Volume 15, Issue 4 (2023)
Iran J War Public Health 2023, 15(4): 361-367 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/10/12 | Accepted: 2023/11/11 | Published: 2023/11/26
Received: 2023/10/12 | Accepted: 2023/11/11 | Published: 2023/11/26
How to cite this article
Ghafil F, Hassan E, Aziz N, Salim M, Majeed S, Rasheed S et al . Cardioprotective Potential of Celastrol in Sepsis-Induced Cardiotoxicity; Mouse Model of Endotoxemia. Iran J War Public Health 2023; 15 (4) :361-367
URL: http://ijwph.ir/article-1-1405-en.html
URL: http://ijwph.ir/article-1-1405-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Cardioprotective Potential of Celastrol in Sepsis-Induced Cardiotoxicity; Mouse Model of Endotoxemia
Authors
F.A. Ghafil1, E.S. Hassan *1, N.D. Aziz2, M.M. Salim3, S.A. Majeed1, S.M.H. Rasheed4, H.W. Mardan5
1- Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa, Najaf, Iraq
2- Department of Clinical Pharmacy, Collage of Pharmacy, University of Kerbala, Kerbala, Iraq
3- Department of Clinical Pathology, Faculty of Medicine, University of Kufa, Najaf, Iraq
4- Department of Clinical Microbiology, Faculty of Medicine, University of Kufa, Najaf, Iraq
5- Middle Euphrates Center of Neurosciences, Al-Sadder Teaching Hospital, Al-Najaf Al-Ashraf Health Directorate, Najaf, Iraq
2- Department of Clinical Pharmacy, Collage of Pharmacy, University of Kerbala, Kerbala, Iraq
3- Department of Clinical Pathology, Faculty of Medicine, University of Kufa, Najaf, Iraq
4- Department of Clinical Microbiology, Faculty of Medicine, University of Kufa, Najaf, Iraq
5- Middle Euphrates Center of Neurosciences, Al-Sadder Teaching Hospital, Al-Najaf Al-Ashraf Health Directorate, Najaf, Iraq
Full-Text (HTML) (459 Views)
Introduction
Sepsis is a critical clinical condition that carries a high rate of morbidity and mortality; occurs when the body's defenses against infection and pathogen invasion are dysregulated and seriously harm its tissues and organs, resulting in end-organ damage and failure [1, 2]. It represents the body's reaction to a massive and severe infection that has failed to be locally controlled due to a dysregulated immune response and overwhelming life-threatening complications, especially in susceptible individuals [3, 4]. Sepsis is a dangerous worldwide health insult with a high rate of mortality and high economic concern [5].
This condition is frequently complicated by cardiac injury in which the heart is the most affected organ by sepsis; this cardiac insult is manifested by an increment in cardiac troponin and creatine-kinase MB (CK-MB) [6, 7]. That situation usually induces depression of cardiac muscle, impairment of coronary arterial circulation, defective cardiac contraction, cardiomyopathy, and mitochondrial damage. Sepsis provokes a systemic inflammatory response, increases pro-inflammatory cytokines production such as interleukins 6 (IL6), tumor necrosis factor (TNF), and toll-like receptor 4 (TLR4) with consequent overt cytokine storm [8], energy depletion with oxidative stress burden together with endothelial dysfunction and apoptosis all are responsible for cellular damage and death [9].
Many factors are involved in myocardial injury during sepsis: cytokines, complement cascade elements, damage associated with variable molecular patterns, ROS, and oxidative stress, dysregulated metabolism of nitric oxide, mitochondrial injury, disturbed calcium homeostasis within cells, and apoptosis [10]. The endothelial dehiscence will also induce vascular leakage, interstitial edema, coagulopathy, and inflammatory response that augment the adverse burden of sepsis [11]. This destruction of the endothelium can disturb microvascular blood flow, increase vascular leakage, and consequent edema [12].
TLR4 is the key member of the toll-like receptor family responsible for pattern recognition. It performs various functions in pathological conditions, such as cardiovascular disease, neuronal degeneration, inflammatory bowel diseases, and allergic, metabolic, and autoimmune disorders [13]. TLR4 has the highest level in the heart compared to other TLRs and plays a vital role in myocardial inflammation, including myocarditis, ischemia-reperfusion injury, atherosclerosis, hypertension, and heart failure [13]. TLR4 has been known as a receptor for Lipopolysaccharides and plays an important role in initiating inflammation, myocardial injury, and dysfunction [5]. Variable signaling pathways are activated upon TLR4 binding to lipopolysaccharides like primary response protein 88: MyD88 [5, 14]. Macrophage scavenger receptor class A is suggested in recent research as a co-receptor for TLR4 to facilitate inflammatory responses, promote cell apoptosis, and suppress cell survival [15].
Isoprostanes are free radical-catalyzed PG-like products. They are broadly documented as a vital marker for oxidative stress and systemic lipid peroxidation in vivo. Formation of these substances is increased in a variety of cardiovascular diseases (CVD) and may predict the risk of cardiovascular complications in patients. Several isoprostanes may openly contribute to the functional concerns of oxidant stress by affecting endothelial cell regeneration and function, tone of vessels, hemostasis, and ischemia/reperfusion injury [16].
Indeed, human and animal studies have researched the correlation between oxidative stress accompanied by increased levels of isoprostane and the outcome of CVD. Therefore, a targeted inhibition of isoprostane formation could help improve outcomes in CVD patients [16].
Despite extensive clinical research dealing with cardiomyopathy and cardiac injury due to endotoxemia regarding its mechanisms, pathophysiological disturbances, and complications, no definite treatment is valuable, and most of the clinical options are uncertain [17].
To understand the pathology and causes of human sepsis, researchers have developed a model of cecal ligation and puncture (CLP), which consists of the cecal ligation and perforation to release the fecal material into the peritoneum; this will generate an exaggerated immune response due to polymicrobial infection, CLP model is of high clinical relevancy [18, 19].
Celastrol is an extract of the Tripterygium wilfordii hook in China. It is a methide triterpenoid with pleiotropic properties, such as antiinflammatory, antioxidant, and immune modulation [20].
