Volume 14, Issue 1 (2022)
Iran J War Public Health 2022, 14(1): 105-109 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/02/26 | Accepted: 2022/04/12 | Published: 2022/04/9
Received: 2022/02/26 | Accepted: 2022/04/12 | Published: 2022/04/9
How to cite this article
Salman Z, Ghudhaib K. Association of Osteopontin and Alkaline Phosphatase in Male Patients with Diabetes Mellitus Type 2 and Periodontitis. Iran J War Public Health 2022; 14 (1) :105-109
URL: http://ijwph.ir/article-1-1138-en.html
URL: http://ijwph.ir/article-1-1138-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
Z.A. Salman *1, K.K. Ghudhaib1
1- Department of Chemistry, College of Science for Women, University of Baghdad, Baghdad, Iraq
Full-Text (HTML) (706 Views)
Introduction
The previous were conducted to study the diabetes complications with kidneys, nerves, and bones [1-4]. Other studies were conducted to investigate the outcome of nano-particles on activities of some selected salivary factors in patients suffering from chronic periodontitis [5-8].
Diabetes disease developed as a result of insulin insufficiency, defect in its action, or both disorders. Periodontitis represents the sixth complication of diabetes, which means that diabetes leads to the stimulation and develop periodontitis. Furthermore, induces of periodontitis causes hyper-inflammation and impairs bone repair, so periodontitis and diabetes possess a bidirectional relationship [9].
Osteopontin is a glycoprotein that was discovered in osteoblast first. It was referred to osteopontin as an active factor in numerous physiological and pathological activities like bone turnover, inflammation, tooth mineralization in addition to osteoporosis and stress. It was reported that a long period of hyperglycemia leads to the formation of oral hygienic problems like periodontal disorders, abscesses, and dry mouth [10].
The major hygienic problem caused by diabetes is periodontitis, which can cause to damage teeth [11]. Also, the host may be vulnerable to pathogenic bacteria, especially gram-negative that lead to an increase in the abnormal condition of teeth. Beside that chronic inflammation that can be occurred affect both connective tissues and bone around the tooth [12, 13].
In the case of periodontitis, several factors can be applied to characterize this case like gingival bleeding, bone resorption, and attachment loss progression [10]. The toxin products that are formed by the action of pathogenic bacteria on dental plaque cause destruction in tooth tissues. the criteria of periodontitis include certain factors like bleeding, loss of clinical attachment, and pocket depth [14]. Levels of periodontitis markers can be estimated after their excreted in blood during the disease period through the inflammation process [15].
Previous studies referred to the elevation of osteopontin level during inflammation conditions in addition to tissue remodeling in compared to its level in normal conditions [16]. Alkaline phosphatase represents one of the hydrolytic enzymes that depend on hydrolyzing of a phosphate group from biomolecule such as protein molecule under dephosphorylating process. Association between diabetes and liver function may be occurred due to Alkaline phosphatase (ALP) relation with both bone disease and diabetes. Subsequently, ALP represents the best clinical marker for both bone and liver diseases [17, 18].
The objectives of the current study are to assess the concentrations of osteopontin and alkaline phosphate in patients with diabetes, periodontitis,
diabetic and periodontitis and healthy subjects to the knowledge of the ability to use these factors as predictor factors for periodontitis and to study the correlation of these factors under each above case, together with Receiver operating characteristic (ROC) curve analysis.
Materials and Methods
Blood samples were obtained from 120 male persons, aged 35-65 years. Patients have been divided into three groups: diabetic patients group (30 patients with diabetes only, periodontitis group (30 patients with periodontitis only and diabetic periodontitis patients group (30 diabetic patients with periodontitis), and 30 healthy subjects as a control group. medical and history of disease data were documented in a special questionnaire approved for this purpose for all patients and healthy persons, all patients, and healthy persons were informed of the study objectives and agreed to be examined when they attended to Albaladyat specialist center for dentistry at a period of November 2020 to April 2021. Vein blood samples (5ml) from each subject(patients and controls) were taken in serum separating gel tubes at room temp., serum was obtained by centrifuging the clotted blood at 400rpm for 10min. The serum was removed then and transferred into Eppendorf tubes and stored at -20C○ until the assayed time. Serum blood glucose and alkaline phosphatase were measured by an enzymatic method, while osteopontin was measured by the ELISA technique.
