Volume 16, Issue 1 (2024)
Iran J War Public Health 2024, 16(1): 91-98 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/03/16 | Accepted: 2024/04/30 | Published: 2024/05/30
Received: 2024/03/16 | Accepted: 2024/04/30 | Published: 2024/05/30
How to cite this article
Aljabiry A, Abdullah M, Almaliky J. Relationship of Colorectal Polyps Emergence with Age and Polyp Histopathology. Iran J War Public Health 2024; 16 (1) :91-98
URL: http://ijwph.ir/article-1-1447-en.html
URL: http://ijwph.ir/article-1-1447-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Karbala Gastroenterology and Hepatology Center, Karbala, Iraq
2- Department of Medicine, Basrah College of Medicine, Basrah Gastroenterology and Hepatology Hospital, Basrah, Iraq
3- Baghdad Gastroenterology and Hepatology Teaching Hospital, Baghdad, Iraq
2- Department of Medicine, Basrah College of Medicine, Basrah Gastroenterology and Hepatology Hospital, Basrah, Iraq
3- Baghdad Gastroenterology and Hepatology Teaching Hospital, Baghdad, Iraq
Full-Text (HTML) (749 Views)
Introduction
Colorectal carcinoma (CRC) is the second-leading cause of cancer-related death in the Western world. It accounts for 8% of all cancer deaths worldwide and is the second most common disease in women and men [1]. Asia and Eastern Europe have experienced the greatest increases in colon cancer incidence [1, 2]. Because more precursor lesions are being detected and removed promptly by colonoscopies, recent cancer data show a declining trend in the incidence of colorectal cancer in the United States [3].
It is well established that adenomas, via the adenoma-carcinoma sequence, are the source of 60-90% of this cancer. Most of the time, this transition happens slowly and can take up to 10-15 years. Endoscopic polyp removal can prevent CRC because of this delayed growth [4]. The adenoma-carcinoma sequence may not be the only mechanism of carcinogenesis in older individuals; Polyps emerging at advanced ages may also have a higher intrinsic propensity for malignant transformation, needing less time for cancer to develop from benign adenomas [5-7]. Because colonic polyps, particularly adenomatous ones, are a risk factor for CRC, they are considered significant [8].
Colonic polyps can be classified into two major groups: Neoplastic (adenomas and carcinomas) and non-neoplastic. Neoplastic polyps can cause ulceration and bleeding, while large polyps rarely obstruct the intestine. A polyp propelled downstream by a peristaltic wave can stretch its blood supply and nerve fibers, resulting in abdominal pain [4]. Screening programs have demonstrated efficacy in reducing the incidence and mortality of CRC, one of the few diseases for which this is true [1]. Repetitive fecal occult blood testing (FOBT) has been shown in randomized controlled studies to reduce CRC mortality by 16%, whereas flexible sigmoidoscopy reduces CRC incidence and mortality by 18% and 28%, respectively. It remains to be established in a randomized study whether full colonoscopy, as opposed to flexible sigmoidoscopy, has a greater potential influence on decreasing the incidence and mortality from colorectal cancer [9]. Colonoscopic polypectomy reduces cancer incidence by 76-90% compared to a general population registry, according to multiple cohort studies and randomized clinical trials [10].
One of the most often carried out medical procedures in the United States is the colonoscopy, which serves as the primary screening test or the follow-up method for all screening strategies [11, 12]. The most frequent neoplasm seen on screening colonoscopies and in diagnostic colonoscopies performed on symptomatic people over 50 is colorectal adenomas. Regarding the likelihood that an adenomatous polyp may proceed to cancer, these lesions can be categorized as low-, moderate, or high-risk [13]. It is deemed advanced when a lesion is larger than 1cm, has a villous component, or has high-grade dysplasia. The occurrence of adenomas and dysplasia is thought to be associated with age, and its frequency rises after reaching the sixth decade of life [14].
This study aimed to estimate the prevalence of colorectal polyps and adenomas and evaluate its relation to patients’ age and polyp histopathology.
Instrument and Materials
This cross-sectional retrospective study was conducted on patients attending Gastroenterology and Hepatology Teaching Hospital, a major tertiary hospital in Iraq (Medical City, Baghdad), who underwent colonoscopy for various indications from January 2020 to April 2022 using a colonoscopic and histopathologic reporting database. G*power statistical software was used in this study to calculate the sample size. All patients underwent colonoscopy for various indications, whose described evidence of polyp/s in their reports was considered for analysis. Those records that had incomplete procedures or were assigned by an endoscopist for re-examination for any reason, as well as those negative for the polyp, were excluded.
The data, including age, sex, symptoms, indications for colonoscopy, polyp types (pedunculated, sessile, or flat), site, size, histopathologic types, and grading of dysplasia, were collected by manually reviewing the files. Polyp sites were classified as proximal (cecum, ascending colon, and transverse colon), distal (descending colon, sigmoid colon, and rectum), and bilateral (proximal and distal). Polyp size was categorized as <1 cm, 1-2 cm, and >2 cm. Polyp size measurement was determined by the endoscopist as compared to open biopsy forceps (≈0.5 cm). The patients with polyp/s were divided into background normal colon (colonoscopic report reveals normal colonic mucosa apart from polyp finding) and background abnormal colon (I. IBD or colitis of any cause; II. CRC, a known case or diagnosed at present colonoscopy; III. Polyposis syndromes of any types) groups. Patients used 4 liters of Polyethylene Glycol in divided doses for bowel preparation on the day before colonoscopy with a clear liquid diet, according to the American Society for Gastrointestinal Endoscopy Guidelines 2009.
Most reviewed reports did not provide a uniform or written score for the quality of colon preparation, so this study did not consider the quality of bowel preparation. Colonoscopy examinations were done under conscious sedation with Phenytoin and/or Diazepam (ASGE guidelines 2008) using EPK-i5000 (Pentax; Japan) and LUCERA CLV 260 (Olympus; Japan) high-image-resolution colonoscopies. They were performed by a board-certified gastroenterologist.
