Volume 14, Issue 2 (2022)
Iran J War Public Health 2022, 14(2): 225-230 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/04/9 | Accepted: 2022/06/20 | Published: 2022/06/25
Received: 2022/04/9 | Accepted: 2022/06/20 | Published: 2022/06/25
How to cite this article
Najim Rasool R, Aboud Khalifa A. Studying the Role of Interleukin-6, C-Reactive Protein, and Nitric Oxide Synthase in Obese, Diabetic, and Sub-Fertile Men. Iran J War Public Health 2022; 14 (2) :225-230
URL: http://ijwph.ir/article-1-1168-en.html
URL: http://ijwph.ir/article-1-1168-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
R. Najim Rasool *1, A. Aboud Khalifa1
1- Department of Biology, College of Science, University of Misan, Maysan, Iraq
Full-Text (HTML) (594 Views)
Introduction
Obesity is characterized by low-grade chronic inflammation, as an acute-phase reactant to inflammation and infection, C-reactive protein (CRP) is the strongest factor associated with obesity, and the chronic elevation of human CRP at baseline level causes obesity [1]. Interleukin-6 (IL-6) is a major pro-inflammatory mediator that contributes significantly to the development of low-grade tissue-specific and/or systemic inflammation, the role of IL-6 in the development of tissue-specific insulin resistance (IR), and impairment of insulin secretion from pancreatic islet β cells, furthermore, IL-6-induced low-grade tissue-specific and systemic inflammation are also responsible for developing of tissue-specific insulin resistance and type 2 diabetes mellitus (T2DM), in the treatment of insulin resistance and T2DM, inhibiting inflammatory reactions is an effective technique for preventing inflammatory diseases [2]. IL-6 level was found to be increased in obese individuals as well as in patients with chronic inflammatory diseases and lipid concentrations abnormalities, the increased IL-6 level in people with obesity may increase the risk of cardiovascular complications, insulin resistance, and type 2 diabetes [3, 4]. The CRP concentration was associated with an increased risk of developing T2DM, and this association was more apparent among the older age group (≥50 years), and CRP and its combination with obesity and hypertension were associated with an increased risk of T2DM [5]. IL-6 and CRP, two sensitive physiological markers of chronic systemic inflammation, have been linked to hyperglycemia, insulin resistance, and overt type 2 diabetes [6, 7].
Nitric oxide synthase (NOS) is a family of enzymes that produced nitric oxide (NO) by the oxidation of L-arginine to L-citrulline [8]. There are three isoforms of NOS, two of these, neuronal NOS (nNOS) and endothelial NOS (eNOS), are permanently expressed, nNOS is largely present in the nervous system and is required for neuronal signaling, eNOS is found in the endothelium and is required for vasodilation and control of blood pressure [9]. These two isoforms create nanomolar amounts of NO for short periods (seconds to minutes), whereas the third inducible NOS (iNOS) is inducible, iNOS is not present in cells all the time and is only expressed when the cell is induced or, stimulated, generally by pro-inflammatory cytokines and/or bacterial lipopolysaccharide (LPS) [10]. iNOS synthesizes a higher amount of NO in chronic inflammatory conditions, therefore, iNOS is primarily responsible for the increased production of NO [11]. NOS is found in Sertoli cells, Leydig cells, spermatocytes, neuronal plexus in the adventitia of arterioles, vascular endothelial, cells, immature sperm head, and smooth muscle, cells, implying that NO / NOS can maintain testicular arteriole tension, regulate testosterone secretion and influence sperm development [12].
Furthermore, nitric oxide synthase was expressed in interstitial cells and blood vessels in vitro culture of interstitial cells of seminiferous tubules, indicating that the testis can create NO [13]. Krause et al. [14] observed that NOS levels were lower in the obese T2DM group compared to the control, T2DM non-obese patients had higher NOS concentrations than controls, as a result, the presence of diabetic comorbidities should be considered when evaluating NOS levels in diabetic patients. However, Foroumandi et al. [15] found NO levels were to be positively associated with body mass index (BMI) in both male and female groups and increased NO levels in obese people may be due to increasing NO production, furthermore, in the animal investigation have revealed that plasma and aorta NO levels in obese animals were significantly greater than in normal-weight animals [16].
The present study is an attempt to investigate the possible role of IL-6, CRP, and NOS in obese, diabetic, and sub-fertile men.
Material and Methods
The current study was conducted in some health centers in Misan province, Iraq, from December 2020 to July 2021. The whole sample included 80 men aged 35-45 years, divided into four groups (20 men/group) as follows: control group, obesity group, diabetic group, and sub-fertility (hyperprolactinemia) group, the samples has been checked medically by a specialist physician and have been diagnosed with obesity, diabetic and sub-fertile (hyperprolactinemia) according to (body mass index BMI, glycated hemoglobinA1c HbA1c and prolactin levels respectively). Men with chronic diseases, tumors, and those whose treatment with hormonal drugs have been excluded.
