Volume 14, Issue 2 (2022)
Iran J War Public Health 2022, 14(2): 165-170 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/02/13 | Accepted: 2022/04/12 | Published: 2022/05/30
Received: 2022/02/13 | Accepted: 2022/04/12 | Published: 2022/05/30
How to cite this article
Mohammed Madfoon Z, Mezher M, Madfoon S. Comparison of Serum Ferritin Levels between Diabetic Patients with COVID-19 and Non-diabetic Patients with COVID-19 Based on Age Groups and Gender. Iran J War Public Health 2022; 14 (2) :165-170
URL: http://ijwph.ir/article-1-1114-en.html
URL: http://ijwph.ir/article-1-1114-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Biology, University of Kufa, Kufa, Iraq
Full-Text (HTML) (770 Views)
Introduction
On December 31, 2019, Chinese Health Authority informed the World Health Organization (WHO) of several cases of pneumonia of unknown cause in Wuhan City, Hubei Province, central China. Cases were reported on December 8, 2019, and several patients worked or resided near the local Huanan Seafood Wholesale Market, but other early cases had no connection to this market [1].
Eventually, a novel virus from the Coronaviridaefamily was detected by the Chinese centers for disease control and prevention from the throat culture of patients with influenza-like manifestations, which was subsequently named Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) [2]. In March of 2020, WHO named this highly contagious disease COVID19 (Coronavirus Disease 19) and declared the outbreak as a global pandemic [3].
Several risk factors, including individuals' underlying diseases, had been shown to be associated with higher vulnerability to get SARS-CoV-2 infection [4-6]. In this regard, Diabetes Mellitus (DM) is reported in a majority of studies to be among the most common comorbidities of patients with Coronavirus disease 2019 (COVID-19) [6]. According to the National Health Commission of China, as of February 4 the fatality rate among confirmed cases in China was 2.1 percent [7].
Based on the initial outbreak cases from China, researchers found a link between COVID-19 with comorbidities [8]. Deng and Peng reported that 25.1% had at least one underlying condition; concurrently hypertension (16.9%) and diabetes (8.2%) were two of the most prevalent comorbidities [9]. This correlated data between diabetes as a comorbidity for COVID-19 is concerning globally as diabetes has been considered the pandemic of the 21st century due to its escalation in the older population and adolescents [10].
Diabetes Mellitus (DM) is a well-known risk factor for worse clinical outcomes in patients with COVID-19. However, the relationship between these two entities seems to be bidirectional [11]. The ongoing pandemic of COVID-19 has significantly affected blood glucose control in patients with diabetes mellitus. The results of this effects can be classified into direct effects (those directly related to the viral infection) and indirect effects (those related to the impact of the pandemic on the management of blood glucose or the use of proposed treatments for the infection that also affect glucose homeostasis) [12].
In comparing COVID-19 patients with and without T2DM, the patients with T2DM tend to develop more severe forms of the disease and have a significant increase in inflammatory markers compared to non-diabetics (i.e., higher levels of C-reactive protein, procalcitonin, ferritin, lactate dehydrogenase, and d-dimer) [13]. Furthermore, the prevalence of diabetes among the patients admitted to intensive care units for COVID-19 is two-to threefold higher, along with the mortality rate which is twice that of non-diabetic patients [14].
Ferritin is a key mediator of immune dysregulation, especially under extreme hyperferritinemia, via direct immune-suppressive and pro-inflammatory effects, contributing to the cytokine storm [15]. It has been reported that fatal outcomes by COVID-19 are accompanied by cytokine storm syndrome, thereby it has been suggested that disease severity is dependent of the cytokine storm syndrome [16]. Many individuals with diabetes exhibit elevated serum ferritin levels [17-19], and it is known that they face a higher probability to experience serious complications from COVID-19 [20].
Hyperferritinemia is more prevalent in diabetic COVID-19 individuals. Serum ferritin can be considered as a valuable biomarker to screen the diabetic and non-diabetic for the presence of hyperinflammation and to predict severity of COVID-19 infection so that it will help the clinician for proper management [21].
