Volume 15, Issue 2 (2023)
Iran J War Public Health 2023, 15(2): 107-114 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/02/26 | Accepted: 2023/04/16 | Published: 2023/05/5
Received: 2023/02/26 | Accepted: 2023/04/16 | Published: 2023/05/5
How to cite this article
Maseer S, Al-Aswad F. Clinical and Sonographic Evaluation of Major Salivary Gland Tumors. Iran J War Public Health 2023; 15 (2) :107-114
URL: http://ijwph.ir/article-1-1321-en.html
URL: http://ijwph.ir/article-1-1321-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Oral Diagnosis, Collage of Dentistry, University of Baghdad, Baghdad, Iraq
Full-Text (HTML) (1588 Views)
Introduction
Pathologies of the salivary glands comprise non-neoplastic lesions, such as inflammations of various causes, cysts, developmental anomalies, and parenchymal lesions in systemic diseases. Another group includes neoplastic lesions, which consist of benign and malignant tumors [1].
Most non-neoplastic swellings are caused by acute or chronic inflammation of the salivary glands. Regarding etiology, acute inflammation is typically caused by infection with viruses and bacteria, and the clinical presentation of this type of swelling is usually associated with sudden onset of induration, erythema, pain, and tenderness, whereas chronic ones are caused by sialolithiasis or autoimmune diseases [2].
Salivary gland neoplasm is a typical head and neck tumor in a clinic, constituting 2-6% of head and neck tumors. Moreover, most tumors involve large glands, such as the parotid glands, in around 70-85%. Those tumors are mostly benign in 80-95% of the cases, and 80% of neoplasms occur in the superficial lobe of the parotid gland, while malignant lesions constitute 10-17%. Slow swelling usually indicates a benign tumor, while rapid swelling is more likely to indicate a malignant tumor or infection, as well as difficulty in movement or weakness of the muscles in one side of the face, known as facial nerve palsy. This can signal a malignant and advanced tumor [3, 4].
Pleomorphic adenoma is ranked as the most common benign tumor and accounts for up to two-thirds of benign salivary gland neoplasms, followed by Warthin Tumor (WT) [5, 6].
Among malignant tumors of the glands, mucoepidermoid carcinoma accounts for 30% of all cases [7], with about 89% occurring in the parotid, followed by 8.4% in the submandibular gland [8].
The lack of specificity in the imaging manifestations and the various features of many pathological subtypes of salivary gland tumors makes it difficult to make a qualitative diagnosis before surgery. The efficacy of gland radiography in identifying benign from malignant tumors is limited [9, 10].
Medical diagnostic ultrasound is widely used worldwide and is considered a harmless, low-cost, and non-invasive technique for anatomical scanning [11, 12]. Consequently, some researchers proposed Multipara metric Ultrasound (MPUS) for salivary gland scanning to overcome a lack of accuracy in differentiating between the imaging patterns of the primary salivary gland tumors [13, 14]. Ultrasound can detect gland tumors' location, size, and internal echogenicity. It examines the morphology, border, internal echo, and posterior echo enhancement of salivary gland tumors and the blood flow signal in salivary gland tumors using Color Doppler Flow Imaging (CDFI) to create a solid foundation for qualitative tumor identification [15].
Although the final diagnosis must be confirmed by histopathology, morphological overlap in ultrasound images prevents accurate differentiation of benign from malignant [14]. This study aimed to determine the association between preoperative ultrasonography and postoperative histopathology in patients with surgically resected major salivary gland neoplasms. The distribution of ultrasound parameters was compared in benign and malignant tumors using the contingency coefficient test.
Instruments and Methods
This descriptive cross-sectional study was performed on 62 patients of both genders complaining of major salivary gland swellings. Clinicopathological and ultrasonic data of patients undergoing surgical treatment for gland neoplasm were collected during the period from December 2021 to July 2022 from two major teachings hospitals in Baghdad “Medical city, hospital of surgical specialties” and “Al-Kindy Hospital, maxillofacial department”. Patients with swelling or enlargement in the parotid salivary gland and submandibular salivary gland region were included in the study. Patients with sublingual salivary gland disorders, autoimmune diseases affecting the salivary gland-like Sjogren syndrome, sarcoidosis or Inflammatory pathogenesis, developmental anomalies of the salivary gland-like agenesis or aplasia, and Stafne bone cyst all were excluded from the study.
A high-frequency linear array probe (7-15 MHz) was used to perform the sonograms. Two machines were used: the Philips HD11XE Ultrasound (Bothell; WA, USA) and the Ultrasound System HS40 (Samsung; South Korea).
The patient was placed in a supine position, with the neck fully exposed and hyperextended and the head slightly turned to the side opposite the examined gland. Conventional US scanning was employed to scan the parotid gland and submandibular masses in sagittal and cross-sections to acquire complete images of the lesions and the adjacent normal tissues. The contrast mode was then turned on to select the section that could demonstrate the characteristics of the tumor. The probe was fixed, and the patient was instructed to relax. Serial scanning with conventional Ultrasound and Color Doppler mode was used to identify the location, size, border, shape, internal echogenicity, calcification, cystic changes, and if there were any lymph node enlargements in the examined area.
The one-sample Kolmogorov-Smirnov test was used to determine the normality of data distribution. Binomial test was used to examine the difference of the frequency of tumors based on diagnosis. The frequency distribution of ultrasound parameters and clinical examination parameters was compared in benign and malignant tumors using the contingency coefficient test. Also, because of the non-normal distribution of the data related to diameter and size of the lesion, the Mann-Whitney U test was used to determine the differences among the studied of independent groups.
Findings
Histopathological examination revealed that 18 of 62 tumors were malignant, and the rest 44 tumors were benign. 68.2% of benign cases were pleomorphic adenoma, and 61.1% of malignant cases were mucoepidermoid carcinoma. The frequency of both benign and malignant tumors was significantly different based on diagnosis (p=0.0001; Table 1).
