Volume 14, Issue 4 (2022)
Iran J War Public Health 2022, 14(4): 401-408 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/08/23 | Accepted: 2022/11/11 | Published: 2022/11/1
Received: 2022/08/23 | Accepted: 2022/11/11 | Published: 2022/11/1
How to cite this article
Jumagaliyeva M, Ayaganov D, Saparbayev S, Tuychibayeva N. Hypoxic Encephalopathy in COVID-19. Iran J War Public Health 2022; 14 (4) :401-408
URL: http://ijwph.ir/article-1-1263-en.html
URL: http://ijwph.ir/article-1-1263-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Neurology with the Course of Psychiatry and Narcology, Faculty of General Medicine, West Kazakhstan Marat Ospanov Medical University, Aktobe, Republic of Kazakhstan
2- West Kazakhstan Marat Ospanov Medical University, Aktobe, Republic of Kazakhstan
3- Department of Neurology with a Course of Medical Psychology and Psychotherapy, Medical Faculty, Tashkent Medical Academy, Tashkent, Republic of Uzbekistan
2- West Kazakhstan Marat Ospanov Medical University, Aktobe, Republic of Kazakhstan
3- Department of Neurology with a Course of Medical Psychology and Psychotherapy, Medical Faculty, Tashkent Medical Academy, Tashkent, Republic of Uzbekistan
Full-Text (HTML) (711 Views)
Introduction
In December 2019, a large number of pneumonia cases were observed in Wuhan (China) with no known cause at the time. Similar symptomatic cases of pneumonia quickly spread to other countries and continents. Over time, it became clear that these cases of pneumonia were caused by a novel Coronavirus (CoV) [1]. In 2003, a novel CoV virus was reported to have symptoms resembling those of Severe Acute Respiratory Syndrome CoV (SARS- CoV) [2]. Both viruses have the same Angiotensin -Converting Enzyme 2 (ACE2) receptor [3], and the new virus was named SARS-CoV-2. In February 2020, the World Health Organization (WHO) defined the disease as COVID-19. Human infection with SARS-CoV-2 often leads to a severe course of the disease and possible high mortality [4]. The worldwide death rate registered as a result of COVID-19 between January 1, 2020, and December 31, 2021, has reached 5.94 million [5]. There are typical clinical symptoms of COVID-19 described in several studies. The disease is manifested by high body temperature, cough, disorders of the gastrointestinal tract, and increased fatigue in the vast majority of cases of the infection. There are also several laboratory test results associated with COVID-19 and clinical diagnostic tests such as computed tomography of the lung [6]. However, scientific studies have rarely reported neurological manifestations in patients with COVID-19.
Hypoxic encephalopathy is one of the neurological disorders among patients with COVID-19. According to some scientific studies, the prevalence of encephalopathy among patients with COVID-19 reaches 31% [7]. In their study, Frontera et al. [8] gave detailed reports of neurological deficits in hospitalized patients with COVID-19. They considered the two types of disease courses in detail and compared the nervous system disorders in mild and severe cases of the disease. Frontera confirmed in another study [9] that mortality in the case of encephalopathy can be very high and reach 70%. Panwar et al. [10] found that hypoxic encephalopathy is observed in 60% of patients with Central Nervous System (CNS) disorders.
Hypoxic encephalopathy is a severe complication of the central nervous system. This disease is caused as a result of acute respiratory distress syndrome, which occurs when infected with the SARS-CoV-2. Hypoxic and metabolic disturbances resulting from the inflammatory response to the virus cause a cytokine storm and subsequently develop acute respiratory distress syndrome and severe multiorgan failure. The presence of concomitant diseases and the high age of the patients are the reasons for the more severe course of the disease and the occurrence of complications related to the nervous system. An analysis was performed of scientific publications in which there were reports of encephalopathy associated with COVID-19. It is known that encephalopathy often occurs in elderly patients, most of whom are over 50 years of age. Patients with encephalopathy were usually in serious or critical condition in the intensive care unit. Most of these patients received mechanical ventilation or oxygenation. There were some improvements after the therapy. However, Barrot et al. [11] and Schjorring et al. [12] did not find a positive effect of the use of artificial oxygenation according to other randomized trials conducted by them.
The severe condition of the patients and the possible unfavorable prognosis of the end of the disease prompted us to investigate the existence of a close relationship between a neurological complication such as hypoxic encephalopathy related to COVID-19 and the high mortality rate in the hospital. In addition, the authors of this study wanted to show the importance and complexity of differential diagnosis of encephalopathies of various etiologies that develop during infection with the SARS-CoV-2. Therefore, this study aimed to investigate the prevalence of hypoxic encephalopathy in patients with COVID-19 and its relationship with in-hospital mortality.
Instruments and Methods
This prospective observational study was conducted on patients hospitalized in four infectious disease hospitals in Aktobe, Kazakhstan, between February 1, 2021, and April 30, 2021. These departments have been identified to treat patients with COVID-19. The researchers analyzed data from patients diagnosed with COVID-19 according to WHO guidelines [13]. Confirmation of SARS-CoV-2 infection was performed by real-time polymerase chain reaction by examining the material of throat swab samples. X-ray examinations were also carried out, which included computed tomography of the chest and brain. Laboratory tests such as complete blood count, biochemical blood test, coagulogram, C-reactive protein, creatine kinase, lactate dehydrogenase, and assessment of liver and kidney function were done according to the needs of the patient's current condition [14]. This study included 1277 hospitalized patients with laboratory-confirmed SARS-CoV-2. Patients were included in the study only after obtaining the consent of themselves or their relatives. The inclusion criteria for this study were as follows: hospitalization, SARS-CoV-2 infection confirmed by PCR, patient age over 18 years, and the presence of neurological symptoms.
The data of the patient's medical history, the results of laboratory tests, and the results of X-ray examinations were taken into account. Data were collected on co-morbidities such as diabetes mellitus, cardiovascular system diseases, oncological diseases, chronic kidney and liver diseases, and neurological and psychological problems. Patients' symptoms and their manifestations from the moment of illness to hospitalization (fever, cough, indigestion, presence, and degree of pain with different positions), nervous system symptoms, and computed tomography results (thorax and brain, if carried out) were considered.
All neurological manifestations were divided into three categories; the first category encompassed manifestations of the central nervous system. Symptoms such as dizziness, headache, and impaired consciousness (drowsiness, stupor, coma, confusion, and delirium) were observed in most patients. Acute cerebrovascular accident, which includes ischemic stroke and cerebral hemorrhage, was diagnosed by clinical symptoms and brain computed tomography. Some patients had ataxia and seizures during the examination. The second category of neurological symptoms included manifestations of peripheral nervous system (PNS) disorders, such as impaired taste, smell, and vision. The third category of neurological symptoms was the manifestations of high morbidity of the musculoskeletal system. If this symptom occurs, the level of serum creatine kinase in patients increased by more than 200 U/L. All patients with neurological symptoms were divided into two groups according to the principle of differential diagnosis of various types of encephalopathy in adults.
Patients with encephalopathy who had a chronic form of other diseases were excluded from the study if they had symptoms before the onset of COVID-19 infection, which included the following:
- Angioencephalopathy (vascular or dyscirculatory form of the syndrome), which occurs in case of impaired blood supply to brain tissues (due to hypertension, arterial atherosclerosis, or vein damage).
- Toxic encephalopathy that occurs as a result of poisoning with toxic substances (for example, lead, carbon monoxide, chloroform), narcotics and certain drugs, and alcohol.
- Metabolic toxic encephalopathy, which occurs in the long-term presence of metabolic products in the body (in violation of the process of decay and their elimination, increased production). Diabetic, hepatic, bilirubin, uremic, and other forms are distinguished.
- Post-traumatic encephalopathy, as a rule, which manifests itself immediately after a brain injury or later.
In this study, special attention was paid to patients with hypoxic encephalopathy, which can be caused by a lack of oxygen, starvation of brain nerve cells, and hypoxia.
Descriptive statistics were performed in Microsoft Excel. The significant level was p<0.05.