Celastrol is a potent antiinflammatory agent via a reduction in IL6, IL1 B, TNF, TLR4, and NFKB, as indicated by previous studies via inhibition of signaling pathways activated by inflammation [21]. It also has an antioxidant property manifested by its role in reducing ROS, lipid peroxidation, and adhesion molecules, together with enhancing antioxidant defenses [22]. Celastrol was found to ameliorate right-sided heart failure by reduction of oxidative stress, inflammation, and remodeling of the pulmonary artery, so a reduction in cardiac failure in mice subjected to hypoxia-induced pulmonary hypertension by reducing right ventricular remodeling, systolic blood pressure, hypertrophy, impaired function, and fibrosis [23]. Celastrol could impede inflammatory reactions induced by sepsis as well as retard the production of variable pro-inflammatory cytokines and chemokines. Celastrol could similarly maintain mitochondrial functions by antagonizing certain oxidative and inflammatory pathways. Celastrol could induce upregulation of certain inflammatory nuclear receptors, so it has an effective anti-inflammatory role, which provides the basis for the development of new agents that can oppose inflammation by maintaining mitochondrial functions and homeostasis [24].
Celastrol enhances strong pro-survival signals that are responsible for the improvement of cardiac cell survival during hypoxia. Continuous celastrol treatment in rats with myocardial ischemia and permanent ligation of the coronary artery for two weeks decreases the size of the infarcted area, improves cardiac performance and function, and protects against deleterious remodeling of the left ventricular wall [25]. Celastrol exerts cardioprotective properties dependent on the amelioration of Reactive Oxygen Species and Heat Shock response since the increased oxidative stress after ischemic insults will trigger Heat Shock protein activation. Celastrol augments several Heat Shock Protein mRNA expressions, such as HSP (70 and 32), leading to their translocation from the cytoplasm to the nucleus [25]. Celastrol could decrease the expression level of pro-inflammatory markers with increased expression of antiinflammatory cytokine IL-10 in the liver and adipose tissue. In addition, celastrol reduced the plasma levels of inflammatory cytokines such as IL-6 and IL-1β in the experimental mouse model [5].
Previous studies have provided celastrol with variable cytoprotective and pleotropic properties, making it a good candidate agent for ameliorating cardiac injury accompanied by sepsis. The study aimed to assess the cardioprotective potential of celastrol against cardiac injury induced by sepsis via amelioration of IL6, TNF, TLR4, IL10, F2-isoprostane, cardiac troponin, and CK-MB, as well as at histological level.
Materials and Methods
In this experimental study, 24 adult male mice were included; they were 6-8 weeks old and weighed 25-30g. The animals were randomly allocated into four groups (n=6);
- Sham: Midline 1-2cm laparotomy under anesthesia without CLP
- Sepsis (CLP): Laparotomy, had their cecum exposed ligated and off by suture below the ileocecal valve and punctured one time by a needle gauge 19 [26]
- Vehicle (DMSO): An equal volume of vehicle IP 1 hour before laparotomy and CLP [27]
- Celastrol: Treated with 2mg/kg in 1-hour IP before the CLP process [27]
All animals were supervised while maintaining good hydration and body temperature, and all procedures were performed under anesthesia. Careful animal supervision was maintained throughout the time of study. Celastrol powder was supplied by (Solarbio; China) and dissolved in diluted DMSO 5% in normal saline, and dimethyl sulfoxide (DMSO) was supplied by (Abu Dhabi Medical; UAE). Complete Protease inhibitor liquid was supplied by (Medchem Express; United States). After 24 hours, they were sacrificed by using ketamine and xylazine to obtain blood and tissue samples. Blood had been aspirated from the heart to assess the serum troponin (Beckman Coulter; USA) and CK-MB (Beckman Coulter; USA) by spectrophotometric assay. Cardiac resection was done, and the heart was divided into two parts; one for homogenate to be used in the ELISA (TNF-α, IL-6, IL-10, F2- isoprostane levels) procedure (BT-LAB; China), another part was preserved in formalin, processed, then fixed in paraffin and prepared for histopathological analysis [28].
Kolmogorov-Smirnov and Schapiro tests were applied after testing the normality of data. ANOVA test was applied for the mean difference of numerical variables among study groups with their bar chart using SPSS 26 software.
Findings
There was a significant elevation in serum troponin in sepsis and vehicle groups compared to the sham group. In contrast, pretreatment of mice with celastrol resulted in a significant reduction in the level of serum troponin (Figure 1a). There was a significant elevation in serum level of CK-MB in sepsis and vehicle groups as compared to the sham group. In contrast, pretreatment of mice with celastrol was resulted in significant reduction in the tissue level of CK-MB (Figure 1b). The tissue level of IL6 was significantly elevated in the sepsis and vehicle groups compared to the sham group. In contrast, pretreatment of mice with celastrol resulted in a significant reduction in the tissue level of IL6 (Figure 1c). There was a significant elevation in the tissue level of TNFα in sepsis and vehicle groups compared to the sham group. In contrast, pretreatment of mice with celastrol resulted in a significant reduction in the tissue level of TNFα (Figure 1d). Compared to the sham group, there was a significant elevation in the tissue level of F2 isoprostane in sepsis and vehicle groups. In contrast, pretreatment of mice with celastrol resulted in a significant reduction in the tissue level of F2 isoprostane (Figure 1e). There was significant reduction in tissue level of IL10 in sepsis and vehicle groups as compared to sham group, whereas pretreatment of mice with celastrol was resulted in significant elevation in the tissue level of IL10 (Figure 1f). There was a significant elevation in the tissue level of TLR4 in sepsis and vehicle groups as compared to the sham group, whereas pretreatment of mice with celastrol resulted in a significant reduction in the tissue level of TLR4 (Figure 1g).