Table 1) The frequency of benign and malignant tumors based on diagnosis

The mean age of the patient with malignant tumors was 49.99±15.65 years, and the mean age of the patient with benign tumors was 39.45_+15.65 years, which there was a significant difference between benign and malignant tumors based on the age (p=0.013). In the benign group, 22 patients were male, and 22 patients were female, while the malignant group consisted of 12 females and only 6 males. A significant difference was also observed regarding the gender (p=0.0001).
Gray-scale ultrasound examination of the echogenicity status in malignant cases showed mixed echogenicity in about 44.4% and hypoechoicity in about 55.6% compared to benign, which showed hypoechoic in 90.9% and mixed echogenicity in only 9.1% (p=0.001; Table 2).
Table 3 shows the estimate of the area of the trade-off between sensitivity and specificity by plotting sensitivity against a complement specificity outcome of studied markers amongst Benign and Malignant tumors to examine that trade-off, which is called a Receiver Operating Characteristic (ROC) curve. The sensitivity and specificity data for all parameters showed that strong differentiation is represented by the studied markers in the studied groups, such as (calcification in the lesion, echogenicity, posterior echo enhancement, and distribution of lesion vascularity).
Table 2) Comparison of the distribution of ultrasound parameters in the studied group's subjects


Given the size of the tumor, the goodness-of-fit test indicated whether the observations could reasonably have come from the specified distribution. Regarding that, the data distribution was non-normal concerning lesion size in the benign group, and concerning the 1st diameters, 2nd diameters, and lesion size in the malignant group, the non-parametric methods were used for the descriptive statistics, such as trimmed mean median and interquartile range, and Mann-Whitney U test was used to determine the differences among the studied of independent groups (Table 4).
Table 3) Receiver Operating Characteristic (ROC) for studied categories markers among studied groups

Table 4) Summary of statistics related to the diameter and size of the lesion distributed in the groups

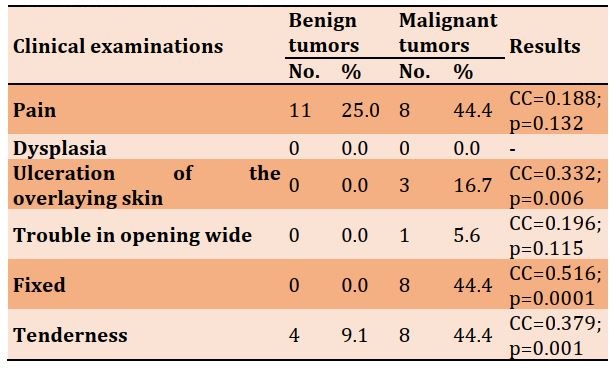
The clinical examination data of various features are represented in Table 5.
Table 5) Frequency distribution of clinical examination parameters in the studied groups
Discussion
Identifying benign and malignant lesions before surgery and knowing the histological types of gland lesions is essential. Sonography, in contrast, clarifies clinically ambiguous salivary gland enlargements. It has an effective accuracy rate for detecting salivary gland stones and distinguishing between inflammation and tumors [16]. Imaging investigations are essential for the preoperative differential diagnosis of gland tumors because they provide information on the precise tumor location within the gland and its relationships to adjacent structures. In the past few years, several researchers have proven that some ultrasound features help differentiate malignant and benign parotid gland tumors, including echotexture, posterior echo enhancement, shape, intra-lesion calcification, margin, and regional lymph enlargement [17].
This study concentrated on the fact that there is no particular sonographic characteristic enough to predict the disease. Therefore, there is a rationale to weigh sonographic characteristics for predicting benign and malignant salivary gland tumors [18]. Furthermore, this study focuses on understanding the epidemiological landscape and distribution of histological subtypes of salivary tumors that are essential for improving the diagnosis of this diverse group of tumors. It would be valuable for future studies to develop international datasets of these tumors used by other researchers.
This study demonstrated an increase in female patients in the malignant group, which is consistent with the findings of Kadhim et al. and Pouloudi et al. [19, 20] and other studies that reported a slight overall female majority among Salivary Gland Tumors (SGTs). Also, Kulhánová et al. reported that females are more affected by malignancy than males in the Middle East [21], as demonstrated by the current study. In contrast, Żurek et al. found that malignant salivary gland tumors have a slite male production, and in Western nations such as the United States, the United Kingdom, and Europe, males are affected by cancer more than females [22].
Regional differences in epidemiological malignancy data have been well-documented and can be attributed to various countries' sociological, socioeconomic, and lifestyle changes [20, 22, 23].
Regarding age, SGTs predominantly occur in adults, but individuals with malignant tumors tend to be older than those with benign tumors. These results are pretty similar to the data found by other studies that showed an increased rate of malignancy in older patients [24-26].
Among the analyzed cases, pleomorphic adenoma was the predominant benign salivary gland tumor, followed by Warthin Tumor (WT) [27]. Among malignant tumors of the glands, mucoepidermoid carcinoma represents the most frequent tumor type and is followed by acinic cell carcinoma. Previous corroborating data of the literature shows the exact distribution of the histological subtype [28].
Mengi et al. concluded that an inverse relationship exists between the incidence of salivary gland tumors by site and the percentage of malignancy. However, this study's result was insignificant when the tumors were evaluated according to their localizations site. Only 11.1 % of malignant tumors affect the submandibular gland, and most salivary tumors are located in the parotid gland, accounting for approximately 80% of all salivary gland tumors [28], most of which (75-80%) are benign [29].
As some studies declared, regional lymphadenopathies are relatively rare in primitive neoplasms of salivary glands. The results showed that no significant relationships were accounted for the distribution of the odds ratio at almost twice as times in the malignant group than in the benign group, compared with studies published in the literature that have shown that pathologic cervical adenopathy and extra glandular spread can be identified. So malignant tumors showed a higher incidence lymph node enlargement [9, 30].
El-Khateeb et al. discussed the shape of salivary gland neoplasm and declared that irregular shape is the most prominent parameter in malignant tumors [31]. The shape of lesions had a significant relationship with malignant tumors. Furthermore, the predominance of the lobulated shape of the lesion was seen in benign tumors. This is explained by the fact that most benign tumors are pleomorphic adenomas. As noted in numerous studies and the present study, this reflected the predominance of lobular shape in benign cases, which accounts for about 43.2%. Many other studies also confirm that the most common morphology of benign tumors is lobular and then oval (31.8%) [32]. Although the lobulated contour is an exciting and valuable feature, and only half of pleomorphic adenoma cases demonstrated lobulation, this feature was not present in any other benign tumor, and it was identified in only a few malignant tumors [33].
