Volume 14, Issue 3 (2022)
Iran J War Public Health 2022, 14(3): 339-345 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/02/23 | Accepted: 2022/07/23 | Published: 2022/10/12
Received: 2022/02/23 | Accepted: 2022/07/23 | Published: 2022/10/12
How to cite this article
Almayahi W, Ali kraidi Q, Saeed Abbas S, Abbas B. Molecular Detection and Histopathological Effect of Infectious Bronchitis Virus Circulating in Vaccinated Broiler Flocks in Basrah, Iraq. Iran J War Public Health 2022; 14 (3) : 100
URL: http://ijwph.ir/article-1-1119-en.html
URL: http://ijwph.ir/article-1-1119-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Pathology and Poultry Diseases, College of Veterinary Medicine, University of Basrsh, Basrsh, Iraq
2- Department of Animal Production, College of Agriculture, University of Misan, Misan, Iraq
3- Department of Biology, College of Education for Pure Science, University of Basrsh, Basrsh, Iraq
4- Department of Microbiology and Parasitology, College of Veterinary Medicine, University of Basrsh, Basrsh, Iraq
2- Department of Animal Production, College of Agriculture, University of Misan, Misan, Iraq
3- Department of Biology, College of Education for Pure Science, University of Basrsh, Basrsh, Iraq
4- Department of Microbiology and Parasitology, College of Veterinary Medicine, University of Basrsh, Basrsh, Iraq
Full-Text (HTML) (525 Views)
Introduction
Avian Infectious Bronchitis Virus (IBV), a member of the family Coronaviridae, order Nidovirales, causes a highly contagious respiratory and sometimes urogenital disease in chickens. IBV is a major poultry pathogen that is endemic worldwide and leads to serious economic losses [1]. The infectious bronchitis virus is highly infectious, and extremely difficult to control because it has extensive genetic diversity, a short generation time, and a high mutation rate [2].
Infectious Bronchitis (IB) is an acute and extremely spreading disease in chickens caused by the infectious bronchitis virus. Such a disease occurs everywhere in the world and affects commercial broilers and layer chickens, which causes huge economic impacts. All ages of birds are susceptible to infectious bronchitis which causes respiratory findings, nephritis, proventriculitis, and a decline in the production of the egg and its quality [3, 4].
The infectious bronchitis virus case was first reported in North Dakota, USA, in 1931 as an acute, highly infectious respiratory disease of chicken [5]. The distribution is currently worldwide. The family of Coronaviridae consists of 2 genera, coronavirus and torovirus. These viruses are linear and positive sense-single-stranded RNA viruses. However, only viruses of the Coronavirus genus have been reported to infect poultry. Coronaviruses are enveloped, pleomorphic but usually spherical virus particles of 120 to 140nm in diameter [6, 7].
IB is often complicated by secondary bacterial infections that cause an elevation in mortality rate, which might be considered the most economically important respiratory viral disease in birds in the regions that are not highly pathogenic with avian influenza or velogenic Newcastle disease [8]. In pathogenesis and vaccination-challenge studies, the trachea has been traditionally highlighted as the primary tissue for IBV infection, immunity, and pathology [9].
Infectious bronchitis has a genome of about 27kb containing 5′ and 3′ Untranslated Regions (UTRs) [10]. Untranslated regions, which have high identification characteristics in reverse of the S1 region of the IBV genome, are used for rapid detection and classification for several IBV strains by generating PCR (Polymerase Chain Reaction) products ranging from 200 bp to 433 bp [11]. The hypervariable region of UTR that includes conserved flanking regions is the main characteristic feature of this region used as a diagnostic tool among different IBV strains [12]. Although this region is used in differentiation between two strains, it is also used to show the similarity between other avian species viruses depending on UTR [13]. UTR also harbors the structural elements involved in replication and translation [14].
Since its first detection in Europe in the 1940s, the infectious bronchitis virus has continued to be one of the major respiratory pathogens affecting the European poultry industry. The development of effective and widely used vaccines for both broilers (live attenuated) and breeders and layers (live attenuated and inactivated) helped improve the health and welfare of poultry but never eliminated the problem. The main reason is the continual emergence of new infectious bronchitis variants (serotypes or genotypes) [15].
There are many serotypes of the IBV and new serotypes are still being discovered. Vaccination is the most common method to prevent IBV infections in chickens, and commercial vaccines include live attenuated vaccines and inactivated vaccines. However, the cross protections of the vaccines are limited, as novel serotypes are continuously emerging, and immune failures are reported frequently. Massachusetts (Mass) and 4/91 (also known as 793B) types are widely used around the world, while local strains have been selected for vaccine development in individual regions [16].
The incidence of infection by infectious bronchitis virus approaches 100% in most locations. At many sites, multiple antigenic types are simultaneously present, requiring the application of multiple vaccines. Although many countries share some common antigenic types, IBV strains within a geographic region are unique and distinct [17].
Chickens and commercially reared pheasants are the only natural hosts for infectious bronchitis virus. Other species are not considered reservoirs of IBV. The majority of IBV strains cause tracheal lesions and respiratory disease with low mortality due to secondary bacterial infections, primarily in broilers. Nephropathogenic strains, in addition to tracheal lesions, also induce prominent kidney lesions with mortality of up to 25% in broilers. Strains of both pathotypes infect adult birds and affect egg production and egg quality to a variable degree. Infected chicks are the major source of viruses in the environment. Contaminated equipment and material are a potential source for indirect transmission over large distances [17].
Infectious bronchitis still causes big economic losses in Iraqi commercial chicken flocks as a result of the capability of available vaccines to supply cross-protection between different serotypes. In Iraq, the diagnosis of infectious bronchitis virus is mostly relying on clinical findings and gross lesions due to the lack of poultry diagnostic laboratories. The virus on the poultry population in Iraq is currently controlled by killed and live attenuated vaccines which contain H120 and 4/91 strains [18, 19].
