Volume 14, Issue 4 (2022)
Iran J War Public Health 2022, 14(4): 367-375 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/07/28 | Accepted: 2022/10/23 | Published: 2022/11/1
Received: 2022/07/28 | Accepted: 2022/10/23 | Published: 2022/11/1
How to cite this article
Kareem D, Sadoon A, Majeed M, Abbas B. Histological and Enzymatic Response of Carbamazepine-induced Liver Injury as A Biomarker in Male Mice. Iran J War Public Health 2022; 14 (4) :367-375
URL: http://ijwph.ir/article-1-1252-en.html
URL: http://ijwph.ir/article-1-1252-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- College of Veterinary Medicine, University of Basrah, Basrah, Iraq
Full-Text (HTML) (612 Views)
Introduction
Harmful and resulting metabolites due to drug interactions and their relationship to hepatotoxicity are a source of concern to many drug manufacturers because they can cause financial drain due to the continuous development of drugs. It has been observed that Cytochrome P450 (CYP) enzyme systems have the ability to produce metabolic reactions accompanying the use of some drugs as a reaction may lead to liver injury either directly or through an immune-related mechanism [1].
Carbamazepine (CBZ) is a white crystalline powder discovered in 1953 AD and is commonly used in the treatment of epilepsy, bipolar affective disorder, schizophrenia, trigeminal neuralgia, and other specific pain disorders such as lingual neuralgia [2]. Carbamazepine is one of the antiepileptic drugs which induces hypersensitivity syndrome, which has a broad spectrum of side effects; however, the differentiation from hypersensitivity syndrome may be difficult, but it has been related to this syndrome affecting the lung, liver, and kidneys in severe cases [3]. Although the drug is generally well tolerated, only a limited number of patients who consumed CBZ develop severe, potentially life-threatening private interactions such as liver [4]. Hepatic toxicity associated with the CBZ dose may be a form of granulomatous hepatitis, as well as the failure of some liver functions, or acute inflammation associated with hepatic cellular necrosis [5].
Several studies have evaluated carbamazepine-induced hepatotoxicity by reactive metabolites in vitro, and they have suggested that CBZ 2,3-epoxide and 3-hydroxy(OH) CBZ may play a role in CBZ induced special drug interactions via covalent linkage with protein or production of Reactive Oxygen Species (ROS) [6, 7]. Antioxidants of Glutathione (GSH) and microsomal epoxide hydrolase are implicated in detoxification [7].
However, there are few articles on CBZ-induced hepatotoxicity in an in vivo animal model. Free radicals result from the metabolism of drugs such as CBZ in the liver and kidney. It is well known that ROS is involved in drug-induced liver damage via mitochondrial dysfunction and degeneration and necrosis of the hepatocytes [8]. Microsomal epoxide hydrolase and Glutathione (GSH) are involved in detoxification [7]. However, there is no confirmation of CBZ-induced hepatotoxicity in an in vivo animal model.
The present study aimed to elucidate important information about the action mechanisms of some drugs, such as CBZ, that induce histological damage and acute inflammation. This information makes it easier for us to understand the mechanical properties of drugs, their action, and how they cause histological, physiological, and metabolic damage to organs, especially the liver.
Materials and Methods
Experimental animals
Twenty-four mature male Balb-C mice (Mus musculus) were used for this study. The animals were distributed in plastic cages covered with metal clamp covers. Water and food were available ad labium during the 30-day trial. Experimental male mice were divided into three groups, each group containing 8 animals: The control group (C) was given 1 ml of normal saline for 30 days, Group II received 2.85 mg/kg/day of CBZ for 30 days, and Group III received 5.7mg/kg/day of CBZ during 30 days. During the 30-day experiment, food and water were available ad libitum.
Biochemical analysis
Alanine Transaminase (ALT) and Aspartate Transaminase (AST) in blood serum were measured at Basrah General Hospital. Liver tissue was prepared as homogenous tissue by mixing 0.1 M phosphate buffer by centrifugation at 12000 rpm for 20 minutes at pH 7.4, however, the enzyme exporter supernatant was kept at 4°C. GSH levels were evaluated according to the study of Mitchell et al. [9]. The level of Glutathione Reductase (GR) was tested by surveillance of the NADPH oxidation at 340 nm according to the method of James et al. [10] so that one unit of GR activity was expressed in 1 nmol/mg protein/min (1 nmol of NADPH oxidized to NADP+ by the enzyme during 1 min per mg of protein). Glutathione-S-Transferase (GST) activity was inspected according to Habig's method [11] so that one unit of GSH activity was equivalent to 1μm of 1-chloro-2,4-dinitrobenzene (CDNB) conjugated per min per mg protein under the inspect conditions.
Histological analysis
Microscopic analysis was done by observing the histological changes in the liver. For microscopic analysis, at the end of the experiment (30 days), all animals were killed and then pieces of liver were removed and fixed in a 10% phosphate-buffered formalin solution. Liver samples were dehydrated, cleared, infiltrated, and embedded in paraffin wax. The blocks were then cut using a rotary microtome. All liver sections were stained with hematoxylin and eosin [12].
Statistical analysis
Data were analyzed using one-way Analysis of Variance (ANOVA) and LSD test by SPSS 21 software. P≤0.05 was considered a significant level for all tests.
Findings
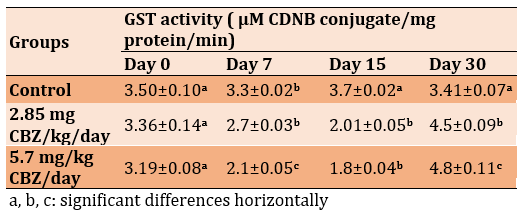
Oral administration at the dose of 2.85 mgCBZ/kg/day and 5.7 mg CBZ/kg/day in experimental groups significantly increased the serum level of AST and ALT compared to the control group (p≤0.05). The highest values were on days 15 and 30 in the 5.7mg CBZ/kg/day group (Tables 1 and 2).
Table 1) Effect of CBZ on serum ALT concentration of mature male mice during the experimental period

