Volume 15, Issue 4 (2023)
Iran J War Public Health 2023, 15(4): 387-394 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/07/5 | Accepted: 2023/12/10 | Published: 2023/12/19
Received: 2023/07/5 | Accepted: 2023/12/10 | Published: 2023/12/19
How to cite this article
Emaduldeen H, Waheeb M, Makki M. Antimicrobial Features of Lepidium sativum L. extract. Iran J War Public Health 2023; 15 (4) :387-394
URL: http://ijwph.ir/article-1-1366-en.html
URL: http://ijwph.ir/article-1-1366-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Environment Research Center, University of Technology, Baghdad, Iraq
2- Department of Biology, College of Science, Al Muthanna University, AL Muthanna, Iraq
2- Department of Biology, College of Science, Al Muthanna University, AL Muthanna, Iraq
Full-Text (HTML) (545 Views)
Introduction
The fast-growing annual herb known as garden cress (Lepidium sativum L.) originates in Egypt and western Asia but is now planted in nearly every region. Garden cress (GC), or Chandrasur as it is known in local languages, is widely cultivated in India for its therapeutic properties. The herbaceous annual grows to a height of 15-45cm and has an upright, glabrous appearance. Small white flowers are carried on long racemes, and the pods might be round or obovate in shape, elliptic without margins, notched at the tip, and wingless. The medicines found in nature are the best there is. Humans have used medicinal plants as a form of disease prevention ever since they developed civilization [1]. Natural chemicals with therapeutic features in the herbs and medicinal plants used for thousands of years are said to be the origin of herbal medicine and traditional healthcare systems like Ayurveda, Siddha, and Unani. 80% of the world's population, mostly in developing nations, rely on medicines made from plants for their health. One-fourth of all prescriptions are formulations based on chemicals derived from plants or plant-derived synthetic analogs. Indigenous wisdom handed down from one generation to the next has significantly impacted traditional medical practices around the globe. Likewise, the exploration of medicinal plants has been aided by this knowledge [2]. The discovery of novel chemical entities has benefited greatly from this investigation of physiologically active natural compounds. This article summarizes the research on Lepidium sativum Linn, including its traditional medical applications, isolated chemicals, and pharmacological studies.
Despite this, many medicinal plants are disappearing, and native civilizations are being destroyed. If the current extinction rate and destruction is not reversed, we stand to lose a significant portion of our biodiversity, the variety of genes, species, ecosystems, and cultural practices. Native peoples have been the guardians of 99 percent of the world's genetic resources, essential to people's economic, agricultural, and health circumstances everywhere. Culture and biological variables are also intrinsically linked [3].
Because so many people worldwide are vulnerable to mosquito-borne illnesses, they threaten human health. The Culex pipiens L. (Diptera: Culicidae) mosquito is a potential vector for a wide variety of diseases, including western equine encephalitis, West Nile virus, Rift Valley fever, St. Louis encephalitis, and bancroftian filariasis. With a flying distance of 1.15 to 2.48km, Culex pipiens may disperse their eggs over a vast area and contribute to the spread of illness. Mosquitoes thrive in poor water drainage and improper waste management [4]. Constant and unchecked use of mosquitocidal chemicals has resulted in mosquito resistance and has negatively affected humans, animals, and the environment.
Lepidium sativum is a tall, annual herb that can reach a height of 50 centimeters. White, little flowers are arranged in racemes, and the leaves can be either lobed or whole. The obovate pods contain two seeds and are around 5 mm in length. A plant's volatile oils can be found in its seeds and leaves. Natives of Saudi Arabia, Sudan, and other Arabic countries know all about the benefits of the Lepidium sativum plant and its seeds for healing bone fractures. Lepidium sativum seed extracts have been utilized historically to treat a broad range of medical conditions. as highlighted by several recent studies [5].
Asthma, a persistent cough, and hemorrhaging piles can all be helped using the plant's aerial parts. The seeds are chewed for various medical purposes, including relieving a sore throat, cough, asthma, or headache. Moreover, they can be used topically as an effective insect repellent. The leaves' mild stimulant and diuretic properties make them excellent for treating scorbutic diseases and liver complaints. Syphilis can be treated with roots. This herb has many medicinal uses in Europe, including as a diuretic, an expectorant, and a treatment for cough and constipation [6].
A rising number of bacteria are becoming antibiotic-resistant, posing a serious health risk. Various biomolecules and metabolites with crucial biological functions are already present in plants. These organic compounds are a gold mine when combating germs that have developed resistance to multiple drugs [7]. Green synthesis employs botanical components and could yield new classes of antibiotics derived from plants. "Lepidium sativum" (L.) LS, also known as garden cress, is a fast-reproducing annual plant. LS seed oil's antimicrobial, antioxidant, and anti-inflammatory properties make it an intriguing therapeutic option. Eliminating Vectors and Individual Safety Limiting your exposure to vectors is presently the most effective way to avoid contracting a disease spread by a vector [8]. It is normal practice to use chemically based intervention strategies for vector control and to reduce the transfer of human infections. Insecticides, repellents, and larvicides made from chemicals were once widely used. Still, their overuse led to the rise of insect-resistant strains, ecological disruption, and human and non-target organism toxicity [9].
Insects are predators, and many plant species have evolved to have defense mechanisms that either eliminate or drive away these pests. These chemicals come in various uses, including as repellants, feeding deterrents, poisons, and growth regulators. Phytophagous insect defense is the primary function of these chemicals. Still, many of them, especially volatile components released as a result of herbivores, are also effective against mosquitoes and other biting Diptera. And, as has been reported, the fact that many of these compounds are also effective against hematophagous insects may be a relic of an ancestral plant-eating lifestyle, given that they evolved as repellents/killers to phytophagous insects.
Material and Methods
Plant material
The Lepidium sativum seeds were purchased from a vendor in Ghaziabad, Uttar Pradesh, India, in May 2019. Using the available ethnobotanical data, a voucher specimen (ML-1/2019) was deposited at the Microbiology Laboratory, Homoeopathic Pharmacopeia Laboratory in Ghaziabad, Uttar Pradesh, India.
Laboratory rearing of Anopheles gambiae
Due to high mortality rates during successive malaria epidemics over the past few years, immature stages of Anopheles gambiae were collected from breeding sites in the Ghaziabad district of Uttar Pradesh. The larval mosquitoes were gathered in September and October 2016 from hoof print, temporary flood accumulation areas. The larval stages were gathered with a kitchen strainer and placed in a wide-mouthed plastic container about three-quarters full of water. We closed the container's hole with muslin so it could be taken to the General Entomology lab on the Maraki campus of the University of Gondar for examination. Larvae from instars I–IV were acclimatized in half-full trays of deionized water. The larvae's size and shape were compared to those described by Walker and Lynch to establish their instar. The adult and larval stages of Anopheles gambiae S. L. are morphologically identical, as shown by using a shared key to identify them. as members of other Anopheles species. Pupae were raised in the lab on a diet of dry yeast and dog biscuit crumbs. Adult emergence cages were set up, and the pupae were collected after the larvae had reached that stage. When mosquitoes matured into adults, they were kept in Bugdorm cages with unrestricted access to cotton soaked in a 10% sucrose solution (w/v). The ideal colony conditions included a constant temperature of 27.1°C, relative humidity of 65-70%, and a light-dark photoperiod of 12 hours daily. An adequate number of field-collected, laboratory-maintained, and bioassayed larvae were employed throughout the trial.
