Volume 17, Issue 1 (2025)
Iran J War Public Health 2025, 17(1): 75-82 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2025/03/11 | Accepted: 2025/04/16 | Published: 2025/04/21
Received: 2025/03/11 | Accepted: 2025/04/16 | Published: 2025/04/21
How to cite this article
Abbas O, Mohammed A. Bacterial Resistance to Disinfectants in Hospitals of the Medical City, Baghdad, Iraq. Iran J War Public Health 2025; 17 (1) :75-82
URL: http://ijwph.ir/article-1-1572-en.html
URL: http://ijwph.ir/article-1-1572-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
O.T. Abbas *1, A.J. Mohammed1
1- Department of Biology, Faculty of Science, University of Baghdad, Baghdad, Iraq
Full-Text (HTML) (557 Views)
Introduction
In some countries, such as Greece and Cyprus, there is a lack of information on the use of disinfectants, and incorrect practices are adopted by workers in both countries regarding the amount of expired or leftover detergents. This issue poses a problem for infection control management in hospitals [1]. Laboratory studies have identified multiple mechanisms, by which bacteria can develop tolerance or resistance to quaternary ammonium compounds (QACs) and antibiotics [2].
Bacterial biofilms are complex communities of bacteria that adhere to surfaces and are held together by a self-produced polymer matrix, primarily composed of polysaccharides, secreted proteins, and extracellular DNA [3]. Biofilms tend to occur in healthcare environments and are linked to healthcare-associated infections (HAIs), making them generally less susceptible to antibacterial agents [4].
The presence of biofilms reduces the vulnerability of both Gram-positive and Gram-negative bacteria to disinfectants, with the greatest impact observed in Gram-negative bacteria [5]. Medical equipment, such as surgical instruments, cardiac prostheses, vascular and urinary catheters, implants, analytical and diagnostic tools, and endotracheal tubes, is particularly vulnerable to the growth of biofilms [6]. The persistence of biofilms on medical devices not only complicates treatment efforts but also increases the risk of severe infections, prolonging hospital stays and elevating healthcare costs [7]. Furthermore, the ineffectiveness of conventional disinfectants against biofilms has led researchers to explore novel antimicrobial agents and strategies, including the use of bacteriophages and enzymatic treatments, which may show increased efficacy against biofilm-associated bacteria [8].
Research indicates that education and training regarding appropriate disinfectant usage are essential to improving infection control practices [9]. For instance, healthcare workers must be aware of the correct concentrations and application methods of disinfectants to enhance their efficacy against biofilm-forming bacteria. Implementing routine assessments and monitoring protocols can also contribute significantly to reducing the risk of HAIs linked to biofilm development [10]. Therefore, addressing the challenges posed by biofilms in healthcare settings is crucial for improving patient safety and infection control outcomes.
Metallic devices and implants, such as stainless steel, titanium, and titanium alloys, are widely used in the medical industry. These materials interact with bodily fluids and microorganisms, which can change their microstructure, induce biocorrosion, and affect the biological properties of the patient [11]. Bacteria form biofilms in response to environmental stresses such as UV radiation, desiccation, limited nutrients, extreme pH, extreme temperatures, high salt concentrations, high pressure, and antimicrobial agents. The events leading to bacterial biofilm formation are complex [12]. Biofilms are believed to account for over 65% of nosocomial infections, around 80% of chronic infections, and 60% of all bacterial infections in humans [13].
Due to the substantial increase in the use of disinfectants containing quaternary ammonium compounds (QACs) in healthcare and community settings during the COVID-19 pandemic, there is growing concern that heavy use might cause bacteria to develop resistance to QACs or contribute to antibiotic resistance [2]. Excessive and high-concentration use of QACs leads to environmental pollution and the generation of resistant strains [14].
Disinfectants are classified into different categories: alcohols, aldehydes, quaternary ammonium compounds, halogens, chlorhexidine, and oxidizing agents [15]. Alcohols, phenols, and quaternary ammonium compounds are widely used disinfectants with varying active ingredients and mechanisms. They operate in two stages: primary and secondary. The effectiveness of these disinfectants is influenced by their shelf life, which can be impacted by temperature, sunlight exposure, and organic matter [16]. Benzalkonium chloride (BAC) is a mixture of alkyl benzyl dimethyl ammonium chloride compounds and is the most common active ingredient in surface cleaning and disinfecting products [17].
Currently, there is a new concern regarding alcohol-tolerant bacteria [18]. A study demonstrated the inhibitory effects of certain disinfectants, such as ethanol and Dettol, on MRSA, Acinetobacter baumannii, E. coli, and Klebsiella species, and significantly revealed that all tested bacteria were resistant to ethanol at all concentrations [19]. This resistance can be attributed to several factors, including the overuse of antiseptics, mechanisms of resistance that may develop in bacteria through mutation and the acquisition of resistance genes from other organisms, and biofilm formation, in which bacteria like Acinetobacter baumannii tend to form biofilms that can protect them from the action of disinfectants [20].
Moreover, the emergence of disinfectant-resistant strains poses a significant challenge to infection control in healthcare settings. Studies have shown that inappropriate use of disinfectants can lead to selective pressure, facilitating the development of resistant bacterial strains [21]. Additionally, the ineffectiveness of traditional disinfectants against biofilm-associated bacteria can complicate treatment protocols and increase the risk of healthcare-associated infections (HAIs) [22].
The aim of this research was to evaluate the biofilm formation capabilities of four healthcare-associated bacteria when exposed to different disinfectant formulations at varying concentrations, assess the efficacy of these disinfectants in disrupting biofilms, and highlight the resilience of biofilms under suboptimal disinfectant conditions. Understanding the biofilm formation capabilities of these bacteria is crucial because biofilms are known to significantly contribute to the persistence of infections in healthcare settings, leading to increased morbidity and healthcare costs. By investigating how different disinfectant formulations perform against biofilms, this study provides valuable insights that can inform infection control protocols and enhance patient safety. Furthermore, the findings from this research could guide the development of more effective disinfectant strategies tailored to combat biofilm-associated infections, ultimately reducing the incidence of HAIs and improving overall clinical outcomes. Given the rising concern over antibiotic resistance, this study also addresses a critical gap in current literature by exploring alternative approaches to infection prevention, emphasizing the need for rigorous evaluation of disinfectants in real-world healthcare environments.
Materials and Methods
Bacterial isolation
This experimental study used bacterial strains collected from various hospital surfaces and medical equipment, such as neonatal incubators, surgical tools, patient beds, bed corners, urinary catheters, and air conditioning openings in Medical City in Baghdad from 2023 to the end of March 2024. A total of 40 bacterial isolates (13 isolates of Acinetobacter baumannii, 9 isolates of Klebsiella pneumoniae, 13 isolates of Pseudomonas aeruginosa, and 5 isolates of Burkholderia cepacia) were identified using the VITEK 2 system.
Disinfectants
Three different disinfectants were used (Table 1). The selection was based on their documented use in sterilization protocols within the hospitals, from which the bacterial samples were sourced. This approach was undertaken to investigate the etiology of disinfectant resistance and to identify the persistence of bacteria despite the implementation of standard sterilization procedures in these healthcare settings.
