Volume 15, Issue 1 (2023)
Iran J War Public Health 2023, 15(1): 67-75 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/01/1 | Accepted: 2023/03/12 | Published: 2023/03/19
Received: 2023/01/1 | Accepted: 2023/03/12 | Published: 2023/03/19
How to cite this article
Abd Uljaleel A, Hassan E. Protective Effect of Ertugliflozin against Acute Lung Injury Caused by Endotoxemia Model in Mice. Iran J War Public Health 2023; 15 (1) :67-75
URL: http://ijwph.ir/article-1-1307-en.html
URL: http://ijwph.ir/article-1-1307-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Pharmacology & Therapeutics, Faculty of Medicine, University of Kufa, Najaf, Iraq
Full-Text (HTML) (1077 Views)
Introduction
The most frequent disease among patients in critical care units is endotoxemia, a Systemic Inflammatory Response Syndrome (SIRS) brought on by infection [1]. A modern study for the Institute of Health Metrics and Evaluation on the widespread of sepsis globally assessed 48.9 million of sepsis conditions and 11 million deaths worldwide, which make up about 20% of all deaths globally [2]. The most serious type of sepsis, endotoxic shock, is spurred on by gram-negative bacterial infections [3].
It is associated with a higher mortality rate of 40%, compared with the 10% mortality observed with sepsis [4]. Acute Respiratory Distress Syndrome (ARDS), Acute Lung Injury (ALI), severe hypotension, rising metabolic acidosis, SIRS, numerous organ failure, and significant mortality are the hallmarks of this shock [3]. The lung is the most exposed and captious organ in sepsis [5], and eventually, lung dysfunction results in lung failure in the form of acute respiratory distress syndrome [6]. ARDS proceed quickly appears within minutes to hours of sepsis onset, where it occurs as a sudden development of severe hypoxemia. The lungs become filled with fluid and are badly compliant, as a result, breathing becomes more difficult. New bilateral diffuse or pulmonary infiltrates will show on chest x-ray, and mechanical ventilation is generally required [7]. Areas of the lung with broken endothelia turn to be filled with neutrophils and macrophages. Interstitial venules develop edema, deposition of fibrin, and surfactant reduction also occurs. Such lung regions become dense and unwell-compliant because gas exchange becomes minimal [8].
Amplification of the host response to infection develops in sepsis and, as a result, variation among pro- and anti-inflammatory responses with a predominance of pro-inflammatory ones [9]. Pro-inflammatory cytokines continue to be produced due to the spreading of inflammation over the bloodstream, which results in increased formation of cytokines in distant spaces [10].
Toll Like Receptors (TLRs) are the most families of the pattern recognition receptors studied, and TLR4 is the most important among this family. TLR4 can recognize Lipopolysaccharide (LPS) and stimulate immune response [11] in order to keep the body against infection. TLR4 and nuclear Factor-κB (NF-κB) are the main regulators of inflammation [12].
The severity of inflammatory response is linked with the expression level of TLR4 and ALI induced by LPS, resulting in activation of NF-κB that leads to stimulation of pulmonary cytokines such as Tumor Necrosis Factor Alpha (TNFα), IL(Interleukin)-1β, IL-6, and in mice with TLR4 [13]. As a response to cytokines produced by immune cells, the endothelial cells manifest adhesion molecules and yield vasoactive compounds, inflammatory mediators, and chemoattractants molecules that cause shifting anticoagulant state to pro-coagulant. Local activation of endothelial cells helps combat the infection source, while systemic activation causes microvascular thrombosis, permeation in capillaries, decreased blood pressure, tissue hypoxia, and lastly tissue damage [14].
Enhanced endothelial vascular permeability in various organs results in plasma extravasations and bacterial translocation, which may play a role in the development of severe tissue damage [15].
The recognition of pro-inflammatory components (e.g., cytokines, endotoxin) give declaration that sepsis may be generated through administration of these molecules into experimental animals to detect particular agents that counteract, prevent or restrict the possible damaging effects of such compounds [16].
The Cecal Ligation and Puncture (CLP) model causes LPS, a gram-negative bacteria element that was considered as a basic regulator of the pathogenesis of bacterial infections and is involved in endotoxic crisis [17]. The illness begins with a localized infection caused by microorganisms such as bacteria, which progresses to tissue invasion via the bloodstream, as a result of which sepsis and endotoxic shock occur, the major symptoms of which are low blood pressure, ischemia, organ damage, and death [18, 19]. CLP model of polymicrobial sepsis is the most widely used model as it thoroughly looks alike the development and features of sepsis in humans because the cecum is filled with bacteria, so its puncture cause polymicrobial peritonitis, bacteremia (bacteria move into the blood), multiple organ malfunction, septic shock, and eventually death [20]. It is usually established that CLP represents clinical actuality more precisely than other practices, such as the administration of endotoxin or bacteria into rodents by injection, so CLP is suggested as a gold standard for the initiation and investigation of sepsis pathogenesis [21].
Ertugliflozin is an efficient and eclectic inhibitor for Sodium-Glucose Cotransporter 2 (SGLT2), which reabsorb about 90% of glucose from glomerulus filtration [22], increasing the urinary excretion of glucose and reducing blood glucose concentration without the need for extreme insulin secretion [23]. It is a novel anti-diabetic medication developed in the latest year. Ertugliflozin was approved by Food and Drug Administration Agency (FDA) in December 2017. This drug is an effective oral medication for patients with type II diabetes mellitus [24], used as an adjuvant to exercise and diet in patients (≥18 years). It is suggested as monotherapy in patients with contraindications and/or intolerant to metformin. Also, it can be combined with other anti-hyperglycemic agents (more commonly metformin and Dipeptidyl Peptidase 4 [DPP-4] inhibitors) to help further reduce HbA1c. Ertugliflozin is not approved for the treatment of type I diabetes and in patients with renal failure and ketoacidosis [25]. Body weight and blood pressure are markedly reduced in diabetic patients using ertugliflozin [26]. Clinical trials show that ertugliflozin is well tolerated and free from significant adverse effects [27]. Its safety is comparable to other agents in the same class (i.e., these agents are associated with urinary tract infection, hypotension, genital mycotic infection, lower limb resection, and ketoacidosis). As ertugliflozin is newly approved, so related risks and adverse effects are under collection [28]. SGLT2 inhibitor drugs were validated in a variety of animal experiments to lower pro-inflammatory cytokine levels [29, 30].
Clinicians lack efficient therapeutic regimens for ALI and ARDS patients. There are many anti-inflammatory drugs available traditionally, but they are associated with systemic adverse effects that make their use for ALI/ARDS limited [30]. According to our knowledge, this is the first study on the potential effect of ertugliflozin in acute lung injury during endotoxemia.
This study aimed to explore how ertugliflozin protects against acute lung injury caused by endotoxemia.
Materials and Methods
This research was conducted in the Department of Pharmacology & the Middle Euphrates Centre for Cancer Research at the Faculty of Medicine, University of Kufa, Iraq, between 10 February and 30 September 2022. The study was approved by the Bioethical Committee at the University of Kufa, as well as its demonstration in the Faculty of Medicine was authorized (ethical approval number 2933 on 2/2/2022). Throughout the proceedings, the Committee’s recommendations were followed.
Animals and study design
Albino Swiss mice (N=20), weighing 20–35 g on average and maturing at 9–13 weeks, were purchased from the Faculty of Science's Animal Resources Centre at the University of Kufa. Animals were housed at a constant temperature of 25°C with a humidity level of 60-65% and a 12 h light/dark cycle. The mice were divided into 4 groups of five mice each:
⦁ Sham group: anaesthetized with laparotomy surgery without CLP
⦁ Sepsis group: CLP was performed.
⦁ Vehicle group: mice were given an equal volume of Dimethyl Sulfoxide (DMSO) orally (PO) daily for one week before CLP
⦁ Ertugliflozin pre-treated group: mice received a 20mg/kg ertugliflozin once daily PO for one week before CLP [31].
Experimental procedure
Mice were sedated intraperitoneally with 100 mg/kg ketamine (Bremer Pharma GMB; Germany) and 10 mg xylazine (V.M.D; Belgium). The cecum was identified after a 1-2 cm median abdominal laparotomy. The cecum then was ligated directly under the ileocecal valve & perforated with a needle (G-20) twice, reverted to its normal position. Then, the abdomen had sutured by 5.0 medical suture. Every 4 h for 24 h, mice were tested for various illness indicators before being returned to their cages [32, 33].
