Volume 14, Issue 2 (2022)
Iran J War Public Health 2022, 14(2): 197-201 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/04/13 | Accepted: 2022/05/25 | Published: 2022/06/28
Received: 2022/04/13 | Accepted: 2022/05/25 | Published: 2022/06/28
How to cite this article
Atiyah Essia I, Mousa Hamza Z, Awad Kadhim S, Alhous S. Comparison of Trace Element Concentrations in Women with Breast Cancer and Lung Cancer and Healthy Women. Iran J War Public Health 2022; 14 (2) :197-201
URL: http://ijwph.ir/article-1-1172-en.html
URL: http://ijwph.ir/article-1-1172-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- AL- Furat Al- Awssat Technical University, Kufa Technical Institute, Kufa, Iraq
2- Department of Physics, Faculty of Science, University of Kufa, Kufa, Iraq
3- Physics Department, Faculty of Education for Girls, University of Kufa, Kufa, Iraq
2- Department of Physics, Faculty of Science, University of Kufa, Kufa, Iraq
3- Physics Department, Faculty of Education for Girls, University of Kufa, Kufa, Iraq
Full-Text (HTML) (752 Views)
Introduction
Exposure to heavy metals is a major threat to human health and the biological system. These minerals have been extensively studied and their effects on human health have been regularly analyzed by the international bodies [1].
The occurrence of two or more primary tumors with different sources in a patient is known as multiple primary malignant tumors (MPMT). At the time of diagnosis, this unusual disease is classified as synchronous or accelerated [2]. The prevalence of MPMT in cancer patients has been observed between 0.73 to 11.7% [3].
Breast cancer (BC) is the most common type of cancer among women worldwide, affecting more than one in ten new cases each year. It is the second leading cause of cancer mortality in women after lung cancer [4]. Over the past three decades, remarkable progress has been made in early detection and treatment and in improving the survival benefit among patients [5]. Because of their lengthy life expectancy, these individuals are at increased risk of having second primary malignancies in their lifetime [6]. 10 years after the diagnosis of BC, approximately 10% of the survivors have developed subsequent metastases, and lung cancer is one of the largest numbers [7]. According to a population research conducted in Taiwan, this country already has the second primary malignancies, which has a detrimental influence on the survival of breast cancer patients in the country [8]. Lung cancer is the most prevalent cause of mortality, so the diagnosing the risks of developing BC patients is in the second place. Lung cancer is more common in women who are diagnosed before the age of 50. In addition, radiation therapy for BC patients who have had a mastectomy can significantly raise their chances of developing a second primary lung cancer. In a study of survivors of British Columbia with second lung cancer, there were 35 patients and in another study, there were 26 other patients [9]. Only one large demographic study compared the outcomes of BC-NSCLC (Non-Small-Cell Lung Carcinoma) patients with those with primary NSCLC [7].
Overall survival does not appear to be affected by BC history. Furthermore, no follow-up investigation was conducted on the second main SCLC (Small Cell Lung Cancer) following BC. Identification the risk factors of developing second lung cancer for BC patients and which factors impact their outcomes are important for identifying those who would benefit from enhanced screening and optimizing treatment [7].
Wang et al. conducted a population-based study using data from the Surveillance Epidemiology, and End Results (SEER) Program to evaluate the characteristics, risk and survival of second primary lung cancer after BC. They concluded that BC patients, especially for TNBC (Triple Negative Breast Cancer), are at a high risk of developing second primary lung cancers. BC history may be a favorable prognostic factor for NSCLC (but not SCLC) patients. Clinicians should closely follow up BC patients with high-risk factors [7].
According to one study, the findings suggest that the molecular subtype and the most common locations may have a substantial effect on the incidence and prediction of breast cancer and lung metastases. The study also discovered a number of prognostic markers that may help physicians choose the best treatment for people with lung metastases [10].
Studies on cadmium, lead, silver and other trace elements in women with breast cancer were also performed and the concentrations of radon and uranium in the blood of these patients were calculated [11-13]. Another research looked at cases of breast cancer diagnosed between January 1, 2010 and December 31, 2015. The researchers then created a cohort of 21,435 individuals with stage IV breast cancer. Lung metastases were found in 6,516 of these individuals [14].
Patients with metastatic breast cancer who have diverse molecular subtypes have a range of clinical characteristics, as well as prognosis and therapeutic options. Lung metastases make up a significant portion of people with metastatic breast cancer. The effect of different tumor subtypes on the survival of individuals with lung metastases is uncertain [15].