Table 1. Disinfectant used in the study
Biofilm formation
Biofilm formation was assessed using the crystal violet assay, following the methods of Hashem et al. [23] and Singh et al. [24] with some modifications. The Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Burkholderia cepacia strains were activated by growing them in double-strength brain heart infusion with 0.5% glucose at 37°C for 24 hours. A sterile flat-bottom 96-well microtiter plate was inoculated with 200 µL of the diluted cultures aseptically (the differences in the three surface disinfectants were measured between isolates according to sub-MIC for each), and each isolate was added in triplicate wells. It is important to mention that the MIC of the three different disinfectants was determined at varying concentrations (Table 2). Additionally, positive and negative controls were prepared.
Table 2. Disinfectant concentration and percentage used in the experiment
The wells were washed with saline three times and left to dry. Methanol was used to fix the adherent cells. Fixed adherent cells were stained with 1% (W/V) crystal violet for 15 minutes, and excess stain was removed by washing (Figure 1). The plates were then left to dry. Crystal violet bound to the adherent cells was re-dissolved in glacial acetic acid. The optical density (OD) was measured at 600nm in a plate reader (Biotek, USA), and the median of three readings was taken. The strength of the biofilm was classified according to the OD readings (Table 3).
Table 3. Biofilm degree of Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa and Burkholderia cepacia isolates
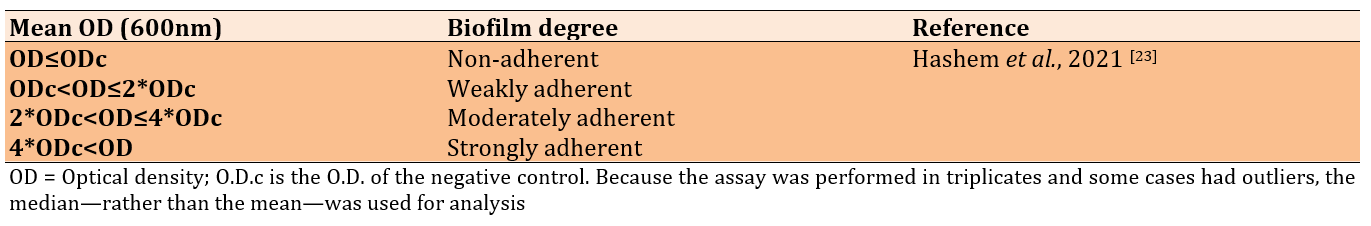
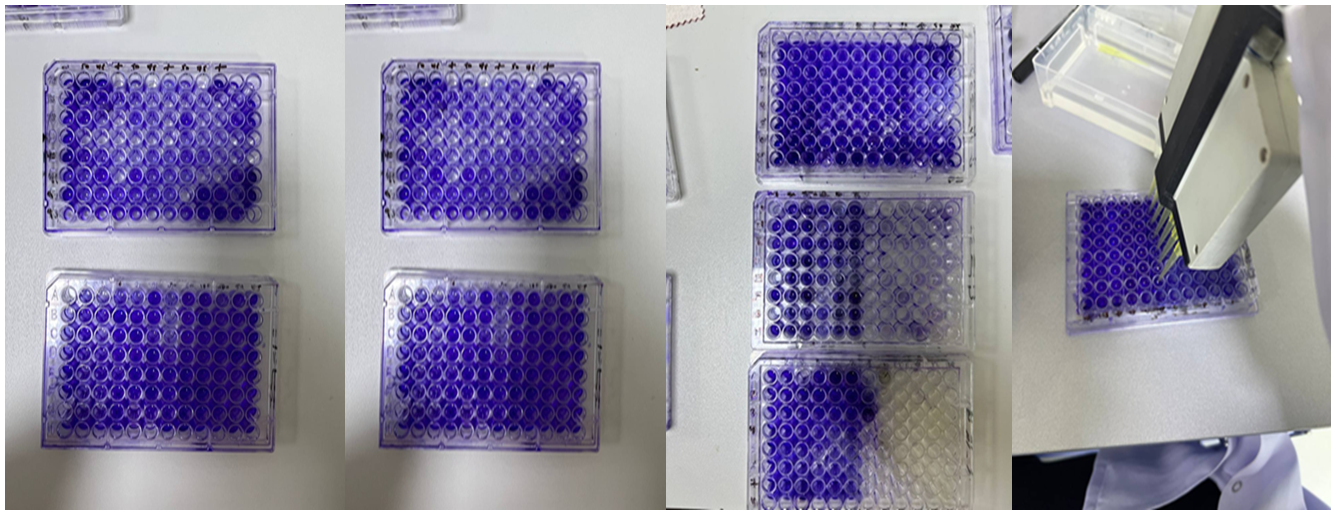
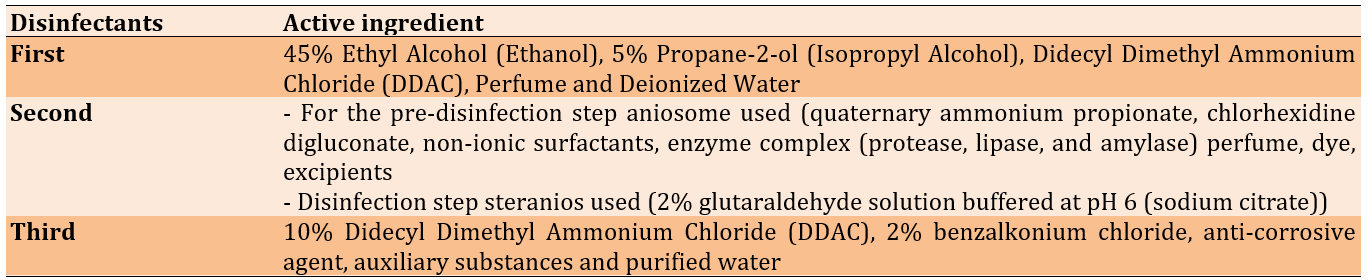
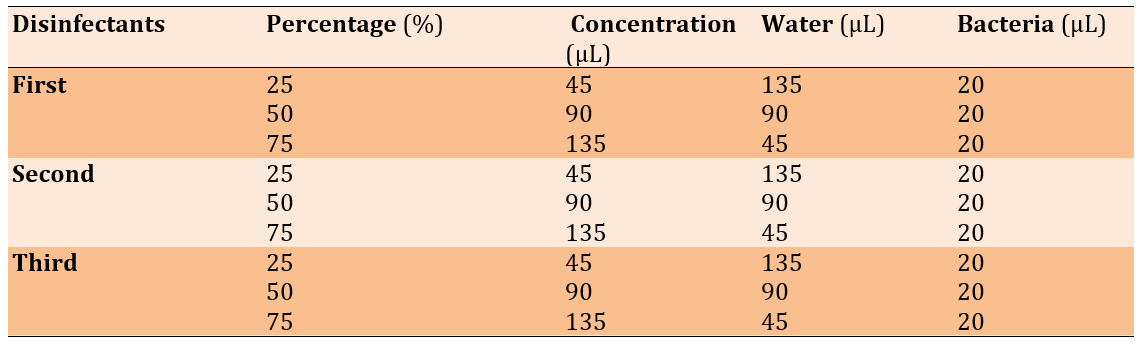
Figure 1. Microtiter plates filled with crystal violet, disinfectants, and different isolates of bacteria.
Data analysis
Data were analyzed by SPSS 20 using the chi-square test to obtain significant differences between the frequency numbers collected regarding the ability of biofilm formation. The results were presented as mean±S.E., and a significant difference was considered at p≤0.05.