Eight to ten milliliters of venous blood samples were drawn at 9 - 11 am, using a disposable needle and plastic syringes for each man. The blood was left at room temperature for 15 minutes for coagulation, centrifuged at 3000rpm for 5 minutes, then serum and plasma were separated and transferred for storage. Serum IL-6 and NOS levels were accurately measured using a highly quantitative enzyme-linked immunosorbent assay (ELISA) kit from Sunlong biotech / China, the range from 2 ng/L -80 ng/L, 0.8 μmol/L - 30 μmol/L respectively. CRP was accurately measured using a mindray automated/China (Spinreact /Spain), the range from 0-5 mg/L. The body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared.
Statistical analysis was performed by IBM SPSS statistics, version 23 (IBM Co., Armonk, NY, USA). The statistical analysis was performed by one-way Analysis of Variance (ANOVA), followed by Duncan's new multiple range tests (DMRT) at a p<0.05 significant level.
Findings
IL-6 level in sub-fertility group (33.93±2.35pg/ml) increased significantly (p<0.05) in comparison with the control (23.65±2.17pg/ml), obesity group (25.02±1.84pg/ml) and diabetic group (24.50±1.09pg/ml).
IL-6 level in the diabetic group increased not significantly in comparison with the control and decreased not significantly in the obesity group, and in the obesity, group increased significantly (p<0.05) in comparison with the control (Figure 1).
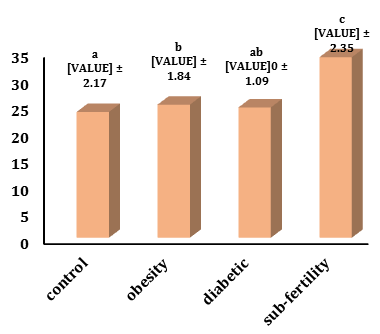
Figure 1) Levels of IL-6 in different groups. The values represent mean±SD. Different small letters represent a significant difference in (p≤0.05) between groups. Similar small letters represent no significant difference.
CRP level in the sub-fertility group (3.60±0.79mg/L) increased significantly (p<0.05) in comparison with the control (2.31±0.84mg/L) and increased not significantly with the obesity group (3.31±0.80mg/L) and decreased not significantly with a diabetic group (3.80±1.02 mg/dl). CRP levels in the diabetic group increased significantly (p<0.05) in comparison with the control and were not significant in the obesity group, and the obesity, group increased not significantly in comparison with the control (Figure 2).
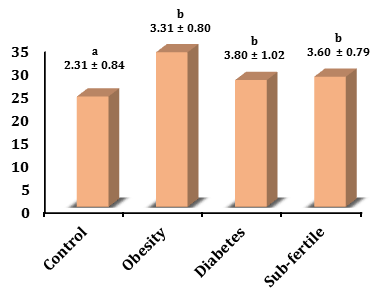
Figure 2) Levels of CRP in different groups. The values represent mean±SD. Different small letters represent a significant difference in (p≤0.05) between groups. Similar small letters represent no significant difference.
NOS levels in the sub-fertility group (12.40±2.14µmol/L) increased not significantly in comparison with the control (14.11±1.42µmol/L) and increased not significantly with the obesity group (12.05±2.31µmol/L) and diabetic group (11.06±2.75µmol/L).
NOS levels in the diabetic group decreased significantly (p<0.05) in comparison with the control and it was not significant in the obesity group, whereas the obesity group decreased significantly (p<0.05) in comparison with the control (Figure 3).
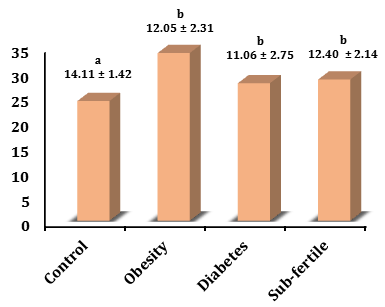
Figure 3) Levels of NOS in different groups. The values represent mean±SD. Different small letters represent a significant difference in (p<0.05) between groups. Similar small letters represent no significant difference.
BMI level in the sub-fertility group (28.36±1.48) increased significantly (p<0.05) in comparison with the control (24.04 ±1.60) and it was not significant in the diabetic group (27.62±2.74) and decreased significantly (p<0.05) with the obesity group (33.67±2.32).
BMI level in the diabetic group increased significantly (p<0.05) in comparison with the control and decreased significantly (p<0.05) with the obesity group, whereas the obesity group increased significantly (p<0.05) in comparison with the control (Figure 4).
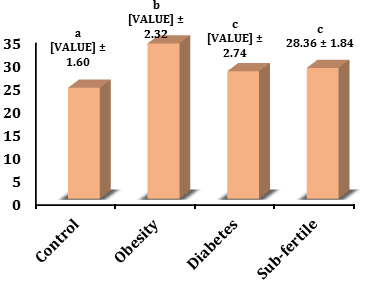
Figure 4) Levels of BMI in different groups. The values represent mean±SD. Different small letters represent a significant difference in (p<0.05) between groups. Similar small letters represent no significant difference.
