Volume 15, Issue 2 (2023)
Iran J War Public Health 2023, 15(2): 177-180 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/02/9 | Accepted: 2023/04/16 | Published: 2023/05/26
Received: 2023/02/9 | Accepted: 2023/04/16 | Published: 2023/05/26
How to cite this article
Hayder Ewad H, Basheer A, Omairi S, Lafta M, Mahdi M. Canagliflozin-associated Peripheral Vascular Ischemia: A Case Report. Iran J War Public Health 2023; 15 (2) :177-180
URL: http://ijwph.ir/article-1-1319-en.html
URL: http://ijwph.ir/article-1-1319-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Acute Medicine, College of Medicine, University of South Wales, Wales, UK
2- Department of Internal Medicine, Collage of Medicine, Wasit University, Kut, Iraq
3- Department of Anatomy and Biology, College of Medicine, Wasit University, Kut, Iraq
4- Department of Internal Medicine, College of Medicine, Baghdad University, Baghdad, Iraq
2- Department of Internal Medicine, Collage of Medicine, Wasit University, Kut, Iraq
3- Department of Anatomy and Biology, College of Medicine, Wasit University, Kut, Iraq
4- Department of Internal Medicine, College of Medicine, Baghdad University, Baghdad, Iraq
Full-Text (HTML) (654 Views)
Introduction
Sodium-Glucose Transporter 2 (SGLT2) inhibitors are a new family of drugs approved by the Food and Drug Administration (FDA) to be used as a treatment for patients with type 2 diabetes mellitus, along with diet and exercise to lower blood glucose levels. There are four drugs in the SGLT2 inhibitors family, including canagliflozin, ertugliflozin, dapagliflozin, and empagliflozin. These drugs are available alone or in combination with other diabetes medications, such as metformin. In the same line of thought, they act on sodium glucose cotransporter in renal proximal convoluted tubule, which is responsible for more than 90% of glucose reabsorption [1]. Previous studies have illustrated that canagliflozin decreases the risk of myocardial infarction, stroke, and cardiovascular mortality. Moreover, this drug reduces hospital admission rate of patients with heart failure, renal failure, as well as decreases blood pressure, body weight, and protein in urine [2, 3]. On the other hand, there are concerns regarding the side effects of these drugs, especially the increase in risk of lower limb amputation, as demonstrated in CANVAS program [3]. In addition, SGLT2 inhibitors have many other side effects on different parts of human body, as they increase the risk of urinary tract infection, diabetic ketoacidosis, and leg ulcer. Several studies have been conducted on this family of drugs to estimate their side effects on patients with various chronic diseases [4-6]. This report is about a patient with type 2 diabetes mellitus who had been using canagliflozin for years and now presented with foot and leg ulcers.
Patient and Methods
The case was a 69-year-old male patient who was admitted to Southmead hospital in Bristol after a fall at home. He was subsequently attended by his careers, who arranged hospital admission through the emergency services as he looked poorly to them.
In the hospital, based on clinical findings, chest X-ray abnormalities, and increased inflammatory markers in blood tests, Community-Acquired Pneumonia (CAP) was diagnosed. Interestingly, we noticed that there was some blackish discoloration on his right big toe with some spreading redness and swelling (Figures 1 & 2). He claimed that these changes existed for the last few weeks prior to his admission. Following a course of Intravenous (IV) antibiotic for CAP, the patient was referred to the vascular team for the new gangrenous changes in his right foot. Arterial duplex study confirmed significant peripheral vascular disease of the lower limb circulation, and the patient was therefore booked for diagnostic and possibly therapeutic angioplasty.
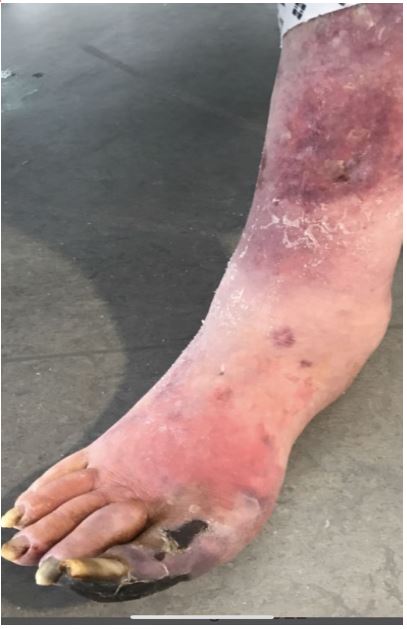
Figure 1) Gross image of the foot with ulcer

Figure 2) Toe ulcer
It should be noted that the patient had a history of ischemic heart disease, multiple sclerosis, chronic kidney disease, and type 2 diabetes mellitus. In addition, he was taking the oral hypoglycemic drug metformin, a sulfonylurea (gliclazide), and the SGLT2 inhibitor canagliflozin, which was started three years ago (2019).