In one study with 20 COVID-19 patients, it was found that individuals with severe and very severe COVID-19 exhibited increased serum ferritin level, being serum ferritin in the very severe COVID-19 group significantly higher than in the severe COVID-19 group [22]. In agreement with this, another study revealed that in patients who died by COVID-19, ferritin levels were high upon hospital admission and throughout the hospital stay. The median values of serum ferritin levels after day 16 of hospitalization exceeded the upper limit of detection in these patients, suggesting that ferritin levels increased non-stop [8]. Also, Chen et al. analyzed the clinical characteristics of 99 patients, in which 63 of them had serum ferritin way above of the normal range [23]. Elevated ferritin levels were found also in autopsies of 12 patients whose cause of death was SARS-CoV-2 infection [24]. An analysis of the peripheral blood of 69 patients with severe COVID-19 revealed elevated levels of ferritin compared with patients with non-severe disease. Therefore, it was concluded that serum ferritin levels were closely related to the severity of COVID-19 [25].
The aim of the present study was to compare ferritin levels in diabetic patients with COVID-19 and non-diabetic patients with COVID-19 based on age and gender groups.
Instruments and Methods
This descriptive cross-sectional study was conducted on 64 patients with COVID-19 and 26 people without COVID-19 (as a control group) referred to the Najaf Hospitals, Najaf, Iraq. Samples were selected by available sampling method.
Clinical tests and laboratory diagnosis were used to investigate the subjects. For this purpose, 5 CC of each person's venous blood was collected in a test tube and after centrifugation at 3500 rpm for 10 minutes, the serum was isolated. IgM and IgG of SARS-CoV-2 (COVID19) were measured using the VIDAS Kit (BioMérieux, France) and serum ferritin levels were measured using a kit (Boditech Med Inc.; South Korea); the test is designed to be used on I-chroma™ devices.
Data were analyzed by SPSS 28 software using Multivariate Analysis of Variance (MANOVA).
Findings
Of the 64 patients with COVID-19, 41 (64.1%) had diabetes and 23 (35.9%) had no diabetes. Also, 21 patients with COVID-19 was female and 43 was male (Table 1).
In diabetic patients with COVID-19, the highest level of ferritin was in the age group of 51-65 years (group D) and the lowest level of ferritin was in the age group of ≤20 years (group A). In non-diabetic patients with COVID-19, the highest level of ferritin was in the age group of 66-80 years (group E) and the lowest level was in the age group of ≤20 years (group A). There was no significant difference between the disease groups in terms of ferritin production based on age groups (p>0.05; Table 2; Diagram 1).
In diabetic patients with COVID-19, ferritin levels were higher in women than men. However, in non-diabetic patients with COVID-19, ferritin levels were higher in men than women. There was no significant difference between the disease groups in terms of ferritin production based on gender (p>0.05; Table 3; Diagram 2)
On December 31, 2019, Chinese Health Authority informed the World Health Organization (WHO) of several cases of pneumonia of unknown cause in Wuhan City, Hubei Province, central China. Cases were reported on December 8, 2019, and several patients worked or resided near the local Huanan Seafood Wholesale Market, but other early cases had no connection to this market [1].
Eventually, a novel virus from the Coronaviridaefamily was detected by the Chinese centers for disease control and prevention from the throat culture of patients with influenza-like manifestations, which was subsequently named Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) [2]. In March of 2020, WHO named this highly contagious disease COVID19 (Coronavirus Disease 19) and declared the outbreak as a global pandemic [3].
Several risk factors, including individuals' underlying diseases, had been shown to be associated with higher vulnerability to get SARS-CoV-2 infection [4-6]. In this regard, Diabetes Mellitus (DM) is reported in a majority of studies to be among the most common comorbidities of patients with Coronavirus disease 2019 (COVID-19) [6]. According to the National Health Commission of China, as of February 4 the fatality rate among confirmed cases in China was 2.1 percent [7].
Based on the initial outbreak cases from China, researchers found a link between COVID-19 with comorbidities [8]. Deng and Peng reported that 25.1% had at least one underlying condition; concurrently hypertension (16.9%) and diabetes (8.2%) were two of the most prevalent comorbidities [9]. This correlated data between diabetes as a comorbidity for COVID-19 is concerning globally as diabetes has been considered the pandemic of the 21st century due to its escalation in the older population and adolescents [10].
Diabetes Mellitus (DM) is a well-known risk factor for worse clinical outcomes in patients with COVID-19. However, the relationship between these two entities seems to be bidirectional [11]. The ongoing pandemic of COVID-19 has significantly affected blood glucose control in patients with diabetes mellitus. The results of this effects can be classified into direct effects (those directly related to the viral infection) and indirect effects (those related to the impact of the pandemic on the management of blood glucose or the use of proposed treatments for the infection that also affect glucose homeostasis) [12].