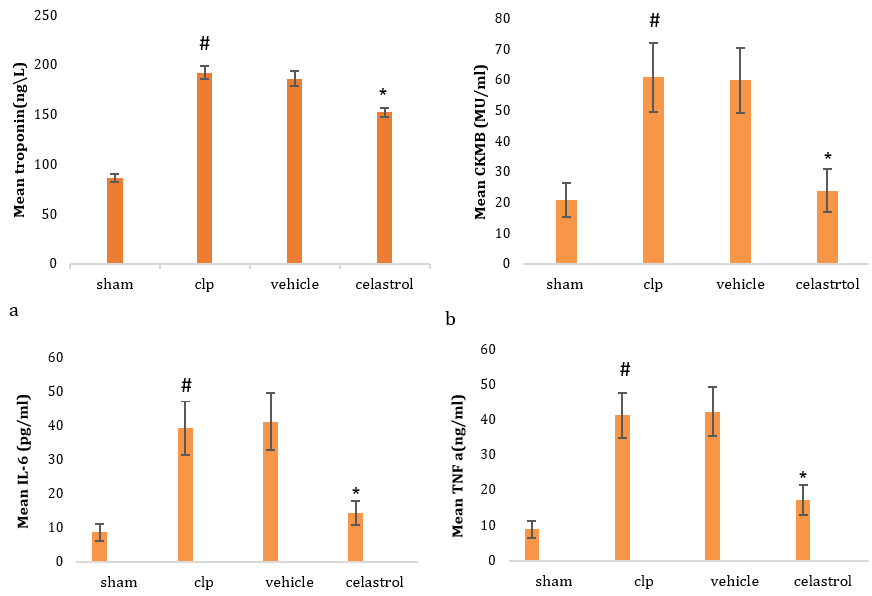
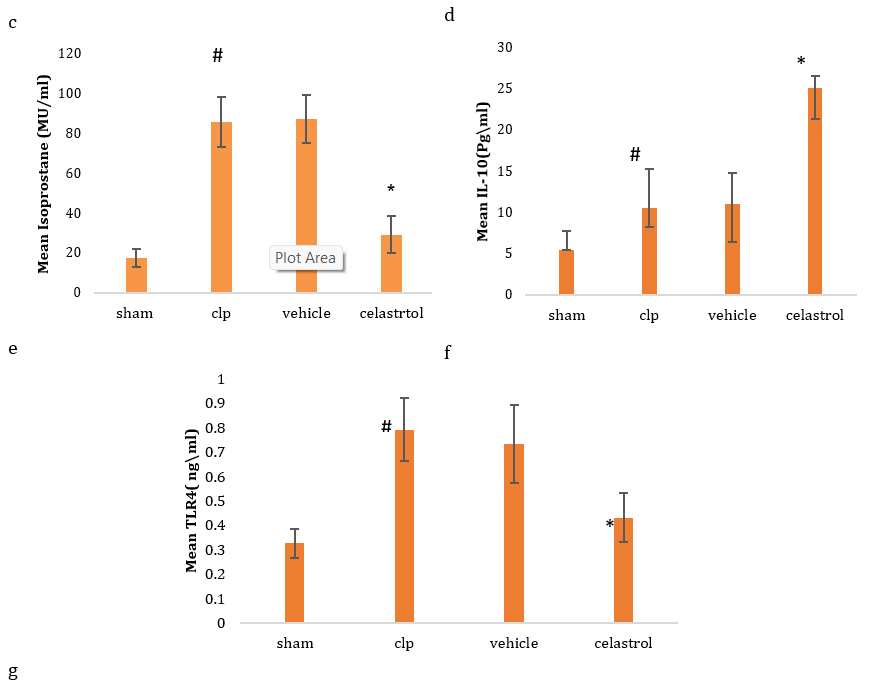
Figure 1. Level of serum troponin (a), CK-MB (b), IL6 (c), TNFα (d), F2 isoprostane (e), IL10 (f), and TLR4 (g) in four groups (#significant difference compared with the sham group; *significant difference compared with the CLP group)
A histological section from a mouse in a sham group exhibited normal cardiac tissue with no evidence of pathological injury (Figure 2a). In contrast, a section from the mouse in the sepsis group manifested significant histological damage via the presence of necrosis, vascular congestion, and inflammatory cell infiltration together with damaged normal tissue architecture (Figure 2b). Pretreatment of mice with celastrol reduced the degree of histological damage via a reduction in inflammatory cell infiltration, vascular congestion, and restoration of near-normal cardiac tissue architecture (Figure 2c).
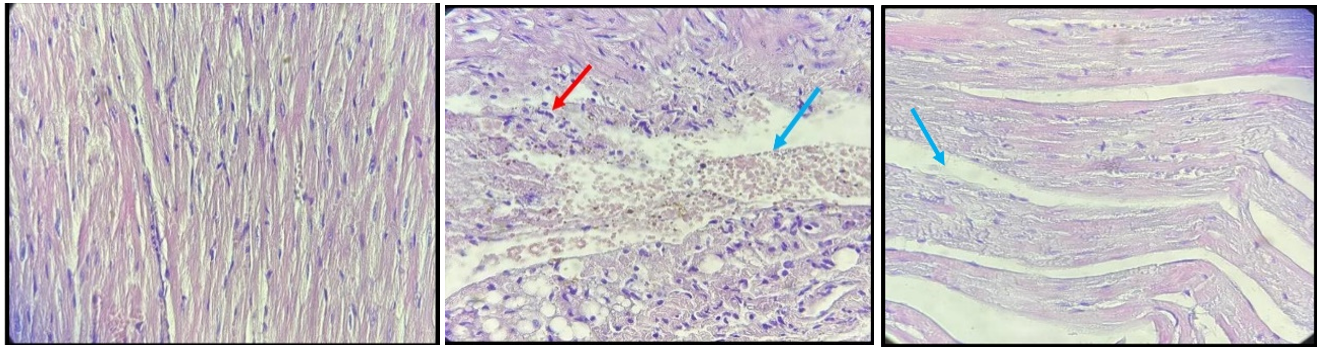
Figure 2. All sections were stained with hematoxylin and eosin in 40X. (a) Myocardial tissue of the sham group demonstrates normal-looking cardiac tissue; (b) CLP group, score four damaged myocardial tissue, hemorrhage (Blue arrow) with acute inflammatory cells (Red arrow); (c) celastrol-treated group, score one mild myocardial damage, interstitial edema (Blue arrow)
Discussion
Acute cardiac injury and cardiomyopathy are recognized fatal complications of sepsis and septic shock in which mortality rate can reach up to 60% among intensive care unit patients. Unfortunately, no definite treatment for this major insult is considered standard.
Many studies and research are directed toward using variable medications, herbal medicines, natural products, and targeted therapeutic modalities that could minimize the cardiac burden due to endotoxins during sepsis.
In this study, CLP and sepsis have led to a significant increment in serum troponin, which is in concordance with Lackner, who found that experimental sepsis can lead to a profound increment in cardiac troponin 1, which might be attributed to increased expression with up-regulation of troponin in the left ventricles mediated via inflammation [29].
In the same manner, CK-MB was significantly elevated in the sepsis group, which is similar to that found by Liu et al. and Sang et al., who found that CK-MB was significantly increased in mice that underwent CLP and sepsis due to cardiac cell damage mediated by overt inflammation, oxidative stress, and tissue destruction [30, 31].
Proinflammatory cytokine IL6 was highly elevated in the sepsis group, similar to previous studies [31-33]. In contrast, counter-regulatory cytokine IL10 was significantly decreased in the sepsis group, which is compatible with Gao S et al., who stated that septic shock would profoundly reduce the level of IL10 via a down-regulatory mechanism [34]. Meanwhile, treatment with celastrol significantly increased the level of IL10, which is in concordance with Zhao J et al., who proved that celastrol ameliorated colitis in mice deficient with IL10, reduced tissue inflammation, decreased ROS concentration together with suppression of inflammatory cytokine [35].