Discussion
In many directions beyond these current results such as the high values of pro-inflammatory cytokines (IL-6 and CRP) associated with adipocyte hypertrophy, these cytokines promote strongly the development of insulin resistance and pathogenesis of type 2 diabetes, and these cytokines positively correlated with hyperprolactinemia and with high levels of prolactin secretion, in addition, these different groups (obesity, diabetic and sub-fertility) be considered as a low-grade inflammation, thus, these high cytokines release may be leading to decreasing the testosterone production, whereas, CRP as a prime inflammatory marker correlated negatively with the testosterone.
Bowker et al. [17] showed that IL-6 mediates in small part the links between obesity, insulin resistance, and cardio-metabolic diseases.
El-Mikkawy et al. [18] mentioned significantly higher levels of IL-6 in subjects with overweight and obesity as compared to those in healthy control, moreover, a significantly positive correlation was found between circulating levels of IL6 and BMI only in subjects with very severe (grade III) obesity.
Amit et al. [19] found that the increasing appreciation that adipose tissue is an active endocrine organ producing a variety of hormones and cytokines that may affect CRP levels, of these, IL-6 is thought to be the principal cytokine involved in CRP release from the liver and up to one-third of circulating IL-6 is derived from adipose tissue.
Mahwati & Nurrika [20] found an association between obesity indicators and CRP levels, there is a positive correlation between BMI, waist circumference (WC), and CRP.
Khaodhiar et al. [21] found that subjects with obesity had significantly higher serum levels of IL-6 and CRP compared to control, and that serum levels of IL-6 and CRP were only positively correlated with BMI in morbidly obese subjects, suggesting that IL-6 could be secreted in an endocrine manner in proportion to fat mass expansion, with an associated increase in CRP hepatic production.
Obese patients, as well as those with chronic inflammatory diseases and abnormal serum lipid concentrations, had higher serum IL-6 levels [22].
Bruun et al. [23] found that the levels of IL-6 were increased and correlated with measures of insulin resistance in male subjects with abdominal obesity.
Increased IL-6 levels in obese people may raise the risk of insulin resistance and type 2 diabetes [ 4].
Elevated circulating IL-6 levels were an independent predictor of type 2 diabetes and were thought to play a role in the development of inflammation, insulin resistance, and cell dysfunction [24].
IL-6 levels were more strongly related to HbA1c, which indicates average glycemic levels in the three months preceding measurement, than with other glycemic traits, infections, and other inflammatory challenges are related to reactive hyperglycemia, which may be aggravated in patients with greater IL-6 levels [25, 26].
The positive association between CRP and T2DM is dependent on insulin resistance and body mass index (BMI), the mechanism of the association between CRP and T2DM is still not known in detail [5].
Phosat et al. [27] mentioned that the role of CRP as an initiated marker for T2DM, where they found that the pre-diabetic and T2DM groups had markedly higher CRP levels than the control, subjects with high CRP levels had an elevated risk of pre-diabetes and T2DM, even after adjusting for confounders (i.e. age, BMI and gender) this strong association remained.
Liu et al. [28] found in a study of 202 patients with hyperprolactinemia that a significantly higher proportion of hyperprolactinemic patients had at least one detectable autoantibody, as well as, higher levels of IL-6 compared to healthy controls.
Li et al. [1] observed that elevated levels of high sensitivity (hs-CRP) were significantly associated with an increased risk of erectile dysfunction (ED) after adjustment for conventional ED risk factors, including BMI, age, testosterone, alcohol, smoking, consumption, physical activity, hypertension, diabetes, and dyslipidemia. Low testosterone levels in men were significantly associated with a high level of inflammatory markers (CRP, IL-6) in different clinical conditions such as obesity, diabetes, and hyperprolactinemia [29-31].
On the other hand, the current decrease of NOS might be attributed to the high adipose tissue as indicated by BMI measurement and/or influence of possible secretion of chemerin that correlated negatively with NOS and/or high level of prolactin secretion [32].
Habib et al. [33] found that serum chemerin levels are elevated in patients with type 2 diabetes compared to control, and are positively correlated with adiposity and insulin resistance in patients with type 2 diabetes. Weigert et al. [34] and Tahir et al. [35] found that chemerin was elevated in T2DM subjects with a higher CRP level and positively correlated with CRP in normal weight, overweight and T2DM subjects after adjusting for BMI. Kraus et al. [14] mentioned that the skeletal muscle eNOS protein was significantly higher in the non-obese compared with the obese subjects.
Insulin resistance may be the cause of the reduced nitric oxide production in type 2 diabetes, insulin insufficiently stimulated NOS activity in skeletal muscle of type 2 diabetic subjects [36].
In humans, insulin resistance is often associated with elevated fasting plasma levels of cholesterol and triglycerides, eNOS deficiency might directly alter lipid metabolism [37].
Kashyap et al. [38] found that the basal and insulin-stimulated muscle NOS activity was impaired in type 2 diabetic subjects, paralleling the severity of insulin resistance.
The inhibition of expressed neuronal NO synthase decreased NO levels and increased basal PRL release, expression of inducible NO synthase also increased NO and inhibited PRL basal, whereas inhibition of this enzyme decreased NO production and recovered PRL release [39].
Duvilanski et al. [40] found that the actions of NO in controlling prolactin release are complex by effects mediated both by the hypothalamus and also by the pituitary gland itself, the actions of NO within the hypothalamustimulate, whereas the action at the pituitary level inhibits prolactin secretion.
