Volume 16, Issue 4 (2024)
Iran J War Public Health 2024, 16(4): 381-387 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/11/1 | Accepted: 2024/12/5 | Published: 2024/12/15
Received: 2024/11/1 | Accepted: 2024/12/5 | Published: 2024/12/15
How to cite this article
Simanjuntak J, Fitriana E. Association between Microalbuminuria and Vitamin D Levels in Elderly Individuals with Tuberculosis, Diabetes Mellitus, and HIV Comorbidities. Iran J War Public Health 2024; 16 (4) :381-387
URL: http://ijwph.ir/article-1-1540-en.html
URL: http://ijwph.ir/article-1-1540-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
J.P. Simanjuntak *1, E. Fitriana1
1- Medical Laboratory Technology Department, Health Polytechnic of Jambi, Jambi, Indonesia
Full-Text (HTML) (439 Views)
Introduction
Tuberculosis (TB) continues to pose a significant global health challenge. In 2021, around 11 million new TB cases were reported [1], with Indonesia reporting the second-largest number of TB cases after India. The TB case-finding rate in Indonesia has shown significant improvement, reaching 74% of total cases based on the latest report [2]. However, this figure also indicates that many undetected TB cases still require further attention [3].
Along with increasing TB case-finding, new challenges are emerging in the form of hard-to-cure TB cases [4-6]. Previous studies have shown that the number of people with TB who develop various complications continues to increase in many countries, including Indonesia. The two most commonly reported conditions accompanying TB are diabetes mellitus (DM) and HIV infection. These two diseases are not only major comorbidities in TB patients but are also known to contribute significantly to difficulties in TB treatment [7, 8].
Impaired immunity due to diabetes mellitus and HIV infection is a key factor that complicates TB treatment in patients with these comorbidities [9, 10]. Vitamin D deficiency is frequently linked to weakened immunity in these conditions, as vitamin D is crucial for regulating inflammation. Decreased blood levels of vitamin D can make TB patients more susceptible to infection and other systemic complications, worsening disease prognosis [11, 12].
In addition, impaired renal function is often reported in TB patients, especially as a result of continued consumption of antituberculosis drugs (OAT). This kidney damage can be detected through laboratory tests, such as measuring microalbumin levels in urine. Microalbuminuria, assessed by the urinary albumin to creatinine ratio (UACR), is an important indicator in detecting the early progression of nephropathy. This ratio is even better than blood creatinine measurements in predicting kidney damage.
Patients with uncontrolled diabetes mellitus have a high risk of nephropathy, while patients with HIV infection face the risk of extensive systemic complications. If these complications occur in TB patients, the risk of disease progression is greater and may increase mortality, as reported in previous studies. This emphasizes the need for special attention to TB patients with comorbid diabetes mellitus (TB-DM) and HIV infection (TB-HIV) [13, 14].
This study presents a more comprehensive approach compared to previous studies, which have generally focused on only one type of TB complication, such as the association of TB with diabetes mellitus (TB-DM) or TB with HIV infection (TB-HIV) [15, 16]. In this study, attention was paid to both categories of complications simultaneously to understand their differential impact on immune function, vitamin D levels, and risk of nephropathy. This combined focus provides a new, more holistic perspective on complications in TB patients [13, 17, 18].
Furthermore, this study comprehensively analyzes vitamin D's role in treating TB. While earlier research has established a connection between vitamin D deficiency and TB infection, this study advances the understanding by examining how vitamin D levels fluctuate during TB treatment, especially in patients with comorbid conditions. Furthermore, this study investigates the potential of intervening vitamin D intake as a preventative measure against the development of nephropathy, thus offering an applicable solution for managing patients [11, 12].
Furthermore, this study used a more specific approach to detecting the risk of nephropathy by measuring the urinary albumin-to-creatinine ratio (UACR). This method is considered more sensitive and accurate in detecting impaired kidney function early compared to blood creatinine measurements, which are often used in previous studies. With this method, this study is expected to provide better insight into renal complications in TB patients while supporting early detection and treatment efforts.
The goal of this study was to emphasize the significance of ensuring adequate vitamin D levels in TB patients who also had diabetes mellitus and HIV infection.
Instrument and Methods
Design & sampling
This cross-sectional study was conducted in health centers in Jambi City, Indonesia, from April to August 2024. Sixty TB patients who were elderly and had been medically diagnosed as active TB patients with or without DM or HIV complications and had complete data regarding vitamin D levels and microalbuminuria were selected based on the reports from several selected health centers that recorded the highest number of TB patients in the region. All patients were categorized into three groups: 30 TB patients without complications (DM or HIV), 25 TB patients with DM complications (TB-DM), and 5 TB patients diagnosed with HIV infection (TB-HIV). Patients with other chronic diseases that could affect the study results (such as severe chronic kidney disease), patients who could not continue treatment, and patients with incomplete data were excluded.
The sample size was calculated using the Slovin formula, with a margin of error set at 𝑑=0.05, ensuring that the sample accurately reflects the population and provides reliable results at the specified confidence level. The sampling technique used was accidental sampling, wherein individuals who happened to meet the researcher were selected as participants if deemed appropriate and suitable data sources.
Data collection
The vitamin D levels and microalbuminuria tests were conducted at the Health Polytechnic of Jambi Laboratory to ensure the quality and accuracy of the laboratory test results. Laboratory data were gathered by collecting urine and blood samples from each participant. Using a photometric method at specific wavelengths (Beckman Coulter AU480 / AU680 / AU5800; USA), the urine samples were analyzed to determine albumin (g/dL) and creatinine (mg/dL) concentrations. The Urine Albumin-to-Creatinine Ratio (UACR) was calculated by dividing the albumin level by the creatinine level and multiplying by a conversion factor of 100 (mg/g). UACR results were classified into Normal (<30mg/g), moderately elevated (30-300mg/g), and significantly elevated (>300mg/g) categories. Blood samples were also analyzed using the Mini VIDAS immunoassay system to assess vitamin D levels (bioMérieux; France). All laboratory procedures adhered to established standards to ensure the reliability and accuracy of the results.