Sepsis will increase oxidative stress burden on different tissues, including the heart, is manifested by an increased level of F2 isoprostane marker, which is similar to that found by Alnfakh et al., who found that endotoxemia had produced a profound increment in the level of F2 isoprostane, which might be attributed to high levels of inflammatory cytokines produced both in systemic circulation and locally in the affected organ in addition to hypoxia [36].
In this research, the tissue level of TNF alpha was highly elevated in the sepsis group, which is similar to that found by Zeng N et al., who found that sepsis increased the level of TNF and NFkB in cardiac tissue and provoked an inflammatory response and destruction of cardiomyocytes [37].
Toll-like receptors are well known as the main receptors for certain microbial pathogens expressed in innate immunological cells and the heart. TLR4 signaling pathway activation during septicemia results in cardiac depression and impairment of cardiac function [38]; TLR4 level was significantly elevated in the sepsis group in this research which is in concordance with Wang Y et al., who found that the tissue level of TLR4 was extremely elevated in mice with septicemia which was attributed mainly to increased its expression by affected tissues [39].
Pretreatment of mice with celastrol has significantly reduced the level of troponin and CK-MB, inflammatory markers, as well as oxidative stress markers, which can be attributed mainly to the powerful cardio-protective properties of this natural product since it can protect cardiomyocytes against damage, hypertrophy, or death via multiple and complicated mechanisms that were hampering inflammation, ROS generation, endoplasmic reticulum stress, and apoptosis [40].
The cardioprotective effects of celastrol in this study were also in agreement with the previous study by Der Sarkissian S et al., who found that during permanent ligation of the coronary artery in rats with celastrol treatment for two weeks, the infarct size was highly reduced with improvement of cardiac function and reduction of left ventricular dilation and remodeling [25].
Another explanation of these cytoprotective properties of celastrol is the up-regulation of tissue survival pathways such as PI3K/AKT/ERK1,2 signaling pathway that promotes cell viability and reduces cell death in addition to enhancement of cellular viability during hypoxia [25].
Pretreatment of mice with celastrol led to a reduction in the level of cardiac TLR4 which is in agreement with Li G et al., who found that celastrol decreased mRNA expression of matrix metalloproteinases in addition to inhibition of certain transcription factors involved in inflammation and NF-κB expression so decrease the level of TLR4 via inhibition of TLR4-NFkB signaling pathway [41].
Sepsis-induced overt histological damage in cardiac tissue manifested by necrosis, inflammatory cell infiltration, and blood vessel congestion, which is in agreement with Zigam et al., who found that sepsis produced contraction areas within cardiac tissue with neutrophilic infiltration, necrosis, and interstitial edema [42]. Whereas pretreatment with celastrol produced a reduction of histological damage and restoration of near normal cardiac tissue architecture with significant cardiac tissue protection, such effect was also documented by Fan J et al., who found that celastrol reduced the histological damage of the myocardium post myocardial infarction via its anti-inflammatory and antioxidant properties [43].
Celastrol was found to have anti-apoptotic, anti-inflammatory, and cardiac protection against diabetic cardiomyopathy in an animal model of diabetes by preventing cardiac remodeling mediated by inhibition of the ACE-ANGR1 pathway [44].
The current study used celastrol in a single dose equal to 2mg/kg, so further work with more different doses and longer duration is feasible. On the other hand, the cardioprotective effect of this natural substance appeared to be mediated by TLR4 downstream signaling pathways, including NF-κB cascades. Other possible mechanisms that need more investigation may be involved.
Conclusion
Celastrol has cardio-protective properties in endotoxemia-induced cardiac injury in mice mediated by its anti-inflammatory, antioxidant, and cellular protective properties and modulating effects on the TLR4 signaling pathway.
Acknowledgments: The researchers would like to thank all the workers in the Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa, for their continuous support in completing the research requirements.
Ethical Permissions: The study was ethically approved by the central laboratory animal use and care committee in the Faculty of Medicine, Kufa University.
Conflicts of Interests: All authors declare no conflict of interest in the study.
Authors’ Contribution: Ghafil FA (First Author), Introduction Writer/Discussion Writer/Main Researcher (30%); Sabah E (Second Author) Assistant Researcher/Statistical Analyst/Methodologist (30%); Aziz N (Third Author), Assistant Researcher (10%); Salim MM (Fourth Author) Assistant Researcher (10%); Majeed SA (Fifth Author) Methodologist (10%); Suaad MH Rasheed (Sixth Author), Statistical Analyst/Assistant Researcher (5%); Mardan HW (Seventh Author), Discussion Writer (5%)
Funding/Support: This is a self-funded study. All authors participated in the costs.
Sepsis is a critical clinical condition that carries a high rate of morbidity and mortality; occurs when the body's defenses against infection and pathogen invasion are dysregulated and seriously harm its tissues and organs, resulting in end-organ damage and failure [1, 2]. It represents the body's reaction to a massive and severe infection that has failed to be locally controlled due to a dysregulated immune response and overwhelming life-threatening complications, especially in susceptible individuals [3, 4]. Sepsis is a dangerous worldwide health insult with a high rate of mortality and high economic concern [5].
This condition is frequently complicated by cardiac injury in which the heart is the most affected organ by sepsis; this cardiac insult is manifested by an increment in cardiac troponin and creatine-kinase MB (CK-MB) [6, 7]. That situation usually induces depression of cardiac muscle, impairment of coronary arterial circulation, defective cardiac contraction, cardiomyopathy, and mitochondrial damage. Sepsis provokes a systemic inflammatory response, increases pro-inflammatory cytokines production such as interleukins 6 (IL6), tumor necrosis factor (TNF), and toll-like receptor 4 (TLR4) with consequent overt cytokine storm [8], energy depletion with oxidative stress burden together with endothelial dysfunction and apoptosis all are responsible for cellular damage and death [9].
Many factors are involved in myocardial injury during sepsis: cytokines, complement cascade elements, damage associated with variable molecular patterns, ROS, and oxidative stress, dysregulated metabolism of nitric oxide, mitochondrial injury, disturbed calcium homeostasis within cells, and apoptosis [10]. The endothelial dehiscence will also induce vascular leakage, interstitial edema, coagulopathy, and inflammatory response that augment the adverse burden of sepsis [11]. This destruction of the endothelium can disturb microvascular blood flow, increase vascular leakage, and consequent edema [12].
TLR4 is the key member of the toll-like receptor family responsible for pattern recognition. It performs various functions in pathological conditions, such as cardiovascular disease, neuronal degeneration, inflammatory bowel diseases, and allergic, metabolic, and autoimmune disorders [13]. TLR4 has the highest level in the heart compared to other TLRs and plays a vital role in myocardial inflammation, including myocarditis, ischemia-reperfusion injury, atherosclerosis, hypertension, and heart failure [13]. TLR4 has been known as a receptor for Lipopolysaccharides and plays an important role in initiating inflammation, myocardial injury, and dysfunction [5]. Variable signaling pathways are activated upon TLR4 binding to lipopolysaccharides like primary response protein 88: MyD88 [5, 14]. Macrophage scavenger receptor class A is suggested in recent research as a co-receptor for TLR4 to facilitate inflammatory responses, promote cell apoptosis, and suppress cell survival [15].