In comparing COVID-19 patients with and without T2DM, the patients with T2DM tend to develop more severe forms of the disease and have a significant increase in inflammatory markers compared to non-diabetics (i.e., higher levels of C-reactive protein, procalcitonin, ferritin, lactate dehydrogenase, and d-dimer) [13]. Furthermore, the prevalence of diabetes among the patients admitted to intensive care units for COVID-19 is two-to threefold higher, along with the mortality rate which is twice that of non-diabetic patients [14].
Ferritin is a key mediator of immune dysregulation, especially under extreme hyperferritinemia, via direct immune-suppressive and pro-inflammatory effects, contributing to the cytokine storm [15]. It has been reported that fatal outcomes by COVID-19 are accompanied by cytokine storm syndrome, thereby it has been suggested that disease severity is dependent of the cytokine storm syndrome [16]. Many individuals with diabetes exhibit elevated serum ferritin levels [17-19], and it is known that they face a higher probability to experience serious complications from COVID-19 [20].
Hyperferritinemia is more prevalent in diabetic COVID-19 individuals. Serum ferritin can be considered as a valuable biomarker to screen the diabetic and non-diabetic for the presence of hyperinflammation and to predict severity of COVID-19 infection so that it will help the clinician for proper management [21].
In one study with 20 COVID-19 patients, it was found that individuals with severe and very severe COVID-19 exhibited increased serum ferritin level, being serum ferritin in the very severe COVID-19 group significantly higher than in the severe COVID-19 group [22]. In agreement with this, another study revealed that in patients who died by COVID-19, ferritin levels were high upon hospital admission and throughout the hospital stay. The median values of serum ferritin levels after day 16 of hospitalization exceeded the upper limit of detection in these patients, suggesting that ferritin levels increased non-stop [8]. Also, Chen et al. analyzed the clinical characteristics of 99 patients, in which 63 of them had serum ferritin way above of the normal range [23]. Elevated ferritin levels were found also in autopsies of 12 patients whose cause of death was SARS-CoV-2 infection [24]. An analysis of the peripheral blood of 69 patients with severe COVID-19 revealed elevated levels of ferritin compared with patients with non-severe disease. Therefore, it was concluded that serum ferritin levels were closely related to the severity of COVID-19 [25].
The aim of the present study was to compare ferritin levels in diabetic patients with COVID-19 and non-diabetic patients with COVID-19 based on age and gender groups.
Instruments and Methods
This descriptive cross-sectional study was conducted on 64 patients with COVID-19 and 26 people without COVID-19 (as a control group) referred to the Najaf Hospitals, Najaf, Iraq. Samples were selected by available sampling method.
Clinical tests and laboratory diagnosis were used to investigate the subjects. For this purpose, 5 CC of each person's venous blood was collected in a test tube and after centrifugation at 3500 rpm for 10 minutes, the serum was isolated. IgM and IgG of SARS-CoV-2 (COVID19) were measured using the VIDAS Kit (BioMérieux, France) and serum ferritin levels were measured using a kit (Boditech Med Inc.; South Korea); the test is designed to be used on I-chroma™ devices.
Data were analyzed by SPSS 28 software using Multivariate Analysis of Variance (MANOVA).
Findings
Of the 64 patients with COVID-19, 41 (64.1%) had diabetes and 23 (35.9%) had no diabetes. Also, 21 patients with COVID-19 was female and 43 was male (Table 1).
In diabetic patients with COVID-19, the highest level of ferritin was in the age group of 51-65 years (group D) and the lowest level of ferritin was in the age group of ≤20 years (group A). In non-diabetic patients with COVID-19, the highest level of ferritin was in the age group of 66-80 years (group E) and the lowest level was in the age group of ≤20 years (group A). There was no significant difference between the disease groups in terms of ferritin production based on age groups (p>0.05; Table 2; Diagram 1).