Findings
Disinfectant resistance among bacteria, such as Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Burkholderia cepacia in hospital environments is a growing concern [25]. These bacteria can exhibit resistance to disinfectants, like ethanol, DDAC, BAC, and quaternary ammonium propionate, even at varying concentrations.
The effect of the three disinfectants (25%, 50%, and 75%) against the biofilm of 40 Gram-negative bacteria (13 isolates of Acinetobacter baumannii, 9 isolates of Klebsiella pneumoniae, 13 isolates of Pseudomonas aeruginosa, and five isolates of Burkholderia cepacia) was tested, and the results showed that all isolates formed biofilms.
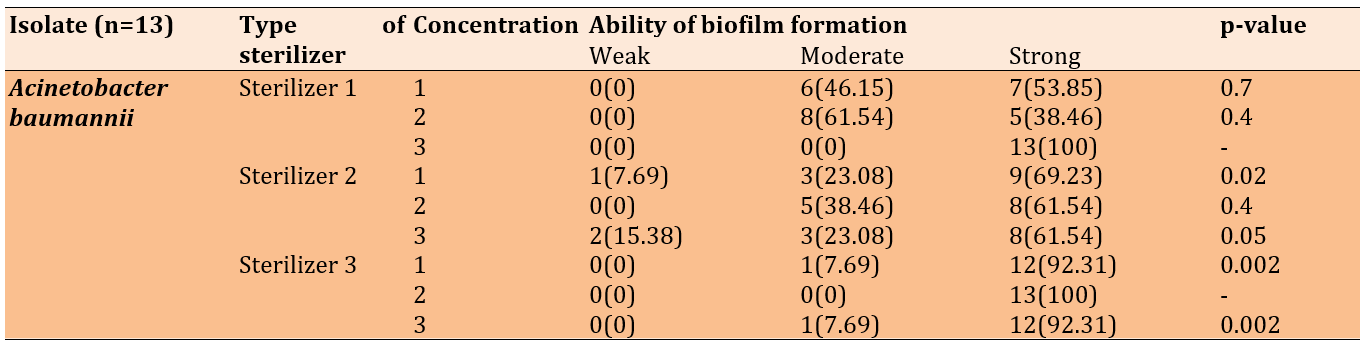
For Acinetobacter baumannii, biofilm formation after treatment with the first disinfectant (ethanol, DDAC) at varying concentrations revealed significant insights into the relationship between disinfectant and biofilm development, notably showing moderate and strong biofilm formation at 25% and 50% concentrations, respectively. All isolates exhibited strong biofilm formation at the highest concentration of 75%.
After treatment with the second disinfectant (quaternary ammonium propionate, chlorhexidine digluconate), there was a significant difference between treatments at 25% and 75% concentrations. Most isolates formed strong biofilms, while fewer formed moderate biofilms, and one isolate formed a weak biofilm at 25%. Additionally, two isolates formed weak biofilms at 75%.
For the third disinfectant (DDAC, benzalkonium chloride), most isolates formed strong biofilms, and one isolate formed a moderate biofilm, indicating a significant difference (Table 4).
Table 4. Biofilm formation of Acinetobacter baumannii after the treatment with disinfectants
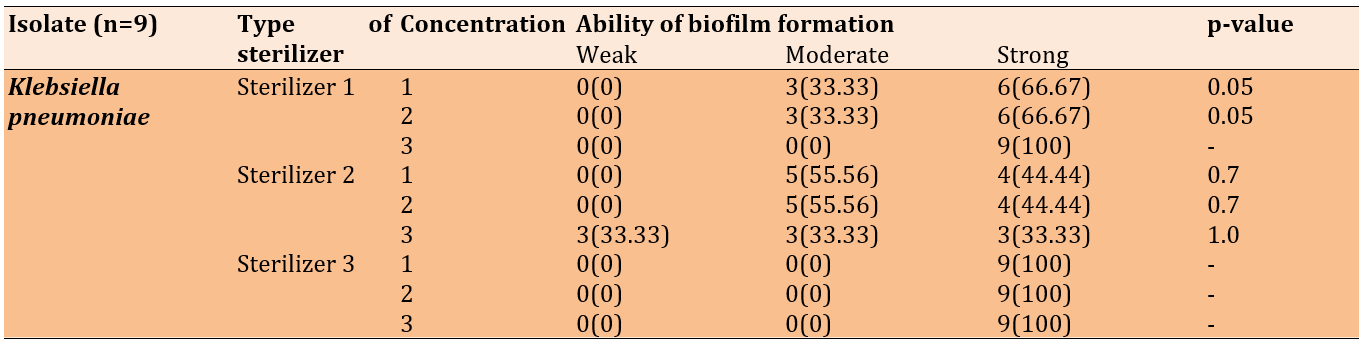
When the nine Klebsiella pneumoniae isolates were treated with the mentioned disinfectants, the first sterilizer (ethanol + DDAC) resulted in six isolates forming strong biofilms and three forming moderate biofilms at concentrations of 25% and 50%. In contrast, all the isolates formed strong biofilms when exposed to the first disinfectant at a concentration of 75% and to all concentrations of the third disinfectant (DDAC, BAC). Following treatment with the second disinfectant (quaternary ammonium propionate), the 25% and 50% concentrations induced moderate to strong biofilm formation, whereas the 75% concentration resulted in weak, moderate, and strong biofilm formation across the isolates (Table 5).
Table 5. Biofilm formation of Klebsiella pneumoniae after the treatment with disinfectants
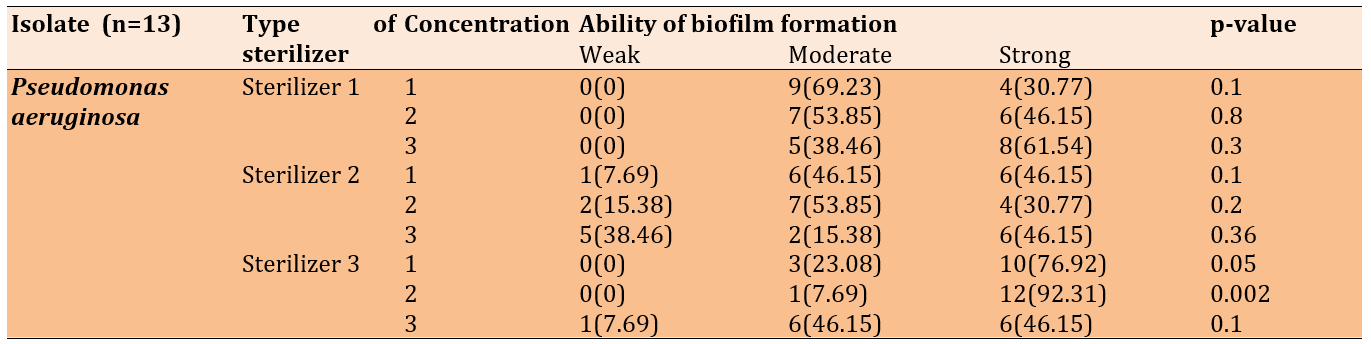
When exposed to disinfectant 1 (ethanol + DDAC) at varying concentrations, the 13 Pseudomonas isolates exhibited moderate to strong biofilm formation. With the second sterilizer (quaternary ammonium propionate), the isolates formed moderate to strong biofilms; however, numerous isolates formed weak biofilms at the 25% and 50% concentrations of the disinfectant. At the 75% concentration, five isolates formed weak biofilms, while six formed strong biofilms.