Isoprostanes are free radical-catalyzed PG-like products. They are broadly documented as a vital marker for oxidative stress and systemic lipid peroxidation in vivo. Formation of these substances is increased in a variety of cardiovascular diseases (CVD) and may predict the risk of cardiovascular complications in patients. Several isoprostanes may openly contribute to the functional concerns of oxidant stress by affecting endothelial cell regeneration and function, tone of vessels, hemostasis, and ischemia/reperfusion injury [16].
Indeed, human and animal studies have researched the correlation between oxidative stress accompanied by increased levels of isoprostane and the outcome of CVD. Therefore, a targeted inhibition of isoprostane formation could help improve outcomes in CVD patients [16].
Despite extensive clinical research dealing with cardiomyopathy and cardiac injury due to endotoxemia regarding its mechanisms, pathophysiological disturbances, and complications, no definite treatment is valuable, and most of the clinical options are uncertain [17].
To understand the pathology and causes of human sepsis, researchers have developed a model of cecal ligation and puncture (CLP), which consists of the cecal ligation and perforation to release the fecal material into the peritoneum; this will generate an exaggerated immune response due to polymicrobial infection, CLP model is of high clinical relevancy [18, 19].
Celastrol is an extract of the Tripterygium wilfordii hook in China. It is a methide triterpenoid with pleiotropic properties, such as antiinflammatory, antioxidant, and immune modulation [20].
Celastrol is a potent antiinflammatory agent via a reduction in IL6, IL1 B, TNF, TLR4, and NFKB, as indicated by previous studies via inhibition of signaling pathways activated by inflammation [21]. It also has an antioxidant property manifested by its role in reducing ROS, lipid peroxidation, and adhesion molecules, together with enhancing antioxidant defenses [22]. Celastrol was found to ameliorate right-sided heart failure by reduction of oxidative stress, inflammation, and remodeling of the pulmonary artery, so a reduction in cardiac failure in mice subjected to hypoxia-induced pulmonary hypertension by reducing right ventricular remodeling, systolic blood pressure, hypertrophy, impaired function, and fibrosis [23]. Celastrol could impede inflammatory reactions induced by sepsis as well as retard the production of variable pro-inflammatory cytokines and chemokines. Celastrol could similarly maintain mitochondrial functions by antagonizing certain oxidative and inflammatory pathways. Celastrol could induce upregulation of certain inflammatory nuclear receptors, so it has an effective anti-inflammatory role, which provides the basis for the development of new agents that can oppose inflammation by maintaining mitochondrial functions and homeostasis [24].
Celastrol enhances strong pro-survival signals that are responsible for the improvement of cardiac cell survival during hypoxia. Continuous celastrol treatment in rats with myocardial ischemia and permanent ligation of the coronary artery for two weeks decreases the size of the infarcted area, improves cardiac performance and function, and protects against deleterious remodeling of the left ventricular wall [25]. Celastrol exerts cardioprotective properties dependent on the amelioration of Reactive Oxygen Species and Heat Shock response since the increased oxidative stress after ischemic insults will trigger Heat Shock protein activation. Celastrol augments several Heat Shock Protein mRNA expressions, such as HSP (70 and 32), leading to their translocation from the cytoplasm to the nucleus [25]. Celastrol could decrease the expression level of pro-inflammatory markers with increased expression of antiinflammatory cytokine IL-10 in the liver and adipose tissue. In addition, celastrol reduced the plasma levels of inflammatory cytokines such as IL-6 and IL-1β in the experimental mouse model [5].
Previous studies have provided celastrol with variable cytoprotective and pleotropic properties, making it a good candidate agent for ameliorating cardiac injury accompanied by sepsis. The study aimed to assess the cardioprotective potential of celastrol against cardiac injury induced by sepsis via amelioration of IL6, TNF, TLR4, IL10, F2-isoprostane, cardiac troponin, and CK-MB, as well as at histological level.
Materials and Methods
In this experimental study, 24 adult male mice were included; they were 6-8 weeks old and weighed 25-30g. The animals were randomly allocated into four groups (n=6);
- Sham: Midline 1-2cm laparotomy under anesthesia without CLP
- Sepsis (CLP): Laparotomy, had their cecum exposed ligated and off by suture below the ileocecal valve and punctured one time by a needle gauge 19 [26]
- Vehicle (DMSO): An equal volume of vehicle IP 1 hour before laparotomy and CLP [27]
- Celastrol: Treated with 2mg/kg in 1-hour IP before the CLP process [27]
All animals were supervised while maintaining good hydration and body temperature, and all procedures were performed under anesthesia. Careful animal supervision was maintained throughout the time of study. Celastrol powder was supplied by (Solarbio; China) and dissolved in diluted DMSO 5% in normal saline, and dimethyl sulfoxide (DMSO) was supplied by (Abu Dhabi Medical; UAE). Complete Protease inhibitor liquid was supplied by (Medchem Express; United States). After 24 hours, they were sacrificed by using ketamine and xylazine to obtain blood and tissue samples. Blood had been aspirated from the heart to assess the serum troponin (Beckman Coulter; USA) and CK-MB (Beckman Coulter; USA) by spectrophotometric assay. Cardiac resection was done, and the heart was divided into two parts; one for homogenate to be used in the ELISA (TNF-α, IL-6, IL-10, F2- isoprostane levels) procedure (BT-LAB; China), another part was preserved in formalin, processed, then fixed in paraffin and prepared for histopathological analysis [28].
Kolmogorov-Smirnov and Schapiro tests were applied after testing the normality of data. ANOVA test was applied for the mean difference of numerical variables among study groups with their bar chart using SPSS 26 software.