Preparation of the extracts
The powder was made from the newly harvested seeds. The seeds were ground into a powder and then extracted using a Soxhlet apparatus with 150ml of chloroform, ethyl acetate, methanol, and dichloromethane for 24 hours. Next, we concentrated the filtrates at 55°C in a rotating evaporator while maintaining a vacuum. The dry extracts were cooled after reconstituting in their respective solvents to a final 100mg/ml concentration. Lepidium sativum seeds are freshly gathered in the Gondar region. The seeds were washed with distilled water and then dried in the shade of the lab. The dry seeds were ground into a powder using an electric blender. The powder utilized in the oil extraction process was obtained by passing the pulverized seeds through a fine mesh sieve. In a conical flask, we combined 200 grams of seed powder from each plant with 1 liter of distilled water. The Clevenger equipment and the hydro-distillation procedure required three hours and a temperature of 100°C to extract the essential oil from the plant components. To prevent the oil from becoming rancid, it was filtered through cotton soaked in anhydrous sodium sulfate and kept in dark, temperature-controlled glass bottles at 4°C. Different concentrations of the stock oil were used in a toxicity bioassay against A. gambiae.
Microorganisms used
Escherichia coli (ATCC 8739), Salmonella typhi (ATCC 23564), Pseudomonas aeruginosa (ATCC 25668), Staphylococcus aureus (ATCC 9144), and Bacillus cereus (ATCC 11778), and Micrococcus luteus were utilized as tests in this study. Bacteria strains were cultured and kept on Nutrient agar at 5-8°C. Bacteria inocula were at a concentration of 106CFU/ml, equivalent to a turbidity of 0.5 McFarland units.
Preparation of stock and experimental concentrations
A stock concentration of 10,000 ppm was achieved by diluting 1ml of pure essential oil with 1mL of acetone in a 250ml conical flask with 100mL of filtered water. After initially testing at 10, 100, and 1000ppm, the researchers narrowed their range and did more focused experiments at 100, 75, 50, and 25ppm. The impregnated filter paper bioassay method was used to test the effectiveness of essential oil in killing and repelling adult mosquitoes. The filter paper was impregnated with 0.5, 2.5, 5, and 10%ppm solutions. In addition, quantities of acetone and distilled water equal to 1mL were generated and utilized as a "control."
Antibacterial activity
Extracts' antibacterial efficacy was evaluated using the agar well diffusion technique. Eight-millimeter-diameter wells were drilled on a petri dish, and 100µl of each extract was poured in, using strict aseptic procedures. The gentamicin antibiotic solution (10.0g/ml) was used to make the positive control. The unadulterated version of the solvent was employed as a reference point (chloroform, ethyl acetate, methanol, and dichloromethane). The plates were allowed to sit at room temperature for one hour to allow the extract to diffuse into the agar. After that, the Petri plates spent 24 hours in an incubator at 37°C. The inhibitory zones were measured in millimeters after the incubation durations. Three passes through the test ensured reliability.
Minimum Inhibitory concentration
Tube dilution was used to determine the MIC of methanol extract. Diluting the methanol extract at various concentrations (0.097-50.0mg/ml) allowed researchers to determine the MIC of the compound. In the course of 18-48 hours, all of the test tubes were kept in an incubator. All development in the tubes was tracked. The minimal inhibitory concentration (MIC) was calculated as the concentration of the extract below, for which no growth was detectable compared to the control tubes.
Minimum bactericidal concentration
Sub-culturing was used to establish the minimum bactericidal concentration. Subcultures were made on fresh nutrient agar plates from the test tubes, and no turbidity or growth was observed during the MIC assays. The bactericidal threshold was determined to be the lowest concentration at which no growth was seen after 24 hours of incubation.
Preparation of stock and experimental concentrations
One milliliter of pure essential oil was combined with one milliliter of acetone, and the resulting solution was diluted with distilled water to a final volume of one hundred milliliters in a two-hundred-fifty milliliter conical flask. This yielded a stock concentration of ten thousand parts per million (ppm) of the extracted oil. After conducting exploratory broad-range tolerance tests at 10, 100, and 1000ppm, the researchers settled on four doses for their experiments: 100, 75, 50, and 25ppm. The impregnated filter paper bioassay method was used to test the effectiveness of essential oil in killing and repelling adult mosquitoes. The filter paper was impregnated with solutions of 0.5, 2.5, 5, and 10%ppm concentrations. Additionally, acetone and distilled water concentrations, each at 1 mL, were prepared and used as a "control."
Testing the essential oil's effectiveness as a larvicidal
Essential oils isolated from L. sativum seeds were tested for larvicidal efficacy using the World Health Organization's gold standard technique. Twenty-five larvae in the second and fourth instars were selected from the culture and placed into 250ml plastic beakers. A constant concentration of essential oil of varying strengths was maintained by changing the total volume of water in each beaker from 200mL to 0.00 (control), 25, 50, 75, and 100ppm. Temperature and humidity in the lab were controlled at 27 1°C (65-80%RH) for the duration of the experiment. Mosquito larvae were released in equal numbers after each concentration was replicated four times. In this study, we measured the mortality of exposed larvae at 24, 48, and 72 hours.
Analysis of the Essential Oil's Puppy-Killing Capacity
We used the newly emerged pupae collected from the culture to test the pupicidal activity of a few different essential oils. When using essential oils for their larvicidal efficacy, the concentrations and methods were as indicated. We released 25 pupae into the plastic beaker to keep the adults from escaping and covered it with nylon mesh. After 24 hours, 48 hours, and 72 hours of exposure, the total number of pupa that had died due to each concentration was recorded across all four replicates. By using Abbott's formula, we were able to accurately determine the pupal mortality rate and adjust for it.
Analysis of the Essential Oil's Adulticiding Capacity
Following World Health Organization guidelines, adult mosquitoes that had not been fed blood were used to test the knockdown action of the essential oil from a select group of plants. Following the preliminary screening, four concentrations (0.5, 2.5, 5.0, and 10.0%ppm) were created and impregnated with Whatman no. 1 filter paper. The 0.1% bendiocarb-treated Whatman filter paper was the positive control, while acetone was the negative control. The WHO toxicity and knockdown bioassays were performed on adult test tubes. Adult mosquitoes were placed in test tubes and fed sugar for three to five days before being exposed for one hour; during this time, the number of dead or recovering mosquitoes was recorded every five minutes. Mosquitoes in this experiment were exposed for 1 hour and then placed in recovery test tubes, where they stayed for 24 hours while researchers watched for any signs of infection. During the restoration period, adults were fed cotton soaked in a 10% sucrose solution and then placed on the mesh screen of the tubes. The temperature and the relative humidity were kept constant at 271°C and 65-70%, respectively, for the duration of the experiment. The proportion of death was computed after the data from four replicates was adjusted using Abbott's technique.
Statistical analysis
The mortality rate was calculated by the ratio of mosquitoes killed in the control group to those killed in the experimental group. The error introduced by this fact has been corrected using Abbott's method, as avoidable deaths exceed 5%. A population's mortality rate after a course correction can be calculated as follows: The median percentage of deaths at different concentrations was compared using one-way ANOVA followed by the LSD post hoc test. We calculated each concentration's LC50, LC90, LCL, and UCL using Probit analysis. Statistical significance in the tested plant essential oil concentrations range was established using a Chi-square test. The 5% statistical significance threshold (p<0.05) was used for all analyses. For the statistical analysis, we utilized SPSS 20 on Windows 7.
Findings
The plant extracts were effective against the organisms tested (Table 1). The methanol extract significantly inhibited the growth of Staphylococcus aureus (22 millimeters), Bacillus cereus (16 millimeters), Escherichia coli (14 millimeters), Pseudomonas aeruginosa (14 millimeters), Micrococcus luteus (16 millimeters), and Salmonella typhi (13 millimeters).