Preparation of Ertugliflozin
Ertugliflozin powder (Med Chem Express; USA) was prepared by dilution in DMSO (Abu Dhabi medical; UAE) and Corn Oil (Sigma-Aldrich Co.; USA) in the concentration of 50 mg/ml and administered orally in a dose of 20mg/kg/day for 7 days before CLP [31].
Measurement of inflammatory and oxidative stress mediators
Tissue homogenization for IL-6, IL-1β, TNF-α, MIF, TLR4 and F2-isoprostane measurements were done as following [34]:
The lung was washed with a 0.9% sodium chloride solution to remove any RBCs or clots before being conserved at -80 C deep freeze. Lung sections were homogenized employing an elevated ultrasonic liquid processing in 1:10 (weight/volume) phosphate buffer saline (PBS; Sigma-Aldrich; USA), PBS with 1% Triton X-100 (Abo LTD; Switzerland) and 1% protease inhibitor cocktail (MedChemExpress MCE®; USA) [35]. The lysates were then centrifuged at 10,000 rpm for 10 min, and the supernatants were then utilized to measure the above inflammatory mediators by Elisa kit for mice specific for each mediator (Bioassay®; China). Instructions of kit manufacture were followed in measurements, and the device used was Bio Elisa Reader (Bio/tek-Instruments. Inc.; USA).
Tissue preparation for histopathology
The other area of the lung tissue sample was immersed in 10% formalin, dehydrated in various alcohols, cleaned in xylene, and embedded in a paraffin block. Tissue slide slices were subsequently cut into 5 mm-thick horizontal lines and stained with Hematoxylin and Eosin (H&E) dye before being sent to a histopathologist for histological examination [36]. Lung tissue injury was assessed blindly by competent pathologists using four randomly selected areas. The sections were graded using a scale constructed to determine the degree of lung injury. The histopathological examination was carried out at original magnifications of X400 and was evaluated based on the percentage of tissue damage in this fashion [37]:
Score zero: normal tissue architecture
Mild, Score 1: <25% damage
Moderate, Score 2: 25-50% damage
Severe, Score 3: 50-75% damage
Highly severe, Score 4: 75-100% damage
Statistical analysis
SPSS software version 26 was used to analyzedata. The information was presented as mean and standard error (SEM). Multiple group comparisons were made using the one way ANOVA, which was followed by a post-hoc analysis using the Bonferroni correction. Differences in mean scores for histopathological alterations of lung tissue were analyzed using the Kruskal-Wallis test. P values under 0.05 were deemed statistically significant in each test.
Findings
Effect of ertugliflozin on inflammatory mediators
The sepsis and vehicle groups were compared to the mice in the sham group, and it was shown that the lung levels of IL-6, IL-1β, TNF-α, Macrophage migration inhibitory factor (MIF), and TLR4 were significantly higher in CLP and DMSO groups (p<0.05). Levels of these mediators in the ertugliflozin pretreated group were significantly (p<0.05) reduced (Diagram 1A-E).
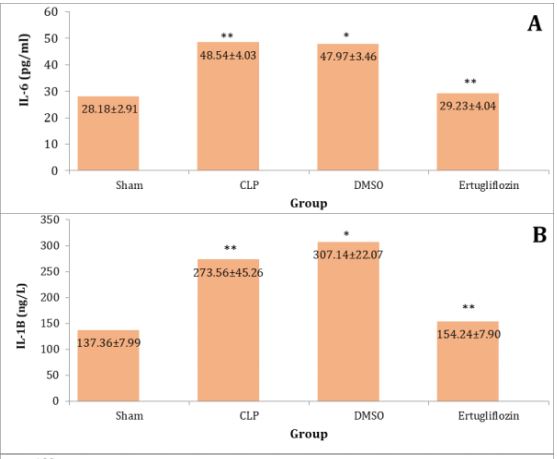
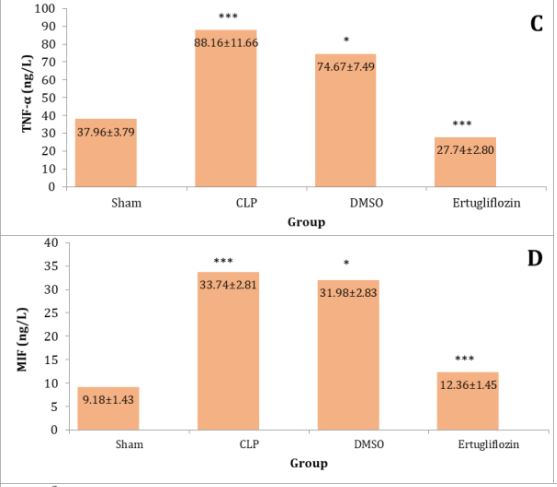
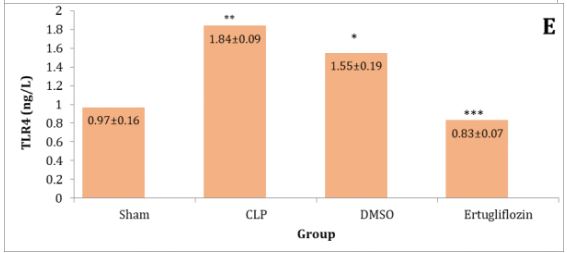
Diagram 1) Effect of ertugliflozin on lung tissue inflammatory mediators. (A) IL-6, (B) IL-1β, (C) TNF-α, (D) MIF, (E) TLR4. Data expressed as mean ± SEM. All comparisons are between CLP & other groups, *p>0.05, **p<0.05, ***p<0.0001.
Ertugliflozin attenuated oxidative stress (8-iso-PGF2α) in lung tissue.
Compared to the sham group, mice in the CLP and DMSO groups showed a significant rise in lung tissue levels of 8-iso-PGF2. The amount of this mediator in the lung tissue was dramatically lowered by ertugliflozin pre-treatment (Diagram 2).

Diagram 2) Effect of ertugliflozin on lung tissue oxidative mediator. All comparisons are between CLP & other groups, *p>0.05, **p<0.05, ***p<0.0001.
Effect of Ertugliflozin on lung histopathology
Lung specimens of mice in sham group appeared without histological changes, and there were no alterations in alveoli and their walls, normal air spaces and bronchioles. Additionally, there were no signs of lung inflammation like edema and hyperemia (Figure 1A). CLP group revealed massive lung damage in which macrophages and neutrophils were seen within the alveoli. Furthermore, lung tissue vessels appeared to be congested with the development of interstitial edema and hyperemia. Additionally, extravasated RBCs in the alveoli and interstitium were also noticed with the highest histopathological score (2.8) (Diagram 3 and Figure 1B). Lung injury was also revealed in mice of the DMSO group with the progression of congestion, hyperemia, and interstitial edema. Besides these changes, perivascular inflammation and accumulation of macrophages and neutrophils in the alveoli also had been observed with a histopathological score of 2.4 (Diagram 3 and Figure 1C).

Diagram 3) Mean histopathological score of lung tissue for the experimental groups. CLP and vehicle groups compared to sham group, p< 0.05 (significant), ertugliflozin compared to CLP and DMSO group, p<0.05 (significant).
Concurring with the potential protective effects of ertugliflozin, the histopathological score (1.2) was significantly diminished in this group (p<0.05). Ertugliflozin pretreated group exhibited mild alterations in lung tissue architecture. Damaging in lung tissue confined to a very mild accumulation of neutrophils and macrophages within the alveoli with focal vascular congestion and hyperemia, suggesting a mild degree of inflammation according to Zahran et al.'s scoring scale of sepsis severity [37] (Diagram 3 and Figure 1D).

