Volume 15, Issue 4 (2023)
Iran J War Public Health 2023, 15(4): 441-446 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/12/19 | Accepted: 2024/01/2 | Published: 2024/02/28
Received: 2023/12/19 | Accepted: 2024/01/2 | Published: 2024/02/28
How to cite this article
AL-Naqeeb A, Bajilan S, Hameedi B, Turki S. Effect of COVID-19 Infection on Kidney Function and Some Related Hormones. Iran J War Public Health 2023; 15 (4) :441-446
URL: http://ijwph.ir/article-1-1425-en.html
URL: http://ijwph.ir/article-1-1425-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Basic Science Department, College of Nursing, University of Baghdad, Baghdad, Iraq
Full-Text (HTML) (380 Views)
Introduction
Over 5 decades, numerous coronaviruses emerged, which led to a wide variety of animal and human diseases. Such viruses are created from genetic substances inside a coating protein that are so small germs that lead to acquainted infectious diseases, i.e., flu, common cold, and warts, or lead to serious illnesses, i.e., Spanish influenza, Ebola, and COVID-19 [1]. Such a virus, which leads to COVID-19, is not the most lethal pandemic in comparison to other viruses. The Coronaviruses that lead to SARS-COV-2 and respiratory syndrome in the Middle East might result in death in a few circumstances. COVID-19 is a newly infectious disease that has extended all the way through the sphere [2]. Although COVID-19 is principally a respiratory disease, the kidney might be among the target infection organs with SARS-COV-2. Kidney involvement records of COVID-19 patients are quite so limited. Nevertheless, there are additional patients who are infected globally [3]. Infection with this virus showed obvious sex-specific severity and mortality greater level of death in males in comparison to females. Differences of sex in their immunity response to the foreign antigen’s distinctions in innate and adaptive immune responses [4].
Many documents have revealed that renal dysfunction is an aggregate clinical COVID-19 propagation indicator. Proteinuria is the most common clinical manifestation that is detected in more than ½ of COVID-19 patients, in addition to hematuria, raised blood urea N, and raised serum creatinine [5]. Numerous endocrine cases of disturbances in relation to all endocrine gland dysfunction after and during infection of SARS-COV-2 were described. Therefore, it has been proposed that infection by SARS-COV-2 might also result in disturbances of the endocrine system. Indeed, angiotensin-converting enzyme 2 (ACE2) receptors that mark the SARS-COV-2 penetration location into cells in large numbers are localized in the endocrine tissues. Therefore, it was proposed that infection with SARS-COV-2 and COVID-19 may result in the development of hormonal disturbances [6].
A study found that physical, emotional, or psychological stresses and pain linked with infections (e.g., COVID-19) stimulate the axis of hypothalamohypophysial, resulting in the release of Antidiuretic hormone (ADH). Otherwise, stress triggers the cortical neurons that encourage the hypothalamus to secrete ADH [7].
ADH or vasopressin or arginine vasopressin (AVP) is a non-peptide made in the hypothalamus. It was documented that it has an essential function in the osmotic body balance control, regulation of BP, Na homeostasis, and functioning of the kidney. Given its essential task in many functions, there is no wonder that ADH has excessive clinical implications. Primarily, ADH affects the kidney's ability to re-absorb H2O. If existing, ADH induces H2O transport protein expression in the collecting duct and late distal tubule to upsurge H2O reabsorption. Numerous states of disease ascend if the body loses ADH secretion control or replies to its attendance [8].
Erythropoietin (EPO) is a produced hormone/cytokine mostly through the kidneys by hypoxia‐inducible factor‐2 as its prime factor of transcription, and over RBC apoptosis of precursors inhibition, upsurges the mass of RBC cells. Nevertheless, EPO has other advantageous cytoprotective effects, i.e., anti‐ischemic, anti-apoptotic, and regenerative effects in a diversity of tissues, i.e., cardiac muscle, lung, nervous system, kidney, pancreas, retina, and endothelial cells. By EPOR‐βcR, as a special receptor; It conducts its defensive influences after trauma and in censoriously ill patients [9].
In COVID-19 patients, responses of disordered inflammation are the cause of end-organ injury. Increased interleukin levels (i.e., IL-6 and IL-1β) are associated independently with severity/mortality of the disease, and therapies targeting IL-6 and IL-1β effects display encouraging results. Studies revealed that the effect of EPO as an immune regulator includes inhibiting IL-6 and IL-1β synthesis by monocytes and prompting regulatory survival of T-cells. Furthermore, growing proof has found global tissue-protective anti-apoptotic EPO effects, particularly in targeted organs by COVID-19. Reliable with such, a report of the current case ascribed amelioration of respiratory distress in an anemic 80-year-old man to the use of EPO [10].
The current work tried to detect the infectious effect of COVID-19 on kidney function and the level of some related hormones.
Instrument and Methods
In this experimental study, some severe patients were admitted to the Ibn Al-Khatib Hospital in Baghdad City, Iraq, from May to October 2021, at the end stage of the pandemic. All positive cases (n=60) were entered into the study, and a sample of 31 healthy individuals was also selected as the control group.