Table 2) Effect of CBZ on serum AST concentration of mature male mice during the experimental period
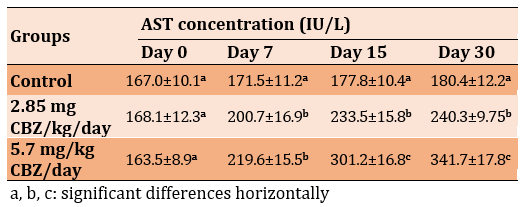
A significant decrease in the level of GSH was observed in the 2.85 mg CBZ group and the 5.7 mg CBZ group after 15 and 30 days compared to the control group (p≤0.05; Table 3).
Table 3) Effect of CBZ on serum GSH level of mature male mice during the experimental period
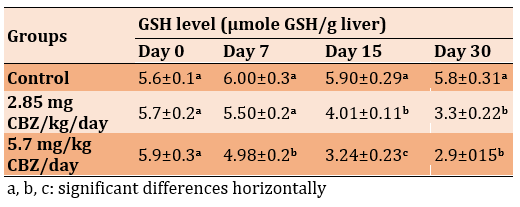
The activity of Glutathione Reductase (GR) in the 2.85 mg CBZ and 5.7 mg CBZ groups showed a significant increase compared to the control group (p≤0.05). The highest values were recorded on day 30 in the 2.85 mg CBZ and 5.7 mg CBZ groups (Table 4).
Table 4) Effect of CBZ on GR activity of mature male mice during the experimental period
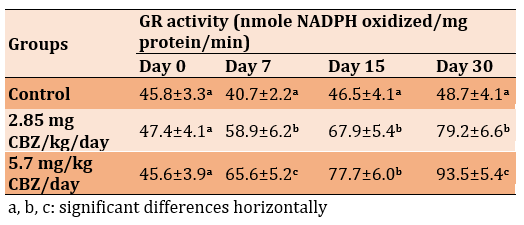
The level of Glutathione-S-Transferase (GST) activity significantly decreased in the 2.85 mg CBZ and 5.7 mg CBZ groups after 7 and 15 days, but a significant increase was observed after 30 days compared to the control group (p≤0.05; Table 5).
Table 5) Effect of CBZ on GST activity of mature male mice during the experimental period
The histological evaluation in the control group showed that normal hepatic cells were arranged as radiating cords from the central vein, and clear histological organization involved normal hepatic cells (Figure 1), while the 2.85 mg CBZ group on days 7 and 15 showed hepatocyte degeneration, vacuolated hepatocytes, and loss of intercellular demarcation in the liver lobule (Figures 2-4).
In addition, on day 30, the experiment showed the presence of Kupffer cells, necrotic hepatocytes, hypertrophy of hepatocytes, pyknotic nuclei of hepatocytes, and loss of intercellular demarcation in liver lobules (Figure 5). Also, sinusoidal expansion, acute necrosis, cytoplasm vacillation of the hepatocytes, and nodal inflammatory cell infiltration were observed (Figures 6 and 7).
The 5.7 mg group on days 7 and 15 showed more acute histopathological differences compared to the other groups, including areas of hydropic degeneration, hypertrophied nuclei, marked complete distortion of the hepatic cell cords, loss of hepatocytes around the central vein, highly dilated sinusoid and highly congested hepatic portal vein, necrosis and damage of hepatocytes, acute congestion of the hepatic portal vein, loss of hepatocytes cytoplasm, loss of normal radial cords regulation of hepatocytes around the hepatic portal vein, hypertrophy of hepatocytes and aggregation Kupffer cells around portal vein (Figures 8-10), While the 5.7 mg group on day 30 showed marked complete distortion of the hepatic cell cords, large areas of hydropic degeneration, nodal inflammatory cell infiltration, acute cytoplasm vacoulation, prominent fragmented pyknotic nuclei, loss of hepatocytes nuclei, highly congested hepatic portal vein, hemolysis blood cells, increased proliferation in highly elongated wall of the bile duct, hypertrophied nuclei, congested blood sinusoids and numerous hemorrhagic areas, and edema (Figures 11-15).
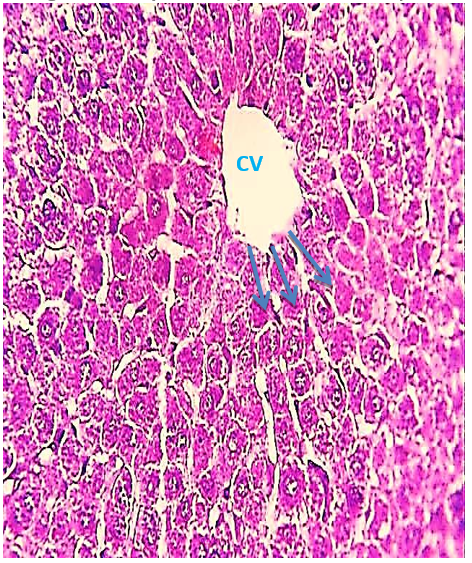
Figure 1) Liver section of the control group showed the Central Vein (CV) and the normal radiating cord of hepatocytes (arrow) (H&E 400X).
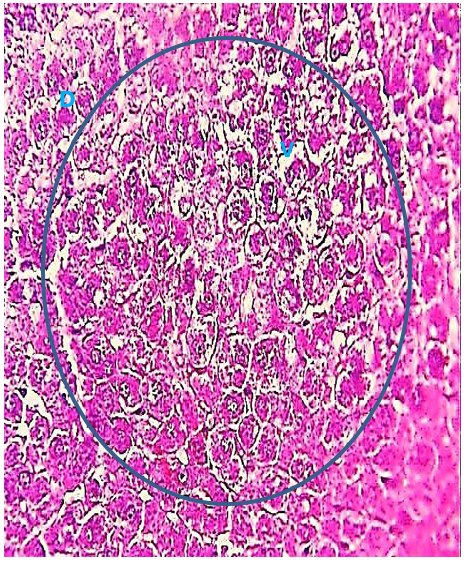
Figure 2) Liver section of the 2.85 mg CBZ group on day 7 showed degeneration of hepatocytes (D) and cytoplasmic vacuolation (V) and loss of intercellular demarcation in the hepatic lobules (circle) (H&E 400X).
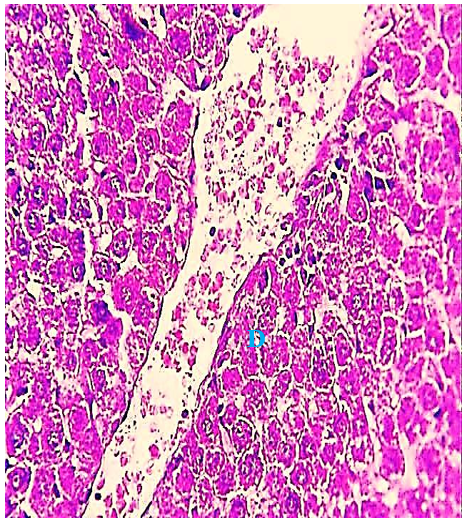
Figure 3) Liver section of the 2.85 mg CBZ group on day 15 showed hepatocyte degeneration (D) and loss of intercellular demarcation of the hepatic lobule. (H&E 400X).
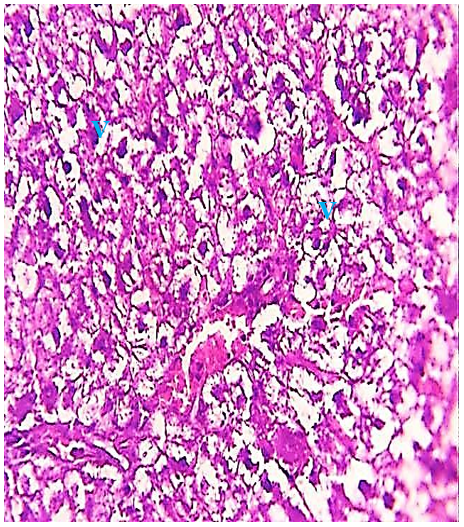
Figure 4) Liver section of the 2.85 mg CBZ group on day 15 showed prevalent cytoplasm vacuolation (V) and loss of intercellular demarcation of the hepatic lobule (H&E 400X).