Findings
A total of 1277 hospitalized patients with confirmed SARS-CoV-2 infection were examined. The mean age of patients was 53.26±0.12 years, 519 patients (40.6%) were male, and 758 patients (59.3%) were female.
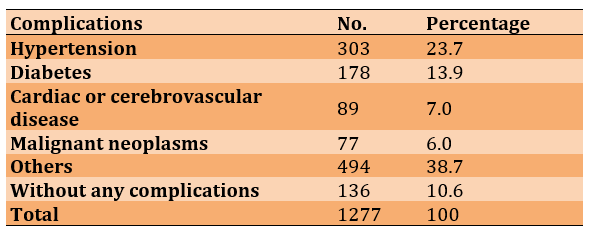
During the study, it was found that a considerable number of patients suffered from complications caused by SARS-CoV-2 (Table 1).
Table 1) Frequency distribution of complications in patients with COVID-19 (n=1277)
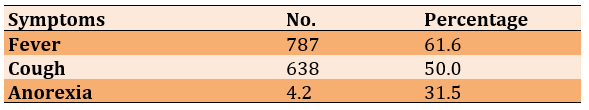
The frequency distribution of symptoms in patients with COVID-19 is shown in Table 2.
Table 2) Frequency distribution of symptoms in patients with COVID-19 (n=1277)
In addition, frequent cases of disease-induced neurological disorders were revealed during the study (Table 3).
Table 3) Frequency distribution of neurological disorders in patients with COVID-19 (n=1277)

In patients with central nervous system involvement, the most common symptoms were dizziness in 19 people (15%) and headache in 15 people (12%). In patients with peripheral nervous system symptoms, the most common symptoms were taste disorder in 3 cases (6%) and smell disorder in 2 cases (4%).
According to the American Thoracic Society guidelines for community-acquired pneumonia [15], 525 patients (41%) had a severe infection (group A), and 752 patients (59%) had a non-severe infection (group B). The mean age of patients with severe infection (Group A) was 58.46±0.33 years. The mean age of patients with milder infections (Group B) was 49.22±0.25 years.
The majority of patients in group A (n=419; 80%) required mechanical ventilation. Group A had more comorbidities, such as hypertension (n=190; 36%) compared to group B (n=113; 15%) and had fewer typical COVID-19 symptoms, such as fever (n=238; 45% versus n=548; 73% in group B) and dry cough (n=178; 34% versus n=459; 61% in group B).
Patients with severe infection had a more pronounced inflammatory reaction in the body. The results of laboratory tests showed a higher number of leukocytes and neutrophils and a lower number of lymphocytes. High levels of C-reactive protein were observed in patients with severe disease compared to patients with non-severe infection.
The mean number of leukocytes in patients with severe infection was 5.5×109/L versus 4.6×109/L in patients with non-severe disease. The mean number of neutrophils in patients with severe infection was 3.9×109/L versus 2.5×109/L in patients with non-severe disease. Also, the mean number of lymphocytes was 0.9×109/L versus 1.4×109/L, and C-reactive protein (median) was 37.1 mg/l versus 9.4 mg/l.
The level of D-dimer in case of patients with severe infection was significantly higher than in patients with non-severe infection (mean 0.8 mg/l vs. 0.5 mg/l), indicating a violation of the blood clotting system.
It should be noted that seriously ill patients had multiple lesions in internal organs. In case of severe liver damage, hepatic markers in blood tests such as lactate dehydrogenase, alanine aminotransferase and aspartate aminotransferase were increased. Renal dysfunction and possible organ dysfunction were confirmed by elevated blood urea nitrogen and creatinine levels. Skeletal muscle involvement was diagnosed through blood tests that showed elevated levels of creatinine kinase.
The probability of symptoms of nervous system disorders was higher in patients with severe type of this disease than in patients who had a milder course of infection. Therefore, in group A, disorders of the nervous system were diagnosed in 238 patients (45%) compared to group B (227; 30%). In group A, 29 patients (6%) suffered from acute cerebrovascular disease, including 20 patients with ischemic stroke and 9 patients with cerebral hemorrhage, who subsequently died because of respiratory failure. A total of 6 (1%) patients with ischemic stroke were in group B. 77 patients (15%) in group A had signs of impaired consciousness compared to 18 patients (2%) in group B.
Patients with central nervous system symptoms had lower levels of lymphocytes and platelets and higher blood urea nitrogen levels compared to the patients without central nervous system symptoms (mean lymphocyte count: 1.2×109/l vs. 1.4×109/l; mean platelet count: 181.0×109/l vs. with 228.0×109/l; mean blood urea nitrogen: 4.6 mmol/l vs. 4.1 mmol/l). Also, in the group of patients with symptoms of the peripheral nervous system, lower levels of lymphocytes and platelets and higher levels of blood urea nitrogen were observed compared to patients without peripheral nervous system symptoms (mean lymphocyte count: 0.8×109/l vs. 0.9×109/l; mean platelet count: 168.0×109/l vs. 221.0×109/l; mean blood urea nitrogen: 5.5 mmol/l vs. 4.5 mmol/l). There was no significant difference in laboratory findings between patients with and without central nervous system disorders in the non-severe disease group. Also, there was no significant difference in the results for patients with and without peripheral nervous system disorders.
In group A, 101 patients (19%) had a high level of skeletal muscle pain syndrome compared to 36 patients (5%) in group B. In patients with musculoskeletal pain syndrome, serum creatine kinase was significantly higher than in patients without it (mean level was 412 U/L compared with 59 U/L). Also, patients with musculoskeletal pain syndrome had lower lymphocyte counts, higher neutrophil counts, higher D-dimer levels, and relatively higher C-reactive protein levels. The results of the analysis showed that these patients have disorders in the blood coagulation system and the body's inflammatory reaction. Also, in patients with musculoskeletal pain, multiple organ damage was observed, such as severe liver dysfunction (increased levels of aspartate aminotransferase, lactate dehydrogenase, and alanine aminotransferase) and impaired renal function (increased levels of creatinine and urea nitrogen in the blood). There was a decrease in the number of lymphocytes and more severe liver dysfunction (increased levels of all diagnostically significant enzymes in the blood) and severe renal impairment (high creatinine level) in the group of patients with severe disease and symptomatic musculoskeletal pain.
In group A, 5 patients experienced seizure-like symptoms characterized by a sudden loss of consciousness, convulsive movements of arms and legs, and foaming at the mouth, which lasted 2-3 minutes. Among the 29 patients (group A) with the acute cerebrovascular accident, 5 patients without typical symptoms of COVID-19, but with symptoms such as diarrhea, anorexia, fever, and cough, were admitted to the intensive care unit. When examining the lungs of these patients by computed tomography, characteristic ground-glass opacities were found. At a later stage, COVID-19 was diagnosed by the detection of the SARS-CoV-2 nucleic acid in a throat scraping by polymerase chain reaction. Some patients were admitted to neurology departments at the very beginning of the disease with symptoms such as headache and fever, but the possibility of contracting COVID-19 was ruled out by using enzyme immunoassay for antibodies and lung computed tomography screening. However, after a few days, typical symptoms of COVID-19 developed, such as sore throat, cough, decreased lymphocyte count, and ground-glass opacities on a CT scan of the lungs. Sometime later, these patients were diagnosed with COVID-19 due to the positive polymerase chain reaction test, and then they were transferred to the infectious department. Some case histories indicated that in the early stages of the disease, patients present with nonspecific symptoms such as dizziness, headache, muscle pain, anosmia, and dysgeusia.
Alteration of consciousness was observed in 92 patients (19%) out of 495 patients with concomitant disorders of the nervous system, which indicated encephalopathy and was more common in elderly patients with severe and advanced disease.
