Volume 16, Issue 1 (2024)
Iran J War Public Health 2024, 16(1): 61-65 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/10/31 | Accepted: 2024/03/4 | Published: 2024/03/25
Received: 2023/10/31 | Accepted: 2024/03/4 | Published: 2024/03/25
How to cite this article
Kazem S, Hussein M. Immunological Role of IL-17A and Biochemical Factors in Patients with Renal Failure Induced by Systemic Lupus Erythematosus. Iran J War Public Health 2024; 16 (1) :61-65
URL: http://ijwph.ir/article-1-1411-en.html
URL: http://ijwph.ir/article-1-1411-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
S.H. Kazem *1, M.H. Hussein1
1- Department of Pathological Analysis, College of Science, Thi-Qar University, Thi-Qar, Iraq
Full-Text (HTML) (374 Views)
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disorder that affects various organ systems, characterized by relapsing and remitting episodes. It predominantly affects women of childbearing age, showing a 9:1 female-to-male ratio [1]. In SLE, different immune cells and inflammatory mediators, especially dysfunctional T and B cells, are harmful. The activation of both the innate and adaptive immune systems is linked with the loss of B- or T-cell tolerance to self-antigens, which can be triggered by hormonal, environmental, or genetic factors [2]. A key characteristic of SLE is the production of autoantibodies by autoreactive B cells that react to self-antigens, initiating a massive inflammatory response [3]. These autoantibodies or immune complex depositions lead to tissue damage in various organs and systems, such as the kidneys, heart, blood vessels, central nervous system, skin, lungs, muscles, and joints, resulting in significant morbidity and mortality rates [4]. Patients with SLE often suffer from lupus nephritis (LN), a kidney condition that is associated with poorer prognoses and is a major cause of death and morbidity in this group [5].
Helper T cells (CD4+ T cells) are fundamental in building the immune system. Based on the cytokines they produce, these cells are classified into several types, including Th1, Th2, Th17, follicular helper T (Tfh) cells, and regulatory T (Treg) cells [6]. Th17 cells secrete interleukin-17 (IL-17), IL-21, and IL-22, which are crucial in initiating inflammation in various organs. Additionally, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F, part of the IL-17 family, are considered type I transmembrane proteins [7]. IL-17A is notably essential in contributing to inflammatory damage [8]. IL-17 stimulates the production of inflammatory cytokines and chemokines, aiding in the recruitment of monocytes and neutrophils to affected organs [9].
The pathogenesis of SLE has not been well elucidated, but it has been reported that interleukin-17 (IL-17) and Th17 cells play important roles in the pathogenesis of SLE. Also, activation of cytokines and low complement levels are common in SLE patients. IL-17A amplifies the immune response by inducing the local generation of chemokines and cytokines, recruiting monocytes and neutrophils, augmenting the generation of autoantibodies, and aggravating the damage and inflammation of target organs, like the kidney in SLE. Hristova et al. indicated that IL-17A rs2275913, IL-17RCrs708567, and TGFB1 rs1800469 polymorphisms are involved in the susceptibility and the clinical manifestations of SLE and serum levels of IL-17A should be monitored in the course of SLE [10]. In addition, Tang et al. assessed the relationship and clinical significance of cytokines and complements with SLE activity and found a positive correlation between the SLEDAI-2K scores and serum IL-6, IL-17, and hsCRP levels. They concluded that the serum IL-6, IL-17, and hsCRP levels were correlated with the disease activity [11].
Accordingly, due to the role of IL-17A and some biochemical factors in SLE-induced renal failure, we investigated the role of IL-17A, creatinine, lymphocytes, and basophils in patients with SLE-related renal failure and compared them with healthy controls.
Materials and Methods
In this clinical controlled study from September to November 2023, all well-diagnosed lupus patients (n=105) blood samples were collected at Al-Hussein Teaching Hospital, Iraq. The samples included 70 females and 35 males aged between 8 to 50 years. For each participant, 5ml of blood was drawn. The first 2ml of each sample was transferred to a tube containing EDTA and gently swirled for a few seconds to prevent clotting, which was used for hematological analysis to determine levels of lymphocytes and basophils. The remaining blood was placed into a gel separator tube and left to coagulate for 10 minutes at 37°C in a water bath (Backman/Counter; Germany), then centrifuged at 3000×g for 10 minutes. The clear serum was aliquoted and stored at -20°C for further IL-17A and creatinine levels analysis. Hemolyzed serum samples were discarded. A group of 50 healthy individuals was also selected and underwent the same procedure as the control.