Slightly high PRL caused a decrease in blood pressure (BP) caused by increased nitric oxide (NO) production, whereas higher PRL leads to increased BP due to decreased NO production [41].
Chemerin was positively correlated with body mass index and serum insulin and was negatively correlated with eNOS [42].
Neves et al. [43] found that chemerin reduces NO production, enhances NO breakdown, and also decreases NO-dependent cGMP signaling, thereby reducing vascular relaxation, the chemerin leads to destabilization of the eNOS dimer, impairing the catalytic function of the enzyme.
Conclusion
The IL-6 and CRP increment and NOS reduction in different groups might be indicated a pro-inflammatory action and low fertility in obesity, diabetic and sub-fertility groups.
Acknowledgments: None declared.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contributions: Najim Rasool R (First Author), Introduction Writer/Methodologist/Main Researcher (50%); Aboud Khalifa A (Second Author), Assistant Researcher/Statistical Analyst/Discussion Writer (50%)
Funding/Support: None declared.
Obesity is characterized by low-grade chronic inflammation, as an acute-phase reactant to inflammation and infection, C-reactive protein (CRP) is the strongest factor associated with obesity, and the chronic elevation of human CRP at baseline level causes obesity [1]. Interleukin-6 (IL-6) is a major pro-inflammatory mediator that contributes significantly to the development of low-grade tissue-specific and/or systemic inflammation, the role of IL-6 in the development of tissue-specific insulin resistance (IR), and impairment of insulin secretion from pancreatic islet β cells, furthermore, IL-6-induced low-grade tissue-specific and systemic inflammation are also responsible for developing of tissue-specific insulin resistance and type 2 diabetes mellitus (T2DM), in the treatment of insulin resistance and T2DM, inhibiting inflammatory reactions is an effective technique for preventing inflammatory diseases [2]. IL-6 level was found to be increased in obese individuals as well as in patients with chronic inflammatory diseases and lipid concentrations abnormalities, the increased IL-6 level in people with obesity may increase the risk of cardiovascular complications, insulin resistance, and type 2 diabetes [3, 4]. The CRP concentration was associated with an increased risk of developing T2DM, and this association was more apparent among the older age group (≥50 years), and CRP and its combination with obesity and hypertension were associated with an increased risk of T2DM [5]. IL-6 and CRP, two sensitive physiological markers of chronic systemic inflammation, have been linked to hyperglycemia, insulin resistance, and overt type 2 diabetes [6, 7].
Nitric oxide synthase (NOS) is a family of enzymes that produced nitric oxide (NO) by the oxidation of L-arginine to L-citrulline [8]. There are three isoforms of NOS, two of these, neuronal NOS (nNOS) and endothelial NOS (eNOS), are permanently expressed, nNOS is largely present in the nervous system and is required for neuronal signaling, eNOS is found in the endothelium and is required for vasodilation and control of blood pressure [9]. These two isoforms create nanomolar amounts of NO for short periods (seconds to minutes), whereas the third inducible NOS (iNOS) is inducible, iNOS is not present in cells all the time and is only expressed when the cell is induced or, stimulated, generally by pro-inflammatory cytokines and/or bacterial lipopolysaccharide (LPS) [10]. iNOS synthesizes a higher amount of NO in chronic inflammatory conditions, therefore, iNOS is primarily responsible for the increased production of NO [11]. NOS is found in Sertoli cells, Leydig cells, spermatocytes, neuronal plexus in the adventitia of arterioles, vascular endothelial, cells, immature sperm head, and smooth muscle, cells, implying that NO / NOS can maintain testicular arteriole tension, regulate testosterone secretion and influence sperm development [12].
Furthermore, nitric oxide synthase was expressed in interstitial cells and blood vessels in vitro culture of interstitial cells of seminiferous tubules, indicating that the testis can create NO [13]. Krause et al. [14] observed that NOS levels were lower in the obese T2DM group compared to the control, T2DM non-obese patients had higher NOS concentrations than controls, as a result, the presence of diabetic comorbidities should be considered when evaluating NOS levels in diabetic patients. However, Foroumandi et al. [15] found NO levels were to be positively associated with body mass index (BMI) in both male and female groups and increased NO levels in obese people may be due to increasing NO production, furthermore, in the animal investigation have revealed that plasma and aorta NO levels in obese animals were significantly greater than in normal-weight animals [16].
The present study is an attempt to investigate the possible role of IL-6, CRP, and NOS in obese, diabetic, and sub-fertile men.
Material and Methods
The current study was conducted in some health centers in Misan province, Iraq, from December 2020 to July 2021. The whole sample included 80 men aged 35-45 years, divided into four groups (20 men/group) as follows: control group, obesity group, diabetic group, and sub-fertility (hyperprolactinemia) group, the samples has been checked medically by a specialist physician and have been diagnosed with obesity, diabetic and sub-fertile (hyperprolactinemia) according to (body mass index BMI, glycated hemoglobinA1c HbA1c and prolactin levels respectively). Men with chronic diseases, tumors, and those whose treatment with hormonal drugs have been excluded.