Figure 5) Liver section of the 2.85 mg CBZ group on day 30 showed aggregation of kupffer cells (circle), cytoplasm vacuolation (V), necrosis of hepatocytes (N), pyknotic of nuclear (arrow), and loss of intercellular demarcation in the hepatic lobules (H&E 400X).
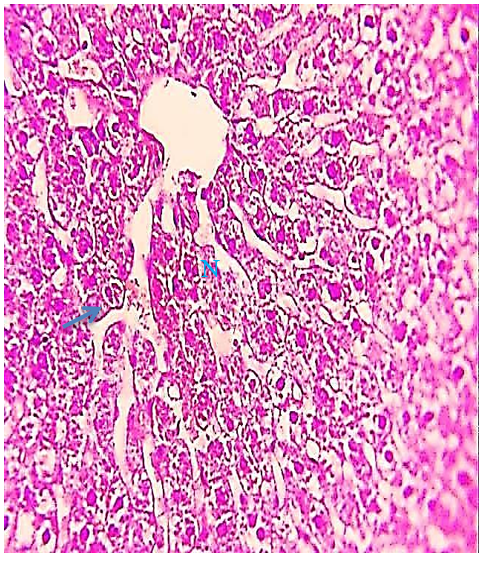
Figure 6) Liver section of the 2.85 mg CBZ group on day 30 showed sinusoidal expansion (arrow) and clear hepatocytes necrosis (N) (H&E 400X).

Figure 7) Liver section of the 2.85 mg CBZ group on day 30 showed nodal inflammation cell infiltration (circle) (H&E 400X).
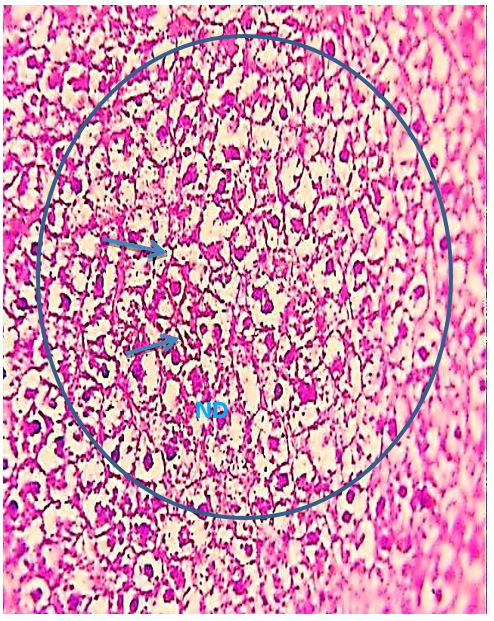
Figure 8) Liver section of the 5.7 mg CBZ group on day 7 showed hydropic degeneration (arrow), necrosis and damage of hepatocytes (ND), and distortion of the hepatic cell cords (circle) (H&E400X)
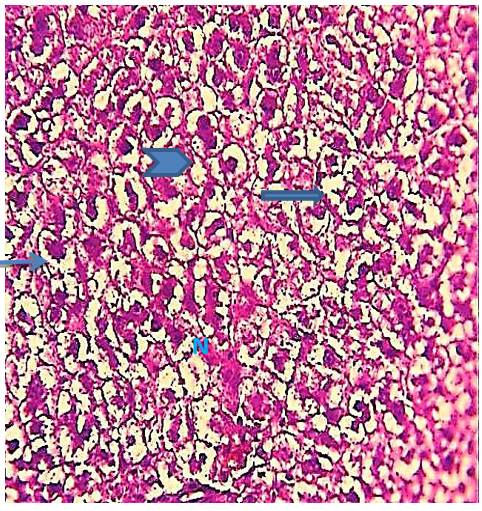
Figure 9) Liver section of the 5.7 mg CBZ group on day 15 showed hypertrophied hepatocytes (arrows), acute necrosis (N), loss of normal radical cords of hepatocytes (head arrow), and loss of hepatocytes cytoplasm (thick arrows) (H&E 400X)
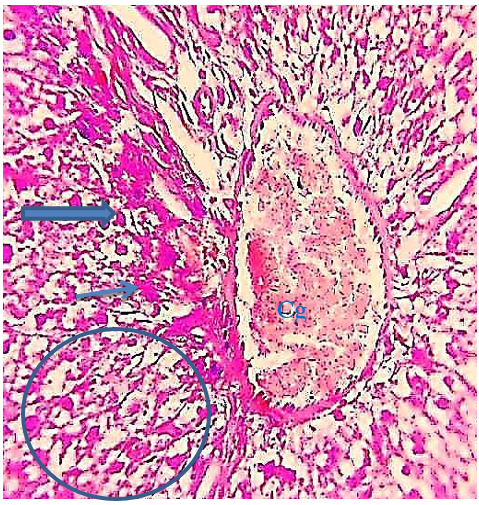
Figure 10) Liver section of the 5.7 mg CBZ group on day 15 showed highly congestion of hepatocytes portal and hemolysis blood vein (Cg), hydropic degeneration (arrows), distortion of hepatic cell cords (thick arrows), sinusoidal dilation (head arrow), and vacuolation and necrosis of hepatocytes (circle)(H&E 400X).
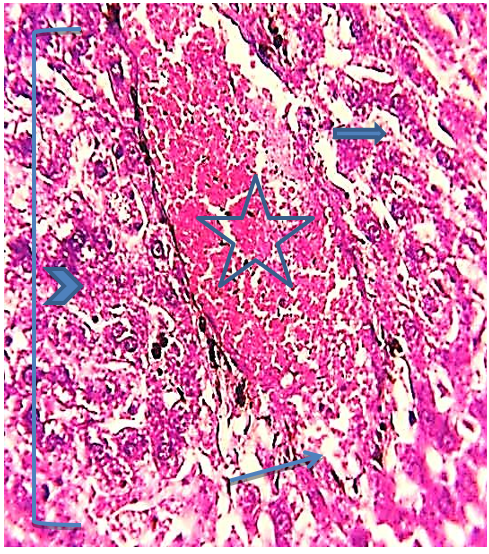
Figure 11) Liver section of the 5.7 mg CBZ group on day 30 showed marked complete distortion of the hepatic cell cords, highly congested hepatic portal vein (star), hemorrhage areas (arrow), hypertrophied hepatocytes (head arrow), congested blood of sinusoids (thick arrow) (H&E 400X)
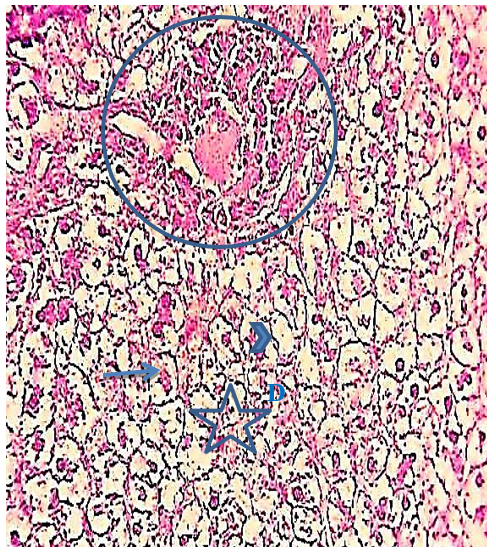
Figure 12) Liver section of the 5.7 mg CBZ group on day 30 showed prominent fragmented pyknotic nuclei (arrows), increased proliferation in a highly elongated wall of the bile duct (circle), areas of hydropic degeneration (D), loss of hepatocytes nuclei (head arrow), and necrosis of hepatocytes (star) (H&E 400X)
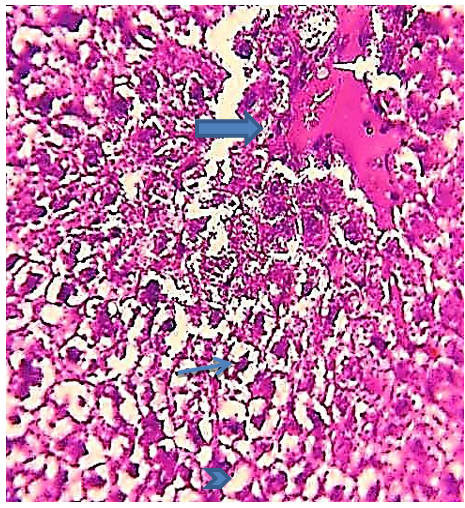
Figure 13) Liver section of the 5.7 mg CBZ group on day 30 showed acute necrosis, prominent fragmented pyknotic nuclei (arrow), loss of hepatocytes nuclei (head arrow), and congested blood sinusoids and numerous hemorrhagic and edema areas (thick arrow)(H&E 400X).