Procedures
The study began with sampling preparation, where the researcher coordinated with the relevant health center to ensure the availability of patients who met the inclusion criteria. Each participating patient gave written consent after receiving an explanation of the study's purpose, procedures, benefits, and risks. Urine and blood samples were taken from each patient for laboratory testing. Using the photometric technique at specific wavelengths, the urine analysis measured albumin (g/dL) and creatinine (mg/dL) concentrations. Test results were carefully recorded for further analysis. Collected data, such as UACR and vitamin D levels, were used to evaluate the patient's condition based on complication categories: uncomplicated TB, TB with diabetes mellitus (TB-DM), or TB with HIV infection (TB-HIV). Results are validated by re-checking during the data collection process to ensure data accuracy and consistency with standardized procedures.
Data analysis
Data was analyzed using SPSS 23.0 software. An ANOVA test was used to assess differences in the mean values across the three groups for each laboratory test. Additionally, a graph was created to visualize the distribution of tuberculosis patients according to the interpretation of the results from the two tests conducted. The Fisher Exact test was applied to examine differences in proportions based on the occurrence of nephropathy and immune status. Spearman's correlation test was also used to explore the relationship between vitamin D levels and UACR values.
Findings
The gender distribution did not reveal significant differences between the groups, although there were notable variations in the TB-HIV group. The majority of patients without diabetes mellitus (DM) and those with DM fell within the 15-64 age range. All groups were primarily patients undergoing advanced treatment (treatment duration >2 months). Among respondents with DM, those with a disease duration exceeding 5 years represented a higher percentage. In contrast, a greater proportion of TB patients with HIV had been diagnosed in less than 5 years (Table 1).
Table 1. Comparing the distribution of tuberculosis patients (TB; n=30), tuberculosis patients with diabetes mellitus (TB-DM. n=25), and tuberculosis patients with diabetes mellitus and HIV (TB-HIV; n=5) groups according to demographic characteristics
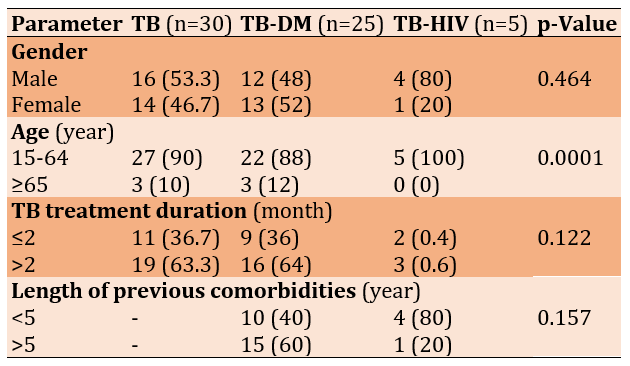
UACR levels were generally higher in TB patients with complications, especially among those with diabetes mellitus (p=0.0067). The TB control group and the TB-DM group had vitamin D levels within the insufficiency range (20-29 ng/mL), while the TB-HIV group displayed deficiency levels (<19 ng/mL). There was a significant difference in vitamin D levels between the groups (p=0.008; Table 2).
Table 2. Comparing the mean of UACR and vitamin D levels between the tuberculosis patients (TB; n=30), tuberculosis patients with diabetes mellitus (TB-DM. n=25), and tuberculosis patients with diabetes mellitus and HIV (TB-HIV; n=5) groups
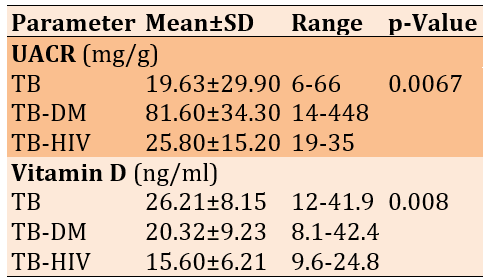
Although severe UACR values were present in the TB-DM group, mild (79.5±51.6mg/g) and normal UACR values (20.2±4.1mg/g) of microalbuminuria occurred more frequently than severe (402.5±64.3mg/g). Mild microalbuminuria was also noted in the TB (49.0±15.1mg/g) and TB-HIV (35.0±9.2mg/g) groups, but most patients in TB (16.4±6.8mg/g), and TB-HIV (23.5±3.9mg/g) groups had normal UACR values (p=0.00016; Figure 1).
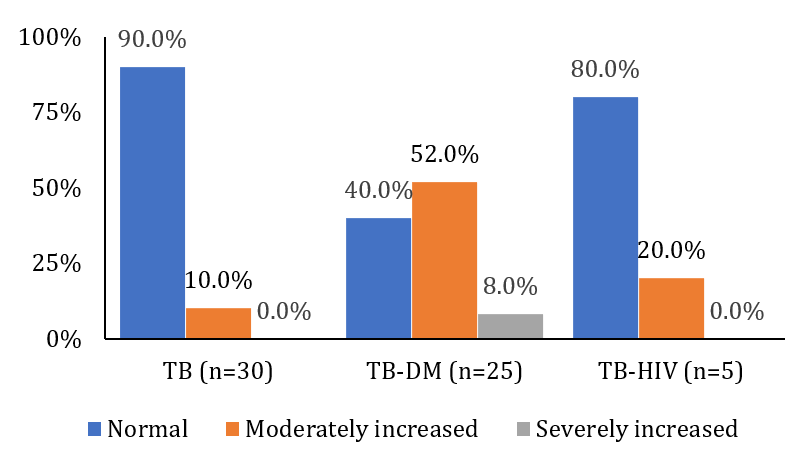
Figure 1. Frequency of urine albumin-creatinine ratio in tuberculosis patients (TB; n=30), tuberculosis patients with diabetes mellitus (TB-DM. n=25), and tuberculosis patients with diabetes mellitus and HIV (TB-HIV; n=5) groups
Normal vitamin D levels were observed in the TB group (35.7±4.5ng/mL) and the TB-DM group (34.0±4.4ng/mL). However, no patients in the TB-HIV group demonstrated normal vitamin D levels.