The primary results of this study were used as inlet data to record the final statistical data in terms of mean and SE by ANOVA, LSD, and ROC analysis to report significant differences and areas under the curve. SAS 10.0. 2 and MedCalc 16.4.3 were used.
Findings
The mean age of the healthy group (control) was 43.63±6.81 years and the mean BMI was 22.43±2.06 kg/m2. In the diabetes mellitus (DM) group, the mean age was 47.43±6.35 years, and the mean BMI was 29.40±3.92kg/m2. In the diabetes mellitus with periodontitis (DM & P) group, the mean age was 47.03±6.11 years, and the mean BMI was 27.26±2.91kg/m2. In Periodontitis (P) group, the mean age was 44.13±5.89 years, and the mean BMI was 28.00±3.63kg/m2. The results showed high importance differences between DM patients and the control groups in both age and BMI. At the same time, there are high considerable differences between groups of DM and DM & P and groups of chronic periodontitis tougher with control groups in age. Also, the results showed no significant difference between groups such as DM and DM & P in the case of age. Furthermore, the results of BMI showed important significance between all patient groups and the control group. (p=0.0391 for age, p=0.0001 for BMI). LSD value for age was 3.222 (p≤0.05) and BMI was 1.645 (p≤0.01).
In the healthy group (control), the mean Fasting Blood Sugar (FBS) level was 94.76±17.06mg/dL, and the mean ALP level was 60.80±17.10U/L. In the DM group, the mean FBS level was 214.56±87.58mg/dL, and the mean ALP level was 89.93±33.40U/L. In DM & P group, the mean FBS level was 248.36±106.42mg/dL, and the mean ALP level was 78.67±34.83U/L. In the P group, the mean FBS level was 102.60±18.74mg/dL, and the mean ALP level was 63.20±16.83U/L. The results of FBS and ALP showed highly significant differences between DM and control groups. Furthermore, the result also showed considerable differences between groups of DM and DM & P related to the periodontitis group in the case of FBS. However, both DM and DM & P groups revealed high significant differences with groups of P and control in the case of ALP. (p=0.0001 for FBS level, p=0.0001 for ALP level). LSD value for FBS level was 35.835 (p≤0.05), and for ALP level was 13.783 (p≤0.01).
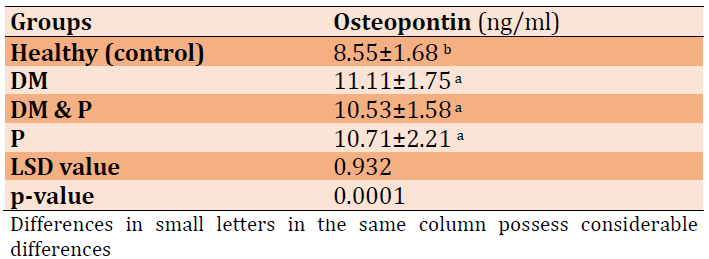
Mean±SD values of osteopontin in patient and control groups showed in Table 1. The outcomes revealed highly significant increases in patient groups in comparison to the healthy group (p=0.0001), while there were no significant differences among other groups.
Table 1) Osteopontin levels for all patients and control groups
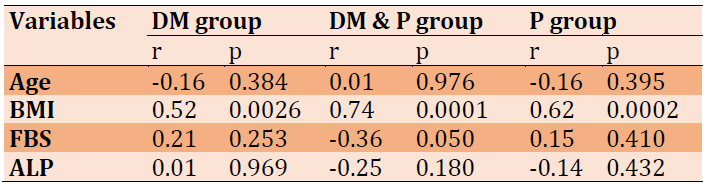
The results of the correlation between osteopontin and the studied parameters in the current work were noticed in Table 2. Outcomes referred to a positive correlation between osteopontin and BMI in all patient groups, while a negative correlation was recorded with FBS in DM & P group. Furthermore, there was a non-significant correlation between osteopontin levels and FBS and ALP levels in the two groups (periodontitis and diabetic multiuse). Also, there is no correlation between osteopontin and age in patient groups as noticed in Table 2.