The majority of the 13 isolates formed strong biofilms, with several forming moderate biofilms at the 25% and 50% concentrations of the third disinfectant (DDAC, BAC). At the 75% concentration, one isolate formed a weak biofilm, while the remaining isolates exhibited moderate to strong biofilms (Table 6).
Table 6. Biofilm formation of Pseudomonas aeruginosa after the treatment with disinfectants
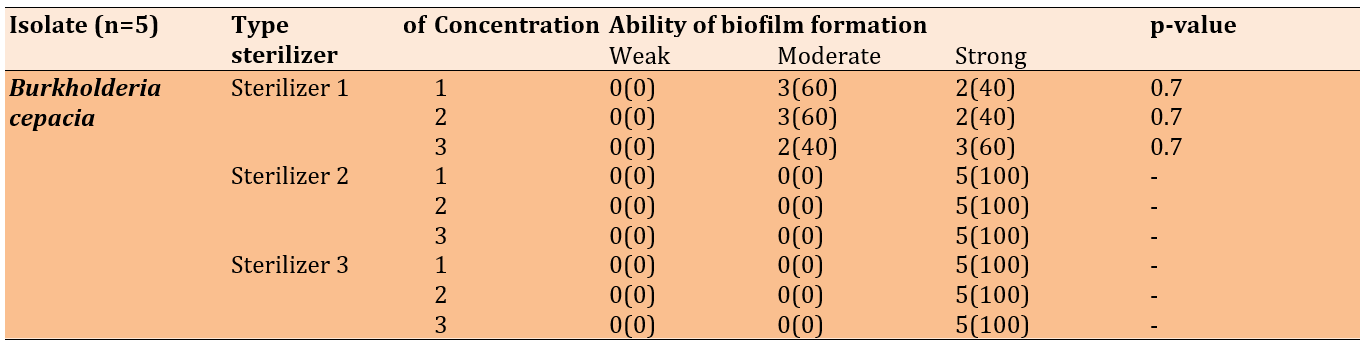
In Burkholderia cepacia, all five isolates formed strong biofilms after treatment with the second disinfectant (quaternary ammonium propionate) and the third disinfectant (DDAC, BAC). Regardless of the concentration used, treatment with the first disinfectant (ethanol + DDAC) resulted in moderate to strong biofilm formation (Table 7).
Table 7. Biofilm formation of Burkholderia cepacia after the treatment with disinfectants
Discussion
Several studies have reported that in high, middle, and low-income healthcare settings, antiseptics, low-level disinfectants, and hand hygiene products can be susceptible to bacterial contamination. Numerous studies assessing the susceptibility of A. baumannii biofilms to disinfectants like benzalkonium chloride and chlorhexidine have shown the ability of both hospital and environmental isolates to form biofilms on surfaces, such as glass and ceramics [26].
Non-fermentative gram-negative bacteria are the predominant contaminants, with Burkholderia cepacia, Pseudomonas aeruginosa, and Achromobacter spp. being the most frequently recognized. These organisms are particularly concerning due to their inherent resistance to multiple antibiotics, which complicates treatment options and increases the risk of severe infections in vulnerable patient populations, such as those in intensive care units. Enterobacterales are also notable contaminants, with Serratia spp. being the most common; these bacteria can lead to a range of healthcare-associated infections, including bloodstream infections and pneumonia. The presence of these pathogens underscores the urgent need for effective infection control measures, as their ability to form biofilms further enhances their persistence in clinical settings and contributes to outbreaks. Moreover, understanding the prevalence and resistance patterns of these contaminants is critical for developing targeted interventions and improving patient safety in healthcare environments [27-29].
In this research, all the isolates were capable of forming biofilms, and most of them had the ability to form strong and moderate biofilms. This finding is consistent with the studies by Mougin et al. [30] and Strempel et al. [31], which demonstrated that the overuse and misuse of disinfectants such as BAC lead to the persistence of opportunistic bacteria like Pseudomonas and stimulate biofilm formation.
This work provides insights into some bacterial adaptation mechanisms and stress responses when exposed to sub-lethal disinfectants. Biofilm formation can be induced by exposure to disinfectants, serving as a protective barrier against antimicrobial agents and leading to increased exopolysaccharide (EPS) production, accelerated cell proliferation, and enhanced adhesion. Bacteria within biofilms are often in a metabolically inactive state, making them less susceptible to both disinfectants and antibiotics [32, 33]. Prior research has indicated that these biofilms exhibit distinct shifts in EPS composition and quantity in response to subinhibitory levels of various disinfectants [34]. Biofilms also facilitate horizontal gene transfer among bacteria, further spreading resistance traits. Additionally, changes in the bacterial cell envelope through various mechanisms, such as increased surface hydrophobicity or changes in surface charge, can reduce permeability to disinfectants, preventing these agents from reaching their targets within the cell. This intrinsic resistance is particularly notable in gram-negative bacteria [35].
Some studies have demonstrated that certain bacterial strains, such as Burkholderia cepacia, exhibit natural resistance to antibiotics, antiseptics, and disinfectants, particularly quaternary ammonium compounds and chlorhexidine, along with biofilm formation [36, 37].
An investigation of surface swab samples from intensive care units (ICUs) revealed that 95.8% of Acinetobacter baumannii isolates produced biofilms; among these, 45.83% exhibited strong biofilm formation, 29.16% showed moderate biofilm formation, and 20.83% demonstrated weak ability for biofilm formation. The capacity of these bacterial isolates, particularly those from bed rails, to form biofilms presents a significant infection risk for hospitalized patients, healthcare workers, and visitors. This is due to the persistent nature of these biofilms, which can harbor and transmit pathogenic microorganisms in hospital environments, thereby complicating infection control measures and increasing the potential for nosocomial infections [3, 38]. Furthermore, the ability of biofilms to resist both antimicrobial agents and host immune responses makes them particularly challenging to eradicate, often leading to prolonged hospital stays and increased healthcare costs. The high prevalence of biofilm-producing Acinetobacter baumannii underscores the necessity for rigorous surface decontamination protocols and routine monitoring to mitigate the risk of infection transmission. Additionally, understanding the biofilm formation dynamics of these pathogens can inform the development of novel therapeutic strategies aimed at disrupting biofilms and enhancing patient safety in critical care settings.
Commonly used disinfectants often exhibit significant limitations when it comes to effectively targeting biofilm-associated bacteria, which presents substantial challenges in healthcare and clinical environmental settings. One major limitation is that biofilms create a protective matrix that shields embedded bacteria from the full effects of disinfectants, significantly reducing their efficacy. Additionally, many disinfectants can be inactivated by organic matter, such as blood and tissue, which frequently accumulate on surfaces in healthcare environments, further compromising their effectiveness. Certain bacterial species, like Pseudomonas aeruginosa and Acinetobacter baumannii, can also develop resistance mechanisms that allow them to survive even in the presence of potent disinfectants. The variability in disinfectant effectiveness against different bacterial strains can lead to inconsistent outcomes, allowing resistant populations to thrive and potentially cause HAIs. Furthermore, the sporadic application and improper dilution of disinfectants can lead to suboptimal conditions for microbial control, resulting in persistent biofilm formation. This situation highlights the urgent necessity for healthcare facilities to implement more comprehensive infection prevention strategies specifically targeting biofilms. Innovative approaches, such as the development of novel antimicrobial agents or the use of combination therapies, should be prioritized to enhance disinfectant effectiveness. Future studies must address the mechanisms of biofilm resilience and the interactions between disinfectants and biofilms to identify more effective interventions. By focusing on these critical areas, researchers can help improve infection control practices, ultimately reducing the incidence of HAIs and safeguarding patient health in clinical settings.