Given the seriousness and association of lung cancer with breast cancer, we want to study some related elements for both types to see if they can be controlled to reduce injury or find a mechanism for early detection.
Copper (Cu) homeostasis is normally well maintained in live organisms with good regulatory systems, and Cu toxicity due to disrupted homeostasis plays a major role in a variety of symptoms and disease situations. Copper deficiency is associated with the inability to synthesize the critical antioxidant enzyme Superoxide Dismutase (SOD), which contains copper and zinc and is an important risk factor for breast cancer. Because this enzyme is so important in defense, lowering these trace elements reduces the effectiveness of the antioxidant system, resulting in the formation of free radicals, which include H+, H2O2, and OH-. These reactive oxygen species (ROS) can damage cellular contents, including DNA, as well as react with almost organic molecules such as lipids and proteins, potentially increasing the risk of tumor development [16]. Its role in human health and disease as an agent or mediator of anti-inflammatory and antioxidant stress. Studies have shown that zinc is an essential co-factor in the production of antioxidants and anti-inflammatory mediators in humans [17-19]. The anti-cancer action of zinc is frequently attributed to its antioxidant properties, which proves the linkbetween breast and lung cancer [20].
Numerous nickel compounds and salts have been demonstrated to cause cancer. The occurrence of cancer appears to be inversely related to the solubility of these chemicals in aqueous media, and the least soluble compounds are the most carcinogenic. Nickel carbonyl [Ni (CO) 4] is associated with lung and nasal sinus cancer in industrial workers, and is also a potential carcinogen in cigarette smoke [21]. Dialysis patients are exposed to nickel and accumulate it in their blood and other organs; this exposure appears to have no negative health effects. Routine monitoring of dialysis patients is not recommended when the toxic values of nickel are more than 10 ng / ml or equivalent [22-25].
The aim of this study was to compare the concentrations of trace elements in women with breast cancer and lung cancer and healthy women.
Instruments and Methods
In this descriptive study, from the beginning of 2017 to the end of 2019, women with breast cancer (n=44), patients with lung cancer (n=44) and healthy individuals (n=80) were sampled from the southern and central provinces of Mosul and Baghdad who referred to the Middle Euphrates Cancer Center and the medical clinics of some physicians in Najaf city, Iraq. These subjects were selected by random sampling method. Many patients with lung cancer have previously been diagnosed with breast cancer with an average age of 30 to 75 years.
Blood samples were taken from the subjects and then centrifuged at 1500 rpm for a maximum of 5 minutes to collect serum and then stored in covered test tubes at room temperature (22±2°C) for 45 minutes until clot formation.
One milliliter of serum was wet digested in a 10 mL (1:1) HNO3/HClO4 acid mixture in a covered glass beaker. Digest was diluted in a 25 ml flask pre-cleaned with twofold deionization water to the required concentration. The sample was then kept for further study. Samples and blank solution were treated and prepared in accordance with a common protocol. The ratio of acid mixture for each sample and blank varied depending on the amount of serum collected from the patients.
To assess the levels of copper, zinc and nickel, a Flame Atomic Absorption Spectrophotometer (FAAS; Shimadzu model AA-670) was utilized with wavelengths of 324.8, 213.9, and 232.0 nm, and widths of 0.7, 0.1, and 0.2 nm, respectively, with the flame type being Air C2H2.
The system was calibrated independently for each of the four impact elements analyzed, and each element was evaluated in sick and healthy samples.
Data were analyzed by SPSS 20 software using one-way Analysis of Variance (ANOVA), Tukey’s post hock test, and Pearson's correlation test.
Findings
The mean age of women with breast cancer (n=44), women with lung cancer (n=44), and healthy women (n=80) were 48.7±13.8, 49.4±15.2, and 51.2±9.12 years, respectively.
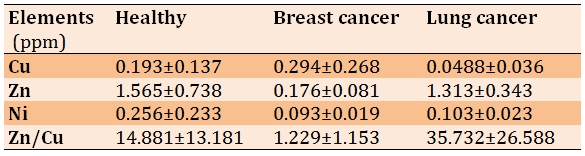
Significant differences were observed in the mean concentrations of trace elements including Cu, Zn, and Ni among the study groups (Table 1).