Table 2) Osteopontin correlation with other parameters in the patient groups
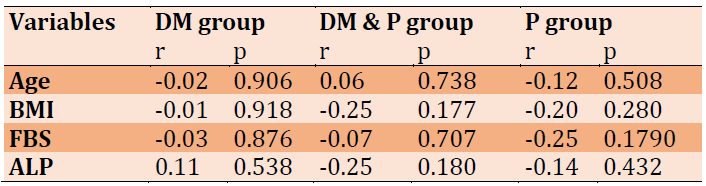
Correlation coefficients of ALP in three patient groups (periodontitis, diabetic and diabetic with periodontitis) were noticed in Table 3. The results revealed that age has a non-significant correlation with all the studied parameters in all patient groups.
Table 3) Correlation coefficient of ALP with other parameters in the patient groups
Data of ROC analysis was demonstrated that osteopontin represents an excellent factor for diagnosis of all the studied cases because the values of AUC were found to be 0.928, 0.969, and 0.911 in the DM group, DM & P group, and P group, respectively, in addition to that CI has the value of 95%, as shown in Table 4 and Figures 1-3.
Table 4) ROC data of osteopontin factor of patient groups
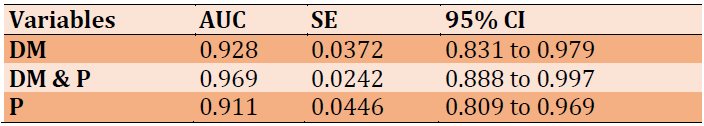
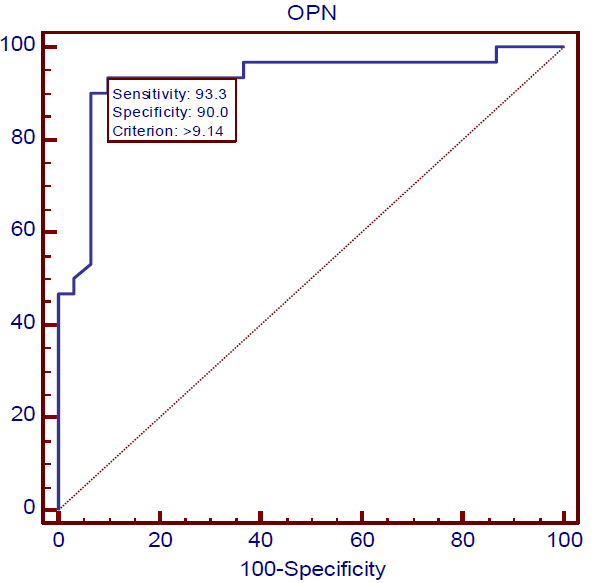
Figure 1) ROC curve of Osteopontin in DM

Figure 2) ROC curve of Osteopontin in DM with Periodontitis
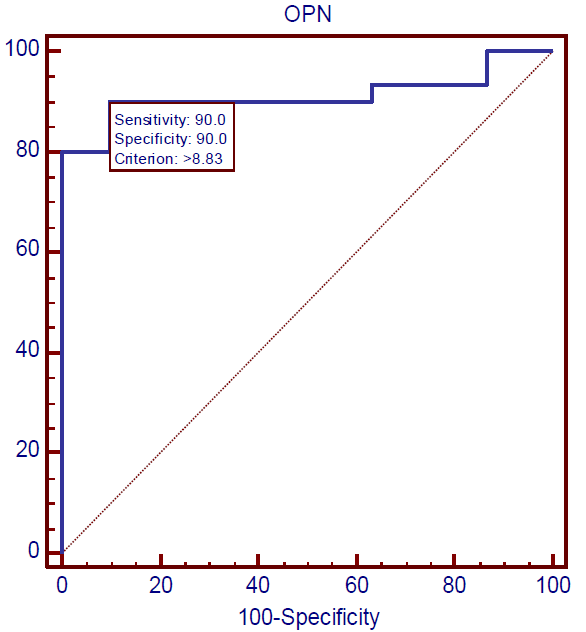
Figure 3) ROC curve of osteopontin in periodontitis
Discussion
The results of the current study were revealed that both age and BMI considerably increased in each of the DM and DM & P groups compared to P and healthy subject groups; however there is no important difference between P and any patients group like DM or DM & P. This result is in agreement with the study [19] that referred to when BMI more than 30 considers a risk factor for insulin resistance; therefore, fatness represents the main threat feature of insulin resistance [20]. Furthermore, BMI was found to be the main risk factor for IR than patients with low BMI [21]. Inversely, several studies showed no significant difference in parameters of BMI and age [22-24].