Figure 14) Liver section of the 5.7 mg CBZ group on day 30 showed acute cytoplasm vacuolation (V), marked complete distortion of the hepatic cell cords, large areas of necrosis and degeneration (circle), highly congested hepatic portal vein and hemolysis blood cells (Ch) (H&E 400X).
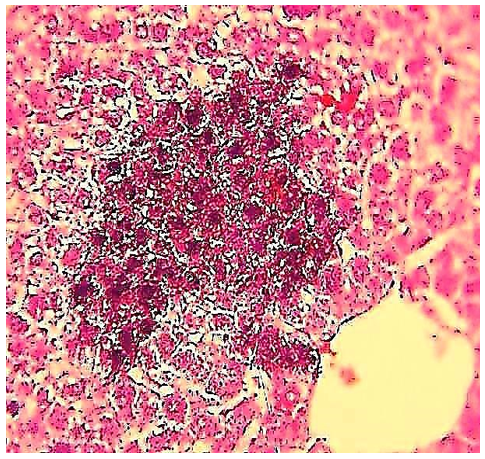
Figure 15) Liver section of the 5.7 mg CBZ group on day 30 showed hyperpigmented and basophilic inflammatory infiltrating cells (circle) (H&E 400X).
Discussion
Advances in the acquaintanceship of the mechanisms of drug-induced liver ecchymosis were hampered by the want of proper animal models. The current study was carried out using the mouse model to provide novel mechanistic information, including aspects of drug metabolism and inflammation in the pathogenesis of CBZ-induced liver injury.
In this study, it was observed that ALT and AST serum levels significantly increased by oral administrations in a dose of 2.85 mg CBZ/kg/day and 5.7 mg CBZ/kg/day compared to the control group. Mullick and Ishak [13] showed that acute hepatic injury with antiepileptic drugs involved primarily hepatocytes degeneration, necrosis, or other damage, which generally caused abnormal serum levels of bilirubin, transaminases, and alkaline phosphatase enzymes. O’Hare et al. [14] showed that there is a statistically significant correlation between the administration duration of carbamazepine and ALP elevation, while other researchers assigned the effect on hepatic enzymes to inducing carbamazepine property, which leads to the creation of microsomal enzymes. Friedrich et al. [15] recorded that there were no significant effects between the therapy duration of CBZ and lowering liver enzymes. A transient and asymptomatic elevation usually of liver enzymes occurs in 25%-61% of patients receiving CBZ [16, 17]. Serious CBZ-associated hepatic toxicity takes the forms of the following: granulomatous hepatitis as a hypersensitive reaction, which caused abnormal hepatocytes functions, or hepatocellular necrosis associated with acute hepatitis, which may cause from effect of direct drug toxicity [18].
The results of the current study showed a significant value decrease in GSH levels at 2.85 mg CBZ and 5.7 mg CBZ groups after days 15 and 30 of the experiment compared with control groups. Some plasma reactive metabolites, such as 3-OH CBZ and 2-OH CBZ play an important role in CBZ-induced liver injury. However, the 3- and 2-OH CBZ, as a reactive metabolite produced by an assortment of CYPs, induced ROS, causing mitochondrial dysfunctions, which causes the suppression of GSH levels and the alteration of oxidative stress signs. CYP3A4 and CYP2B6 are generally liable for the arrangement of 3-OH CBZ, and these enzymes are initiated by CBZ treatment [19]. Subsequently, the repeated overdose administration of CBZ might cause the height of the 3-OH CBZ plane in the plasma [20]. The cytotoxicity of certain chemical substances can be enhanced either by depleting glutathione level or by blocking the reduction of the oxidized form of Glutathione disulfide (GSSG) to its reduced form the glutathione reduction antioxidant (GSH) through inhibition of Glutathione-Disulfide Reductase (GSR). GSTs of mammalian are divided into four groups [21, 22], however, the GSTπ considered one of these groups, and its serves as biomarkers of hepatic toxicity in the rodent system [23]. Our results showed a significant increase in GST in the treatment groups after 30 days, unlike the control group. It may be because of physiological responses and adaptive mechanisms of the hepatocytes to enhance the GSH conjugation capacity with the toxicity of the metabolites due to drugs to reduce their toxicity. However, GSH concentration does not directly relate to regulating intracellular levels of GST activity [21], which agreement with our study results.
The present study reports the importance of understanding the direct relationship between some liver enzymes (ALT and AST), in addition to antioxidant factor (GSH), also GR enzymes, and GSTs isozymes in potential drug toxicity of pharmacological metabolites of carbamazepine, as bio-indicator of liver toxicity. Our study deals with the importance of understanding alterations in GSH levels and GSH-related enzymes as biomarkers in the assessment, and toxicity of carbamazepine drug overdose and other anticonvulsants and antiepileptic drug metabolism.
The histological study of the liver tissue section showed that the 2.85 and 5.7 mg CBZ/kg/day groups showed histological alterations between degeneration, inflammation and necrosis. The cause of the histopathological liver disorder can be concluded as follows: CBZ is metabolized in hepatocytes by Cytochromes P450 (CYPs) enzymes; ROS production in macrophages is stimulated by reactive receptors produced by CBZ metabolism, and then activates macrophage-activated danger signals, Toll-Like Receptor 4 (TLR4) and Receptors for Advanced Glycation End products (RAGE), which activates the secretion of immunoregulatory chemokines and cytokines, thereby causing liver inflammation and hepatocellular necrosis, secretes RAGE and TLR4 ligands, which cause liver inflammation.
Evidence suggests that liver inflammation due to CBZ exposure is a hypersensitivity reaction possibly mediated by immunological mechanisms [24]. Carbamazepine hepatotoxicity is due to the immunological response to a metabolically produced drug-protein complex and the effect on liver, which ranges from mild to severe depending on time and dose [5]. Carbamazepine-induced hepatitis was confirmed due to an allergic immune mechanism with increasing carbamazepine dosage [25]. However, carbamazepine-induced hepatocyte abnormalities have been reported by several researchers [26].
Conclusion