Polyp prevalence was defined as the number of colonoscopies in which one or more polyps had been detected divided by the total number of colonoscopies. Adenoma prevalence (synonymous with adenoma detection rate) was defined as the number of colonoscopies in which one or more adenomas had been detected divided by the total number of colonoscopies. Adenoma of advanced pathology (AAP) was defined as adenoma >1cm or with villoglandular histology or high-grade dysplasia.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The collected data was handled and analyzed by SPSS 23 software. The chi-square test was used for categorical data. All analyses were done with 95% confidence intervals, and p-values less than 0.05 were considered statistically significant.
Findings
The total number of colonoscopies from January 1st, 2020, to March 30th, 2022, was 3,893. After excluding poorly prepared or failed colonoscopies for any reason, 399 patients were found; thus, the number of patients with colonoscopies eligible in this study was 3494. Males represented 1908 (54.6%), while females represented 1586 (45.4%). There were 3003 patients with colonoscopies with no polyp seen (including 820 with IBD or colitis and 141 with CRC) and 491 positive colonoscopies (with polyp). Those with polyps on background abnormal colon (IBD, CRC, and polyposis syndromes) were 93 (18.9%). The other 398 (81.1%) were patients with polyp/s on background normal colon. Of 491 (total no. of patients with polyp), patients with inconclusive histopathology (normal mucosa), and patients with no histopathological report, which totaled 187 (38.1%), were excluded. From 141 (46.4%) adenomatous polyps, there were 103 (73%) with normal and 38 (27%) with abnormal colonic background. Non-adenomatous polyps were 163 (53.6%), 133 (69.3%) with normal and 50 (30.7%) with abnormal colonic background. There were 73 (51.8%) advanced adenomas, compared with 68 (48.2%) non-advanced adenomatous polyps (Figure 1).
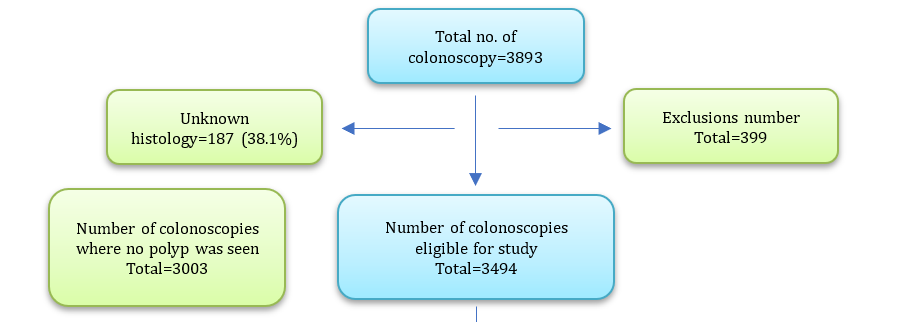
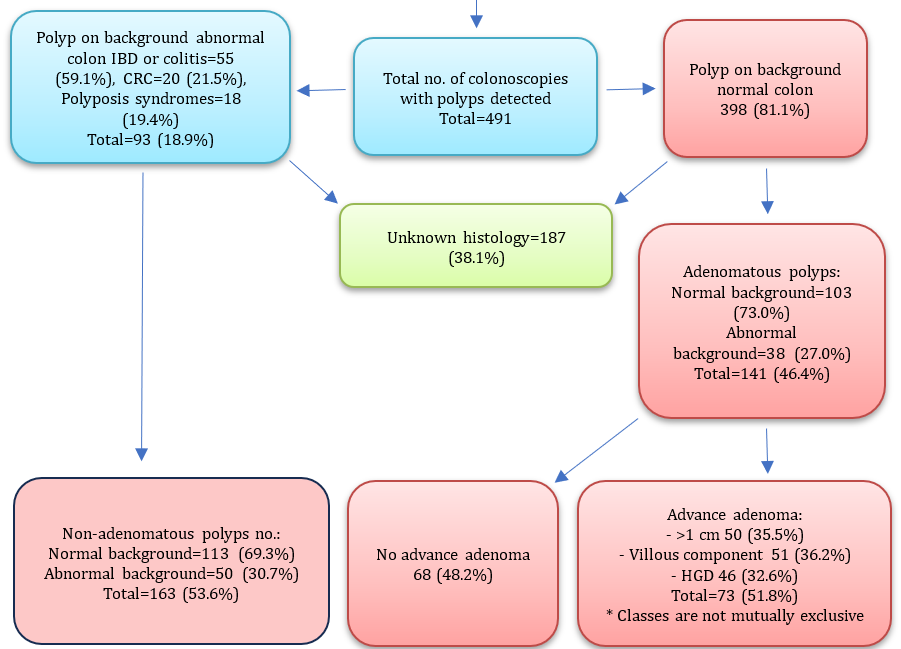
Figure 1. Baseline summary of the study characteristics
The most frequent presenting complaint in patients with polyps was bleeding per rectum (39.5%), followed by unexplained abdominal pain (19.6%), IBD or colitis (11.2%), constipation (6.9%), chronic diarrhea (5.1%), colorectal carcinoma (4.1%), polyposis syndrome (3.7%), altered bowel motion (3.5%), unexplained anemia (2.2%), family history of CA colon screening (2.0%), weight loss (1.0%), treated TB follow-up (0.6%), abdominal distension (0.4%), and colonic GIST on CT scan (0.2%).
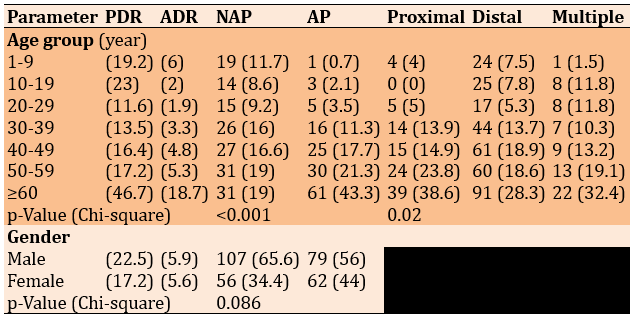
The polyp detection rate was 16.3%, which increased with age, reaching the peak of 46.7% at 60 and above, with a disputing increase in PDR for patients under 20. The polyp detection rate in males (22.5%) was higher than in females (17.2%). The adenoma detection rate was 5.8%, with the highest 18.7% in patients ≥60 years old; in males, ADR was 5.9%, while in females, it was 5.6%. 187 patients (38.1%) with polyps had no defining histopathology. There was a significant relationship between age groups and type of lesion (p<0.001). Still, there was no significant relationship between gender and type of lesion (p=0.086). Also, there was a significant relationship (p=0.02) between age groups and sites of lesions, as younger patients tended to have more distal lesions, some with multiple sites, and less proximal lesions, compared with older patients who had mostly distal, proximal, and multiple lesions (Table 1).