Table 1) Comparison of the mean concentrations of trace elements between the studied groups (P<0.01)
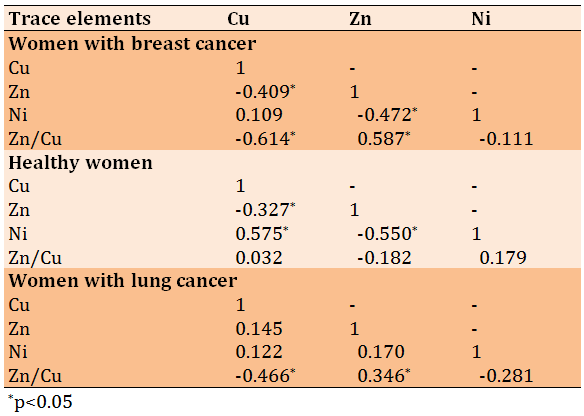
In the breast cancer group, the correlation between Cu and Zn (r=-0.409, p=0.007), as well as between Cu and Zn/Cu ratio (r=-0.614, p=0.0001) was inversely significant. The inverse correlation between Zn and Ni (r=-0.472, p=0.002) and the direct correlation between Zn and Zn/Cu ratio (r=0.587, p=0.0001) were significant. In the healthy group, the correlation between Cu and Zn (r=-0.327, p=0.001), as well as between Zn and Ni (r=-0.550, p=0.0001) was inversely significant, while the correlation between Cu and Ni (r=0.575, p=0.0001) was direct and significant. Finally, in the lung cancer group, the correlation between Cu and Zn/Cu ratio was invers (r=-0.550, p=0.0001) and between Zn and Zn/Cu ratio (r=0.575, p=0.0001) was direct (Table 2).
Table 2) Pearson correlation matrix of mean trace element concentrations between study groups
Discussion
The aim of this study was to compare the concentrations of trace elements in women with breast cancer and lung cancer and healthy women.
Serum Cu levels in women with lung cancer and healthy women were significantly lower than women with breast cancer. Furthermore, serum Cu levels in women with lung cancer were significantly lower than healthy women.
Copper is a trace metal that helps preserve DNA integrity by preventing oxidative DNA [26], damage, and gene alterations. Cu toxicity has recently been linked to changes in lipid metabolism, gene expression, alpha-syncline aggregation, activation of Acidic sphingomyelinase and ceramide production, temporal and geographic distribution of Cu in hepatocytes, and Cu-protein interaction in the nervous system [27].
Healthy women had the highest levels of zinc and nickel compared to the groups of women with breast cancer and lung cancer (p<0.01). Moreover, serum zinc and nickel levels were significantly higher in women with lung cancer compared to women with breast cancer (p<0.05).
Decreased zinc content in cell membranes highlights some of the problems associated with zinc deficiency and loss of zinc from membranes leads to increased vulnerability to oxidative damage, structural stresses and other problems [18].
In a study by Abu-Seif and Yousef, plasma Cu levels increased in diabetic patients, but Zn, Mg and Ca levels decreased significantly compared to controls [28].
Gagandeep et al.'s research showed an imbalance in levels of serum copper, magnesium and zinc in patients with type 2 diabetes mellitus. Fasting sugar, lipids, glycated hemoglobin and copper were significantly elevated in diabetic patients as compared to healthy controls (p<0.05) whereas serum magnesium and zinc decreased significantly in diabetic patients (p<0.05). Glycated hemoglobin correlated positively with copper and negatively with magnesium and zinc. The correlations were statistically significant (p<0.05) [29].
Durak et al. in their study investigated the trace element contents in the blood serum of type II diabetic patients with and without complication as compared to non-diabetic healthy controls. Mean Ca, Cu and Se concentrations in both diabetic patients with and without complication were significantly lower than those in healthy controls [30].
In a previous study, it was shown that increasing the level of nickel concentration impairs lung function [25]. Templeton et al. in their study evaluated the tentative reference values for nickel concentrations in human serum according to the TRACY protocol and the mean values for serum Ni concentration were <0.3 μg/l [31].