The concentration of FBS revealed a considerable increase (p≤0.01) in both of DM & P and DM groups (248.36±106.42), (214.56±87.58) respectively, in comparison with the control and periodontitis groups (94.76±17.06), (102.60±18.74), respectively. This result conforms with other studies which referred that the level of FBS increase in diabetic and diabetic nephropathy comparison with control groups [23, 24]. Furthermore, a study found that periodontitis leads to elevate sugar levels in diabetic patients, and management of periodontitis causes enhancement of hyperglycemic control in this case [25]. Other studies reported that patients with periodontitis have more risk factors in the development of diabetes complications like nephropathy, retinopathy, increase glucose levels as well as poor metabolic control in comparison with diabetic patients without periodontitis [26, 27]. Moreover, patients with diabetic periodontitis are more vulnerable to infection and diseases due to the risk to the immune system that occurred by the action of disease complications [28]. Therefore, bone damage may be occurred by the action of numerous pathologies that can be developed by the diabetic periodontitis effect [29].
Alkaline phosphatase results in the current study revealed high levels in patient groups of diabetic and diabetic periodontitis related to control and periodontitis groups, these results may be attributed to the nature of alkaline phosphatase which considers as the marker for bone and liver disorders. Subsequently, the results reflect the patient clinical cases and agreement with the study which found an increased level of ALK significantly in DM and DM & P groups compared to control and P groups [16]. Furthermore, it was reported that the ALP level was increased in the DM & P than in the control group [30, 31]. The level of serum osteopontin show also significantly higher in DM groups than in the control group. This result can be elucidated based on the damage to bone cells which is in agreement with a previous study that recorded a high significance in ALP level in diabetic patients than in healthy subjects [32].
Conclusion
Serum levels of Osteopontin and ALP are raised in the development of DM and its complications such as periodontitis. Subsequently, osteopontin is the best indicator that can be used for predicting diabetes and diagnosis of periodontitis.
Acknowledgments: We appreciate all the participants who participated in the present study.
Ethical permissions: There is no ethical code.
Conflicts of Interest: No conflict of interest was reported.
Authors' contribution: Salman ZA (First Author), Main Researcher (50%); Ghudhaib KK (Second Author), Main Researcher (50%)
Funding/Support: The present study did not have the financial support of any institution or organization.
The previous were conducted to study the diabetes complications with kidneys, nerves, and bones [1-4]. Other studies were conducted to investigate the outcome of nano-particles on activities of some selected salivary factors in patients suffering from chronic periodontitis [5-8].
Diabetes disease developed as a result of insulin insufficiency, defect in its action, or both disorders. Periodontitis represents the sixth complication of diabetes, which means that diabetes leads to the stimulation and develop periodontitis. Furthermore, induces of periodontitis causes hyper-inflammation and impairs bone repair, so periodontitis and diabetes possess a bidirectional relationship [9].
Osteopontin is a glycoprotein that was discovered in osteoblast first. It was referred to osteopontin as an active factor in numerous physiological and pathological activities like bone turnover, inflammation, tooth mineralization in addition to osteoporosis and stress. It was reported that a long period of hyperglycemia leads to the formation of oral hygienic problems like periodontal disorders, abscesses, and dry mouth [10].
The major hygienic problem caused by diabetes is periodontitis, which can cause to damage teeth [11]. Also, the host may be vulnerable to pathogenic bacteria, especially gram-negative that lead to an increase in the abnormal condition of teeth. Beside that chronic inflammation that can be occurred affect both connective tissues and bone around the tooth [12, 13].
In the case of periodontitis, several factors can be applied to characterize this case like gingival bleeding, bone resorption, and attachment loss progression [10]. The toxin products that are formed by the action of pathogenic bacteria on dental plaque cause destruction in tooth tissues. the criteria of periodontitis include certain factors like bleeding, loss of clinical attachment, and pocket depth [14]. Levels of periodontitis markers can be estimated after their excreted in blood during the disease period through the inflammation process [15].