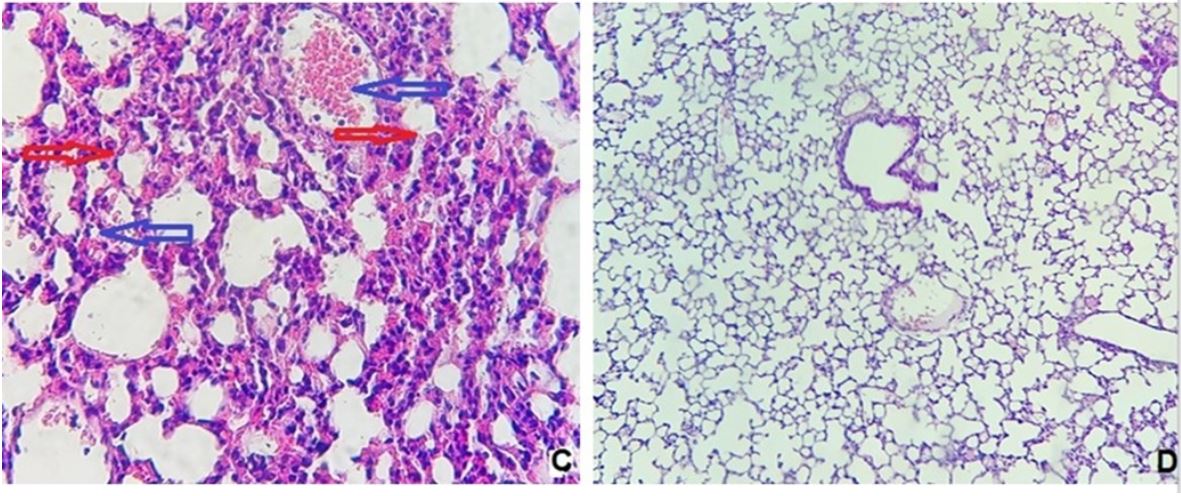
Figure 1) Histological examination of lung tissue
A: sham group (400X). B: CLP group showed severe mixed inflammatory cells infiltration mainly neutrophils and macrophages (red arrows) with hemorrhage, hyperemia &congestion (blue arrows) (100X). C: DMSO group with severe intra-alveolar inflammation rich in macrophages & neutrophils (red arrows) with perivascular inflammation, hyperemia (blue arrows) and extravasation of RBCs (400X). D: Ertugliflozin treated mice, the lung tissue showed very mild, focal interstitial inflammation (400X).
Discussion
The condition known as sepsis describes the body's systemic immunological response to an invading pathogen, which may result in organ damage or even death [38]. Progressive lung function impairment and susceptibility to intrapulmonary infection are prominent sepsis complications among multi-organ failure. In similar circumstances, ARDS frequently develops [39]. In this novel study, we assessed for the first time the potential protective effects of ertugliflozin on ameliorating lung dysfunction due to sepsis caused by the CLP model.
According to our results, inflammatory mediators (IL- 6, TNF-α, IL-1β, MIF, TLR4 & 8-iso-PGF2α) were elevated in lungs of sepsis and vehicle groups. Our results are in line with a study conducted by Wang et al. and clarified that paclitaxel protects the mice lung from acute injury following CLP where the concentrations of IL-6, IL-1B, and TNF- α were markedly elevated in the sepsis group [40].
Furthermore, Xue and Li [41] found that the lung levels of IL-6 and TNF-α were considerably increased in the sepsis group of rats after the CLP procedure. Also, Kong et al. found that the levels of IL-6, IL-1B, and TNF-α in CLP-challenged mice were significantly increased in Bronchoalveolar Lavage Fluid (BALF) [42]. Xu et al. showed that the level of MIF was markedly high in BALF of mice due to LPS sepsis which is consistent with our investigation [43].
TLR4 is essential for LPS responsiveness and is included in the body's defense against G -ve organisms [44]. Through myeloid differentiation factor 88, the TLR4 activates the NF-κB protein, which stimulates the genes that encode pro-inflammatory mediators like TNF- α, NO, and IL-6. They play a vital role in the regulation of inflammatory reactions [45].
In our current study, TLR4 was significantly elevated following CLP induction of sepsis. These findings continued with many other types of research. Yuqing et al., in their investigation on the prophylactic effect of dexmedetomidine on rat's lung, showed that TLR4/MyD88 expressions were noticeably enhanced following CLP [46].
8-iso-PGF2-α level in lung tissue homogenates was dramatically increased in the CLP mice group. Our finding is in settlement with the study performed by Duan et al. in which oxidative stress markers were elevated considerably on LPS stimulation [47]. This marker also appeared to be increased in the lung after CLP injury while studying the preserving effects of zileuton on the lung by Al-Nafakh et al. [48].
SGLT-2 inhibitors, in addition to their effectiveness in lowering blood sugar, also have protective impacts in many disorders like CVD and renal injury [49, 50].
Our study demonstrated that pro-inflammatory mediators were reduced by ertugliflozin. This, in line with Saxena et al., showed that ertugliflozin effectively decreases LPS induced IL-1β secretion in lung cells [29]. Lin et al. investigated that serum and Broncho-alveolar lavage levels of TNF-alpha, IL-1β, and IL-6 were obviously reduced in the canagliflozin-treated group as compared to the LPS group [30]. A meta-analysis of 23 clinical studies (15 randomized plus 8 observational) of SGLT-2I agents exhibited a consistent decline in biomarkers of inflammation (IL-6 and TNF-α) and oxidative stress (8-iso-PGF2α and 8-hydroxy-20-deoxyguanosine) [51].
Abd El-Fattah et al. revealed that dapagliflozin used in LPS-produced ALI results in a substantial decrease in the level of P65 subunit of NF-κB that cause inhibition of NF-kB signaling pathway involving TNF-α, level of NLRP3 in addition to IL-1β [52]. Huang et al. demonstrated a cross-link between AMPK and NF-κB signaling, where found that AMPK signaling results in the suppression of the NF-κB pathway and, as a result, the expression of pro-inflammatory markers also depressed [53]. Ertugliflozin may also suppress the pro-inflammatory mediators by the same mechanisms above. Based on previous findings about ertugliflozin and other SGLT-2Is, we hypothesize that ertugliflozin can modulate AMPK/NF-κB pathway and NLRP3 inflammosome, which may be responsible for its anti-inflammatory effects. To the best of our knowledge, no data existing about the effect of ertugliflozin on these pro-inflammatory cytokines in lung injury sepsis.
In this novel study, ertugliflozin decreased TLR4 in lung tissue. There were insufficient studies about the protective and anti-inflammatory effects of ertugliflozin on different organs as a drug were approved by FDA in 2017, and so the exact mechanism by which it decreases the TLR4 and the other pro-inflammatory cytokines was not fully understood and requires further investigations. However, other SGLT-2 inhibitors exert their anti-inflammatory effects by inhibiting of TLR4/NF- κB pathway. For instance, Panchapakesan et al. showed that empagliflozin reverse glucose-induced increase in TLR4/NF-κB levels in human tubular cells [54]. Ashrafi Jigheh et al. investigated that empagliflozin effectively reduced TLR4 in kidney tissues, and this reduction goes together with diminished NF-κB activity [55]. To the best of our knowledge, no data exist about the effect of ertugliflozin on TLR4 in sepsis-induced lung injury.
SGLT2Is newly recognized as a powerful antioxidant drug that can save the tissues versus oxidative damage, not only due to their anti-hyperglycemic effects but also by depressing the free radical production as presented by the outcomes in kidney tissues of streptozocin-induced diabetic rats [56, 57]. Sugizaki et al. showed that gliflozins enhanced the redox condition and reduced oxidative impairment in diabetic mice nourished high-fat diet [58]. Gliflozins can affect the production of free radicals via direct and indirect mechanisms [59]. Free radicals are generated as a result of the action of a pro-oxidant enzyme, lipid peroxidation, mitochondrial impairment, hemodynamic alterations, and activation of protein kinase C [60]. Dapagliflozin decreased the production of free radicals through the inhibition of NADPH oxidase and enhanced the hemodynamic state, as exhibited in other studies [61, 62]. Kawanami et al. found that Nox4 expression is suppressed following SGLT2Is administration, which results in a decrease in a free radical generation that leads to the inhibition of diabetic nephropathy due to the decline of oxidative stress [63]. Reno-protective potentials of empagliflozin suggested by Gangadharan Komala et al. versus oxidative stress may be due to its glucose-lowering effects [64]. Thus, previous studies supported the influence of SGLT2Is in reducing oxidative stress via adjustment of pro-oxidant enzymes like Nox, nitric oxide synthase of the endothelium, and xanthine oxidase via modification of their expression [63, 65]. Furthermore, there were 23 clinical studies recognized involving 1654 contributors (in 4 studies) that 201 patients with oxidative stress treated with gliflozins. In 75% of these examinations, 8-iso-PGF2α levels were significantly decreased following the use of SGLT-2 inhibitors [66-68]. Data from this meta-analysis study support our finding that SGLT2 inhibitors (Ertugliflozin) reduce oxidative stress markers. To the best of our knowledge, no data exists about the effect of ertugliflozin on 8-isoPGF2a in lung injury sepsis.