A significant proportion of the TB-HIV group exhibited vitamin D deficiency (13.3±4.0ng/mL), reflecting the compromised immunity associated with this viral infection. Similarly, a high deficiency prevalence was observed in the TB-DM group (12.8±3.9ng/mL). Vitamin D insufficiency (23.9±2.9ng/mL) rather than deficiency (15.9±2.4ng/mL) was observed in the TB group (p=0.05427; Figure 2).
Figure 2. Frequency of vitamin D in tuberculosis patients (TB; n=30), tuberculosis patients with diabetes mellitus (TB-DM. n=25), and tuberculosis patients with diabetes mellitus and HIV (TB-HIV; n=5) groups
The Flix plot showed a broad spread of data points that did not closely follow the linear trend line (Figure 3), suggesting a weak correlation between the UACR and Vitamin D (r=-0.239). This indicated a weak negative relationship that was not statistically significant (p=0.066).
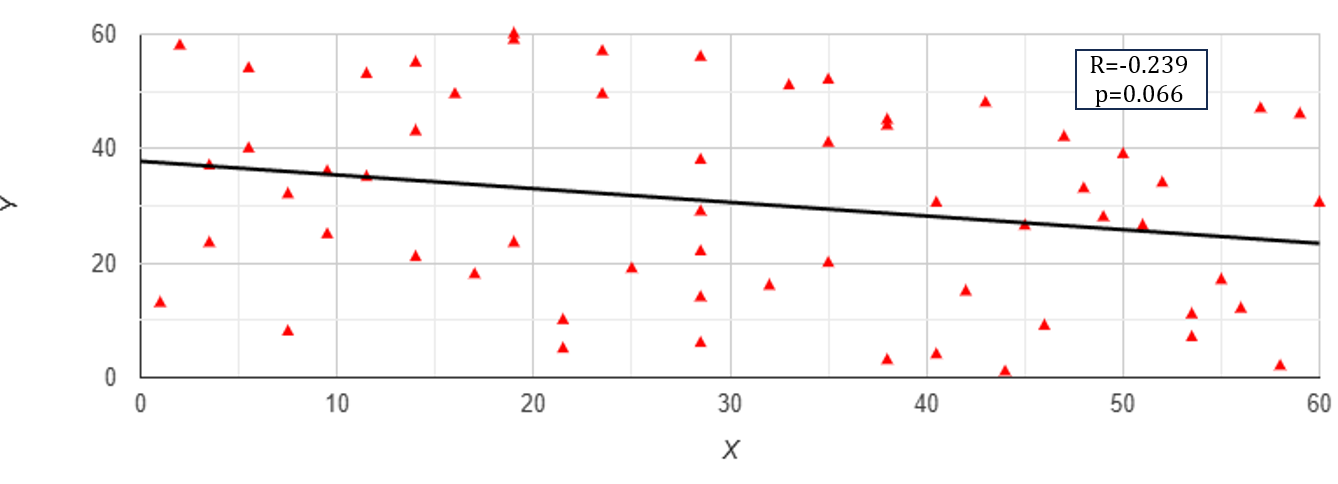
Figure 3. Flix plot of UACR (X; mg/g) and Vitamin D (Y; ng/mL) levels in TB patients
Discussion
The findings revealed that most observed TB cases were among middle-aged individuals, aligning with the trends reported by the Ministry of Health. Despite the relatively high overall treatment success rate across all forms of TB, the incidence of drug-susceptible TB cases has shown an increase [19]. This condition frequently necessitates hospitalization, significantly elevating healthcare costs [20, 21].
Research has shown that individuals infected with HIV are 29 times more likely to contract tuberculosis than those without HIV in the same country. TB remains the primary cause of both hospitalization and death among individuals with HIV, contributing to one in every five HIV-related deaths worldwide. To address this, the integration of HIV and TB services is anticipated to lower the number of deaths caused by HIV-related TB. Government initiatives are working to expand programs that combine the treatment of both conditions in a unified approach [22].
Similarly, diabetes mellitus triples the likelihood of developing tuberculosis. While the connection between diabetes and TB has been recognized for some time, recent studies over the past 10-15 years have emphasized that diabetes heightens the risk of contracting active TB. Additionally, individuals with both conditions tend to experience worse TB treatment outcomes than those with TB alone. To address this, effective strategies are crucial to ensure patients with both diabetes and TB receive the best possible care [23, 24].
As part of this study, all patients diagnosed with tuberculosis were screened for diabetes, while diabetic patients were offered TB screening. The same approach was applied to individuals with HIV-associated TB. The increasing number of diabetes-related TB cases poses a threat to the global progress made in combating TB [25-27]. The presence of both TB-DM and TB-HIV patients in this study reflects the high incidence of these conditions in Indonesia, particularly in Jambi City. The most frequently reported cases involved TB complications in diabetic patients, although some TB patients were later diagnosed with diabetes. Although TB-HIV cases were identified, the researcher faced challenges in gaining consent from patients to participate, leading to a smaller sample of such cases in the study.
Both tuberculosis (TB) and its complications, such as diabetes and HIV, are known to exacerbate immune system decline and complicate patient management. Vitamin D deficiency has been widely recognized as a factor in TB activation, with TB patients typically exhibiting lower serum vitamin D levels than healthy individuals [11, 12]. Additionally, prolonged TB treatment can further deplete vitamin D levels in the body. Research suggests that vitamin D is an immunomodulator, enhancing the body's innate immune response by promoting antimycobacterial activity. Specifically, vitamin D has been shown to inhibit the growth of Mycobacterium tuberculosis (MTB) and strengthen the host's natural defense mechanisms [28, 29]. Vitamin D exerts its effects by binding to the vitamin D receptor (VDR) on monocytes and macrophages, which is essential for MTB replication control and destruction via antigen-presenting cells (APCs). While vitamin D deficiency has long been associated with an increased risk of TB, varying definitions of deficiency exist across studies. For instance, some studies set the threshold for vitamin D deficiency at 50nmol/L, while others use 25nmol/L or even 30nmol/L. This study found lower vitamin D levels, most notably in TB patients with diabetic complications (TB-DM).