Evaluation of IL-17A by ELISA
Serum IL-17A levels in the 105 patients with confirmed SLE-related renal failure and 50 healthy controls were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Bioassay Technology Laboratory; China). The protocol for the IL-17A ELISA kit involved bringing the frozen serum samples to room temperature and mixing thoroughly. Initially, 50μl of the standard was added to the standard wells (excluding the blank control well). Then, 40μl of sample or standard was added to the appropriate wells, followed by 10μl of anti-IL-17A antibody and 50μl of streptavidin-HRP. The wells were mixed well, sealed with a plate sealer, and incubated for 60 minutes at 37°C. After incubation, the plate was washed five times using a wash buffer. For each wash, 0.35ml of wash buffer was added to each well and left for 30-60 seconds before being aspirated thoroughly using an automated washing system. This was repeated five times, and the plate was then blotted dry using absorbent material such as paper towels. Subsequently, 50μl of substrate solution A and 50μl of substrate solution B were added to each well. The plate was resealed and incubated for another 10 minutes at 37°C in the dark. Following incubation, 50μl of Stop Solution was added to each well, changing the solution from blue to yellow. Each well's optical density (OD) was read within 10 minutes of adding the stop solution using a Thermo Scientific microplate reader set to 450nm.
Detection of creatinine
In this method, picric acid and creatinine react in alkaline solutions to form an orange-red creatinine-picric complex. The absorbance of this complex, which indicates creatinine levels, is measured at a mercury wavelength of 492nm, within a spectral range of 490-510nm. The optical path length is set at 1cm, and the temperature for the reaction may be maintained at either 25 or 37°C. Enhancing the anti-air measurement improves absorption. The sample volume used is 100µl, with 1000µl of reagent added. The process begins by starting a stopwatch simultaneously with mixing the sample and reagent. The initial absorbance (A1) was observed and recorded after 30 seconds, followed by the measurement of absorbance A2 exactly two minutes later.
Detection of lymphocyte and basophile
Lymphocytes and basophils were measured using a complete blood count (CBC) with 2ml of venous blood collected in an EDTA tube (HumaCount; Germany).
Statistical analysis
The data was analyzed using SPSS 23 software using the Chi-square and Welch's T-tests. A receiver operating characteristic (ROC) curve was used to show all the model's classification thresholds.
Findings
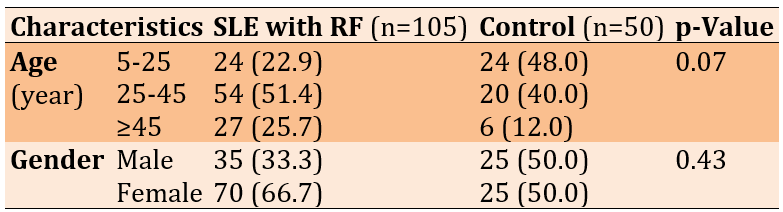
Females were more likely than men to get infected. There was a significant difference in the infection rate between sexes (p<0.05). A higher percentage of infection (51.44%) was found within the age group 25-45 years, followed by the age group 5-25, and the lowest one was (22.86%) within the age group 5-10 (Table 1).
Table 1. Frequency of baseline characteristics of the study participants in the systemic lupus erythematosus (SLE) with renal failure (RF) and control groups
The levels of IL-17A in infected patients (70.94g/mL) were significantly (p=0.004) higher than those in the control group (28.20g/mL). IL-17A had 100% sensitivity, 83.0% specificity, and 92.0% accuracy at the cutoff point (13.42) to predict SLE positively (86.4%) and negatively (100%; Figure 1).