Because of the financial losses caused by the virus, a fast and sensitive virus detection method is of interest to the poultry industry. There are, however, no standardized methods for the detection and classification of IBV strains; each laboratory must rely on its own methods. Virus detection is most commonly achieved by enzyme-linked immunosorbent assays, using either monoclonal or polyclonal antibodies. A disadvantage of serological tests is that new serotypes might be missed [20].
Infectious bronchitis can be diagnosed by clinical signs, post-mortem, and laboratory examination such as molecular assays using the Restriction Fragment Length Polymorphism (RFLP) or nucleic acid sequencing to identify the type of the virus [21-25]. Since Polymerase Chain Reaction (PCR) is more sensitive and faster than other methods for the detection of pathogens, this method is a fundamental instrument in the laboratory. At this time, no information on IB infection is available in such areas and the situation is not completely clear.
As the potential hazard of transition of the virus to humans and causes respirational disorders, the aim of the current study was to detect Infectious Bronchitis Virus (IBV) in Basrah Province using molecular techniques that target the 5' untranslated region gene of IBV, which is less variable than other genes and find the histopathological changes caused by these types of viruses.
Materials and Methods
Samples collection
In this study, 80 tracheal and kidney samples were collected from 8 broiler flocks located in different regions in Basrah province, the southern part of Iraq (where the major industrial farms are located in the studied regions) from January 2019 to March 2019. These tissue samples taken from the farms showed respiratory signs including rales, gasping, sneezing, nasal discharge, swollen face, and swollen kidney. The sampled tissues were later collected with the aseptic technique and transferred under cold chain conditions for histopathology and PCR detection. The H120 and 4/91 vaccines were used as IB vaccines in all studied farms.
Homogenization
Tracheal and kidney samples were taken into a sterile pestle and homogenized by sterile scissors and mortar and Phosphate-Buffered Saline (PBS) was added to prepare a suspension. The suspension was then clarified by centrifugation (1000rpm for 10 minutes). Penicillin (1000IU/ml) and gentamicin (500µm/ml) were used for suspension treatment. Total RNA was extracted from the kidney and tracheal tissue by viral gene-spin/viral DNA/RNA extraction kit (iNtRON Biotechnology Inc.; South Korea) according to manufacturer’s instructions and then stored at -70°C.
One-step quantitative PCR (qPCR)
The qRT-PCR reaction was performed on the Cepheid smart cycler Real Time-PCR (RT-PCR) system, based on IBV 5’-UTR gene primers as probe: FAM IBV 5’G BHQ15’FAM-CACCACCAGAACCTGTCACCTC-BHQ3’, PRIMER: IBV 5’ GU391 5’-GCTTTTGAGCCTAGCGTT-3’ and PRIMER: IBV 5’ GL533 5’-GCCATGTTGTCACTGTCTATTG-3’; under the following conditions: 40 cycles of 10-sec denaturation at 95°C, annealing for 30 sec at 60°C and extension 30 sec at 72°C, dissociation for 1 cycle at 60-95°C. The assay included negative controls for the qRT-PCR and RNA extraction.
Histopathological preparation
The representative tissue samples of the trachea and kidney were collected from suspected dead or sacrificed birds and then fixed in 10% neutral buffered formalin. The tissues were then processed and 5-μm-thick tissue sections were cut from paraffin-embedded tissue blocks and stained with hematoxylin and eosin staining.
Findings
qPCR results
The molecular part of the study explained infectious bronchitis in all studied farms. The specificity of the primer-probe set was tested with positive control of IB. Positive samples to IB were detected with real-time PCR (Figure 1).
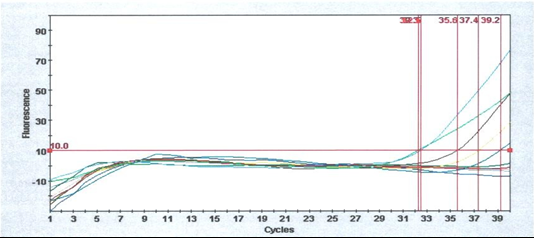
Figure 1) The IB primer-probe set directed to the UTR gene. There are positive samples for IB detection by real-time PCR.
Histopathological results
The mortality rate in the studied farms was 10%-50%, and the clinical signs included gasping, sneezing swelling face, and depression. The main gross lesions were tracheitis, lung congestion, air-sacculitis, and enlarged kidneys. The histopathological changes included excessive vacuolation mostly in subcapsular renal tubules, aggregation of inflammatory cells in subcapsular renal tubules, hemorrhage and inflammatory cells in mucosal and submucosal layers of the trachea, and discharge in the tracheal lumen. In addition to being pathogenic for the respiratory tract, this IBV has the capability to replicate in epithelial layers of different organs (Figure 2).

Avian Infectious Bronchitis Virus (IBV), a member of the family Coronaviridae, order Nidovirales, causes a highly contagious respiratory and sometimes urogenital disease in chickens. IBV is a major poultry pathogen that is endemic worldwide and leads to serious economic losses [1]. The infectious bronchitis virus is highly infectious, and extremely difficult to control because it has extensive genetic diversity, a short generation time, and a high mutation rate [2].
Infectious Bronchitis (IB) is an acute and extremely spreading disease in chickens caused by the infectious bronchitis virus. Such a disease occurs everywhere in the world and affects commercial broilers and layer chickens, which causes huge economic impacts. All ages of birds are susceptible to infectious bronchitis which causes respiratory findings, nephritis, proventriculitis, and a decline in the production of the egg and its quality [3, 4].
The infectious bronchitis virus case was first reported in North Dakota, USA, in 1931 as an acute, highly infectious respiratory disease of chicken [5]. The distribution is currently worldwide. The family of Coronaviridae consists of 2 genera, coronavirus and torovirus. These viruses are linear and positive sense-single-stranded RNA viruses. However, only viruses of the Coronavirus genus have been reported to infect poultry. Coronaviruses are enveloped, pleomorphic but usually spherical virus particles of 120 to 140nm in diameter [6, 7].