HIV infection induces a chronic inflammatory state that involves Th1-like responses, which may be compounded by insufficient local production of 1,25(OH)2D. This deficiency could contribute to the enhanced production of pro-inflammatory cytokines, leading to tissue damage and dysfunction. Restoring normal levels of extracellular 25(OH)D might help reduce persistent inflammation, potentially alleviating complications linked to both HIV and antiretroviral therapy (ART). Additionally, low 25(OH)D levels may influence the severity of infections and cancers frequently observed in individuals with HIV [10, 30].
There is increasing evidence that vitamin D deficiency may play a role in the onset of both type 1 and type 2 diabetes. Insulin-secreting β-cells in the pancreas are found to have both the vitamin D receptor (VDR) and the enzyme 1-alpha hydroxylase. Research suggests that vitamin D supplementation improves glucose tolerance and insulin sensitivity, while its deficiency impairs insulin secretion. Animal studies have shown that vitamin D supplementation can restore insulin secretion [31-33]. Additionally, vitamin D may indirectly influence insulin secretion by regulating calcium levels, essential for proper insulin release. Vitamin D helps maintain normal extracellular calcium levels, ensuring calcium can efficiently affect insulin secretion. Other proposed mechanisms linking vitamin D to diabetes include enhancing insulin action by increasing the expression of insulin receptors, promoting glucose transport, and modulating systemic inflammation through direct effects on cytokines [34-36].
This study revealed a higher incidence of nephropathy among TB patients with diabetes mellitus (DM) complications compared to those with HIV complications. However, the researchers suggest that the relatively small number of TB-HIV patients in the study may have limited the ability to assess the prevalence of nephropathy in this group accurately. Factors such as nutritional status and vitamin D deficiency also impair immunity in patients with renal dysfunction. Huang et al. [37] found that administering 50,000 IU of vitamin D to patients with diabetic nephropathy significantly improved immune function. Despite this, the analysis in this study did not demonstrate a significant correlation between vitamin D levels and UACR values, which are used as markers for nephropathy.
Conclusion
The immune status in the TB group with comorbidities is lower than in the control group, especially in Vitamin D deficiency status. The combination of TB with both comorbidities leads to an increasing incidence of nephropathy, especially in TB-DM patients.
Acknowledgments: With great respect and sincerity, we thank the Director of Jambi Polytechnic for the support and facilities provided during this research process. Without the assistance and direction provided, the implementation of this research would not have run smoothly. We greatly appreciate the contribution of Jambi Polytechnic in supporting the development of science, especially in the health sector.
Ethical Permissions: This study received ethical approval from Health Research Ethics Commission of the Ministry of Health, Jambi, under protocol number LB.02.06/5/62/2023. All procedures adhered to established research ethics standards, ensuring the protection of participants' rights and privacy.
Conflicts of Interests: The authors reported no conflicts of intersts.
Authors' Contribution: Simanjuntak JP (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (70%); Fitriana E (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (30%)
Funding/Support: This research was not funded; no financial support was received from any government, private, or non-profit organizations.
Tuberculosis (TB) continues to pose a significant global health challenge. In 2021, around 11 million new TB cases were reported [1], with Indonesia reporting the second-largest number of TB cases after India. The TB case-finding rate in Indonesia has shown significant improvement, reaching 74% of total cases based on the latest report [2]. However, this figure also indicates that many undetected TB cases still require further attention [3].
Along with increasing TB case-finding, new challenges are emerging in the form of hard-to-cure TB cases [4-6]. Previous studies have shown that the number of people with TB who develop various complications continues to increase in many countries, including Indonesia. The two most commonly reported conditions accompanying TB are diabetes mellitus (DM) and HIV infection. These two diseases are not only major comorbidities in TB patients but are also known to contribute significantly to difficulties in TB treatment [7, 8].
Impaired immunity due to diabetes mellitus and HIV infection is a key factor that complicates TB treatment in patients with these comorbidities [9, 10]. Vitamin D deficiency is frequently linked to weakened immunity in these conditions, as vitamin D is crucial for regulating inflammation. Decreased blood levels of vitamin D can make TB patients more susceptible to infection and other systemic complications, worsening disease prognosis [11, 12].
In addition, impaired renal function is often reported in TB patients, especially as a result of continued consumption of antituberculosis drugs (OAT). This kidney damage can be detected through laboratory tests, such as measuring microalbumin levels in urine. Microalbuminuria, assessed by the urinary albumin to creatinine ratio (UACR), is an important indicator in detecting the early progression of nephropathy. This ratio is even better than blood creatinine measurements in predicting kidney damage.
Patients with uncontrolled diabetes mellitus have a high risk of nephropathy, while patients with HIV infection face the risk of extensive systemic complications. If these complications occur in TB patients, the risk of disease progression is greater and may increase mortality, as reported in previous studies. This emphasizes the need for special attention to TB patients with comorbid diabetes mellitus (TB-DM) and HIV infection (TB-HIV) [13, 14].
This study presents a more comprehensive approach compared to previous studies, which have generally focused on only one type of TB complication, such as the association of TB with diabetes mellitus (TB-DM) or TB with HIV infection (TB-HIV) [15, 16]. In this study, attention was paid to both categories of complications simultaneously to understand their differential impact on immune function, vitamin D levels, and risk of nephropathy. This combined focus provides a new, more holistic perspective on complications in TB patients [13, 17, 18].