In the current study, we found that the sepsis and vehicle groups had significantly more lung tissue damage than the sham group. Sepsis and the vehicle group had the highest histopathological damage scores. Histopathological observations in the CLP and DMSO groups were related to acute and extensive infiltration of inflammatory cells in the alveolar spaces and interstitium, in addition to congestion and extravasation of RBCs. The alveolar wall became thicker with edema and patchy hemorrhage, and also hyaline membrane was formed compared to the sham group.
Our findings are consistent with the research of Zhou et al., which showed that LPS-challenged mice exhibited severe grades of inflammatory cell penetration, interstitial edema, and within the alveoli in addition to thickening in the septum of alveoli [69]. Ertugliflozin pretreatment considerably decreased mononuclear inflammation and another pathological abrasion resulted from the LPS challenge. In the current study, the lung injury score of the drug in the CLP group was 1.6 times higher than that of the ertugliflozin group. This indicated that ertugliflozin pretreatment efficiently attenuated the injury score of the lung. So the histopathological score was significantly reduced (p<0.05) in mice receiving the drug. This diminished pathological score in CLP+ ertugliflozin group supported our novel finding that this drug potentially has lung protective effects where it decreased penetration of inflammatory cells into the lungs, hyperemia, edema, and congestion. As far as we know to date, no sufficient studies are available about protective effects of ertugliflozin on lungs with sepsis. However, many SGLT2I agents produced the same effects as our drug on the lungs. Lin et al. demonstrated that canagliflozin, an SGLT2I analog, manifested normal morphology of the lung with decreased lung pathological score in LPS+ Cana than in the LPS group alone [30]. Abd El-Fattah et al. presented that lung specimens with dapagliflozin, an SGLT2I analog, exhibited no histological changes in bronchioles, airspaces, and alveoli compared to rats exposed to LPS [52].
Limitations of the study: This experimental study in a mouse model used ertugliflozin in a dose of 20 mg/kg/day, so further research with other doses and longer duration are recommended. The pulmonary protective effect of this drug demonstrated in our study was mediated by TLR4 downstream signaling pathways, including NF-κB cascades. Additional studies for other possible mechanisms are needed. Future studies must investigate diabetic patients with sepsis, already under treatment with ertugliflozin.
Conclusion
Ertugliflozin attenuates lung dysfunction during endotoxemia in male mice via downstream inflammatory and oxidative stress signaling pathways.
Acknowledgements: The authors thank the Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa.
Ethical Permission: The study was approved by the Bioethical Committee at the University of Kufa, as well as its demonstration in the Faculty of Medicine was authorized (ethical approval number 2933 on 2/2/2022). Throughout the proceedings, the Committee’s recommendations were followed.
Conflict of Interests: All authors declare that there is no conflict of interest in the study.
Authors’ Contribution: Abd Uljaleel AQ (First Author), Introduction Writer/Main Researcher/Discussion Writer (50%); Hassan ES (Second Author), Methodologist/Assistant Researcher/Statistical Analyst (50%)
Funding: This is a self-funded study. All authors participated in the costs.
The most frequent disease among patients in critical care units is endotoxemia, a Systemic Inflammatory Response Syndrome (SIRS) brought on by infection [1]. A modern study for the Institute of Health Metrics and Evaluation on the widespread of sepsis globally assessed 48.9 million of sepsis conditions and 11 million deaths worldwide, which make up about 20% of all deaths globally [2]. The most serious type of sepsis, endotoxic shock, is spurred on by gram-negative bacterial infections [3].
It is associated with a higher mortality rate of 40%, compared with the 10% mortality observed with sepsis [4]. Acute Respiratory Distress Syndrome (ARDS), Acute Lung Injury (ALI), severe hypotension, rising metabolic acidosis, SIRS, numerous organ failure, and significant mortality are the hallmarks of this shock [3]. The lung is the most exposed and captious organ in sepsis [5], and eventually, lung dysfunction results in lung failure in the form of acute respiratory distress syndrome [6]. ARDS proceed quickly appears within minutes to hours of sepsis onset, where it occurs as a sudden development of severe hypoxemia. The lungs become filled with fluid and are badly compliant, as a result, breathing becomes more difficult. New bilateral diffuse or pulmonary infiltrates will show on chest x-ray, and mechanical ventilation is generally required [7]. Areas of the lung with broken endothelia turn to be filled with neutrophils and macrophages. Interstitial venules develop edema, deposition of fibrin, and surfactant reduction also occurs. Such lung regions become dense and unwell-compliant because gas exchange becomes minimal [8].
Amplification of the host response to infection develops in sepsis and, as a result, variation among pro- and anti-inflammatory responses with a predominance of pro-inflammatory ones [9]. Pro-inflammatory cytokines continue to be produced due to the spreading of inflammation over the bloodstream, which results in increased formation of cytokines in distant spaces [10].
Toll Like Receptors (TLRs) are the most families of the pattern recognition receptors studied, and TLR4 is the most important among this family. TLR4 can recognize Lipopolysaccharide (LPS) and stimulate immune response [11] in order to keep the body against infection. TLR4 and nuclear Factor-κB (NF-κB) are the main regulators of inflammation [12].
The severity of inflammatory response is linked with the expression level of TLR4 and ALI induced by LPS, resulting in activation of NF-κB that leads to stimulation of pulmonary cytokines such as Tumor Necrosis Factor Alpha (TNFα), IL(Interleukin)-1β, IL-6, and in mice with TLR4 [13]. As a response to cytokines produced by immune cells, the endothelial cells manifest adhesion molecules and yield vasoactive compounds, inflammatory mediators, and chemoattractants molecules that cause shifting anticoagulant state to pro-coagulant. Local activation of endothelial cells helps combat the infection source, while systemic activation causes microvascular thrombosis, permeation in capillaries, decreased blood pressure, tissue hypoxia, and lastly tissue damage [14].
Enhanced endothelial vascular permeability in various organs results in plasma extravasations and bacterial translocation, which may play a role in the development of severe tissue damage [15].
The recognition of pro-inflammatory components (e.g., cytokines, endotoxin) give declaration that sepsis may be generated through administration of these molecules into experimental animals to detect particular agents that counteract, prevent or restrict the possible damaging effects of such compounds [16].
The Cecal Ligation and Puncture (CLP) model causes LPS, a gram-negative bacteria element that was considered as a basic regulator of the pathogenesis of bacterial infections and is involved in endotoxic crisis [17]. The illness begins with a localized infection caused by microorganisms such as bacteria, which progresses to tissue invasion via the bloodstream, as a result of which sepsis and endotoxic shock occur, the major symptoms of which are low blood pressure, ischemia, organ damage, and death [18, 19]. CLP model of polymicrobial sepsis is the most widely used model as it thoroughly looks alike the development and features of sepsis in humans because the cecum is filled with bacteria, so its puncture cause polymicrobial peritonitis, bacteremia (bacteria move into the blood), multiple organ malfunction, septic shock, and eventually death [20]. It is usually established that CLP represents clinical actuality more precisely than other practices, such as the administration of endotoxin or bacteria into rodents by injection, so CLP is suggested as a gold standard for the initiation and investigation of sepsis pathogenesis [21].
Ertugliflozin is an efficient and eclectic inhibitor for Sodium-Glucose Cotransporter 2 (SGLT2), which reabsorb about 90% of glucose from glomerulus filtration [22], increasing the urinary excretion of glucose and reducing blood glucose concentration without the need for extreme insulin secretion [23]. It is a novel anti-diabetic medication developed in the latest year. Ertugliflozin was approved by Food and Drug Administration Agency (FDA) in December 2017. This drug is an effective oral medication for patients with type II diabetes mellitus [24], used as an adjuvant to exercise and diet in patients (≥18 years). It is suggested as monotherapy in patients with contraindications and/or intolerant to metformin. Also, it can be combined with other anti-hyperglycemic agents (more commonly metformin and Dipeptidyl Peptidase 4 [DPP-4] inhibitors) to help further reduce HbA1c. Ertugliflozin is not approved for the treatment of type I diabetes and in patients with renal failure and ketoacidosis [25]. Body weight and blood pressure are markedly reduced in diabetic patients using ertugliflozin [26]. Clinical trials show that ertugliflozin is well tolerated and free from significant adverse effects [27]. Its safety is comparable to other agents in the same class (i.e., these agents are associated with urinary tract infection, hypotension, genital mycotic infection, lower limb resection, and ketoacidosis). As ertugliflozin is newly approved, so related risks and adverse effects are under collection [28]. SGLT2 inhibitor drugs were validated in a variety of animal experiments to lower pro-inflammatory cytokine levels [29, 30].