In addition, malignant tumors showed a higher incidence of irregular shapes, with an increase of more than three times compared with benign. Kim et al. believe that tumors have an irregular shape that occurs due to the invasion of the tumors to the surrounding structures [34]. This result is also assured by Guiban et al., who validate this outcome and combine it with the aggressive behavior of malignant neoplasm and its infiltration into the surrounding adjacent structure [35].
Some studies hypothesized that the borders of malignant salivary gland tumors are indistinct, but the boundaries are clear (well-defined) in benign tumors of the salivary gland [17, 18]. In this study, rather than simply stating that a significant level was not achieved, it could be said that a meaningful relationship is accounted for because the odd ratio of the unclear border increased almost about four times in malignant than in benign. In contrast to benign tumors, malignant tumors have partial capsules or none at all, which is the primary cause of the unclear border [15]. This may be because ultrasonography frequently misdiagnoses tumors as benign, especially malignant ones less than 2 cm in diameter, mainly low-grade malignant ones. Because these tumors appear with smooth, clear borders and a homogeneous structure, when it comes to more extensive lesions, the echo pattern is typically heterogeneous, and the margins are irregular. Ultrasound is highly accurate in diagnosing these tumors as malignant [24].
The presence of necrotic and hemorrhagic regions in the lesion gives the tumors different internal echo as hypoechoic or mixed-echoic pattern compared to the surrounding normal salivary gland tissue echo, and the mixed echo increased about eight times in malignant than in benign in this study. Lesions were analyzed as heterogeneous, which may be because of above mention cause [36]. The conclusion of the above-described data is very compatible with the research published in this field regarding this parameter [37, 38].
In ultrasound, calcification in the lesion is displayed as hyperechoic because of its high-intensity reflectors. Yoon et al. hypothesized the methods that help understand intra-tumoral calcification in the salivary gland as a result of either metastatic calcification due to hypercalcemia, dystrophic calcification in necrotic areas, or a constituent of the tumor, finally as calcinations of the substance generated by cancerous cells [39]. Sonographic evidence of calcification or microcalcifications showed the odds ratio of occurrence calcification in lesions was increased about 5.6 times in the malignant group than in the benign group, which is consistent with Lo et al.'s study that considers calcification as one of the sonographic characteristics for distinguishing malignant salivary gland tumors from benign [18].
Echotexture is either homogenous or Heterogeneous. Heterogeneity in the malignant group increased by 3.4 times more than in benign lesions, so malignancy is generally associated with a heterogeneous echotexture appearance; more aggressive malignant lesions have a more typically heterogeneous appearance. The results of this study confirm previous studies, reporting that heterogeneity is more associated with malignant tumors. In addition, the formation of internal cystic degeneration or dystrophic calcification is more associated with persistent lesions [13].
Posterior echo enhancement is a benign ultrasonic characteristic. Simultaneously evaluating all US parameters can boost the technique's sensitivity. There is a significant association between posterior echo enhancement and benign salivary glands. According to previous research, the present investigation indicated that the absence of posterior enhancement is suggestive of malignancy [9, 40].
When the color Doppler mode was activated, the vascularization pattern was classified depending on which pattern of vascularity is the most predominant internal or peripheral vessel formation [41]. Benign tumors are poorly or mainly peripherally vascularized and have little or no central flow because the dominant type of benign tumor is pleomorphic adenoma, which displays diminished central vasculature [30, 42]. Depending on their size, they may occasionally exhibit increased vascularization [13]. Malignancies show abundant capsular and internal vascularization on Doppler US (with a high number of micro angiogenesis, which is the primary source of sustenance for the tumor).
The parotid gland was the most afflicted, primarily by benign tumors, and symptoms were more typical in malignant tumors when the behavior of the lesions was analyzed. Benign and malignant salivary tumors differ in size, except for pleomorphic adenoma, which can grow to enormous proportions. It has been found that the duration of the swelling, in years, does not provide any certainty as to whether the mass is benign or malignant. As demonstrated in other research, huge tumors are more likely to be malignant than benign [43], and the bigger malignant tumors (>4 cm) are linked to a higher incidence of facial paralysis [44].
Clinically, salivary gland neoplasms are typically asymptomatic lumps. In addition, Benign and malignant salivary gland tumors generally have comparable clinical symptoms. In this study, symptoms were most prevalent in malignant tumors. However, some clinical traits, such as evolutionary history, facial palsy, ulceration, and advanced cancers, can have trismus, skin ulceration, and fistulas. The slow growth of a painless mass, however, does not rule out a malignant character [45]. Benign tumors grow slowly and do not cause any symptoms. Malignant tumors, on the other hand, grow quickly and may cause symptoms. The facial nerve is unaffected by the growth of benign tumors regardless of their size. Pain is a rare presenting symptom but can be devastating when combined with salivary gland cancer [46].
Limitation
This technique is operator dependent, lacks relatively uniform sections, and has difficulty evaluating tumors in the deep lobe of the parotid gland or posterior to bone structures. Therefore, establishing a dependable non-invasive diagnostic tool for identifying benign and malignant tumors would be helpful.
Conclusion
Several ultrasonic features of salivary gland tumors, such as irregular morphology, internal calcification, internal or central vascularity, and heterogeneity, are frequently linked with malignant than benign tumors. Regarding histopathological examination of tumor subtypes, pleomorphic adenoma ranks as the commonly occurring benign tumor and constitutes up to two-thirds of all benign salivary gland neoplasms. Among malignant tumors, mucoepidermoid carcinoma is the most common type. Older age and female gender are associated with a higher frequency of malignant salivary gland tumors.
Acknowledgements: We are grateful to the Maxillofacial Department at Baghdad Medical City, Specialized Surgical Hospital, and Al-Kindi Teaching Hospital.
Ethical Permission: This study was approved by ethical code 428721.