In diabetic patients with COVID-19, ferritin levels were higher in women than men. However, in non-diabetic patients with COVID-19, ferritin levels were higher in men than women. There was no significant difference between the disease groups in terms of ferritin production based on gender (p>0.05; Table 3; Diagram 2)
Table 1) Frequency distribution of subjects based on age group and gender
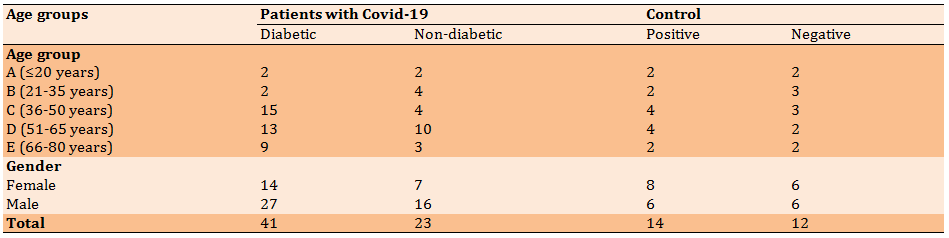
Table 2) The mean of serum ferritin levels (ngml) in different groups according to age
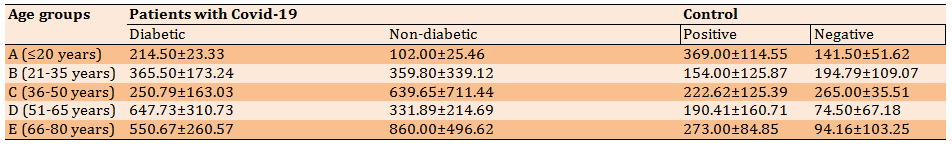

Diagram 1) Comparison of serum ferritin levels (ngml) in different groups based on age groups; Group A: ≤20 years; Group B: 21-35 years; Group C: 36-50 years; Group D: 51-65 years; Group E: 66-80 years
Table 3) The mean of serum ferritin levels (ngml) in different groups according to gender

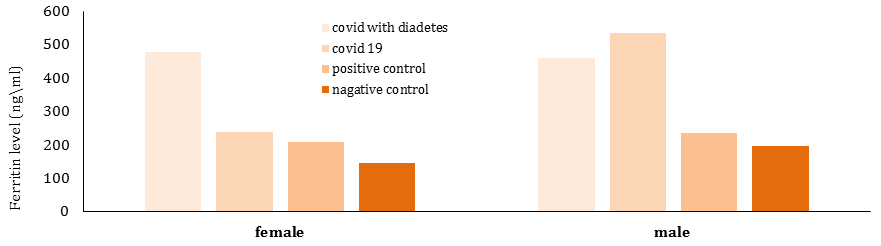
Diagram 2) Comparison of serum ferritin levels (ngml) in different groups based on gender
Discussion
The emerging COVID-19 virus (SARS COV 2) is believed to affect most people with chronic diseases, including diabetics.
This study examined some parameters of immunity in patients with COVID-19 and compared the results of patients with COVID19 and diabetes according to age groups and gender.
No significant difference was observed in the disease groups in terms of the rate of ferritin production in the blood, as there is a relationship between the age of ferritin overproduction and age among COVID-19 patients.
The highest rate among non-diabetic COVID patients was in the age group of 66-80 years compared to other disease and age groups in which ferritin production was 860.00±496.62 and based on this, we examine the data supporting this concept that the ferritin levels may be a major component of the severity of COVID-19 disease.
Perhaps the high ferritin in COVID patients is a result of the good nutrition that is recommended for COVID-19 patients, as this nutrition depends on foods that increase the percentage of iron in the blood, which leads to a high concentration of ferritin and this may lead to serious complications.
As shown by one study, high levels of blood ferritin are attributed to inflammatory disease pathways, and ferritin synthesis actively occurs at the site of infection by various inflammatory stimuli such as cytokines [26]. On the other hand, increased ferritin levels may be the result of a subsequent bacterial infection and be associated with poor clinical outcomes [27].
Many people infected with COVID-19 have elevated ferritin levels in their blood and have been shown to be at risk for significant COVID-19 consequences [28].
A study by Italian scientists showed that ferritin is able to activate macrophages (macrocytes), a type of white blood cell in the immune system, when they are activated, they begin to secrete cytokines. This is a class of signaling molecules that mediate and regulate immunity. When it is secreted in low concentrations, it is considered safe for the body and helps protect it from viruses and bacteria. When it is secreted in high concentrations, a so-called “cytokine storm” develops, which can be fatal for half of patients, especially the elderly. These findings are consistent with the results of our study [29].