In the breast cancer group, the correlation between Cu and Zn (r=-0.409, p=0.007), as well as between Cu and Zn/Cu ratio (r=-0.614, p=0.0001) was inversely significant. The inverse correlation between Zn and Ni (r=-0.472, p=0.002) and the direct correlation between Zn and Zn/Cu ratio (r=0.587, p=0.0001) were significant. In the healthy group, the correlation between Cu and Zn (r=-0.327, p=0.001), as well as between Zn and Ni (r=-0.550, p=0.0001) was inversely significant, while the correlation between Cu and Ni (r=0.575, p=0.0001) was direct and significant. Finally, in the lung cancer group, the correlation between Cu and Zn/Cu ratio was invers (r=-0.550, p=0.0001) and between Zn and Zn/Cu ratio (r=0.575, p=0.0001) was direct.
Mirończuk et al. found positive correlations between the concentrations of Cu and the Cu/Zn, Cu/Se ratios (r=0.53, p<0.001; r=0.61, p<0.001), as well as the Se and Zn concentrations (r=0.43, p<0.001) and Cu/Zn and Cu/Se ratios (r=0.60; p<0.001) in patients with Acute Ischemic Stroke (AIS). They observed negative correlations in patients with AIS between concentrations of Zn, Se, and the Cu/Zn ratio (r=−0.71, p<0.001; r=−0.34, p=0.001, respectively), as well as Zn, Se, and the Cu/Se molar ratio (r=−0.25, p=0.003; r=−0.73, p < 0.001, respectively) [32].
This study can be considered as the first study of its kind in Najaf province of Iraq as a basic data for future studies.
Conclusion
Serum concentrations of trace elements increase in some groups and decrease in others, and there is a link between some elements that can be used as a means of early detection.
Acknowledgments: The authors would like to thank Prof. Dr. Murtadha Sh. Aswood for his valuable advice that helped us to complete this study.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contributions: Atiyah Essia IN (First Author), Methodologist/Main Researcher (25%); Mousa Hamza Z (Second Author), Introduction Writer/Discussion Writer (25%); Awad Kadhim Sh (Third Author), Assistant Researcher (25%); Alhous SF (Forth author), Assistant Researcher (25%)
Funding/Support: None declared.
Exposure to heavy metals is a major threat to human health and the biological system. These minerals have been extensively studied and their effects on human health have been regularly analyzed by the international bodies [1].
The occurrence of two or more primary tumors with different sources in a patient is known as multiple primary malignant tumors (MPMT). At the time of diagnosis, this unusual disease is classified as synchronous or accelerated [2]. The prevalence of MPMT in cancer patients has been observed between 0.73 to 11.7% [3].
Breast cancer (BC) is the most common type of cancer among women worldwide, affecting more than one in ten new cases each year. It is the second leading cause of cancer mortality in women after lung cancer [4]. Over the past three decades, remarkable progress has been made in early detection and treatment and in improving the survival benefit among patients [5]. Because of their lengthy life expectancy, these individuals are at increased risk of having second primary malignancies in their lifetime [6]. 10 years after the diagnosis of BC, approximately 10% of the survivors have developed subsequent metastases, and lung cancer is one of the largest numbers [7]. According to a population research conducted in Taiwan, this country already has the second primary malignancies, which has a detrimental influence on the survival of breast cancer patients in the country [8]. Lung cancer is the most prevalent cause of mortality, so the diagnosing the risks of developing BC patients is in the second place. Lung cancer is more common in women who are diagnosed before the age of 50. In addition, radiation therapy for BC patients who have had a mastectomy can significantly raise their chances of developing a second primary lung cancer. In a study of survivors of British Columbia with second lung cancer, there were 35 patients and in another study, there were 26 other patients [9]. Only one large demographic study compared the outcomes of BC-NSCLC (Non-Small-Cell Lung Carcinoma) patients with those with primary NSCLC [7].
Overall survival does not appear to be affected by BC history. Furthermore, no follow-up investigation was conducted on the second main SCLC (Small Cell Lung Cancer) following BC. Identification the risk factors of developing second lung cancer for BC patients and which factors impact their outcomes are important for identifying those who would benefit from enhanced screening and optimizing treatment [7].
Wang et al. conducted a population-based study using data from the Surveillance Epidemiology, and End Results (SEER) Program to evaluate the characteristics, risk and survival of second primary lung cancer after BC. They concluded that BC patients, especially for TNBC (Triple Negative Breast Cancer), are at a high risk of developing second primary lung cancers. BC history may be a favorable prognostic factor for NSCLC (but not SCLC) patients. Clinicians should closely follow up BC patients with high-risk factors [7].