Clinicians lack efficient therapeutic regimens for ALI and ARDS patients. There are many anti-inflammatory drugs available traditionally, but they are associated with systemic adverse effects that make their use for ALI/ARDS limited [30]. According to our knowledge, this is the first study on the potential effect of ertugliflozin in acute lung injury during endotoxemia.
This study aimed to explore how ertugliflozin protects against acute lung injury caused by endotoxemia.
Materials and Methods
This research was conducted in the Department of Pharmacology & the Middle Euphrates Centre for Cancer Research at the Faculty of Medicine, University of Kufa, Iraq, between 10 February and 30 September 2022. The study was approved by the Bioethical Committee at the University of Kufa, as well as its demonstration in the Faculty of Medicine was authorized (ethical approval number 2933 on 2/2/2022). Throughout the proceedings, the Committee’s recommendations were followed.
Animals and study design
Albino Swiss mice (N=20), weighing 20–35 g on average and maturing at 9–13 weeks, were purchased from the Faculty of Science's Animal Resources Centre at the University of Kufa. Animals were housed at a constant temperature of 25°C with a humidity level of 60-65% and a 12 h light/dark cycle. The mice were divided into 4 groups of five mice each:
⦁ Sham group: anaesthetized with laparotomy surgery without CLP
⦁ Sepsis group: CLP was performed.
⦁ Vehicle group: mice were given an equal volume of Dimethyl Sulfoxide (DMSO) orally (PO) daily for one week before CLP
⦁ Ertugliflozin pre-treated group: mice received a 20mg/kg ertugliflozin once daily PO for one week before CLP [31].
Experimental procedure
Mice were sedated intraperitoneally with 100 mg/kg ketamine (Bremer Pharma GMB; Germany) and 10 mg xylazine (V.M.D; Belgium). The cecum was identified after a 1-2 cm median abdominal laparotomy. The cecum then was ligated directly under the ileocecal valve & perforated with a needle (G-20) twice, reverted to its normal position. Then, the abdomen had sutured by 5.0 medical suture. Every 4 h for 24 h, mice were tested for various illness indicators before being returned to their cages [32, 33].
Preparation of Ertugliflozin
Ertugliflozin powder (Med Chem Express; USA) was prepared by dilution in DMSO (Abu Dhabi medical; UAE) and Corn Oil (Sigma-Aldrich Co.; USA) in the concentration of 50 mg/ml and administered orally in a dose of 20mg/kg/day for 7 days before CLP [31].
Measurement of inflammatory and oxidative stress mediators
Tissue homogenization for IL-6, IL-1β, TNF-α, MIF, TLR4 and F2-isoprostane measurements were done as following [34]:
The lung was washed with a 0.9% sodium chloride solution to remove any RBCs or clots before being conserved at -80 C deep freeze. Lung sections were homogenized employing an elevated ultrasonic liquid processing in 1:10 (weight/volume) phosphate buffer saline (PBS; Sigma-Aldrich; USA), PBS with 1% Triton X-100 (Abo LTD; Switzerland) and 1% protease inhibitor cocktail (MedChemExpress MCE®; USA) [35]. The lysates were then centrifuged at 10,000 rpm for 10 min, and the supernatants were then utilized to measure the above inflammatory mediators by Elisa kit for mice specific for each mediator (Bioassay®; China). Instructions of kit manufacture were followed in measurements, and the device used was Bio Elisa Reader (Bio/tek-Instruments. Inc.; USA).
Tissue preparation for histopathology
The other area of the lung tissue sample was immersed in 10% formalin, dehydrated in various alcohols, cleaned in xylene, and embedded in a paraffin block. Tissue slide slices were subsequently cut into 5 mm-thick horizontal lines and stained with Hematoxylin and Eosin (H&E) dye before being sent to a histopathologist for histological examination [36]. Lung tissue injury was assessed blindly by competent pathologists using four randomly selected areas. The sections were graded using a scale constructed to determine the degree of lung injury. The histopathological examination was carried out at original magnifications of X400 and was evaluated based on the percentage of tissue damage in this fashion [37]:
Score zero: normal tissue architecture
Mild, Score 1: <25% damage
Moderate, Score 2: 25-50% damage
Severe, Score 3: 50-75% damage
Highly severe, Score 4: 75-100% damage
Statistical analysis
SPSS software version 26 was used to analyzedata. The information was presented as mean and standard error (SEM). Multiple group comparisons were made using the one way ANOVA, which was followed by a post-hoc analysis using the Bonferroni correction. Differences in mean scores for histopathological alterations of lung tissue were analyzed using the Kruskal-Wallis test. P values under 0.05 were deemed statistically significant in each test.
Findings
Effect of ertugliflozin on inflammatory mediators
The sepsis and vehicle groups were compared to the mice in the sham group, and it was shown that the lung levels of IL-6, IL-1β, TNF-α, Macrophage migration inhibitory factor (MIF), and TLR4 were significantly higher in CLP and DMSO groups (p<0.05). Levels of these mediators in the ertugliflozin pretreated group were significantly (p<0.05) reduced (Diagram 1A-E).



Diagram 1) Effect of ertugliflozin on lung tissue inflammatory mediators. (A) IL-6, (B) IL-1β, (C) TNF-α, (D) MIF, (E) TLR4. Data expressed as mean ± SEM. All comparisons are between CLP & other groups, *p>0.05, **p<0.05, ***p<0.0001.
Ertugliflozin attenuated oxidative stress (8-iso-PGF2α) in lung tissue.
Compared to the sham group, mice in the CLP and DMSO groups showed a significant rise in lung tissue levels of 8-iso-PGF2. The amount of this mediator in the lung tissue was dramatically lowered by ertugliflozin pre-treatment (Diagram 2).

Diagram 2) Effect of ertugliflozin on lung tissue oxidative mediator. All comparisons are between CLP & other groups, *p>0.05, **p<0.05, ***p<0.0001.
Effect of Ertugliflozin on lung histopathology
Lung specimens of mice in sham group appeared without histological changes, and there were no alterations in alveoli and their walls, normal air spaces and bronchioles. Additionally, there were no signs of lung inflammation like edema and hyperemia (Figure 1A). CLP group revealed massive lung damage in which macrophages and neutrophils were seen within the alveoli. Furthermore, lung tissue vessels appeared to be congested with the development of interstitial edema and hyperemia. Additionally, extravasated RBCs in the alveoli and interstitium were also noticed with the highest histopathological score (2.8) (Diagram 3 and Figure 1B). Lung injury was also revealed in mice of the DMSO group with the progression of congestion, hyperemia, and interstitial edema. Besides these changes, perivascular inflammation and accumulation of macrophages and neutrophils in the alveoli also had been observed with a histopathological score of 2.4 (Diagram 3 and Figure 1C).

Diagram 3) Mean histopathological score of lung tissue for the experimental groups. CLP and vehicle groups compared to sham group, p< 0.05 (significant), ertugliflozin compared to CLP and DMSO group, p<0.05 (significant).
Concurring with the potential protective effects of ertugliflozin, the histopathological score (1.2) was significantly diminished in this group (p<0.05). Ertugliflozin pretreated group exhibited mild alterations in lung tissue architecture. Damaging in lung tissue confined to a very mild accumulation of neutrophils and macrophages within the alveoli with focal vascular congestion and hyperemia, suggesting a mild degree of inflammation according to Zahran et al.'s scoring scale of sepsis severity [37] (Diagram 3 and Figure 1D).


Figure 1) Histological examination of lung tissue
A: sham group (400X). B: CLP group showed severe mixed inflammatory cells infiltration mainly neutrophils and macrophages (red arrows) with hemorrhage, hyperemia &congestion (blue arrows) (100X). C: DMSO group with severe intra-alveolar inflammation rich in macrophages & neutrophils (red arrows) with perivascular inflammation, hyperemia (blue arrows) and extravasation of RBCs (400X). D: Ertugliflozin treated mice, the lung tissue showed very mild, focal interstitial inflammation (400X).
Discussion
The condition known as sepsis describes the body's systemic immunological response to an invading pathogen, which may result in organ damage or even death [38]. Progressive lung function impairment and susceptibility to intrapulmonary infection are prominent sepsis complications among multi-organ failure. In similar circumstances, ARDS frequently develops [39]. In this novel study, we assessed for the first time the potential protective effects of ertugliflozin on ameliorating lung dysfunction due to sepsis caused by the CLP model.