Table 1. Comparing the frequency (numbers in parentheses are percentages) of the patients according to age group and gender according to Polyp detection rate (PDR) and adenoma detection rate (ADR), type of lesion (non-adenomatous polyp-NAP/adenomatous polyp-AP) and location of the lesion (patients with colitis, colorectal carcinoma and polyposis syndrome were excluded)
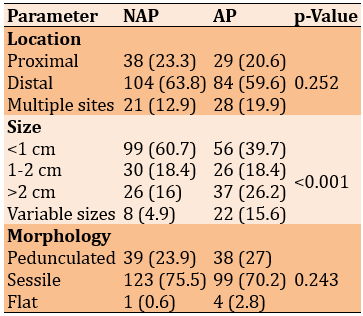
Lesion types had no significant relationship with location (p=0.252) and shape (p=0.243). However, there was a significant statistical relationship between lesion types and corresponding sizes of lesions (p<0.001; Table 2).
Table 2. Comparing the frequency (numbers in parentheses are percentages) of lesion types (non-adenomatous polyp-NAP=163; adenomatous polyp-AP=141) with their corresponding location, size, and morphology (Chi-square test)
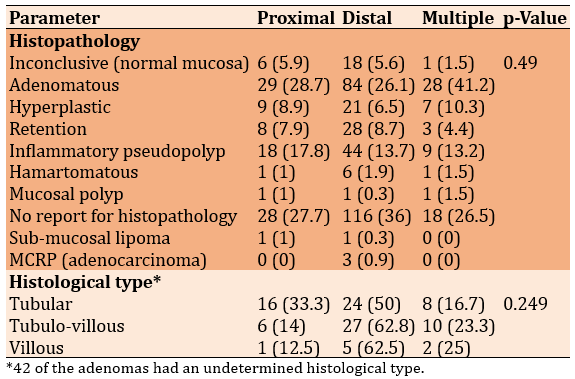
No significant relationship existed between sites and their corresponding histopathology (p=0.49). All types of polyps were found most commonly in the distal colon. Three cases of malignant colorectal polyp MCRP (adenocarcinoma; 0.9%) were also found in the distal colon. Also, there was no significant relationship between types of adenomas and their corresponding sites (p=0.249), as again, all types were commonly seen in the distal colon (Table 3).
Table 3. Comparing the frequency (numbers in parentheses are percentages) of lesion location with their histopathology and histological type (Chi-square test)
The commonest lesions were retention polyps in 1-9 years (55.2%), inflammatory pseudopolyp in 10-19 years (21.2%) and 20-29 years (23.3%), and adenomatous polyps in 30-39 (24.6%), 40-49 (29.4%), 50-59 (30.9%), and over 60 (40.1%) groups.
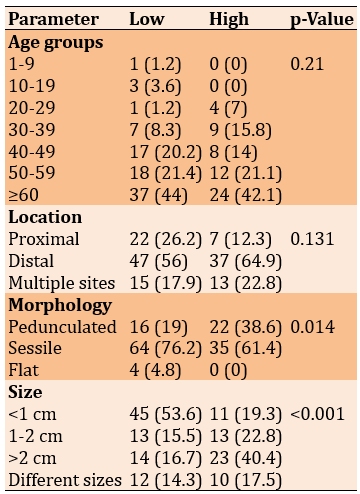
There was no significant relationship between the grade of adenomas and age groups (p=0.249). Also, there was no significant relationship between the grade of adenomas and their corresponding sites (p=0.131). However, the grade of dysplasia had significant relationships with their corresponding shapes (p=0.014) and their corresponding sizes (p<0.001; Table 4).
Table 4. Comparing the frequency (numbers in parentheses are percentages) of the grade of adenomatous polyps’ dysplasia (low=84; high=57) with age, location, morphology, and size of the lesion (Chi-square test)
Discussion
This study aimed to estimate the prevalence of colorectal polyps and adenomas and evaluate its relation to patients’ age and polyp histopathology. Two-thirds of all colon polyps are adenomas, which, by definition, are dysplastic and have the potential for malignant transformation. Nearly all CRCs arise from adenomas, but only a small minority of adenomas will progress to cancer [15].
The outcome of our study showed that PDR was 16.3%, increasing with increasing age, reaching a peak of 46.7% at age 60 and old and slightly more in males (22.5%) than in females (17.2%), which is comparable with Almadi et al. [16], that PDR was 20.8% and 31.8% in patients ≥60 years old. Among the Iranians, a PDR of 16.5% was recorded [17], still much lower in Western countries than in the United States of America; the Mayo Clinic revealed a mean PDR of 49% [18].
Worldwide varies in adenoma prevalence with different populations [19]; in this study, adenoma detection rates were 5.8% highest (18.7%) at age ≥60 years old, with significant association with increasing age and can be regarded as risk factors independently associated with increase adenoma prevalence [19], with no difference between male and female. This is comparable with Almadi et al. [16], that report ADR was 8.1% with not much gender difference, and this was partially consistent with Sohrabi et al. [17], where ADR was 14.3% and the new study in Mainland China by Hong et al. [19], ADR was 13.3% highest at age ≥65 years old (27.3%) and increase with age was similar in both sex; But in another study which report colorectal adenoma (CRA) in all age group combined was more likely to occur in men than in women as shown by Corley et al. [20]. In the Western population, the benchmark ADR is 25% for males and 15% for females in those >50 years old [21]; it’s much higher than what’s reported in our locality.
This discrepancy and low rates might be due to different reasons. PDR is influenced by several factors [22] that are not noted in the database used in our study, e.g., withdrawal time and overall procedure time, the quality of bowel preparation, the quality of the endoscopic devices, and the expert endoscopist. Variability in indications for colonoscopy, asymptomatic (screening) or symptomatic, and high or low risk for CRC in our study low level of screening colonoscopy (2% from all indications). All age group was included, and the bulk of patients included in this study are between ages 30-59 years (64%), in contrast to (13.3%) of those ≥60 years old, as adenoma prevalence increases with age, especially those ≥60 years old [23]. It seems that adenomas are less prevalent in Asian populations compared to Western ones [16].