The consultant medical staff carried out a clinical diagnosis and examination for every patient, and the examination results of the biochemical laboratory (serum creatinine, blood urea, serum calcium, and uric acid) were measured as routine work. Serum separated from 5ml of venous blood was obtained from the patients and control groups to assess EPO and Anti-diuretic hormones using an ELISA kit (Elab Science; China).
The Xpert Xpress SARS-COVID-2 kit (Cepheid; USA) was used to detect the disease in throat and nasal swabs. The in vitro test is an automated diagnostic for qualitative nucleic acid detection from SARS-COVID-2. It is performed on a system of gene pert instruments that integrate and automate sample preparation, extraction of nucleic acids, amplification, and target sequence detection in samples utilizing RT-PCR assays in the clinical laboratory to detect the presence of SARS-CoV-2 RNA with AgPath-IDTM One-Step RT-PCR Reagents (ThermoFisher Scientific; USA) in a Bio-Rad CFX 96 qPCR machine with WHO primers and probe (R: ATATTGCAGCAGTACGCACACA; F: ACAGGTACGTTAATAGTTAATAGCGT). Reactions were heated to 50°C for 30 minutes for reverse transcription, denatured at 95°C for 10 minutes, and then 46 cycles of amplification were carried at 95°C for 15 seconds and 55°C for 32 seconds. Fluorescence was measured using the FAM parameters.
The laboratory RT-qPCR procedure was performed according to the procedure for individual samples in the clinical laboratory, on an identical qPCR machine and program, and with reagents donated from the Rambam Health Care Campus. To conserve resources and allow multiple pooling and duplicates of the same sample, each sample was diluted by X0.4 prior to mixing with reagents.
The data were evaluated in SPSS 20 software; as the data was found normal according to the Kolmogrov-Smirnov test, the Independent T-test and Pearson correlation coefficient were used for the analysis. A p-value<0.05 was considered as significant.
Findings
The mean age of the patient group was 35.1±7.5 (20-54 years), and the control group was 37.3±6.8 (22-57 years; p>0.05). There were 31 males and 29 females in the patient group and 17 males and 14 females in the control group.
There were significant differences between the patient and control groups in the total levels of urea (p=0.04), creatinine (p=0.009), uric acid (p=0.009), erythropoietin (p=0.0001), and antidiuretic hormone (p=0.0001) except calcium (p=0.085; Figure 1).
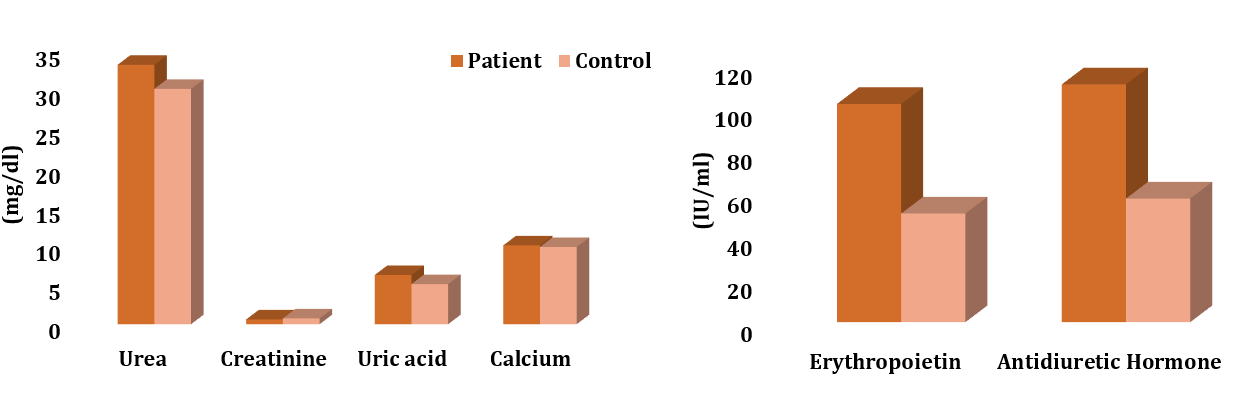
Figure 1. The comparison of the total levels of urea, creatinine, uric acid, calcium, erythropoietin, and antidiuretic hormone between the patient (n=60) and control (n=31) groups
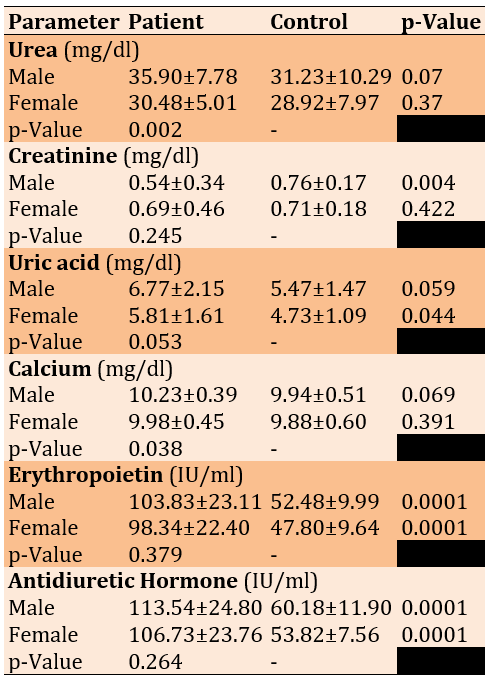
Female patients and controls significantly differed in uric acid levels and the two other hormones. Male patients and controls revealed significant differences in Creatinine and both hormones. Calcium and urea represented a significantly higher elevation in male than female patients (Table 1).