Previous studies referred to the elevation of osteopontin level during inflammation conditions in addition to tissue remodeling in compared to its level in normal conditions [16]. Alkaline phosphatase represents one of the hydrolytic enzymes that depend on hydrolyzing of a phosphate group from biomolecule such as protein molecule under dephosphorylating process. Association between diabetes and liver function may be occurred due to Alkaline phosphatase (ALP) relation with both bone disease and diabetes. Subsequently, ALP represents the best clinical marker for both bone and liver diseases [17, 18].
The objectives of the current study are to assess the concentrations of osteopontin and alkaline phosphate in patients with diabetes, periodontitis,
diabetic and periodontitis and healthy subjects to the knowledge of the ability to use these factors as predictor factors for periodontitis and to study the correlation of these factors under each above case, together with Receiver operating characteristic (ROC) curve analysis.
Materials and Methods
Blood samples were obtained from 120 male persons, aged 35-65 years. Patients have been divided into three groups: diabetic patients group (30 patients with diabetes only, periodontitis group (30 patients with periodontitis only and diabetic periodontitis patients group (30 diabetic patients with periodontitis), and 30 healthy subjects as a control group. medical and history of disease data were documented in a special questionnaire approved for this purpose for all patients and healthy persons, all patients, and healthy persons were informed of the study objectives and agreed to be examined when they attended to Albaladyat specialist center for dentistry at a period of November 2020 to April 2021. Vein blood samples (5ml) from each subject(patients and controls) were taken in serum separating gel tubes at room temp., serum was obtained by centrifuging the clotted blood at 400rpm for 10min. The serum was removed then and transferred into Eppendorf tubes and stored at -20C○ until the assayed time. Serum blood glucose and alkaline phosphatase were measured by an enzymatic method, while osteopontin was measured by the ELISA technique.
The primary results of this study were used as inlet data to record the final statistical data in terms of mean and SE by ANOVA, LSD, and ROC analysis to report significant differences and areas under the curve. SAS 10.0. 2 and MedCalc 16.4.3 were used.
Findings
The mean age of the healthy group (control) was 43.63±6.81 years and the mean BMI was 22.43±2.06 kg/m2. In the diabetes mellitus (DM) group, the mean age was 47.43±6.35 years, and the mean BMI was 29.40±3.92kg/m2. In the diabetes mellitus with periodontitis (DM & P) group, the mean age was 47.03±6.11 years, and the mean BMI was 27.26±2.91kg/m2. In Periodontitis (P) group, the mean age was 44.13±5.89 years, and the mean BMI was 28.00±3.63kg/m2. The results showed high importance differences between DM patients and the control groups in both age and BMI. At the same time, there are high considerable differences between groups of DM and DM & P and groups of chronic periodontitis tougher with control groups in age. Also, the results showed no significant difference between groups such as DM and DM & P in the case of age. Furthermore, the results of BMI showed important significance between all patient groups and the control group. (p=0.0391 for age, p=0.0001 for BMI). LSD value for age was 3.222 (p≤0.05) and BMI was 1.645 (p≤0.01).
In the healthy group (control), the mean Fasting Blood Sugar (FBS) level was 94.76±17.06mg/dL, and the mean ALP level was 60.80±17.10U/L. In the DM group, the mean FBS level was 214.56±87.58mg/dL, and the mean ALP level was 89.93±33.40U/L. In DM & P group, the mean FBS level was 248.36±106.42mg/dL, and the mean ALP level was 78.67±34.83U/L. In the P group, the mean FBS level was 102.60±18.74mg/dL, and the mean ALP level was 63.20±16.83U/L. The results of FBS and ALP showed highly significant differences between DM and control groups. Furthermore, the result also showed considerable differences between groups of DM and DM & P related to the periodontitis group in the case of FBS. However, both DM and DM & P groups revealed high significant differences with groups of P and control in the case of ALP. (p=0.0001 for FBS level, p=0.0001 for ALP level). LSD value for FBS level was 35.835 (p≤0.05), and for ALP level was 13.783 (p≤0.01).
Mean±SD values of osteopontin in patient and control groups showed in Table 1. The outcomes revealed highly significant increases in patient groups in comparison to the healthy group (p=0.0001), while there were no significant differences among other groups.