Conclusion
Most bacterial strains exhibit moderate to strong biofilm formation across all treatments.
Acknowledgments: The authors thank Dr. Ridha D.A. for assisting them in collecting the samples, as well as the staff at the Medical City in Baghdad for their support.
Ethical Permissions: Approval was obtained from the hospitals of the Medical City in Baghdad to collect environmental swabs from within the hospital environment and medical equipment (code: 22970-4/7/2024)
Conflicts of Interests: The authors declared no conflicts of interests.
Authors' Contribution: Abbas OT (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (80%); Mohammed AJ (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (20%)
Funding/Support: This research was not funded by any grant.
In some countries, such as Greece and Cyprus, there is a lack of information on the use of disinfectants, and incorrect practices are adopted by workers in both countries regarding the amount of expired or leftover detergents. This issue poses a problem for infection control management in hospitals [1]. Laboratory studies have identified multiple mechanisms, by which bacteria can develop tolerance or resistance to quaternary ammonium compounds (QACs) and antibiotics [2].
Bacterial biofilms are complex communities of bacteria that adhere to surfaces and are held together by a self-produced polymer matrix, primarily composed of polysaccharides, secreted proteins, and extracellular DNA [3]. Biofilms tend to occur in healthcare environments and are linked to healthcare-associated infections (HAIs), making them generally less susceptible to antibacterial agents [4].
The presence of biofilms reduces the vulnerability of both Gram-positive and Gram-negative bacteria to disinfectants, with the greatest impact observed in Gram-negative bacteria [5]. Medical equipment, such as surgical instruments, cardiac prostheses, vascular and urinary catheters, implants, analytical and diagnostic tools, and endotracheal tubes, is particularly vulnerable to the growth of biofilms [6]. The persistence of biofilms on medical devices not only complicates treatment efforts but also increases the risk of severe infections, prolonging hospital stays and elevating healthcare costs [7]. Furthermore, the ineffectiveness of conventional disinfectants against biofilms has led researchers to explore novel antimicrobial agents and strategies, including the use of bacteriophages and enzymatic treatments, which may show increased efficacy against biofilm-associated bacteria [8].
Research indicates that education and training regarding appropriate disinfectant usage are essential to improving infection control practices [9]. For instance, healthcare workers must be aware of the correct concentrations and application methods of disinfectants to enhance their efficacy against biofilm-forming bacteria. Implementing routine assessments and monitoring protocols can also contribute significantly to reducing the risk of HAIs linked to biofilm development [10]. Therefore, addressing the challenges posed by biofilms in healthcare settings is crucial for improving patient safety and infection control outcomes.
Metallic devices and implants, such as stainless steel, titanium, and titanium alloys, are widely used in the medical industry. These materials interact with bodily fluids and microorganisms, which can change their microstructure, induce biocorrosion, and affect the biological properties of the patient [11]. Bacteria form biofilms in response to environmental stresses such as UV radiation, desiccation, limited nutrients, extreme pH, extreme temperatures, high salt concentrations, high pressure, and antimicrobial agents. The events leading to bacterial biofilm formation are complex [12]. Biofilms are believed to account for over 65% of nosocomial infections, around 80% of chronic infections, and 60% of all bacterial infections in humans [13].
Due to the substantial increase in the use of disinfectants containing quaternary ammonium compounds (QACs) in healthcare and community settings during the COVID-19 pandemic, there is growing concern that heavy use might cause bacteria to develop resistance to QACs or contribute to antibiotic resistance [2]. Excessive and high-concentration use of QACs leads to environmental pollution and the generation of resistant strains [14].
Disinfectants are classified into different categories: alcohols, aldehydes, quaternary ammonium compounds, halogens, chlorhexidine, and oxidizing agents [15]. Alcohols, phenols, and quaternary ammonium compounds are widely used disinfectants with varying active ingredients and mechanisms. They operate in two stages: primary and secondary. The effectiveness of these disinfectants is influenced by their shelf life, which can be impacted by temperature, sunlight exposure, and organic matter [16]. Benzalkonium chloride (BAC) is a mixture of alkyl benzyl dimethyl ammonium chloride compounds and is the most common active ingredient in surface cleaning and disinfecting products [17].
Currently, there is a new concern regarding alcohol-tolerant bacteria [18]. A study demonstrated the inhibitory effects of certain disinfectants, such as ethanol and Dettol, on MRSA, Acinetobacter baumannii, E. coli, and Klebsiella species, and significantly revealed that all tested bacteria were resistant to ethanol at all concentrations [19]. This resistance can be attributed to several factors, including the overuse of antiseptics, mechanisms of resistance that may develop in bacteria through mutation and the acquisition of resistance genes from other organisms, and biofilm formation, in which bacteria like Acinetobacter baumannii tend to form biofilms that can protect them from the action of disinfectants [20].
Moreover, the emergence of disinfectant-resistant strains poses a significant challenge to infection control in healthcare settings. Studies have shown that inappropriate use of disinfectants can lead to selective pressure, facilitating the development of resistant bacterial strains [21]. Additionally, the ineffectiveness of traditional disinfectants against biofilm-associated bacteria can complicate treatment protocols and increase the risk of healthcare-associated infections (HAIs) [22].
The aim of this research was to evaluate the biofilm formation capabilities of four healthcare-associated bacteria when exposed to different disinfectant formulations at varying concentrations, assess the efficacy of these disinfectants in disrupting biofilms, and highlight the resilience of biofilms under suboptimal disinfectant conditions. Understanding the biofilm formation capabilities of these bacteria is crucial because biofilms are known to significantly contribute to the persistence of infections in healthcare settings, leading to increased morbidity and healthcare costs. By investigating how different disinfectant formulations perform against biofilms, this study provides valuable insights that can inform infection control protocols and enhance patient safety. Furthermore, the findings from this research could guide the development of more effective disinfectant strategies tailored to combat biofilm-associated infections, ultimately reducing the incidence of HAIs and improving overall clinical outcomes. Given the rising concern over antibiotic resistance, this study also addresses a critical gap in current literature by exploring alternative approaches to infection prevention, emphasizing the need for rigorous evaluation of disinfectants in real-world healthcare environments.
Materials and Methods
Bacterial isolation
This experimental study used bacterial strains collected from various hospital surfaces and medical equipment, such as neonatal incubators, surgical tools, patient beds, bed corners, urinary catheters, and air conditioning openings in Medical City in Baghdad from 2023 to the end of March 2024. A total of 40 bacterial isolates (13 isolates of Acinetobacter baumannii, 9 isolates of Klebsiella pneumoniae, 13 isolates of Pseudomonas aeruginosa, and 5 isolates of Burkholderia cepacia) were identified using the VITEK 2 system.
Disinfectants
Three different disinfectants were used (Table 1). The selection was based on their documented use in sterilization protocols within the hospitals, from which the bacterial samples were sourced. This approach was undertaken to investigate the etiology of disinfectant resistance and to identify the persistence of bacteria despite the implementation of standard sterilization procedures in these healthcare settings.