Conflict of Interests: There is no conflict of interests.
Authors’ Contributions: Maseer SK (First Author), Introduction Writer/Main Researcher/Methodologist/Statistical Analyst/Discussion Writer (60%); Al-Aswad FD (Second Author), Assistant Researcher (40%)
Funding: There are no financial resources.
Pathologies of the salivary glands comprise non-neoplastic lesions, such as inflammations of various causes, cysts, developmental anomalies, and parenchymal lesions in systemic diseases. Another group includes neoplastic lesions, which consist of benign and malignant tumors [1].
Most non-neoplastic swellings are caused by acute or chronic inflammation of the salivary glands. Regarding etiology, acute inflammation is typically caused by infection with viruses and bacteria, and the clinical presentation of this type of swelling is usually associated with sudden onset of induration, erythema, pain, and tenderness, whereas chronic ones are caused by sialolithiasis or autoimmune diseases [2].
Salivary gland neoplasm is a typical head and neck tumor in a clinic, constituting 2-6% of head and neck tumors. Moreover, most tumors involve large glands, such as the parotid glands, in around 70-85%. Those tumors are mostly benign in 80-95% of the cases, and 80% of neoplasms occur in the superficial lobe of the parotid gland, while malignant lesions constitute 10-17%. Slow swelling usually indicates a benign tumor, while rapid swelling is more likely to indicate a malignant tumor or infection, as well as difficulty in movement or weakness of the muscles in one side of the face, known as facial nerve palsy. This can signal a malignant and advanced tumor [3, 4].
Pleomorphic adenoma is ranked as the most common benign tumor and accounts for up to two-thirds of benign salivary gland neoplasms, followed by Warthin Tumor (WT) [5, 6].
Among malignant tumors of the glands, mucoepidermoid carcinoma accounts for 30% of all cases [7], with about 89% occurring in the parotid, followed by 8.4% in the submandibular gland [8].
The lack of specificity in the imaging manifestations and the various features of many pathological subtypes of salivary gland tumors makes it difficult to make a qualitative diagnosis before surgery. The efficacy of gland radiography in identifying benign from malignant tumors is limited [9, 10].
Medical diagnostic ultrasound is widely used worldwide and is considered a harmless, low-cost, and non-invasive technique for anatomical scanning [11, 12]. Consequently, some researchers proposed Multipara metric Ultrasound (MPUS) for salivary gland scanning to overcome a lack of accuracy in differentiating between the imaging patterns of the primary salivary gland tumors [13, 14]. Ultrasound can detect gland tumors' location, size, and internal echogenicity. It examines the morphology, border, internal echo, and posterior echo enhancement of salivary gland tumors and the blood flow signal in salivary gland tumors using Color Doppler Flow Imaging (CDFI) to create a solid foundation for qualitative tumor identification [15].
Although the final diagnosis must be confirmed by histopathology, morphological overlap in ultrasound images prevents accurate differentiation of benign from malignant [14]. This study aimed to determine the association between preoperative ultrasonography and postoperative histopathology in patients with surgically resected major salivary gland neoplasms. The distribution of ultrasound parameters was compared in benign and malignant tumors using the contingency coefficient test.
Instruments and Methods
This descriptive cross-sectional study was performed on 62 patients of both genders complaining of major salivary gland swellings. Clinicopathological and ultrasonic data of patients undergoing surgical treatment for gland neoplasm were collected during the period from December 2021 to July 2022 from two major teachings hospitals in Baghdad “Medical city, hospital of surgical specialties” and “Al-Kindy Hospital, maxillofacial department”. Patients with swelling or enlargement in the parotid salivary gland and submandibular salivary gland region were included in the study. Patients with sublingual salivary gland disorders, autoimmune diseases affecting the salivary gland-like Sjogren syndrome, sarcoidosis or Inflammatory pathogenesis, developmental anomalies of the salivary gland-like agenesis or aplasia, and Stafne bone cyst all were excluded from the study.
A high-frequency linear array probe (7-15 MHz) was used to perform the sonograms. Two machines were used: the Philips HD11XE Ultrasound (Bothell; WA, USA) and the Ultrasound System HS40 (Samsung; South Korea).
The patient was placed in a supine position, with the neck fully exposed and hyperextended and the head slightly turned to the side opposite the examined gland. Conventional US scanning was employed to scan the parotid gland and submandibular masses in sagittal and cross-sections to acquire complete images of the lesions and the adjacent normal tissues. The contrast mode was then turned on to select the section that could demonstrate the characteristics of the tumor. The probe was fixed, and the patient was instructed to relax. Serial scanning with conventional Ultrasound and Color Doppler mode was used to identify the location, size, border, shape, internal echogenicity, calcification, cystic changes, and if there were any lymph node enlargements in the examined area.
The one-sample Kolmogorov-Smirnov test was used to determine the normality of data distribution. Binomial test was used to examine the difference of the frequency of tumors based on diagnosis. The frequency distribution of ultrasound parameters and clinical examination parameters was compared in benign and malignant tumors using the contingency coefficient test. Also, because of the non-normal distribution of the data related to diameter and size of the lesion, the Mann-Whitney U test was used to determine the differences among the studied of independent groups.
Findings
Histopathological examination revealed that 18 of 62 tumors were malignant, and the rest 44 tumors were benign. 68.2% of benign cases were pleomorphic adenoma, and 61.1% of malignant cases were mucoepidermoid carcinoma. The frequency of both benign and malignant tumors was significantly different based on diagnosis (p=0.0001; Table 1).
Table 1) The frequency of benign and malignant tumors based on diagnosis

The mean age of the patient with malignant tumors was 49.99±15.65 years, and the mean age of the patient with benign tumors was 39.45_+15.65 years, which there was a significant difference between benign and malignant tumors based on the age (p=0.013). In the benign group, 22 patients were male, and 22 patients were female, while the malignant group consisted of 12 females and only 6 males. A significant difference was also observed regarding the gender (p=0.0001).
Gray-scale ultrasound examination of the echogenicity status in malignant cases showed mixed echogenicity in about 44.4% and hypoechoicity in about 55.6% compared to benign, which showed hypoechoic in 90.9% and mixed echogenicity in only 9.1% (p=0.001; Table 2).