Table 1. Extraction of Lepidium sativum seeds has antibacterial properties
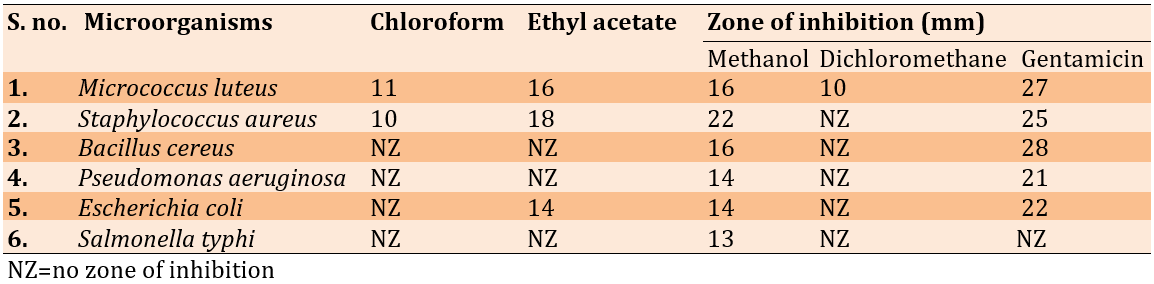
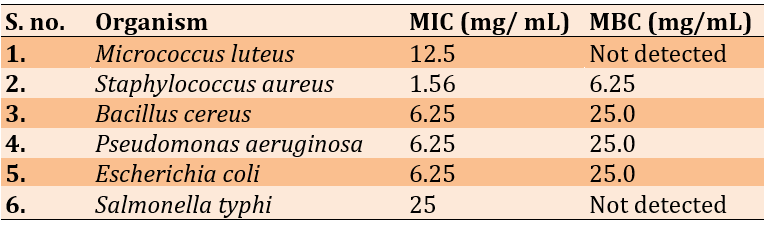
The ethyl acetate and the importance of the inhibitory zone in methanol extract, MIC, and MBC analyses were performed. Staphylococcus aureus had an MIC of 1.56mg/ml and a minimum inhibitory concentration (MBC) of 6.52mg/ml, while Salmonella typhi had an MIC of 25mg/ml (Table 2).
Table 2. Evaluation of Lepidium sativum seed methanol extract for minimum inhibitory and bactericidal concentrations
The effect of Lepidium sativum essential oil on the mortality of Anopheles gambiae larvae
A. gambiae pupae and larvae of the second and fourth instars exposed to essential oil from L. sativum for 24, 48, and 72 hours were evaluated. Second-instar larvae exposed to 75ppm, the highest exposure tested, had the highest mortality rate (96.0% after 48 hours). After 72 hours of exposure, 97% of those exposed to 100ppm died. After testing for 24 hours, the LC50 and LC90 concentrations were calculated to be 317.8 and 437.1ppm, respectively (Table 3).
Table 3. The median percentage of immature Anopheles gambiae that died after being exposed to essential oil from the plant Lepidium sativum
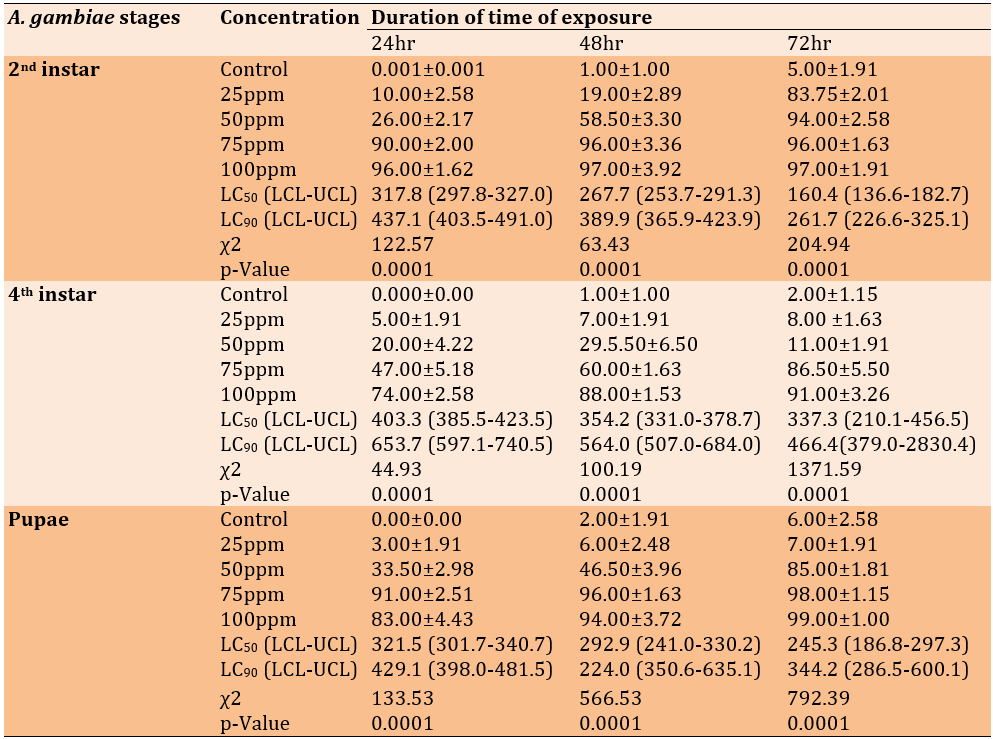
The LC50 was the concentration at which 50% of mosquito larvae exposed to a certain substance die. The confidence interval bounds for the essential oil concentration were lower and higher.
Knock downtime (KDT50) and Essential oils can be toxic to adult gambiae
The median knockdown time (KDT50) following 1 hour of exposure to L. sativum essential oils for adult, non-blood-fed female A. gambiae is shown in Table 4. At a concentration of 10% L. sativum, the half-life (KDT50) was determined to be 7.34 minutes. Conversely, the half-life of L. sativum oil was only 10.05 minutes when used at the same concentration. Compared to the L. sativum concentrations that resulted in the KDT50 (50 and 10%, respectively), the bendiocarb 0.1% impregnated filter paper performed poorly. The X-axis shows the percentage of adult A. gambiae that died after exposure to a 10% solution of L. sativum essential oils. According to the findings, bendiocarb, the positive control, showed a 100% death rate. Nonetheless, 95.66±2.40 and 82.66±3.48% L. sativum mortality rates were significantly higher than the negative control's (7.33±0.88%).
Table 4. The essential oils of Lepidium sativum were tested for their ability to kill 50% of female adult Anopheles gambiae after exposing them to them for 1 hour (the KDT50)
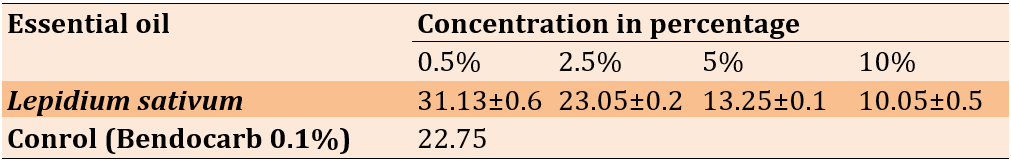
KDT50 was the length of time required to kill 50% of an exposed population. This data was derived by averaging four samples and adding their respective standard deviations.