Findings
There was a significant elevation in serum troponin in sepsis and vehicle groups compared to the sham group. In contrast, pretreatment of mice with celastrol resulted in a significant reduction in the level of serum troponin (Figure 1a). There was a significant elevation in serum level of CK-MB in sepsis and vehicle groups as compared to the sham group. In contrast, pretreatment of mice with celastrol was resulted in significant reduction in the tissue level of CK-MB (Figure 1b). The tissue level of IL6 was significantly elevated in the sepsis and vehicle groups compared to the sham group. In contrast, pretreatment of mice with celastrol resulted in a significant reduction in the tissue level of IL6 (Figure 1c). There was a significant elevation in the tissue level of TNFα in sepsis and vehicle groups compared to the sham group. In contrast, pretreatment of mice with celastrol resulted in a significant reduction in the tissue level of TNFα (Figure 1d). Compared to the sham group, there was a significant elevation in the tissue level of F2 isoprostane in sepsis and vehicle groups. In contrast, pretreatment of mice with celastrol resulted in a significant reduction in the tissue level of F2 isoprostane (Figure 1e). There was significant reduction in tissue level of IL10 in sepsis and vehicle groups as compared to sham group, whereas pretreatment of mice with celastrol was resulted in significant elevation in the tissue level of IL10 (Figure 1f). There was a significant elevation in the tissue level of TLR4 in sepsis and vehicle groups as compared to the sham group, whereas pretreatment of mice with celastrol resulted in a significant reduction in the tissue level of TLR4 (Figure 1g).


Figure 1. Level of serum troponin (a), CK-MB (b), IL6 (c), TNFα (d), F2 isoprostane (e), IL10 (f), and TLR4 (g) in four groups (#significant difference compared with the sham group; *significant difference compared with the CLP group)
A histological section from a mouse in a sham group exhibited normal cardiac tissue with no evidence of pathological injury (Figure 2a). In contrast, a section from the mouse in the sepsis group manifested significant histological damage via the presence of necrosis, vascular congestion, and inflammatory cell infiltration together with damaged normal tissue architecture (Figure 2b). Pretreatment of mice with celastrol reduced the degree of histological damage via a reduction in inflammatory cell infiltration, vascular congestion, and restoration of near-normal cardiac tissue architecture (Figure 2c).

Figure 2. All sections were stained with hematoxylin and eosin in 40X. (a) Myocardial tissue of the sham group demonstrates normal-looking cardiac tissue; (b) CLP group, score four damaged myocardial tissue, hemorrhage (Blue arrow) with acute inflammatory cells (Red arrow); (c) celastrol-treated group, score one mild myocardial damage, interstitial edema (Blue arrow)
Discussion
Acute cardiac injury and cardiomyopathy are recognized fatal complications of sepsis and septic shock in which mortality rate can reach up to 60% among intensive care unit patients. Unfortunately, no definite treatment for this major insult is considered standard.
Many studies and research are directed toward using variable medications, herbal medicines, natural products, and targeted therapeutic modalities that could minimize the cardiac burden due to endotoxins during sepsis.
In this study, CLP and sepsis have led to a significant increment in serum troponin, which is in concordance with Lackner, who found that experimental sepsis can lead to a profound increment in cardiac troponin 1, which might be attributed to increased expression with up-regulation of troponin in the left ventricles mediated via inflammation [29].
In the same manner, CK-MB was significantly elevated in the sepsis group, which is similar to that found by Liu et al. and Sang et al., who found that CK-MB was significantly increased in mice that underwent CLP and sepsis due to cardiac cell damage mediated by overt inflammation, oxidative stress, and tissue destruction [30, 31].
Proinflammatory cytokine IL6 was highly elevated in the sepsis group, similar to previous studies [31-33]. In contrast, counter-regulatory cytokine IL10 was significantly decreased in the sepsis group, which is compatible with Gao S et al., who stated that septic shock would profoundly reduce the level of IL10 via a down-regulatory mechanism [34]. Meanwhile, treatment with celastrol significantly increased the level of IL10, which is in concordance with Zhao J et al., who proved that celastrol ameliorated colitis in mice deficient with IL10, reduced tissue inflammation, decreased ROS concentration together with suppression of inflammatory cytokine [35].
Sepsis will increase oxidative stress burden on different tissues, including the heart, is manifested by an increased level of F2 isoprostane marker, which is similar to that found by Alnfakh et al., who found that endotoxemia had produced a profound increment in the level of F2 isoprostane, which might be attributed to high levels of inflammatory cytokines produced both in systemic circulation and locally in the affected organ in addition to hypoxia [36].
In this research, the tissue level of TNF alpha was highly elevated in the sepsis group, which is similar to that found by Zeng N et al., who found that sepsis increased the level of TNF and NFkB in cardiac tissue and provoked an inflammatory response and destruction of cardiomyocytes [37].
Toll-like receptors are well known as the main receptors for certain microbial pathogens expressed in innate immunological cells and the heart. TLR4 signaling pathway activation during septicemia results in cardiac depression and impairment of cardiac function [38]; TLR4 level was significantly elevated in the sepsis group in this research which is in concordance with Wang Y et al., who found that the tissue level of TLR4 was extremely elevated in mice with septicemia which was attributed mainly to increased its expression by affected tissues [39].
Pretreatment of mice with celastrol has significantly reduced the level of troponin and CK-MB, inflammatory markers, as well as oxidative stress markers, which can be attributed mainly to the powerful cardio-protective properties of this natural product since it can protect cardiomyocytes against damage, hypertrophy, or death via multiple and complicated mechanisms that were hampering inflammation, ROS generation, endoplasmic reticulum stress, and apoptosis [40].
The cardioprotective effects of celastrol in this study were also in agreement with the previous study by Der Sarkissian S et al., who found that during permanent ligation of the coronary artery in rats with celastrol treatment for two weeks, the infarct size was highly reduced with improvement of cardiac function and reduction of left ventricular dilation and remodeling [25].
Another explanation of these cytoprotective properties of celastrol is the up-regulation of tissue survival pathways such as PI3K/AKT/ERK1,2 signaling pathway that promotes cell viability and reduces cell death in addition to enhancement of cellular viability during hypoxia [25].
Pretreatment of mice with celastrol led to a reduction in the level of cardiac TLR4 which is in agreement with Li G et al., who found that celastrol decreased mRNA expression of matrix metalloproteinases in addition to inhibition of certain transcription factors involved in inflammation and NF-κB expression so decrease the level of TLR4 via inhibition of TLR4-NFkB signaling pathway [41].
Sepsis-induced overt histological damage in cardiac tissue manifested by necrosis, inflammatory cell infiltration, and blood vessel congestion, which is in agreement with Zigam et al., who found that sepsis produced contraction areas within cardiac tissue with neutrophilic infiltration, necrosis, and interstitial edema [42]. Whereas pretreatment with celastrol produced a reduction of histological damage and restoration of near normal cardiac tissue architecture with significant cardiac tissue protection, such effect was also documented by Fan J et al., who found that celastrol reduced the histological damage of the myocardium post myocardial infarction via its anti-inflammatory and antioxidant properties [43].
Celastrol was found to have anti-apoptotic, anti-inflammatory, and cardiac protection against diabetic cardiomyopathy in an animal model of diabetes by preventing cardiac remodeling mediated by inhibition of the ACE-ANGR1 pathway [44].