Table 3 shows the estimate of the area of the trade-off between sensitivity and specificity by plotting sensitivity against a complement specificity outcome of studied markers amongst Benign and Malignant tumors to examine that trade-off, which is called a Receiver Operating Characteristic (ROC) curve. The sensitivity and specificity data for all parameters showed that strong differentiation is represented by the studied markers in the studied groups, such as (calcification in the lesion, echogenicity, posterior echo enhancement, and distribution of lesion vascularity).
Table 2) Comparison of the distribution of ultrasound parameters in the studied group's subjects


Given the size of the tumor, the goodness-of-fit test indicated whether the observations could reasonably have come from the specified distribution. Regarding that, the data distribution was non-normal concerning lesion size in the benign group, and concerning the 1st diameters, 2nd diameters, and lesion size in the malignant group, the non-parametric methods were used for the descriptive statistics, such as trimmed mean median and interquartile range, and Mann-Whitney U test was used to determine the differences among the studied of independent groups (Table 4).
Table 3) Receiver Operating Characteristic (ROC) for studied categories markers among studied groups

Table 4) Summary of statistics related to the diameter and size of the lesion distributed in the groups

The clinical examination data of various features are represented in Table 5.
Table 5) Frequency distribution of clinical examination parameters in the studied groups

Discussion
Identifying benign and malignant lesions before surgery and knowing the histological types of gland lesions is essential. Sonography, in contrast, clarifies clinically ambiguous salivary gland enlargements. It has an effective accuracy rate for detecting salivary gland stones and distinguishing between inflammation and tumors [16]. Imaging investigations are essential for the preoperative differential diagnosis of gland tumors because they provide information on the precise tumor location within the gland and its relationships to adjacent structures. In the past few years, several researchers have proven that some ultrasound features help differentiate malignant and benign parotid gland tumors, including echotexture, posterior echo enhancement, shape, intra-lesion calcification, margin, and regional lymph enlargement [17].
This study concentrated on the fact that there is no particular sonographic characteristic enough to predict the disease. Therefore, there is a rationale to weigh sonographic characteristics for predicting benign and malignant salivary gland tumors [18]. Furthermore, this study focuses on understanding the epidemiological landscape and distribution of histological subtypes of salivary tumors that are essential for improving the diagnosis of this diverse group of tumors. It would be valuable for future studies to develop international datasets of these tumors used by other researchers.
This study demonstrated an increase in female patients in the malignant group, which is consistent with the findings of Kadhim et al. and Pouloudi et al. [19, 20] and other studies that reported a slight overall female majority among Salivary Gland Tumors (SGTs). Also, Kulhánová et al. reported that females are more affected by malignancy than males in the Middle East [21], as demonstrated by the current study. In contrast, Żurek et al. found that malignant salivary gland tumors have a slite male production, and in Western nations such as the United States, the United Kingdom, and Europe, males are affected by cancer more than females [22].
Regional differences in epidemiological malignancy data have been well-documented and can be attributed to various countries' sociological, socioeconomic, and lifestyle changes [20, 22, 23].
Regarding age, SGTs predominantly occur in adults, but individuals with malignant tumors tend to be older than those with benign tumors. These results are pretty similar to the data found by other studies that showed an increased rate of malignancy in older patients [24-26].
Among the analyzed cases, pleomorphic adenoma was the predominant benign salivary gland tumor, followed by Warthin Tumor (WT) [27]. Among malignant tumors of the glands, mucoepidermoid carcinoma represents the most frequent tumor type and is followed by acinic cell carcinoma. Previous corroborating data of the literature shows the exact distribution of the histological subtype [28].
Mengi et al. concluded that an inverse relationship exists between the incidence of salivary gland tumors by site and the percentage of malignancy. However, this study's result was insignificant when the tumors were evaluated according to their localizations site. Only 11.1 % of malignant tumors affect the submandibular gland, and most salivary tumors are located in the parotid gland, accounting for approximately 80% of all salivary gland tumors [28], most of which (75-80%) are benign [29].
As some studies declared, regional lymphadenopathies are relatively rare in primitive neoplasms of salivary glands. The results showed that no significant relationships were accounted for the distribution of the odds ratio at almost twice as times in the malignant group than in the benign group, compared with studies published in the literature that have shown that pathologic cervical adenopathy and extra glandular spread can be identified. So malignant tumors showed a higher incidence lymph node enlargement [9, 30].
El-Khateeb et al. discussed the shape of salivary gland neoplasm and declared that irregular shape is the most prominent parameter in malignant tumors [31]. The shape of lesions had a significant relationship with malignant tumors. Furthermore, the predominance of the lobulated shape of the lesion was seen in benign tumors. This is explained by the fact that most benign tumors are pleomorphic adenomas. As noted in numerous studies and the present study, this reflected the predominance of lobular shape in benign cases, which accounts for about 43.2%. Many other studies also confirm that the most common morphology of benign tumors is lobular and then oval (31.8%) [32]. Although the lobulated contour is an exciting and valuable feature, and only half of pleomorphic adenoma cases demonstrated lobulation, this feature was not present in any other benign tumor, and it was identified in only a few malignant tumors [33].
In addition, malignant tumors showed a higher incidence of irregular shapes, with an increase of more than three times compared with benign. Kim et al. believe that tumors have an irregular shape that occurs due to the invasion of the tumors to the surrounding structures [34]. This result is also assured by Guiban et al., who validate this outcome and combine it with the aggressive behavior of malignant neoplasm and its infiltration into the surrounding adjacent structure [35].
Some studies hypothesized that the borders of malignant salivary gland tumors are indistinct, but the boundaries are clear (well-defined) in benign tumors of the salivary gland [17, 18]. In this study, rather than simply stating that a significant level was not achieved, it could be said that a meaningful relationship is accounted for because the odd ratio of the unclear border increased almost about four times in malignant than in benign. In contrast to benign tumors, malignant tumors have partial capsules or none at all, which is the primary cause of the unclear border [15]. This may be because ultrasonography frequently misdiagnoses tumors as benign, especially malignant ones less than 2 cm in diameter, mainly low-grade malignant ones. Because these tumors appear with smooth, clear borders and a homogeneous structure, when it comes to more extensive lesions, the echo pattern is typically heterogeneous, and the margins are irregular. Ultrasound is highly accurate in diagnosing these tumors as malignant [24].