Discussion
Seeds include 25% protein, 14%-24% lipids, 33-54% carbs, and 8% crude fiber. Non-starch polysaccharides make up the other 90% of the carbohydrates. The seed bran is high in fiber and may absorb large amounts of liquid. GC bran is a great option for those looking to enhance their fiber consumption. Semilepidinosides A and B, sinapin 15, and sinapic acid are only some of the seven distinct imidazole alkaloids in the seeds [10]. These are in addition to the previously described monomeric alkaloids lepidine B, C, D, E, and F. Carotene, cellulose, calcium, phosphorus, iron, thiamine, riboflavin, niacin, uric acid, and uric acid are present; so are N, N'- dibenzyl urea, N, N'- dibenzyl thiourea, Sinapic Acid and its choline esters (sinapin), and glucotropaeoline. As stated by several researchers [11]. Seed oil is primarily made up of alpha-linolenic acid (32-40%), which gives it a yellowish appearance and makes it semi-drying. In addition to natural antioxidants like tocopherols and carotenoids, its 46.8% polyunsaturated fatty acid (PUFA) content and 37.6% monounsaturated fatty acid (MUFA) content help keep it from going rancid. Benzyl isothiocyanate, benzyl cyanide, sterol, and sitosterol are present in addition to palmitic, stearic, oleic, linolenic, arachidic, behenic, and lignoceric acids. Protein, fat, carbohydrates, minerals, phosphorus, calcium, trace elements, nickel, cobalt, iodine, and vitamin C; thiamine, riboflavin, niacin, ascorbic acid, and vitamin A are just some of the nutrients that can be found in the leaf. The plant as a whole may include protein, minerals, vitamins, and other substances. Chemicals like glucotropoeolin, 4-methoxyglucobrassicin, caffeic acid esters, -sitosterol, benzyl cyanide, calmodulin, sinapoyglucose, p-coumaric acid, ferulic acid, quinic acid, and many more have been identified. This chemical occurs in three different isomers, including 5-3'-dihydroxy-6,7,4'-tetramethoxyflavone and 5-4'-dihydroxy-7,8,3',5-tetramethoxyflavone.
The effectiveness of the plant's extracts as antibiotics against Gram-negative and Gram-positive bacteria was investigated, and the results were promising. The ethanolic extract was also more effective against bacteria than the aqueous extracts [12]. Seed extracts in petroleum ether, methanol, and water were tested against opportunistic pathogens like Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Proteus vulgaris, Pseudomonas aeruginosa, and Candida albicans. They were put through a battery of tests to determine whether or not plant seed extracts might be used as an alternative to the antibiotics Gentamicin and Ketoconazole. The findings also showed that petroleum ether was a more effective solvent than methanol and water for removing the plant's antibacterial compounds during extraction [13]. Leaves aqueous-methanolic extract N-butanol fraction was tested for antioxidant activity using DPPH and ABTS free radicals assay. Extracting the N-hexane fraction yielded three flavonol glycosides with anti-oxidant properties. The ethanolic seed extract has shown promising in vitro antioxidant activity in the DPPH radical scavenging assay, the ferric chloride radical scavenging assay, and the phosphor-molybdenum radical scavenging assay. According to the data, the seeds' ethanolic extract has strong potential in-vitro antioxidant activity. Albino rats developed pedal inflammation after suffering from carrageenan-induced paw edema. The volume of each foot was determined using the displacement technique and a plethysmometer. Using oxyphenbutazone as a reference, we find that the ethanolic extract inhibits the activity of the drug [14].
Nayak et al. report that the approximate composition of L. sativum seeds reveals sizable amounts of protein, lipids, carbohydrates, fiber, ash, and moisture. Varietal characteristics, agricultural methods, the time of year when seeds are harvested, and the local climate and geology all play a role in the final proximate composition of a crop. It is crucial for determining the nutritional value of plant and crop fruits and seeds, and it mandates more research into the more intriguing constituents. Higher ash contents suggest that the GCS are a healthy source of minerals. The stability, quality, and lengthened storage life of seeds can be gauged by their low moisture content. GCS have high food energy because their protein and lipid contents are higher [15].
As a result, it's reasonable to assume that the recovered antibacterial chemicals may kill bacteria via a mechanism distinct from that of conventional antibiotics, enhancing their potential therapeutic effectiveness. A literature review revealed no information on Lepidium sativum's potential antibacterial effects against various pathogens found in food [16-22]. As a result, this study has the potential to be the first to show that extracts of Lepidium sativum seeds have antibacterial properties when tested against a broad spectrum of food-borne bacteria. Eco-friendly vector control measures are necessary now due to the need to minimize environmental contamination from synthetic pesticides, mitigate the potential harm to non-target creatures and manage the spread of insecticide resistance in mosquito populations. One beneficial reason to focus on the mosquito's larval stage is that it decreases the number of adult mosquitoes that can develop into disease carriers. Small ponds, marshes, ditches, pools, drains, and water containers should all be emptied because they collect water that mosquitoes can use for breeding. Lab tests using essential oils extracted from L. sativum against A. gambiae larvae and adults provided valuable information for the development of future eco-products.
The present findings corroborated prior findings about the toxicity of L. sativum essential oils [23-29]. Depending on the dosage of the plant extracts and the length of time the larvae and pupae were exposed to the extracts, the mortality rate changed dramatically between the second and fourth in stars. Possibly due to the accumulation of secondary metabolites in seeds, L. sativum oil has a high mortality rate. These findings are consistent with those of Andemo et al., who discovered that treating adult A. arabiensis with a methanol extract of L. sativum seeds completely eradicated the pest population. This study suggests that phytochemicals and the solubility of individual phytochemicals in their respective solvents may be responsible for the high mortality rate observed among A. gambiae exposed to L. sativum essential oil. Producing pesticides with rotenoids dates back to 1848.
Using standard antibiotics to treat infections caused by these germs is notoriously difficult when the relevant microorganisms have become resistant to many medicines. L. essential oils from the sativum plant had a strong mosquito-killing effect on the An. Mosquitoes of the genus Culex are responsible for spreading malaria. To counter an in the field, widespread use of this tool is required. gambiae and thereby lessen the likelihood of malaria transmission in places like impoverished nations; however, its efficacy relies on accurate formulation and field assessment.
Conclusion
Antibacterial chemicals are widely distributed across therapeutic plants. Methanol extract is more effective against Gram-positive bacteria than Gram-negative bacteria. Benzyl isothiocyanate in Lepidium sativum seeds may account for their potent antimicrobial action against various pathogenic bacteria responsible for life-threatening diseases.
Acknowledgments: None declared.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contribution: Emaduldeen HA (First Author), Introduction Writer/Main Researcher/Discussion Writer/ (40%); Waheeb MQ (Second Author), Methodologist/Assistant Researcher/Discussion Writer (30%); Makki MA (Thired Author), Statistical Analyst (30%)
Funding/Support: None declared.
The fast-growing annual herb known as garden cress (Lepidium sativum L.) originates in Egypt and western Asia but is now planted in nearly every region. Garden cress (GC), or Chandrasur as it is known in local languages, is widely cultivated in India for its therapeutic properties. The herbaceous annual grows to a height of 15-45cm and has an upright, glabrous appearance. Small white flowers are carried on long racemes, and the pods might be round or obovate in shape, elliptic without margins, notched at the tip, and wingless. The medicines found in nature are the best there is. Humans have used medicinal plants as a form of disease prevention ever since they developed civilization [1]. Natural chemicals with therapeutic features in the herbs and medicinal plants used for thousands of years are said to be the origin of herbal medicine and traditional healthcare systems like Ayurveda, Siddha, and Unani. 80% of the world's population, mostly in developing nations, rely on medicines made from plants for their health. One-fourth of all prescriptions are formulations based on chemicals derived from plants or plant-derived synthetic analogs. Indigenous wisdom handed down from one generation to the next has significantly impacted traditional medical practices around the globe. Likewise, the exploration of medicinal plants has been aided by this knowledge [2]. The discovery of novel chemical entities has benefited greatly from this investigation of physiologically active natural compounds. This article summarizes the research on Lepidium sativum Linn, including its traditional medical applications, isolated chemicals, and pharmacological studies.