In our study, more than one-third of 38.1% of patients with polyp detection had unknown histology, which surely affects adenoma prevalence. Older patients ≥50 years had mostly distal as well as proximal and multiple sites of lesions, in contrast to younger age groups, which tend to have more distal lesions and less proximal; this finding is consistent with what was reported by Sohrabi et al. A remarkable portion (54%) of adenomas was detected beyond the rectosigmoid and was not detected by sigmoidoscopy alone [17].
All histopathological types are common, and three MCRPs (all of them) were found in the distal colon. This is the same finding by Hong et al. [19] and comparable with Yamaji et al. [24], who found that the malignant tendency of polyps in old people was generally stronger on the left-side colon than on the right-side colon. However, at the follow-up examinations, the malignant potential of neoplasms on the right-side colon increased substantially in the old to reach the same level as that on the left-side colon [25-42].
Non-adenomatous polyp tends to have small <1 cm size, while most polyps >1 cm are adenomatous; despite adenomas showing higher variability in sizes compared to non-adenomatous polyps, these findings are consistent with Hong et al. [19] finding that patients with polyps >2 cm were higher in patients with adenomatous polyps than patients without and show the proportion of adenomatous polyps was 99.8% in patient groups with ≥1 cm polyps.
The commonest polyps seen in patients <30 years are retention and inflammatory pseudopolyp; in contrast, adenomatous one emerges as the most frequent polyp detected at age ≥30. This is a comparable finding with Almadi et al. [16] and Hong et al. [19].
We report that tubular as well as tubulovillous adenomas (48.4 and 43.4%, respectively) are nearly equally common and more frequent findings than villous type; this is inconsistent with Al-AlKhazraji et al. [43], which showed that tubular type much more common than the tubulovillous type (40% vs. 20%), but our result is similar to what was found in two previous Iraqi studies [44, 45] were tubular and tubulovillous are both common (34.4 and 62.4%, respectively). These findings should be considered and might need to be endorsed by large multicenter studies.
There was a significant association between the grade of dysplasia with morphological type and the size of the polyp, as pedunculated polyps and polyp size ≥1 cm were strongly associated with high-grade dysplasia (p<0.0003). This was consistent with Silva et al. [44] in Brazil, who showed that polyps >1 cm tended to be pedunculated and were more likely to exhibit an adenomatous component, a villous component, and dysplasia and also with Hong et al. [19], showed that the proportion of advanced-stage adenomatous polyps was 100% in patient with ≥1 cm polyp size.
This study recommends proper polyp handling during and after polypectomy and through specimen processing in the histopathological department; multicenter studies are needed to confirm the presence of a high percentage of advanced adenoma and to know more about adenoma characteristics and to estimate the cutoff age eligible for screening in Iraqi population. There is a need to develop a screening program in Iraq with colonoscopy as the first modality used because polyp/s in the proximal colon are common at age ≥50 years.
Conclusion
Increasing age is associated with an increased prevalence of colorectal polyps, especially adenoma, with male predominance. Tubulovillous adenoma and adenoma with advanced pathology are common findings. Over 1cm pedunculated polyps are associated with high-grade dysplasia.
Acknowledgments: The authors are grateful to all patients who agreed to participate in this study and all the medical staff in the endoscopy unit and the histopathology department.
Ethical Permissions: The study was approved by the Ethics Committee of the Iraqi Council of Gastroenterology and Hepatology, Baghdad, Iraq, and by the Ethics Committee of the College of Medicine, University of Basrah, Basrah, Iraq.
Conflicts of Interests: Authors have declared that no competing interests exist.
Authors’ Contributions: Aljabiry AH (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (40%); Abdullah MA (Second Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (30%); Almaliky JM (Third Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (30%)
Funding/Support: This research did not receive a specific grant from any public, commercial, or not-for-profit funding agency.
Colorectal carcinoma (CRC) is the second-leading cause of cancer-related death in the Western world. It accounts for 8% of all cancer deaths worldwide and is the second most common disease in women and men [1]. Asia and Eastern Europe have experienced the greatest increases in colon cancer incidence [1, 2]. Because more precursor lesions are being detected and removed promptly by colonoscopies, recent cancer data show a declining trend in the incidence of colorectal cancer in the United States [3].
It is well established that adenomas, via the adenoma-carcinoma sequence, are the source of 60-90% of this cancer. Most of the time, this transition happens slowly and can take up to 10-15 years. Endoscopic polyp removal can prevent CRC because of this delayed growth [4]. The adenoma-carcinoma sequence may not be the only mechanism of carcinogenesis in older individuals; Polyps emerging at advanced ages may also have a higher intrinsic propensity for malignant transformation, needing less time for cancer to develop from benign adenomas [5-7]. Because colonic polyps, particularly adenomatous ones, are a risk factor for CRC, they are considered significant [8].
Colonic polyps can be classified into two major groups: Neoplastic (adenomas and carcinomas) and non-neoplastic. Neoplastic polyps can cause ulceration and bleeding, while large polyps rarely obstruct the intestine. A polyp propelled downstream by a peristaltic wave can stretch its blood supply and nerve fibers, resulting in abdominal pain [4]. Screening programs have demonstrated efficacy in reducing the incidence and mortality of CRC, one of the few diseases for which this is true [1]. Repetitive fecal occult blood testing (FOBT) has been shown in randomized controlled studies to reduce CRC mortality by 16%, whereas flexible sigmoidoscopy reduces CRC incidence and mortality by 18% and 28%, respectively. It remains to be established in a randomized study whether full colonoscopy, as opposed to flexible sigmoidoscopy, has a greater potential influence on decreasing the incidence and mortality from colorectal cancer [9]. Colonoscopic polypectomy reduces cancer incidence by 76-90% compared to a general population registry, according to multiple cohort studies and randomized clinical trials [10].