Biomarkers can be used as a warning about the pre-sensitivity of some patients to carbamazepine. Also, carbamazepine treatment may change the capacity of the liver to detoxify many toxic compounds.
Acknowledgements: None declared by the authors.
Ethical Permission: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: Kareem DA (First Author), Introduction Writer/Main Researcher (25%); Sadoon AH (Second Author), Methodologist/Assistant Researcher (25%); Majeed MF (Third Author), Discussion Writer (25%); Abbas BA (Fourth Author), Statistical Analyst (25%)
Funding/Support: None declared by the authors.
Harmful and resulting metabolites due to drug interactions and their relationship to hepatotoxicity are a source of concern to many drug manufacturers because they can cause financial drain due to the continuous development of drugs. It has been observed that Cytochrome P450 (CYP) enzyme systems have the ability to produce metabolic reactions accompanying the use of some drugs as a reaction may lead to liver injury either directly or through an immune-related mechanism [1].
Carbamazepine (CBZ) is a white crystalline powder discovered in 1953 AD and is commonly used in the treatment of epilepsy, bipolar affective disorder, schizophrenia, trigeminal neuralgia, and other specific pain disorders such as lingual neuralgia [2]. Carbamazepine is one of the antiepileptic drugs which induces hypersensitivity syndrome, which has a broad spectrum of side effects; however, the differentiation from hypersensitivity syndrome may be difficult, but it has been related to this syndrome affecting the lung, liver, and kidneys in severe cases [3]. Although the drug is generally well tolerated, only a limited number of patients who consumed CBZ develop severe, potentially life-threatening private interactions such as liver [4]. Hepatic toxicity associated with the CBZ dose may be a form of granulomatous hepatitis, as well as the failure of some liver functions, or acute inflammation associated with hepatic cellular necrosis [5].
Several studies have evaluated carbamazepine-induced hepatotoxicity by reactive metabolites in vitro, and they have suggested that CBZ 2,3-epoxide and 3-hydroxy(OH) CBZ may play a role in CBZ induced special drug interactions via covalent linkage with protein or production of Reactive Oxygen Species (ROS) [6, 7]. Antioxidants of Glutathione (GSH) and microsomal epoxide hydrolase are implicated in detoxification [7].
However, there are few articles on CBZ-induced hepatotoxicity in an in vivo animal model. Free radicals result from the metabolism of drugs such as CBZ in the liver and kidney. It is well known that ROS is involved in drug-induced liver damage via mitochondrial dysfunction and degeneration and necrosis of the hepatocytes [8]. Microsomal epoxide hydrolase and Glutathione (GSH) are involved in detoxification [7]. However, there is no confirmation of CBZ-induced hepatotoxicity in an in vivo animal model.
The present study aimed to elucidate important information about the action mechanisms of some drugs, such as CBZ, that induce histological damage and acute inflammation. This information makes it easier for us to understand the mechanical properties of drugs, their action, and how they cause histological, physiological, and metabolic damage to organs, especially the liver.
Materials and Methods
Experimental animals
Twenty-four mature male Balb-C mice (Mus musculus) were used for this study. The animals were distributed in plastic cages covered with metal clamp covers. Water and food were available ad labium during the 30-day trial. Experimental male mice were divided into three groups, each group containing 8 animals: The control group (C) was given 1 ml of normal saline for 30 days, Group II received 2.85 mg/kg/day of CBZ for 30 days, and Group III received 5.7mg/kg/day of CBZ during 30 days. During the 30-day experiment, food and water were available ad libitum.
Biochemical analysis
Alanine Transaminase (ALT) and Aspartate Transaminase (AST) in blood serum were measured at Basrah General Hospital. Liver tissue was prepared as homogenous tissue by mixing 0.1 M phosphate buffer by centrifugation at 12000 rpm for 20 minutes at pH 7.4, however, the enzyme exporter supernatant was kept at 4°C. GSH levels were evaluated according to the study of Mitchell et al. [9]. The level of Glutathione Reductase (GR) was tested by surveillance of the NADPH oxidation at 340 nm according to the method of James et al. [10] so that one unit of GR activity was expressed in 1 nmol/mg protein/min (1 nmol of NADPH oxidized to NADP+ by the enzyme during 1 min per mg of protein). Glutathione-S-Transferase (GST) activity was inspected according to Habig's method [11] so that one unit of GSH activity was equivalent to 1μm of 1-chloro-2,4-dinitrobenzene (CDNB) conjugated per min per mg protein under the inspect conditions.
Histological analysis
Microscopic analysis was done by observing the histological changes in the liver. For microscopic analysis, at the end of the experiment (30 days), all animals were killed and then pieces of liver were removed and fixed in a 10% phosphate-buffered formalin solution. Liver samples were dehydrated, cleared, infiltrated, and embedded in paraffin wax. The blocks were then cut using a rotary microtome. All liver sections were stained with hematoxylin and eosin [12].
Statistical analysis
Data were analyzed using one-way Analysis of Variance (ANOVA) and LSD test by SPSS 21 software. P≤0.05 was considered a significant level for all tests.
Findings
Oral administration at the dose of 2.85 mgCBZ/kg/day and 5.7 mg CBZ/kg/day in experimental groups significantly increased the serum level of AST and ALT compared to the control group (p≤0.05). The highest values were on days 15 and 30 in the 5.7mg CBZ/kg/day group (Tables 1 and 2).
Table 1) Effect of CBZ on serum ALT concentration of mature male mice during the experimental period