Despite this, many medicinal plants are disappearing, and native civilizations are being destroyed. If the current extinction rate and destruction is not reversed, we stand to lose a significant portion of our biodiversity, the variety of genes, species, ecosystems, and cultural practices. Native peoples have been the guardians of 99 percent of the world's genetic resources, essential to people's economic, agricultural, and health circumstances everywhere. Culture and biological variables are also intrinsically linked [3].
Because so many people worldwide are vulnerable to mosquito-borne illnesses, they threaten human health. The Culex pipiens L. (Diptera: Culicidae) mosquito is a potential vector for a wide variety of diseases, including western equine encephalitis, West Nile virus, Rift Valley fever, St. Louis encephalitis, and bancroftian filariasis. With a flying distance of 1.15 to 2.48km, Culex pipiens may disperse their eggs over a vast area and contribute to the spread of illness. Mosquitoes thrive in poor water drainage and improper waste management [4]. Constant and unchecked use of mosquitocidal chemicals has resulted in mosquito resistance and has negatively affected humans, animals, and the environment.
Lepidium sativum is a tall, annual herb that can reach a height of 50 centimeters. White, little flowers are arranged in racemes, and the leaves can be either lobed or whole. The obovate pods contain two seeds and are around 5 mm in length. A plant's volatile oils can be found in its seeds and leaves. Natives of Saudi Arabia, Sudan, and other Arabic countries know all about the benefits of the Lepidium sativum plant and its seeds for healing bone fractures. Lepidium sativum seed extracts have been utilized historically to treat a broad range of medical conditions. as highlighted by several recent studies [5].
Asthma, a persistent cough, and hemorrhaging piles can all be helped using the plant's aerial parts. The seeds are chewed for various medical purposes, including relieving a sore throat, cough, asthma, or headache. Moreover, they can be used topically as an effective insect repellent. The leaves' mild stimulant and diuretic properties make them excellent for treating scorbutic diseases and liver complaints. Syphilis can be treated with roots. This herb has many medicinal uses in Europe, including as a diuretic, an expectorant, and a treatment for cough and constipation [6].
A rising number of bacteria are becoming antibiotic-resistant, posing a serious health risk. Various biomolecules and metabolites with crucial biological functions are already present in plants. These organic compounds are a gold mine when combating germs that have developed resistance to multiple drugs [7]. Green synthesis employs botanical components and could yield new classes of antibiotics derived from plants. "Lepidium sativum" (L.) LS, also known as garden cress, is a fast-reproducing annual plant. LS seed oil's antimicrobial, antioxidant, and anti-inflammatory properties make it an intriguing therapeutic option. Eliminating Vectors and Individual Safety Limiting your exposure to vectors is presently the most effective way to avoid contracting a disease spread by a vector [8]. It is normal practice to use chemically based intervention strategies for vector control and to reduce the transfer of human infections. Insecticides, repellents, and larvicides made from chemicals were once widely used. Still, their overuse led to the rise of insect-resistant strains, ecological disruption, and human and non-target organism toxicity [9].
Insects are predators, and many plant species have evolved to have defense mechanisms that either eliminate or drive away these pests. These chemicals come in various uses, including as repellants, feeding deterrents, poisons, and growth regulators. Phytophagous insect defense is the primary function of these chemicals. Still, many of them, especially volatile components released as a result of herbivores, are also effective against mosquitoes and other biting Diptera. And, as has been reported, the fact that many of these compounds are also effective against hematophagous insects may be a relic of an ancestral plant-eating lifestyle, given that they evolved as repellents/killers to phytophagous insects.
Material and Methods
Plant material
The Lepidium sativum seeds were purchased from a vendor in Ghaziabad, Uttar Pradesh, India, in May 2019. Using the available ethnobotanical data, a voucher specimen (ML-1/2019) was deposited at the Microbiology Laboratory, Homoeopathic Pharmacopeia Laboratory in Ghaziabad, Uttar Pradesh, India.
Laboratory rearing of Anopheles gambiae
Due to high mortality rates during successive malaria epidemics over the past few years, immature stages of Anopheles gambiae were collected from breeding sites in the Ghaziabad district of Uttar Pradesh. The larval mosquitoes were gathered in September and October 2016 from hoof print, temporary flood accumulation areas. The larval stages were gathered with a kitchen strainer and placed in a wide-mouthed plastic container about three-quarters full of water. We closed the container's hole with muslin so it could be taken to the General Entomology lab on the Maraki campus of the University of Gondar for examination. Larvae from instars I–IV were acclimatized in half-full trays of deionized water. The larvae's size and shape were compared to those described by Walker and Lynch to establish their instar. The adult and larval stages of Anopheles gambiae S. L. are morphologically identical, as shown by using a shared key to identify them. as members of other Anopheles species. Pupae were raised in the lab on a diet of dry yeast and dog biscuit crumbs. Adult emergence cages were set up, and the pupae were collected after the larvae had reached that stage. When mosquitoes matured into adults, they were kept in Bugdorm cages with unrestricted access to cotton soaked in a 10% sucrose solution (w/v). The ideal colony conditions included a constant temperature of 27.1°C, relative humidity of 65-70%, and a light-dark photoperiod of 12 hours daily. An adequate number of field-collected, laboratory-maintained, and bioassayed larvae were employed throughout the trial.
Preparation of the extracts
The powder was made from the newly harvested seeds. The seeds were ground into a powder and then extracted using a Soxhlet apparatus with 150ml of chloroform, ethyl acetate, methanol, and dichloromethane for 24 hours. Next, we concentrated the filtrates at 55°C in a rotating evaporator while maintaining a vacuum. The dry extracts were cooled after reconstituting in their respective solvents to a final 100mg/ml concentration. Lepidium sativum seeds are freshly gathered in the Gondar region. The seeds were washed with distilled water and then dried in the shade of the lab. The dry seeds were ground into a powder using an electric blender. The powder utilized in the oil extraction process was obtained by passing the pulverized seeds through a fine mesh sieve. In a conical flask, we combined 200 grams of seed powder from each plant with 1 liter of distilled water. The Clevenger equipment and the hydro-distillation procedure required three hours and a temperature of 100°C to extract the essential oil from the plant components. To prevent the oil from becoming rancid, it was filtered through cotton soaked in anhydrous sodium sulfate and kept in dark, temperature-controlled glass bottles at 4°C. Different concentrations of the stock oil were used in a toxicity bioassay against A. gambiae.
Microorganisms used
Escherichia coli (ATCC 8739), Salmonella typhi (ATCC 23564), Pseudomonas aeruginosa (ATCC 25668), Staphylococcus aureus (ATCC 9144), and Bacillus cereus (ATCC 11778), and Micrococcus luteus were utilized as tests in this study. Bacteria strains were cultured and kept on Nutrient agar at 5-8°C. Bacteria inocula were at a concentration of 106CFU/ml, equivalent to a turbidity of 0.5 McFarland units.