85 patients (92%) of 92 patients with altered consciousness were identified as patients with hypoxic COVID-19-associated encephalopathy. Among patients with encephalopathy, patients with hypoxic encephalopathy showed more markers of severe disease (Maximum score in SOFA; Sequential Organ Failure Assessment Scale), hospitalization in intensive care units, and intubation compared to patients with encephalopathy with other causes. Only 10 of 85 (12%) patients with hypoxic encephalopathy experienced cardiac arrest, the rest had severe or prolonged hypoxemia. Among patients with hypoxic encephalopathy who did not experience cardiac arrest, the average level of minimum oxygen saturation was 80% (range minimum oxygen saturation level 67-87%) compared to 88% (range minimum oxygen saturation level 81-92%) among patients without hypoxic encephalopathy. Hypotension was more common in patients with hypoxic encephalopathy. Mean arterial pressure determines tissue perfusion better than systolic blood pressure and allows the evaluation of the most complete amount of blood supply to the tissue. The norm of mean arterial pressure is considered to be indicators from 70 to 110 mmHg. Mean arterial pressure was 55 mmHg in patients with hypoxic encephalopathy vs. 67 mmHg in patients with encephalopathy of another genesis. 80% of patients had a mean arterial pressure of less than 65 mmHg vs. 42% of patients with encephalopathy of other etiologies. Hypoxic encephalopathy was often associated with an oxygen saturation level of less than 88% and a mean arterial pressure of less than 65 mmHg. Hypoxic encephalopathy was observed in these patients with different degrees of severity.
In the presence of a computed tomogram of the brain, it is possible to observe the manifestations of hypoxic encephalopathy, such as diffuse edema of the brain substance, loss of differentiation of gray and white matter, and others. A close relationship between hypoxic encephalopathy and the high mortality rate of patients was revealed. At the same time, despite the severe course of the infection, no association was found with the high mortality rate in encephalopathy from other causes, such as uremic encephalopathy and sepsis-related encephalopathy. Compared with patients with encephalopathy from other causes, patients with hypoxic encephalopathy had a high level of poor disease prognosis (78; 92%) among all patients with encephalopathy. A total of 204 of 495 (41%) patients with neurological disorders died compared to 146 of 813 (18%) patients without concomitant neurological disorders.
Discussion
This study reports on the neurological manifestations of SARS-CoV-2 infection in 495 out of 1277 laboratory-confirmed COVID -19 hospitalized patients who were admitted between February 1, 2021, and April 30, 2021, at four infectious diseases hospitals in the city of Aktobe (Kazakhstan).
Hypoxic encephalopathy was identified as the most severe complication in this prospective study among patients with neurological disorders, affecting 20% of 495 patients.
In most cases, metabolic encephalopathy occurs with COVID-19 due to the lack of nutrients or oxygen (hypoglycemia, ischemia, hypoxia, hypercapnia). The course of the disease is complicated, and it is important to differentiate these complications from encephalopathy related to organ dysfunction (hepatic, uremic, diabetic), which has also been observed in some patients.
Cerebral ischemia and hypoxia lead to a decrease in energy metabolism and provoke the development of neurodegeneration and neurological deficit. As a result, the course of the disease becomes severe and has a poor prognosis very often [16]. Since acute respiratory distress syndrome is the main cause of respiratory failure in COVID-19, hypoxic encephalopathy may be a common complication factor in the course of this disease. Acute respiratory distress syndrome in COVID-19 is diagnosed in the presence of bilateral pulmonary infiltrates in more than 50% of lung fields, severe hypoxemia, and symptoms of pulmonary edema. The diagnostic criteria for respiratory failure are also a respiratory rate of more than 30 per minute and oxygen saturation of the blood less than 93%. Respiratory failure is often severe enough to cause cerebral hypoxia [17, 18]. Hypoxic brain injury was the result of insufficient cerebral blood flow as a result of reduced oxygen diffusion in the lungs, reduced blood oxygen delivery, and increasing metabolic disturbances that impair the use of available oxygen.
It was found in the study described in this article that almost one in three patients hospitalized with COVID-19 had various disorders of the nervous system. The course of the disease in 27% of patients with disorders of the nervous system had an unfavorable prognosis. In general, encephalopathy is considered to be a reversible condition, however, in COVID-19, hypoxic encephalopathy is an aggravating factor for the course of the disease and contributes to a poor outcome of the disease. According to the studies of Frontera [9] and Young et al. [19], similar to this study, one of the common causes of encephalopathy in COVID-19 were hypoxic encephalopathy, sepsis-related encephalopathy, and uremic encephalopathy. In some studies, sepsis-associated encephalopathy has been reported in up to 70% of patients with viremia. Other studies have shown a connection between viral encephalopathy and increased mortality [20, 21]. No such connection was observed among the total number of patients (1277 people) hospitalized with COVID-19 in the present study.
Patients with impaired renal function most often acquire uremic encephalopathy as a complication, which is usually related to the toxicity of nitrogen-containing substances and other osmotically active toxins [22]. Meta-analyses conducted by Frontera [9] and Hansrivijit et al. [23] showed that uremic encephalopathy is significantly related to mortality after SARS-CoV-2 infection. In this study, mortality was also observed about patients with uremic encephalopathy, however, a clear pattern between its development and poor prognosis of the disease was not found. In one study, hypoxic encephalopathy was observed in 60% of patients with nervous system disorders.
Interesting data on the relationship between the reduction in-hospital mortality rates and the level of artificial blood oxygen saturation (from 88 to 92%) aimed at preventing brain damage associated with hypoxia [10] have been noted. However, other randomized trials have not found benefits from the use of artificial oxygen [11, 12]. Although oxygen deficiency in the blood is a common symptom in hospitalized patients with COVID-19, it is still unknown how long and to what extent oxygen deficiency can lead to irreversible brain damage. The current study identified the relationship between hypotension and hypoxemia for the development of hypoxic encephalopathy. There are arguments from Robbins-Juarez et al. [24] and Busl and Greer [25] that only limited hypoxic brain injury without hypotension or cardiac arrest can be considered benign and not related to ischemic disorders (this study was done in animals and at autopsy) [26]. However, there are studies that state that reduced blood oxygen levels can also be dangerous. Studies in which transient cognitive impairment was observed related to low blood oxygen levels for a short period of time (oxygen saturation less than 66%) have been conducted. At the same time, the pressure remained normal for the volunteers [27-30].
There are cases of long-term cognitive impairment after the reduction of the level of oxygen in the blood sometime later, described in various sources of scientific literature devoted to the disclosure of the topic of acute respiratory distress syndrome; such cognitive impairment occurs on average in case of 40% of survivors of acute respiratory distress syndrome [31-33]. Di Paola et al. [32] found that low blood oxygen saturation over a long time was strongly related to severe cognitive impairment, while mean arterial pressure did not. One of the strengths of this study was that the authors carried out a thorough differential diagnosis of the etiology of encephalopathy, which was a rather complicated process due to its prospective nature and the low level of access of patients to more detailed examinations in a pandemic. There is a similar study by Frontera et al. [8], which outlines detailed reports of neurological manifestations in patients hospitalized with COVID-19.
Frontera et al. [8] examined in a similar study 214 patients, of whom 89 (42%) had a severe course of the disease and 127 (59%) had a milder course of the infection. 79 (37%) of them faced various neurological disorders of varying severity. Severe disease has been observed in older patients who have prominent manifestations of their long-term chronic illnesses such as hypertension, diabetes mellitus, and kidney and liver dysfunction, and typical symptoms for COVID-19 in case of these patients were less pronounced or were completely absent. During the disease, neurological manifestations developed more often in these patients, including encephalopathy, which most often led to death. Frontera et al. [8] concluded that patients with COVID-19 need to pay special attention to their neurological status as an unfavorable factor in terms of the possible development of the severity of the disease and the possible adverse outcome of the disease. This study, according to the authors, had several limitations. Firstly, only 214 patients were examined, so the results of the study could have an inaccurate interpretation. Secondly, all patient data were obtained from electronic medical records, and the study itself was a prospective analysis of clinical records. Thirdly, some information about the results of laboratory and clinical examinations was not available at the time of the study, so it was difficult for the authors to assess the impact of neurological manifestations on the results of analyzes and examinations. Fourth, during the SARS-CoV-2 epidemic, it was quite difficult to carry out the most necessary detailed diagnostics for patients with serious condition, such as computed tomography of the brain, lumbar puncture, and others.