Table 2) Effect of CBZ on serum AST concentration of mature male mice during the experimental period

A significant decrease in the level of GSH was observed in the 2.85 mg CBZ group and the 5.7 mg CBZ group after 15 and 30 days compared to the control group (p≤0.05; Table 3).
Table 3) Effect of CBZ on serum GSH level of mature male mice during the experimental period

The activity of Glutathione Reductase (GR) in the 2.85 mg CBZ and 5.7 mg CBZ groups showed a significant increase compared to the control group (p≤0.05). The highest values were recorded on day 30 in the 2.85 mg CBZ and 5.7 mg CBZ groups (Table 4).
Table 4) Effect of CBZ on GR activity of mature male mice during the experimental period

The level of Glutathione-S-Transferase (GST) activity significantly decreased in the 2.85 mg CBZ and 5.7 mg CBZ groups after 7 and 15 days, but a significant increase was observed after 30 days compared to the control group (p≤0.05; Table 5).
Table 5) Effect of CBZ on GST activity of mature male mice during the experimental period

The histological evaluation in the control group showed that normal hepatic cells were arranged as radiating cords from the central vein, and clear histological organization involved normal hepatic cells (Figure 1), while the 2.85 mg CBZ group on days 7 and 15 showed hepatocyte degeneration, vacuolated hepatocytes, and loss of intercellular demarcation in the liver lobule (Figures 2-4).
In addition, on day 30, the experiment showed the presence of Kupffer cells, necrotic hepatocytes, hypertrophy of hepatocytes, pyknotic nuclei of hepatocytes, and loss of intercellular demarcation in liver lobules (Figure 5). Also, sinusoidal expansion, acute necrosis, cytoplasm vacillation of the hepatocytes, and nodal inflammatory cell infiltration were observed (Figures 6 and 7).
The 5.7 mg group on days 7 and 15 showed more acute histopathological differences compared to the other groups, including areas of hydropic degeneration, hypertrophied nuclei, marked complete distortion of the hepatic cell cords, loss of hepatocytes around the central vein, highly dilated sinusoid and highly congested hepatic portal vein, necrosis and damage of hepatocytes, acute congestion of the hepatic portal vein, loss of hepatocytes cytoplasm, loss of normal radial cords regulation of hepatocytes around the hepatic portal vein, hypertrophy of hepatocytes and aggregation Kupffer cells around portal vein (Figures 8-10), While the 5.7 mg group on day 30 showed marked complete distortion of the hepatic cell cords, large areas of hydropic degeneration, nodal inflammatory cell infiltration, acute cytoplasm vacoulation, prominent fragmented pyknotic nuclei, loss of hepatocytes nuclei, highly congested hepatic portal vein, hemolysis blood cells, increased proliferation in highly elongated wall of the bile duct, hypertrophied nuclei, congested blood sinusoids and numerous hemorrhagic areas, and edema (Figures 11-15).

Figure 1) Liver section of the control group showed the Central Vein (CV) and the normal radiating cord of hepatocytes (arrow) (H&E 400X).

Figure 2) Liver section of the 2.85 mg CBZ group on day 7 showed degeneration of hepatocytes (D) and cytoplasmic vacuolation (V) and loss of intercellular demarcation in the hepatic lobules (circle) (H&E 400X).

Figure 3) Liver section of the 2.85 mg CBZ group on day 15 showed hepatocyte degeneration (D) and loss of intercellular demarcation of the hepatic lobule. (H&E 400X).

Figure 4) Liver section of the 2.85 mg CBZ group on day 15 showed prevalent cytoplasm vacuolation (V) and loss of intercellular demarcation of the hepatic lobule (H&E 400X).

Figure 5) Liver section of the 2.85 mg CBZ group on day 30 showed aggregation of kupffer cells (circle), cytoplasm vacuolation (V), necrosis of hepatocytes (N), pyknotic of nuclear (arrow), and loss of intercellular demarcation in the hepatic lobules (H&E 400X).

Figure 6) Liver section of the 2.85 mg CBZ group on day 30 showed sinusoidal expansion (arrow) and clear hepatocytes necrosis (N) (H&E 400X).

Figure 7) Liver section of the 2.85 mg CBZ group on day 30 showed nodal inflammation cell infiltration (circle) (H&E 400X).

Figure 8) Liver section of the 5.7 mg CBZ group on day 7 showed hydropic degeneration (arrow), necrosis and damage of hepatocytes (ND), and distortion of the hepatic cell cords (circle) (H&E400X)

Figure 9) Liver section of the 5.7 mg CBZ group on day 15 showed hypertrophied hepatocytes (arrows), acute necrosis (N), loss of normal radical cords of hepatocytes (head arrow), and loss of hepatocytes cytoplasm (thick arrows) (H&E 400X)

Figure 10) Liver section of the 5.7 mg CBZ group on day 15 showed highly congestion of hepatocytes portal and hemolysis blood vein (Cg), hydropic degeneration (arrows), distortion of hepatic cell cords (thick arrows), sinusoidal dilation (head arrow), and vacuolation and necrosis of hepatocytes (circle)(H&E 400X).