Preparation of stock and experimental concentrations
A stock concentration of 10,000 ppm was achieved by diluting 1ml of pure essential oil with 1mL of acetone in a 250ml conical flask with 100mL of filtered water. After initially testing at 10, 100, and 1000ppm, the researchers narrowed their range and did more focused experiments at 100, 75, 50, and 25ppm. The impregnated filter paper bioassay method was used to test the effectiveness of essential oil in killing and repelling adult mosquitoes. The filter paper was impregnated with 0.5, 2.5, 5, and 10%ppm solutions. In addition, quantities of acetone and distilled water equal to 1mL were generated and utilized as a "control."
Antibacterial activity
Extracts' antibacterial efficacy was evaluated using the agar well diffusion technique. Eight-millimeter-diameter wells were drilled on a petri dish, and 100µl of each extract was poured in, using strict aseptic procedures. The gentamicin antibiotic solution (10.0g/ml) was used to make the positive control. The unadulterated version of the solvent was employed as a reference point (chloroform, ethyl acetate, methanol, and dichloromethane). The plates were allowed to sit at room temperature for one hour to allow the extract to diffuse into the agar. After that, the Petri plates spent 24 hours in an incubator at 37°C. The inhibitory zones were measured in millimeters after the incubation durations. Three passes through the test ensured reliability.
Minimum Inhibitory concentration
Tube dilution was used to determine the MIC of methanol extract. Diluting the methanol extract at various concentrations (0.097-50.0mg/ml) allowed researchers to determine the MIC of the compound. In the course of 18-48 hours, all of the test tubes were kept in an incubator. All development in the tubes was tracked. The minimal inhibitory concentration (MIC) was calculated as the concentration of the extract below, for which no growth was detectable compared to the control tubes.
Minimum bactericidal concentration
Sub-culturing was used to establish the minimum bactericidal concentration. Subcultures were made on fresh nutrient agar plates from the test tubes, and no turbidity or growth was observed during the MIC assays. The bactericidal threshold was determined to be the lowest concentration at which no growth was seen after 24 hours of incubation.
Preparation of stock and experimental concentrations
One milliliter of pure essential oil was combined with one milliliter of acetone, and the resulting solution was diluted with distilled water to a final volume of one hundred milliliters in a two-hundred-fifty milliliter conical flask. This yielded a stock concentration of ten thousand parts per million (ppm) of the extracted oil. After conducting exploratory broad-range tolerance tests at 10, 100, and 1000ppm, the researchers settled on four doses for their experiments: 100, 75, 50, and 25ppm. The impregnated filter paper bioassay method was used to test the effectiveness of essential oil in killing and repelling adult mosquitoes. The filter paper was impregnated with solutions of 0.5, 2.5, 5, and 10%ppm concentrations. Additionally, acetone and distilled water concentrations, each at 1 mL, were prepared and used as a "control."
Testing the essential oil's effectiveness as a larvicidal
Essential oils isolated from L. sativum seeds were tested for larvicidal efficacy using the World Health Organization's gold standard technique. Twenty-five larvae in the second and fourth instars were selected from the culture and placed into 250ml plastic beakers. A constant concentration of essential oil of varying strengths was maintained by changing the total volume of water in each beaker from 200mL to 0.00 (control), 25, 50, 75, and 100ppm. Temperature and humidity in the lab were controlled at 27 1°C (65-80%RH) for the duration of the experiment. Mosquito larvae were released in equal numbers after each concentration was replicated four times. In this study, we measured the mortality of exposed larvae at 24, 48, and 72 hours.
Analysis of the Essential Oil's Puppy-Killing Capacity
We used the newly emerged pupae collected from the culture to test the pupicidal activity of a few different essential oils. When using essential oils for their larvicidal efficacy, the concentrations and methods were as indicated. We released 25 pupae into the plastic beaker to keep the adults from escaping and covered it with nylon mesh. After 24 hours, 48 hours, and 72 hours of exposure, the total number of pupa that had died due to each concentration was recorded across all four replicates. By using Abbott's formula, we were able to accurately determine the pupal mortality rate and adjust for it.
Analysis of the Essential Oil's Adulticiding Capacity
Following World Health Organization guidelines, adult mosquitoes that had not been fed blood were used to test the knockdown action of the essential oil from a select group of plants. Following the preliminary screening, four concentrations (0.5, 2.5, 5.0, and 10.0%ppm) were created and impregnated with Whatman no. 1 filter paper. The 0.1% bendiocarb-treated Whatman filter paper was the positive control, while acetone was the negative control. The WHO toxicity and knockdown bioassays were performed on adult test tubes. Adult mosquitoes were placed in test tubes and fed sugar for three to five days before being exposed for one hour; during this time, the number of dead or recovering mosquitoes was recorded every five minutes. Mosquitoes in this experiment were exposed for 1 hour and then placed in recovery test tubes, where they stayed for 24 hours while researchers watched for any signs of infection. During the restoration period, adults were fed cotton soaked in a 10% sucrose solution and then placed on the mesh screen of the tubes. The temperature and the relative humidity were kept constant at 271°C and 65-70%, respectively, for the duration of the experiment. The proportion of death was computed after the data from four replicates was adjusted using Abbott's technique.
Statistical analysis
The mortality rate was calculated by the ratio of mosquitoes killed in the control group to those killed in the experimental group. The error introduced by this fact has been corrected using Abbott's method, as avoidable deaths exceed 5%. A population's mortality rate after a course correction can be calculated as follows: The median percentage of deaths at different concentrations was compared using one-way ANOVA followed by the LSD post hoc test. We calculated each concentration's LC50, LC90, LCL, and UCL using Probit analysis. Statistical significance in the tested plant essential oil concentrations range was established using a Chi-square test. The 5% statistical significance threshold (p<0.05) was used for all analyses. For the statistical analysis, we utilized SPSS 20 on Windows 7.
Findings
The plant extracts were effective against the organisms tested (Table 1). The methanol extract significantly inhibited the growth of Staphylococcus aureus (22 millimeters), Bacillus cereus (16 millimeters), Escherichia coli (14 millimeters), Pseudomonas aeruginosa (14 millimeters), Micrococcus luteus (16 millimeters), and Salmonella typhi (13 millimeters).
Table 1. Extraction of Lepidium sativum seeds has antibacterial properties

The ethyl acetate and the importance of the inhibitory zone in methanol extract, MIC, and MBC analyses were performed. Staphylococcus aureus had an MIC of 1.56mg/ml and a minimum inhibitory concentration (MBC) of 6.52mg/ml, while Salmonella typhi had an MIC of 25mg/ml (Table 2).
Table 2. Evaluation of Lepidium sativum seed methanol extract for minimum inhibitory and bactericidal concentrations

The effect of Lepidium sativum essential oil on the mortality of Anopheles gambiae larvae
A. gambiae pupae and larvae of the second and fourth instars exposed to essential oil from L. sativum for 24, 48, and 72 hours were evaluated. Second-instar larvae exposed to 75ppm, the highest exposure tested, had the highest mortality rate (96.0% after 48 hours). After 72 hours of exposure, 97% of those exposed to 100ppm died. After testing for 24 hours, the LC50 and LC90 concentrations were calculated to be 317.8 and 437.1ppm, respectively (Table 3).
Table 3. The median percentage of immature Anopheles gambiae that died after being exposed to essential oil from the plant Lepidium sativum

The LC50 was the concentration at which 50% of mosquito larvae exposed to a certain substance die. The confidence interval bounds for the essential oil concentration were lower and higher.