Table 1. Disinfectant used in the study

Biofilm formation
Biofilm formation was assessed using the crystal violet assay, following the methods of Hashem et al. [23] and Singh et al. [24] with some modifications. The Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Burkholderia cepacia strains were activated by growing them in double-strength brain heart infusion with 0.5% glucose at 37°C for 24 hours. A sterile flat-bottom 96-well microtiter plate was inoculated with 200 µL of the diluted cultures aseptically (the differences in the three surface disinfectants were measured between isolates according to sub-MIC for each), and each isolate was added in triplicate wells. It is important to mention that the MIC of the three different disinfectants was determined at varying concentrations (Table 2). Additionally, positive and negative controls were prepared.
Table 2. Disinfectant concentration and percentage used in the experiment

The wells were washed with saline three times and left to dry. Methanol was used to fix the adherent cells. Fixed adherent cells were stained with 1% (W/V) crystal violet for 15 minutes, and excess stain was removed by washing (Figure 1). The plates were then left to dry. Crystal violet bound to the adherent cells was re-dissolved in glacial acetic acid. The optical density (OD) was measured at 600nm in a plate reader (Biotek, USA), and the median of three readings was taken. The strength of the biofilm was classified according to the OD readings (Table 3).
Table 3. Biofilm degree of Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa and Burkholderia cepacia isolates


Figure 1. Microtiter plates filled with crystal violet, disinfectants, and different isolates of bacteria.
Data analysis
Data were analyzed by SPSS 20 using the chi-square test to obtain significant differences between the frequency numbers collected regarding the ability of biofilm formation. The results were presented as mean±S.E., and a significant difference was considered at p≤0.05.
Findings
Disinfectant resistance among bacteria, such as Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Burkholderia cepacia in hospital environments is a growing concern [25]. These bacteria can exhibit resistance to disinfectants, like ethanol, DDAC, BAC, and quaternary ammonium propionate, even at varying concentrations.
The effect of the three disinfectants (25%, 50%, and 75%) against the biofilm of 40 Gram-negative bacteria (13 isolates of Acinetobacter baumannii, 9 isolates of Klebsiella pneumoniae, 13 isolates of Pseudomonas aeruginosa, and five isolates of Burkholderia cepacia) was tested, and the results showed that all isolates formed biofilms.
For Acinetobacter baumannii, biofilm formation after treatment with the first disinfectant (ethanol, DDAC) at varying concentrations revealed significant insights into the relationship between disinfectant and biofilm development, notably showing moderate and strong biofilm formation at 25% and 50% concentrations, respectively. All isolates exhibited strong biofilm formation at the highest concentration of 75%.
After treatment with the second disinfectant (quaternary ammonium propionate, chlorhexidine digluconate), there was a significant difference between treatments at 25% and 75% concentrations. Most isolates formed strong biofilms, while fewer formed moderate biofilms, and one isolate formed a weak biofilm at 25%. Additionally, two isolates formed weak biofilms at 75%.
For the third disinfectant (DDAC, benzalkonium chloride), most isolates formed strong biofilms, and one isolate formed a moderate biofilm, indicating a significant difference (Table 4).
Table 4. Biofilm formation of Acinetobacter baumannii after the treatment with disinfectants

When the nine Klebsiella pneumoniae isolates were treated with the mentioned disinfectants, the first sterilizer (ethanol + DDAC) resulted in six isolates forming strong biofilms and three forming moderate biofilms at concentrations of 25% and 50%. In contrast, all the isolates formed strong biofilms when exposed to the first disinfectant at a concentration of 75% and to all concentrations of the third disinfectant (DDAC, BAC). Following treatment with the second disinfectant (quaternary ammonium propionate), the 25% and 50% concentrations induced moderate to strong biofilm formation, whereas the 75% concentration resulted in weak, moderate, and strong biofilm formation across the isolates (Table 5).
Table 5. Biofilm formation of Klebsiella pneumoniae after the treatment with disinfectants

When exposed to disinfectant 1 (ethanol + DDAC) at varying concentrations, the 13 Pseudomonas isolates exhibited moderate to strong biofilm formation. With the second sterilizer (quaternary ammonium propionate), the isolates formed moderate to strong biofilms; however, numerous isolates formed weak biofilms at the 25% and 50% concentrations of the disinfectant. At the 75% concentration, five isolates formed weak biofilms, while six formed strong biofilms.
The majority of the 13 isolates formed strong biofilms, with several forming moderate biofilms at the 25% and 50% concentrations of the third disinfectant (DDAC, BAC). At the 75% concentration, one isolate formed a weak biofilm, while the remaining isolates exhibited moderate to strong biofilms (Table 6).
Table 6. Biofilm formation of Pseudomonas aeruginosa after the treatment with disinfectants

In Burkholderia cepacia, all five isolates formed strong biofilms after treatment with the second disinfectant (quaternary ammonium propionate) and the third disinfectant (DDAC, BAC). Regardless of the concentration used, treatment with the first disinfectant (ethanol + DDAC) resulted in moderate to strong biofilm formation (Table 7).
Table 7. Biofilm formation of Burkholderia cepacia after the treatment with disinfectants

Discussion
Several studies have reported that in high, middle, and low-income healthcare settings, antiseptics, low-level disinfectants, and hand hygiene products can be susceptible to bacterial contamination. Numerous studies assessing the susceptibility of A. baumannii biofilms to disinfectants like benzalkonium chloride and chlorhexidine have shown the ability of both hospital and environmental isolates to form biofilms on surfaces, such as glass and ceramics [26].
Non-fermentative gram-negative bacteria are the predominant contaminants, with Burkholderia cepacia, Pseudomonas aeruginosa, and Achromobacter spp. being the most frequently recognized. These organisms are particularly concerning due to their inherent resistance to multiple antibiotics, which complicates treatment options and increases the risk of severe infections in vulnerable patient populations, such as those in intensive care units. Enterobacterales are also notable contaminants, with Serratia spp. being the most common; these bacteria can lead to a range of healthcare-associated infections, including bloodstream infections and pneumonia. The presence of these pathogens underscores the urgent need for effective infection control measures, as their ability to form biofilms further enhances their persistence in clinical settings and contributes to outbreaks. Moreover, understanding the prevalence and resistance patterns of these contaminants is critical for developing targeted interventions and improving patient safety in healthcare environments [27-29].
In this research, all the isolates were capable of forming biofilms, and most of them had the ability to form strong and moderate biofilms. This finding is consistent with the studies by Mougin et al. [30] and Strempel et al. [31], which demonstrated that the overuse and misuse of disinfectants such as BAC lead to the persistence of opportunistic bacteria like Pseudomonas and stimulate biofilm formation.
This work provides insights into some bacterial adaptation mechanisms and stress responses when exposed to sub-lethal disinfectants. Biofilm formation can be induced by exposure to disinfectants, serving as a protective barrier against antimicrobial agents and leading to increased exopolysaccharide (EPS) production, accelerated cell proliferation, and enhanced adhesion. Bacteria within biofilms are often in a metabolically inactive state, making them less susceptible to both disinfectants and antibiotics [32, 33]. Prior research has indicated that these biofilms exhibit distinct shifts in EPS composition and quantity in response to subinhibitory levels of various disinfectants [34]. Biofilms also facilitate horizontal gene transfer among bacteria, further spreading resistance traits. Additionally, changes in the bacterial cell envelope through various mechanisms, such as increased surface hydrophobicity or changes in surface charge, can reduce permeability to disinfectants, preventing these agents from reaching their targets within the cell. This intrinsic resistance is particularly notable in gram-negative bacteria [35].