Table 1. Comparing the Renal function biomarker levels between COVID-19 patients (n=60) and control (n=31) according to sex by the independent T-test
There were positive significant correlations between age/creatinine, urea/uric acid, urea/EPO, urea/ADH, uric acid/calcium, and EPO/ADH in the patient group (p<0.05).
Discussion
The kidneys are a fundamental part of the human body's functioning; damage can impair such cycles and disturb human metabolism. The kidney is among the diverse organs significantly afflicted by the infection by SARS-COV-2. Reports have revealed that numerous COVID-19 pneumonia patients have presented multiple kidney injury types, whereas others who passed away from illness of COVID-19 presented austere kidney injury [11].
Regarding Table 2, a significant increase was there in Serum Urea Nitrogen, Creatinine, and Uric acids levels of patients except in Calcium. These findings were consistent with the documents that revealed that kidney dysfunction is a growing clinical COVID-19 propagation indicator. proteinuria is the most common clinical manifestation detected in more than ½ of COVID-19 patients, besides hematuria, raised blood urea N, and elevated serum creatinine [5].
The mechanism involved in the BUN increase level following infection by SARS-COV-2 has not been fully elucidated. Given that ACE2 is the principal cellular SARS-COV-2 receptor and is expressed highly in epithelial renal cells, it is probable that the viral infection might lead directly to a SARS-COV-2 interaction with its receptor in the kidney to decrease ACE2 expression, leading to the abnormal renin-angiotensin-aldosterone system (RAAS) activation [12, 13]. The RAAS, as activated, can significantly increase water absorption by the kidney tubules, enhancing urea resorption and causing elevated levels of BUN [14]. The BUN level elevation is not just a dysfunction of kidney indicator; nevertheless, it also can reflect the status of inflammation, N equilibrium, catabolism, sepsis, and renal hypo-perfusion from hypovolemia or minimized cardiac output, numerous of which have been stated to be associated closely with the opposing outcomes in COVID-19 patients [15, 16].
The high serum creatinine (SCr) levels may be due to the austere cases across the disease course, backing the level of SCr as a danger factor foreseeing mortality in coronavirus-infected [17].
Also, the elevation of serum uric acid (SUA) can be attributed to patients with a history of other diseases, such as type 1 diabetes or smoking, as SUA is the greatest plentiful antioxidant molecule in the plasma, and this result agrees with [18]. High levels of SUA in humans signify an evolutionary benefit that can enhance antioxidant defense and increase levels of SUA, which is related to the upsurge in the capacity of serum antioxidants. UA returns endothelial tasks in patients with T1DM and smokers as regular through the response to antioxidant stress. Consequently, the antioxidant SAU effect might be advantageous in the circumstances considered by OS, even though the molecular mechanisms are not fully understood. SUA is believed to have a defensive effect on both the prime angle-closure glaucoma and the central nervous system against oxidative injury.
Additionally, there were high significant elevations in EPO and ADH hormones serum level in comparison to the control group. Elevation of high significance in the serum ADH level may be associated with fever, pain, stress, and dehydration that the patient may suffer during the infection with COVID-19. This result agrees with [19], who studied Vasopressin System Activation throughout COVID-19. Their findings indicate that the vasopressin system activation plays a key function in the osmotic maintenance, cardiovascular, and stress concerning the serum EPO level increment. This can be attributed to the kidney's attempt to compensate for the lack of oxygen, especially since this hormone is responsible for the RBC production that is responsible for transporting O2 to all body parts. The finding comes along with [20], who found that hypoxia and EPO increased EPO receptors, increased protein levels, and EPOR gene expression in human microvascular endothelial cells (HMVEC-L). Furthermore, EPO dose- and time-dependently stimulated NO production. Such NO stimulation was obvious although hypoxia induced endothelial NO synthase eNOS gene expression reduction.
With respect to gender, all parameters showed a significant elevation between male and female patients in Calcium and urea and a non-significant elevation in the level of uric acid in the male than female group. This is in line with the study of [21], who revealed that rates of infectious are greater in males than in females, and that might be because of sex hormones that contribute to diverse immunologic responses in males and females: As an overall rule, estrogens encourage both adaptive and innate immune responses that resulting in faster pathogens clearance and more vaccine efficacy. Contrariwise, testosterone has mainly suppressive influences on immune function that might elucidate the susceptibility to infectious diseases perceived in males.
A study reported that COVID-19 has tended to be a global health disaster since its 1st advent in Wuhan, China. Epidemiological reports propose that COVID-19 influences elderly people with numerous comorbidities, i.e., obesity, hypertension, and diseases of chronic lung [22]. The variances in the COVID-19 severity and incidence are probable to be multi-faceted and reliant on numerous social, biological, and economic aspects. Precisely, the socio-economic variances and psychological COVID-19 impact affecting females and males are vital in pandemic preparedness and mitigation. Preceding clinical reports have revealed that women are less vulnerable to viral infections and minimized cytokine synthesis [23]. Females have greater neutrophil and macrophage activity besides antibody creation and response [24].