Furthermore, this study comprehensively analyzes vitamin D's role in treating TB. While earlier research has established a connection between vitamin D deficiency and TB infection, this study advances the understanding by examining how vitamin D levels fluctuate during TB treatment, especially in patients with comorbid conditions. Furthermore, this study investigates the potential of intervening vitamin D intake as a preventative measure against the development of nephropathy, thus offering an applicable solution for managing patients [11, 12].
Furthermore, this study used a more specific approach to detecting the risk of nephropathy by measuring the urinary albumin-to-creatinine ratio (UACR). This method is considered more sensitive and accurate in detecting impaired kidney function early compared to blood creatinine measurements, which are often used in previous studies. With this method, this study is expected to provide better insight into renal complications in TB patients while supporting early detection and treatment efforts.
The goal of this study was to emphasize the significance of ensuring adequate vitamin D levels in TB patients who also had diabetes mellitus and HIV infection.
Instrument and Methods
Design & sampling
This cross-sectional study was conducted in health centers in Jambi City, Indonesia, from April to August 2024. Sixty TB patients who were elderly and had been medically diagnosed as active TB patients with or without DM or HIV complications and had complete data regarding vitamin D levels and microalbuminuria were selected based on the reports from several selected health centers that recorded the highest number of TB patients in the region. All patients were categorized into three groups: 30 TB patients without complications (DM or HIV), 25 TB patients with DM complications (TB-DM), and 5 TB patients diagnosed with HIV infection (TB-HIV). Patients with other chronic diseases that could affect the study results (such as severe chronic kidney disease), patients who could not continue treatment, and patients with incomplete data were excluded.
The sample size was calculated using the Slovin formula, with a margin of error set at 𝑑=0.05, ensuring that the sample accurately reflects the population and provides reliable results at the specified confidence level. The sampling technique used was accidental sampling, wherein individuals who happened to meet the researcher were selected as participants if deemed appropriate and suitable data sources.
Data collection
The vitamin D levels and microalbuminuria tests were conducted at the Health Polytechnic of Jambi Laboratory to ensure the quality and accuracy of the laboratory test results. Laboratory data were gathered by collecting urine and blood samples from each participant. Using a photometric method at specific wavelengths (Beckman Coulter AU480 / AU680 / AU5800; USA), the urine samples were analyzed to determine albumin (g/dL) and creatinine (mg/dL) concentrations. The Urine Albumin-to-Creatinine Ratio (UACR) was calculated by dividing the albumin level by the creatinine level and multiplying by a conversion factor of 100 (mg/g). UACR results were classified into Normal (<30mg/g), moderately elevated (30-300mg/g), and significantly elevated (>300mg/g) categories. Blood samples were also analyzed using the Mini VIDAS immunoassay system to assess vitamin D levels (bioMérieux; France). All laboratory procedures adhered to established standards to ensure the reliability and accuracy of the results.
Procedures
The study began with sampling preparation, where the researcher coordinated with the relevant health center to ensure the availability of patients who met the inclusion criteria. Each participating patient gave written consent after receiving an explanation of the study's purpose, procedures, benefits, and risks. Urine and blood samples were taken from each patient for laboratory testing. Using the photometric technique at specific wavelengths, the urine analysis measured albumin (g/dL) and creatinine (mg/dL) concentrations. Test results were carefully recorded for further analysis. Collected data, such as UACR and vitamin D levels, were used to evaluate the patient's condition based on complication categories: uncomplicated TB, TB with diabetes mellitus (TB-DM), or TB with HIV infection (TB-HIV). Results are validated by re-checking during the data collection process to ensure data accuracy and consistency with standardized procedures.
Data analysis
Data was analyzed using SPSS 23.0 software. An ANOVA test was used to assess differences in the mean values across the three groups for each laboratory test. Additionally, a graph was created to visualize the distribution of tuberculosis patients according to the interpretation of the results from the two tests conducted. The Fisher Exact test was applied to examine differences in proportions based on the occurrence of nephropathy and immune status. Spearman's correlation test was also used to explore the relationship between vitamin D levels and UACR values.
Findings
The gender distribution did not reveal significant differences between the groups, although there were notable variations in the TB-HIV group. The majority of patients without diabetes mellitus (DM) and those with DM fell within the 15-64 age range. All groups were primarily patients undergoing advanced treatment (treatment duration >2 months). Among respondents with DM, those with a disease duration exceeding 5 years represented a higher percentage. In contrast, a greater proportion of TB patients with HIV had been diagnosed in less than 5 years (Table 1).
Table 1. Comparing the distribution of tuberculosis patients (TB; n=30), tuberculosis patients with diabetes mellitus (TB-DM. n=25), and tuberculosis patients with diabetes mellitus and HIV (TB-HIV; n=5) groups according to demographic characteristics

UACR levels were generally higher in TB patients with complications, especially among those with diabetes mellitus (p=0.0067). The TB control group and the TB-DM group had vitamin D levels within the insufficiency range (20-29 ng/mL), while the TB-HIV group displayed deficiency levels (<19 ng/mL). There was a significant difference in vitamin D levels between the groups (p=0.008; Table 2).
Table 2. Comparing the mean of UACR and vitamin D levels between the tuberculosis patients (TB; n=30), tuberculosis patients with diabetes mellitus (TB-DM. n=25), and tuberculosis patients with diabetes mellitus and HIV (TB-HIV; n=5) groups

Although severe UACR values were present in the TB-DM group, mild (79.5±51.6mg/g) and normal UACR values (20.2±4.1mg/g) of microalbuminuria occurred more frequently than severe (402.5±64.3mg/g). Mild microalbuminuria was also noted in the TB (49.0±15.1mg/g) and TB-HIV (35.0±9.2mg/g) groups, but most patients in TB (16.4±6.8mg/g), and TB-HIV (23.5±3.9mg/g) groups had normal UACR values (p=0.00016; Figure 1).