Figure 11) Liver section of the 5.7 mg CBZ group on day 30 showed marked complete distortion of the hepatic cell cords, highly congested hepatic portal vein (star), hemorrhage areas (arrow), hypertrophied hepatocytes (head arrow), congested blood of sinusoids (thick arrow) (H&E 400X)

Figure 12) Liver section of the 5.7 mg CBZ group on day 30 showed prominent fragmented pyknotic nuclei (arrows), increased proliferation in a highly elongated wall of the bile duct (circle), areas of hydropic degeneration (D), loss of hepatocytes nuclei (head arrow), and necrosis of hepatocytes (star) (H&E 400X)

Figure 13) Liver section of the 5.7 mg CBZ group on day 30 showed acute necrosis, prominent fragmented pyknotic nuclei (arrow), loss of hepatocytes nuclei (head arrow), and congested blood sinusoids and numerous hemorrhagic and edema areas (thick arrow)(H&E 400X).

Figure 14) Liver section of the 5.7 mg CBZ group on day 30 showed acute cytoplasm vacuolation (V), marked complete distortion of the hepatic cell cords, large areas of necrosis and degeneration (circle), highly congested hepatic portal vein and hemolysis blood cells (Ch) (H&E 400X).

Figure 15) Liver section of the 5.7 mg CBZ group on day 30 showed hyperpigmented and basophilic inflammatory infiltrating cells (circle) (H&E 400X).
Discussion
Advances in the acquaintanceship of the mechanisms of drug-induced liver ecchymosis were hampered by the want of proper animal models. The current study was carried out using the mouse model to provide novel mechanistic information, including aspects of drug metabolism and inflammation in the pathogenesis of CBZ-induced liver injury.
In this study, it was observed that ALT and AST serum levels significantly increased by oral administrations in a dose of 2.85 mg CBZ/kg/day and 5.7 mg CBZ/kg/day compared to the control group. Mullick and Ishak [13] showed that acute hepatic injury with antiepileptic drugs involved primarily hepatocytes degeneration, necrosis, or other damage, which generally caused abnormal serum levels of bilirubin, transaminases, and alkaline phosphatase enzymes. O’Hare et al. [14] showed that there is a statistically significant correlation between the administration duration of carbamazepine and ALP elevation, while other researchers assigned the effect on hepatic enzymes to inducing carbamazepine property, which leads to the creation of microsomal enzymes. Friedrich et al. [15] recorded that there were no significant effects between the therapy duration of CBZ and lowering liver enzymes. A transient and asymptomatic elevation usually of liver enzymes occurs in 25%-61% of patients receiving CBZ [16, 17]. Serious CBZ-associated hepatic toxicity takes the forms of the following: granulomatous hepatitis as a hypersensitive reaction, which caused abnormal hepatocytes functions, or hepatocellular necrosis associated with acute hepatitis, which may cause from effect of direct drug toxicity [18].
The results of the current study showed a significant value decrease in GSH levels at 2.85 mg CBZ and 5.7 mg CBZ groups after days 15 and 30 of the experiment compared with control groups. Some plasma reactive metabolites, such as 3-OH CBZ and 2-OH CBZ play an important role in CBZ-induced liver injury. However, the 3- and 2-OH CBZ, as a reactive metabolite produced by an assortment of CYPs, induced ROS, causing mitochondrial dysfunctions, which causes the suppression of GSH levels and the alteration of oxidative stress signs. CYP3A4 and CYP2B6 are generally liable for the arrangement of 3-OH CBZ, and these enzymes are initiated by CBZ treatment [19]. Subsequently, the repeated overdose administration of CBZ might cause the height of the 3-OH CBZ plane in the plasma [20]. The cytotoxicity of certain chemical substances can be enhanced either by depleting glutathione level or by blocking the reduction of the oxidized form of Glutathione disulfide (GSSG) to its reduced form the glutathione reduction antioxidant (GSH) through inhibition of Glutathione-Disulfide Reductase (GSR). GSTs of mammalian are divided into four groups [21, 22], however, the GSTπ considered one of these groups, and its serves as biomarkers of hepatic toxicity in the rodent system [23]. Our results showed a significant increase in GST in the treatment groups after 30 days, unlike the control group. It may be because of physiological responses and adaptive mechanisms of the hepatocytes to enhance the GSH conjugation capacity with the toxicity of the metabolites due to drugs to reduce their toxicity. However, GSH concentration does not directly relate to regulating intracellular levels of GST activity [21], which agreement with our study results.
The present study reports the importance of understanding the direct relationship between some liver enzymes (ALT and AST), in addition to antioxidant factor (GSH), also GR enzymes, and GSTs isozymes in potential drug toxicity of pharmacological metabolites of carbamazepine, as bio-indicator of liver toxicity. Our study deals with the importance of understanding alterations in GSH levels and GSH-related enzymes as biomarkers in the assessment, and toxicity of carbamazepine drug overdose and other anticonvulsants and antiepileptic drug metabolism.
The histological study of the liver tissue section showed that the 2.85 and 5.7 mg CBZ/kg/day groups showed histological alterations between degeneration, inflammation and necrosis. The cause of the histopathological liver disorder can be concluded as follows: CBZ is metabolized in hepatocytes by Cytochromes P450 (CYPs) enzymes; ROS production in macrophages is stimulated by reactive receptors produced by CBZ metabolism, and then activates macrophage-activated danger signals, Toll-Like Receptor 4 (TLR4) and Receptors for Advanced Glycation End products (RAGE), which activates the secretion of immunoregulatory chemokines and cytokines, thereby causing liver inflammation and hepatocellular necrosis, secretes RAGE and TLR4 ligands, which cause liver inflammation.