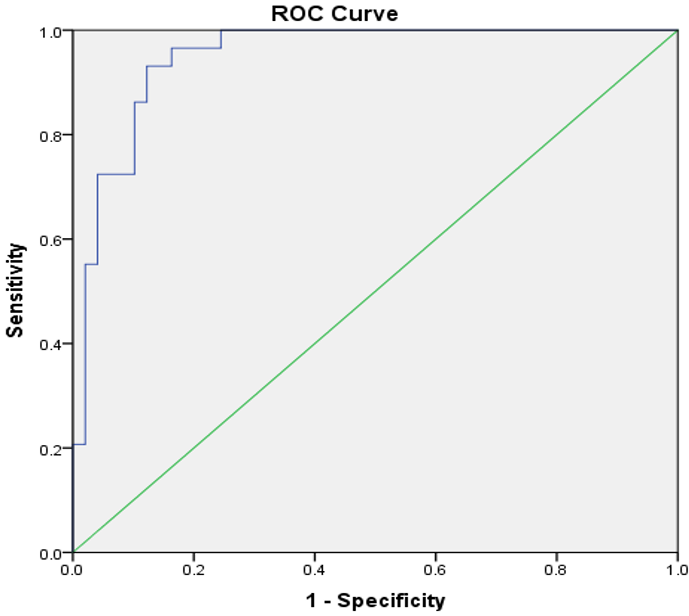
Figure 1. Area under the receiver operating characteristic curve of IL-17A in subjects with SLE and controls (standard error under the nonparametric assumption=0.023; asymptotic 95% confidence interval=0.904-0.995; and asymptotic significance level=0.0001)
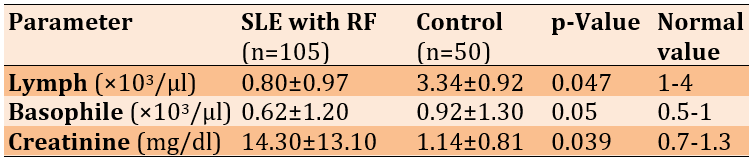
The levels of lymphocytes and basophils in patients were lower than those of controls, and the creatinine levels in patients were significantly higher than in controls (Table 2).
Table 2. Comparing the mean inflammatory and hemostatic parameters between the systemic lupus erythematosus (SLE) with renal failure (RF) and control groups using Welch's t-test
Discussion
This study compared the role of IL-17A, creatinine, lymphocytes, and basophils in patients with SLE-related renal failure with healthy controls. Chronic autoimmune illness SLE has several symptoms. Race and nationality affect SLE prevalence differentially [12]. The initial documentation of SLE in Iraq dates back to 1971 [13]. Variation in incidence rates was observed globally, with North America reporting the highest incidence [14] and Africa and Ukraine reporting the lowest incidence [15, 16]. The occurrence of SLE was often lower in Europe compared to Asia, Australasia, and the Americas [17, 18]. Our research findings indicated variations in disease rates between the sexes, with females having a higher incidence than males. This finding is consistent with multiple investigations [19-25].
All our cases were adults, which aligns with previous research, explaining that females are more likely to be affected with SLE after puberty than males due to high circulating estrogen levels. This suggests that the observed sex difference may result from the interaction of genetics, environment, and sex hormones during individual development [26]. Lupus patients' low progesterone levels might indicate that their immune systems are too sensitive to estrogen [27]. Regarding the effect of age on the prevalence of the disease, most cases of SLE occurred in the age group of 13-45 years, followed by the age group of >45 years. These results agreed with other studies [28, 29].
Serum IL-17A concentrations were greater in SLE-related renal failure patients than in healthy controls. The current study's findings were consistent with those of other studies [30-32]. Analysis of human kidney biopsies revealed that IL-17A+ T cells are associated with some diseases [33].
Serum creatinine levels were reported as one of the significant predictors of end-stage renal disease in patients with SLE. Our study's results revealed increased creatinine levels in SLE patients, which agrees with other research [34]. In addition, Ge et al. found a significant association between decreased creatinine clearance rate (Ccr) and increased risk for mortality in patients with SLE. They concluded that early clinical interventions to modulate the Ccr of patients with SLE can benefit their survival [35]. Also, the results of Yang et al. showed that serum urea, creatinine, and uric acid levels were associated with several laboratory and clinical characteristics of SLE [36].
Lymphocyte and basophile levels in patients were lower than those of controls. This result was consistent with previous research [37]. Basophils contribute to the immunopathogenesis of SLE. A study assessing the blood basophil count as a potential marker of SLE activity revealed that basophil counts were significantly lower in patients with SLE than in healthy controls [38]. Therefore, blood basophil counts may be useful in assessing SLE activity. The function of lymphocytes in the immunological pathways that lead to SLE development is well-known. Despite their critical involvement in the immune response [39, 40], lymphocytes in SLE have only lately been the subject of extensive research. The study shows SLE patients' lymphocyte levels have decreased [40, 41]. Also, Dossybayeva et al. assessed circulating basophils in children affected with pediatric SLE and declared a significant reduction in peripherally circulating basophils in children with SLE patients [42].
This study is restricted by the brief duration of the study and limited number of samples. Hence, it is recommended to extend the duration of the study and employ more samples. It is also possible to use IL17a as early biomarkers for diagnosis of SLE complications & future prevention. The use of renal function tests (such as Creatinine) is suggested to evaluate the importance of them in early detection of impaired renal function in SLE patients.