According to one study, the findings suggest that the molecular subtype and the most common locations may have a substantial effect on the incidence and prediction of breast cancer and lung metastases. The study also discovered a number of prognostic markers that may help physicians choose the best treatment for people with lung metastases [10].
Studies on cadmium, lead, silver and other trace elements in women with breast cancer were also performed and the concentrations of radon and uranium in the blood of these patients were calculated [11-13]. Another research looked at cases of breast cancer diagnosed between January 1, 2010 and December 31, 2015. The researchers then created a cohort of 21,435 individuals with stage IV breast cancer. Lung metastases were found in 6,516 of these individuals [14].
Patients with metastatic breast cancer who have diverse molecular subtypes have a range of clinical characteristics, as well as prognosis and therapeutic options. Lung metastases make up a significant portion of people with metastatic breast cancer. The effect of different tumor subtypes on the survival of individuals with lung metastases is uncertain [15].
Given the seriousness and association of lung cancer with breast cancer, we want to study some related elements for both types to see if they can be controlled to reduce injury or find a mechanism for early detection.
Copper (Cu) homeostasis is normally well maintained in live organisms with good regulatory systems, and Cu toxicity due to disrupted homeostasis plays a major role in a variety of symptoms and disease situations. Copper deficiency is associated with the inability to synthesize the critical antioxidant enzyme Superoxide Dismutase (SOD), which contains copper and zinc and is an important risk factor for breast cancer. Because this enzyme is so important in defense, lowering these trace elements reduces the effectiveness of the antioxidant system, resulting in the formation of free radicals, which include H+, H2O2, and OH-. These reactive oxygen species (ROS) can damage cellular contents, including DNA, as well as react with almost organic molecules such as lipids and proteins, potentially increasing the risk of tumor development [16]. Its role in human health and disease as an agent or mediator of anti-inflammatory and antioxidant stress. Studies have shown that zinc is an essential co-factor in the production of antioxidants and anti-inflammatory mediators in humans [17-19]. The anti-cancer action of zinc is frequently attributed to its antioxidant properties, which proves the linkbetween breast and lung cancer [20].
Numerous nickel compounds and salts have been demonstrated to cause cancer. The occurrence of cancer appears to be inversely related to the solubility of these chemicals in aqueous media, and the least soluble compounds are the most carcinogenic. Nickel carbonyl [Ni (CO) 4] is associated with lung and nasal sinus cancer in industrial workers, and is also a potential carcinogen in cigarette smoke [21]. Dialysis patients are exposed to nickel and accumulate it in their blood and other organs; this exposure appears to have no negative health effects. Routine monitoring of dialysis patients is not recommended when the toxic values of nickel are more than 10 ng / ml or equivalent [22-25].
The aim of this study was to compare the concentrations of trace elements in women with breast cancer and lung cancer and healthy women.
Instruments and Methods
In this descriptive study, from the beginning of 2017 to the end of 2019, women with breast cancer (n=44), patients with lung cancer (n=44) and healthy individuals (n=80) were sampled from the southern and central provinces of Mosul and Baghdad who referred to the Middle Euphrates Cancer Center and the medical clinics of some physicians in Najaf city, Iraq. These subjects were selected by random sampling method. Many patients with lung cancer have previously been diagnosed with breast cancer with an average age of 30 to 75 years.
Blood samples were taken from the subjects and then centrifuged at 1500 rpm for a maximum of 5 minutes to collect serum and then stored in covered test tubes at room temperature (22±2°C) for 45 minutes until clot formation.
One milliliter of serum was wet digested in a 10 mL (1:1) HNO3/HClO4 acid mixture in a covered glass beaker. Digest was diluted in a 25 ml flask pre-cleaned with twofold deionization water to the required concentration. The sample was then kept for further study. Samples and blank solution were treated and prepared in accordance with a common protocol. The ratio of acid mixture for each sample and blank varied depending on the amount of serum collected from the patients.
To assess the levels of copper, zinc and nickel, a Flame Atomic Absorption Spectrophotometer (FAAS; Shimadzu model AA-670) was utilized with wavelengths of 324.8, 213.9, and 232.0 nm, and widths of 0.7, 0.1, and 0.2 nm, respectively, with the flame type being Air C2H2.
The system was calibrated independently for each of the four impact elements analyzed, and each element was evaluated in sick and healthy samples.