Additionally, in vivo ACE2 studies presented greater expression in the male than female patients’ kidneys, which might clarify the variances in COVID-19 progression and susceptibility between male and female patients. Nevertheless, it remains unidentified if the ACE2 expression varies in female or male patients’ lungs. Differences in the socio-economic status and access to healthcare among ethnic groups might affect the rates of COVID-19 [25]. Ethnic groups frequently have greater medical comorbidities levels and lower socio-economic status, which might elevate their contracting COVID-19 risk by weak immunity as cell-mediated. A study examined the existing literature on the racial differences in gender among patients of COVID-19 and an additional exam for the conceivable biological mechanisms underlying such variances [26]. Such is consistent with our results.
Table 3 shows the correlations between renal function parameters in both groups. It revealed a positive relationship between age and creatinine, and this reflects that age affects kidney function, especially in the case of infection with the Coronavirus, and some routinely measured biochemical parameters, and it is expected that creatinine in the blood is one of them.
The present study is consistent with a study by [17], which exposed that the high serum creatinine (SCr) levels may be due to the austere cases across the disease course. The study backed the level of SCr as a threat factor for death in COVID-19-infected patients.
Also, there was a positive relationship level between urea and uric acid, which indicates that the clearance of uric acid and urea relies upon an operative intravascular volume. In the unsuitable SIADH (a state syndrome augmented intravascular volume), uric acid clearance is elevated, and the area is elevated just if the excretion of salt is low [27].
Finally, uric acid had a positive correlation with calcium. Perhaps the reason is that corona patients suffer from inflammation in the kidneys and joints, which is linked to the relationship between uric acid and calcium.
The present study agreed with a study by [28], which revealed that the concentration of calcium is correlated positively with inflammation. Few reports presented that hypercalcemia is associated with diseases of inflammation. A few critical inflammatory cytokines, i.e., IL-1β and IL-6, are able to upregulate the receptor of calcium-sensing, which is able to control the homeostasis of blood calcium and is an inflammation responder and promoter. For the moment, TNF-a and IL-6 are necessary cytokines of inflammation, associated positively with levels of SUA. Consequently, if UA crystallizes in joints, an increased SUA level might result in inflammatory arthritis. Based on the foregoing analysis, we guess that the mechanism of inflammation might affect the +ve association between SUA and total calcium. Generally, additional studies must be directed to find out the association mechanism between SUA and total calcium.
Disturbance diagnostics of an endocrine system according to clinical symptoms must be considered in both patients with COVID-19 and post-COVID-19 syndrome. Supplementary studies are required to correlate the incidence and pathogenesis of ADH in COVID-19. The well‐organized designation of clinical trials along with careful EPO administration consideration in anemic COVID‐19 patients to evaluate its clinical assistance in such a life-threatening patient population group.
Conclusion
Recombinant human erythropoietin weakens distress syndrome of the respiratory system and opposes SARS-COV-2.
Acknowledgments: Nothing declared by the authors.
Ethical Permission: The study was approved by the University of Baghdad Ethical Committee (Code: MOH348909MC).
Conflicts of Interests: There were no conflicts.
Authors’ Contribution: AL-Naqeeb AA (First Author), Introduction Writer/Methodologist (40%); Bajilan SI (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (40%); Hameedi BH (Third Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (10%); Turki SG (Fourth Author), Statistical Analyst (10%)
Funding/Support: This study was funded privately by researchers without external support.
Over 5 decades, numerous coronaviruses emerged, which led to a wide variety of animal and human diseases. Such viruses are created from genetic substances inside a coating protein that are so small germs that lead to acquainted infectious diseases, i.e., flu, common cold, and warts, or lead to serious illnesses, i.e., Spanish influenza, Ebola, and COVID-19 [1]. Such a virus, which leads to COVID-19, is not the most lethal pandemic in comparison to other viruses. The Coronaviruses that lead to SARS-COV-2 and respiratory syndrome in the Middle East might result in death in a few circumstances. COVID-19 is a newly infectious disease that has extended all the way through the sphere [2]. Although COVID-19 is principally a respiratory disease, the kidney might be among the target infection organs with SARS-COV-2. Kidney involvement records of COVID-19 patients are quite so limited. Nevertheless, there are additional patients who are infected globally [3]. Infection with this virus showed obvious sex-specific severity and mortality greater level of death in males in comparison to females. Differences of sex in their immunity response to the foreign antigen’s distinctions in innate and adaptive immune responses [4].
Many documents have revealed that renal dysfunction is an aggregate clinical COVID-19 propagation indicator. Proteinuria is the most common clinical manifestation that is detected in more than ½ of COVID-19 patients, in addition to hematuria, raised blood urea N, and raised serum creatinine [5]. Numerous endocrine cases of disturbances in relation to all endocrine gland dysfunction after and during infection of SARS-COV-2 were described. Therefore, it has been proposed that infection by SARS-COV-2 might also result in disturbances of the endocrine system. Indeed, angiotensin-converting enzyme 2 (ACE2) receptors that mark the SARS-COV-2 penetration location into cells in large numbers are localized in the endocrine tissues. Therefore, it was proposed that infection with SARS-COV-2 and COVID-19 may result in the development of hormonal disturbances [6].