Figure 1. Frequency of urine albumin-creatinine ratio in tuberculosis patients (TB; n=30), tuberculosis patients with diabetes mellitus (TB-DM. n=25), and tuberculosis patients with diabetes mellitus and HIV (TB-HIV; n=5) groups
Normal vitamin D levels were observed in the TB group (35.7±4.5ng/mL) and the TB-DM group (34.0±4.4ng/mL). However, no patients in the TB-HIV group demonstrated normal vitamin D levels.
A significant proportion of the TB-HIV group exhibited vitamin D deficiency (13.3±4.0ng/mL), reflecting the compromised immunity associated with this viral infection. Similarly, a high deficiency prevalence was observed in the TB-DM group (12.8±3.9ng/mL). Vitamin D insufficiency (23.9±2.9ng/mL) rather than deficiency (15.9±2.4ng/mL) was observed in the TB group (p=0.05427; Figure 2).

Figure 2. Frequency of vitamin D in tuberculosis patients (TB; n=30), tuberculosis patients with diabetes mellitus (TB-DM. n=25), and tuberculosis patients with diabetes mellitus and HIV (TB-HIV; n=5) groups
The Flix plot showed a broad spread of data points that did not closely follow the linear trend line (Figure 3), suggesting a weak correlation between the UACR and Vitamin D (r=-0.239). This indicated a weak negative relationship that was not statistically significant (p=0.066).

Figure 3. Flix plot of UACR (X; mg/g) and Vitamin D (Y; ng/mL) levels in TB patients
Discussion
The findings revealed that most observed TB cases were among middle-aged individuals, aligning with the trends reported by the Ministry of Health. Despite the relatively high overall treatment success rate across all forms of TB, the incidence of drug-susceptible TB cases has shown an increase [19]. This condition frequently necessitates hospitalization, significantly elevating healthcare costs [20, 21].
Research has shown that individuals infected with HIV are 29 times more likely to contract tuberculosis than those without HIV in the same country. TB remains the primary cause of both hospitalization and death among individuals with HIV, contributing to one in every five HIV-related deaths worldwide. To address this, the integration of HIV and TB services is anticipated to lower the number of deaths caused by HIV-related TB. Government initiatives are working to expand programs that combine the treatment of both conditions in a unified approach [22].
Similarly, diabetes mellitus triples the likelihood of developing tuberculosis. While the connection between diabetes and TB has been recognized for some time, recent studies over the past 10-15 years have emphasized that diabetes heightens the risk of contracting active TB. Additionally, individuals with both conditions tend to experience worse TB treatment outcomes than those with TB alone. To address this, effective strategies are crucial to ensure patients with both diabetes and TB receive the best possible care [23, 24].
As part of this study, all patients diagnosed with tuberculosis were screened for diabetes, while diabetic patients were offered TB screening. The same approach was applied to individuals with HIV-associated TB. The increasing number of diabetes-related TB cases poses a threat to the global progress made in combating TB [25-27]. The presence of both TB-DM and TB-HIV patients in this study reflects the high incidence of these conditions in Indonesia, particularly in Jambi City. The most frequently reported cases involved TB complications in diabetic patients, although some TB patients were later diagnosed with diabetes. Although TB-HIV cases were identified, the researcher faced challenges in gaining consent from patients to participate, leading to a smaller sample of such cases in the study.
Both tuberculosis (TB) and its complications, such as diabetes and HIV, are known to exacerbate immune system decline and complicate patient management. Vitamin D deficiency has been widely recognized as a factor in TB activation, with TB patients typically exhibiting lower serum vitamin D levels than healthy individuals [11, 12]. Additionally, prolonged TB treatment can further deplete vitamin D levels in the body. Research suggests that vitamin D is an immunomodulator, enhancing the body's innate immune response by promoting antimycobacterial activity. Specifically, vitamin D has been shown to inhibit the growth of Mycobacterium tuberculosis (MTB) and strengthen the host's natural defense mechanisms [28, 29]. Vitamin D exerts its effects by binding to the vitamin D receptor (VDR) on monocytes and macrophages, which is essential for MTB replication control and destruction via antigen-presenting cells (APCs). While vitamin D deficiency has long been associated with an increased risk of TB, varying definitions of deficiency exist across studies. For instance, some studies set the threshold for vitamin D deficiency at 50nmol/L, while others use 25nmol/L or even 30nmol/L. This study found lower vitamin D levels, most notably in TB patients with diabetic complications (TB-DM).
HIV infection induces a chronic inflammatory state that involves Th1-like responses, which may be compounded by insufficient local production of 1,25(OH)2D. This deficiency could contribute to the enhanced production of pro-inflammatory cytokines, leading to tissue damage and dysfunction. Restoring normal levels of extracellular 25(OH)D might help reduce persistent inflammation, potentially alleviating complications linked to both HIV and antiretroviral therapy (ART). Additionally, low 25(OH)D levels may influence the severity of infections and cancers frequently observed in individuals with HIV [10, 30].
There is increasing evidence that vitamin D deficiency may play a role in the onset of both type 1 and type 2 diabetes. Insulin-secreting β-cells in the pancreas are found to have both the vitamin D receptor (VDR) and the enzyme 1-alpha hydroxylase. Research suggests that vitamin D supplementation improves glucose tolerance and insulin sensitivity, while its deficiency impairs insulin secretion. Animal studies have shown that vitamin D supplementation can restore insulin secretion [31-33]. Additionally, vitamin D may indirectly influence insulin secretion by regulating calcium levels, essential for proper insulin release. Vitamin D helps maintain normal extracellular calcium levels, ensuring calcium can efficiently affect insulin secretion. Other proposed mechanisms linking vitamin D to diabetes include enhancing insulin action by increasing the expression of insulin receptors, promoting glucose transport, and modulating systemic inflammation through direct effects on cytokines [34-36].