IB is often complicated by secondary bacterial infections that cause an elevation in mortality rate, which might be considered the most economically important respiratory viral disease in birds in the regions that are not highly pathogenic with avian influenza or velogenic Newcastle disease [8]. In pathogenesis and vaccination-challenge studies, the trachea has been traditionally highlighted as the primary tissue for IBV infection, immunity, and pathology [9].
Infectious bronchitis has a genome of about 27kb containing 5′ and 3′ Untranslated Regions (UTRs) [10]. Untranslated regions, which have high identification characteristics in reverse of the S1 region of the IBV genome, are used for rapid detection and classification for several IBV strains by generating PCR (Polymerase Chain Reaction) products ranging from 200 bp to 433 bp [11]. The hypervariable region of UTR that includes conserved flanking regions is the main characteristic feature of this region used as a diagnostic tool among different IBV strains [12]. Although this region is used in differentiation between two strains, it is also used to show the similarity between other avian species viruses depending on UTR [13]. UTR also harbors the structural elements involved in replication and translation [14].
Since its first detection in Europe in the 1940s, the infectious bronchitis virus has continued to be one of the major respiratory pathogens affecting the European poultry industry. The development of effective and widely used vaccines for both broilers (live attenuated) and breeders and layers (live attenuated and inactivated) helped improve the health and welfare of poultry but never eliminated the problem. The main reason is the continual emergence of new infectious bronchitis variants (serotypes or genotypes) [15].
There are many serotypes of the IBV and new serotypes are still being discovered. Vaccination is the most common method to prevent IBV infections in chickens, and commercial vaccines include live attenuated vaccines and inactivated vaccines. However, the cross protections of the vaccines are limited, as novel serotypes are continuously emerging, and immune failures are reported frequently. Massachusetts (Mass) and 4/91 (also known as 793B) types are widely used around the world, while local strains have been selected for vaccine development in individual regions [16].
The incidence of infection by infectious bronchitis virus approaches 100% in most locations. At many sites, multiple antigenic types are simultaneously present, requiring the application of multiple vaccines. Although many countries share some common antigenic types, IBV strains within a geographic region are unique and distinct [17].
Chickens and commercially reared pheasants are the only natural hosts for infectious bronchitis virus. Other species are not considered reservoirs of IBV. The majority of IBV strains cause tracheal lesions and respiratory disease with low mortality due to secondary bacterial infections, primarily in broilers. Nephropathogenic strains, in addition to tracheal lesions, also induce prominent kidney lesions with mortality of up to 25% in broilers. Strains of both pathotypes infect adult birds and affect egg production and egg quality to a variable degree. Infected chicks are the major source of viruses in the environment. Contaminated equipment and material are a potential source for indirect transmission over large distances [17].
Infectious bronchitis still causes big economic losses in Iraqi commercial chicken flocks as a result of the capability of available vaccines to supply cross-protection between different serotypes. In Iraq, the diagnosis of infectious bronchitis virus is mostly relying on clinical findings and gross lesions due to the lack of poultry diagnostic laboratories. The virus on the poultry population in Iraq is currently controlled by killed and live attenuated vaccines which contain H120 and 4/91 strains [18, 19].
Because of the financial losses caused by the virus, a fast and sensitive virus detection method is of interest to the poultry industry. There are, however, no standardized methods for the detection and classification of IBV strains; each laboratory must rely on its own methods. Virus detection is most commonly achieved by enzyme-linked immunosorbent assays, using either monoclonal or polyclonal antibodies. A disadvantage of serological tests is that new serotypes might be missed [20].
Infectious bronchitis can be diagnosed by clinical signs, post-mortem, and laboratory examination such as molecular assays using the Restriction Fragment Length Polymorphism (RFLP) or nucleic acid sequencing to identify the type of the virus [21-25]. Since Polymerase Chain Reaction (PCR) is more sensitive and faster than other methods for the detection of pathogens, this method is a fundamental instrument in the laboratory. At this time, no information on IB infection is available in such areas and the situation is not completely clear.
As the potential hazard of transition of the virus to humans and causes respirational disorders, the aim of the current study was to detect Infectious Bronchitis Virus (IBV) in Basrah Province using molecular techniques that target the 5' untranslated region gene of IBV, which is less variable than other genes and find the histopathological changes caused by these types of viruses.
Materials and Methods
Samples collection
In this study, 80 tracheal and kidney samples were collected from 8 broiler flocks located in different regions in Basrah province, the southern part of Iraq (where the major industrial farms are located in the studied regions) from January 2019 to March 2019. These tissue samples taken from the farms showed respiratory signs including rales, gasping, sneezing, nasal discharge, swollen face, and swollen kidney. The sampled tissues were later collected with the aseptic technique and transferred under cold chain conditions for histopathology and PCR detection. The H120 and 4/91 vaccines were used as IB vaccines in all studied farms.
Homogenization
Tracheal and kidney samples were taken into a sterile pestle and homogenized by sterile scissors and mortar and Phosphate-Buffered Saline (PBS) was added to prepare a suspension. The suspension was then clarified by centrifugation (1000rpm for 10 minutes). Penicillin (1000IU/ml) and gentamicin (500µm/ml) were used for suspension treatment. Total RNA was extracted from the kidney and tracheal tissue by viral gene-spin/viral DNA/RNA extraction kit (iNtRON Biotechnology Inc.; South Korea) according to manufacturer’s instructions and then stored at -70°C.
One-step quantitative PCR (qPCR)
The qRT-PCR reaction was performed on the Cepheid smart cycler Real Time-PCR (RT-PCR) system, based on IBV 5’-UTR gene primers as probe: FAM IBV 5’G BHQ15’FAM-CACCACCAGAACCTGTCACCTC-BHQ3’, PRIMER: IBV 5’ GU391 5’-GCTTTTGAGCCTAGCGTT-3’ and PRIMER: IBV 5’ GL533 5’-GCCATGTTGTCACTGTCTATTG-3’; under the following conditions: 40 cycles of 10-sec denaturation at 95°C, annealing for 30 sec at 60°C and extension 30 sec at 72°C, dissociation for 1 cycle at 60-95°C. The assay included negative controls for the qRT-PCR and RNA extraction.