A study found that physical, emotional, or psychological stresses and pain linked with infections (e.g., COVID-19) stimulate the axis of hypothalamohypophysial, resulting in the release of Antidiuretic hormone (ADH). Otherwise, stress triggers the cortical neurons that encourage the hypothalamus to secrete ADH [7].
ADH or vasopressin or arginine vasopressin (AVP) is a non-peptide made in the hypothalamus. It was documented that it has an essential function in the osmotic body balance control, regulation of BP, Na homeostasis, and functioning of the kidney. Given its essential task in many functions, there is no wonder that ADH has excessive clinical implications. Primarily, ADH affects the kidney's ability to re-absorb H2O. If existing, ADH induces H2O transport protein expression in the collecting duct and late distal tubule to upsurge H2O reabsorption. Numerous states of disease ascend if the body loses ADH secretion control or replies to its attendance [8].
Erythropoietin (EPO) is a produced hormone/cytokine mostly through the kidneys by hypoxia‐inducible factor‐2 as its prime factor of transcription, and over RBC apoptosis of precursors inhibition, upsurges the mass of RBC cells. Nevertheless, EPO has other advantageous cytoprotective effects, i.e., anti‐ischemic, anti-apoptotic, and regenerative effects in a diversity of tissues, i.e., cardiac muscle, lung, nervous system, kidney, pancreas, retina, and endothelial cells. By EPOR‐βcR, as a special receptor; It conducts its defensive influences after trauma and in censoriously ill patients [9].
In COVID-19 patients, responses of disordered inflammation are the cause of end-organ injury. Increased interleukin levels (i.e., IL-6 and IL-1β) are associated independently with severity/mortality of the disease, and therapies targeting IL-6 and IL-1β effects display encouraging results. Studies revealed that the effect of EPO as an immune regulator includes inhibiting IL-6 and IL-1β synthesis by monocytes and prompting regulatory survival of T-cells. Furthermore, growing proof has found global tissue-protective anti-apoptotic EPO effects, particularly in targeted organs by COVID-19. Reliable with such, a report of the current case ascribed amelioration of respiratory distress in an anemic 80-year-old man to the use of EPO [10].
The current work tried to detect the infectious effect of COVID-19 on kidney function and the level of some related hormones.
Instrument and Methods
In this experimental study, some severe patients were admitted to the Ibn Al-Khatib Hospital in Baghdad City, Iraq, from May to October 2021, at the end stage of the pandemic. All positive cases (n=60) were entered into the study, and a sample of 31 healthy individuals was also selected as the control group.
The consultant medical staff carried out a clinical diagnosis and examination for every patient, and the examination results of the biochemical laboratory (serum creatinine, blood urea, serum calcium, and uric acid) were measured as routine work. Serum separated from 5ml of venous blood was obtained from the patients and control groups to assess EPO and Anti-diuretic hormones using an ELISA kit (Elab Science; China).
The Xpert Xpress SARS-COVID-2 kit (Cepheid; USA) was used to detect the disease in throat and nasal swabs. The in vitro test is an automated diagnostic for qualitative nucleic acid detection from SARS-COVID-2. It is performed on a system of gene pert instruments that integrate and automate sample preparation, extraction of nucleic acids, amplification, and target sequence detection in samples utilizing RT-PCR assays in the clinical laboratory to detect the presence of SARS-CoV-2 RNA with AgPath-IDTM One-Step RT-PCR Reagents (ThermoFisher Scientific; USA) in a Bio-Rad CFX 96 qPCR machine with WHO primers and probe (R: ATATTGCAGCAGTACGCACACA; F: ACAGGTACGTTAATAGTTAATAGCGT). Reactions were heated to 50°C for 30 minutes for reverse transcription, denatured at 95°C for 10 minutes, and then 46 cycles of amplification were carried at 95°C for 15 seconds and 55°C for 32 seconds. Fluorescence was measured using the FAM parameters.
The laboratory RT-qPCR procedure was performed according to the procedure for individual samples in the clinical laboratory, on an identical qPCR machine and program, and with reagents donated from the Rambam Health Care Campus. To conserve resources and allow multiple pooling and duplicates of the same sample, each sample was diluted by X0.4 prior to mixing with reagents.
The data were evaluated in SPSS 20 software; as the data was found normal according to the Kolmogrov-Smirnov test, the Independent T-test and Pearson correlation coefficient were used for the analysis. A p-value<0.05 was considered as significant.
Findings
The mean age of the patient group was 35.1±7.5 (20-54 years), and the control group was 37.3±6.8 (22-57 years; p>0.05). There were 31 males and 29 females in the patient group and 17 males and 14 females in the control group.
There were significant differences between the patient and control groups in the total levels of urea (p=0.04), creatinine (p=0.009), uric acid (p=0.009), erythropoietin (p=0.0001), and antidiuretic hormone (p=0.0001) except calcium (p=0.085; Figure 1).