The current study used celastrol in a single dose equal to 2mg/kg, so further work with more different doses and longer duration is feasible. On the other hand, the cardioprotective effect of this natural substance appeared to be mediated by TLR4 downstream signaling pathways, including NF-κB cascades. Other possible mechanisms that need more investigation may be involved.
Conclusion
Celastrol has cardio-protective properties in endotoxemia-induced cardiac injury in mice mediated by its anti-inflammatory, antioxidant, and cellular protective properties and modulating effects on the TLR4 signaling pathway.
Acknowledgments: The researchers would like to thank all the workers in the Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa, for their continuous support in completing the research requirements.
Ethical Permissions: The study was ethically approved by the central laboratory animal use and care committee in the Faculty of Medicine, Kufa University.
Conflicts of Interests: All authors declare no conflict of interest in the study.
Authors’ Contribution: Ghafil FA (First Author), Introduction Writer/Discussion Writer/Main Researcher (30%); Sabah E (Second Author) Assistant Researcher/Statistical Analyst/Methodologist (30%); Aziz N (Third Author), Assistant Researcher (10%); Salim MM (Fourth Author) Assistant Researcher (10%); Majeed SA (Fifth Author) Methodologist (10%); Suaad MH Rasheed (Sixth Author), Statistical Analyst/Assistant Researcher (5%); Mardan HW (Seventh Author), Discussion Writer (5%)
Funding/Support: This is a self-funded study. All authors participated in the costs.
References
1. Jawad AS, Hassan ES, Mohammad AR. Protective effect of empagliflozin from acute renal injury during endotoxemia in mice model. Lat Am J Pharm. 2022;41(2):463-71. [Link]
2. Hamza RT, Majeed SA, Ghafil FA, Hassan, E. S. Hadi NR. Nephroprotective effect of melatonin in sepsis induces renal injury : CLP mice model. Lat Am J Pharm. 2022;41(3):589-96. [Link]
3. Abd Uljaleel AQ, Hassan ES, Mohammad AR, Hadi NR. Protective effect of dulaglutide on lung injury in endotoxemia mouse model. Iran J War Public Health 2023;15(1):35-42. [Link]
4. Ghafil FA, Majeed SA, Qassam H, Mardan HW, Hadi NR. Nephroprotective effect of gamma-secretase inhibitor on sepsis- induced renal injury in mouse model of clp. Wiad Lek. 2023;76(1):122-30. [Link] [DOI:10.36740/WLek202301117]
5. Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y, et al. Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS One. 2013;8(6):e65477. [Link] [DOI:10.1371/journal.pone.0065477]
6. Garg N, Soni KD, Aggarwal R. Unstable cardiac injury complicated with septic shock-a challenge. Burns Trauma. 2016:4:11. [Link] [DOI:10.1186/s41038-016-0035-y]
7. Mohammed Hussain Hadi S, Majeed S, Ghafil FA, Altoraihi K, Hadi NR. Effect of sulforaphane on cardiac injury induced by sepsis in a mouse model : Role of toll-like receptor 4. J Med Life. 2023;16(7):1120-6. [Link] [DOI:10.25122/jml-2023-0015]
8. Abdul Kadhim SA, Ghafil FA, Majeed SA, Hadi NR. Nephroprotective effects of curcumin against cyclosporine a-induced nephrotoxicity in rat model. Wiad Lek. 2021;74(12):3135-46. [Link] [DOI:10.36740/WLek202112103]
9. Abd Uljaleel A, Hassan E. Protective effect of ertugliflozin against acute lung injury caused by endotoxemia model in mice. Iran J War Public Health. 2023;15(1):67-75. [Link]
10. Ehrman RR, Sullivan AN, Favot MJ, Sherwin RL, Reynolds CA, Abidov A, et al. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: A review of the literature. Crit Care. 2018;22(1):112. [Link] [DOI:10.1186/s13054-018-2043-8]
11. Smart L, Bosio E, Macdonald SPJ, Dull R, Fatovich DM, Neil C, et al. Glycocalyx biomarker syndecan-1 is a stronger predictor of respiratory failure in patients with sepsis due to pneumonia, compared to endocan. J Crit Care. 2018;47:93-8. [Link] [DOI:10.1016/j.jcrc.2018.06.015]
12. Vasques-Nóvoa F, Laundos TL, Madureira A, Bettencourt N, Nunes JPL, Carneiro F, et al. Myocardial edema: An overlooked mechanism of septic cardiomyopathy?. Shock. 2020;53(5):616-9. [Link] [DOI:10.1097/SHK.0000000000001395]
13. Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W, et ak. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016;7(5):e2234. [Link] [DOI:10.1038/cddis.2016.140]
14. Ghazi A, Majeed SA, Metib NJ, Abood SH, Alaqouli H, Hadi NR. Ibudilast ameliorates acute pancreatitis through downregulation of interleukin-1 beta and lipase Enzyme. Asian J Pharm. 2020;14(1):9-11. [Link]
15. Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794-9. [Link] [DOI:10.1073/pnas.0609671104]
16. Bauer J, Ripperger A, Frantz S, Ergün S, Schwedhelm E, Benndorf RA. Pathophysiology of isoprostanes in the cardiovascular system: implications of isoprostane-mediated thromboxane A2 receptor activation. Br J Pharmacol. 2014;171(13):3115-31. [Link] [DOI:10.1111/bph.12677]
17. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18(3):169-93. [Link] [DOI:10.1038/s41569-020-00435-x]
18. Blangy-Letheule A, Persello A, Rozec B, Waard M De, Lauzier B. New approaches to identify sepsis biomarkers: The importance of model and sample source for mass spectrometry. Oxid Med Cell Longev. 2020;2020:6681073. [Link] [DOI:10.1155/2020/6681073]
19. Hadi SMH, Majeed S, Ghafil FAS, Altoraihi K, Hadi NR. Xanthohumol ameliorates cardiac injury induced by sepsis in a mice model: role of toll-like receptor 4. J Med Life. 2023;16(7):1105-10. [Link] [DOI:10.25122/jml-2023-0016]
20. Mohammad AR, Hadi AR, Hassan ES. Potential protective effect of ibrutinib from acute brain injury during endotoxemia in mice. Lat Am J Pharm. 2022;41 (2):472-80. [Link]
21. Hussein SN, Majeed SA, Ghafil FA, Hassan ES, Abdulkadim AH, Ghazi A, et al. Nephroprotective effect of Celastrol in an experimental model of Endotoxemia. Bulletin of national institute of health. 2022;140(6):2865-74.
