly higher in the Betaferon and Tysabri groups compared to the healthy group. However, when comparing both the Betaferon and Tysabri groups with the naive MS group, the results were not significant. Thus, future studies are needed to resolve this matter.
The difficulty of finding a laboratory to determine the quantity of bacteria and the short study period were the limitations of this research. It is suggested that more studies be done in the future to explain why the Bacteroides fragilis does not affect MS.
Conclusion
The prevalence of oral bacteria (Staphylococcus aureus, Prophyromonos gingivalis, and Bacteroides fragilis) increases in naive MS patients compared to healthy individuals. Therefore, oral bacteria are involved in MS development. Furthermore, MS patients are more susceptible to periodontal disease due to high P. gingivalis presence compared to healthy individuals, and these patients need to receive extra care to prevent periodontal disease. Contrarily, Bacteroides fragilis is not affected in any of the MS treatments. However, other bacterial species (S. aureus and P. gingivalis) increase in the MS treatment groups, and more bacterial infections occurs in the Tysabri treatment.
Acknowledgements: Special thanks to Dr. Haider Jaafar Chilabi for his help and guidance in doing the work. We are also very grateful to Dr. Hiba Murtadha Al-Saadi, Dr. Mohammed Murtadha Al-Saadi, and Dr. Ahmed Mustafa Sadiq for their help and guidance.
Ethical Permission: The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (Research Ethics Committee) of College of Dentistry, Baghdad University (Protocol code: 461722 19/01/2022).
Informed consent was obtained from all subjects involved in the study. Informed written consent was obtained from the patient(s) for publication of this article.
Conflict of Interests: The authors declare no conflict of interests.
Authors’ Contribution: Al-Saadi ZM (First Author), Introduction Writer/Methodologist/Main Researcher/Statistical Analyst (40%); Al-Aswad FD (Second Author), Introduction Writer/Assistant Researcher/Statistical Analyst/Discussion Writer (30%); Sheaheed NM (Third Author), Methodologist/Assistant Researcher/Statistical Analyst/Discussion Writer (20%)
Funding: This research did not receive any external funding (self-funded).
Keywords:
References
1. Murúa SR, Farez MF, Quintana FJ. The immune response in multiple sclerosis. Annu Rev Pathol. 2022;17:121-39. [Link] [DOI:10.1146/annurev-pathol-052920-040318]
2. Soud SA, Al-Rubaei SHN. Study of ABO system and multiple sclerosis disease in Iraq. Iraq J Sci.2022;63:2345-53. [Link] [DOI:10.24996/ijs.2022.63.6.3]
3. Hassoun HK, Al-Mahadawi A, Sheaheed NM, Sami SM, Jamal A, Allebban Z. Epidemiology of multiple sclerosis in Iraq: Retrospective review of 4355 cases and literature review. Neurol Res.2022;44(1):14-23. [Link] [DOI:10.1080/01616412.2021.1952511]
4. da Cunha ETS, Figueiredo-Godoi LMA, Santos DH, Carneiro RPCD, do Olival GS, de Barros PP, et al. Oral colonization by candida species in patients with multiple sclerosis. Mycopathologia. 2020;185(6):983-91. [Link] [DOI:10.1007/s11046-020-00486-1]
5. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-73. [Link] [DOI:10.1016/S1474-4422(17)30470-2]
6. Marvin M. Goldenberg multiple sclerosis review. Pharm Ther. 2012;37(3):175-84. [Link]
7. Luna G, Alping P, Burman J, Fink K, Fogdell-Hahn A, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184-91. [Link] [DOI:10.1001/jamaneurol.2019.3365]
8. Loma I, Heyman R. Multiple sclerosis: Pathogenesis and treatment. Curr Neuropharmacol. 2011;9(3):409-16. [Link] [DOI:10.2174/157015911796557911]