Knock downtime (KDT50) and Essential oils can be toxic to adult gambiae
The median knockdown time (KDT50) following 1 hour of exposure to L. sativum essential oils for adult, non-blood-fed female A. gambiae is shown in Table 4. At a concentration of 10% L. sativum, the half-life (KDT50) was determined to be 7.34 minutes. Conversely, the half-life of L. sativum oil was only 10.05 minutes when used at the same concentration. Compared to the L. sativum concentrations that resulted in the KDT50 (50 and 10%, respectively), the bendiocarb 0.1% impregnated filter paper performed poorly. The X-axis shows the percentage of adult A. gambiae that died after exposure to a 10% solution of L. sativum essential oils. According to the findings, bendiocarb, the positive control, showed a 100% death rate. Nonetheless, 95.66±2.40 and 82.66±3.48% L. sativum mortality rates were significantly higher than the negative control's (7.33±0.88%).
Table 4. The essential oils of Lepidium sativum were tested for their ability to kill 50% of female adult Anopheles gambiae after exposing them to them for 1 hour (the KDT50)

KDT50 was the length of time required to kill 50% of an exposed population. This data was derived by averaging four samples and adding their respective standard deviations.
Discussion
Seeds include 25% protein, 14%-24% lipids, 33-54% carbs, and 8% crude fiber. Non-starch polysaccharides make up the other 90% of the carbohydrates. The seed bran is high in fiber and may absorb large amounts of liquid. GC bran is a great option for those looking to enhance their fiber consumption. Semilepidinosides A and B, sinapin 15, and sinapic acid are only some of the seven distinct imidazole alkaloids in the seeds [10]. These are in addition to the previously described monomeric alkaloids lepidine B, C, D, E, and F. Carotene, cellulose, calcium, phosphorus, iron, thiamine, riboflavin, niacin, uric acid, and uric acid are present; so are N, N'- dibenzyl urea, N, N'- dibenzyl thiourea, Sinapic Acid and its choline esters (sinapin), and glucotropaeoline. As stated by several researchers [11]. Seed oil is primarily made up of alpha-linolenic acid (32-40%), which gives it a yellowish appearance and makes it semi-drying. In addition to natural antioxidants like tocopherols and carotenoids, its 46.8% polyunsaturated fatty acid (PUFA) content and 37.6% monounsaturated fatty acid (MUFA) content help keep it from going rancid. Benzyl isothiocyanate, benzyl cyanide, sterol, and sitosterol are present in addition to palmitic, stearic, oleic, linolenic, arachidic, behenic, and lignoceric acids. Protein, fat, carbohydrates, minerals, phosphorus, calcium, trace elements, nickel, cobalt, iodine, and vitamin C; thiamine, riboflavin, niacin, ascorbic acid, and vitamin A are just some of the nutrients that can be found in the leaf. The plant as a whole may include protein, minerals, vitamins, and other substances. Chemicals like glucotropoeolin, 4-methoxyglucobrassicin, caffeic acid esters, -sitosterol, benzyl cyanide, calmodulin, sinapoyglucose, p-coumaric acid, ferulic acid, quinic acid, and many more have been identified. This chemical occurs in three different isomers, including 5-3'-dihydroxy-6,7,4'-tetramethoxyflavone and 5-4'-dihydroxy-7,8,3',5-tetramethoxyflavone.
The effectiveness of the plant's extracts as antibiotics against Gram-negative and Gram-positive bacteria was investigated, and the results were promising. The ethanolic extract was also more effective against bacteria than the aqueous extracts [12]. Seed extracts in petroleum ether, methanol, and water were tested against opportunistic pathogens like Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Proteus vulgaris, Pseudomonas aeruginosa, and Candida albicans. They were put through a battery of tests to determine whether or not plant seed extracts might be used as an alternative to the antibiotics Gentamicin and Ketoconazole. The findings also showed that petroleum ether was a more effective solvent than methanol and water for removing the plant's antibacterial compounds during extraction [13]. Leaves aqueous-methanolic extract N-butanol fraction was tested for antioxidant activity using DPPH and ABTS free radicals assay. Extracting the N-hexane fraction yielded three flavonol glycosides with anti-oxidant properties. The ethanolic seed extract has shown promising in vitro antioxidant activity in the DPPH radical scavenging assay, the ferric chloride radical scavenging assay, and the phosphor-molybdenum radical scavenging assay. According to the data, the seeds' ethanolic extract has strong potential in-vitro antioxidant activity. Albino rats developed pedal inflammation after suffering from carrageenan-induced paw edema. The volume of each foot was determined using the displacement technique and a plethysmometer. Using oxyphenbutazone as a reference, we find that the ethanolic extract inhibits the activity of the drug [14].
Nayak et al. report that the approximate composition of L. sativum seeds reveals sizable amounts of protein, lipids, carbohydrates, fiber, ash, and moisture. Varietal characteristics, agricultural methods, the time of year when seeds are harvested, and the local climate and geology all play a role in the final proximate composition of a crop. It is crucial for determining the nutritional value of plant and crop fruits and seeds, and it mandates more research into the more intriguing constituents. Higher ash contents suggest that the GCS are a healthy source of minerals. The stability, quality, and lengthened storage life of seeds can be gauged by their low moisture content. GCS have high food energy because their protein and lipid contents are higher [15].
As a result, it's reasonable to assume that the recovered antibacterial chemicals may kill bacteria via a mechanism distinct from that of conventional antibiotics, enhancing their potential therapeutic effectiveness. A literature review revealed no information on Lepidium sativum's potential antibacterial effects against various pathogens found in food [16-22]. As a result, this study has the potential to be the first to show that extracts of Lepidium sativum seeds have antibacterial properties when tested against a broad spectrum of food-borne bacteria. Eco-friendly vector control measures are necessary now due to the need to minimize environmental contamination from synthetic pesticides, mitigate the potential harm to non-target creatures and manage the spread of insecticide resistance in mosquito populations. One beneficial reason to focus on the mosquito's larval stage is that it decreases the number of adult mosquitoes that can develop into disease carriers. Small ponds, marshes, ditches, pools, drains, and water containers should all be emptied because they collect water that mosquitoes can use for breeding. Lab tests using essential oils extracted from L. sativum against A. gambiae larvae and adults provided valuable information for the development of future eco-products.
The present findings corroborated prior findings about the toxicity of L. sativum essential oils [23-29]. Depending on the dosage of the plant extracts and the length of time the larvae and pupae were exposed to the extracts, the mortality rate changed dramatically between the second and fourth in stars. Possibly due to the accumulation of secondary metabolites in seeds, L. sativum oil has a high mortality rate. These findings are consistent with those of Andemo et al., who discovered that treating adult A. arabiensis with a methanol extract of L. sativum seeds completely eradicated the pest population. This study suggests that phytochemicals and the solubility of individual phytochemicals in their respective solvents may be responsible for the high mortality rate observed among A. gambiae exposed to L. sativum essential oil. Producing pesticides with rotenoids dates back to 1848.
Using standard antibiotics to treat infections caused by these germs is notoriously difficult when the relevant microorganisms have become resistant to many medicines. L. essential oils from the sativum plant had a strong mosquito-killing effect on the An. Mosquitoes of the genus Culex are responsible for spreading malaria. To counter an in the field, widespread use of this tool is required. gambiae and thereby lessen the likelihood of malaria transmission in places like impoverished nations; however, its efficacy relies on accurate formulation and field assessment.
Conclusion
Antibacterial chemicals are widely distributed across therapeutic plants. Methanol extract is more effective against Gram-positive bacteria than Gram-negative bacteria. Benzyl isothiocyanate in Lepidium sativum seeds may account for their potent antimicrobial action against various pathogenic bacteria responsible for life-threatening diseases.