Histopathological preparation
The representative tissue samples of the trachea and kidney were collected from suspected dead or sacrificed birds and then fixed in 10% neutral buffered formalin. The tissues were then processed and 5-μm-thick tissue sections were cut from paraffin-embedded tissue blocks and stained with hematoxylin and eosin staining.
Findings
qPCR results
The molecular part of the study explained infectious bronchitis in all studied farms. The specificity of the primer-probe set was tested with positive control of IB. Positive samples to IB were detected with real-time PCR (Figure 1).

Figure 1) The IB primer-probe set directed to the UTR gene. There are positive samples for IB detection by real-time PCR.
Histopathological results
The mortality rate in the studied farms was 10%-50%, and the clinical signs included gasping, sneezing swelling face, and depression. The main gross lesions were tracheitis, lung congestion, air-sacculitis, and enlarged kidneys. The histopathological changes included excessive vacuolation mostly in subcapsular renal tubules, aggregation of inflammatory cells in subcapsular renal tubules, hemorrhage and inflammatory cells in mucosal and submucosal layers of the trachea, and discharge in the tracheal lumen. In addition to being pathogenic for the respiratory tract, this IBV has the capability to replicate in epithelial layers of different organs (Figure 2).

a: Excessive vacuolation mostly in subcapsular renal tubules (H&E, 10X)

b: Excessive vacuolation mostly in subcapsular renal tubules (H&E, 40X)

c: Aggregation of inflammatory cells in subcapsular renal tubules (H&E, 40X)
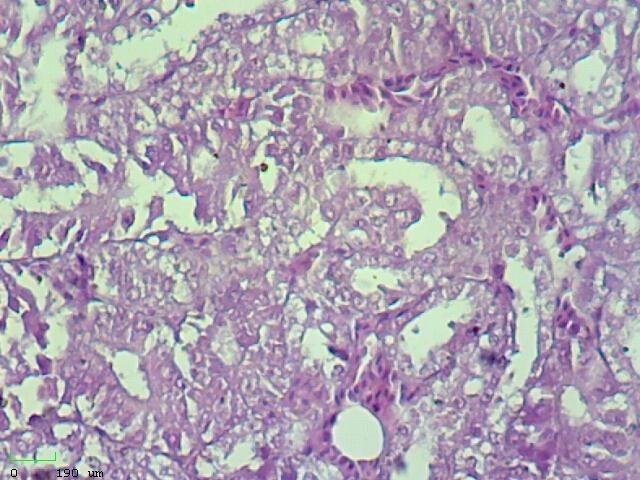
d: Papillary projection into the lumen of renal tubules (H&E, 40X)

e: Increase of goblet cells in intestinal villi and vacuolation (hydropic degeneration) of epithelial cells (H&E, 10X)
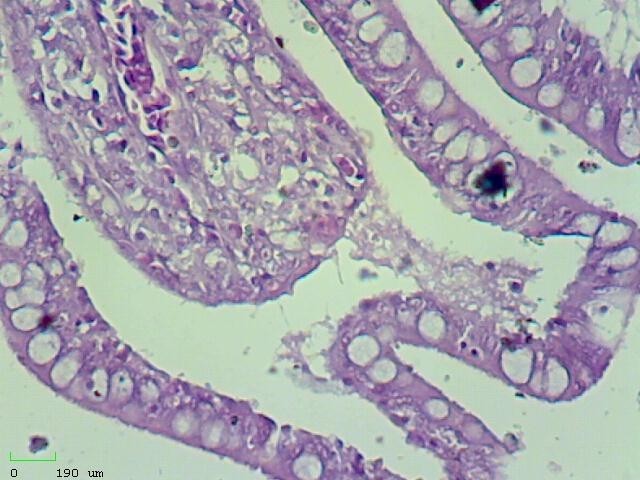
f: Increase of goblet cells in intestinal villi and vacuolation (hydropic degeneration) of epithelial cells (H&E, 40X)
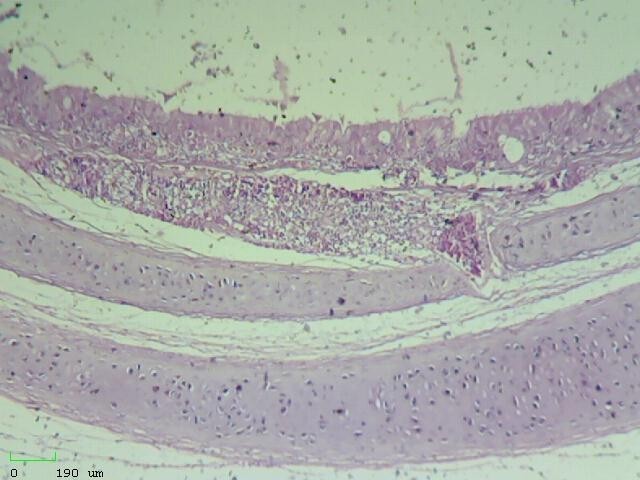
g: Hemorrhage and inflammatory cells in mucosal and submucosal layers of trachea; discharge in the lumen (H&E, 10X)

h: Hemorrhage and inflammatory cells in mucosal and submucosal layers of trachea; discharge in the lumen (H&E, 40X)
Figure 2) Microscopic sections of different tissues

b: Excessive vacuolation mostly in subcapsular renal tubules (H&E, 40X)

c: Aggregation of inflammatory cells in subcapsular renal tubules (H&E, 40X)

d: Papillary projection into the lumen of renal tubules (H&E, 40X)

e: Increase of goblet cells in intestinal villi and vacuolation (hydropic degeneration) of epithelial cells (H&E, 10X)

f: Increase of goblet cells in intestinal villi and vacuolation (hydropic degeneration) of epithelial cells (H&E, 40X)

g: Hemorrhage and inflammatory cells in mucosal and submucosal layers of trachea; discharge in the lumen (H&E, 10X)

h: Hemorrhage and inflammatory cells in mucosal and submucosal layers of trachea; discharge in the lumen (H&E, 40X)
Figure 2) Microscopic sections of different tissues
Discussion
Infectious bronchitis disease is one of the viral diseases that affect poultry worldwide, causing economic losses, especially in small ages leading to growth retardation, loss of appetite, and mortality [26]. Chicken and other avian species are considered the natural host of the virus [27]. The disease has many pathological effects on vital organs that lead to low egg production and quality; the virus also causing renal damage and is considered a predisposing factor for respiratory bacterial infection specially airsacculitis. Lack of cross-protection between IBV genotypes and emerging of new genotypes was always a problem in controlling and preventing the disease [28]. The aim of the current study was to detect Infectious Bronchitis Virus (IBV) in Basrah Province using molecular techniques that target the 5' untranslated region gene of IBV, which is less variable than other genes and find the histopathological changes caused by these types of viruses.