Therefore, the fact indicated in such a study is that most of the analyzed symptoms were subjective descriptions of the patients themselves. In some cases, it was not possible to determine the etiology of neurological manifestations, in particular encephalopathy, and to conduct a differential diagnosis to find out what caused neurological manifestations, a virus, or a lesion of internal organs related to a long-term chronic pathology. The authors of the current study described in this article also faced some of the identified problems. According to the authors, it is required to study not only the relationship between brain damage in COVID-19 and possible prognosis for patients but also to develop algorithms for rapid differential diagnosis with the involvement of neurologists for possible effective correction of the patient's condition and to improve the prognosis of the course of the disease. Also, the rehabilitation of people who survived after hypoxic encephalopathy is important.
Brain damage caused by low blood oxygen levels also called hypoxic COVID-19-associated encephalopathy is a severe complication of the brain oxygen deprivation that can be caused by pathogenetic mechanisms of the SARS-CoV-2 virus. There are a large number of studies that describe the causes of hypoxic encephalopathy. One of the main causes of brain damage from oxygen deprivation can be cardiac arrest due to cardiovascular disease (82%) [34]. About 300000 people in America (slightly fewer in Europe) die because of sudden cardiac death every year. The number of patients surviving after severe hypoxic brain injury is increasing every year due to effective and urgent emergency resuscitation [35]. The prognosis of hypoxic encephalopathy can range from complete recovery to coma or death. With COVID-19, the unfavorable outcome of the disease in patients with hypoxic encephalopathy significantly prevails over recovery. Some clinical studies indicate that 26% of patients were in a coma for 28 days as a result of cerebral hypoxia and then regained consciousness, 8% of patients were in a coma for a longer time or it was in a state of unresponsive wakefulness, and 65% of patients died [36]. Several studies have attempted to define outcome criteria for hypoxic encephalopathy [37-40]. COVID-19-associated hypoxic encephalopathy is a complex serious problem. Further research is needed to fully understand the whole picture and develop measures for prevention, early diagnosis, therapy, and rehabilitation of patients.
This study’s results are important, because it encompasses confirmed statistical data on the relationship between the severity of the disease and the possible adverse outcome in patients with hypoxic encephalopathy. This information may help clinicians raise awareness of COVID-19 and its involvement in neurological complications. It is particularly important to be aware that for patients with severe COVID-19, rapid clinical deterioration may be related to a neurological event such as hypoxic encephalopathy, which may contribute to a high mortality rate. Moreover, this study may contribute to the development of methods for the prevention, early diagnosis, treatment, and subsequent rehabilitation of patients with hypoxic encephalopathy pertained to COVID-19.
Conclusion
There is a relationship between the severity of the disease and the possible adverse outcome in patients with hypoxic encephalopathy. The course of the COVID-19 disease can significantly complicate the development of hypoxic encephalopathy and, with a high percentage of probability, lead to the death of the patient.
Acknowledgements: Not declared.
Ethical Permission: This study was approved by the local ethical commission No. 1, dated January 20, 2021.
Conflict of Interests: The authors declare no conflict of interests.
Authors’ Contributions: Jumagaliyeva M (First Author), Introduction Writer/Main Researcher (25%); Ayaganov D (Second Author), Methodologist/Statistical Analyst (25%); Saparbayev S (Third Author), Discussion Writer/Statistical Analyst (25%); Tuychibayeva N (Fourth Author), Assistant Researcher/Discussion Writer (25%)
Funding: Not declared.
In December 2019, a large number of pneumonia cases were observed in Wuhan (China) with no known cause at the time. Similar symptomatic cases of pneumonia quickly spread to other countries and continents. Over time, it became clear that these cases of pneumonia were caused by a novel Coronavirus (CoV) [1]. In 2003, a novel CoV virus was reported to have symptoms resembling those of Severe Acute Respiratory Syndrome CoV (SARS- CoV) [2]. Both viruses have the same Angiotensin -Converting Enzyme 2 (ACE2) receptor [3], and the new virus was named SARS-CoV-2. In February 2020, the World Health Organization (WHO) defined the disease as COVID-19. Human infection with SARS-CoV-2 often leads to a severe course of the disease and possible high mortality [4]. The worldwide death rate registered as a result of COVID-19 between January 1, 2020, and December 31, 2021, has reached 5.94 million [5]. There are typical clinical symptoms of COVID-19 described in several studies. The disease is manifested by high body temperature, cough, disorders of the gastrointestinal tract, and increased fatigue in the vast majority of cases of the infection. There are also several laboratory test results associated with COVID-19 and clinical diagnostic tests such as computed tomography of the lung [6]. However, scientific studies have rarely reported neurological manifestations in patients with COVID-19.
Hypoxic encephalopathy is one of the neurological disorders among patients with COVID-19. According to some scientific studies, the prevalence of encephalopathy among patients with COVID-19 reaches 31% [7]. In their study, Frontera et al. [8] gave detailed reports of neurological deficits in hospitalized patients with COVID-19. They considered the two types of disease courses in detail and compared the nervous system disorders in mild and severe cases of the disease. Frontera confirmed in another study [9] that mortality in the case of encephalopathy can be very high and reach 70%. Panwar et al. [10] found that hypoxic encephalopathy is observed in 60% of patients with Central Nervous System (CNS) disorders.
Hypoxic encephalopathy is a severe complication of the central nervous system. This disease is caused as a result of acute respiratory distress syndrome, which occurs when infected with the SARS-CoV-2. Hypoxic and metabolic disturbances resulting from the inflammatory response to the virus cause a cytokine storm and subsequently develop acute respiratory distress syndrome and severe multiorgan failure. The presence of concomitant diseases and the high age of the patients are the reasons for the more severe course of the disease and the occurrence of complications related to the nervous system. An analysis was performed of scientific publications in which there were reports of encephalopathy associated with COVID-19. It is known that encephalopathy often occurs in elderly patients, most of whom are over 50 years of age. Patients with encephalopathy were usually in serious or critical condition in the intensive care unit. Most of these patients received mechanical ventilation or oxygenation. There were some improvements after the therapy. However, Barrot et al. [11] and Schjorring et al. [12] did not find a positive effect of the use of artificial oxygenation according to other randomized trials conducted by them.
The severe condition of the patients and the possible unfavorable prognosis of the end of the disease prompted us to investigate the existence of a close relationship between a neurological complication such as hypoxic encephalopathy related to COVID-19 and the high mortality rate in the hospital. In addition, the authors of this study wanted to show the importance and complexity of differential diagnosis of encephalopathies of various etiologies that develop during infection with the SARS-CoV-2. Therefore, this study aimed to investigate the prevalence of hypoxic encephalopathy in patients with COVID-19 and its relationship with in-hospital mortality.
Instruments and Methods
This prospective observational study was conducted on patients hospitalized in four infectious disease hospitals in Aktobe, Kazakhstan, between February 1, 2021, and April 30, 2021. These departments have been identified to treat patients with COVID-19. The researchers analyzed data from patients diagnosed with COVID-19 according to WHO guidelines [13]. Confirmation of SARS-CoV-2 infection was performed by real-time polymerase chain reaction by examining the material of throat swab samples. X-ray examinations were also carried out, which included computed tomography of the chest and brain. Laboratory tests such as complete blood count, biochemical blood test, coagulogram, C-reactive protein, creatine kinase, lactate dehydrogenase, and assessment of liver and kidney function were done according to the needs of the patient's current condition [14]. This study included 1277 hospitalized patients with laboratory-confirmed SARS-CoV-2. Patients were included in the study only after obtaining the consent of themselves or their relatives. The inclusion criteria for this study were as follows: hospitalization, SARS-CoV-2 infection confirmed by PCR, patient age over 18 years, and the presence of neurological symptoms.