Some studies have demonstrated that certain bacterial strains, such as Burkholderia cepacia, exhibit natural resistance to antibiotics, antiseptics, and disinfectants, particularly quaternary ammonium compounds and chlorhexidine, along with biofilm formation [36, 37].
An investigation of surface swab samples from intensive care units (ICUs) revealed that 95.8% of Acinetobacter baumannii isolates produced biofilms; among these, 45.83% exhibited strong biofilm formation, 29.16% showed moderate biofilm formation, and 20.83% demonstrated weak ability for biofilm formation. The capacity of these bacterial isolates, particularly those from bed rails, to form biofilms presents a significant infection risk for hospitalized patients, healthcare workers, and visitors. This is due to the persistent nature of these biofilms, which can harbor and transmit pathogenic microorganisms in hospital environments, thereby complicating infection control measures and increasing the potential for nosocomial infections [3, 38]. Furthermore, the ability of biofilms to resist both antimicrobial agents and host immune responses makes them particularly challenging to eradicate, often leading to prolonged hospital stays and increased healthcare costs. The high prevalence of biofilm-producing Acinetobacter baumannii underscores the necessity for rigorous surface decontamination protocols and routine monitoring to mitigate the risk of infection transmission. Additionally, understanding the biofilm formation dynamics of these pathogens can inform the development of novel therapeutic strategies aimed at disrupting biofilms and enhancing patient safety in critical care settings.
Commonly used disinfectants often exhibit significant limitations when it comes to effectively targeting biofilm-associated bacteria, which presents substantial challenges in healthcare and clinical environmental settings. One major limitation is that biofilms create a protective matrix that shields embedded bacteria from the full effects of disinfectants, significantly reducing their efficacy. Additionally, many disinfectants can be inactivated by organic matter, such as blood and tissue, which frequently accumulate on surfaces in healthcare environments, further compromising their effectiveness. Certain bacterial species, like Pseudomonas aeruginosa and Acinetobacter baumannii, can also develop resistance mechanisms that allow them to survive even in the presence of potent disinfectants. The variability in disinfectant effectiveness against different bacterial strains can lead to inconsistent outcomes, allowing resistant populations to thrive and potentially cause HAIs. Furthermore, the sporadic application and improper dilution of disinfectants can lead to suboptimal conditions for microbial control, resulting in persistent biofilm formation. This situation highlights the urgent necessity for healthcare facilities to implement more comprehensive infection prevention strategies specifically targeting biofilms. Innovative approaches, such as the development of novel antimicrobial agents or the use of combination therapies, should be prioritized to enhance disinfectant effectiveness. Future studies must address the mechanisms of biofilm resilience and the interactions between disinfectants and biofilms to identify more effective interventions. By focusing on these critical areas, researchers can help improve infection control practices, ultimately reducing the incidence of HAIs and safeguarding patient health in clinical settings.
Conclusion
Most bacterial strains exhibit moderate to strong biofilm formation across all treatments.
Acknowledgments: The authors thank Dr. Ridha D.A. for assisting them in collecting the samples, as well as the staff at the Medical City in Baghdad for their support.
Ethical Permissions: Approval was obtained from the hospitals of the Medical City in Baghdad to collect environmental swabs from within the hospital environment and medical equipment (code: 22970-4/7/2024)
Conflicts of Interests: The authors declared no conflicts of interests.
Authors' Contribution: Abbas OT (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (80%); Mohammed AJ (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (20%)
Funding/Support: This research was not funded by any grant.
Keywords:
References
1. Ntelezos K, Karavasili M, Kyriakopoulou G, Delitzakis D, Giannakas AM, Vergadou C. Comparative study of disinfectants' management in hospitals of Greece and Cyprus. Sci Chron. 2020;25(2):358. [Link]
2. Boyce JM. Quaternary ammonium disinfectants and antiseptics: Tolerance, resistance and potential impact on antibiotic resistance. Antimicrob Resist Infect Control. 2023;12(1):32. [Link] [DOI:10.1186/s13756-023-01241-z]
3. Muhammad MH, Idris AL, Fan X, Guo Y, Yu Y, Jin X, et al. Beyond risk: Bacterial biofilms and their regulating approaches. Front Microbiol. 2020;11:928. [Link] [DOI:10.3389/fmicb.2020.00928]
4. Maillard JY, Centeleghe I. How biofilm changes our understanding of cleaning and disinfection. Antimicrob Resist Infect Control. 2023;12(1):95. [Link] [DOI:10.1186/s13756-023-01290-4]
5. Pagedar A, Singh J, Batish VK. Adaptation to benzalkonium chloride and ciprofloxacin affects biofilm formation potential, efflux pump and haemolysin activity of Escherichia coli of dairy origin. J Dairy Res. 2012;79(4):383-9. [Link] [DOI:10.1017/S0022029912000295]
6. Vishwakarma V. Impact of environmental biofilms: Industrial components and its remediation. J Basic Microbiol. 2020;60(3):198-206. [Link] [DOI:10.1002/jobm.201900569]
7. Jiao Y, Tay FR, Niu LN, Chen JH. Advancing antimicrobial strategies for managing oral biofilm infections. International journal of oral science. 2019;11(3):28. [Link] [DOI:10.1038/s41368-019-0062-1]
8. Pires DP, Melo LD, Boas DV, Sillankorva S, Azeredo J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Current opinion in microbiology. 2017;39:48-56. [Link] [DOI:10.1016/j.mib.2017.09.004]
9. Gaikwad UN, Basak S, Kulkarni P, Sande S, Cahavan S, Mudey G, Tankhiwale NS, Fule RP, Gaikwad NR. Educational intervention to foster best infection control practices among nursing staff. Int J Infect. 2018;5(3):e81531. [Link] [DOI:10.5812/iji.81531]
10. O'grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II. Guidelines for the prevention of intravascular catheter-related infections. Clinical infectious diseases. 2011;52(9):e162-93. [Link] [DOI:10.1093/cid/cir257]
11. Finšgar M, Uzunalić AP, Stergar J, Gradišnik L, Maver U. Novel chitosan/diclofenac coatings on medical grade stainless steel for hip replacement applications. Sci Rep. 2016;6:26653. [Link] [DOI:10.1038/srep26653]