Table 1) Osteopontin levels for all patients and control groups

The results of the correlation between osteopontin and the studied parameters in the current work were noticed in Table 2. Outcomes referred to a positive correlation between osteopontin and BMI in all patient groups, while a negative correlation was recorded with FBS in DM & P group. Furthermore, there was a non-significant correlation between osteopontin levels and FBS and ALP levels in the two groups (periodontitis and diabetic multiuse). Also, there is no correlation between osteopontin and age in patient groups as noticed in Table 2.
Table 2) Osteopontin correlation with other parameters in the patient groups

Correlation coefficients of ALP in three patient groups (periodontitis, diabetic and diabetic with periodontitis) were noticed in Table 3. The results revealed that age has a non-significant correlation with all the studied parameters in all patient groups.
Table 3) Correlation coefficient of ALP with other parameters in the patient groups

Data of ROC analysis was demonstrated that osteopontin represents an excellent factor for diagnosis of all the studied cases because the values of AUC were found to be 0.928, 0.969, and 0.911 in the DM group, DM & P group, and P group, respectively, in addition to that CI has the value of 95%, as shown in Table 4 and Figures 1-3.
Table 4) ROC data of osteopontin factor of patient groups


Figure 1) ROC curve of Osteopontin in DM

Figure 2) ROC curve of Osteopontin in DM with Periodontitis

Figure 3) ROC curve of osteopontin in periodontitis
Discussion
The results of the current study were revealed that both age and BMI considerably increased in each of the DM and DM & P groups compared to P and healthy subject groups; however there is no important difference between P and any patients group like DM or DM & P. This result is in agreement with the study [19] that referred to when BMI more than 30 considers a risk factor for insulin resistance; therefore, fatness represents the main threat feature of insulin resistance [20]. Furthermore, BMI was found to be the main risk factor for IR than patients with low BMI [21]. Inversely, several studies showed no significant difference in parameters of BMI and age [22-24].
The concentration of FBS revealed a considerable increase (p≤0.01) in both of DM & P and DM groups (248.36±106.42), (214.56±87.58) respectively, in comparison with the control and periodontitis groups (94.76±17.06), (102.60±18.74), respectively. This result conforms with other studies which referred that the level of FBS increase in diabetic and diabetic nephropathy comparison with control groups [23, 24]. Furthermore, a study found that periodontitis leads to elevate sugar levels in diabetic patients, and management of periodontitis causes enhancement of hyperglycemic control in this case [25]. Other studies reported that patients with periodontitis have more risk factors in the development of diabetes complications like nephropathy, retinopathy, increase glucose levels as well as poor metabolic control in comparison with diabetic patients without periodontitis [26, 27]. Moreover, patients with diabetic periodontitis are more vulnerable to infection and diseases due to the risk to the immune system that occurred by the action of disease complications [28]. Therefore, bone damage may be occurred by the action of numerous pathologies that can be developed by the diabetic periodontitis effect [29].
Alkaline phosphatase results in the current study revealed high levels in patient groups of diabetic and diabetic periodontitis related to control and periodontitis groups, these results may be attributed to the nature of alkaline phosphatase which considers as the marker for bone and liver disorders. Subsequently, the results reflect the patient clinical cases and agreement with the study which found an increased level of ALK significantly in DM and DM & P groups compared to control and P groups [16]. Furthermore, it was reported that the ALP level was increased in the DM & P than in the control group [30, 31]. The level of serum osteopontin show also significantly higher in DM groups than in the control group. This result can be elucidated based on the damage to bone cells which is in agreement with a previous study that recorded a high significance in ALP level in diabetic patients than in healthy subjects [32].
Conclusion
Serum levels of Osteopontin and ALP are raised in the development of DM and its complications such as periodontitis. Subsequently, osteopontin is the best indicator that can be used for predicting diabetes and diagnosis of periodontitis.
Acknowledgments: We appreciate all the participants who participated in the present study.
Ethical permissions: There is no ethical code.
Conflicts of Interest: No conflict of interest was reported.
Authors' contribution: Salman ZA (First Author), Main Researcher (50%); Ghudhaib KK (Second Author), Main Researcher (50%)
Funding/Support: The present study did not have the financial support of any institution or organization.