Several methods are used for the diagnosis of IBV, including the reverse transcriptase-polymerase chain reaction (RT-PCR) which consider a useful tool in comparison with serological methods [29].
The use of the polymerase chain reaction (PCR) in molecular diagnostics has increased to the point where it is now accepted as the gold standard for detecting nucleic acids from a number of origins and it has become an essential tool in the research laboratory. Real-time PCR has engendered wider acceptance of the PCR due to its improved rapidity, sensitivity, reproducibility, and reduced risk of carry-over contamination [30].
In the present study, a real-time Taqman®-based RT-PCR targeting the 5´UTR region of the viral genome was utilized to detect and quantify IBV genomic RNA, which were directly collected from tissue samples (trachea & kidney) of infected broilers, with primer and probe specificity for IB isolates.
Regarding the detection of some types of IBV, the qRT-PCR assay has been reported to be more sensitive [31]. Despite the use of primers targeting the S1 gene of the IBV genome, the extensive variability and mutation rates in such genes make it easier to produce false-negative results in RT-PCR techniques compared to the 5´UTR of the IBV genome, which is known as highly conserved [32].
Our results from Real-time PCR were consistent with the results obtained by Najafi et al. [33], who reported the positive samples using the real-time PCR which targeted 5´ untranslated region of IBV successfully due to detect the IBVs directly from tissue samples infected with different European isolates.
On other hand, the present study showed the histopathological changes in the kidney and trachea of infected broiler chicks, such as excessive vacuolation mostly in subcapsular renal tubules, aggregation of inflammatory cells in the subcapsular renal tubules, hemorrhage and inflammatory cells in the mucosal and submucosal layers of the trachea and discharge in the tracheal lumen.
In addition to being pathogenic for the respiratory tract, this IBV also has the ability to replicate in the epithelial layers of different organs [22]. Australia categorized the major IBV strains as respiratory and nephropathogenic. However, the major strains are undergoing variation in their pathogenicity [34].
The current results were similar to those of Gola et al. [35]. They mentioned that histopathological examination of infected chicken trachea shows desiclation, desquamation of epithelial cells, and serous exudation. The trachea of some chickens revealed hemorrhage. The kidney showed tubular necrosis, hyaline degeneration, and desquamation of epithelial cells.
Najimudeen et al. [36] designed an in vivo experiment, using Specific Pathogen Free (SPF) chickens, to study the pathogenesis of, and host response to, Canadian (CAN) 4/91 IBV infection. At one week of age, the chickens were infected with 4/91 IBV/Ck/Can/17-038913 isolate. At one week of age, the chickens were infected with 4/91 IBV/Ck/Can/17-038913 isolate and were euthanized at 3, 7- and 10-days post-infection to collect lung and kidney tissues. The results indicated IBV replication in these tissues at all three-time points with prominent histological lesions, significant immune cell recruitment, and upregulation of proinflammatory mediators [36].
In Hassan et al.'s study [37], in the histopathological examination of IBV-infected chickens, the trachea showed epithelial cell necrosis with deciliation, and the lamina propria was infiltrated with focal lymphoplasmacytic aggregates. The lung demonstrated hyperplasia of the epithelial cells lining the secondary bronchi together with mononuclear cell infiltrations in the lamina propria. Furthermore, the tertiary bronchi and atria were occupied by homogenous eosinophilic material, and the interstitial connective tissue was infiltrated with mononuclear cell infiltrations. The kidney showed tubular cell necrosis with cellular exfoliation. Dilated renal tubules were occluded by intra-luminal casts containing a mixture of necrosed epithelia, mucus, and disintegrated heterophils [37].
Hasan et al. [13] investigated the pathological effects of the infectious bronchitis virus (IBV) on the chicken trachea and kidney tissues and also desired to diagnose the virus genome using a molecular tool. Histopathological study of renal tissue showed signs of inflammation and cellular degeneration. The signs included infiltration of neutrophils, disruption of glomeruli, different stages of corruption to the renal tubules. The renal tubules conducted a cellular swelling and narrowing of the lumen with cellular necrosis. In some locations, tubular endothelial cells were replaced by fibrin. Pathological study to the tissue of the trachea reveled a remarkable effect of IBV on the mucosal and submucosal area of the trachea. These changes included loss of epithelial cells with its cilia, sever hyperplasia leads to thinking in large epithelial areas, infiltration of neutrophils, and disappearances of goblet cells, which replaced by empty vacuoles with an increased amount of fibrin and congestion in the sub-epithelial layer [13].
The current study results also agreed with other groups of researchers who showed that the IBV causes cellular necrosis and damage after it replicates inside the cells [34, 38].
Jang et al. agreed with the current study results of in terms of the renal tubular degeneration and acute necrosis of epithelial layer of the trachea, and concluded that the high inflammatory response induced by cytokines during IBV infection attributed in local renal tissue damage [39].