Figure 1. The comparison of the total levels of urea, creatinine, uric acid, calcium, erythropoietin, and antidiuretic hormone between the patient (n=60) and control (n=31) groups
Female patients and controls significantly differed in uric acid levels and the two other hormones. Male patients and controls revealed significant differences in Creatinine and both hormones. Calcium and urea represented a significantly higher elevation in male than female patients (Table 1).
Table 1. Comparing the Renal function biomarker levels between COVID-19 patients (n=60) and control (n=31) according to sex by the independent T-test

There were positive significant correlations between age/creatinine, urea/uric acid, urea/EPO, urea/ADH, uric acid/calcium, and EPO/ADH in the patient group (p<0.05).
Discussion
The kidneys are a fundamental part of the human body's functioning; damage can impair such cycles and disturb human metabolism. The kidney is among the diverse organs significantly afflicted by the infection by SARS-COV-2. Reports have revealed that numerous COVID-19 pneumonia patients have presented multiple kidney injury types, whereas others who passed away from illness of COVID-19 presented austere kidney injury [11].
Regarding Table 2, a significant increase was there in Serum Urea Nitrogen, Creatinine, and Uric acids levels of patients except in Calcium. These findings were consistent with the documents that revealed that kidney dysfunction is a growing clinical COVID-19 propagation indicator. proteinuria is the most common clinical manifestation detected in more than ½ of COVID-19 patients, besides hematuria, raised blood urea N, and elevated serum creatinine [5].
The mechanism involved in the BUN increase level following infection by SARS-COV-2 has not been fully elucidated. Given that ACE2 is the principal cellular SARS-COV-2 receptor and is expressed highly in epithelial renal cells, it is probable that the viral infection might lead directly to a SARS-COV-2 interaction with its receptor in the kidney to decrease ACE2 expression, leading to the abnormal renin-angiotensin-aldosterone system (RAAS) activation [12, 13]. The RAAS, as activated, can significantly increase water absorption by the kidney tubules, enhancing urea resorption and causing elevated levels of BUN [14]. The BUN level elevation is not just a dysfunction of kidney indicator; nevertheless, it also can reflect the status of inflammation, N equilibrium, catabolism, sepsis, and renal hypo-perfusion from hypovolemia or minimized cardiac output, numerous of which have been stated to be associated closely with the opposing outcomes in COVID-19 patients [15, 16].
The high serum creatinine (SCr) levels may be due to the austere cases across the disease course, backing the level of SCr as a danger factor foreseeing mortality in coronavirus-infected [17].
Also, the elevation of serum uric acid (SUA) can be attributed to patients with a history of other diseases, such as type 1 diabetes or smoking, as SUA is the greatest plentiful antioxidant molecule in the plasma, and this result agrees with [18]. High levels of SUA in humans signify an evolutionary benefit that can enhance antioxidant defense and increase levels of SUA, which is related to the upsurge in the capacity of serum antioxidants. UA returns endothelial tasks in patients with T1DM and smokers as regular through the response to antioxidant stress. Consequently, the antioxidant SAU effect might be advantageous in the circumstances considered by OS, even though the molecular mechanisms are not fully understood. SUA is believed to have a defensive effect on both the prime angle-closure glaucoma and the central nervous system against oxidative injury.
Additionally, there were high significant elevations in EPO and ADH hormones serum level in comparison to the control group. Elevation of high significance in the serum ADH level may be associated with fever, pain, stress, and dehydration that the patient may suffer during the infection with COVID-19. This result agrees with [19], who studied Vasopressin System Activation throughout COVID-19. Their findings indicate that the vasopressin system activation plays a key function in the osmotic maintenance, cardiovascular, and stress concerning the serum EPO level increment. This can be attributed to the kidney's attempt to compensate for the lack of oxygen, especially since this hormone is responsible for the RBC production that is responsible for transporting O2 to all body parts. The finding comes along with [20], who found that hypoxia and EPO increased EPO receptors, increased protein levels, and EPOR gene expression in human microvascular endothelial cells (HMVEC-L). Furthermore, EPO dose- and time-dependently stimulated NO production. Such NO stimulation was obvious although hypoxia induced endothelial NO synthase eNOS gene expression reduction.
With respect to gender, all parameters showed a significant elevation between male and female patients in Calcium and urea and a non-significant elevation in the level of uric acid in the male than female group. This is in line with the study of [21], who revealed that rates of infectious are greater in males than in females, and that might be because of sex hormones that contribute to diverse immunologic responses in males and females: As an overall rule, estrogens encourage both adaptive and innate immune responses that resulting in faster pathogens clearance and more vaccine efficacy. Contrariwise, testosterone has mainly suppressive influences on immune function that might elucidate the susceptibility to infectious diseases perceived in males.
A study reported that COVID-19 has tended to be a global health disaster since its 1st advent in Wuhan, China. Epidemiological reports propose that COVID-19 influences elderly people with numerous comorbidities, i.e., obesity, hypertension, and diseases of chronic lung [22]. The variances in the COVID-19 severity and incidence are probable to be multi-faceted and reliant on numerous social, biological, and economic aspects. Precisely, the socio-economic variances and psychological COVID-19 impact affecting females and males are vital in pandemic preparedness and mitigation. Preceding clinical reports have revealed that women are less vulnerable to viral infections and minimized cytokine synthesis [23]. Females have greater neutrophil and macrophage activity besides antibody creation and response [24].