One of the most often carried out medical procedures in the United States is the colonoscopy, which serves as the primary screening test or the follow-up method for all screening strategies [11, 12]. The most frequent neoplasm seen on screening colonoscopies and in diagnostic colonoscopies performed on symptomatic people over 50 is colorectal adenomas. Regarding the likelihood that an adenomatous polyp may proceed to cancer, these lesions can be categorized as low-, moderate, or high-risk [13]. It is deemed advanced when a lesion is larger than 1cm, has a villous component, or has high-grade dysplasia. The occurrence of adenomas and dysplasia is thought to be associated with age, and its frequency rises after reaching the sixth decade of life [14].
This study aimed to estimate the prevalence of colorectal polyps and adenomas and evaluate its relation to patients’ age and polyp histopathology.
Instrument and Materials
This cross-sectional retrospective study was conducted on patients attending Gastroenterology and Hepatology Teaching Hospital, a major tertiary hospital in Iraq (Medical City, Baghdad), who underwent colonoscopy for various indications from January 2020 to April 2022 using a colonoscopic and histopathologic reporting database. G*power statistical software was used in this study to calculate the sample size. All patients underwent colonoscopy for various indications, whose described evidence of polyp/s in their reports was considered for analysis. Those records that had incomplete procedures or were assigned by an endoscopist for re-examination for any reason, as well as those negative for the polyp, were excluded.
The data, including age, sex, symptoms, indications for colonoscopy, polyp types (pedunculated, sessile, or flat), site, size, histopathologic types, and grading of dysplasia, were collected by manually reviewing the files. Polyp sites were classified as proximal (cecum, ascending colon, and transverse colon), distal (descending colon, sigmoid colon, and rectum), and bilateral (proximal and distal). Polyp size was categorized as <1 cm, 1-2 cm, and >2 cm. Polyp size measurement was determined by the endoscopist as compared to open biopsy forceps (≈0.5 cm). The patients with polyp/s were divided into background normal colon (colonoscopic report reveals normal colonic mucosa apart from polyp finding) and background abnormal colon (I. IBD or colitis of any cause; II. CRC, a known case or diagnosed at present colonoscopy; III. Polyposis syndromes of any types) groups. Patients used 4 liters of Polyethylene Glycol in divided doses for bowel preparation on the day before colonoscopy with a clear liquid diet, according to the American Society for Gastrointestinal Endoscopy Guidelines 2009.
Most reviewed reports did not provide a uniform or written score for the quality of colon preparation, so this study did not consider the quality of bowel preparation. Colonoscopy examinations were done under conscious sedation with Phenytoin and/or Diazepam (ASGE guidelines 2008) using EPK-i5000 (Pentax; Japan) and LUCERA CLV 260 (Olympus; Japan) high-image-resolution colonoscopies. They were performed by a board-certified gastroenterologist.
Polyp prevalence was defined as the number of colonoscopies in which one or more polyps had been detected divided by the total number of colonoscopies. Adenoma prevalence (synonymous with adenoma detection rate) was defined as the number of colonoscopies in which one or more adenomas had been detected divided by the total number of colonoscopies. Adenoma of advanced pathology (AAP) was defined as adenoma >1cm or with villoglandular histology or high-grade dysplasia.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The collected data was handled and analyzed by SPSS 23 software. The chi-square test was used for categorical data. All analyses were done with 95% confidence intervals, and p-values less than 0.05 were considered statistically significant.
Findings
The total number of colonoscopies from January 1st, 2020, to March 30th, 2022, was 3,893. After excluding poorly prepared or failed colonoscopies for any reason, 399 patients were found; thus, the number of patients with colonoscopies eligible in this study was 3494. Males represented 1908 (54.6%), while females represented 1586 (45.4%). There were 3003 patients with colonoscopies with no polyp seen (including 820 with IBD or colitis and 141 with CRC) and 491 positive colonoscopies (with polyp). Those with polyps on background abnormal colon (IBD, CRC, and polyposis syndromes) were 93 (18.9%). The other 398 (81.1%) were patients with polyp/s on background normal colon. Of 491 (total no. of patients with polyp), patients with inconclusive histopathology (normal mucosa), and patients with no histopathological report, which totaled 187 (38.1%), were excluded. From 141 (46.4%) adenomatous polyps, there were 103 (73%) with normal and 38 (27%) with abnormal colonic background. Non-adenomatous polyps were 163 (53.6%), 133 (69.3%) with normal and 50 (30.7%) with abnormal colonic background. There were 73 (51.8%) advanced adenomas, compared with 68 (48.2%) non-advanced adenomatous polyps (Figure 1).


Figure 1. Baseline summary of the study characteristics
The most frequent presenting complaint in patients with polyps was bleeding per rectum (39.5%), followed by unexplained abdominal pain (19.6%), IBD or colitis (11.2%), constipation (6.9%), chronic diarrhea (5.1%), colorectal carcinoma (4.1%), polyposis syndrome (3.7%), altered bowel motion (3.5%), unexplained anemia (2.2%), family history of CA colon screening (2.0%), weight loss (1.0%), treated TB follow-up (0.6%), abdominal distension (0.4%), and colonic GIST on CT scan (0.2%).
The polyp detection rate was 16.3%, which increased with age, reaching the peak of 46.7% at 60 and above, with a disputing increase in PDR for patients under 20. The polyp detection rate in males (22.5%) was higher than in females (17.2%). The adenoma detection rate was 5.8%, with the highest 18.7% in patients ≥60 years old; in males, ADR was 5.9%, while in females, it was 5.6%. 187 patients (38.1%) with polyps had no defining histopathology. There was a significant relationship between age groups and type of lesion (p<0.001). Still, there was no significant relationship between gender and type of lesion (p=0.086). Also, there was a significant relationship (p=0.02) between age groups and sites of lesions, as younger patients tended to have more distal lesions, some with multiple sites, and less proximal lesions, compared with older patients who had mostly distal, proximal, and multiple lesions (Table 1).
Table 1. Comparing the frequency (numbers in parentheses are percentages) of the patients according to age group and gender according to Polyp detection rate (PDR) and adenoma detection rate (ADR), type of lesion (non-adenomatous polyp-NAP/adenomatous polyp-AP) and location of the lesion (patients with colitis, colorectal carcinoma and polyposis syndrome were excluded)

Lesion types had no significant relationship with location (p=0.252) and shape (p=0.243). However, there was a significant statistical relationship between lesion types and corresponding sizes of lesions (p<0.001; Table 2).