The data of the patient's medical history, the results of laboratory tests, and the results of X-ray examinations were taken into account. Data were collected on co-morbidities such as diabetes mellitus, cardiovascular system diseases, oncological diseases, chronic kidney and liver diseases, and neurological and psychological problems. Patients' symptoms and their manifestations from the moment of illness to hospitalization (fever, cough, indigestion, presence, and degree of pain with different positions), nervous system symptoms, and computed tomography results (thorax and brain, if carried out) were considered.
All neurological manifestations were divided into three categories; the first category encompassed manifestations of the central nervous system. Symptoms such as dizziness, headache, and impaired consciousness (drowsiness, stupor, coma, confusion, and delirium) were observed in most patients. Acute cerebrovascular accident, which includes ischemic stroke and cerebral hemorrhage, was diagnosed by clinical symptoms and brain computed tomography. Some patients had ataxia and seizures during the examination. The second category of neurological symptoms included manifestations of peripheral nervous system (PNS) disorders, such as impaired taste, smell, and vision. The third category of neurological symptoms was the manifestations of high morbidity of the musculoskeletal system. If this symptom occurs, the level of serum creatine kinase in patients increased by more than 200 U/L. All patients with neurological symptoms were divided into two groups according to the principle of differential diagnosis of various types of encephalopathy in adults.
Patients with encephalopathy who had a chronic form of other diseases were excluded from the study if they had symptoms before the onset of COVID-19 infection, which included the following:
- Angioencephalopathy (vascular or dyscirculatory form of the syndrome), which occurs in case of impaired blood supply to brain tissues (due to hypertension, arterial atherosclerosis, or vein damage).
- Toxic encephalopathy that occurs as a result of poisoning with toxic substances (for example, lead, carbon monoxide, chloroform), narcotics and certain drugs, and alcohol.
- Metabolic toxic encephalopathy, which occurs in the long-term presence of metabolic products in the body (in violation of the process of decay and their elimination, increased production). Diabetic, hepatic, bilirubin, uremic, and other forms are distinguished.
- Post-traumatic encephalopathy, as a rule, which manifests itself immediately after a brain injury or later.
In this study, special attention was paid to patients with hypoxic encephalopathy, which can be caused by a lack of oxygen, starvation of brain nerve cells, and hypoxia.
Descriptive statistics were performed in Microsoft Excel. The significant level was p<0.05.
Findings
A total of 1277 hospitalized patients with confirmed SARS-CoV-2 infection were examined. The mean age of patients was 53.26±0.12 years, 519 patients (40.6%) were male, and 758 patients (59.3%) were female.
During the study, it was found that a considerable number of patients suffered from complications caused by SARS-CoV-2 (Table 1).
Table 1) Frequency distribution of complications in patients with COVID-19 (n=1277)

The frequency distribution of symptoms in patients with COVID-19 is shown in Table 2.
Table 2) Frequency distribution of symptoms in patients with COVID-19 (n=1277)

In addition, frequent cases of disease-induced neurological disorders were revealed during the study (Table 3).
Table 3) Frequency distribution of neurological disorders in patients with COVID-19 (n=1277)

In patients with central nervous system involvement, the most common symptoms were dizziness in 19 people (15%) and headache in 15 people (12%). In patients with peripheral nervous system symptoms, the most common symptoms were taste disorder in 3 cases (6%) and smell disorder in 2 cases (4%).
According to the American Thoracic Society guidelines for community-acquired pneumonia [15], 525 patients (41%) had a severe infection (group A), and 752 patients (59%) had a non-severe infection (group B). The mean age of patients with severe infection (Group A) was 58.46±0.33 years. The mean age of patients with milder infections (Group B) was 49.22±0.25 years.
The majority of patients in group A (n=419; 80%) required mechanical ventilation. Group A had more comorbidities, such as hypertension (n=190; 36%) compared to group B (n=113; 15%) and had fewer typical COVID-19 symptoms, such as fever (n=238; 45% versus n=548; 73% in group B) and dry cough (n=178; 34% versus n=459; 61% in group B).
Patients with severe infection had a more pronounced inflammatory reaction in the body. The results of laboratory tests showed a higher number of leukocytes and neutrophils and a lower number of lymphocytes. High levels of C-reactive protein were observed in patients with severe disease compared to patients with non-severe infection.
The mean number of leukocytes in patients with severe infection was 5.5×109/L versus 4.6×109/L in patients with non-severe disease. The mean number of neutrophils in patients with severe infection was 3.9×109/L versus 2.5×109/L in patients with non-severe disease. Also, the mean number of lymphocytes was 0.9×109/L versus 1.4×109/L, and C-reactive protein (median) was 37.1 mg/l versus 9.4 mg/l.
The level of D-dimer in case of patients with severe infection was significantly higher than in patients with non-severe infection (mean 0.8 mg/l vs. 0.5 mg/l), indicating a violation of the blood clotting system.
It should be noted that seriously ill patients had multiple lesions in internal organs. In case of severe liver damage, hepatic markers in blood tests such as lactate dehydrogenase, alanine aminotransferase and aspartate aminotransferase were increased. Renal dysfunction and possible organ dysfunction were confirmed by elevated blood urea nitrogen and creatinine levels. Skeletal muscle involvement was diagnosed through blood tests that showed elevated levels of creatinine kinase.
The probability of symptoms of nervous system disorders was higher in patients with severe type of this disease than in patients who had a milder course of infection. Therefore, in group A, disorders of the nervous system were diagnosed in 238 patients (45%) compared to group B (227; 30%). In group A, 29 patients (6%) suffered from acute cerebrovascular disease, including 20 patients with ischemic stroke and 9 patients with cerebral hemorrhage, who subsequently died because of respiratory failure. A total of 6 (1%) patients with ischemic stroke were in group B. 77 patients (15%) in group A had signs of impaired consciousness compared to 18 patients (2%) in group B.
Patients with central nervous system symptoms had lower levels of lymphocytes and platelets and higher blood urea nitrogen levels compared to the patients without central nervous system symptoms (mean lymphocyte count: 1.2×109/l vs. 1.4×109/l; mean platelet count: 181.0×109/l vs. with 228.0×109/l; mean blood urea nitrogen: 4.6 mmol/l vs. 4.1 mmol/l). Also, in the group of patients with symptoms of the peripheral nervous system, lower levels of lymphocytes and platelets and higher levels of blood urea nitrogen were observed compared to patients without peripheral nervous system symptoms (mean lymphocyte count: 0.8×109/l vs. 0.9×109/l; mean platelet count: 168.0×109/l vs. 221.0×109/l; mean blood urea nitrogen: 5.5 mmol/l vs. 4.5 mmol/l). There was no significant difference in laboratory findings between patients with and without central nervous system disorders in the non-severe disease group. Also, there was no significant difference in the results for patients with and without peripheral nervous system disorders.
In group A, 101 patients (19%) had a high level of skeletal muscle pain syndrome compared to 36 patients (5%) in group B. In patients with musculoskeletal pain syndrome, serum creatine kinase was significantly higher than in patients without it (mean level was 412 U/L compared with 59 U/L). Also, patients with musculoskeletal pain syndrome had lower lymphocyte counts, higher neutrophil counts, higher D-dimer levels, and relatively higher C-reactive protein levels. The results of the analysis showed that these patients have disorders in the blood coagulation system and the body's inflammatory reaction. Also, in patients with musculoskeletal pain, multiple organ damage was observed, such as severe liver dysfunction (increased levels of aspartate aminotransferase, lactate dehydrogenase, and alanine aminotransferase) and impaired renal function (increased levels of creatinine and urea nitrogen in the blood). There was a decrease in the number of lymphocytes and more severe liver dysfunction (increased levels of all diagnostically significant enzymes in the blood) and severe renal impairment (high creatinine level) in the group of patients with severe disease and symptomatic musculoskeletal pain.