Additionally, in vivo ACE2 studies presented greater expression in the male than female patients’ kidneys, which might clarify the variances in COVID-19 progression and susceptibility between male and female patients. Nevertheless, it remains unidentified if the ACE2 expression varies in female or male patients’ lungs. Differences in the socio-economic status and access to healthcare among ethnic groups might affect the rates of COVID-19 [25]. Ethnic groups frequently have greater medical comorbidities levels and lower socio-economic status, which might elevate their contracting COVID-19 risk by weak immunity as cell-mediated. A study examined the existing literature on the racial differences in gender among patients of COVID-19 and an additional exam for the conceivable biological mechanisms underlying such variances [26]. Such is consistent with our results.
Table 3 shows the correlations between renal function parameters in both groups. It revealed a positive relationship between age and creatinine, and this reflects that age affects kidney function, especially in the case of infection with the Coronavirus, and some routinely measured biochemical parameters, and it is expected that creatinine in the blood is one of them.
The present study is consistent with a study by [17], which exposed that the high serum creatinine (SCr) levels may be due to the austere cases across the disease course. The study backed the level of SCr as a threat factor for death in COVID-19-infected patients.
Also, there was a positive relationship level between urea and uric acid, which indicates that the clearance of uric acid and urea relies upon an operative intravascular volume. In the unsuitable SIADH (a state syndrome augmented intravascular volume), uric acid clearance is elevated, and the area is elevated just if the excretion of salt is low [27].
Finally, uric acid had a positive correlation with calcium. Perhaps the reason is that corona patients suffer from inflammation in the kidneys and joints, which is linked to the relationship between uric acid and calcium.
The present study agreed with a study by [28], which revealed that the concentration of calcium is correlated positively with inflammation. Few reports presented that hypercalcemia is associated with diseases of inflammation. A few critical inflammatory cytokines, i.e., IL-1β and IL-6, are able to upregulate the receptor of calcium-sensing, which is able to control the homeostasis of blood calcium and is an inflammation responder and promoter. For the moment, TNF-a and IL-6 are necessary cytokines of inflammation, associated positively with levels of SUA. Consequently, if UA crystallizes in joints, an increased SUA level might result in inflammatory arthritis. Based on the foregoing analysis, we guess that the mechanism of inflammation might affect the +ve association between SUA and total calcium. Generally, additional studies must be directed to find out the association mechanism between SUA and total calcium.
Disturbance diagnostics of an endocrine system according to clinical symptoms must be considered in both patients with COVID-19 and post-COVID-19 syndrome. Supplementary studies are required to correlate the incidence and pathogenesis of ADH in COVID-19. The well‐organized designation of clinical trials along with careful EPO administration consideration in anemic COVID‐19 patients to evaluate its clinical assistance in such a life-threatening patient population group.
Conclusion
Recombinant human erythropoietin weakens distress syndrome of the respiratory system and opposes SARS-COV-2.
Acknowledgments: Nothing declared by the authors.
Ethical Permission: The study was approved by the University of Baghdad Ethical Committee (Code: MOH348909MC).
Conflicts of Interests: There were no conflicts.
Authors’ Contribution: AL-Naqeeb AA (First Author), Introduction Writer/Methodologist (40%); Bajilan SI (Second Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (40%); Hameedi BH (Third Author), Introduction Writer/Methodologist/Assistant Researcher/Discussion Writer (10%); Turki SG (Fourth Author), Statistical Analyst (10%)
Funding/Support: This study was funded privately by researchers without external support.