Table 2. Comparing the frequency (numbers in parentheses are percentages) of lesion types (non-adenomatous polyp-NAP=163; adenomatous polyp-AP=141) with their corresponding location, size, and morphology (Chi-square test)

No significant relationship existed between sites and their corresponding histopathology (p=0.49). All types of polyps were found most commonly in the distal colon. Three cases of malignant colorectal polyp MCRP (adenocarcinoma; 0.9%) were also found in the distal colon. Also, there was no significant relationship between types of adenomas and their corresponding sites (p=0.249), as again, all types were commonly seen in the distal colon (Table 3).
Table 3. Comparing the frequency (numbers in parentheses are percentages) of lesion location with their histopathology and histological type (Chi-square test)

The commonest lesions were retention polyps in 1-9 years (55.2%), inflammatory pseudopolyp in 10-19 years (21.2%) and 20-29 years (23.3%), and adenomatous polyps in 30-39 (24.6%), 40-49 (29.4%), 50-59 (30.9%), and over 60 (40.1%) groups.
There was no significant relationship between the grade of adenomas and age groups (p=0.249). Also, there was no significant relationship between the grade of adenomas and their corresponding sites (p=0.131). However, the grade of dysplasia had significant relationships with their corresponding shapes (p=0.014) and their corresponding sizes (p<0.001; Table 4).
Table 4. Comparing the frequency (numbers in parentheses are percentages) of the grade of adenomatous polyps’ dysplasia (low=84; high=57) with age, location, morphology, and size of the lesion (Chi-square test)

Discussion
This study aimed to estimate the prevalence of colorectal polyps and adenomas and evaluate its relation to patients’ age and polyp histopathology. Two-thirds of all colon polyps are adenomas, which, by definition, are dysplastic and have the potential for malignant transformation. Nearly all CRCs arise from adenomas, but only a small minority of adenomas will progress to cancer [15].
The outcome of our study showed that PDR was 16.3%, increasing with increasing age, reaching a peak of 46.7% at age 60 and old and slightly more in males (22.5%) than in females (17.2%), which is comparable with Almadi et al. [16], that PDR was 20.8% and 31.8% in patients ≥60 years old. Among the Iranians, a PDR of 16.5% was recorded [17], still much lower in Western countries than in the United States of America; the Mayo Clinic revealed a mean PDR of 49% [18].
Worldwide varies in adenoma prevalence with different populations [19]; in this study, adenoma detection rates were 5.8% highest (18.7%) at age ≥60 years old, with significant association with increasing age and can be regarded as risk factors independently associated with increase adenoma prevalence [19], with no difference between male and female. This is comparable with Almadi et al. [16], that report ADR was 8.1% with not much gender difference, and this was partially consistent with Sohrabi et al. [17], where ADR was 14.3% and the new study in Mainland China by Hong et al. [19], ADR was 13.3% highest at age ≥65 years old (27.3%) and increase with age was similar in both sex; But in another study which report colorectal adenoma (CRA) in all age group combined was more likely to occur in men than in women as shown by Corley et al. [20]. In the Western population, the benchmark ADR is 25% for males and 15% for females in those >50 years old [21]; it’s much higher than what’s reported in our locality.
This discrepancy and low rates might be due to different reasons. PDR is influenced by several factors [22] that are not noted in the database used in our study, e.g., withdrawal time and overall procedure time, the quality of bowel preparation, the quality of the endoscopic devices, and the expert endoscopist. Variability in indications for colonoscopy, asymptomatic (screening) or symptomatic, and high or low risk for CRC in our study low level of screening colonoscopy (2% from all indications). All age group was included, and the bulk of patients included in this study are between ages 30-59 years (64%), in contrast to (13.3%) of those ≥60 years old, as adenoma prevalence increases with age, especially those ≥60 years old [23]. It seems that adenomas are less prevalent in Asian populations compared to Western ones [16].
In our study, more than one-third of 38.1% of patients with polyp detection had unknown histology, which surely affects adenoma prevalence. Older patients ≥50 years had mostly distal as well as proximal and multiple sites of lesions, in contrast to younger age groups, which tend to have more distal lesions and less proximal; this finding is consistent with what was reported by Sohrabi et al. A remarkable portion (54%) of adenomas was detected beyond the rectosigmoid and was not detected by sigmoidoscopy alone [17].
All histopathological types are common, and three MCRPs (all of them) were found in the distal colon. This is the same finding by Hong et al. [19] and comparable with Yamaji et al. [24], who found that the malignant tendency of polyps in old people was generally stronger on the left-side colon than on the right-side colon. However, at the follow-up examinations, the malignant potential of neoplasms on the right-side colon increased substantially in the old to reach the same level as that on the left-side colon [25-42].
Non-adenomatous polyp tends to have small <1 cm size, while most polyps >1 cm are adenomatous; despite adenomas showing higher variability in sizes compared to non-adenomatous polyps, these findings are consistent with Hong et al. [19] finding that patients with polyps >2 cm were higher in patients with adenomatous polyps than patients without and show the proportion of adenomatous polyps was 99.8% in patient groups with ≥1 cm polyps.
The commonest polyps seen in patients <30 years are retention and inflammatory pseudopolyp; in contrast, adenomatous one emerges as the most frequent polyp detected at age ≥30. This is a comparable finding with Almadi et al. [16] and Hong et al. [19].
We report that tubular as well as tubulovillous adenomas (48.4 and 43.4%, respectively) are nearly equally common and more frequent findings than villous type; this is inconsistent with Al-AlKhazraji et al. [43], which showed that tubular type much more common than the tubulovillous type (40% vs. 20%), but our result is similar to what was found in two previous Iraqi studies [44, 45] were tubular and tubulovillous are both common (34.4 and 62.4%, respectively). These findings should be considered and might need to be endorsed by large multicenter studies.
There was a significant association between the grade of dysplasia with morphological type and the size of the polyp, as pedunculated polyps and polyp size ≥1 cm were strongly associated with high-grade dysplasia (p<0.0003). This was consistent with Silva et al. [44] in Brazil, who showed that polyps >1 cm tended to be pedunculated and were more likely to exhibit an adenomatous component, a villous component, and dysplasia and also with Hong et al. [19], showed that the proportion of advanced-stage adenomatous polyps was 100% in patient with ≥1 cm polyp size.