In group A, 5 patients experienced seizure-like symptoms characterized by a sudden loss of consciousness, convulsive movements of arms and legs, and foaming at the mouth, which lasted 2-3 minutes. Among the 29 patients (group A) with the acute cerebrovascular accident, 5 patients without typical symptoms of COVID-19, but with symptoms such as diarrhea, anorexia, fever, and cough, were admitted to the intensive care unit. When examining the lungs of these patients by computed tomography, characteristic ground-glass opacities were found. At a later stage, COVID-19 was diagnosed by the detection of the SARS-CoV-2 nucleic acid in a throat scraping by polymerase chain reaction. Some patients were admitted to neurology departments at the very beginning of the disease with symptoms such as headache and fever, but the possibility of contracting COVID-19 was ruled out by using enzyme immunoassay for antibodies and lung computed tomography screening. However, after a few days, typical symptoms of COVID-19 developed, such as sore throat, cough, decreased lymphocyte count, and ground-glass opacities on a CT scan of the lungs. Sometime later, these patients were diagnosed with COVID-19 due to the positive polymerase chain reaction test, and then they were transferred to the infectious department. Some case histories indicated that in the early stages of the disease, patients present with nonspecific symptoms such as dizziness, headache, muscle pain, anosmia, and dysgeusia.
Alteration of consciousness was observed in 92 patients (19%) out of 495 patients with concomitant disorders of the nervous system, which indicated encephalopathy and was more common in elderly patients with severe and advanced disease.
85 patients (92%) of 92 patients with altered consciousness were identified as patients with hypoxic COVID-19-associated encephalopathy. Among patients with encephalopathy, patients with hypoxic encephalopathy showed more markers of severe disease (Maximum score in SOFA; Sequential Organ Failure Assessment Scale), hospitalization in intensive care units, and intubation compared to patients with encephalopathy with other causes. Only 10 of 85 (12%) patients with hypoxic encephalopathy experienced cardiac arrest, the rest had severe or prolonged hypoxemia. Among patients with hypoxic encephalopathy who did not experience cardiac arrest, the average level of minimum oxygen saturation was 80% (range minimum oxygen saturation level 67-87%) compared to 88% (range minimum oxygen saturation level 81-92%) among patients without hypoxic encephalopathy. Hypotension was more common in patients with hypoxic encephalopathy. Mean arterial pressure determines tissue perfusion better than systolic blood pressure and allows the evaluation of the most complete amount of blood supply to the tissue. The norm of mean arterial pressure is considered to be indicators from 70 to 110 mmHg. Mean arterial pressure was 55 mmHg in patients with hypoxic encephalopathy vs. 67 mmHg in patients with encephalopathy of another genesis. 80% of patients had a mean arterial pressure of less than 65 mmHg vs. 42% of patients with encephalopathy of other etiologies. Hypoxic encephalopathy was often associated with an oxygen saturation level of less than 88% and a mean arterial pressure of less than 65 mmHg. Hypoxic encephalopathy was observed in these patients with different degrees of severity.
In the presence of a computed tomogram of the brain, it is possible to observe the manifestations of hypoxic encephalopathy, such as diffuse edema of the brain substance, loss of differentiation of gray and white matter, and others. A close relationship between hypoxic encephalopathy and the high mortality rate of patients was revealed. At the same time, despite the severe course of the infection, no association was found with the high mortality rate in encephalopathy from other causes, such as uremic encephalopathy and sepsis-related encephalopathy. Compared with patients with encephalopathy from other causes, patients with hypoxic encephalopathy had a high level of poor disease prognosis (78; 92%) among all patients with encephalopathy. A total of 204 of 495 (41%) patients with neurological disorders died compared to 146 of 813 (18%) patients without concomitant neurological disorders.
Discussion
This study reports on the neurological manifestations of SARS-CoV-2 infection in 495 out of 1277 laboratory-confirmed COVID -19 hospitalized patients who were admitted between February 1, 2021, and April 30, 2021, at four infectious diseases hospitals in the city of Aktobe (Kazakhstan).
Hypoxic encephalopathy was identified as the most severe complication in this prospective study among patients with neurological disorders, affecting 20% of 495 patients.
In most cases, metabolic encephalopathy occurs with COVID-19 due to the lack of nutrients or oxygen (hypoglycemia, ischemia, hypoxia, hypercapnia). The course of the disease is complicated, and it is important to differentiate these complications from encephalopathy related to organ dysfunction (hepatic, uremic, diabetic), which has also been observed in some patients.
Cerebral ischemia and hypoxia lead to a decrease in energy metabolism and provoke the development of neurodegeneration and neurological deficit. As a result, the course of the disease becomes severe and has a poor prognosis very often [16]. Since acute respiratory distress syndrome is the main cause of respiratory failure in COVID-19, hypoxic encephalopathy may be a common complication factor in the course of this disease. Acute respiratory distress syndrome in COVID-19 is diagnosed in the presence of bilateral pulmonary infiltrates in more than 50% of lung fields, severe hypoxemia, and symptoms of pulmonary edema. The diagnostic criteria for respiratory failure are also a respiratory rate of more than 30 per minute and oxygen saturation of the blood less than 93%. Respiratory failure is often severe enough to cause cerebral hypoxia [17, 18]. Hypoxic brain injury was the result of insufficient cerebral blood flow as a result of reduced oxygen diffusion in the lungs, reduced blood oxygen delivery, and increasing metabolic disturbances that impair the use of available oxygen.
It was found in the study described in this article that almost one in three patients hospitalized with COVID-19 had various disorders of the nervous system. The course of the disease in 27% of patients with disorders of the nervous system had an unfavorable prognosis. In general, encephalopathy is considered to be a reversible condition, however, in COVID-19, hypoxic encephalopathy is an aggravating factor for the course of the disease and contributes to a poor outcome of the disease. According to the studies of Frontera [9] and Young et al. [19], similar to this study, one of the common causes of encephalopathy in COVID-19 were hypoxic encephalopathy, sepsis-related encephalopathy, and uremic encephalopathy. In some studies, sepsis-associated encephalopathy has been reported in up to 70% of patients with viremia. Other studies have shown a connection between viral encephalopathy and increased mortality [20, 21]. No such connection was observed among the total number of patients (1277 people) hospitalized with COVID-19 in the present study.
Patients with impaired renal function most often acquire uremic encephalopathy as a complication, which is usually related to the toxicity of nitrogen-containing substances and other osmotically active toxins [22]. Meta-analyses conducted by Frontera [9] and Hansrivijit et al. [23] showed that uremic encephalopathy is significantly related to mortality after SARS-CoV-2 infection. In this study, mortality was also observed about patients with uremic encephalopathy, however, a clear pattern between its development and poor prognosis of the disease was not found. In one study, hypoxic encephalopathy was observed in 60% of patients with nervous system disorders.
Interesting data on the relationship between the reduction in-hospital mortality rates and the level of artificial blood oxygen saturation (from 88 to 92%) aimed at preventing brain damage associated with hypoxia [10] have been noted. However, other randomized trials have not found benefits from the use of artificial oxygen [11, 12]. Although oxygen deficiency in the blood is a common symptom in hospitalized patients with COVID-19, it is still unknown how long and to what extent oxygen deficiency can lead to irreversible brain damage. The current study identified the relationship between hypotension and hypoxemia for the development of hypoxic encephalopathy. There are arguments from Robbins-Juarez et al. [24] and Busl and Greer [25] that only limited hypoxic brain injury without hypotension or cardiac arrest can be considered benign and not related to ischemic disorders (this study was done in animals and at autopsy) [26]. However, there are studies that state that reduced blood oxygen levels can also be dangerous. Studies in which transient cognitive impairment was observed related to low blood oxygen levels for a short period of time (oxygen saturation less than 66%) have been conducted. At the same time, the pressure remained normal for the volunteers [27-30].
There are cases of long-term cognitive impairment after the reduction of the level of oxygen in the blood sometime later, described in various sources of scientific literature devoted to the disclosure of the topic of acute respiratory distress syndrome; such cognitive impairment occurs on average in case of 40% of survivors of acute respiratory distress syndrome [31-33]. Di Paola et al. [32] found that low blood oxygen saturation over a long time was strongly related to severe cognitive impairment, while mean arterial pressure did not. One of the strengths of this study was that the authors carried out a thorough differential diagnosis of the etiology of encephalopathy, which was a rather complicated process due to its prospective nature and the low level of access of patients to more detailed examinations in a pandemic. There is a similar study by Frontera et al. [8], which outlines detailed reports of neurological manifestations in patients hospitalized with COVID-19.