According to our results, inflammatory mediators (IL- 6, TNF-α, IL-1β, MIF, TLR4 & 8-iso-PGF2α) were elevated in lungs of sepsis and vehicle groups. Our results are in line with a study conducted by Wang et al. and clarified that paclitaxel protects the mice lung from acute injury following CLP where the concentrations of IL-6, IL-1B, and TNF- α were markedly elevated in the sepsis group [40].
Furthermore, Xue and Li [41] found that the lung levels of IL-6 and TNF-α were considerably increased in the sepsis group of rats after the CLP procedure. Also, Kong et al. found that the levels of IL-6, IL-1B, and TNF-α in CLP-challenged mice were significantly increased in Bronchoalveolar Lavage Fluid (BALF) [42]. Xu et al. showed that the level of MIF was markedly high in BALF of mice due to LPS sepsis which is consistent with our investigation [43].
TLR4 is essential for LPS responsiveness and is included in the body's defense against G -ve organisms [44]. Through myeloid differentiation factor 88, the TLR4 activates the NF-κB protein, which stimulates the genes that encode pro-inflammatory mediators like TNF- α, NO, and IL-6. They play a vital role in the regulation of inflammatory reactions [45].
In our current study, TLR4 was significantly elevated following CLP induction of sepsis. These findings continued with many other types of research. Yuqing et al., in their investigation on the prophylactic effect of dexmedetomidine on rat's lung, showed that TLR4/MyD88 expressions were noticeably enhanced following CLP [46].
8-iso-PGF2-α level in lung tissue homogenates was dramatically increased in the CLP mice group. Our finding is in settlement with the study performed by Duan et al. in which oxidative stress markers were elevated considerably on LPS stimulation [47]. This marker also appeared to be increased in the lung after CLP injury while studying the preserving effects of zileuton on the lung by Al-Nafakh et al. [48].
SGLT-2 inhibitors, in addition to their effectiveness in lowering blood sugar, also have protective impacts in many disorders like CVD and renal injury [49, 50].
Our study demonstrated that pro-inflammatory mediators were reduced by ertugliflozin. This, in line with Saxena et al., showed that ertugliflozin effectively decreases LPS induced IL-1β secretion in lung cells [29]. Lin et al. investigated that serum and Broncho-alveolar lavage levels of TNF-alpha, IL-1β, and IL-6 were obviously reduced in the canagliflozin-treated group as compared to the LPS group [30]. A meta-analysis of 23 clinical studies (15 randomized plus 8 observational) of SGLT-2I agents exhibited a consistent decline in biomarkers of inflammation (IL-6 and TNF-α) and oxidative stress (8-iso-PGF2α and 8-hydroxy-20-deoxyguanosine) [51].
Abd El-Fattah et al. revealed that dapagliflozin used in LPS-produced ALI results in a substantial decrease in the level of P65 subunit of NF-κB that cause inhibition of NF-kB signaling pathway involving TNF-α, level of NLRP3 in addition to IL-1β [52]. Huang et al. demonstrated a cross-link between AMPK and NF-κB signaling, where found that AMPK signaling results in the suppression of the NF-κB pathway and, as a result, the expression of pro-inflammatory markers also depressed [53]. Ertugliflozin may also suppress the pro-inflammatory mediators by the same mechanisms above. Based on previous findings about ertugliflozin and other SGLT-2Is, we hypothesize that ertugliflozin can modulate AMPK/NF-κB pathway and NLRP3 inflammosome, which may be responsible for its anti-inflammatory effects. To the best of our knowledge, no data existing about the effect of ertugliflozin on these pro-inflammatory cytokines in lung injury sepsis.
In this novel study, ertugliflozin decreased TLR4 in lung tissue. There were insufficient studies about the protective and anti-inflammatory effects of ertugliflozin on different organs as a drug were approved by FDA in 2017, and so the exact mechanism by which it decreases the TLR4 and the other pro-inflammatory cytokines was not fully understood and requires further investigations. However, other SGLT-2 inhibitors exert their anti-inflammatory effects by inhibiting of TLR4/NF- κB pathway. For instance, Panchapakesan et al. showed that empagliflozin reverse glucose-induced increase in TLR4/NF-κB levels in human tubular cells [54]. Ashrafi Jigheh et al. investigated that empagliflozin effectively reduced TLR4 in kidney tissues, and this reduction goes together with diminished NF-κB activity [55]. To the best of our knowledge, no data exist about the effect of ertugliflozin on TLR4 in sepsis-induced lung injury.
SGLT2Is newly recognized as a powerful antioxidant drug that can save the tissues versus oxidative damage, not only due to their anti-hyperglycemic effects but also by depressing the free radical production as presented by the outcomes in kidney tissues of streptozocin-induced diabetic rats [56, 57]. Sugizaki et al. showed that gliflozins enhanced the redox condition and reduced oxidative impairment in diabetic mice nourished high-fat diet [58]. Gliflozins can affect the production of free radicals via direct and indirect mechanisms [59]. Free radicals are generated as a result of the action of a pro-oxidant enzyme, lipid peroxidation, mitochondrial impairment, hemodynamic alterations, and activation of protein kinase C [60]. Dapagliflozin decreased the production of free radicals through the inhibition of NADPH oxidase and enhanced the hemodynamic state, as exhibited in other studies [61, 62]. Kawanami et al. found that Nox4 expression is suppressed following SGLT2Is administration, which results in a decrease in a free radical generation that leads to the inhibition of diabetic nephropathy due to the decline of oxidative stress [63]. Reno-protective potentials of empagliflozin suggested by Gangadharan Komala et al. versus oxidative stress may be due to its glucose-lowering effects [64]. Thus, previous studies supported the influence of SGLT2Is in reducing oxidative stress via adjustment of pro-oxidant enzymes like Nox, nitric oxide synthase of the endothelium, and xanthine oxidase via modification of their expression [63, 65]. Furthermore, there were 23 clinical studies recognized involving 1654 contributors (in 4 studies) that 201 patients with oxidative stress treated with gliflozins. In 75% of these examinations, 8-iso-PGF2α levels were significantly decreased following the use of SGLT-2 inhibitors [66-68]. Data from this meta-analysis study support our finding that SGLT2 inhibitors (Ertugliflozin) reduce oxidative stress markers. To the best of our knowledge, no data exists about the effect of ertugliflozin on 8-isoPGF2a in lung injury sepsis.
In the current study, we found that the sepsis and vehicle groups had significantly more lung tissue damage than the sham group. Sepsis and the vehicle group had the highest histopathological damage scores. Histopathological observations in the CLP and DMSO groups were related to acute and extensive infiltration of inflammatory cells in the alveolar spaces and interstitium, in addition to congestion and extravasation of RBCs. The alveolar wall became thicker with edema and patchy hemorrhage, and also hyaline membrane was formed compared to the sham group.
Our findings are consistent with the research of Zhou et al., which showed that LPS-challenged mice exhibited severe grades of inflammatory cell penetration, interstitial edema, and within the alveoli in addition to thickening in the septum of alveoli [69]. Ertugliflozin pretreatment considerably decreased mononuclear inflammation and another pathological abrasion resulted from the LPS challenge. In the current study, the lung injury score of the drug in the CLP group was 1.6 times higher than that of the ertugliflozin group. This indicated that ertugliflozin pretreatment efficiently attenuated the injury score of the lung. So the histopathological score was significantly reduced (p<0.05) in mice receiving the drug. This diminished pathological score in CLP+ ertugliflozin group supported our novel finding that this drug potentially has lung protective effects where it decreased penetration of inflammatory cells into the lungs, hyperemia, edema, and congestion. As far as we know to date, no sufficient studies are available about protective effects of ertugliflozin on lungs with sepsis. However, many SGLT2I agents produced the same effects as our drug on the lungs. Lin et al. demonstrated that canagliflozin, an SGLT2I analog, manifested normal morphology of the lung with decreased lung pathological score in LPS+ Cana than in the LPS group alone [30]. Abd El-Fattah et al. presented that lung specimens with dapagliflozin, an SGLT2I analog, exhibited no histological changes in bronchioles, airspaces, and alveoli compared to rats exposed to LPS [52].
Limitations of the study: This experimental study in a mouse model used ertugliflozin in a dose of 20 mg/kg/day, so further research with other doses and longer duration are recommended. The pulmonary protective effect of this drug demonstrated in our study was mediated by TLR4 downstream signaling pathways, including NF-κB cascades. Additional studies for other possible mechanisms are needed. Future studies must investigate diabetic patients with sepsis, already under treatment with ertugliflozin.