The presence of necrotic and hemorrhagic regions in the lesion gives the tumors different internal echo as hypoechoic or mixed-echoic pattern compared to the surrounding normal salivary gland tissue echo, and the mixed echo increased about eight times in malignant than in benign in this study. Lesions were analyzed as heterogeneous, which may be because of above mention cause [36]. The conclusion of the above-described data is very compatible with the research published in this field regarding this parameter [37, 38].
In ultrasound, calcification in the lesion is displayed as hyperechoic because of its high-intensity reflectors. Yoon et al. hypothesized the methods that help understand intra-tumoral calcification in the salivary gland as a result of either metastatic calcification due to hypercalcemia, dystrophic calcification in necrotic areas, or a constituent of the tumor, finally as calcinations of the substance generated by cancerous cells [39]. Sonographic evidence of calcification or microcalcifications showed the odds ratio of occurrence calcification in lesions was increased about 5.6 times in the malignant group than in the benign group, which is consistent with Lo et al.'s study that considers calcification as one of the sonographic characteristics for distinguishing malignant salivary gland tumors from benign [18].
Echotexture is either homogenous or Heterogeneous. Heterogeneity in the malignant group increased by 3.4 times more than in benign lesions, so malignancy is generally associated with a heterogeneous echotexture appearance; more aggressive malignant lesions have a more typically heterogeneous appearance. The results of this study confirm previous studies, reporting that heterogeneity is more associated with malignant tumors. In addition, the formation of internal cystic degeneration or dystrophic calcification is more associated with persistent lesions [13].
Posterior echo enhancement is a benign ultrasonic characteristic. Simultaneously evaluating all US parameters can boost the technique's sensitivity. There is a significant association between posterior echo enhancement and benign salivary glands. According to previous research, the present investigation indicated that the absence of posterior enhancement is suggestive of malignancy [9, 40].
When the color Doppler mode was activated, the vascularization pattern was classified depending on which pattern of vascularity is the most predominant internal or peripheral vessel formation [41]. Benign tumors are poorly or mainly peripherally vascularized and have little or no central flow because the dominant type of benign tumor is pleomorphic adenoma, which displays diminished central vasculature [30, 42]. Depending on their size, they may occasionally exhibit increased vascularization [13]. Malignancies show abundant capsular and internal vascularization on Doppler US (with a high number of micro angiogenesis, which is the primary source of sustenance for the tumor).
The parotid gland was the most afflicted, primarily by benign tumors, and symptoms were more typical in malignant tumors when the behavior of the lesions was analyzed. Benign and malignant salivary tumors differ in size, except for pleomorphic adenoma, which can grow to enormous proportions. It has been found that the duration of the swelling, in years, does not provide any certainty as to whether the mass is benign or malignant. As demonstrated in other research, huge tumors are more likely to be malignant than benign [43], and the bigger malignant tumors (>4 cm) are linked to a higher incidence of facial paralysis [44].
Clinically, salivary gland neoplasms are typically asymptomatic lumps. In addition, Benign and malignant salivary gland tumors generally have comparable clinical symptoms. In this study, symptoms were most prevalent in malignant tumors. However, some clinical traits, such as evolutionary history, facial palsy, ulceration, and advanced cancers, can have trismus, skin ulceration, and fistulas. The slow growth of a painless mass, however, does not rule out a malignant character [45]. Benign tumors grow slowly and do not cause any symptoms. Malignant tumors, on the other hand, grow quickly and may cause symptoms. The facial nerve is unaffected by the growth of benign tumors regardless of their size. Pain is a rare presenting symptom but can be devastating when combined with salivary gland cancer [46].
Limitation
This technique is operator dependent, lacks relatively uniform sections, and has difficulty evaluating tumors in the deep lobe of the parotid gland or posterior to bone structures. Therefore, establishing a dependable non-invasive diagnostic tool for identifying benign and malignant tumors would be helpful.
Conclusion
Several ultrasonic features of salivary gland tumors, such as irregular morphology, internal calcification, internal or central vascularity, and heterogeneity, are frequently linked with malignant than benign tumors. Regarding histopathological examination of tumor subtypes, pleomorphic adenoma ranks as the commonly occurring benign tumor and constitutes up to two-thirds of all benign salivary gland neoplasms. Among malignant tumors, mucoepidermoid carcinoma is the most common type. Older age and female gender are associated with a higher frequency of malignant salivary gland tumors.
Acknowledgements: We are grateful to the Maxillofacial Department at Baghdad Medical City, Specialized Surgical Hospital, and Al-Kindi Teaching Hospital.
Ethical Permission: This study was approved by ethical code 428721.
Conflict of Interests: There is no conflict of interests.
Authors’ Contributions: Maseer SK (First Author), Introduction Writer/Main Researcher/Methodologist/Statistical Analyst/Discussion Writer (60%); Al-Aswad FD (Second Author), Assistant Researcher (40%)
Funding: There are no financial resources.