This study revealed a higher incidence of nephropathy among TB patients with diabetes mellitus (DM) complications compared to those with HIV complications. However, the researchers suggest that the relatively small number of TB-HIV patients in the study may have limited the ability to assess the prevalence of nephropathy in this group accurately. Factors such as nutritional status and vitamin D deficiency also impair immunity in patients with renal dysfunction. Huang et al. [37] found that administering 50,000 IU of vitamin D to patients with diabetic nephropathy significantly improved immune function. Despite this, the analysis in this study did not demonstrate a significant correlation between vitamin D levels and UACR values, which are used as markers for nephropathy.
Conclusion
The immune status in the TB group with comorbidities is lower than in the control group, especially in Vitamin D deficiency status. The combination of TB with both comorbidities leads to an increasing incidence of nephropathy, especially in TB-DM patients.
Acknowledgments: With great respect and sincerity, we thank the Director of Jambi Polytechnic for the support and facilities provided during this research process. Without the assistance and direction provided, the implementation of this research would not have run smoothly. We greatly appreciate the contribution of Jambi Polytechnic in supporting the development of science, especially in the health sector.
Ethical Permissions: This study received ethical approval from Health Research Ethics Commission of the Ministry of Health, Jambi, under protocol number LB.02.06/5/62/2023. All procedures adhered to established research ethics standards, ensuring the protection of participants' rights and privacy.
Conflicts of Interests: The authors reported no conflicts of intersts.
Authors' Contribution: Simanjuntak JP (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (70%); Fitriana E (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (30%)
Funding/Support: This research was not funded; no financial support was received from any government, private, or non-profit organizations.
Keywords:
References
1. WHO. Tuberculosis [Internet]. Geneva: World Health Organization; 2023 [cited 2023, Mar, 23rd]. Available from: https://www.who.int/indonesia/news/campaign/tb-day-2022/fact-sheets. [Link]
2. Kemenkes RI. Indonesia Health Profile. Jakarta: Kementerian Kesehatan Republik Indonesia; 2023. [Indonesian] [Link]
3. Putra IGNE, Rahmaniati M, Eryando T, Sipahutar T. Modeling the prevalence of tuberculosis in Java, Indonesia: An ecological study using geographically weighted regression. J Popul Soc Stud. 2022;30:741-63. [Link] [DOI:10.25133/JPSSv302022.041]
4. Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893-902. [Link] [DOI:10.1056/NEJMoa1901814]
5. Peloquin CA, Davies GR. The treatment of tuberculosis. Clin Pharmacol Ther. 2021;110(6):1455-66. [Link] [DOI:10.1002/cpt.2261]
6. Koo HK, Min J, Kim HW, Lee J, Kim JS, Park JS, et al. Prediction of treatment failure and compliance in patients with tuberculosis. BMC Infect Dis. 2020;20:622. [Link] [DOI:10.1186/s12879-020-05350-7]
7. Abrar MN, Syafa'Ah I, Mudjanarko SW, Permatasari A. Impact of comorbidity in pulmonary tuberculosis patients with acute respiratory failure: Literature review. Int J Sci Adv. 2024;5(6):1286-93. [Link] [DOI:10.51542/ijscia.v5i6.35]
8. Anasulfalah H, Tamtomo DG, Murti B. Effect of diabetes mellitus comorbidity on mortality risk in tuberculosis patients who received tuberculosis treatment: A meta-analysis. J Epidemiol Public Health. 2022;7(4):441-53. [Link] [DOI:10.26911/jepublichealth.2022.07.04.03]
9. Singh A, Prasad R, Balasubramanian V, Gupta N. Drug-resistant tuberculosis and HIV infection: Current perspectives. HIV AIDS. 2020;12:9-31. [Link] [DOI:10.2147/HIV.S193059]
10. Hamada Y, Getahun H, Tadesse BT, Ford N. HIV-associated tuberculosis. Int J STD AIDS. 2021;32(9):780-90. [Link] [DOI:10.1177/0956462421992257]
11. Ayelign B, Workneh M, Molla MD, Dessie G. Role of vitamin-D supplementation in TB/HIV co-infected patients. Infect Drug Resist. 2020;13:111-8. [Link] [DOI:10.2147/IDR.S228336]
12. Sudfeld CR, Mugusi F, Muhihi A, Aboud S, Nagu TJ, Ulenga N, et al. Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: A randomised, double-blind, placebo-controlled trial. Lancet HIV. 2020;7(7):e463-71. [Link] [DOI:10.1016/S2352-3018(20)30108-9]
13. Cerrone M, Bracchi M, Wasserman S, Pozniak A, Meintjes G, Cohen K, et al. Safety implications of combined antiretroviral and anti-tuberculosis drugs. Expert Opin Drug Saf. 2020;19(1):23-41. [Link] [DOI:10.1080/14740338.2020.1694901]
14. Pooranagangadevi N, Padmapriyadarsini C. Treatment of tuberculosis and the drug interactions associated with HIV-TB co-infection treatment. Front Trop Dis. 2022;3:834013. [Link] [DOI:10.3389/fitd.2022.834013]
15. Krishna S, Jacob JJ. Diabetes mellitus and tuberculosis. In: Endotext. South Dartmouth (MA): MDText.com, Inc; 2021. [Link]
16. Huang D, Wang Y, Wang Y, Liang Z. The impact of diabetes mellitus on drug resistance in patients with newly diagnosed tuberculosis: A systematic review and meta-analysis. Ann Palliat Med. 2020;9(2):152-62. [Link] [DOI:10.21037/apm.2020.02.16]