Conclusion
Ertugliflozin attenuates lung dysfunction during endotoxemia in male mice via downstream inflammatory and oxidative stress signaling pathways.
Acknowledgements: The authors thank the Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa.
Ethical Permission: The study was approved by the Bioethical Committee at the University of Kufa, as well as its demonstration in the Faculty of Medicine was authorized (ethical approval number 2933 on 2/2/2022). Throughout the proceedings, the Committee’s recommendations were followed.
Conflict of Interests: All authors declare that there is no conflict of interest in the study.
Authors’ Contribution: Abd Uljaleel AQ (First Author), Introduction Writer/Main Researcher/Discussion Writer (50%); Hassan ES (Second Author), Methodologist/Assistant Researcher/Statistical Analyst (50%)
Funding: This is a self-funded study. All authors participated in the costs.
Keywords:
References
1. Hamza RT, Majeed SA. Nephroprotective effect of melatonin in sepsis induces renal injury: CLP mice model. Lat Am J Pharm. 2022;40(4):589-96. [Link]
2. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med. 2021;47(11):1181-247. [Link] [DOI:10.1007/s00134-021-06506-y]
3. Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014;5(1):213-8. [Link] [DOI:10.4161/viru.27024]
4. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75-87. [Link] [DOI:10.1016/S0140-6736(18)30696-2]
5. Hasan Z, Palani K, Rahman M, Thorlacius H. Targeting CD44 expressed on neutrophils inhibits lung damage in abdominal sepsis. Shock. 2011;35(6):567-72. [Link] [DOI:10.1097/SHK.0b013e3182144935]
6. Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):337-49. [Link] [DOI:10.1055/s-2006-948288]
7. Diamond M, Peniston HL, Sanghavi D, Mahapatra S, et al. Acute respiratory distress syndrome. [Updated 2022 Oct 30-. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [Link]
8. Nugent K, Dobbe L, Rahman R, Elmassry M, Paz P. Lung morphology and surfactant function in cardiogenic pulmonary edema: a narrative review. J Thorac Dis. 2019;11(9):4031-8. [Link] [DOI:10.21037/jtd.2019.09.02]
9. Ginsburg I, Korem M, Koren E, Varani J. Pro-inflammatory agents released by pathogens, dying host cells, and neutrophils act synergistically to destroy host tissues: a working hypothesis. J Inflamm Res. 2019;12:35-47. [Link] [DOI:10.2147/JIR.S190007]
10. Kang SS, Ren Y, Liu CC, Kurti A, Baker KE, Bu G, et al. Lipocalin-2 protects the brain during inflammatory conditions. Mol Psychiatry. 2018;23(2):344-50. [Link] [DOI:10.1038/mp.2016.243]
11. Hussein SN, Majeed SA, Ghafil FA, Hassan ES, Hadi NR. Toll-like receptors 4 antagonist, Ibudilast, ameliorates acute renal impairment induced by sepsis in an experimental model. Bull Nation Institut Health. 2022;140(7):2899-909. [Link]
12. Xing H, Li R, Qing Y, Ying B, Qin Y. Biomaterial-based osteoimmunomodulatory strategies via the TLR4-NF-κB signaling pathway: a review. Appl Mater Today. 2021;22:100969. [Link] [DOI:10.1016/j.apmt.2021.100969]
13. Hu R, Xu H, Jiang H, Zhang Y, Sun Y. The role of TLR4 in the pathogenesis of indirect acute lung injury. Front Biosci (Landmark Ed). 2013;18(4):1244-55. [Link] [DOI:10.2741/4176]
14. Dolmatova EV, Wang K, Mandavilli R, Griendling KK. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res. 2021;117(1):60-73. [Link] [DOI:10.1093/cvr/cvaa070]
15. Kwon S-Y, Ro M, Kim JH. Mediatory roles of leukotriene B4 receptors in LPS-induced endotoxic shock. Sci Rep. 2019;9:5936. [Link] [DOI:10.1038/s41598-019-42410-8]
16. Balk RA. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. 2014;5(1):20-6. [Link] [DOI:10.4161/viru.27135]
17. Jawad AS, Hassan ES, Mohammad AR. Protective effect of empagliflozin from acute renal injury during endotoxemia in mice model. Lat Am J Pharm. 2022;41(2):463-71. [Link]
18. Mohammad AR, Hadi AR, Hassan ES. Potential protective effect of ibrutinib from acute brain injury during endotoxemia in mice. Lat Am J Pharm. 2022;41(2):472-80. [Link]
19. Hassan ES, Jawad AS. Mohammad AR. Protective effect of liraglutide from acute renal injury during endotoxemia in mice mode. Lat Am J Pharm. 2022;41(2):428-36. [Link]
20. Deitch EA. Rodent models of intra-abdominal infection. Shock. 2005;24(Suppl 1):19-23. [Link] [DOI:10.1097/01.shk.0000191386.18818.0a]
21. Raven K. Rodent models of sepsis found shockingly lacking. Nat Med. 2012;18:998. [Link] [DOI:10.1038/nm0712-998a]
22. Miao Z, Nucci G, Amin N, Sharma R, Mascitti V, Tugnait M, et al. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF-04971729) in healthy male subjects. Drug Metab Dispos. 2013;41(2):445-56. [Link] [DOI:10.1124/dmd.112.049551]
23. Kovacich N, Chavez B. Ertugliflozin (Steglatro): a new option for SGLT2 inhibition. P T. 2018;43(12):736-42. [Link]
24. Nguyen VK, White JR. Overview of Ertugliflozin. Clin Diabetes. 2019;37(2):176-8. [Link] [DOI:10.2337/cd18-0097]
25. Ahrén B. Novel combination treatment of type 2 diabetes DPP-4 inhibition + metformin. Vasc Health Risk Manag. 2008;4(2):383-94. [Link] [DOI:10.2147/VHRM.S1944]
26. Kalgutkar AS, Tugnait M, Zhu T, Kimoto E, Miao Z, Mascitti V, et al. Preclinical species and human disposition of PF-04971729, a selective inhibitor of the sodium-dependent glucose cotransporter 2 and clinical candidate for the treatment of type 2 diabetes mellitus. Drug Metab Dispos. 2011;39(9):1609-19. [Link] [DOI:10.1124/dmd.111.040675]
27. Cinti F, Moffa S, Impronta F, Cefalo CM, Sun VA, Sorice GP, et a. Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: evidence to date. Drug Des Devel Ther. 2017;11:2905-19. [Link] [DOI:10.2147/DDDT.S114932]
28. Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health. 2019;16(16):2965. [Link] [DOI:10.3390/ijerph16162965]
29. Saxena S, Meher K, Rotella M, Vangala S, Chandran S, Malhotra N, et al. Identification of SGLT2 inhibitor Ertugliflozin as a treatment for COVID-19 using computational and experimental paradigm. Biorexiv, 2021. [Link] [DOI:10.1101/2021.06.18.448921]
30. Lin F, Song C, Zeng Y, Li Y, Li H, Liu B, et al. Canagliflozin alleviates LPS-induced acute lung injury by modulating alveolar macrophage polarization. Int Immunopharmacol. 2020;88:106969. [Link] [DOI:10.1016/j.intimp.2020.106969]
31. Basalay MV, Arjun S, Davidson SM, Yellon DM. The role of parasympathetic mechanisms in the infarct-limiting effect of SGLT2 inhibitor ertugliflozin. Biorxiv 2021. [Link] [DOI:10.1101/2021.10.01.462765]
32. Drosatos K, Khan RS, Trent CM, Jiang H, Son N-H, Blaner WS, et al. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail. 2013;6(3):550-62. [Link] [DOI:10.1161/CIRCHEARTFAILURE.112.000177]
33. Wellington D, Mikaelian I, Singer L. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci. 2013;52(4):481-7. [Link]
34. Yousif NG, Hadi NR, Al-Amran FG, Zigam QA. The cardioprotective effect of irbesartan in polymicrobial sepsis: the role of the P38 MAPK/ NF- ĸB signaling pathway. Herz. 2018;43(2):140-5. [Linkvvvv] [DOI:10.1007/s00059-017-4537-6]
35. Hadi NR, Al-amran FG, Hussein AA. Effects of thyroid hormone analogue and a leukotrienes pathway-blocker on renal ischemia/reperfusion injury in mice. BMC Nephrol. 2011;12:70. [Link] [DOI:10.1186/1471-2369-12-70]