The results of our study also showed that COVID patients with diabetes have higher levels of ferritin production in the blood, which is higher in the age group of 51 to 65 years (647.73±310.73), and that the percentage of ferritin in non-infected COVID patients with diabetes is higher than COVID-19 patients. For diabetes, in general, the level of ferritin in patients with COVID is higher than in healthy people. This is because ferritin is high in diabetics, as iron stores levels of abnormal glucose metabolism, which makes the surrounding cells less sensitive to insulin, as high ferritin concentrations indicate the secretion of free radicals that destroy beta cells in the liver [30].
The results of our study showed higher levels of ferritin in men than in women, where the mean ferritin in men was 535.14±415.77, but these differences were not statistically significant (p>0.05).
This suggests that men are more likely to develop severe disease and liver damage than women, and this may be partly due to the increased expression of Angiotensin-Converting Enzyme-2 (ACE-2), a cell-associated receptor for SARS-CoV-2 that mediates virus entry. The lungs of men are more than that of women. High levels of ferritin indicate liver damage and serious disease, and this was confirmed by a study [31].
Rane & Bhadade in their study showed that high levels of ferritin in the blood lead to weak liver activity or as a result of metabolic syndrome [32].
The cause of death may be acute inflammation of the virus, which is associated with high levels of ferritin. However, blood ferritin levels can not only show a strong phase reaction, but also play an important role in inflammation [33].
The results of our study agreed with Perricone et al. [34], as well as with Hussein et al. [35] and Lau et al. [36] which showed that ferritin concentration increases more in men than women.
Conclusion
Chronic diseases, including diabetes, greatly affect the susceptibility to infection with the COVID virus, and blood ferritin levels increase significantly in diabetic patients with COVID infection than in non-diabetic COVID-19 patients, and the elderly produce higher levels of ferritin. In addition, ferritin levels increase in women more than men with COVID-19.
The emerging COVID-19 virus (SARS COV 2) is believed to affect most people with chronic diseases, including diabetics.
This study examined some parameters of immunity in patients with COVID-19 and compared the results of patients with COVID19 and diabetes according to age groups and gender.
No significant difference was observed in the disease groups in terms of the rate of ferritin production in the blood, as there is a relationship between the age of ferritin overproduction and age among COVID-19 patients.
The highest rate among non-diabetic COVID patients was in the age group of 66-80 years compared to other disease and age groups in which ferritin production was 860.00±496.62 and based on this, we examine the data supporting this concept that the ferritin levels may be a major component of the severity of COVID-19 disease.
Perhaps the high ferritin in COVID patients is a result of the good nutrition that is recommended for COVID-19 patients, as this nutrition depends on foods that increase the percentage of iron in the blood, which leads to a high concentration of ferritin and this may lead to serious complications.
As shown by one study, high levels of blood ferritin are attributed to inflammatory disease pathways, and ferritin synthesis actively occurs at the site of infection by various inflammatory stimuli such as cytokines [26]. On the other hand, increased ferritin levels may be the result of a subsequent bacterial infection and be associated with poor clinical outcomes [27].
Many people infected with COVID-19 have elevated ferritin levels in their blood and have been shown to be at risk for significant COVID-19 consequences [28].
A study by Italian scientists showed that ferritin is able to activate macrophages (macrocytes), a type of white blood cell in the immune system, when they are activated, they begin to secrete cytokines. This is a class of signaling molecules that mediate and regulate immunity. When it is secreted in low concentrations, it is considered safe for the body and helps protect it from viruses and bacteria. When it is secreted in high concentrations, a so-called “cytokine storm” develops, which can be fatal for half of patients, especially the elderly. These findings are consistent with the results of our study [29].
The results of our study also showed that COVID patients with diabetes have higher levels of ferritin production in the blood, which is higher in the age group of 51 to 65 years (647.73±310.73), and that the percentage of ferritin in non-infected COVID patients with diabetes is higher than COVID-19 patients. For diabetes, in general, the level of ferritin in patients with COVID is higher than in healthy people. This is because ferritin is high in diabetics, as iron stores levels of abnormal glucose metabolism, which makes the surrounding cells less sensitive to insulin, as high ferritin concentrations indicate the secretion of free radicals that destroy beta cells in the liver [30].