Eight to ten milliliters of venous blood samples were drawn at 9 - 11 am, using a disposable needle and plastic syringes for each man. The blood was left at room temperature for 15 minutes for coagulation, centrifuged at 3000rpm for 5 minutes, then serum and plasma were separated and transferred for storage. Serum IL-6 and NOS levels were accurately measured using a highly quantitative enzyme-linked immunosorbent assay (ELISA) kit from Sunlong biotech / China, the range from 2 ng/L -80 ng/L, 0.8 μmol/L - 30 μmol/L respectively. CRP was accurately measured using a mindray automated/China (Spinreact /Spain), the range from 0-5 mg/L. The body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared.
Statistical analysis was performed by IBM SPSS statistics, version 23 (IBM Co., Armonk, NY, USA). The statistical analysis was performed by one-way Analysis of Variance (ANOVA), followed by Duncan's new multiple range tests (DMRT) at a p<0.05 significant level.
Findings
IL-6 level in sub-fertility group (33.93±2.35pg/ml) increased significantly (p<0.05) in comparison with the control (23.65±2.17pg/ml), obesity group (25.02±1.84pg/ml) and diabetic group (24.50±1.09pg/ml).
IL-6 level in the diabetic group increased not significantly in comparison with the control and decreased not significantly in the obesity group, and in the obesity, group increased significantly (p<0.05) in comparison with the control (Figure 1).

Figure 1) Levels of IL-6 in different groups. The values represent mean±SD. Different small letters represent a significant difference in (p≤0.05) between groups. Similar small letters represent no significant difference.
CRP level in the sub-fertility group (3.60±0.79mg/L) increased significantly (p<0.05) in comparison with the control (2.31±0.84mg/L) and increased not significantly with the obesity group (3.31±0.80mg/L) and decreased not significantly with a diabetic group (3.80±1.02 mg/dl). CRP levels in the diabetic group increased significantly (p<0.05) in comparison with the control and were not significant in the obesity group, and the obesity, group increased not significantly in comparison with the control (Figure 2).

Figure 2) Levels of CRP in different groups. The values represent mean±SD. Different small letters represent a significant difference in (p≤0.05) between groups. Similar small letters represent no significant difference.
NOS levels in the sub-fertility group (12.40±2.14µmol/L) increased not significantly in comparison with the control (14.11±1.42µmol/L) and increased not significantly with the obesity group (12.05±2.31µmol/L) and diabetic group (11.06±2.75µmol/L).
NOS levels in the diabetic group decreased significantly (p<0.05) in comparison with the control and it was not significant in the obesity group, whereas the obesity group decreased significantly (p<0.05) in comparison with the control (Figure 3).

Figure 3) Levels of NOS in different groups. The values represent mean±SD. Different small letters represent a significant difference in (p<0.05) between groups. Similar small letters represent no significant difference.
BMI level in the sub-fertility group (28.36±1.48) increased significantly (p<0.05) in comparison with the control (24.04 ±1.60) and it was not significant in the diabetic group (27.62±2.74) and decreased significantly (p<0.05) with the obesity group (33.67±2.32).
BMI level in the diabetic group increased significantly (p<0.05) in comparison with the control and decreased significantly (p<0.05) with the obesity group, whereas the obesity group increased significantly (p<0.05) in comparison with the control (Figure 4).

Figure 4) Levels of BMI in different groups. The values represent mean±SD. Different small letters represent a significant difference in (p<0.05) between groups. Similar small letters represent no significant difference.
Discussion
In many directions beyond these current results such as the high values of pro-inflammatory cytokines (IL-6 and CRP) associated with adipocyte hypertrophy, these cytokines promote strongly the development of insulin resistance and pathogenesis of type 2 diabetes, and these cytokines positively correlated with hyperprolactinemia and with high levels of prolactin secretion, in addition, these different groups (obesity, diabetic and sub-fertility) be considered as a low-grade inflammation, thus, these high cytokines release may be leading to decreasing the testosterone production, whereas, CRP as a prime inflammatory marker correlated negatively with the testosterone.
Bowker et al. [17] showed that IL-6 mediates in small part the links between obesity, insulin resistance, and cardio-metabolic diseases.
El-Mikkawy et al. [18] mentioned significantly higher levels of IL-6 in subjects with overweight and obesity as compared to those in healthy control, moreover, a significantly positive correlation was found between circulating levels of IL6 and BMI only in subjects with very severe (grade III) obesity.
Amit et al. [19] found that the increasing appreciation that adipose tissue is an active endocrine organ producing a variety of hormones and cytokines that may affect CRP levels, of these, IL-6 is thought to be the principal cytokine involved in CRP release from the liver and up to one-third of circulating IL-6 is derived from adipose tissue.
Mahwati & Nurrika [20] found an association between obesity indicators and CRP levels, there is a positive correlation between BMI, waist circumference (WC), and CRP.
Khaodhiar et al. [21] found that subjects with obesity had significantly higher serum levels of IL-6 and CRP compared to control, and that serum levels of IL-6 and CRP were only positively correlated with BMI in morbidly obese subjects, suggesting that IL-6 could be secreted in an endocrine manner in proportion to fat mass expansion, with an associated increase in CRP hepatic production.