Angiography of the right lower limb showed significant proximal and distal arterial disease (Figures 3 & 4). Angioplasty helped the proximal lesion in the superficial femoral artery but failed to improve the distal circulation. Unfortunately, despite all attempts to optimize his medical management, there was an inadequate response to all interventions, and he subsequently died.
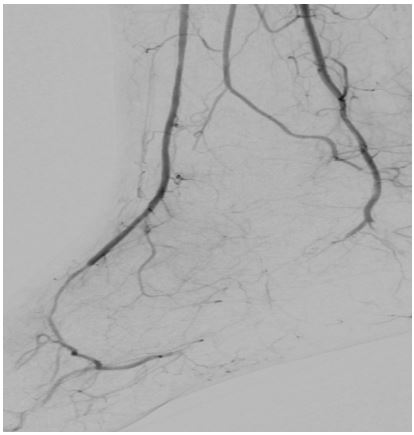
Figure 3) Angiogram of lower limb
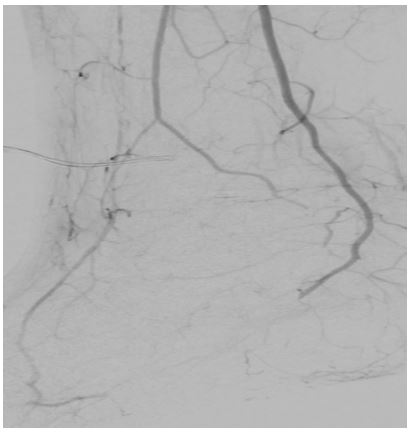
Figure 4) Angiogram of lower limb
Findings
It seems that the risk of peripheral vascular ischemia is increased in type 2 diabetes patients taking continuous medications containing SGL2 inhibitors, especially canagliflozin.
This case was mainly highlighted to confirm the fact that using relatively newly introduced SGLT2 inhibitors is associated with increased incidence of lower limb amputation, particularly toes. Importantly, even though this patient was recognized at the beginning of his medical admission to have gangrenous changes in his big toe and second toe, no serous attempts were made to stop it till a few days later.
Discussion
Our case report states that the risk of peripheral vascular ischemia is increased in type 2 diabetic patients taking continuous medications containing SGL2 inhibitors, especially canagliflozin. Although there is no approved evidence, this case report raises suspicions about other SGL2 inhibitors, such as empagliflozin and dapagliflozin, which is consistent with a previous study in the US. It has been reported that amputations in diabetic patients over 65 years of age with pre-existing cardiovascular disease, especially taking canagliflozin, are higher than in patients with similar disease profile but taking glucagon-like peptide-1 receptor agonists [7, 8]. The aim of this report was to establish new data for the currently used medication, such as commonly prescribed SGL2 inhibitors. Therefore, it is necessary to raise awareness about the potential side effects behind some of the new drug classes that are commonly prescribed today, although the main drugs used are dapagliflozin and empagliflozin, which have not yet been evidently proven to have the same risk as canagliflozin [9, 10].
Despite the harmful psychological effects, amputation of the lower limb is associated with a high risk of morbidity and mortality because most of these patients have depression and anxiety. Our interpretation of the main reason behind this ischemic process is that canagliflozin has stronger diuretic effects than dapagliflozin and empagliflozin, thus it induces blood concentration, and hyper viscosity supports the process of ischemia and gangrenous [11]. Interestingly, cardiovascular outcome trials for empagliflozin and dapagliflozin found no increased risk of amputation compared with placebo [12, 13]. Some speculate that the unique pharmacologic properties of canagliflozin could explain the increased risk of amputation that has not been seen for empagliflozin or dapagliflozin [13].
As these drugs are now widely used not only in the management of patients with diabetes mellitus but also in patients with heart failure, this case is a reminder to look into this relatively rare but serious side effect association with these drugs. A careful look into the indications of SGLT2 inhibitor drugs and follow-up of patients using them in order to intervene early to prevent these unexpected complications is highly recommended.
Conclusion
There may be is a relationship between canagliflozin and lower limb ulcers in patients with type 2 diabetes mellitus who are older than 65 years.
Acknowledgements: We would like to thank all teachers who helped us with this case report.