9. Dhib-Jalbut S, Marks S. Interferon- mechanisms of action in multiple sclerosis. Neurology. 2010;74:S17-24. [Link] [DOI:10.1212/WNL.0b013e3181c97d99]
10. Zorba M, Melidou A, Patsatsi A, Ioannou E, Kolokotronis A. The possible role of oral microbiome in autoimmunity. Int J Womens Dermatol. 2020;6(5):357-64. [Link] [DOI:10.1016/j.ijwd.2020.07.011]
11. Zangeneh Z, Abdi-Ali A, Khamooshian K, Alvandi A, Abiri R. Bacterial variation in the oral microbiota in multiple sclerosis patients. PLoS One. 2021;16(11):e0260384. [Link] [DOI:10.1371/journal.pone.0260384]
12. Franciotti R, Pignatelli P, Carrarini C, Romei FM, Mastrippolito M, Gentile A, et al. Exploring the connection between Porphyromonas gingivalis and neurodegenerative diseases: A pilot quantitative study on the bacterium abundance in oral cavity and the amount of antibodies in serum. Biomolecules. 2021;11(6):845. [Link] [DOI:10.3390/biom11060845]
13. González-Sanmiguel J, Schuh CMAP, Muñoz-Montesin C, Contreras-Kallens P, Aguayo LG, Aguayo S. Complex Interaction between Resident Microbiota and Misfolded Proteins: Role in Neuroinflammation and Neurodegeneration. Cells. 2020;9(11):2476. [Link] [DOI:10.3390/cells9112476]
14. Li J, Yang J, Lu Y, Wu S, Wang M, Zhu J. Possible role of staphylococcal enterotoxin B in the pathogenesis of autoimmune diseases. Viral Immunol. 2015;28(7):354-9. [Link] [DOI:10.1089/vim.2015.0017]
15. Mulvey MR, Doupe M, Prout M, Leong C, Hizon R, Grossberndt A, et al. Staphylococcus aureus harbouring Enterotoxin A as a possible risk factor for multiple sclerosis exacerbations. Mult Scler J. 2011;17(4):397-403. [Link] [DOI:10.1177/1352458510391343]
16. Ballah FM, Islam MS, Rana ML, Ferdous FB, Ahmed R, Pramanik PK, et al. Phenotypic and genotypic detection of biofilm-forming Staphylococcus aureus from different food sources in Bangladesh. Biology (Basel). 2022;11(7):949. [Link] [DOI:10.3390/biology11070949]
17. Krishnan M. Detection of Porphyromonas gingivalis fimA type I genotype in gingivitis by real-time PCR- a pilot study. J Clin Diagnostic Res. 2016;10(6):ZC32-5. [Link] [DOI:10.7860/JCDR/2016/17938.7979]
18. Bakuradze N, Merabishvili M, Makalatia K, Kakabadze E, Grdzelishvili N, Wagemans J, et al. In vitro evaluation of the therapeutic potential of phage VA7 against enterotoxigenic Bacteroides fragilis infection. Viruses. 2021;13(10):2044. [Link] [DOI:10.3390/v13102044]
19. Shahi SK, Freedman SN, Mangalam AK. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes. 2017;8(6):607-15. [Link] [DOI:10.1080/19490976.2017.1349041]
20. Jangi S, Gandhi R, Cox LM, Li N, Von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1):12015. [Link] [DOI:10.1038/ncomms12015]
21. Trott S, King IL. An introduction to the microbiome and MS. Mult Scler J. 2018;24(1):53-7. [Link] [DOI:10.1177/1352458517737391]
22. Amini ME, Shomali N, Bakhshi A, Rezaei S, Hemmatzadeh M, Hosseinzadeh R, et al. Gut microbiome and multiple sclerosis: New insights and perspective. Int. Immunopharmacol. 2020;88:107024. [Link] [DOI:10.1016/j.intimp.2020.107024]
23. Wu YF, Lee WF, Salamanca E, Yao WL, Su JN, Wang SY, et al. Oral microbiota changes in elderly patients, an indicator of alzheimer's disease. Int J Environ Res Public Health. 2021;18(8):4211. [Link] [DOI:10.3390/ijerph18084211]
24. Li Z, Peres AG, Damian AC, Madrenas J. Immunomodulation and disease tolerance to Staphylococcus aureus. Pathogens. 2015;4(4):793-815. [Link] [DOI:10.3390/pathogens4040793]
25. Marrodan M, Alessandro L, Farez MF, Correale J. The role of infections in multiple sclerosis. Mult Scler J. 2019;25(7):891-901. [Link] [DOI:10.1177/1352458518823940]