Keywords:
References
1. Hamid GS, Ghudhaib KK, Allawi AA. Association of type IV collagen with progression of diabetic nephropathy disease in Iraqi patients under typical criteria. Biochem Cell Arch. 2021;21(1):1471-5. [Link]
2. Hamid GS, Allawi AA, Ghudhaib KK. Correlation of pentosidine with kidney diseases in Iraqi patients with diabetic nephropathy. Iraqi J Sci. 2021:3436-42. [Link] [DOI:10.24996/ijs.2021.62.10.2]
3. Ghudhaib KK. Effect of alcoholic Catechin extract on hyperglycemia, hyperlipidemia and liver functions in Alloxan diabetic mice. Baghdad Sci J. 2014;11(3). [Link] [DOI:10.21123/bsj.11.3.1192-1200]
4. Ghudhaib KK, Turaki KM, Muzal SA. Estimation of serum Osteocalcin levels in osteoporotic postmenopausal Iraqi women with type 2 diabetes mellitus. Baghdad Sci J. 2014;11(4). [Link] [DOI:10.21123/bsj.11.4.1549-1555]
5. Ghudhaib KK, Ibrahim LM, Salman EA, Salman ZA. Effect of Zno nanoparticles on salivary peroxidase activity in chronic periodontitis patients (in vitro study). IOSR J Appl Chem. 2016;9(4). [Link]
6. Ibrahim L, Ghudhaib KK, Al-Rubaee EA, Salman ZA. Estimation of ZnO nanoparticles effect on salivary alp activity in chronic periodontitis patients: in vitro study. Int J Adv Res Biol Sci. 2016;3(4):152-9. [Link]
7. Ghudhaib KK, Ibrahim L, Al-Rubaee EA, Salman ZA. Assessment of ZnO nanoparticles effect on AST activity in Saliva of patients with chronic periodontitis: in vitro study. Int J Adv Res Biol Sci. 2016;3(6):179-86. [Link]
8. Ghudhaib KK, Ibrahim LM, Al-Rubaee EA, Salman ZA. Determination of Vmax and Km of ALP, AST and Peroxidase in Saliva of Chronic Periodontitis Patients with and without ZnO NPs: A Kinetic Study. Int J Adv Res Biol Sci. 2016;3(7):85-91. [Link]
9. Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20:204. [Link] [DOI:10.1186/s12903-020-01180-w]
10. Salman ZA, Mezil SA, ALI VS, Kadim NM. Assessment of ZnO nanoparticles effect on peroxidase activity in serum of patients with type2 diabetes millets: in vitro study. Int J Pharmaceutical Res. 2019;11(2):76-80. [Link] [DOI:10.31838/ijpr/2019.11.02.014]
11. Fadhil R, Abdulameer LA, Salman ZA, Raheem ZJ. Effect of silicon dioxide nanoparticles in detection of serum peroxidase activity in severe periodontitis patients. Biochem Cell Arch. 2020;20(2):4971-5. [Link]
12. Panezai J, Altamash M, Engstrӧm PE, Larsson A. Association of Glycated proteins with inflammatory proteins and periodontal disease parameters. J Diabetes Res. 2020;2020:6450742. [Link] [DOI:10.1155/2020/6450742]
13. Koppolu P, Sirisha S, Mishra A, Deshpande K, Lingam AS, Alotaibi DH, et al. Alkaline phosphatase and acid phosphatase levels in saliva and serum of patients with healthy periodontium, gingivitis, and periodontitis before and after scaling with root planing: A clinico-biochemical study. Saudi J Biol Sci. 2021;28(1):380-5. [Link] [DOI:10.1016/j.sjbs.2020.10.016]
14. Salman ZA, Fadhil R, Kadim NM, Al-Rubaee EA. Effect of ZnO Nanoparticles on AST activity in gingival cervicular fluid of smokers and nonsmokers chronic periodontitis patients: in vitro study. J Glob Pharm Technol. 2018;10(3):375-80. [Link]
15. De A, Puttannavar R, Rahman F, Adak A, Sahoo R, Prakash BR. Estimation of salivary and serum alkaline phosphatase level as a diagnostic marker in type-2 diabetes mellitus with periodontal health and disease: a clinico-biochemical study. J Oral Maxillofac Pathol. 2018;22(3):445. [Link] [DOI:10.4103/jomfp.JOMFP_212_18]