22. Venkatesha SH, Dudics S, Astry B, Moudgil KD. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog Dis. 2016;74(6):ftw059. [Link] [DOI:10.1093/femspd/ftw059]
23. Kurosawa R, Satoh K, Nakata T, Shindo T, Kikuchi N, Satoh T, et al. Identification of celastrol as a novel therapeutic agent for pulmonary arterial hypertension and right ventricular failure through suppression of Bsg (Basigin)/CyPA (Cyclophilin A). Arterioscler Thromb Vasc Biol. 2021;41(3):1205-17. [Link] [DOI:10.1161/ATVBAHA.120.315731]
24. Tao Z, Xiao Q, Che X, Zhang H, Geng N, Shao Q. Regulating mitochondrial homeostasis and inhibiting inflammatory responses through Celastrol. Ann Transl Med. 2022;10(7):400. [Link] [DOI:10.21037/atm-21-7015]
25. Der Sarkissian S, Cailhier JF, Borie M, Stevens LM, Gaboury L, Mansour S, et al. Celastrol protects ischaemic myocardium through a heat shock response with up-regulation of haeme oxygenase-1. Br J Pharmacol. 2014;171(23):5265-79. [Link] [DOI:10.1111/bph.12838]
26. Hassan ES, Jawad AS, Mohammad AR. Protective effect of liraglutide from acute renal injury during endotoxemia in mice mode. Lat Am J Pharm. 2022;41(2):428-36. [Link]
27. Yu X, Meng X, Xu M, Zhang X, Zhang Y, Ding G, et al. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-κB and improving mitochondrial function. EBioMedicine. 2018;36:266-80. [Link] [DOI:10.1016/j.ebiom.2018.09.031]
28. Hussein SN, Majeed SA, Ghafil FA, Hassan ES, Hadi NR. Toll-like receptors 4 antagonist, Ibudilast, ameliorates acute renal impairment induced by sepsis in an experimental model. BNJHS. 2022;140(7):2900-9. [Link]
29. Lackner I, Weber B, Chakraborty S, Braumüller S, Huber-Lang M, Gebhard F, et al. Toll-like receptor-mediated cardiac injury during experimental sepsis. Mediators Inflamm. 2020;2020:6051983. [Link] [DOI:10.1155/2020/6051983]
30. Sang Z, Zhang P, Wei Y, Dong S. MiR-214-3p attenuates sepsis-induced myocardial dysfunction in mice by inhibiting autophagy through PTEN/AKT/mTOR Pathway. Biomed Res Int. 2020;2020:1409038.. [Link] [DOI:10.1155/2020/1409038]
31. Liu Z, Zeng Z, Wu C, Liu H. Tropisetron inhibits sepsis by repressing hyper-inflammation and regulating the cardiac action potential in rat models. Biomed Pharmacother. 2019;110:380-8. [Link] [DOI:10.1016/j.biopha.2018.11.142]
32. Mohammad AR, Shnaien AA, Alabsawy SK, Hassan ES. Protective effect of ipragliflozin in acute brain injury induced by endotoxemia in mice. Iran J War Public Health 2023;15(3):1001-8. [Link] [DOI:10.58209/ijwph.15.3.225]
33. Shnaien A, Mohammad A, Hassan E. Neuroprotective effects of semaglutide in endotoxemia mouse model. Iran J War Public Health 2023;15(2):199-205. [Link] [DOI:10.58209/ijwph.15.3.225]
34. Gao S, Li H, Xie H, Wu S, Yuan Y, Chu L, et al. Therapeutic efficacy of Schistosoma japonicum cystatin on sepsis-induced cardiomyopathy in a mouse model. Parasit Vectors. 2020;13(1):260. [Link] [DOI:10.1186/s13071-020-04104-3]
35. Zhao J, Sun Y, Shi P, Dong JN, Zuo LG, Wang HG, et al. Celastrol ameliorates experimental colitis in IL-10 deficient mice via the up-regulation of autophagy. Int Immunopharmacol. 2015;26(1):221-8. [Link] [DOI:10.1016/j.intimp.2015.03.033]
36. Alnfakh ZA, Al-Mudhafar DH, Al-Nafakh RT, Jasim AE, Hadi NR. The anti-inflammatory and antioxidant effects of Montelukast on lung sepsis in adult mice. J Med Life. 2022;15(6):819-27. [Link] [DOI:10.25122/jml-2021-0269]
37. Zeng N, Jian Z, Zhu W, Xu J, Fan Y, Xiao F. KLF13 overexpression protects sepsis-induced myocardial injury and LPS-induced inflammation and apoptosis. Int J Exp Pathol. 2023;104(1):23-32. [Link] [DOI:10.1111/iep.12459]
38. Sameer AS, Nissar S. Toll-Like Receptors (TLRs): Structure, functions, signaling, and role of their polymorphisms in colorectal cancer susceptibility. Biomed Res Int. 2021;2021:1157023. [Link] [DOI:10.1155/2021/1157023]
39. Afrazi A, Branca MF, Sodhi CP, Good M, Yamaguchi Y, Egan CE, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. 2014;289(14):9584-99. [Link] [DOI:10.1074/jbc.M113.526517]
40. Li Z, Zhang J, Duan X, Zhao G, Zhang M. Celastrol: A promising agent fighting against cardiovascular diseases. Antioxidants. 2022;11(8):1597. [Link] [DOI:10.3390/antiox11081597]
41. Li G, Liu D, Zhang Y, Qian Y, Zhang H, Guo S, et al. Celastrol inhibits lipopolysaccharide-stimulated rheumatoid fibroblast-like synoviocyte invasion through suppression of TLR4/NF-κB-mediated matrix metalloproteinase-9 expression. PLoS One. 2013;8(7):e68905. [Link] [DOI:10.1371/journal.pone.0068905]
42. Zigam QA, Al-Zubaidy AA, Sami Z, Abbas WJ. The effects of levosimendan against sepsis-induced cardiotoxicity in mice model. J Med Chem Sci. 2023;6(3):634-44. [Link]
43. Fan J, Ren M, Chen W, Wang H, He Y. Celastrol relieves myocardial infarction-induced cardiac fibrosis by inhibiting NLRP3 inflammasomes in rats. Int Immunopharmacol. 2023;121:110511. [Link] [DOI:10.1016/j.intimp.2023.110511]
44. Zhao X, Huang B, Zhang J, Xiang W, Zhu N. Celastrol attenuates streptozotocin-induced diabetic cardiomyopathy in mice by inhibiting the ACE/Ang II/AGTR1 signaling pathway. Diabetol Metab Syndr. 2023;15(1):1-12. [Link] [DOI:10.1186/s13098-023-01159-x]