Keywords:
References
1. Kucharska E, Rzepakowska A, Cieślik M, Wilemska S, Bara M, Osuch-Wójcikiewicz E, et al. Indications for surgical treatment of major salivary gland pathologies with epidemiology analysis in adults-cohort study of 1173 cases. Otolaryngol Pol. 2022;76(4):1-5. [Link] [DOI:10.5604/01.3001.0015.8056]
2. Lindburg M, Ogden MA. Infectious Sialadenitis. Curr Otorhinolaryngol Rep. 2021;9(1):87-91. [Link] [DOI:10.1007/s40136-020-00315-5]
3. Iyer J, Hariharan A, Cao UMN, Mai CTT, Wang A, Khayambashi P, et al. An overview on the histogenesis and morphogenesis of salivary gland neoplasms and evolving diagnostic approaches. Cancers. 2021;13(15):3910. [Link] [DOI:10.3390/cancers13153910]
4. Cunha JLS, de Sousa SF, Mota CP, Rocha Silva JV, Cavalcante IL, Marinho EB, et al. Sialolipomas of minor salivary glands: A multi-institutional study and literature review. J Oral Pathol Med. 2021;50(2):210-9. [Link] [DOI:10.1111/jop.13124]
5. Luers J, Guntinas‐Lichius O, Klussmann J, Küsgen C, Beutner D, Grosheva M. The incidence of Warthin tumours and pleomorphic adenomas in the parotid gland over a 25‐year period. Clin Otolaryngol. 2016;41(6):793-7. [Link] [DOI:10.1111/coa.12694]
6. Ratnakumari V, Krishna KV. A clinical study of parotid tumours. Int J Surg. 2021;5(1):592-7. [Link] [DOI:10.33545/surgery.2021.v5.i1g.788]
7. Al-Ani LS, Al-Azzawi LM. Evaluation of immunohistochemical expression of P53 and Pcna in pleomorphic adenoma, mucoepidermoid and adenoid cystic carcinomas of salivary glands. Tikrit J Dent Sci. 2014;3(1):1-8. [Link] [DOI:10.12816/0015037]
8. Young A, Okuyemi OT. Malignant salivary gland tumors. StatPearls [Internet]: StatPearls Publishing; 2022. [Link]
9. Stoia S, Băciuț G, Lenghel M, Badea R, Băciuț M, Bran S, et al. Ultrasonography techniques in the preoperative diagnosis of parotid gland tumors-an updated review of the literature. Med Ultrason. 2021;23(2):194-202. [Link] [DOI:10.11152/mu-2652]
10. Yan M, Xu D, Chen L, Zhou L. Comparative study of qualitative and quantitative analyses of contrast-enhanced ultrasound and the diagnostic value of B-mode and color Doppler for common benign tumors in the parotid gland. Front Oncol. 2021;11:669542. [Link] [DOI:10.3389/fonc.2021.669542]
11. Ramsha A, Keskool P, Ongard S, Metheetrairut C. Outcome of the management of salivary gland diseases by sialendoscopy: a university hospital's experience. J Oral Maxillofac Surg. 2023;81(3):344-9. [Link] [DOI:10.1016/j.joms.2022.11.010]
12. Al-jaburi AA, Al-sudani AH. Medical ultrasound image quality enhancement and regions segmentation. Iraq J Sci. 2022;63(10):4518-33. [Link] [DOI:10.24996/ijs.2022.63.10.35]
13. Martino M, Fodor D, Fresilli D, Guiban O, Rubini A, Cassoni A, et al. Narrative review of multiparametric ultrasound in parotid gland evaluation. Gland Surg. 2020;9(6):2295-311. [Link] [DOI:10.21037/gs-20-530]
14. Wang Y, Xie W, Huang S, Feng M, Ke X, Zhong Z, et al. The diagnostic value of ultrasound-based deep learning in differentiating parotid gland tumors. J Oncol. 2022;2022:8192999. [Link] [DOI:10.1155/2022/8192999]
15. Chen H, Bao X, Wan L. Application of contrast-enhanced ultrasound combined with elastic imaging technology in differential diagnosis of salivary gland tumors. J Healthc Eng. 2022;2022:4600751. [Link] [DOI:10.1155/2022/4600751]
16. Bhatia KSS, Dai Y-L. Routine and advanced ultrasound of major salivary glands. Neuroimaging Clin N Am. 2018;28(2):273-93. [Link] [DOI:10.1016/j.nic.2018.01.007]
17. Haidar YM, Moshtaghi O, Mahmoodi A, Helmy M, Goddard JA, Armstrong WB. The utility of in-office ultrasound in the diagnosis of parotid lesions. Otolaryngol Head Neck Surg. 2017;156(3):511-7. [Link] [DOI:10.1177/0194599816687744]
18. Lo WC, Chang CM, Wang CT, Cheng PW, Liao LJ. A novel sonographic scoring model in the prediction of major salivary gland tumors. Laryngoscope. 2021;131(1):E157-62. [Link] [DOI:10.1002/lary.28591]
19. Kadhim NJ, Bede SY. Surgical treatment outcome of salivary gland tumors. J Baghdad Coll Dent. 2019;31(3):34-8. [Link] [DOI:10.26477/jbcd.v31i3.2698]
20. Pouloudi D, Sotiriadis A, Theodorakidou M, Sarantis P, Pergaris A, Karamouzis MV, et al. The impact of angiogenesis in the most common salivary gland malignant tumors. Int J Mol Sci. 2020;21(24):9335. [Link] [DOI:10.3390/ijms21249335]
21. Kulhánová I, Bray F, Fadhil I, Al-Zahrani AS, El-Basmy A, Anwar WA, et al. Profile of cancer in the Eastern Mediterranean region: The need for action. Cancer Epidemiol. 2017;47:125-32. [Link] [DOI:10.1016/j.canep.2017.01.009]
22. Żurek M, Rzepakowska A, Jasak K, Niemczyk K. The epidemiology of salivary glands pathologies in adult population over 10 years in Poland-cohort study. Int J Environ Res Public Health. 2021;19(1):179. [Link] [DOI:10.3390/ijerph19010179]
23. Karwan M, Abdullah OS, Amin AM, Mohamed ZA, Bestoon B, Shekha M, et al. Cancer incidence in the Kurdistan region of Iraq: Results of a seven-year cancer registration in Erbil and Duhok Governorates. Asian Pac J Cancer Prev. 2022;23(2):601-15. [Link] [DOI:10.31557/APJCP.2022.23.2.601]