Conclusion
IL-17A, creatinine, lymphocytes, and basophils play a fundamental role in the pathogenesis of SLE.
Acknowledgments: Authors are deeply grateful to all the people who helped them, especially the staff of AL-Hussein Teaching Hospital
Ethical Permission: This study is subjected to the qualifications of ethical considerations and according to the form prepared for this purpose by the Iraqi Ministry of Health. The research also got the agreement of the committee of ethical standards at the College of Science, Thi-Qar University, Iraq. In addition, informed consent was obtained from all patients before we took samples.
Conflicts of Interests: There is no conflicts of interests.
Authors’ Contribution: Kazem SH (First Author), Introduction Writer/Main Researcher/Statistical Analyst (50%); Hussein MH (Second Author), Methodologist/Main Researcher/Discussion Writer (50%)
Funding/Support: None declared by the authors.
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disorder that affects various organ systems, characterized by relapsing and remitting episodes. It predominantly affects women of childbearing age, showing a 9:1 female-to-male ratio [1]. In SLE, different immune cells and inflammatory mediators, especially dysfunctional T and B cells, are harmful. The activation of both the innate and adaptive immune systems is linked with the loss of B- or T-cell tolerance to self-antigens, which can be triggered by hormonal, environmental, or genetic factors [2]. A key characteristic of SLE is the production of autoantibodies by autoreactive B cells that react to self-antigens, initiating a massive inflammatory response [3]. These autoantibodies or immune complex depositions lead to tissue damage in various organs and systems, such as the kidneys, heart, blood vessels, central nervous system, skin, lungs, muscles, and joints, resulting in significant morbidity and mortality rates [4]. Patients with SLE often suffer from lupus nephritis (LN), a kidney condition that is associated with poorer prognoses and is a major cause of death and morbidity in this group [5].
Helper T cells (CD4+ T cells) are fundamental in building the immune system. Based on the cytokines they produce, these cells are classified into several types, including Th1, Th2, Th17, follicular helper T (Tfh) cells, and regulatory T (Treg) cells [6]. Th17 cells secrete interleukin-17 (IL-17), IL-21, and IL-22, which are crucial in initiating inflammation in various organs. Additionally, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F, part of the IL-17 family, are considered type I transmembrane proteins [7]. IL-17A is notably essential in contributing to inflammatory damage [8]. IL-17 stimulates the production of inflammatory cytokines and chemokines, aiding in the recruitment of monocytes and neutrophils to affected organs [9].
The pathogenesis of SLE has not been well elucidated, but it has been reported that interleukin-17 (IL-17) and Th17 cells play important roles in the pathogenesis of SLE. Also, activation of cytokines and low complement levels are common in SLE patients. IL-17A amplifies the immune response by inducing the local generation of chemokines and cytokines, recruiting monocytes and neutrophils, augmenting the generation of autoantibodies, and aggravating the damage and inflammation of target organs, like the kidney in SLE. Hristova et al. indicated that IL-17A rs2275913, IL-17RCrs708567, and TGFB1 rs1800469 polymorphisms are involved in the susceptibility and the clinical manifestations of SLE and serum levels of IL-17A should be monitored in the course of SLE [10]. In addition, Tang et al. assessed the relationship and clinical significance of cytokines and complements with SLE activity and found a positive correlation between the SLEDAI-2K scores and serum IL-6, IL-17, and hsCRP levels. They concluded that the serum IL-6, IL-17, and hsCRP levels were correlated with the disease activity [11].
Accordingly, due to the role of IL-17A and some biochemical factors in SLE-induced renal failure, we investigated the role of IL-17A, creatinine, lymphocytes, and basophils in patients with SLE-related renal failure and compared them with healthy controls.
Materials and Methods
In this clinical controlled study from September to November 2023, all well-diagnosed lupus patients (n=105) blood samples were collected at Al-Hussein Teaching Hospital, Iraq. The samples included 70 females and 35 males aged between 8 to 50 years. For each participant, 5ml of blood was drawn. The first 2ml of each sample was transferred to a tube containing EDTA and gently swirled for a few seconds to prevent clotting, which was used for hematological analysis to determine levels of lymphocytes and basophils. The remaining blood was placed into a gel separator tube and left to coagulate for 10 minutes at 37°C in a water bath (Backman/Counter; Germany), then centrifuged at 3000×g for 10 minutes. The clear serum was aliquoted and stored at -20°C for further IL-17A and creatinine levels analysis. Hemolyzed serum samples were discarded. A group of 50 healthy individuals was also selected and underwent the same procedure as the control.