Data were analyzed by SPSS 20 software using one-way Analysis of Variance (ANOVA), Tukey’s post hock test, and Pearson's correlation test.
Findings
The mean age of women with breast cancer (n=44), women with lung cancer (n=44), and healthy women (n=80) were 48.7±13.8, 49.4±15.2, and 51.2±9.12 years, respectively.
Significant differences were observed in the mean concentrations of trace elements including Cu, Zn, and Ni among the study groups (Table 1).
Table 1) Comparison of the mean concentrations of trace elements between the studied groups (P<0.01)

In the breast cancer group, the correlation between Cu and Zn (r=-0.409, p=0.007), as well as between Cu and Zn/Cu ratio (r=-0.614, p=0.0001) was inversely significant. The inverse correlation between Zn and Ni (r=-0.472, p=0.002) and the direct correlation between Zn and Zn/Cu ratio (r=0.587, p=0.0001) were significant. In the healthy group, the correlation between Cu and Zn (r=-0.327, p=0.001), as well as between Zn and Ni (r=-0.550, p=0.0001) was inversely significant, while the correlation between Cu and Ni (r=0.575, p=0.0001) was direct and significant. Finally, in the lung cancer group, the correlation between Cu and Zn/Cu ratio was invers (r=-0.550, p=0.0001) and between Zn and Zn/Cu ratio (r=0.575, p=0.0001) was direct (Table 2).
Table 2) Pearson correlation matrix of mean trace element concentrations between study groups

Discussion
The aim of this study was to compare the concentrations of trace elements in women with breast cancer and lung cancer and healthy women.
Serum Cu levels in women with lung cancer and healthy women were significantly lower than women with breast cancer. Furthermore, serum Cu levels in women with lung cancer were significantly lower than healthy women.
Copper is a trace metal that helps preserve DNA integrity by preventing oxidative DNA [26], damage, and gene alterations. Cu toxicity has recently been linked to changes in lipid metabolism, gene expression, alpha-syncline aggregation, activation of Acidic sphingomyelinase and ceramide production, temporal and geographic distribution of Cu in hepatocytes, and Cu-protein interaction in the nervous system [27].
Healthy women had the highest levels of zinc and nickel compared to the groups of women with breast cancer and lung cancer (p<0.01). Moreover, serum zinc and nickel levels were significantly higher in women with lung cancer compared to women with breast cancer (p<0.05).
Decreased zinc content in cell membranes highlights some of the problems associated with zinc deficiency and loss of zinc from membranes leads to increased vulnerability to oxidative damage, structural stresses and other problems [18].
In a study by Abu-Seif and Yousef, plasma Cu levels increased in diabetic patients, but Zn, Mg and Ca levels decreased significantly compared to controls [28].
Gagandeep et al.'s research showed an imbalance in levels of serum copper, magnesium and zinc in patients with type 2 diabetes mellitus. Fasting sugar, lipids, glycated hemoglobin and copper were significantly elevated in diabetic patients as compared to healthy controls (p<0.05) whereas serum magnesium and zinc decreased significantly in diabetic patients (p<0.05). Glycated hemoglobin correlated positively with copper and negatively with magnesium and zinc. The correlations were statistically significant (p<0.05) [29].
Durak et al. in their study investigated the trace element contents in the blood serum of type II diabetic patients with and without complication as compared to non-diabetic healthy controls. Mean Ca, Cu and Se concentrations in both diabetic patients with and without complication were significantly lower than those in healthy controls [30].
In a previous study, it was shown that increasing the level of nickel concentration impairs lung function [25]. Templeton et al. in their study evaluated the tentative reference values for nickel concentrations in human serum according to the TRACY protocol and the mean values for serum Ni concentration were <0.3 μg/l [31].
In the breast cancer group, the correlation between Cu and Zn (r=-0.409, p=0.007), as well as between Cu and Zn/Cu ratio (r=-0.614, p=0.0001) was inversely significant. The inverse correlation between Zn and Ni (r=-0.472, p=0.002) and the direct correlation between Zn and Zn/Cu ratio (r=0.587, p=0.0001) were significant. In the healthy group, the correlation between Cu and Zn (r=-0.327, p=0.001), as well as between Zn and Ni (r=-0.550, p=0.0001) was inversely significant, while the correlation between Cu and Ni (r=0.575, p=0.0001) was direct and significant. Finally, in the lung cancer group, the correlation between Cu and Zn/Cu ratio was invers (r=-0.550, p=0.0001) and between Zn and Zn/Cu ratio (r=0.575, p=0.0001) was direct.
