Volume 15, Issue 3 (2023)
Iran J War Public Health 2023, 15(3): 219-224 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/12/22 | Accepted: 2023/07/22 | Published: 2023/07/29
Received: 2022/12/22 | Accepted: 2023/07/22 | Published: 2023/07/29
How to cite this article
Al Kaabi Z. Histo-morphological Study of Dorsal Skin of Hemidactylus frenatus (Asian House Gecko) in Iraq. Iran J War Public Health 2023; 15 (3) :219-224
URL: http://ijwph.ir/article-1-1243-en.html
URL: http://ijwph.ir/article-1-1243-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
Z.S.M. Al Kaabi *
Department of Laboratory Investigations, Faculty of Science, University of Kufa, Kufa, Iraq
Full-Text (HTML) (1476 Views)
Introduction
The reptile family, alongside fish, birds, mammals, amphibians, invertebrates, and invertebrates, is one of the six major animal groupings. They gained two characteristics that are still highly significant to reptiles today when early reptiles split from amphibians: amniotic eggs and scales (eggs that have a fluid membrane on the inside of them). The four orders of lizards and reptiles include the following: Squamata, Crocodilia, Chelonia, Rhynchocephalia, and the lizard, snake, and worm-lizard clade [1, 2].
The most diversity and the best skull movement can be seen in Squamaes compared to other groups. The only species that exhibits this unusual skull movement is the tuatara, which is almost extinct and only found on a few islands in New Zealand. A prominent parietal eye is present on top of the head of this reptile, which also possesses a non-joined skull, slow growth, and slow reproduction [3, 4].
Gecko is one of the lizards that belongs to the sub-order Gekkota, and prevails in warm environments all over the world [5]. It ranges from 1.6 cm to 60 cm in length, and it is unlike other lizards, because it is nocturnal and has huge potential for attachment. Geckos appear in multiple colors and shapes, and are one of the most common lizards in nature [6]. This genus includes approximately 20 species spread in Africa, the Middle East, and the Canary Islands, but it has few species in Europe and Equatorial America [7].
The gecko lizard (Hemidactylus frenatus), which is found in the family Gekkonidae, belongs there (infra-order Gekkota). It is regularly observed in several Asian countries, including Iraq, and is thought to have started in Southeast Asia. Even though “House Lizard” is a more precise name for this animal, its common name is “Asian House Gecko”. The Asian house gecko is an invading lizard that has thrived. It was first discovered in Asia and the Indo-Pacific region, and it has since spread to many tropical and subtropical areas across the world. It frequently develops itself in these situations due to its excellent adaptation to human settlements and heavily altered ecosystems [8, 9]. Hemidactylus frenatus is characterized by being nocturnal activity and has great ability to climb. It is widely found in human homes and feeds on insects, spiders and worms [7].
Reptiles represent the first amniotes. From stem reptiles, extant reptiles, birds and mammals have evolved. The early reptilian integument had to adapt to the challenges of terrestrial life, developing a multi-layered stratum corneum capable of barrier function and ultraviolet protection. For better mechanical protection, diverse reptilian scale types have evolved. The evolution of endothermy has driven the convergent evolution of hair and feather follicles: both form multiple localized growth units with stem cells and transient amplifying cells protected in the proximal follicle. This topological arrangement allows them to elongate, molt and regenerate without structural constraints. Another unique feature of reptile skin is the exquisite arrangement of scales and pigment patterns, making them testable models for mechanisms of pattern formation. Since they face the constant threat of damage on land, different strategies were developed to accommodate skin homeostasis and regeneration. Temporally, they can be under continuous renewal or sloughing cycles. Spatially, they can be diffuse or form discrete localized growth units (follicles) [2, 10-12].
Reptiles' skin is composed of a dermis on the inside and a typical epidermis on the surface. The epidermis is divided into stratum germinativum, which is germinative, and stratum corneum, which is keratinized. The stratum germinativum is composed of a single or double layer of epithelial cells above the basement membrane. According to Rödder et al. [13] and Szydowski et al. [14], the dermis is composed of chromatophores, connective tissue cells such fibrocytes, collagen fibers, and fibroblasts, which are located in the extracellular matrix.
This layer, which also prevents water loss from the epidermis distinguishes their skin from other people's skin in a significant way. This characteristic shows their greater commitment to terrestrial life. Even if there are scales, they fundamentally differ from fish dermal scales. Reptiles' scales are a permanent part of their skin, unlike fish, which can have their scales removed. The structural support of the bony understructure of the reptile scale is typically not greatly aided by the dermis. A surface epidermal scale is a fold in the epidermis. The articulation between adjacent epidermal scales serves as the flexible hinge of the epidermis [10, 15].