Evaluation of IL-17A by ELISA
Serum IL-17A levels in the 105 patients with confirmed SLE-related renal failure and 50 healthy controls were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Bioassay Technology Laboratory; China). The protocol for the IL-17A ELISA kit involved bringing the frozen serum samples to room temperature and mixing thoroughly. Initially, 50μl of the standard was added to the standard wells (excluding the blank control well). Then, 40μl of sample or standard was added to the appropriate wells, followed by 10μl of anti-IL-17A antibody and 50μl of streptavidin-HRP. The wells were mixed well, sealed with a plate sealer, and incubated for 60 minutes at 37°C. After incubation, the plate was washed five times using a wash buffer. For each wash, 0.35ml of wash buffer was added to each well and left for 30-60 seconds before being aspirated thoroughly using an automated washing system. This was repeated five times, and the plate was then blotted dry using absorbent material such as paper towels. Subsequently, 50μl of substrate solution A and 50μl of substrate solution B were added to each well. The plate was resealed and incubated for another 10 minutes at 37°C in the dark. Following incubation, 50μl of Stop Solution was added to each well, changing the solution from blue to yellow. Each well's optical density (OD) was read within 10 minutes of adding the stop solution using a Thermo Scientific microplate reader set to 450nm.
Detection of creatinine
In this method, picric acid and creatinine react in alkaline solutions to form an orange-red creatinine-picric complex. The absorbance of this complex, which indicates creatinine levels, is measured at a mercury wavelength of 492nm, within a spectral range of 490-510nm. The optical path length is set at 1cm, and the temperature for the reaction may be maintained at either 25 or 37°C. Enhancing the anti-air measurement improves absorption. The sample volume used is 100µl, with 1000µl of reagent added. The process begins by starting a stopwatch simultaneously with mixing the sample and reagent. The initial absorbance (A1) was observed and recorded after 30 seconds, followed by the measurement of absorbance A2 exactly two minutes later.
Detection of lymphocyte and basophile
Lymphocytes and basophils were measured using a complete blood count (CBC) with 2ml of venous blood collected in an EDTA tube (HumaCount; Germany).
Statistical analysis
The data was analyzed using SPSS 23 software using the Chi-square and Welch's T-tests. A receiver operating characteristic (ROC) curve was used to show all the model's classification thresholds.
Findings
Females were more likely than men to get infected. There was a significant difference in the infection rate between sexes (p<0.05). A higher percentage of infection (51.44%) was found within the age group 25-45 years, followed by the age group 5-25, and the lowest one was (22.86%) within the age group 5-10 (Table 1).
Table 1. Frequency of baseline characteristics of the study participants in the systemic lupus erythematosus (SLE) with renal failure (RF) and control groups

The levels of IL-17A in infected patients (70.94g/mL) were significantly (p=0.004) higher than those in the control group (28.20g/mL). IL-17A had 100% sensitivity, 83.0% specificity, and 92.0% accuracy at the cutoff point (13.42) to predict SLE positively (86.4%) and negatively (100%; Figure 1).

Figure 1. Area under the receiver operating characteristic curve of IL-17A in subjects with SLE and controls (standard error under the nonparametric assumption=0.023; asymptotic 95% confidence interval=0.904-0.995; and asymptotic significance level=0.0001)
The levels of lymphocytes and basophils in patients were lower than those of controls, and the creatinine levels in patients were significantly higher than in controls (Table 2).
Table 2. Comparing the mean inflammatory and hemostatic parameters between the systemic lupus erythematosus (SLE) with renal failure (RF) and control groups using Welch's t-test

Discussion
This study compared the role of IL-17A, creatinine, lymphocytes, and basophils in patients with SLE-related renal failure with healthy controls. Chronic autoimmune illness SLE has several symptoms. Race and nationality affect SLE prevalence differentially [12]. The initial documentation of SLE in Iraq dates back to 1971 [13]. Variation in incidence rates was observed globally, with North America reporting the highest incidence [14] and Africa and Ukraine reporting the lowest incidence [15, 16]. The occurrence of SLE was often lower in Europe compared to Asia, Australasia, and the Americas [17, 18]. Our research findings indicated variations in disease rates between the sexes, with females having a higher incidence than males. This finding is consistent with multiple investigations [19-25].