Mirończuk et al. found positive correlations between the concentrations of Cu and the Cu/Zn, Cu/Se ratios (r=0.53, p<0.001; r=0.61, p<0.001), as well as the Se and Zn concentrations (r=0.43, p<0.001) and Cu/Zn and Cu/Se ratios (r=0.60; p<0.001) in patients with Acute Ischemic Stroke (AIS). They observed negative correlations in patients with AIS between concentrations of Zn, Se, and the Cu/Zn ratio (r=−0.71, p<0.001; r=−0.34, p=0.001, respectively), as well as Zn, Se, and the Cu/Se molar ratio (r=−0.25, p=0.003; r=−0.73, p < 0.001, respectively) [32].
This study can be considered as the first study of its kind in Najaf province of Iraq as a basic data for future studies.
Conclusion
Serum concentrations of trace elements increase in some groups and decrease in others, and there is a link between some elements that can be used as a means of early detection.
Acknowledgments: The authors would like to thank Prof. Dr. Murtadha Sh. Aswood for his valuable advice that helped us to complete this study.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contributions: Atiyah Essia IN (First Author), Methodologist/Main Researcher (25%); Mousa Hamza Z (Second Author), Introduction Writer/Discussion Writer (25%); Awad Kadhim Sh (Third Author), Assistant Researcher (25%); Alhous SF (Forth author), Assistant Researcher (25%)
Funding/Support: None declared.
Keywords:
References
1. Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60-72. [Link] [DOI:10.2478/intox-2014-0009]
2. Wang D, Xu Y, Tu Y, Tan X, Zhu Z, et al. Comparison analysis in synchronous and metachronous metastatic colorectal cancer based on microarray expression profile. Hepatogastroenterology. 2014;61(136):2215-8. [Link]
3. Jin B, Zhang S, Chuang X, Yu P, Chen Y, Teng Y, et al. Breast cancer and synchronous multiple primary lung adenocarcinomas with heterogeneous mutations: a case report. BMC Cancer. 2018;18:1138. [Link] [DOI:10.1186/s12885-018-5011-4]
4. Witjes JA, Lebret T, Compérat EM, Cowan NC, De Santis M, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462-75. [Link] [DOI:10.1016/j.eururo.2016.06.020]
5. Meier‐Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4(8):1289-95. [Link] [DOI:10.1111/j.1600-6143.2004.00515.x]
6. Davis EJ, Beebe-Dimmer JL, Yee CL, Cooney KA. Risk of second primary tumors in men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2014;120(17):2735-41. [Link] [DOI:10.1002/cncr.28769]
7. Wang R, Yin Z, Liu L, Gao W, Li W, et al. Second primary lung cancer after breast cancer: a population-based study of 6,269 women. Front Oncol. 2018;8:427. [Link] [DOI:10.3389/fonc.2018.00427]
8. Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C, et al. Pathologic complete response (PCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33(5):534-43. [Link] [DOI:10.1016/j.annonc.2022.02.004]
9. Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Semin Cancer Boil. 2004;14(6):473-86. [Link] [DOI:10.1016/j.semcancer.2004.06.010]
10. Chen S, Yang J, Liu Y, You H, Dong Y, Lyu J. Prognostic factors and survival outcomes according to tumor subtype in patients with breast cancer lung metastases. Peer J. 2019;7:e8298. [Link] [DOI:10.7717/peerj.8298]
11. Alshebly SAK, Hussain HH, Trier SH, Kadhim BA. Serum levels of lead, cadmium and silver in patients with breast cancer compared with healthy females in Iraq. AIP Conference Proceedings. 2019;2086:030048. [Link] [DOI:10.1063/1.5095133]
12. Hussein HH, Alsabari EK, Kadhim BA, Hatif KH, AL-Khafaji QS, Hamidi SAK. Study the impact of the trace elements between the healthy females and who take chemotherapy for samples of Sera. Res J Pharm Technol. 2017;10(10):3323-5. [Link] [DOI:10.5958/0974-360X.2017.00589.3]
13. Kadhim SA, Harjan AH, Alhous SF, AL-Khafaji QS. Study of the difference between uranium concentrations in blood samples of healthy, newly infected and women who took chemotherapy in Iraq, Najaf. AIP Conf Proc. 2022;2386. [Link] [DOI:10.1063/5.0067446]