Acknowledgments: None declared.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contribution: Emaduldeen HA (First Author), Introduction Writer/Main Researcher/Discussion Writer/ (40%); Waheeb MQ (Second Author), Methodologist/Assistant Researcher/Discussion Writer (30%); Makki MA (Thired Author), Statistical Analyst (30%)
Funding/Support: None declared.
Keywords:
References
1. Painuli S, Quispe C, Herrera-Bravo J, Semwal P, Martorell M, Almarhoon ZM, et al. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxid Med. Cell Longev. 2021;2022:2910411. [Link] [DOI:10.1155/2022/2910411]
2. Imade OV, Smith OF, Gazal O, Adekunle EO, Beshel JA. Effects of Lepidium sativum seed on reproductive characteristics in rabbit bucks. J Phytopharm. 2020;9(2):89-95. [Link] [DOI:10.31254/phyto.2020.9203]
3. Razaq S, Ibraheem MR, Hashim SS. Effect of Lepidium sativum aqueous crude extract in some fertility parameters in mice. Int J Sci Res. 2017;6(9):260-6. [Link]
4. Getahun T, Sharma V, Gupta N. Chemical composition, antibacterial and antioxidant activities of oils obtained by different extraction methods from Lepidium sativum L. seeds. Ind Crops Prod. 2020;156:112876. [Link] [DOI:10.1016/j.indcrop.2020.112876]
5. Alqahtani FY, Aleanizy FS, Mahmoud AZ, Fashori NN, Alraraj R, Alsarra IA, et al. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J Biol Sci. 2019;26(5):1089-92. [Link] [DOI:10.1016/j.sjbs.2018.05.007]
6. Ullah MA, Tungmunnithum D, Garros L, Drouet S, Hano C, Abbasi BH. Effect of ultraviolet-c radiation and melatonin stress on biosynthesis of antioxidant and antidiabetic metabolites produced in in vitro callus culture of Lepidium sativum L. Int J Mol.Sci. 2019;20(7):1787. [Link] [DOI:10.3390/ijms20071787]
7. Al-Snafi AE. Chemical constituents and pharmacological effects of Lepidium sativum: A review. Int J Curr Pharm Res. 2019;11(6):1-10. [Link] [DOI:10.22159/ijcpr.2019v11i6.36338]
8. Elspeiy M, Adbella M, Khalifah A. Effects of oral administration of Lepidium sativum, moringa oleifera oils and aqueous extract of Vitex agnnus cactus on reproductive performance and blood biochemical of doe rabbits. Egypt J Rabb Sci. 2021;31(1):1-24. [Link] [DOI:10.21608/ejrs.2021.127380]
9. Aslani E, Naghsh N, Ranjbar M. Cytotoxic effects of hydro-alcoholic extracts of cress (Lepidium sativum)-made from different stages of the plant-on k562 Leukemia cell line. Hormozgan Med J. 2014;18(5):411-9. [Link]
10. Balgoon MJ. Assessment of the protective effect of Lepidium sativum against aluminium/induced liver and kidney effects in albino rat. Biomed Res Int. 2019;2019:4516730. [Link] [DOI:10.1155/2019/4516730]
11. Raval ND, Pandya TN. Pharmacognostic study of Lepidium sativum Linn (Chandrashura). AYU. 2012;32(1):116-9. [Link] [DOI:10.4103/0974-8520.85742]
12. Bigoniya P, Singh CS, Shukla A. Pharmacognostical and physico-chemical standardization of ethnopharmacologically important seeds L. sativum and W. tinctoria in Indian system of medicine. India J Nat Prod Resource. 2011;2(4):464-71. [Link]
13. Mathews S, Singhal RS, Kulkarni PR. Some physicochemical properties of Lepidium sativum (haliv) seeds. Die Nahrung.1993;37(1):69-71. [Link] [DOI:10.1002/food.19930370113]
14. Gokavi SS, Malleshi NG, Guo M. Chemical Composition of Garden Cress (Lepidium sativum) seeds and its fractions and use of bran as a functional ingredient. Plant Foods Human Nutr. 2004;59(3):105-11. [Link] [DOI:10.1007/s11130-004-4308-4]
15. Nayak PS, Upadhyay A, Dwivedi SK, Rao S. Hplc analysis of sinapic acid in Lepidium sativum. Electronic J Environ Agricultural Food Chem. 2012;11(3):156‐62. [Link]
16. Divekar VB, Kalaskar MG, Chougule PD, Redasani VK, Baheti DG. Isolation and characterization of mucilage from Lepidium sativum linn. seeds. Int J Pharma Res Dev. 2010;2(1):1-5. [Link]
17. Diwakar BT, Dutta PK, Lokesh BR, Naidu KA. Physicochemical properties garden cress (Lepidium sativum) seed oil. J Am Oil Chem Soc. 2010;87:539-48. [Link] [DOI:10.1007/s11746-009-1523-z]
18. Datta PK, Diwakar BT, Viswanatha S, Murthy KN, Naidu KA. Safety evaluation studies on Garden cress (Lepidium sativum L.) seeds in Wistar rats. Int J Appl Res Nat Prod. 2011;4(1):37-43. [Link]
19. Patel R, Kumar S, Jaiswal R, Rai S, Sahu A, Dwivedi S. Quantitative estimation of fixed oil obtained from seeds of Lepidium sativum Linn. Int J Chem Anal Sci. 2010;1(1):6-9.
20. Mirza M, Navaei MN. Essential oil composition of Lepidium sativum L. Iran J Med Aromat Plants. 2006;21(4):481-8. [Persian] [Link]
21. Agarwal J, Verma D.L. Antioxidant activity- guided fractionation of aqueous extracts from Lepidium sativum and identification of active flavonol glycosides. Academia Arena. 2011;3(12):14-8. [Link]
22. Hero FS, Akrayi JD. Anti bacterial activity of Lepidium sativum and Allium porrum extracts and juices against some gram positive and gram negative extracts. Pharmacognosia. Med J Islamic World Acad Sci. 2012;20(1):10-6. [Link]
23. Shama IY, Shayma AM, Salih S, Warda S. Abdelgadir. in vitro antimicrobial assessment of Lepidium sativum L. seeds extracts. Asian J Med Sci. 2011;3(6):261-6. [Link]
24. Yadav YC, Srivastava DN, Saini V, Seth AK, Tejas K, Malik A, et al. In-vitro antioxidant activities of ethanolic extract of Lepidium sativum seeds. Int J Pharmaceutical Sci. 2011;2(3):244-53. [Link]
25. Shukla A, Chandra SS, Papiya B. Phytochemical and CNS activity of Lepidium sativum Linn. Seeds total alkaloid. Der Pharmacia Lettre. 2011;3(2):226-37. [Link]
26. Abdullah HA. The effects of Lepidium sativum seeds on fracture-induced healing in rabbits. MedGenMed. 2007;9(2):23. [Link]
27. Afaf I, Nuha HS, Mohammed AH. hepatoprotective effect of Lepidium sativum against carbon tetrachloride induced damage in rats. Res J Animal Veterinary Sci. 2008;3:20-3. [Link]
28. Shinde N, Jagtap A, Undale V, Kakade S, Kotwal S, Patil R. Protective effect of Lepidium sativum against doxorubicin-induced nephrotoxicity in rats. Res J Pharma Biol Chem Sci. 2010;1(3):42-9. [Link]
29. Najeeb UR, Arif UK, Khalid MA, Anwarul HG. Pharmacological basis for the medicinal use of Lepidium sativum in airways disorders. Evid Based Complementary Alternative Med. 2012:1-8. [Link] [DOI:10.1155/2012/596524]