Grgić et al. [40] suggest that the IBV is well associated with different stages of trachea hyperplasia. Other researcher showed that some of IBV strains cause tissue damage not only in the respiratory tract but it also causes tissue damage in both renal and tracheal tissues [41].
Conclusion
Avian Infectious Bronchitis Virus (IBV) is still circulating with devastating effects on the poultry industry in Basrah Province, and this might be due to the lack of awareness among the owners of the farms in matters related to biosecurity and vaccination program.
Acknowledgments: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contributions: Waleed Majeed Almayahi (First Author), Introduction Writer/Main Researcher (25%); Qayssar Ali Kraidi (Second Author), Methodologist (25%); Salma Saeed Abbas (Third Author), Assistant Researcher (25%); Basil A. Abbas (Fourth Author), Discussion Writer (25%)
Funding/Support: None declared by the authors.
Infectious bronchitis disease is one of the viral diseases that affect poultry worldwide, causing economic losses, especially in small ages leading to growth retardation, loss of appetite, and mortality [26]. Chicken and other avian species are considered the natural host of the virus [27]. The disease has many pathological effects on vital organs that lead to low egg production and quality; the virus also causing renal damage and is considered a predisposing factor for respiratory bacterial infection specially airsacculitis. Lack of cross-protection between IBV genotypes and emerging of new genotypes was always a problem in controlling and preventing the disease [28]. The aim of the current study was to detect Infectious Bronchitis Virus (IBV) in Basrah Province using molecular techniques that target the 5' untranslated region gene of IBV, which is less variable than other genes and find the histopathological changes caused by these types of viruses.
Several methods are used for the diagnosis of IBV, including the reverse transcriptase-polymerase chain reaction (RT-PCR) which consider a useful tool in comparison with serological methods [29].
The use of the polymerase chain reaction (PCR) in molecular diagnostics has increased to the point where it is now accepted as the gold standard for detecting nucleic acids from a number of origins and it has become an essential tool in the research laboratory. Real-time PCR has engendered wider acceptance of the PCR due to its improved rapidity, sensitivity, reproducibility, and reduced risk of carry-over contamination [30].
In the present study, a real-time Taqman®-based RT-PCR targeting the 5´UTR region of the viral genome was utilized to detect and quantify IBV genomic RNA, which were directly collected from tissue samples (trachea & kidney) of infected broilers, with primer and probe specificity for IB isolates.
Regarding the detection of some types of IBV, the qRT-PCR assay has been reported to be more sensitive [31]. Despite the use of primers targeting the S1 gene of the IBV genome, the extensive variability and mutation rates in such genes make it easier to produce false-negative results in RT-PCR techniques compared to the 5´UTR of the IBV genome, which is known as highly conserved [32].
Our results from Real-time PCR were consistent with the results obtained by Najafi et al. [33], who reported the positive samples using the real-time PCR which targeted 5´ untranslated region of IBV successfully due to detect the IBVs directly from tissue samples infected with different European isolates.
On other hand, the present study showed the histopathological changes in the kidney and trachea of infected broiler chicks, such as excessive vacuolation mostly in subcapsular renal tubules, aggregation of inflammatory cells in the subcapsular renal tubules, hemorrhage and inflammatory cells in the mucosal and submucosal layers of the trachea and discharge in the tracheal lumen.
In addition to being pathogenic for the respiratory tract, this IBV also has the ability to replicate in the epithelial layers of different organs [22]. Australia categorized the major IBV strains as respiratory and nephropathogenic. However, the major strains are undergoing variation in their pathogenicity [34].
The current results were similar to those of Gola et al. [35]. They mentioned that histopathological examination of infected chicken trachea shows desiclation, desquamation of epithelial cells, and serous exudation. The trachea of some chickens revealed hemorrhage. The kidney showed tubular necrosis, hyaline degeneration, and desquamation of epithelial cells.
Najimudeen et al. [36] designed an in vivo experiment, using Specific Pathogen Free (SPF) chickens, to study the pathogenesis of, and host response to, Canadian (CAN) 4/91 IBV infection. At one week of age, the chickens were infected with 4/91 IBV/Ck/Can/17-038913 isolate. At one week of age, the chickens were infected with 4/91 IBV/Ck/Can/17-038913 isolate and were euthanized at 3, 7- and 10-days post-infection to collect lung and kidney tissues. The results indicated IBV replication in these tissues at all three-time points with prominent histological lesions, significant immune cell recruitment, and upregulation of proinflammatory mediators [36].
In Hassan et al.'s study [37], in the histopathological examination of IBV-infected chickens, the trachea showed epithelial cell necrosis with deciliation, and the lamina propria was infiltrated with focal lymphoplasmacytic aggregates. The lung demonstrated hyperplasia of the epithelial cells lining the secondary bronchi together with mononuclear cell infiltrations in the lamina propria. Furthermore, the tertiary bronchi and atria were occupied by homogenous eosinophilic material, and the interstitial connective tissue was infiltrated with mononuclear cell infiltrations. The kidney showed tubular cell necrosis with cellular exfoliation. Dilated renal tubules were occluded by intra-luminal casts containing a mixture of necrosed epithelia, mucus, and disintegrated heterophils [37].
Hasan et al. [13] investigated the pathological effects of the infectious bronchitis virus (IBV) on the chicken trachea and kidney tissues and also desired to diagnose the virus genome using a molecular tool. Histopathological study of renal tissue showed signs of inflammation and cellular degeneration. The signs included infiltration of neutrophils, disruption of glomeruli, different stages of corruption to the renal tubules. The renal tubules conducted a cellular swelling and narrowing of the lumen with cellular necrosis. In some locations, tubular endothelial cells were replaced by fibrin. Pathological study to the tissue of the trachea reveled a remarkable effect of IBV on the mucosal and submucosal area of the trachea. These changes included loss of epithelial cells with its cilia, sever hyperplasia leads to thinking in large epithelial areas, infiltration of neutrophils, and disappearances of goblet cells, which replaced by empty vacuoles with an increased amount of fibrin and congestion in the sub-epithelial layer [13].