16. Assy MH, El Ashmawy HM, Soliman JS, Abd El Hamed AB. Osteopontin as a marker of diabetic nephropathy in patients with type 2 diabetes mellitus. Egypt J Hospital Med. 2020;81(7):2439-44. [Link] [DOI:10.21608/ejhm.2020.133956]
17. Zhang Y, Zhou C, Li J, Zhang Y, Xie D, Liang M, Wang B, et al. Serum alkaline phosphatase levels and the risk of new-onset diabetes in hypertensive adults. Cardiovasc Diabetol. 2020;19(1):186. [Link] [DOI:10.1186/s12933-020-01161-x]
18. Salman ZA, Azeez RA, Yaseen AK, AL-Rubaee EA. Effect of zinc oxide nanoparticles on serum alkaline phosphatase activity in male mice. Biochem Cell Arch. 2018;18(2):1715-9. [Link]
19. Dennis M, Lee AR, McCarthy T. Nutritional considerations of the gluten-free diet. Gastroenterol Clin North Am. 2019;48(1):53-72 [Link] [DOI:10.1016/j.gtc.2018.09.002]
20. Kim K, Park SM. Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: a cross-sectional study. Sci Rep. 2018;8(1):2703. [Link] [DOI:10.1038/s41598-018-21168-5]
21. Ghaib ZJ, Ghudhaib KK, Mohsen FY. Assessment of neuron specific enolase level and some related biochemical factors in patients with diabetic peripheral nerve disorders. Indian J Forensic Med Toxicol. 2021;15(3). [Link]
22. Mahmood IH, Abed MN, Merkhan MM. Effects of blocking of angiotensin system on the prevalence of metabolic syndrome in type 2 diabetic patients. Pak J Med Sci. 2013;29(1):140-3. [Link] [DOI:10.12669/pjms.291.2782]
23. Hassan KM, Balaji R, Gnaneswaran S, Kumar JS. To Study the association of HbA1C with retinopathy, neuropathy and high risk foot among diabetic patients attending rural tertiary care Hospital of Tamil Nadu, India. Int J Ophthalmol Eye Res. 2016;4(4):206-11. [Link] [DOI:10.19070/2332-290X-1600042]
24. Pan HZ, Zhang L, Guo MY, Sui H, Li H, Wu WH, et al. The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol. 2010; 47(Suppl 1):71-6. [Link] [DOI:10.1007/s00592-009-0128-1]
25. Preshaw PM, Bissett SM. Periodontitis and diabetes. Br Dent J. 2019;227(7):577-84. [Link] [DOI:10.1038/s41415-019-0794-5]
26. Khanuja PK, Narula SC, Rajput R, Sharma RK, Tewari S. Association of periodontal disease with glycemic control in patients with type 2 diabetes in Indian population. Front Med. 2017;11(1):110-9. [Link] [DOI:10.1007/s11684-016-0484-5]
27. Jeftha A, Roberts T, Kimmie-Dhansay F. The effect of periodontal disease on metabolic control in patients with diabetes mellitus in South Africa: Protocol for a Systematic Review. JMIR Res Protoc. 2021;10(7):e27471. [Link] [DOI:10.2196/27471]
28. Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non‐insulin‐dependent diabetes mellitus. J Periodontol. 1991;62(2):123-31. [Link] [DOI:10.1902/jop.1991.62.2.123]
29. Mochida Y, Duarte WR, Tanzawa H, Paschalis EP, Yamauchi M. Decorin modulates matrix mineralization in vitro. Biochem Biophys Res Commun. 2003;305(1):6-9. [Link] [DOI:10.1016/S0006-291X(03)00693-4]
30. Nakamura M, Slots J. Salivary enzymes: origin and relationship to periodontal disease. J Periodontal Res. 1983;18(6):559-69. [Link] [DOI:10.1111/j.1600-0765.1983.tb00393.x]
31. Chen J, Singh K, Mukherjee BB, Sodek J. Developmental expression of osteopontin (OPN) mRNA in rat tissues: evidence for a role for OPN in bone formation and resorption. Matrix. 1993;13(2):113-23. [Link] [DOI:10.1016/S0934-8832(11)80070-3]
32. Yan X, Sano M, Lu L, Wang W, Zhang Q, Zhang R, et al. Plasma concentrations of osteopontin, but not thrombin-cleaved osteopontin, are associated with the presence and severity of nephropathy and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2010;9:70. [Link] [DOI:10.1186/1475-2840-9-70]