Frontera et al. [8] examined in a similar study 214 patients, of whom 89 (42%) had a severe course of the disease and 127 (59%) had a milder course of the infection. 79 (37%) of them faced various neurological disorders of varying severity. Severe disease has been observed in older patients who have prominent manifestations of their long-term chronic illnesses such as hypertension, diabetes mellitus, and kidney and liver dysfunction, and typical symptoms for COVID-19 in case of these patients were less pronounced or were completely absent. During the disease, neurological manifestations developed more often in these patients, including encephalopathy, which most often led to death. Frontera et al. [8] concluded that patients with COVID-19 need to pay special attention to their neurological status as an unfavorable factor in terms of the possible development of the severity of the disease and the possible adverse outcome of the disease. This study, according to the authors, had several limitations. Firstly, only 214 patients were examined, so the results of the study could have an inaccurate interpretation. Secondly, all patient data were obtained from electronic medical records, and the study itself was a prospective analysis of clinical records. Thirdly, some information about the results of laboratory and clinical examinations was not available at the time of the study, so it was difficult for the authors to assess the impact of neurological manifestations on the results of analyzes and examinations. Fourth, during the SARS-CoV-2 epidemic, it was quite difficult to carry out the most necessary detailed diagnostics for patients with serious condition, such as computed tomography of the brain, lumbar puncture, and others.
Therefore, the fact indicated in such a study is that most of the analyzed symptoms were subjective descriptions of the patients themselves. In some cases, it was not possible to determine the etiology of neurological manifestations, in particular encephalopathy, and to conduct a differential diagnosis to find out what caused neurological manifestations, a virus, or a lesion of internal organs related to a long-term chronic pathology. The authors of the current study described in this article also faced some of the identified problems. According to the authors, it is required to study not only the relationship between brain damage in COVID-19 and possible prognosis for patients but also to develop algorithms for rapid differential diagnosis with the involvement of neurologists for possible effective correction of the patient's condition and to improve the prognosis of the course of the disease. Also, the rehabilitation of people who survived after hypoxic encephalopathy is important.
Brain damage caused by low blood oxygen levels also called hypoxic COVID-19-associated encephalopathy is a severe complication of the brain oxygen deprivation that can be caused by pathogenetic mechanisms of the SARS-CoV-2 virus. There are a large number of studies that describe the causes of hypoxic encephalopathy. One of the main causes of brain damage from oxygen deprivation can be cardiac arrest due to cardiovascular disease (82%) [34]. About 300000 people in America (slightly fewer in Europe) die because of sudden cardiac death every year. The number of patients surviving after severe hypoxic brain injury is increasing every year due to effective and urgent emergency resuscitation [35]. The prognosis of hypoxic encephalopathy can range from complete recovery to coma or death. With COVID-19, the unfavorable outcome of the disease in patients with hypoxic encephalopathy significantly prevails over recovery. Some clinical studies indicate that 26% of patients were in a coma for 28 days as a result of cerebral hypoxia and then regained consciousness, 8% of patients were in a coma for a longer time or it was in a state of unresponsive wakefulness, and 65% of patients died [36]. Several studies have attempted to define outcome criteria for hypoxic encephalopathy [37-40]. COVID-19-associated hypoxic encephalopathy is a complex serious problem. Further research is needed to fully understand the whole picture and develop measures for prevention, early diagnosis, therapy, and rehabilitation of patients.
This study’s results are important, because it encompasses confirmed statistical data on the relationship between the severity of the disease and the possible adverse outcome in patients with hypoxic encephalopathy. This information may help clinicians raise awareness of COVID-19 and its involvement in neurological complications. It is particularly important to be aware that for patients with severe COVID-19, rapid clinical deterioration may be related to a neurological event such as hypoxic encephalopathy, which may contribute to a high mortality rate. Moreover, this study may contribute to the development of methods for the prevention, early diagnosis, treatment, and subsequent rehabilitation of patients with hypoxic encephalopathy pertained to COVID-19.
Conclusion
There is a relationship between the severity of the disease and the possible adverse outcome in patients with hypoxic encephalopathy. The course of the COVID-19 disease can significantly complicate the development of hypoxic encephalopathy and, with a high percentage of probability, lead to the death of the patient.
Acknowledgements: Not declared.
Ethical Permission: This study was approved by the local ethical commission No. 1, dated January 20, 2021.
Conflict of Interests: The authors declare no conflict of interests.
Authors’ Contributions: Jumagaliyeva M (First Author), Introduction Writer/Main Researcher (25%); Ayaganov D (Second Author), Methodologist/Statistical Analyst (25%); Saparbayev S (Third Author), Discussion Writer/Statistical Analyst (25%); Tuychibayeva N (Fourth Author), Assistant Researcher/Discussion Writer (25%)
Funding: Not declared.
Keywords:
References
1. Dmytriiev D, Dobrovanov O. Post-COVID pain syndrome. Anaesth Pain Intens Care. 2021;25(4):505-12. [Link]
2. Lokshin VN, Sharman AT, Mirzakhmetova DD, Terlikbaeva AT, Aimbetova AR, Karibaeva SK, et al. The modern organizational principles of specialized medical care for pregnant and puerperant women during the coronavirus pandemic in the republic of Kazakhstan. Akush Ginekol (Russian Federation). 2020;2020(12):34-43. [Link] [DOI:10.18565/aig.2020.12.34-43]
3. Polatova DSh, Madaminov AYu. Molecular and clinical aspects of oropharyngeal squamous cell carcinoma associated with human Papillomavirus. Opuhol Golov Sei. 2021;11(2):31-40. [Link] [DOI:10.17650/2313-805X-2021-11-2-31-40]
4. Sahalevych A, Sergiychuk R, Ozhohin V, Vozianov O, Khrapchuk A, Dubovyi Y, et al. Mini-percutaneous nephrolithotomy in surgery of Nephrolithiasis. Ukrain J Nephrol Dialys. 2021;71(3):44-52. [Link] [DOI:10.31450/ukrjnd.3(71).2021.06]
5. Polaka I, Bhandari MP, Mezmale L, Anarkulova L, Veliks V, Sivins A, et al. Modular point-of-care breath analyzer and shape taxonomy-based machine learning for gastric cancer detection. Diagnostics. 2022;12(2):491. [Link] [DOI:10.3390/diagnostics12020491]
6. Sahalevych AI, Sergiychuk RV, Ozhohin VV, Khrapchuk AYu, Dubovyi YO, Frolov OS. The modified procedure of totally tubeless PNL. Inter J Biol Biomed Engin. 2022;16:82-9. [Link] [DOI:10.46300/91011.2022.16.10]