The current study results also agreed with other groups of researchers who showed that the IBV causes cellular necrosis and damage after it replicates inside the cells [34, 38].
Jang et al. agreed with the current study results of in terms of the renal tubular degeneration and acute necrosis of epithelial layer of the trachea, and concluded that the high inflammatory response induced by cytokines during IBV infection attributed in local renal tissue damage [39].
Grgić et al. [40] suggest that the IBV is well associated with different stages of trachea hyperplasia. Other researcher showed that some of IBV strains cause tissue damage not only in the respiratory tract but it also causes tissue damage in both renal and tracheal tissues [41].
Conclusion
Avian Infectious Bronchitis Virus (IBV) is still circulating with devastating effects on the poultry industry in Basrah Province, and this might be due to the lack of awareness among the owners of the farms in matters related to biosecurity and vaccination program.
Acknowledgments: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contributions: Waleed Majeed Almayahi (First Author), Introduction Writer/Main Researcher (25%); Qayssar Ali Kraidi (Second Author), Methodologist (25%); Salma Saeed Abbas (Third Author), Assistant Researcher (25%); Basil A. Abbas (Fourth Author), Discussion Writer (25%)
Funding/Support: None declared by the authors.
Keywords:
References
1. Feng J, Hu Y, Ma Z, Yu Q, Zhao J, Xiaodong Liu X, Zhang G. Virulent avian infectious bronchitis virus, people's Republic of China. Emerg Infect Dis. 2012;18(12):1994-2001. [Link] [DOI:10.3201/eid1812.120552]
2. Khataby K, Fellahi S, Loutfi C, Mustapha EM. Avian infectious bronchitis virus in Africa: a review. Vet Q. 2016;36(2):71-5. [Link] [DOI:10.1080/01652176.2015.1126869]
3. Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38(2):281-97. [Link] [DOI:10.1051/vetres:2006055]
4. Han Z, Sun C, Yan B, Zhang X, Wang Y, Li C, et al. A 15-year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infect Genet Evol. 2011;11(1):190-200. [Link] [DOI:10.1016/j.meegid.2010.09.002]
5. Sjaak de Wit JJ, Cook JKA, van der Heijden HMJF. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40(3):223-35. [Link] [DOI:10.1080/03079457.2011.566260]
6. Fadhilah AS, Kai TH, Lokman HI, Yasmin NAR, Hafandi A, et al. Molecular and pathogenicity of infectious bronchitis virus (Gammacoronavirus) in Japanese quail (Coturnix japonica). Poult Sci. 2020;99(6):2937-43. [Link] [DOI:10.1016/j.psj.2020.01.026]
7. Abdel-Moneim AS, El-Kady MF, Ladman BS, and Gelb J. S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol J. 2006;3:78. [Link] [DOI:10.1186/1743-422X-3-78]
8. Cavanagh D. Coronaviruses in poultry and other birds. Avian pathol. 2005;34(6):439-48. [Link] [DOI:10.1080/03079450500367682]
9. Al-Rasheed M, Manswra B, Leeminga G, Ganapathya K. Infectious bronchitis virus infection in chicken: viral load and immune responses in Harderian gland, choanal cleft and turbinate tissues compared to trachea. B Poultry Sci. 2022;63(4):484-92. [Link] [DOI:10.1080/00071668.2022.2035675]
10. Boursnell ME, Brown TD, Foulds, IJ, Green PF, Tomley FM, Binns MM. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J Gen Virol. 1987;68(Pt 1):57-77. [Link] [DOI:10.1099/0022-1317-68-1-57]
11. Hewson K, Noormohammadi AH, Devlin JM, Mardani K, Ignjatovic J. Rapid detection and non-subjective characterisation of infectious bronchitis virus isolates using high-resolution melt curve analysis and a mathematical model. Arch Virol. 2009;154(4):649-60. [Link] [DOI:10.1007/s00705-009-0357-1]
12. Okino CH, de Fátima Silva Montassier M, de Oliveira AP, Montassier HJ. Rapid detection and differentiation of avian infectious bronchitis virus:An application of mass genotype by melting temperature analysis in RT-qPCR using SYBR green I. J Vet Med Sci. 2018;80(4):725-30. [Link] [DOI:10.1292/jvms.17-0566]
13. Hasan II, Rasheed ST, Jasim NA, Shakor MK. Pathological effect of infectious bronchitis disease virus on broiler chicken trachea and kidney tissues. Vet World. 2020;13(10):2203-8. [Link] [DOI:10.14202/vetworld.2020.2203-2208]
14. Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193-292. [Link] [DOI:10.1016/S0065-3527(06)66005-3]
15. de Wit JJ, de Wit MK, Cook JKA. Infectious bronchitis virus types affecting European countries-A review. Avian Dis. 2021;65(4):643-8. [Link] [DOI:10.1637/aviandiseases-D-21-00106]
16. Lin S-Y, Chen HW. Infectious bronchitis virus variants: molecular analysis and pathogenicity investigation. Int J Mol Sci. 2017;18(10):2030. [Link] [DOI:10.3390/ijms18102030]
17. Ignjatović J, Sapats S. Avian infectious bronchitis virus. Rev Sci Tech. 2000;19(2):493-508. [Link] [DOI:10.20506/rst.19.2.1228]
18. Al-Dabhawe AH, Kadhim HM, Samaka HM. Molecular detection of infectious bronchitis virus and it is relation with avian influenza virus (H9) and Mycoplasma gallisepticum from different geographical regions in Iraq. Iraqi J Vet Sci. 2013;27(2):97-101. [Link] [DOI:10.33899/ijvs.2013.82811]