17. Bisson GP, Bastos M, Campbell JR, Bang D, Brust JC, Isaakidis P, et al. Mortality in adults with multidrug-resistant tuberculosis and HIV by antiretroviral therapy and tuberculosis drug use: An individual patient data meta-analysis. Lancet. 2020;396(10248):402-11. [Link] [DOI:10.1016/S0140-6736(20)31316-7]
18. Cummings C. Cross-sectional design. In: The SAGE Encyclopedia of Communication Research Methods. Thousand Oaks: SAGE Publications; 2017. [Link]
19. Assebe LF, Negussie EK, Jbaily A, Tolla MTT, Johansson KA. Financial burden of HIV and TB among patients in Ethiopia: A cross-sectional survey. BMJ Open. 2020;10(6):e036892. [Link] [DOI:10.1136/bmjopen-2020-036892]
20. Long Q, Jiang W, Dong D, Chen J, Xiang L, Li Q, et al. A new financing model for tuberculosis (TB) care in China: Challenges of policy development and lessons learned from the implementation. Int J Environ Res Public Health. 2020;17(4):1400. [Link] [DOI:10.3390/ijerph17041400]
21. Long Q, Jiang WX, Zhang H, Cheng J, Tang SL, Wang WB. Multi-source financing for tuberculosis treatment in China: Key issues and challenges. Infect Dis Poverty. 2021;10(2):17. [Link] [DOI:10.1186/s40249-021-00809-4]
22. Canetti D, Riccardi N, Martini M, Villa S, Di Biagio A, Codecasa L, et al. HIV and tuberculosis: The paradox of dual illnesses and the challenges of their fighting in the history. Tuberculosis. 2020;122:101921. [Link] [DOI:10.1016/j.tube.2020.101921]
23. Mave V, Gaikwad S, Barthwal M, Chandanwale A, Lokhande R, Kadam D, et al. Diabetes mellitus and tuberculosis treatment outcomes in Pune, India. Open Forum Infect Dis. 2021;8(4):ofab097. [Link] [DOI:10.1093/ofid/ofab097]
24. Gautam S, Shrestha N, Mahato S, Nguyen TPA, Mishra SR, Berg-Beckhoff G. Diabetes among tuberculosis patients and its impact on tuberculosis treatment in South Asia: A systematic review and meta-analysis. Sci Rep. 2021;11(1):2113. [Link] [DOI:10.1038/s41598-021-81057-2]
25. Tulu B, Amsalu E, Zenebe Y, Abebe M, Fetene Y, Agegn M, et al. Diabetes mellitus and HIV infection among active tuberculosis patients in Northwest Ethiopia: Health facility-based cross-sectional study. Trop Med Health. 2021;49(1):68. [Link] [DOI:10.1186/s41182-021-00358-4]
26. Nyirenda JLZ, Wagner D, Ngwira B, Lange B. Bidirectional screening and treatment outcomes of diabetes mellitus (DM) and Tuberculosis (TB) patients in hospitals with measures to integrate care of DM and TB and those without integration measures in Malawi. BMC Infect Dis. 2022;22(1):28. [Link] [DOI:10.1186/s12879-021-07017-3]
27. Dabhi PA, Thangakunam B, Gupta R, James P, Thomas N, Naik D, et al. Screening for prevalence of current TB disease and latent TB infection in type 2 diabetes mellitus patients attending a diabetic clinic in an Indian tertiary care hospital. PLoS One. 2020;15(6):e0233385. [Link] [DOI:10.1371/journal.pone.0233385]
28. Rizwan M, Cheng K, Gang Y, Hou Y, Wang C. Immunomodulatory effects of vitamin D and Zinc on viral infection. Biol Trace Elem Res. 2025;203(1):1-17. [Link] [DOI:10.1007/s12011-024-04139-y]
29. Vaccaro J. Role of vitamin D, folate, and cobalamin deficiency in mycobacterium avium paratuberculosis infection and inflammation [dissertation]. Orlando, FL: University of Central Florida; 2023. [Link]
30. Periyasamy KM, Ranganathan UD, Tripathy SP, Bethunaickan R. Vitamin D-A host directed autophagy mediated therapy for tuberculosis. Mol Immunol. 2020;127:238-44. [Link] [DOI:10.1016/j.molimm.2020.08.007]
31. Singh M, Vaughn C, Sasaninia K, Yeh C, Mehta D, Khieran I, et al. Understanding the relationship between glutathione, TGF-β, and vitamin D in combating Mycobacterium tuberculosis infections. J Clin Med. 2020;9(9):2757. [Link] [DOI:10.3390/jcm9092757]
32. Blanc FX, Badje AD, Bonnet M, Gabillard D, Messou E, Muzoora C, et al. Systematic or test-guided treatment for tuberculosis in HIV-infected adults. N Engl J Med. 2020;382(25):2397-410. [Link] [DOI:10.1056/NEJMoa1910708]
33. Szymczak-Pajor I, Miazek K, Selmi A, Balcerczyk A, Śliwińska A. The action of vitamin D in adipose tissue: Is there the link between vitamin D deficiency and adipose tissue-related metabolic disorders?. Int J Mol Sci. 2022;23(2):956. [Link] [DOI:10.3390/ijms23020956]
34. Contreras-Bolívar V, García-Fontana B, García-Fontana C, Muñoz-Torres M. Mechanisms involved in the relationship between vitamin D and insulin resistance: Impact on clinical practice. Nutrients. 2021;13(10):3491. [Link] [DOI:10.3390/nu13103491]
35. Szymczak-Pajor I, Drzewoski J, Śliwińska A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int J Mol Sci. 2020;21(18):6644. [Link] [DOI:10.3390/ijms21186644]
36. Ehrampoush E, Razzaz JM, Ghaemi A, Shahraki HR, Babaei AE, Osati S, et al. The association of vitamin D levels and insulin resistance. Clin Nutr ESPEN. 2021;42:325-32. [Link] [DOI:10.1016/j.clnesp.2021.01.012]
37. Huang HY, Lin TW, Hong ZX, Lim LM. Vitamin D and diabetic kidney disease. Int J Mol Sci. 2023;24(4):3751. [Link] [DOI:10.3390/ijms24043751]