All our cases were adults, which aligns with previous research, explaining that females are more likely to be affected with SLE after puberty than males due to high circulating estrogen levels. This suggests that the observed sex difference may result from the interaction of genetics, environment, and sex hormones during individual development [26]. Lupus patients' low progesterone levels might indicate that their immune systems are too sensitive to estrogen [27]. Regarding the effect of age on the prevalence of the disease, most cases of SLE occurred in the age group of 13-45 years, followed by the age group of >45 years. These results agreed with other studies [28, 29].
Serum IL-17A concentrations were greater in SLE-related renal failure patients than in healthy controls. The current study's findings were consistent with those of other studies [30-32]. Analysis of human kidney biopsies revealed that IL-17A+ T cells are associated with some diseases [33].
Serum creatinine levels were reported as one of the significant predictors of end-stage renal disease in patients with SLE. Our study's results revealed increased creatinine levels in SLE patients, which agrees with other research [34]. In addition, Ge et al. found a significant association between decreased creatinine clearance rate (Ccr) and increased risk for mortality in patients with SLE. They concluded that early clinical interventions to modulate the Ccr of patients with SLE can benefit their survival [35]. Also, the results of Yang et al. showed that serum urea, creatinine, and uric acid levels were associated with several laboratory and clinical characteristics of SLE [36].
Lymphocyte and basophile levels in patients were lower than those of controls. This result was consistent with previous research [37]. Basophils contribute to the immunopathogenesis of SLE. A study assessing the blood basophil count as a potential marker of SLE activity revealed that basophil counts were significantly lower in patients with SLE than in healthy controls [38]. Therefore, blood basophil counts may be useful in assessing SLE activity. The function of lymphocytes in the immunological pathways that lead to SLE development is well-known. Despite their critical involvement in the immune response [39, 40], lymphocytes in SLE have only lately been the subject of extensive research. The study shows SLE patients' lymphocyte levels have decreased [40, 41]. Also, Dossybayeva et al. assessed circulating basophils in children affected with pediatric SLE and declared a significant reduction in peripherally circulating basophils in children with SLE patients [42].
This study is restricted by the brief duration of the study and limited number of samples. Hence, it is recommended to extend the duration of the study and employ more samples. It is also possible to use IL17a as early biomarkers for diagnosis of SLE complications & future prevention. The use of renal function tests (such as Creatinine) is suggested to evaluate the importance of them in early detection of impaired renal function in SLE patients.
Conclusion
IL-17A, creatinine, lymphocytes, and basophils play a fundamental role in the pathogenesis of SLE.
Acknowledgments: Authors are deeply grateful to all the people who helped them, especially the staff of AL-Hussein Teaching Hospital
Ethical Permission: This study is subjected to the qualifications of ethical considerations and according to the form prepared for this purpose by the Iraqi Ministry of Health. The research also got the agreement of the committee of ethical standards at the College of Science, Thi-Qar University, Iraq. In addition, informed consent was obtained from all patients before we took samples.
Conflicts of Interests: There is no conflicts of interests.
Authors’ Contribution: Kazem SH (First Author), Introduction Writer/Main Researcher/Statistical Analyst (50%); Hussein MH (Second Author), Methodologist/Main Researcher/Discussion Writer (50%)
Funding/Support: None declared by the authors.
Keywords:
References
1. Justiz Vaillant AA, Goyal A, Varacallo M. Systemic lupus erythematosus. Treasure Island (FL): StatPearls Publishing; 2023. [Link]