14. Lin S, Ji R, Yan C, Zhang B, Cao L, et al. Towards optimal structured CNN pruning via generative adversarial learning. Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition. Long Beach, CA, USA; 2019. [Link] [DOI:10.1109/CVPR.2019.00290]
15. Gong Y, Liu YR, Ji P, Hu X, Shao ZM. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep. 2017;7:45411. [Link] [DOI:10.1038/srep45411]
16. Ahmad SI. Reactive oxygen species in biology and human health. Boca Raton, Florida: CRC press; 2017. [Link] [DOI:10.1201/b20228]
17. Prasad AS, Bao B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants. 2019;8(6):164. [Link] [DOI:10.3390/antiox8060164]
18. Bjørklund G, Dadar M, Pivina L, Doşa MD, Semenova Y, Aaseth J. The role of zinc and copper in insulin resistance and diabetes mellitus. Curr Med Chem. 2020;27(39):6643-57. [Link] [DOI:10.2174/0929867326666190902122155]
19. Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316-28. [Link] [DOI:10.1016/j.numecd.2005.05.003]
20. Skrajnowska D, Bobrowska-Korczak B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients. 2019;11(10):2273. [Link] [DOI:10.3390/nu11102273]
21. Salahuddin Ahammad SK. Air pollutants source tracking [Dissertation]. Dhaka: Bangladesh University of Engineering and Technology; 2008. [Link]
22. Brunet M, van Gelder T, Åsberg A, Haufroid V, Hesselink DA, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261-307. [Link] [DOI:10.1097/FTD.0000000000000640]
23. Amini MK, Kabiri M. Determination of trace amounts of nickel by differential pulse adsorptive cathodic stripping voltammetry using calconcarboxylic acid as a chelating agent. J Iran Chem Soc. 2005;2(1):32-9. [Link] [DOI:10.1007/BF03245777]
24. Mudjari S, Achmad MH. Comparison between nickel and chromium levels in serum and urine in patients treated with fixed orthodontic appliances: a longitudinal study. Pesquisa Brasileira em Odontopediatria e Clínica Integrada. 2018;18(1):4071. [Portuguese] [Link] [DOI:10.4034/PBOCI.2018.181.72]
25. Jabbar U, Mushtaq M, Qureshi JA. Serum nickel levels compromise the oxidative status and lung functions in ceramic workers. Lung Cancer. 2021;5(1):1-6. [Link] [DOI:10.11648/j.ijec.20210501.11]
26. Mahabir S, Forman MR, Barerra SL, Dong YQ, Spitz MR, Wei Q. Joint effects of dietary trace metals and DNA repair capacity in lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2756-62. [Link] [DOI:10.1158/1055-9965.EPI-07-0324]
27. Gaetke LM, Chow-Johnson HS, Chow CK. Copper: toxicological relevance and mechanisms. Arch Toxicol. 2014;88(11):1929-38. [Link] [DOI:10.1007/s00204-014-1355-y]
28. Abou-Seif MA, Youssef AA. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346(2):161-70. [Link] [DOI:10.1016/j.cccn.2004.03.030]
29. Gagandeep D, Shailaza S, Rahul R. Evaluation of trace elements and glycated hemoglobin in type 2 diabetes mellitus. World J Pharm Pharm Sci. 2015;4(5):940-7. [Link]
30. Durak R, Gülen Y, Kurudirek M, Kaçal M, Capoğlu I. Determination of trace element levels in human blood serum from patients with type II diabetes using WDXRF technique: a comparative study. J Xray Sci Technol. 2010;18(2):111-20. [Link] [DOI:10.3233/XST-2010-0247]
31. Templeton DM, Sunderman Jr FW, Herber RF. Tentative reference values for nickel concentrations in human serum, plasma, blood, and urine: evaluation according to the TRACY protocol. Sci Total Environ. 1994;148(2-3):243-51. [Link] [DOI:10.1016/0048-9697(94)90400-6]
32. Mirończuk A, Kapica-Topczewska K, Socha K. Selenium, copper, zinc concentrations and Cu/Zn, Cu/Se molar ratios in the serum of patients with acute ischemic stroke in Northeastern Poland-a new insight into stroke pathophysiology. Nutrients. 2021;13(7):2139. [Link] [DOI:10.3390/nu13072139]