24. Ribeiro A, de Carvalho ALSH, Koth VS, Campos MM. Salivary gland tumors: a ten-year retrospective analysis in a Brazilian Teaching Hospital. Revista Brasileira de Cancerologia. 2021;67(4):041452. [Link] [DOI:10.32635/2176-9745.RBC.2021v67n4.1452]
25. Zuo H. The clinical characteristics and CT findings of parotid and submandibular gland tumours. J Oncol. 2021;2021:8874100. [Link] [DOI:10.1155/2021/8874100]
26. Rutt AL, Hawkshaw MJ, Lurie D, Sataloff RT. Salivary gland cancer in patients younger than 30 years. Ear Nose Throat J. 2011;90(4):174-84. [Link] [DOI:10.1177/014556131109000409]
27. Kucharska E, Rzepakowska A, Cieślik M, Wilemska S, Bara M, Osuch-Wójcikiewicz E, et al. Indications for surgical treatment of major salivary gland pathologies with epidemiology analysis in adults-cohort study of 1173 cases. Otolaryngol Pol. 2022;76(4):1-5. [Link] [DOI:10.5604/01.3001.0015.8056]
28. Mengi E, Kara CO, Tumkaya F, Ardic FN, Topuz B, Bir F. Salivary gland tumors: A 15-year experience of a university hospital in Turkey. North Clin Istanb. 2020;7(4):366-71. [Link] [DOI:10.14744/nci.2020.57767]
29. Tretiakow D, Mikaszewski B, Skorek A. The role of fine‑needle aspiration biopsy (FNAB) in the diagnostic management of parotid gland masses with emphasis on potential pitfalls. Eur Arch Otorhinolaryngol. 2020;277(10):2939-40. [Link] [DOI:10.1007/s00405-020-05923-x]
30. David E, Cantisani V, De Vincentiis M, Sidhu P, Greco A, Tombolini M, et al. Contrast-enhanced ultrasound in the evaluation of parotid gland lesions: an update of the literature. Ultrasound. 2016;24(2):104-10. [Link] [DOI:10.1177/1742271X15626611]
31. El-Khateeb SM, Abou-Khalaf AE, Farid MM, Nassef MA. A prospective study of three diagnostic sonographic methods in differentiation between benign and malignant salivary gland tumours. Dentomaxillofac Radiol. 2011;40(8):476-85. [Link] [DOI:10.1259/dmfr/18407834]
32. Petrovan C, Nekula DM, Mocan SL, Voidăzan TS, Coşarcă A. Ultrasonography-histopathology correlation in major salivary glands lesions. Rom J Morphol Embryol. 2015;56(2):491-7. [Link]
33. Dumitriu D, Dudea SM, Botar-Jid C, Băciuţ G. Ultrasonographic and sonoelastographic features of pleomorphic adenomas of the salivary glands. Med Ultrason. 2010;12(3):175-83. [Link]
34. Kim S-Y, Kim E-K, Moon HJ, Yoon JH, Kwak JY. Application of texture analysis in the differential diagnosis of benign and malignant thyroid nodules: comparison with gray-scale ultrasound and elastography. Am J Roentgenol. 2015;205(3):W343-51. [Link] [DOI:10.2214/AJR.14.13825]
35. Guiban O, Rubini A, Fresilli D, Lucarelli GT, Ralli M, Cassoni A, et al. Preoperative multiparametric ultrasound and fine needle aspiration cytology evaluation of parotid gland tumors: which is the best technique? Med Ultrason. 2021;23(4):402-9. [Link] [DOI:10.11152/mu-3068]
36. Angang D, Jia L, Xia G, Ping X, Jiang L. Gray scale and Doppler ultrasonography features of the carcinoma ex pleomorphic adenoma. Dentomaxillofac Radiol. 2018;47(4):20170268. [Link] [DOI:10.1259/dmfr.20170268]
37. Peng L, Li N, Luo Y, Fei X, Li Q, Zhao X. Ultrasonographic prediction model for benign and malignant salivary gland tumors: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;134(6):758-67. [Link] [DOI:10.1016/j.oooo.2022.07.017]
38. Zhao Y, Jiang T, Lv K, Pan M, Wen Q, Huang P. Application of ultrasound and contrast-enhanced ultrasound to distinguish salivary focal inflammatory masses from malignant masses: A retrospective observational study. Clin Hemorheol Microcirc. 2021;79(3):423-34. [Link] [DOI:10.3233/CH-211151]
39. Yoon JH, Ahn SG, Kim SG, Kim J. Calcifications in a clear cell mucoepidermoid carcinoma of the hard palate. Int J Oral Maxillofac Surg. 2005;34(8):927-9. [Link] [DOI:10.1016/j.ijom.2005.03.012]
40. Gerwel A, Kosik K, Jurkiewicz D. US in preoperative evaluation of parotid gland neoplasms. Otolaryngol Pol. 2015;69(2):27-33. [Link] [DOI:10.5604/00306657.1149638]
41. Mansour N, Bas M, Stock KF, Strassen U, Hofauer B, Knopf A. Multimodal ultrasonographic pathway of parotid gland lesions. Ultraschall Med. 2017;38(2):166-73. [Link] [DOI:10.1055/s-0035-1553267]
42. Zhao L, Mao Y, Mu J, Zhao J, Li F, Zhang S, et al. The diagnostic value of Superb Microvascular Imaging in identifying benign tumors of parotid gland. BMC Med Imaging. 2020;20(1):107. [Link] [DOI:10.1186/s12880-020-00506-y]
43. Bradley PJ. Frequency and histopathology by site, major pathologies, symptoms and signs of salivary gland neoplasms. Adv Otorhinolaryngol. 2016;78:9-16. [Link] [DOI:10.1159/000442120]
44. da Silva LP, Serpa MS, Viveiros SK, Sena DAC, de Carvalho Pinho RF, de Abreu Guimaraes LD, et al. Salivary gland tumors in a Brazilian population: A 20-year retrospective and multicentric study of 2292 cases. J Craniomaxillofac Surg. 2018;46(12):2227-33. [Link] [DOI:10.1016/j.jcms.2018.09.028]
45. Gatta G, Guzzo M, Locati LD, McGurk M, Prott FJ. Major and minor salivary gland tumours. Crit Rev Oncol Hematol. 2020;152:102959. [Link] [DOI:10.1016/j.critrevonc.2020.102959]
46. Fomete B, Adebayo E, Ononiwu C. Management of salivary gland tumors in a Nigerian tertiary institution. Ann Afr Med. 2015;14(3):148-54. [Link] [DOI:10.4103/1596-3519.152071]