Ethical Permission: Permission was obtained from the patient at the time of admission to the hospital.
Conflict of Interests: There is no conflict of interests.
Authors’ Contribution: Hayder Ewad H (First Author), Main Researcher (20%); Basheer A (Second Author), Introduction Writer (20%); Omairi S (Third Author), Discussion Writer (20%); Lafta MS (Fourth Author), Discussion Writer (20%); Mahdi MG (Fifth Author), Introduction Writer (20%)
Funding: There was no financial support for this case report.
Sodium-Glucose Transporter 2 (SGLT2) inhibitors are a new family of drugs approved by the Food and Drug Administration (FDA) to be used as a treatment for patients with type 2 diabetes mellitus, along with diet and exercise to lower blood glucose levels. There are four drugs in the SGLT2 inhibitors family, including canagliflozin, ertugliflozin, dapagliflozin, and empagliflozin. These drugs are available alone or in combination with other diabetes medications, such as metformin. In the same line of thought, they act on sodium glucose cotransporter in renal proximal convoluted tubule, which is responsible for more than 90% of glucose reabsorption [1]. Previous studies have illustrated that canagliflozin decreases the risk of myocardial infarction, stroke, and cardiovascular mortality. Moreover, this drug reduces hospital admission rate of patients with heart failure, renal failure, as well as decreases blood pressure, body weight, and protein in urine [2, 3]. On the other hand, there are concerns regarding the side effects of these drugs, especially the increase in risk of lower limb amputation, as demonstrated in CANVAS program [3]. In addition, SGLT2 inhibitors have many other side effects on different parts of human body, as they increase the risk of urinary tract infection, diabetic ketoacidosis, and leg ulcer. Several studies have been conducted on this family of drugs to estimate their side effects on patients with various chronic diseases [4-6]. This report is about a patient with type 2 diabetes mellitus who had been using canagliflozin for years and now presented with foot and leg ulcers.
Patient and Methods
The case was a 69-year-old male patient who was admitted to Southmead hospital in Bristol after a fall at home. He was subsequently attended by his careers, who arranged hospital admission through the emergency services as he looked poorly to them.
In the hospital, based on clinical findings, chest X-ray abnormalities, and increased inflammatory markers in blood tests, Community-Acquired Pneumonia (CAP) was diagnosed. Interestingly, we noticed that there was some blackish discoloration on his right big toe with some spreading redness and swelling (Figures 1 & 2). He claimed that these changes existed for the last few weeks prior to his admission. Following a course of Intravenous (IV) antibiotic for CAP, the patient was referred to the vascular team for the new gangrenous changes in his right foot. Arterial duplex study confirmed significant peripheral vascular disease of the lower limb circulation, and the patient was therefore booked for diagnostic and possibly therapeutic angioplasty.

Figure 1) Gross image of the foot with ulcer

Figure 2) Toe ulcer
It should be noted that the patient had a history of ischemic heart disease, multiple sclerosis, chronic kidney disease, and type 2 diabetes mellitus. In addition, he was taking the oral hypoglycemic drug metformin, a sulfonylurea (gliclazide), and the SGLT2 inhibitor canagliflozin, which was started three years ago (2019).
Angiography of the right lower limb showed significant proximal and distal arterial disease (Figures 3 & 4). Angioplasty helped the proximal lesion in the superficial femoral artery but failed to improve the distal circulation. Unfortunately, despite all attempts to optimize his medical management, there was an inadequate response to all interventions, and he subsequently died.

Figure 3) Angiogram of lower limb

Figure 4) Angiogram of lower limb
Findings
It seems that the risk of peripheral vascular ischemia is increased in type 2 diabetes patients taking continuous medications containing SGL2 inhibitors, especially canagliflozin.
This case was mainly highlighted to confirm the fact that using relatively newly introduced SGLT2 inhibitors is associated with increased incidence of lower limb amputation, particularly toes. Importantly, even though this patient was recognized at the beginning of his medical admission to have gangrenous changes in his big toe and second toe, no serous attempts were made to stop it till a few days later.