19. Mahmood ZH, Sleman RR, Uthman AU. Isolation and molecular characterization of Sul/01/09 avian infectious bronchitis virus, indicates the emergence of a new genotype in the Middle East. Vet Microbiol. 2011;150(1-2):21-7. [Link] [DOI:10.1016/j.vetmic.2010.12.015]
20. Zwaagstra KA, van der Zeijst BA, Kusters JG. Rapid detection and identification of avian infectious bronchitis virus. J Clin Microbiol. 1992;30(1):79-84. [Link] [DOI:10.1128/jcm.30.1.79-84.1992]
21. Cavanagh D, Nyagi SA. Infectious bronchitis. In: Calnek BW, editor. Diseases of poultry. 10th Edition. IOWA State University Press; 1997. [Link]
22. Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32(6):567-82. [Link] [DOI:10.1080/03079450310001621198]
23. Jackwood MW, Hilt DA, Lee CW, Kwon HM, Callison SA, et al. Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Dis. 2005;49(4):614-8. [Link] [DOI:10.1637/7389-052905R.1]
24. Keeler Jr CL, Reed KL, Nix WA, Gelb Jr J. Serotype identification of avian infectious bronchitis virus by RT-PCR of the peplomer (S-1) gene. Avian Dis. 1998;42(2):275-84. [Link] [DOI:10.2307/1592477]
25. Kingham BF, Keeler Jr CL, Nix WA, Ladman BS, Gelb Jr J. Identification of avian infectious bronchitis virus by direct automated cycle sequencing of the S-1 gene. Avian Dis. 2000;44(2):325-35. [Link] [DOI:10.2307/1592547]
26. Bande F, Arshad SS, Omar AR, Hair-Bejo M, Mahmuda A, Nair V. Global distributions and strain diversity of avian infectious bronchitis virus: A review. Anim Health Res Rev. 2017;18(1):70-83. [Link] [DOI:10.1017/S1466252317000044]
27. Suryaman GK, Soejoedono RD, Setiyono A, Poetri ON, Handharyani E. Isolation and characterisation of avian coronavirus from healthy Eclectus parrots (Eclectus roratus) from Indonesia. Vet World. 2019;12(11):1797-805. [Link] [DOI:10.14202/vetworld.2019.1797-1805]
28. Bande F, Arshad SS, Omar AR, Bejo MH, Abubakar MS, Abba Y. Pathogenesis and diagnostic approaches of avian infectious bronchitis. Adv Virol. 2016;2016:4621659. [Link] [DOI:10.1155/2016/4621659]
29. Lounas A, Oumouna-Benachour K, Medkour H, Oumouna M. The first evidence of a new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and unvaccinated broiler flocks in Algeria. Vet World. 2018;11(11):1630-6. [Link] [DOI:10.14202/vetworld.2018.1630-1636]
30. Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res. 2002;30(6):1292-305. [Link] [DOI:10.1093/nar/30.6.1292]
31. Roh HJ, Hilt DA, Jackwood MW. Detection of infectious bronchitis virus with the use of real-time quantitative reverse transcriptase-PCR and correlation with virus detection in embryonated eggs. Avian Dis. 2014;58(3):398-403. [Link] [DOI:10.1637/10764-010914-Reg.1]
32. Callison SA, Hilt DA, Boynton TO, Sample BF, Robison R, Swayne DE, Jackwood MW. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods. 2006;138(1-2):60-5. [Link] [DOI:10.1016/j.jviromet.2006.07.018]
33. Najafi H, Nofrarías M, Cortey M, Pina S, Valle R, Sánchez R, Majó N. Rapid detection and quantification of differentEuropean IBV strains by quantitative real-time RT-PCR. 2015. [Link]
34. Ignjatovic J, Ashton DF, Reece R, Scott P, Hooper P. Pathogenicity of Australian strains of avian infectious bronchitis virus. J Comparat Pathol. 2002;126(2-3):115-23. [Link] [DOI:10.1053/jcpa.2001.0528]
35. Gola S, Shukla SK, Shekhar S, Kumar M. Prevalence serodiagnosis and histopathological changes in field cases of infectious bronchitis in chickens. Int J Curr Microbiol App Sci. 2017;6(8):1591-7. [Link] [DOI:10.20546/ijcmas.2017.608.190]
36. Najimudeen SM, Barboza-Solis C, Ali A, Buharideen SM, Isham IM, et al. Pathogenesis and host responses in lungs and kidneys following Canadian 4/91 infectious bronchitis virus (IBV) infection in chickens. Virology. 2022;566:75-88. [Link] [DOI:10.1016/j.virol.2021.11.013]
37. Hassan MSH, Buharideen SM, Ali A, Najimudeen S, Goldsmith D, et al. Efficacy of commercial infectious bronchitis vaccines against Canadian Delmarva (DMV/1639) infectious bronchitis virus infection in layers. Vaccines. 2022;10(8):1-14. [Link] [DOI:10.3390/vaccines10081194]
38. Lee CW, Brown C, Hilt DA, Jackwood MW. Nephropathogenesis of chickens experimentally infected with various strains of infectious bronchitis virus. J Vet Med Sci. 2004;66(7):835-40. [Link] [DOI:10.1292/jvms.66.835]
39. Jang H, Koo B.S, Jeon E.O, Lee H.R, Lee SM, Mo IP. Altered pro-inflammatory cytokine mRNA levels in chickens infected with infectious bronchitis virus. Poult Sci. 2013;92(9):2290-8. [Link] [DOI:10.3382/ps.2013-03116]
40. Grgić H, Hunter DB, Hunton P, Nagy É. Pathogenicity of infectious bronchitis virus isolates from Ontario chickens. Can J Vet Res. 2008;72(5):403-10. [Link]
41. Dolz R, Vergara-Alert J, Pérez M, Pujols J, Majó N. New insights on infectious bronchitis virus pathogenesis:Characterisation of Italy 02 serotype in chicks and adult hens. Vet Microbiol. 2012;156(3-4):256-64. [Link] [DOI:10.1016/j.vetmic.2011.11.001]