This study recommends proper polyp handling during and after polypectomy and through specimen processing in the histopathological department; multicenter studies are needed to confirm the presence of a high percentage of advanced adenoma and to know more about adenoma characteristics and to estimate the cutoff age eligible for screening in Iraqi population. There is a need to develop a screening program in Iraq with colonoscopy as the first modality used because polyp/s in the proximal colon are common at age ≥50 years.
Conclusion
Increasing age is associated with an increased prevalence of colorectal polyps, especially adenoma, with male predominance. Tubulovillous adenoma and adenoma with advanced pathology are common findings. Over 1cm pedunculated polyps are associated with high-grade dysplasia.
Acknowledgments: The authors are grateful to all patients who agreed to participate in this study and all the medical staff in the endoscopy unit and the histopathology department.
Ethical Permissions: The study was approved by the Ethics Committee of the Iraqi Council of Gastroenterology and Hepatology, Baghdad, Iraq, and by the Ethics Committee of the College of Medicine, University of Basrah, Basrah, Iraq.
Conflicts of Interests: Authors have declared that no competing interests exist.
Authors’ Contributions: Aljabiry AH (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (40%); Abdullah MA (Second Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (30%); Almaliky JM (Third Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (30%)
Funding/Support: This research did not receive a specific grant from any public, commercial, or not-for-profit funding agency.
Keywords:
References
1. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893-907. [Link] [DOI:10.1158/1055-9965.EPI-10-0437]
2. Yiu HY, Whittemore AS, Shibata A. Increasing colorectal cancer incidence rates in Japan. Int J Cancer. 2004;109(5):777-81. [Link] [DOI:10.1002/ijc.20030]
3. Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975‐2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-73. [Link] [DOI:10.1002/cncr.24760]
4. Garborg K, Holme Ø, Løberg M, Kalager M, Adami HO, Bretthauer M. Current status of screening for colorectal cancer. Ann Oncol. 2013;24(8):1963-72. [Link] [DOI:10.1093/annonc/mdt157]
5. Rostirolla RA, Pereira-Lima JC, Teixeira CR, Schuch AW, Perazzoli C, Saul C. Development of colorectal advanced neoplasia/adenomas in the long-term follow-up of patients submitted to colonoscopy with polipectomy. Arquivos de gastroenterologia. 2009;46(3):167-72. [Portuguese] [Link] [DOI:10.1590/S0004-28032009000300005]
6. Winawer SJ, Zauber AG, Ho MN, O'brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329(27):1977-81. [Link] [DOI:10.1056/NEJM199312303292701]
7. Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi‐Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130-60. [Link] [DOI:10.3322/CA.2007.0018]
8. Zauber AG, Winawer SJ, O'brien MJ, Lansdorp-Vogelaar I, Van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687-96. [Link] [DOI:10.1056/NEJMoa1100370]
9. Kim EC, Lance P. Colorectal polyps and their relationship to cancer. Gastroenterol Clin North Am. 1997;26(1):1-17. [Link] [DOI:10.1016/S0889-8553(05)70280-6]
10. Okamoto M, Shiratori Y, Yamaji Y, Kato J, Ikenoue T, Togo G, et al. Relationship between age and site of colorectal cancer based on colonoscopy findings. Gastrointest Endosc. 2002;55(4):548-51. [Link] [DOI:10.1067/mge.2002.122335]
11. Togo G, Toda N, Kanai F, Kato N, Shiratori Y, Kishi K, et al. A transforming growth factor β type II receptor gene mutation common in sporadic cecum cancer with microsatellite instability. Cancer Res. 1996;56(24):5620-3. [Link]
12. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816-9. [Link] [DOI:10.1126/science.8484122]
13. Bond JH. Clinical evidence for the adenoma-carcinoma sequence, and the management of patients with colorectal adenomas. Semin Gastrointest Dis. 2000;11(4):176-84. [Link]
14. Itzkowitz SH, Potac J. Colonic polyps and polyposis syndromes. In: Sleisenger and fordtran's gastrointestinal and liver disease-2 volume set. Netherlands: Elsevier; 2016. p. 2155-89. [Link] [DOI:10.1016/B978-1-4160-6189-2.00122-0]
15. Hodadoostan MK, Reza F, Elham M, Mohammad Alizade AH, Molaie M, Mashaiekhy R, et al. Clinical and pathology characteristics of colorectal polyps in Iranian population. Asian Pac J Cancer Prev. 2010;11(2):557-60. [Link]
16. Almadi MA, Alharbi O, Azzam N, Wadera J, Sadaf N, Aljebreen AM. Prevalence and characteristics of colonic polyps and adenomas in 2654 colonoscopies in Saudi Arabia. Saudi J Gastroenterol. 2014;20(3):154-61. [Link] [DOI:10.4103/1319-3767.132986]
17. Sohrabi M, Zamani F, Ajdarkosh H, Rakhshani N, Ameli M, Mohamadnejad M, et al. Prevalence of colorectal polyps in a group of subjects at average-risk of colorectal cancer undergoing colonoscopic screening in Tehran, Iran between 2008 and 2013. Asian Pac J Cancer Prev. 2014;15(22):9773-9. [Link] [DOI:10.7314/APJCP.2014.15.22.9773]
18. Boroff ES, Gurudu SR, Hentz JG, Leighton JA, Ramirez FC. Polyp and adenoma detection rates in the proximal and distal colon. Am J Gastroenterol. 2013;108(6):993-9. [Link] [DOI:10.1038/ajg.2013.68]
19. Hong W, Dong L, Stock S, Basharat Z, Zippi M, Zhou M. Prevalence and characteristics of colonic adenoma in mainland China. Cancer Manag Res. 2018;10:2743-55. [Link] [DOI:10.2147/CMAR.S166186]
20. Corley DA, Jensen CD, Marks AR, Zhao WK, De Boer J, Levin TR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: Implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11(2):172-80. [Link] [DOI:10.1016/j.cgh.2012.09.010]
21. Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362(19):1795-803. [Link] [DOI:10.1056/NEJMoa0907667]
22. Francis DL, Rodriguez-Correa DT, Buchner A, Harewood GC, Wallace M. Application of a conversion factor to estimate the adenoma detection rate from the polyp detection rate. Gastrointest Endosc. 2011;73(3):493-7. [Link] [DOI:10.1016/j.gie.2011.01.005]
23. Salmo E, Haboubi N. Adenoma and malignant colorectal polyp: Pathological considerations and clinical applications. EMJ Gastroenterol. 2018;7(1):92-102. [Link] [DOI:10.33590/emjgastroenterol/10313443]
24. Yamaji Y, Mitsushima T, Yoshida H, Watabe H, Okamoto M, Wada R, et al. The malignant potential of freshly developed colorectal polyps according to age. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2418-21. [Link] [DOI:10.1158/1055-9965.EPI-06-0136]
25. Foss FA, West KP, McGregor AH. Pathology of polyps detected in the bowel cancer screening program. Diagn Histopathol. 2011;17(11):495-504. [Link] [DOI:10.1016/j.mpdhp.2011.08.002]
26. Cooper GS, Yuan Z, Landefeld CS, Johanson JF, Rimtn AA. A national population‐based study of incidence of colorectal cancer and age. Implications for screening in older Americans. Cancer. 1995;75(3):775-81.