Obese patients, as well as those with chronic inflammatory diseases and abnormal serum lipid concentrations, had higher serum IL-6 levels [22].
Bruun et al. [23] found that the levels of IL-6 were increased and correlated with measures of insulin resistance in male subjects with abdominal obesity.
Increased IL-6 levels in obese people may raise the risk of insulin resistance and type 2 diabetes [ 4].
Elevated circulating IL-6 levels were an independent predictor of type 2 diabetes and were thought to play a role in the development of inflammation, insulin resistance, and cell dysfunction [24].
IL-6 levels were more strongly related to HbA1c, which indicates average glycemic levels in the three months preceding measurement, than with other glycemic traits, infections, and other inflammatory challenges are related to reactive hyperglycemia, which may be aggravated in patients with greater IL-6 levels [25, 26].
The positive association between CRP and T2DM is dependent on insulin resistance and body mass index (BMI), the mechanism of the association between CRP and T2DM is still not known in detail [5].
Phosat et al. [27] mentioned that the role of CRP as an initiated marker for T2DM, where they found that the pre-diabetic and T2DM groups had markedly higher CRP levels than the control, subjects with high CRP levels had an elevated risk of pre-diabetes and T2DM, even after adjusting for confounders (i.e. age, BMI and gender) this strong association remained.
Liu et al. [28] found in a study of 202 patients with hyperprolactinemia that a significantly higher proportion of hyperprolactinemic patients had at least one detectable autoantibody, as well as, higher levels of IL-6 compared to healthy controls.
Li et al. [1] observed that elevated levels of high sensitivity (hs-CRP) were significantly associated with an increased risk of erectile dysfunction (ED) after adjustment for conventional ED risk factors, including BMI, age, testosterone, alcohol, smoking, consumption, physical activity, hypertension, diabetes, and dyslipidemia. Low testosterone levels in men were significantly associated with a high level of inflammatory markers (CRP, IL-6) in different clinical conditions such as obesity, diabetes, and hyperprolactinemia [29-31].
On the other hand, the current decrease of NOS might be attributed to the high adipose tissue as indicated by BMI measurement and/or influence of possible secretion of chemerin that correlated negatively with NOS and/or high level of prolactin secretion [32].
Habib et al. [33] found that serum chemerin levels are elevated in patients with type 2 diabetes compared to control, and are positively correlated with adiposity and insulin resistance in patients with type 2 diabetes. Weigert et al. [34] and Tahir et al. [35] found that chemerin was elevated in T2DM subjects with a higher CRP level and positively correlated with CRP in normal weight, overweight and T2DM subjects after adjusting for BMI. Kraus et al. [14] mentioned that the skeletal muscle eNOS protein was significantly higher in the non-obese compared with the obese subjects.
Insulin resistance may be the cause of the reduced nitric oxide production in type 2 diabetes, insulin insufficiently stimulated NOS activity in skeletal muscle of type 2 diabetic subjects [36].
In humans, insulin resistance is often associated with elevated fasting plasma levels of cholesterol and triglycerides, eNOS deficiency might directly alter lipid metabolism [37].
Kashyap et al. [38] found that the basal and insulin-stimulated muscle NOS activity was impaired in type 2 diabetic subjects, paralleling the severity of insulin resistance.
The inhibition of expressed neuronal NO synthase decreased NO levels and increased basal PRL release, expression of inducible NO synthase also increased NO and inhibited PRL basal, whereas inhibition of this enzyme decreased NO production and recovered PRL release [39].
Duvilanski et al. [40] found that the actions of NO in controlling prolactin release are complex by effects mediated both by the hypothalamus and also by the pituitary gland itself, the actions of NO within the hypothalamustimulate, whereas the action at the pituitary level inhibits prolactin secretion.
Slightly high PRL caused a decrease in blood pressure (BP) caused by increased nitric oxide (NO) production, whereas higher PRL leads to increased BP due to decreased NO production [41].
Chemerin was positively correlated with body mass index and serum insulin and was negatively correlated with eNOS [42].
Neves et al. [43] found that chemerin reduces NO production, enhances NO breakdown, and also decreases NO-dependent cGMP signaling, thereby reducing vascular relaxation, the chemerin leads to destabilization of the eNOS dimer, impairing the catalytic function of the enzyme.
Conclusion
The IL-6 and CRP increment and NOS reduction in different groups might be indicated a pro-inflammatory action and low fertility in obesity, diabetic and sub-fertility groups.
Acknowledgments: None declared.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contributions: Najim Rasool R (First Author), Introduction Writer/Methodologist/Main Researcher (50%); Aboud Khalifa A (Second Author), Assistant Researcher/Statistical Analyst/Discussion Writer (50%)
Funding/Support: None declared.