12. Toyofuku M, Inaba T, Kiyokawa T, Obana N, Yawata Y, Nomura N. Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem. 2016;80(1):7-12. [Link] [DOI:10.1080/09168451.2015.1058701]
13. Preda VG, Săndulescu O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries. 2019;7(3):e100. [Link] [DOI:10.15190/d.2019.13]
14. Bureš F. Quaternary ammonium compounds: Simple in structure, complex in application. Top Curr Chem. 2019;377(3):14. [Link] [DOI:10.1007/s41061-019-0239-2]
15. Dvorak G. Disinfection 101. Ames: The Center for Food Security and Public Health; 2008. [Link]
16. Mehmood MD, Sabir S, Ghani MU, Ul-Haq HA, Khalid R, Sharif N. An assessment of various disinfectants using the Kirby-Bauer Method with disc diffusion to determine their effectiveness against locally isolated pathogens. J Drug Deliv Ther. 2024;14(6):143-9. [Link] [DOI:10.22270/jddt.v14i6.6612]
17. Pena SA, Salas JG, Gautam N, Ramos AM, Frantz AL. Sublethal exposure to common benzalkonium chloride leads to antimicrobial tolerance and antibiotic cross-resistance in commensal and opportunistic bacterial species. Appl Microbiol. 2023;3(2):580-91. [Link] [DOI:10.3390/applmicrobiol3020041]
18. Yeung W, Ma Y, Liu Y, Pun H, Chua L. Prevalence of alcohol-tolerant and antibiotic-resistant bacterial pathogens on public hand sanitizer dispensers. J Hosp Infect. 2022;127:26-33. [Link] [DOI:10.1016/j.jhin.2022.05.017]
19. Hassanain T, Alyaa R, Ahmad R, Nadia Z, Syakirah H, Nur M. Effectiveness of commonly used antiseptics on bacteria causing nosocomial infections in tertiary hospital in Malaysia. Afr J Microbiol Res. 2019;13(10):188-94. [Link]
20. Denysko TV, Nazarchuk OA, Gruzevskyi O, Bahniuk NÀ, Dmytriiev DV, Chornopyschuk RM, et al. In vitro evaluation of the antimicrobial activity of antiseptics against clinical Acinetobacter baumannii strains isolated from combat wounds. Front Microbiol. 2022;13:932467. [Link] [DOI:10.3389/fmicb.2022.932467]
21. Rozman U, Pušnik M, Kmetec S, Duh D, Šostar Turk S. Reduced susceptibility and increased resistance of bacteria against disinfectants: A systematic review. Microorganisms. 2021;9(12):2550. [Link] [DOI:10.3390/microorganisms9122550]
22. Caselli E, Brusaferro S, Coccagna M, Arnoldo L, Berloco F, Antonioli P, et al. Reducing healthcare-associated infections incidence by a probiotic-based sanitation system: A multicentre, prospective, intervention study. PLoS One. 2018;13(7):e0199616. [Link] [DOI:10.1371/journal.pone.0199616]
23. Hashem YA, Abdelrahman KA, Aziz RK. Phenotype-genotype correlations and distribution of key virulence factors in enterococcus faecalis isolated from patients with urinary tract infections. Infect Drug Resist. 2021;14:1713-23. [Link] [DOI:10.2147/IDR.S305167]
24. Singh AK, Prakash P, Achra A, Singh GP, Das A, Singh RK. Standardization and classification of in vitro biofilm formation by clinical isolates of Staphylococcus aureus. J Glob Infect Dis. 2017;9(3):93-101. [Link] [DOI:10.4103/jgid.jgid_91_16]
25. Biedenbach DJ, Giao PT, Hung Van P, Su Minh Tuyet N, Thi Thanh Nga T, Phuong DM, et al. Antimicrobial-resistant Pseudomonas aeruginosa and acinetobacter baumannii from patients with hospital-acquired or ventilator-associated pneumonia in Vietnam. Clin Ther. 2016;38(9):2098-105. [Link] [DOI:10.1016/j.clinthera.2016.07.172]
26. Elkheloui R, Laktib A, Mimouni R, Aitalla A, Hassi M, Elboulani A, et al. Acinetobacter baumannii Biofilm: Intervening factors, persistence, drug resistance, and strategies of treatment. Mediterr J Infect Microbes Antimicrob. 2020;9(1):7. [Link] [DOI:10.4274/mjima.galenos.2020.2020.7]
27. Lompo P, Heroes AS, Agbobli E, Kühne V, Tinto H, Affolabi D, et al. Bacterial contamination of antiseptics, disinfectants and hand hygiene products in healthcare facilities in high-income countries: A scoping review. Hygiene. 2023;3(2):136-75. [Link] [DOI:10.3390/hygiene3020012]
28. Ogunsola FT, Mehtar S. Challenges regarding the control of environmental sources of contamination in healthcare settings in low-and middle-income countries-A narrative review. Antimicrob Resist Infect Control. 2020;9(1):81. [Link] [DOI:10.1186/s13756-020-00747-0]
29. Lompo P, Heroes AS, Ouédraogo K, Okitale P, Wakpo A, Kalema J, et al. Knowledge, awareness, and risk practices related to bacterial contamination of antiseptics, disinfectants, and hand hygiene products among healthcare workers in sub-saharan Africa: A cross-sectional survey in three tertiary care hospitals (Benin, Burkina Faso, and DR Congo). Antimicrob Resist Infect Control. 2024;13(1):441. [Link] [DOI:10.1186/s13756-024-01396-3]
30. Mougin J, Midelet G, Leterme S, Best G, Ells T, Joyce A, et al. Benzalkonium chloride disinfectant residues stimulate biofilm formation and increase survival of vibrio bacterial pathogens. Front Microbiol. 2024;14:1309032. [Link] [DOI:10.3389/fmicb.2023.1309032]
31. Strempel N, Nusser M, Neidig A, Brenner-Weiss G, Overhage J. The oxidative stress agent hypochlorite stimulates c-di-GMP synthesis and biofilm formation in pseudomonas aeruginosa. Front Microbiol. 2017;8:2311. [Link] [DOI:10.3389/fmicb.2017.02311]
32. Van Dijk HFG, Verbrugh HA, Ad Hoc Advisory Committee on Disinfectants of the Health Council of the Netherlands. Resisting disinfectants. Commun Med. 2022;2:6. [Link] [DOI:10.1038/s43856-021-00070-8]
33. Rakshit P, Singh A, Singh R, Banerjee T. An in-depth study on survival mechanism of bacterial isolates in disinfectants within the hospital environment. Front Cell Infect Microbiol. 2024;14:1442914. [Link] [DOI:10.3389/fcimb.2024.1442914]
34. Dynes JJ, Lawrence JR, Korber DR, Swerhone GD, Leppard GG, Hitchcock AP. Morphological and biochemical changes in pseudomonas fluorescens biofilms induced by sub-inhibitory exposure to antimicrobial agents. Can J Microbiol. 2009;55(2):163-78. [Link] [DOI:10.1139/W08-109]
35. Russell AD. Bacterial resistance to disinfectants: Present knowledge and future problems. J Hosp Infect. 1999;43(Suppl 1):S57-68. [Link] [DOI:10.1016/S0195-6701(99)90066-X]
36. Meena S, Bir R, Sood S, Das BK, Kapil A. Emergence of Burkholderia cepacia in ICU setting. Indian J Crit Care Med. 2019;23(9):423-6. [Link] [DOI:10.5005/jp-journals-10071-23237]
37. Tavares M, Kozak M, Balola A, Sá-Correia I. Burkholderia cepacia complex bacteria: A feared contamination risk in water-based pharmaceutical products. Clin Microbiol Rev. 2020;33(3):e00139-19. [Link] [DOI:10.1128/CMR.00139-19]
38. Ababneh Q, Abulaila S, Jaradat Z. Isolation of extensively drug resistant acinetobacter baumannii from environmental surfaces inside intensive care units. Am J Infect Control. 2022;50(2):159-65. [Link] [DOI:10.1016/j.ajic.2021.09.001]