36. Chandrashekhar VM, Ranpariya VL, Ganapaty S, Parashar A, Muchandi AA. Neuroprotective activity of Matricaria recutita Linn against global model of ischemia in rats. J Ethnopharmacol. 2010;127(3):645-51. [Link] [DOI:10.1016/j.jep.2009.12.009]
37. Zahran R, Ghozy A, Elkholy SS, El-Taweel F, El-Magd MA. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia-reperfusion injury in a rat model. Int J Urol. 2020;27(11):1039-49. [Link] [DOI:10.1111/iju.14345]
38. Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7:2050312119835043. [Link] [DOI:10.1177/2050312119835043]
39. Costa ELV, Schettino IAL, Schettino GPP. The lung in sepsis: guilty or innocent? Endocr Metab Immune Disord Drug Targets. 2006;6(2):213-6. [Link] [DOI:10.2174/187153006777442413]
40. Wang YM, Ji R, Chen WW, Huang SW, Zheng YJ, Yang ZT, et al. Paclitaxel alleviated sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-κB pathway. Drug Des Devel Ther. 2019;13:3391-404. [Link] [DOI:10.2147/DDDT.S222296]
41. Xue H, Li M. Protective effect of pterostilbene on sepsis-induced acute lung injury in a rat model via the JAK2/STAT3 pathway. Ann Transl Med. 2020;8(21):1452. [Link] [DOI:10.21037/atm-20-5814]
42. 42 Kong Q, Wu X, Qiu Z, Huang Q, Xia Z, Song X. Protective effect of dexmedetomidine on acute lung injury via the upregulation of tumour necrosis factor-α-induced protein-8-like 2 in septic mice. Inflammation. 2020;43(3):833-46. [Link] [DOI:10.1007/s10753-019-01169-w]
43. Xu J, Wei G, Wang J, Zhu J, Yu M, Zeng X, et al. Glucagon-like peptide-1 receptor activation alleviates lipopolysaccharide-induced acute lung injury in mice via maintenance of endothelial barrier function. Lab Investig. 2019;99(4):577-87. [Link] [DOI:10.1038/s41374-018-0170-0]
44. Luo L, Lucas RM, Liu L, Stow JL. Signalling, sorting and scaffolding adaptors for Toll-like receptors. J Cell Sci. 2019;133(5):jcs239194. [Link] [DOI:10.1242/jcs.239194]
45. Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell. 2017;168(1-2):37-57. [Link] [DOI:10.1016/j.cell.2016.12.012]
46. Wu Y, Liu Y, Huang H, Zhu Y, Zhang Y, Lu F, et al. Dexmedetomidine inhibits inflammatory reaction in lung tissues of septic rats by suppressing TLR4/NF-κB pathway. Mediators Inflamm. 2013;2013:562154. [Link] [DOI:10.1155/2013/562154]
47. Duan Q, Jia Y, Qin Y, Jin Y, Hu H, Chen J. Narciclasine attenuates LPS-induced acute lung injury in neonatal rats through suppressing inflammation and oxidative stress. Bioengineered. 2020;11(1):801-10. [Link] [DOI:10.1080/21655979.2020.1795424]
48. Alnfakh ZA, Al-Nafakh RT, Hameed AMA, Abdelhussain MA, Hadi NR. Lung protective potential effect of zileuton during endotoxaemia model in male mice. Wiad Lek. 2022;75(12):3066-73. [Link] [DOI:10.36740/WLek202212130]
49. Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154-66. [Link] [DOI:10.1007/s00125-019-4859-4]
50. Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65(9): 2784-94. [Link] [DOI:10.2337/db16-0058]
51. Bray JJ, Foster-Davies H, Stephens JW. A systematic review examining the effects of sodium-glucose cotransporter-2 inhibitors (SGLT2is) on biomarkers of inflammation and oxidative stress. Diabetes Res Clin Pract. 2020;168: 108368. [Link] [DOI:10.1016/j.diabres.2020.108368]
52. Abd El-Fattah EE, Saber S, Mourad AAE, El-Ahwany E, Amin NA, Cavalu S, et al. The dynamic interplay between AMPK/NFκB signaling and NLRP3 is a new therapeutic target in inflammation: Emerging role of dapagliflozin in overcoming lipopolysaccharide-mediated lung injury. Biomed Pharmacother. 2022;147:112628. [Link] [DOI:10.1016/j.biopha.2022.112628]
53. Huang BP, Lin CH, Chen HM, Lin JT, Cheng YF, Kao SH. AMPK activation inhibits expression of proinflammatory mediators through downregulation of PI3K/p38 MAPK and NF-κB signaling in murine macrophages. DNA Cell Biol. 2015;34(2):133-41. [Link] [DOI:10.1089/dna.2014.2630]
54. Panchapakesan U, Pegg K, Gross S, Komala Mg, Mudaliar H, orbes J, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells-renoprotection in diabetic nephropathy? PLoS One. 2013;8(2):e54442. [Link] [DOI:10.1371/journal.pone.0054442]
55. Ashrafi Jigheh Z, Ghorbani Haghjo A, Argani H, Roshangar L, Rashtchizadeh N, Sanajou D, et al. Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iran J Basic Med Sci. 2019;22(4):384-90. [Link]
56. Ishibashi Y, Matsui T, Yamagishi SI. Tofogliflozin, a highly selective inhibitor of SGLT2 blocks proinflammatory and proapoptotic effects of glucose overload on proximal tubular cells partly by suppressing oxidative stress generation. Horm Metab Res. 2016;48(3):191-5. [Link] [DOI:10.1055/s-0035-1555791]
57. Osorio H, Coronel I, Arellano A, Pacheco U, Bautista R, Franco M, et al. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med Cell Longev. 2012;2012:542042. [Link] [DOI:10.1155/2012/542042]
58. Sugizaki T, Zhu S, Guo G, Matsumoto A, Zhao J, Endo M, et al. Treatment of diabetic mice with the SGLT2 inhibitor TA‐1887 antagonizes diabetic cachexia and decreases mortality. NPJ Aging Mech Dis. 2017;3:12. [Link] [DOI:10.1038/s41514-017-0012-0]
59. Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. European Journal of Pharmacology. 2013;715(1‐3): 246-55. [Link] [DOI:10.1016/j.ejphar.2013.05.014]
60. Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180-3. [Link] [DOI:10.1016/j.redox.2015.01.002]
61. Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, et al. Long‐term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;9(6):e100777. [Link] [DOI:10.1371/journal.pone.0100777]
62. Steven S, Oelze M, Hanf A, Kröller‐Schön S, Kashani F, Roohani S, et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370-85. [Link] [DOI:10.1016/j.redox.2017.06.009]
63. Kawanami D, Matoba K, Takeda Y, Nagai Y, Akamine T, Yokota T, et al. SGLT2 inhibitors as a therapeutic option for diabetic nephropathy. Int J Mol Sci. 2017;18(5):1083. [Link] [DOI:10.3390/ijms18051083]
64. Gangadharan Komala M, Gross S, Mudaliar H, Huang C, Pegg K, Mather A, et al. Inhibition of kidney proximal tubular glucose reabsorption does not prevent against diabetic nephropathy in type 1 diabetic eNOS knockout mice. PLoS One. 2014;9(11):e108994. [Link] [DOI:10.1371/journal.pone.0108994]
65. Oelze M, Kröller‐Schön S, Welschof P, Jansen T, Hausding M, Mikhed Y, et al. The sodium-glucose co‐transporter 2 inhibitor empagliflozin improves diabetes‐induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One. 2014;9(11):e112394. [Link] [DOI:10.1371/journal.pone.0112394]
66. Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:13. [Link] [DOI:10.1186/s12933-014-0169-9]
67. Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. [Link] [DOI:10.1186/s12933-017-0621-8]
68. Pignatelli P, Baratta F, Buzzetti R, D'Amico A, Castellani V, Bartimoccia et al. The Sodium-Glucose Co-Transporter-2 (SGLT2) inhibitors reduce platelet activation and thrombus formation by lowering NOX2-related oxidative stress: A pilot study. Antioxidants (Basel). 2022;11(10):1878. [Link] [DOI:10.3390/antiox11101878]
69. Zhou W, Shao W, Zhang Y, Liu D, Liu M, Jin T. Glucagon-like peptide-1 receptor mediates the beneficial effect of Liraglutide in an acute lung injury mouse model involving the thioredoxin interacting protein. American Journal of Physiology-Endocrinology and Metabolism. Am J Physiol Endocrinol Metab. 2020;319:E568-78. [Link] [DOI:10.1152/ajpendo.00292.2020]