2. Dema B, Charles N. Advances in mechanisms of systemic lupus erythematosus. Discov Med. 2014;17(95):247-55. [Link]
3. Bag-Ozbek A, Hui-Yuen JS. Emerging B-cell therapies in systemic lupus erythematosus. Ther Clin Risk Manag. 2021;17:39-54. [Link] [DOI:10.2147/TCRM.S252592]
4. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80(1):14-25. [Link] [DOI:10.1136/annrheumdis-2020-218272]
5. Rovin BH, Ayoub I, Arora S. The clinical evaluation of kidney disease in systemic lupus erythematosus. In: Systemic lupus erythematosus. Cambridge: Academic Press; 2021. p. 379-88. [Link] [DOI:10.1016/B978-0-12-814551-7.00042-8]
6. Koga T, Ichinose K, Kawakami A, Tsokos GC. The role of IL-17 in systemic lupus erythematosus and its potential as a therapeutic target. Expert Rev Clin Immunol. 2019;15(6):629-37. [Link] [DOI:10.1080/1744666X.2019.1593141]
7. Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467-76. [Link] [DOI:10.1016/j.immuni.2004.08.018]
8. Barath S, Aleksza M, Tarr T, Sipka S, Szegedi G, Kiss E. Measurement of natural (CD4+CD25high) and inducible (CD4+IL-10+) regulatory T cells in patients with systemic lupus erythematosus. Lupus. 2007;16(7):489-96. [Link] [DOI:10.1177/0961203307080226]
9. Burkett PR, Zu Horste GM, Kuchroo VK. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest. 2015;125(6):2211-9. [Link] [DOI:10.1172/JCI78085]
10. Hristova M, Kamenarska Z, Dzhebir G, Nikolova S, Hristova R, Mihova K, et al. The role of IL-17 rs2275913, IL-17RC rs708567 and TGFB1 rs1800469 SNPs and IL-17A serum levels in patients with lupus nephritis. Rheumatol Int. 2021;41(12):2205-13. [Link] [DOI:10.1007/s00296-021-04996-z]
11. Tang Y, Tao H, Gong Y, Chen F, Li C, Yang X. Changes of serum IL-6, IL-17, and complements in systemic lupus erythematosus patients. Journal of Interferon & Cytokine Research. 2019;39(7):410-5. [Link] [DOI:10.1089/jir.2018.0169]
12. Siegel M, Lee SL. The epidemiology of systemic lupus erythematosus. Semin Arthritis Rheum. 1973;3(1):1-54. [Link] [DOI:10.1016/0049-0172(73)90034-6]
13. Al-Rawi Z, Al-Shaarbaf H, Al-Raheem E, Khalifa SJ. Clinical features of early cases of systemic lupus erythematosus in Iraqi patients. Rheumatology. 1983;22(3):165-71. [Link] [DOI:10.1093/rheumatology/22.3.165]
14. Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcón GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum. 2013;65(3):753-63. [Link] [DOI:10.1002/art.37795]
15. Taylor HG, Stein CM. Systemic lupus erythematosus in Zimbabwe. Ann Rheum Dis. 1986;45(8):645-8. [Link] [DOI:10.1136/ard.45.8.645]
16. Nasonov E, Soloviev S, Davidson JE, Lila A, Ivanova R, Togizbayev G, et al. The prevalence and incidence of Systemic Lupus Erythematosus (SLE) in selected cities from three Commonwealth of Independent States countries (the Russian Federation, Ukraine, and Kazakhstan). Lupus. 2014;23(2):213-9. [Link] [DOI:10.1177/0961203313512881]
17. Tian J, Zhang D, Yao X, Huang Y, Lu Q. Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modeling study. Ann Rheum Dis. 2023;82(3):351-6. [Link] [DOI:10.1136/ard-2022-223035]
18. Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology. 2017;56(11):1945-61. [Link] [DOI:10.1093/rheumatology/kex260]
19. Osio-Salido E, Manapat-Reyes H. Epidemiology of systemic lupus erythematosus in Asia. Lupus. 2010;19(12):1365-73. [Link] [DOI:10.1177/0961203310374305]
20. Pradgan V, Patwardhan M, Rajadhyaksha A, Ghosh K. Clinical and immunological profile of systemic lupus erythematosus. Indian Pediatr. 2013;50(4):405-7. [Link] [DOI:10.1007/s13312-013-0115-z]
21. Abdulridha RH, Saud AM, Alosami MH. Evaluation of interferon-alpha (IFN-α) in women with systemic lupus erythematosus in Iraq. Iraqi J Sci. 2022;63(10):4225-33. [Link] [DOI:10.24996/ijs.2022.63.10.9]
22. Duarte-García A, Hocaoglu M, Valenzuela-Almada M, Osei-Onomah SA, Dabit JY, Sanchez-Rodriguez A, et al. Rising incidence and prevalence of systemic lupus erythematosus: a population-based study over four decades. Rheumat Dis. 2022;81(9):1260-6. [Link] [DOI:10.1136/annrheumdis-2022-222276]