Keywords:
Interleukin-6 [MeSH], C - Reactive Protein [MeSH], Nitric Oxide Synthase [MeSH], Obesity [MeSH], Diabetes [MeSH], Subfertility [MeSH]
References
1. Li Q, Wang QI, Xu W, Ma Y, Wang Q, Eatman D, et al. C-reactive protein causes adult-onset obesity through chronic inflammatory mechanism. Front Cell Dev Biol. 2020;8:18. [Link] [DOI:10.3389/fcell.2020.00018]
2. Rehman K, Akash MS, Liaqat A, Kamal S, Qadir MI, Rasul A. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2017;27(3):229-36. [Link] [DOI:10.1615/CritRevEukaryotGeneExpr.2017019712]
3. Stenlöf K, Wernstedt I, Fjällman T, Wallenius V, Wallenius K, Jansson JO. Interleukin-6 levels in the central nervous system are negatively correlated with fat mass in overweight/obese subjects. J Clin Endocrinol Metab. 2003;88(9):4379-83. [Link] [DOI:10.1210/jc.2002-021733]
4. Takumansang R, Warouw SM, Lestari H. Interleukin-6 and insulin resistance in obese adolescents. Paediatrica Indonesiana. 2013;53(5):268-72. [Link] [DOI:10.14238/pi53.5.2013.06]
5. Kanmani S, Kwon M, Shin MK, Kim MK. Association of C-reactive protein with risk of developing type 2 diabetes mellitus, and role of obesity and hypertension: a large population-based Korean cohort study. Sci Rep. 2019;9:4573. [Link] [DOI:10.1038/s41598-019-40987-8]
6. Sandler S, Bendtzen K, Eizirik DL, Welsh M. Interleukin-6 affects insulin secretion and glucose metabolism of rat pancreatic islets in vitro. Endocrinology. 1990;126(2):1288-94. [Link] [DOI:10.1210/endo-126-2-1288]
7. Fröhlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing HE, et al. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes care. 2000;23(12):1835-9. [Link] [DOI:10.2337/diacare.23.12.1835]
8. Cinelli MA, Do HT, Miley GP, Silverman RB. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Medicin Res Rev. 2020;40(1):158-89. [Link] [DOI:10.1002/med.21599]
9. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(Pt 3):593-615. [Link] [DOI:10.1042/bj3570593]
10. Kone BC, Kuncewicz T, Zhang W, Yu ZY. Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol Renal Physiol. 2003;285(2):F178-90. [Link] [DOI:10.1152/ajprenal.00048.2003]
11. Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915-8. [Link] [DOI:10.1016/0092-8674(94)90266-6]
12. Luo Y, Zhu Y, Basang W, Wang X, Li C, Zhou X. Roles of nitric oxide in the regulation of reproduction: a review. Front endocrinol. 2021;12. [Link] [DOI:10.3389/fendo.2021.752410]
13. O'Bryan MK, Schlatt S, Gerdprasert O, Phillips DJ, de Kretser DM, Hedger MP. Inducible nitric oxide synthase in the rat testis: evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod. 2000;63(5):1285-93. [Link] [DOI:10.1095/biolreprod63.5.1285]
14. Krause M, Rodrigues-Krause J, O'Hagan C, De Vito G, Boreham C, Susta D, et al. Differential nitric oxide levels in the blood and skeletal muscle of type 2 diabetic subjects may be consequence of adiposity: a preliminary study. Metabolism. 2012;61(11):1528-37. [Link] [DOI:10.1016/j.metabol.2012.05.003]
15. Foroumandi E, Alizadeh M, Kheirouri S, Asghari Jafarabadi M. Exploring the role of body mass index in relationship of serum nitric oxide and advanced glycation end products in apparently healthy subjects. PLoS One. 2019;14(3):e0213307. [Link] [DOI:10.1371/journal.pone.0213307]
16. Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, et al. TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116(10):2791-8. [Link] [DOI:10.1172/JCI28570.]