26. Sadeghi J, Alizadeh N, Ahangar Oskouei M, Laghusi D, Savadi Oskouei D, Nikanfar M, et al. Frequency of superantigen encoding genes of Staphylococcus aureus isolates collected from multiple sclerosis (MS) patients and nasal carriers. Microb Pathog. 2019;127:316-9. [Link] [DOI:10.1016/j.micpath.2018.12.010]
27. Sheykhsaran E, Abbasi A, Baghi HB, Ghotaslou R, Sharifi Y, Sefidan FY, et al. Staphylococcus aureus: A bacterial candidate for multiple sclerosis incidence and progression. Rev Res Med Microbiol. 2022;33:212-20. [Link]
28. Jassim SA, Kandala NJ. Molecular detection of enterotoxin genes of multiresistant Staphylococcus aureus isolates from different sources of food. Iraq J. Sci. 2021;62(1):61-74. [Link] [DOI:10.24996/ijs.2021.62.1.6]
29. Winkelmann A, Loebermann M, Reisinger EC, Zettl UK. Multiple sclerosis treatment and infectious issues: Update 2013. Clin Exp Immunol. 2014;175(3):425-38. [Link] [DOI:10.1111/cei.12226]
30. Taylor TA, Unakal CG. Staphylococcus aureus Infection. Florida: StatPearls; 2022. [Link]
31. Cuesta AI, Jewtuchowicz V, Brusca MI, Nastri ML, Rosa AC. Prevalence of Staphylococcus spp and Candida spp in the oral cavity and periodontal pockets of periodontal disease patients. Acta Odontol Latinoam. 2010;23(1):20-6. [Link]
32. Morrow SA, Clift F, Devonshire V, Lapointe E, Schneider R, Stefanelli M, et al. Use of natalizumab in persons with multiple sclerosis: 2022 update. Mult Scler Relat Disord. 2022;65:103995. [Link] [DOI:10.1016/j.msard.2022.103995]
33. Conlon BP. Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells: An investigation of persister cells, their formation and their role in S. aureus disease. Bioessays. 2014;36(10):991-6. [Link] [DOI:10.1002/bies.201400080]
34. Hirai K, Yamaguchi-Tomikawa T, Eguchi T, Maeda H, Takashiba S. Identification and modification of Porphyromonas gingivalis cysteine protease, gingipain, ideal for screening periodontitis. Front Immunol. 2020;11:1-15. [Link] [DOI:10.3389/fimmu.2020.01017]
35. Grollmus ZCN, Chávez MCM, Donat FJS. Periodontal disease associated to systemic genetic disorder. Med Oral Patol Oral Cir Bucal. 2007;12(3):E211-5. [Link]
36. Julkunen A, Heikkinen A, Söder B, Söder PÖ, Toppila-Salmi S, Meurman J. Autoimmune diseases and oral health: 30-year follow-up of a swedish cohort. Dent J. 2017;6(1):1. [Linkv] [DOI:10.3390/dj6010001]
37. Kareem HH, Al-ghurabi BH, Albadri C. Molecular detection of Porphyromonas gingivalis in COVID-19 Patients. J Baghdad Coll Dentistry. 2022;34(2):52-61. [Link] [DOI:10.26477/jbcd.v34i2.3145]
38. Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333. [Link] [DOI:10.1126/sciadv.aau3333]
39. Li Y, Guo R, Oduro PK, Sun T, Chen H, Yi Y, et al. The relationship between Porphyromonas gingivalis and rheumatoid arthritis: A meta-analysis. Front Cell Infect Microbiol. 2022;12:956417. [Link] [DOI:10.3389/fcimb.2022.956417]
40. Qadir MIA, Al-Waheb AM. Salivary C- reactive protein in relation to periodontal health among a group of patients with rheumatoid arthritis in Iraq. J Baghdad Coll Dentistry. 2014;26(3):138-43. [Link] [DOI:10.12816/0015239]
41. Mei F, Xie M, Huang X, Long Y, Lu X, Wang X, et al. Porphyromonas gingivalis and its systemic impact: Current status. Pathogens. 2020;9(11):944. [Link] [DOI:10.3390/pathogens9110944]
42. Lukiw WJ. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer's disease. Front Microbiol. 2016;7:1544. [Link] [DOI:10.3389/fmicb.2016.01544]
43. Loria K. Common Gut Bacteria Linked to Autoimmune Diseases [Internet]. New Jersey: MJH Life Sciences. 2019 Feb- [cited 2022 Nov 3].Available from: https://www.managedhealthcareexecutive.com/view/common-gut-bacteria-linked-autoimmune-diseases [Link]