Keywords:
Urea [MeSH], Uric Acid [MeSH], Creatinine [MeSH], Antidiuretic [MeSH], Erythropoietin [MeSH], COV-19 [MeSH]
References
1. Li C, Zhao C, Bao J, Tang B, Wang Y, Gu B. Laboratory diagnosis of coronavirus disease-2019 (COVID-19). Clin Chim Acta. 2020;510:35-46. [Link] [DOI:10.1016/j.cca.2020.06.045]
2. Al-Jumaili MHA. The impact of COVID-19 on Iraqi community: A descriptive study based on data reported from the Ministry of Health in Iraq. J Infect Dev Ctries. 2021;15(9):1244-51. [Link] [DOI:10.3855/jidc.15010]
3. Liakopoulos V, Roumeliotis S, Papachristou S, Papanas N. COVID-19 and the kidney: Time to take a closer look. Int Urol Nephrol. 2022;54(5):1053-7. [Link] [DOI:10.1007/s11255-021-02976-7]
4. Brakas SA, Turki SG, Khalid ZS, Jalil AJ, Hashim AF, Alnaser O, et al. Serum level of IL-1 and IL-6 in Iraqi patients with COVID-19. Biochem Cell Arch. 2022;22(2):3639-43. [Link] [DOI:10.51470/bca.2022.22.2.3639]
5. Faour WH, Choaib A, Issa E, Choueiry FE, Shbaklo K, Alhajj M, et al. Mechanisms of COVID-19-induced kidney injury and current pharmacotherapies. Inflamm Res. 2022;71(1):39-56. [Link] [DOI:10.1007/s00011-021-01520-8]
6. Ogarek N, Obozai P, Olszanecka-Glinianowicz M, Kocelak P. The endocrine system function disturbances during and after SARS-COV-2 infection. Eur Rev Med Pharmacol Sci. 2022;26(6):2171-8. [Link]
7. Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-associated SIADH: A clue in the times of pandemic!. Am J Physiol Endocrinol Metab. 2020;318(6):E882-5. [Link] [DOI:10.1152/ajpendo.00178.2020]
8. Cuzzo B, Padala SA, Lappin SL. Physiology, vasopressin. Treasure Island (FL): StatPearls Publishing; 2023. [Link]
9. Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects?. J Med Virol. 2020;92(7):915-8. [Link] [DOI:10.1002/jmv.25839]
10. Leventhal J, Angeletti A, Cravedi P. EPO in patients with COVID-19: More than an erythropoietic hormone. Am J Kidney Dis. 2020;76(3):441. [Link] [DOI:10.1053/j.ajkd.2020.06.002]
11. Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380-3. [Link] [DOI:10.1681/ASN.2020040419]
12. Soleimani M. Acute kidney injury in SARS-COV-2 infection: Direct effect of virus on kidney proximal tubule cells. Int J Mol Sci. 2020;21(9):3275. [Link] [DOI:10.3390/ijms21093275]
13. Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and functional basis of SARS-COV-2 entry by using human ACE2. Cell. 2020;181(4):894-904. [Link] [DOI:10.1016/j.cell.2020.03.045]
14. Macedo E. Blood urea nitrogen beyond estimation of renal function. Crit Care Med. 2011;39(2):405-6. [Link] [DOI:10.1097/CCM.0b013e318205c33a]
15. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671-81. [Link] [DOI:10.1161/CIRCRESAHA.120.317134]
16. Ye B, Deng H, Zhao H, Liang J, Ke L, Li W. Association between an increase in blood urea nitrogen at 24 h and worse outcomes in COVID-19 pneumonia. Ren Fail. 2021;43(1):347-50. [Link] [DOI:10.21203/rs.3.rs-74258/v1]
17. Li Z, Wu M, Yao J, Guo J, Liao X, Song S, et al. Caution on kidney dysfunctions of COVID-19 patients. MedRxiv. 2020. [Link] [DOI:10.1101/2020.02.08.20021212]
18. Hu F, Guo Y, Lin J, Zeng Y, Wang J, Li M, et al. Association of serum uric acid levels with COVID-19 severity. BMC Endocr Disord. 2021;21:97. [Link] [DOI:10.1186/s12902-021-00745-2]
19. Gregoriano C, Molitor A, Haag E, Kutz A, Koch D, Haubitz S, et al. Activation of vasopressin system during COVID-19 is associated with adverse clinical outcomes: An observational study. J Endocr Soc. 2021;5(6):bvab045. [Link] [DOI:10.1210/jendso/bvab045]
20. Beleslin-Čokić BB, Cokić VP, Wang L, Piknova B, Teng R, Schechter AN, et al. Erythropoietin and hypoxia increase erythropoietin receptor and nitric oxide levels in lung microvascular endothelial cells. Cytokine. 2011;54(2):129-35. [Link] [DOI:10.1016/j.cyto.2011.01.015]
21. Mohammed AA, Mustafa MN, Abdulsattar SA, Al-Zaidi AS. COVID 19: Evaluate of liver and renal function tests in Iraqi patients. Medi Leg Update. 2021;21(1):7-10. [Link]
22. Kopel J, Perisetti A, Roghani A, Aziz M, Gajendran M, Goyal H. Racial and gender-based differences in COVID-19. Front Public Health. 2020;8:418. [Link] [DOI:10.3389/fpubh.2020.00418]
23. Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10(5):509-16. [Link] [DOI:10.1038/gene.2009.12]
24. Xia HJ, Zhang GH, Wang RR, Zheng YT. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese rhesus macaques. Cell Mol Immunol. 2009;6(6):433-40. [Link] [DOI:10.1038/cmi.2009.55]
25. Kaseb AO, Mohamed YI, Malek AE, Raad II, Altameemi L, Li D, et al. The impact of Angiotensin-Converting Enzyme 2 (ACE2) expression on the incidence and severity of COVID-19 infection. Pathogens. 2021;10(3):379. [Link] [DOI:10.3390/pathogens10030379]
26. Khanijahani A, Iezadi S, Gholipour K, Azami-Aghdash S, Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. 2021;20:248. [Link] [DOI:10.1186/s12939-021-01582-4]
27. Decaux G, Prospert F, Namias B, Schlesser M, Soupart A. Raised urea clearance in cirrhotic patients with high uric acid clearance is related to low salt excretion. Gut. 1992;33(8):1105-8. [Link] [DOI:10.1136/gut.33.8.1105]
28. Gu F, Luo X, Jin X, Cai C, Zhao W. Association of total calcium with serum uric acid levels among United States adolescents aged 12-19 years: A cross-sectional study. Front Med. 2022;9:915371. [Link] [DOI:10.3389/fmed.2022.915371]