The results of our study showed higher levels of ferritin in men than in women, where the mean ferritin in men was 535.14±415.77, but these differences were not statistically significant (p>0.05).
This suggests that men are more likely to develop severe disease and liver damage than women, and this may be partly due to the increased expression of Angiotensin-Converting Enzyme-2 (ACE-2), a cell-associated receptor for SARS-CoV-2 that mediates virus entry. The lungs of men are more than that of women. High levels of ferritin indicate liver damage and serious disease, and this was confirmed by a study [31].
Rane & Bhadade in their study showed that high levels of ferritin in the blood lead to weak liver activity or as a result of metabolic syndrome [32].
The cause of death may be acute inflammation of the virus, which is associated with high levels of ferritin. However, blood ferritin levels can not only show a strong phase reaction, but also play an important role in inflammation [33].
The results of our study agreed with Perricone et al. [34], as well as with Hussein et al. [35] and Lau et al. [36] which showed that ferritin concentration increases more in men than women.
Conclusion
Chronic diseases, including diabetes, greatly affect the susceptibility to infection with the COVID virus, and blood ferritin levels increase significantly in diabetic patients with COVID infection than in non-diabetic COVID-19 patients, and the elderly produce higher levels of ferritin. In addition, ferritin levels increase in women more than men with COVID-19.
Keywords:
Ferritin [MeSH], Hyperferritinemia [MeSH], COVID-19 [MeSH], Diabetes Mellitus [MeSH], Patients [MeSH]
References
1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92(4):401-2. [Link] [DOI:10.1002/jmv.25678]
2. Mohammadi M, Meskini M, do Nascimento Pinto AL. 2019 Novel coronavirus (COVID-19) overview. Gesundh Wiss. 2020;1-9. [Link] [DOI:10.1007/s10389-020-01258-3]
3. Reshad RA, Riana SH, Chowdhury MA, Moin AT, Miah F, et al. Diabetes in COVID-19 patients: challenges and possible management strategies. Egypt J Bronchol. 2021;15(53):1-13. [Link] [DOI:10.1186/s43168-021-00099-2]
4. Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, et al. Obesity ‑a risk factor for increased COVID‑19 prevalence, severity and lethality. Mol Med Rep. 2020;22(1):9-19. [Link] [DOI:10.3892/mmr.2020.11127]
5. Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766-73. [Link] [DOI:10.14309/ajg.0000000000000620]
6. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32-40. [Link] [DOI:10.1148/radiol.2020200642]
7. Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health. 2020;13(5):667-73. [Link] [DOI:10.1016/j.jiph.2020.03.019]
8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, china: A retrospective cohort study. Lancet. 2020;395(10229):1054-62. [Link] [DOI:10.1016/S0140-6736(20)30566-3]
9. Deng SQ, Peng HJ. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9(2):575. [Link] [DOI:10.3390/jcm9020575]
10. Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56-64. [Link] [DOI:10.1016/S2213-8587(13)70112-8]
11. Elamari S, Motaib I, Zbiri S, Elaidaoui K, Chadli A, Elkettani C. Characteristics and outcomes of diabetic patients infected by the SARS-CoV-2. Pan Afri Med J. 2020;37:32. [Link] [DOI:10.11604/pamj.2020.37.32.25192]
12. Nassar M, Daoud A, Nso N, Medina L, Ghernautan V, et al. Diabetes mellitus and COVID-19: review article. Diabetes Metab Syndr. 2021;15(6):102268. [Link] [DOI:10.1016/j.dsx.2021.102268]
13. Wang X, Liu Z, Li J, Zhang J, Tian S, et al. Impacts of type 2 diabetes on disease severity, therapeutic effect, and mortality of patients with COVID-19. J Clin Endocrinol Metab. 2020;105(12):dgaa535. [Link] [DOI:10.1210/clinem/dgaa535]
14. Scheen AJ, Marre M, Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46(4):265-71. [Link] [DOI:10.1016/j.diabet.2020.05.008]
15. Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164-74. [Link]
16. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 20d20;395(10223):497-506. [Link] [DOI:10.1016/S0140-6736(20)30183-5]
17. Khalil UA, Seliem FO, Alnahal A, Awad M, Sadek AM, Fawzy MS. Association of serum ferritin with insulin resistance in offsprings of type 2 diabetes. Egypt J Intern Med. 2018;30:13-7. [Link] [DOI:10.4103/ejim.ejim_70_17]
18. Momeni A, Behradmanesh MS, Kheiri S, Abasi F. Serum ferritin has correlation with HbA1c in type 2 diabetic patients. Adv Biomed Res. 2015;4:74. [Link] [DOI:10.4103/2277-9175.153900]
19. Son NE. Influence of ferritin levels and inflammatory markers on HbA1c in the Type 2 Diabetes mellitus patients. Pak J Med Sci. 2019;35(4):1030-5. [Link] [DOI:10.12669/pjms.35.4.1003]
20. American Diabetes Association. How COVID-19 impacts people with diabetes [Internet]. American Diabetes Association; 2020 [cited 2021 May 22]. Available from: https://www.diabetes.org/coronavirus-COVID-19/how-coronavirus-impacts-people-with-diabetes. [Link]
21. Kumar MCM, Bindu AC, Shyam, RR. Ferritin - The key model inflammatory marker in diabetic and non-diabetic COVID-19. Asian J Med Sci. 2021;12(12):23-31. [Link] [DOI:10.3126/ajms.v12i12.39717]
22. Zhou B, She J, Wang Y, Ma X. Utility of ferritin, procalcitonin, and C-reactive protein in severe patients with 2019 novel coronavirus disease [Internet]. Research Square;2020 [cited 2021 May 22]. Available from: https://www.researchsquare.com/article/rs-18079/v1. [Link] [DOI:10.21203/rs.3.rs-18079/v1]
23. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-13. [Link] [DOI:10.1016/S0140-6736(20)30211-7]
24. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. 2020. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681-6. [Link] [DOI:10.1016/S2213-2600(20)30243-5]
25. Liu T, Zhang J, Yang Y, Ma H, Li Z, et al. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019.medRxiv. 2020. [Link] [DOI:10.1101/2020.03.01.20029769]
26. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. [Link] [DOI:10.1056/NEJMoa2002032]
27. Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D'Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. [Link] [DOI:10.1186/1741-7015-11-185]
28. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33. [Link] [DOI:10.1056/NEJMoa2001017]
29. Lanser L, Burkert FR, Bellmann-Weiler R, Schroll A, Wildner S, Fritsche G, Weiss G. Dynamics in anemia development and dysregulation of iron homeostasis in hospitalized patients with COVID-19- Metabolites. 2021;11(10):653. [Link] [DOI:10.3390/metabo11100653]
30. Backe MB, Moen IW, Ellervik C, Hansen JB, Mandrup-Poulsen T. Iron regulation of pancreatic beta-cell functions and oxidative stress. Annu Rev Of Nutr. 2016;36:241-73. [Link] [DOI:10.1146/annurev-nutr-071715-050939]
31. Qeadan F, Tingey B, Gu LY, Packard AH, Erdei E, Saeed AI. Prognostic values of serum ferritin and d-dimer trajectory in patients with COVID-19. Viruses. 2021;13(3):419. [Link] [DOI:10.3390/v13030419]
32. Rane M, Bhadade U. Multimodal biometric identification using feature fusion. Test Eng Manage. 2020;83:26444-54. [Link]
33. Xie J, Ding, C, Li J, Wang Y, Guo H, et al. Characteristics of patients with coronavirus disease (COVID‐ 19) confirmed using an IgM‐ IgG antibody test. J Med Virol. 2020;92(10):2004-10. [Link] [DOI:10.1002/jmv.25930]
34. Perricone C, Bartoloni E, Bursi R, Cafaro G, Guidelli GM, Shoenfeld Y, Gerli R. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol Res. 2020;68(4):213-24. [Link] [DOI:10.1007/s12026-020-09145-5]
35. Hussein AM, Taha ZB, Malek AG, Rasul KA, Kazim DH, Ahmed RJ, Mohamed UB. D-dimer and serum ferritin as an independent risk factor for severity in COVID-19 patients. Mater Today Proce. 2021 [In press]. [Link] [DOI:10.1016/j.matpr.2021.04.009]
36. Lau ES, McNeill JN, Paniagua SM, Liu EE, Wang JK, et al. Sex differences in inflammatory markers in patients hospitalized with COVID-19 infection: insights from the MGH COVID-19 patient registry. PLoS One. 2021;16(4):e0250774. [Link] [DOI:10.1371/journal.pone.0250774]