23. Ambrose N, Morgan TA, Galloway J, Ionnoau Y, Beresford MW, Isenberg DA. Differences in disease phenotype and severity in SLE across age groups. Lupus. 2016;25(14):1542-50. [Link] [DOI:10.1177/0961203316644333]
24. Sharifzadeh M, Naeimi S, Nasiri MM, Ariannia S, Farrokhseresht R. IL-17A gene polymorphism at position G197A and systemic lupus erythematosus. Rheumatol Res. 2018;3(3):107-12. [Link] [DOI:10.22631/rr.2018.69997.1050]
25. Mok CC, Mak A, Chu WP, To CH, Wong SN. Long-term survival of southern Chinese patients with systemic lupus erythematosus: A prospective study of all age-groups. Medicine. 2005;84(4):218-24. [Link] [DOI:10.1097/01.md.0000170022.44998.d1]
26. McKiernan PJ, McElvaney NG, Greene CM. SLPI and inflammatory lung disease in females. Biochem Soc Trans. 2011;39(5):1421-6. [Link] [DOI:10.1042/BST0391421]
27. Shabanova SS, Ananieva LP, Alekberova ZS, Guzov II. Ovarian function and disease activity in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2008;26(3):436-41. [Link]
28. AlSaleh J, Jassim V, ElSayed M, Saleh N, Harb D. Clinical and immunological manifestations in 151 SLE patients living in Dubai. Lupus. 2008;17(1):62-6. [Link] [DOI:10.1177/0961203307084297]
29. Wang WS, Chen PM, Hsiao HL, Wang HS, Liang WY, Su Y. Overexpression of the thymosin β-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene. 2004;23(39):6666-71. [Link] [DOI:10.1038/sj.onc.1207888]
30. Abdul-Razzaq LN, Jassim MMA, Mahmood MM. The high presence of HCMV pp71 proteins, correlate with P63 expression in pancreatic cancer tumor tissue. Asian Pac J Cancer Prev. 2023;24(4):1443-7. [Link] [DOI:10.31557/APJCP.2023.24.4.1443]
31. Chen M, Chen X, Wan Q. Altered frequency of Th17 and Treg cells in new‐onset systemic lupus erythematosus patients. Eur J Clin Invest. 2018;48(11):e13012. [Link] [DOI:10.1111/eci.13012]
32. Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthrit Res Therap. 2013;15:R97. [Link] [DOI:10.1186/ar4277]
33. Wang Y, Ito S, Chino Y, Goto D, Matsumoto I, Murata H, et al. Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clin Exp Immunol. 2010;159(1):1-10. [Link] [DOI:10.1111/j.1365-2249.2009.04031.x]
34. Harley JB, Sestak AL, Willis LG, Fu SM, Hansen JA, Reichlin M. A model for disease heterogeneity in systemic lupus erythematosus: Relationships between histocompatibility antigens, autoantibodies, and lymphopenia or renal disease. Arthritis Rheum. 1989;32(7):826-36. [Link] [DOI:10.1002/j.2326-5205.1989.tb00013.x]
35. Ge J, Jin Z, Feng X, Pan W, Liu L, Wu M, et al. Creatinine clearance rate predicts prognosis of patients with systemic lupus erythematosus: a large retrospective cohort study. Clin Rheumatol. 2021;40:2221-31. [Link] [DOI:10.1007/s10067-020-05485-7]
36. Yang Z, Liang Y, Li C, Xi W, Zhong R. Associations of serum urea, creatinine and uric acid with clinical and laboratory features in patients with systemic lupus erythematosus. Rheumatol Int. 2012;32:2715-23. [Link] [DOI:10.1007/s00296-011-1987-7]
37. Hussein MH, Khalaf MM. Association of interleukin-6 and interleukin-1-β levels in patients with toxoplasmosis. Ann Parasitol. 2022;68(2):317-21. [Link]
38. Liang P, Tang Y, Fu S, Lv J, Liu B, Feng M, et al. Basophil count, a marker for disease activity in systemic lupus erythematosus. Clin Rheumatol. 2015;34(5):891-6. [Link] [DOI:10.1007/s10067-014-2822-9]
39. Pan Q, Gong L, Xiao H, Feng Y, Li L, Deng Z, et al. Basophil activation-dependent autoantibody and interleukin-17 production exacerbate systemic lupus erythematosus. Front Immunol. 2017;8:348. [Link] [DOI:10.3389/fimmu.2017.00348]
40. Rowlands DT, Daniele RP. Surface receptors in the immune response. N Engl J Med. 1975;293(1):26-32. [Link] [DOI:10.1056/NEJM197507032930108]
41. Rivero SJ, Díaz‐Jouanen E, Alarcón‐Segovia D. Lymphopenia in systemic lupus erythematosus. Arthritis Rheum. 1978;21(3):295-305. [Link] [DOI:10.1002/art.1780210302]
42. Dossybayeva K, Bexeitov Y, Mukusheva Z, Almukhamedova Z, Assylbekova M, Abdukhakimova D, et al. Analysis of peripheral blood basophils in pediatric systemic lupus erythematosus. Diagnostics. 2022;12(7):1701. [Link] [DOI:10.3390/diagnostics12071701]