17. Bowker N, Shah RL, Sharp SJ, Stewart ID, Wheeler E, Ferreira MA, et al. Meta-analysis investigating the role of interleukin-6 mediated inflammation in type 2 diabetes. EBioMedicine. 2020;61:103062. [Link] [DOI:10.1016/j.ebiom.2020.103062]
18. El-Mikkawy DM, EL-Sadek MA, EL-Badawy MA, Samaha D. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egyp Rheumatol Rehabil. 2020;47:7. [Link] [DOI:10.1186/s43166-020-00003-8]
19. Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94(9):3251-8. [Link] [DOI:10.1210/jc.2008-2406]
20. Mahwati Y, Nurrika D. Obesity indicators and C-reactive protein in Indonesian adults (more than equal to 40 years old): the Indonesian family life survey 5. Nat Public Health J. 2020;15(4). [Link] [DOI:10.21109/kesmas.v15i4.3296]
21. Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin‐6 and C‐reactive protein correlate with body mass index across the broad range of obesity. J Parenter Enteral Nutr. 2004;28(6):410-5. [Link] [DOI:10.1177/0148607104028006410]
22. Galcheva SV, Iotova VM, Yotov YT, Bernasconi S, Street ME. Circulating proinflammatory peptides related to abdominal adiposity and cardiometabolic risk factors in healthy prepubertal children. Eur J Endocrinol. 2011;164(4):553-8. [Link] [DOI:10.1530/EJE-10-1124]
23. Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148(5):535-42. [Link] [DOI:10.1530/eje.0.1480535]
24. Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26(3):685-98. [Link] [DOI:10.1007/s10787-018-0458-0]
25. Dungan KM, Braithwaite SS. Preiser. Stress Hyperglycaemia. Lancet. 2009;373(9677):1798-807. [Link] [DOI:10.1016/S0140-6736(09)60553-5]
26. Nakamura M, Oda S, Sadahiro T, Watanabe E, Abe R, Nakada TA, et al. Correlation between high blood IL-6 level, hyperglycemia, and glucose control in septic patients. Crit Care. 2012;16(2):R58. [Link] [DOI:10.1186/cc11301]
27. Phosat C, Panprathip P, Chumpathat N, Prangthip P, Chantratita N, Soonthornworasiri N, et al. Elevated C-reactive protein, interleukin 6, tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural Thais: a cross-sectional study. BMC Endocr Disord. 2017;17(1):44. [Link] [DOI:10.1186/s12902-017-0189-z]
28. Liu Y, Zhang Z, Jin Q, Liu Y, Kang Z, Huo Y, et al. Hyperprolactinemia is associated with a high prevalence of serum autoantibodies, high levels of inflammatory cytokines and an abnormal distribution of peripheral B-cell subsets. Endocrine. 2019;64(3):648-56. [Link] [DOI:10.1007/s12020-019-01896-y]
29. Tremellen K, McPhee N, Pearce K. Metabolic endotoxaemia related inflammation is associated with hypogonadism in overweight men. Basic Clin Androl. 2017;27:5. [Link] [DOI:10.1186/s12610-017-0049-8]
30. Langer C, Gansz B, Goepfert C, Engel T, Uehara Y, von Dehn G, Jansen H, Assmann G, von Eckardstein A. Testosterone up-regulates scavenger receptor BI and stimulates cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2002;296(5):1051-7. [Link] [DOI:10.1016/S0006-291X(02)02038-7]
31. Benjamin UO, Akhere TI, Orhue AA. The prevalence and patterns of endocrinopathies amongs azoospermic male partners at a fertility clinic in Benin city. Endocrinol Metab Int J. 2014;1(1):8-13. [Link] [DOI:10.15406/emij.2014.01.00003]
32. Qusay Falih I, AH Alobeady M, Banoon SR, Saleh MY. Role of oxidized low-density lipoprotein in human diseases: a review. J Chem Health Risks. 2021;11:71-83. [Link]
33. Habib SS, Eshki A, AlTassan B, Fatani D, Helmi H, AlSaif S. Relationship of serum novel adipokine chemerin levels with body composition, insulin resistance, dyslipidemia and diabesity in Saudi women. Eur Rev Med Pharmacol Sci. 2017;21(6):1296-302. [Link]
34. Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol. 2010;72(3):342-8. [Link] [DOI:10.1111/j.1365-2265.2009.03664.x]
35. Tahir NT, Falih IQ, AL_Husaini FK, Zeghair SA. Study the effect of chemerin level in type ii diabetic patients with and without retinopathy. Syst Rev Pharm. 2020;11(11):1856-63. [Link]
36. Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes. 2010;59(9):2152-9. [Link] [DOI:10.2337/db09-1772]
37. Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104(3):342-5. [Link] [DOI:10.1161/01.CIR.104.3.342]
38. Kashyap SR, Roman LJ, Lamont J, Masters BS, Bajaj M, Suraamornkul S, et al. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J Clin Endocrinol Metab. 2005;90(2):1100-5. [Link] [DOI:10.1210/jc.2004-0745]
39. Andric SA, Gonzalez-Iglesias AE, Van Goor F, Tomić M, Stojilkovic SS. Nitric oxide inhibits prolactin secretion in pituitary cells downstream of voltage-gated calcium influx. Endocrinology. 2003;144(7):2912-21. [Link] [DOI:10.1210/en.2002-0147]
40. Duvilanski BH, Zambruno C, Seilicovich A, Pisera D, Lasaga M, Diaz MD, et al. Role of nitric oxide in control of prolactin release by the adenohypophysis. Proc Natl Acad Sci U S A. 1995;92(1):170-4. [Link] [DOI:10.1073/pnas.92.1.170]
41. Dourado M, Cavalcanti F, Vilar L, Cantilino A. Relationship between prolactin, chronic kidney disease, and cardiovascular risk. Int J Endocrinol. 2020;2020. [Link] [DOI:10.1155/2020/9524839]
42. Wang L, Yang T, Ding Y, Zhong Y, Yu L, Peng M. Chemerin plays a protective role by regulating human umbilical vein endothelial cell-induced nitric oxide signaling in preeclampsia. Endocrine. 2015;48(1):299-308. [Link] [DOI:10.1007/s12020-014-0286-y]
43. Neves KB, Lobato NS, Lopes RA, Filgueira FP, Zanotto CZ, Oliveira AM, et al. Chemerin reduces vascular nitric oxide/cGMP signalling in rat aorta: a link to vascular dysfunction in obesity?. Clin Sci. 2014;127(2):111-22. [Link] [DOI:10.1042/CS20130286]