7. Crimi A, Dodero L, Sambataro F, Murino V, Sona D. Structurally constrained effective brain connectivity. Neuroimage. 2021;239:118288. [Link] [DOI:10.1016/j.neuroimage.2021.118288]
8. Frontera JA, Melmed K, Fang T, Granger A, Lin J, Yaghi S, et al. Toxic metabolic encephalopathy in hospitalized patients with COVID-19. Neurocrit Care. 2021;35(3):693-706. [Link] [DOI:10.1007/s12028-021-01220-5]
9. Frontera JA. Metabolic encephalopathies in the critical care unit. Continuum. 2012;18(3):611-39. [Link] [DOI:10.1212/01.CON.0000415431.07019.c2]
10. Panwar R, Hardie M, Bellomo R, Barrot L, Eastwood GM, Young PJ, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193(1):43-51. [Link] [DOI:10.1164/rccm.201505-1019OC]
11. Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999-1008. [Link] [DOI:10.1056/NEJMoa1916431]
12. Schjørring OL, Klitgaard TL, Perner A, Wetterslev J, Lange T, Siegemund M, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384(14):1301-11. [Link] [DOI:10.1056/NEJMoa2032510]
13. Bezshapochnyy S, Podovzhnii O, Polianska V, Zachepylo S, Fedorchenko V. Opportunities and prospects of microbiological diagnosis of ENT organs mycoses (review). Georgian Med News. 2020;308:36-43. [Link]
14. Svyatova GS, Mirzakhmetova DD, Berezina GM, Murtazaliyeva AV. Genetic factors of idiopathic recurrent miscarriage in kazakh population. J Reprod Infertil. 2022;23(1):39-45. [Link] [DOI:10.18502/jri.v23i1.8451]
15. Dmytriiev D, Dobrovanov O, Kralinsky K, Dmytriiev K, Melnychenko M. A case report of successful experience of using adaptive support ventilation in the pediatric patient with viral interstitial pneumonia covid-19 positive. Lekarsky Obzor. 2021;70(3):119-23. [Link]
16. Zhanaidarova G, Nauryzov N, Nurseitova K, Arystan L, Dyussembekov R, Turdunova G, et al. Development of the heart muscle after antenatal ethanol intoxication during the neonatal period. Bangladesh J Med Sci. 2022;21(2):344-53. [Link] [DOI:10.3329/bjms.v21i2.58067]
17. Navruzov SN, Polatova DS, Geldieva MS, Nurieva EI. Possibilities of study of the main cytokines of the immune system in patients with osteogenic sarcoma. Vopr Onkol. 2013;59(5):599-602. [Russian] [Link]
18. Jabbarov OO, Daminov BT, Boboev KT, Tursunova LD, Tashpulatova MX, Maksudova LI. Associations of polymorphic markers aluins/deli>D Ace T-786C gene Enos3 in diabetic nefropate progressing for type 2 diabetes mellitus. Int J Res Pharm Sci. 2020;11(4):6028-32. [Link] [DOI:10.26452/ijrps.v11i4.3268]
19. Young GB, Bolton CF, Austin TW, Archibald YM, Gonder J, Wells GA. The encephalopathy associated with septic illness. Clin Invest Med. 1990;13(6):297-304. [Link]
20. Dobrovanov OY. Efficacy and sensitivity of prenatal and postnatal ultrasound screening of congenital developmental anomalies of kidneys in Slovakia. Wiad Lek. 2021;74(3 cz 1):450-4. [Link] [DOI:10.36740/WLek202103112]
21. Bezshapochnyy SB, Zachepylo SV, Polianskaya VP, Bobrova NA, Fedorchenko VI. Opportunistic fungal infections of ENT organs. Part 2. Vestn Otorinolaringol. 2019;84(3):74-81. [Russian] [Link] [DOI:10.17116/otorino20198403174]
22. Molcan J, Dobrovanov A, Koren R, Kralinsky K, Balaz V. Unilateral scrotal hernia with dual ureter herniation: The first experience of successful surgical correction. Pediatr - Zhur im GN Speranskogo. 2021;100(4):171-5. [Slovak] [Link] [DOI:10.24110/0031-403X-2021-100-4-171-175]
23. Hansrivijit P, Qian C, Boonpheng B, Thongprayoon C, Vallabhajosyula S, Cheungpasitporn W, et al. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med. 2020;68(7):1261-70. [Link] [DOI:10.1136/jim-2020-001407]
24. Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, et al. Outcomes for patients with COVID-19 and acute kidney injury: A systematic review and meta-analysis. Kidney Int Rep. 2020;5(8):1149-60. [Link] [DOI:10.1016/j.ekir.2020.06.013]
25. Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation. 2010;26(1):5-13. [Link] [DOI:10.3233/NRE-2010-0531]
26. Elsheikh SSM, Chimusa ER, Mulder NJ, Crimi A. Relating global and local connectome changes to dementia and targeted gene expression in Alzheimer's disease. Front Hum Neurosci. 2021;15:761424. [Link] [DOI:10.3389/fnhum.2021.761424]
27. Chekhovska IV, Balynska OM, Blahuta RI, Sereda VV, Mosondz SO. Euthanasia or palliative care: legal principles of the implementation in the context of the realization of human rights to life. Wiad Lek. 2019;72(4):677-81. [Link] [DOI:10.36740/WLek201904133]
28. Okassova AK, Ilderbayev OZ, Nursafina AZh, Zharmakhanova GM, Rakhimova BB, Bayan YT, et al. Evaluation of lipid peroxidation under immobilization stress in irradiated animals in experiment. Open Access Maced J Med Sci. 2021;9:119-22. [Link] [DOI:10.3889/oamjms.2021.5781]
29. Chulenbayeva L, Ilderbayev O, Taldykbayev Z, Ilderbayeva G, Argynbekova A. Phytocorrection of immunological and biochemical changes in the combined impact of coal dust and high dose of radiation. Georgian Med News. 2018;(Issue):141-50. [Russian] [Link]
30. Tołodziecki MM, Chudański MM, Ponikowska I, Adamczyk P. Nonalcoholic fatty liver disease in obese patients. Wiad Lek. 2014;67(2):76-9. [Polish] [Link]
31. Dobrovanov O, Králinský K. The role of prenatal diagnostics in the identification congenital malformations of urogenital system in Slovakia. Lek Obz. 2019;68(2):59-62. [Russian] [Link]
32. Di Paola M, Bozzali M, Fadda L, Musicco M, Sabatini U, Caltagirone C. Reduced oxygen due to high-altitude exposure relates to atrophy in motor-function brain areas. Eur J Neurol. 2008;15(10):1050-7. [Link] [DOI:10.1111/j.1468-1331.2008.02243.x]
33. Bezshapochny SB, Zachepilo SV, Polyanskaya VP, Bobrova NA, Fedorchenko VI. Opportunistic mycoses of ENT organs. Part 1. Vestn Otorinolaringol. 2018;83(6):67-71. [Russian] [Link] [DOI:10.17116/otorino20188306167]
34. Ilderbayeva G, Zhetpisbaev B, Ilderbayev О, Taldykbayev Zh, Bekeeva S. Metabolic processes of organism in remote period after the combined effects of radiation and emotional stress. Georgian Med News. 2016;250:76-82. [Russian] [Link]
35. Balynska O, Blahuta R, Sereda V, Shelukhin N, Kharaberiush I. Neurolaw: Branch or section of new siences, a complex branch of law or a way to justify criminals (review). Georgian Med News. 2019;289:162-8. [Link]
36. Crimi A, Giancardo L, Sambataro F, Gozzi A, Murino V, Sona D. MultiLink analysis: Brain network comparison via sparse connectivity analysis. Sci. Rep. 2019;9(1):65. [Link] [DOI:10.1038/s41598-018-37300-4]
37. Bersimbaev R, Bulgakova O, Aripova A, Kussainova A, Ilderbayev O. Role of microRNAs in lung carcinogenesis induced by asbestos. J Pers Med. 2021;11(2):1-23. [Link] [DOI:10.3390/jpm11020097]
38. Stankiewicz R, Firszt-Adamczyk A, Czarnecki J, Adamczyk-Kipigroch H, Adamczyk P, Sinica W, et al. Recombinant tissue plasminogen activator for therapy of right atrial thrombus in a 2-year old child with nephrotic syndrome. Przeglad Pediatr. 2007;37(4):413-7. [Link]
39. Fedorchenko V, Polyanskaya V, Zachepilo S, Bobrova N, Loban G. Comprehensive study of anti-fungal effect of evgenol emulsion in polysorbat-80 on reference strain of candida albicans atcc 885-653. Georgian Med News. 2018;282:166-70. [Russian] [Link]
40. Dobrovanov AE, Dmytriev D, Dmytrieva KYu, Hustavova L. Difficulties in Kawasaki disease diagnosis and treatment in children. Ross Vestn Perinat Pediatr. 2020;65(6):122-8. [Link] [DOI:10.21508/1027-4065-2020-65-6-122-128]