Discussion
Our case report states that the risk of peripheral vascular ischemia is increased in type 2 diabetic patients taking continuous medications containing SGL2 inhibitors, especially canagliflozin. Although there is no approved evidence, this case report raises suspicions about other SGL2 inhibitors, such as empagliflozin and dapagliflozin, which is consistent with a previous study in the US. It has been reported that amputations in diabetic patients over 65 years of age with pre-existing cardiovascular disease, especially taking canagliflozin, are higher than in patients with similar disease profile but taking glucagon-like peptide-1 receptor agonists [7, 8]. The aim of this report was to establish new data for the currently used medication, such as commonly prescribed SGL2 inhibitors. Therefore, it is necessary to raise awareness about the potential side effects behind some of the new drug classes that are commonly prescribed today, although the main drugs used are dapagliflozin and empagliflozin, which have not yet been evidently proven to have the same risk as canagliflozin [9, 10].
Despite the harmful psychological effects, amputation of the lower limb is associated with a high risk of morbidity and mortality because most of these patients have depression and anxiety. Our interpretation of the main reason behind this ischemic process is that canagliflozin has stronger diuretic effects than dapagliflozin and empagliflozin, thus it induces blood concentration, and hyper viscosity supports the process of ischemia and gangrenous [11]. Interestingly, cardiovascular outcome trials for empagliflozin and dapagliflozin found no increased risk of amputation compared with placebo [12, 13]. Some speculate that the unique pharmacologic properties of canagliflozin could explain the increased risk of amputation that has not been seen for empagliflozin or dapagliflozin [13].
As these drugs are now widely used not only in the management of patients with diabetes mellitus but also in patients with heart failure, this case is a reminder to look into this relatively rare but serious side effect association with these drugs. A careful look into the indications of SGLT2 inhibitor drugs and follow-up of patients using them in order to intervene early to prevent these unexpected complications is highly recommended.
Conclusion
There may be is a relationship between canagliflozin and lower limb ulcers in patients with type 2 diabetes mellitus who are older than 65 years.
Acknowledgements: We would like to thank all teachers who helped us with this case report.
Ethical Permission: Permission was obtained from the patient at the time of admission to the hospital.
Conflict of Interests: There is no conflict of interests.
Authors’ Contribution: Hayder Ewad H (First Author), Main Researcher (20%); Basheer A (Second Author), Introduction Writer (20%); Omairi S (Third Author), Discussion Writer (20%); Lafta MS (Fourth Author), Discussion Writer (20%); Mahdi MG (Fifth Author), Introduction Writer (20%)
Funding: There was no financial support for this case report.
Keywords:
References
1. Wilcox T, De Block C, Schwartzbard AZ, Newman JD. Diabetic agents, from metformin to SGLT2 inhibitors and GLP1 receptor agonists: JACC focus seminar. J Am Coll Cardiol. 2020;75(16):1956-74. [Link] [DOI:10.1016/j.jacc.2020.02.056]
2. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Credence trial investigators canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295-306. [Link] [DOI:10.1056/NEJMoa1811744]
3. Neal B, Perkovic V, Matthews DR, Mahaffey KW, de Zeeuw D, Fulcher G, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. [Link] [DOI:10.1056/NEJMc1712572]
4. Heerspink H, Stefánsson BV, Correa Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-46. [Link] [DOI:10.1056/NEJMoa2024816]
5. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-24. [Link] [DOI:10.1056/NEJMoa2022190]
6. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425-35. [Link] [DOI:10.1056/NEJMoa2004967]
7. Fralick M, Kim SC, Schneeweiss S, Everett BM, Glynn RJ, Patorno E. Risk of amputation with canagliflozin across categories of age and cardiovascular risk in three US nationwide databases: Cohort study. BMJ. 2020;370:m2812. [Link] [DOI:10.1136/bmj.m2812]
8. Ueda P, Svanström H, Melbye M, Melbye M, Eliasson B, Svensson A, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious ad- verse events: nationwide register based cohort study. BMJ. 2018;363:k4365. [Link] [DOI:10.1136/bmj.k4365]
9. Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes Care. 2001;24(5):860-4. [Link] [DOI:10.2337/diacare.24.5.860]
10. Canavan RJ, Unwin NC, Kelly WF, Connolly VM. Diabetes and nondiabetes-related lower extremity amputation incidence before and after the introduction of better organized diabetes foot care: Continuous longitudinal monitoring using a standard method. Diabetes Care 2008;31(3):459-63. [Link] [DOI:10.2337/dc07-1159]
11. Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: Potential mechanisms, clinical applications, and summary of clinical trials. Circulation 2017;136(17):1643-58. [Link] [DOI:10.1161/CIRCULATIONAHA.117.030012]
12. Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care 2018;41(1):e4-5. [Link] [DOI:10.2337/dc17-1551]
13. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG outcome investigators empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-28. [Link] [DOI:10.1056/NEJMoa1504720]