Evidence suggests that liver inflammation due to CBZ exposure is a hypersensitivity reaction possibly mediated by immunological mechanisms [24]. Carbamazepine hepatotoxicity is due to the immunological response to a metabolically produced drug-protein complex and the effect on liver, which ranges from mild to severe depending on time and dose [5]. Carbamazepine-induced hepatitis was confirmed due to an allergic immune mechanism with increasing carbamazepine dosage [25]. However, carbamazepine-induced hepatocyte abnormalities have been reported by several researchers [26].
Conclusion
Biomarkers can be used as a warning about the pre-sensitivity of some patients to carbamazepine. Also, carbamazepine treatment may change the capacity of the liver to detoxify many toxic compounds.
Acknowledgements: None declared by the authors.
Ethical Permission: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: Kareem DA (First Author), Introduction Writer/Main Researcher (25%); Sadoon AH (Second Author), Methodologist/Assistant Researcher (25%); Majeed MF (Third Author), Discussion Writer (25%); Abbas BA (Fourth Author), Statistical Analyst (25%)
Funding/Support: None declared by the authors.
Keywords:
References
1. Park BK, Kitteringham NR, Powell H, Pirmohamed M. Advances in molecular toxicology-towards understanding idiosyncratic drug toxicity. Toxicology. 2000;153(1-3):39-60. [Link] [DOI:10.1016/S0300-483X(00)00303-6]
2. Hollander P, Gupta A, Plodkowski R, Greenway F, Bays H. Effects of naltrexone sustained-release bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12): 4022-9. [Link] [DOI:10.2337/dc13-0234]
3. Schlienger RG, Shear NH. Antiepileptic drug hypersensitivity syndrome. Epilepsia. 1998;39 Suppl 7:S3-7. [Link] [DOI:10.1111/j.1528-1157.1998.tb01678.x]
4. Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42(2):481-9. [Link] [DOI:10.1002/hep.20800]
5. Bjornsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118(5):281-90. [Link] [DOI:10.1111/j.1600-0404.2008.01009.x]
6. Lu W, Uetrecht JP. Peroxidase-mediated bioactivation of hydroxylated metabolites of carbamazepine and phenytoin. Drug Metab Dispos. 2008;36(8):1624-36. [Link] [DOI:10.1124/dmd.107.019554]
7. Pirmohamed M, Kitteringham NR, Guenthner TM, Breckenridge AM, Park BK. An investigation of the formation of cytotoxic, protein-reactive and stable metabolites from carbamazepine in vitro. Biochem Pharmacol. 1992;43(8):1675-82. [Link] [DOI:10.1016/0006-2952(92)90696-G]
8. Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65(2):166-76. [Link] [DOI:10.1093/toxsci/65.2.166]
9. Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necroses. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187(1):211-7. [Link]
10. Suojanen JN, Gay RJ, Hilf R. Influence of estrogen on glutathione levels and glutathione-metabolizing enzymes in uteri and R3230AC mammary tumors of rats. Biochim Biophys Acta. 1980;630(4):485-96. [Link] [DOI:10.1016/0304-4165(80)90003-3]
11. Habig W, Pabs TM, Jakoby W. Glutathione S-transferases. The rest enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130-9. [Link] [DOI:10.1016/S0021-9258(19)42083-8]
12. Luna LG. Manual of histologic staining methods of the armed forces institute of pathology. 3rd Edition. Blakiston Division, McGraw-Hill, New York: Blakiston Division, McGraw-Hill; 1968. [Link]
13. Mullick FG, Ishak KG. Hepatic injury associated with diphenyllhydantoin therapy: A clinicopahtological study of 20 cases. Am J Clin Pathol.71980;74(4):442-52. [Link] [DOI:10.1093/ajcp/74.4.442]
14. O'Hare JA, Duggan B, O'Driscoll D, Callaghan N. Biochemical evidence for osteomalacia with carbamazepine therapy. Acta Neurol Scand. 1980;62(5):282-6. [Link] [DOI:10.1111/j.1600-0404.1980.tb03037.x]
15. Friedrich M-E, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkle D, et al. Drug-induced liver injury during antidepressant treatment: Results of AMSP, a drug surveillance program. Int J Neuropsychopharmacol. 2016;19(4):pyv126. [Link] [DOI:10.1093/ijnp/pyv126]
16. Cepelak I, Grubisic TZ, Mandusic A, Rekic B, Lenicek J. Valproate and carbamazepine communication changes hepatic enzyme activities in the sera of epileptic children. Clin Chim Acta. 1998;276(2):121-7. [Link] [DOI:10.1016/S0009-8981(98)00094-1]
17. Benedetti MS, Ruty B, Baltes E. Induction of endogenous pathways by antiepileptics and clinical implications. Fundam Clin Pharmacol. 2005;19(5):511-29. [Link] [DOI:10.1111/j.1472-8206.2005.00341.x]
18. Driefus FE, Langer DH. Hepatic considerations in the use of antiepileptic drugs. Epilepsia. 1987;28 Suppl 2:S23-9. [Link] [DOI:10.1111/j.1528-1157.1987.tb05768.x]
19. Pearce RE, Lu W, Wang Y, Uetrecht JP, Correia MA, Leeder JS. Pathways of carbamazepine bioactivation in vitro. III. The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine. Drug Metab Dispos. 2008;36(8):1637-49. [Link] [DOI:10.1124/dmd.107.019562]
20. Pearce RE, Vakkalagadda GR, Leeder JS. Pathways of carbamazepine P450 responsible for the formation of 2- and 3-hydroxylated metabolites. Drug Metab Dispos 2002;30(11):1170-9. [Link] [DOI:10.1124/dmd.30.11.1170]
21. Boyer TD, Kenney WCm Zakim D. Structural, functional and hybridization studies of the glutathione S-transferases of rat liver. Biochem Pharmacol. 1983;32(12):1843-50. [Link] [DOI:10.1016/0006-2952(83)90048-5]
22. Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357-417. [Link] [DOI:10.1002/9780470123034.ch5]
23. Sato K. Glutathione transferasesas markers of preneoplasia and neoplasia. Adv Cancer Res. 1989;52:205-55. [Link] [DOI:10.1016/S0065-230X(08)60214-6]
24. Gram L, Bensten KD. Hepatic toxicity of antiepileptic drugs: a review. Acta Neurol Scand Suppl. 1983;63:81-90. [Link] [DOI:10.1111/j.1600-0404.1983.tb01537.x]
25. Davion T, Capron JP, Andrejak M, Geoffroy P, Capron-Chivrac D, Quénum C. Acute hepatitis due to carbamazepine (Tegretol). Study of a case and review of the literature. Gastroenterol Clin Biol. 1984;8(1):52-6. [French] [Link]
26. Jeavons PM. Hepatotoxicity of antiepileptic drugs In: Oxley J, Janz D, Meinardi H, editors. Antiepileptic Therapy: Chronic toxicity of antiepileptic drugs. New York: Raven Press; 1983. [Link]