https://doi.org/10.1002/1097-0142(19950201)75:3<775::AID-CNCR2820750305>3.0.CO;2-D [Link] [DOI:10.1002/1097-0142(19950201)75:33.0.CO;2-D]
27. Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, Von Wagner C. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut. 2012;61(10):1439-46. [Link] [DOI:10.1136/gutjnl-2011-300843]
28. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-57. [Link] [DOI:10.1053/j.gastro.2012.06.001]
29. Weston AP, Campbell DR. Diminutive colonic polyps: Histopathology, spatial distribution, concomitant significant lesions, and treatment complications. Am J Gastroenterol. 1995;90(1):24-8. [Link]
30. Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am J Gastroenterol. 2012;107(9):1315-29. [Link] [DOI:10.1038/ajg.2012.161]
31. Frayling IM, Beck NE, Ilyas M, Dove-Edwin I, Goodman P, Pack K, et al. The APC variants I1307K and E1317Q are associated with colorectal tumors but not always with a family history. Proc Natl Acad Sci USA. 1998;95(18):10722-7. [Link] [DOI:10.1073/pnas.95.18.10722]
32. Rex DK, Lehman GA, Hawes RH, Ulbright TM, Smith JJ. Screening colonoscopy in asymptomatic average-risk persons with negative fecal occult blood tests. Gastroenterology. 1991;100(1):64-7. [Link] [DOI:10.1016/0016-5085(91)90583-7]
33. Foutch PG, DiSario JA, Pardy K, Mai HD, Manne RK. The sentinel hyperplastic polyp: A marker for synchronous neoplasia in the proximal colon. Am J Gastroenterol. 1991;86(10):1482-5. [Link]
34. Nugent KP, Talbot IC, Hodgson SV, Phillips RK. Solitary juvenile polyps: Not a marker for subsequent malignancy. Gastroenterology. 1993;105(3):698-700. [Link] [DOI:10.1016/0016-5085(93)90885-G]
35. Teague RH, Read AE. Polyposis in ulcerative colitis. Gut. 1975;16(10):792-5. [Link] [DOI:10.1136/gut.16.10.792]
36. Berkowitz D, Bernstein LH. Colonic pseudopolyps in association with amebic colitis. Gastroenterology. 1975;68(4):786-9. [Link] [DOI:10.1016/S0016-5085(75)80291-5]
37. Huang CS, O'brien MJ, Yang S, Farraye FA. Hyperplastic polyps, serrated adenomas, and the serrated polyp neoplasia pathway. Am J Gastroenterol. 2004;99(11):2242-55. [Link] [DOI:10.1111/j.1572-0241.2004.40131.x]
38. Kudo S, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68(Suppl 4):S3-47. [Link] [DOI:10.1016/j.gie.2008.07.052]
39. Kubota O, Kino I, Kimura T, Harada Y. Nonpolypoid adenomas and adenocarcinomas found in background mucosa of surgically resected colons. Cancer. 1996;77(4):621-6.
https://doi.org/10.1002/(SICI)1097-0142(19960215)77:4<621::AID-CNCR6>3.0.CO;2-J [Link] [DOI:10.1002/(SICI)1097-0142(19960215)77:43.0.CO;2-J]
40. Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, et al. Flat and depressed colonic neoplasms: A prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355(9211):1211-4. [Link] [DOI:10.1016/S0140-6736(00)02086-9]
41. Saitoh Y, Waxman I, West AB, Popnikolov NK, Gatalica Z, Watari J, et al. Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology. 2001;120(7):1657-65. [Link] [DOI:10.1053/gast.2001.24886]
42. Tsuda S, Veress B, Toth E, Fork FT. Flat and depressed colorectal tumors in a southern Swedish population: A prospective chromoendoscopic and histopathological study. Gut. 2002;51(4):550-5. [Link] [DOI:10.1136/gut.51.4.550]
43. Al-Khazraji K, Hashim M, Abbas W, Dhahir M. Histopathology of polyps and its clinical correlation in a sample of Iraqi patients undergoing colonoscopic examination. Glob J Health Sci. 2021;13(4):106-14. [Link] [DOI:10.5539/gjhs.v13n4p106]
44. Abdulhadi A, Alkhalidi N, Abd-Alhusain S. A clinical study of newly-diagnosed colorectal cancer over 2 years in a gastroenterology center in Iraq. J Coloproctol. 2019;39(3):217-22. [Link] [DOI:10.1016/j.jcol.2019.05.010]
45. Falih Soliman N, Jasim Mohamad B. Clinical and histopathological characteristics of colorectal cancer in Iraq between 2015-2021. Arch Razi Inst. 2022;77(6):2407-13. [Link]
46. Silva SM, Rosa VF, Santos AC, Almeida RM, Oliveira PG, Sousa JB. Influence of patient age and colorectal polyp size on histopathology findings. ARQUIVOS BRASILEIROS DE CIRURGIA DIGESTIVA. 2014;27(2):109-13. [Portuguese] [Link] [DOI:10.1590/S0102-67202014000200006]