The term “scutes” refers to large, oblong epidermal scales that resemble plates. Some species' epidermal scales may overgrow, and numerous skin protrusions, including sensory receptors, plastron, micro ornamentation, spines, crests, scutes, horn-like processes, pits, and carapace, may form in various body regions. With no dermal involvement, these protrusions are primarily epidermal in origin. The stratum basale (germinativum), stratum granulosum, and stratum corneum, which are the three major layers of the epidermis in its optimal resting phase, are made up of a basal layer of living keratinocytes and four layers of dead yet fully differentiated keratinocytes [2, 16].
The dermis in reptiles is composed of lymphatic and blood vessels, pigmentary cells, nerves, and fibrous connective tissue. The subcutaneous layer is a tissue layer located beneath the dermis that primarily consists of macrophages, fat cells, and fibroblasts (hypoderm, hypodermis, and subcutis). Reptiles frequently have a weaker form of subcutaneous fat than mammals do. Although certain species of snakes and lizards have paired abdominal ‘fat bodies” (corpora adiposa) that serve as the central place for the storage of fat in adipose tissue, other snakes and lizards do not have this structure [17].
The Mediterranean house gecko, or Hemidactylus turcicus, is one illustration of this. It is distinguished by having enormous subcutaneous fat pads. A considerable deposit of subcutaneous tissue may also be present in the tail, particularly in the case of geckos [18].
The more common overlapping variants of gecko scales are only noticeable on the ventral surface and the animal's foot. Modified tuberculate scales are virtually always present. There are numerous color variations for gecko scales. In spite of this, the pattern of keratinization on the exterior of the scales is constant throughout [19, 20].
The present study was conducted with the aim of histomorphological examination of the dorsal skin of Hemidactylus farnatus in Iraq.
Materials and Methods
In this exprimental study, 10 Hemidactylus frenatus (Asian House Gecko) were examined, which were gathered from various parts of the Najaf Province, Iraq. They were fixed by adhesive and were killed by chloroform. The length of the animal's body, including the tail, was between 3cm and 5cm.
Histo-morphological examination
A light microscopic analysis of the skin was done. In the following 48 hours, 10% formalin was used to heal the dorsal skin. Another histological technique was carried out. At first, the tissues were washed by tap water, then dehydrated with graduated alcohol concentrations (50%, 70%, 90%, and 100%). After that, the tissues were cleared with xylol, embedded in paraffin wax, and a paraffin block was cut with a rotary microtome using Mayer's albumin at a thickness of 5 micrometres. In the next stage, the sections were stained with hematoxylin and eosin (H & E) to adhere to the glass slides. Sections were mounted with DPX, covered with a cover slip. Then, they were examined with a light microscope at magnifications between 10x and 40x and took pictures with a camera [21].
Findings
The epidermis and dermis of the skin were examined in vertical sections of the skin of the Asian House Gecko (Hemidactylus frenatus), a species of reptile. In comparison to the ventral region, the dorsal region's skin was more keratinized and rougher (Figure 1).
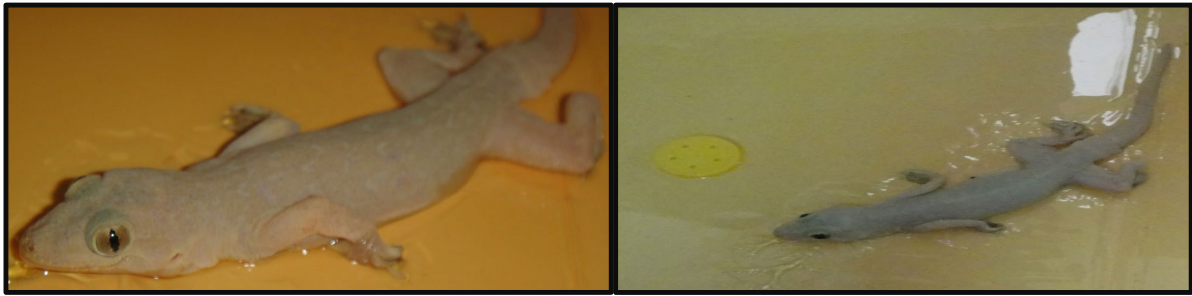
Figure 1) An adult Common House Gecko (Hemidactylus frenatus)
Hemidactylus frenatus had a thick epidermis made of several cell layers. The base of the epidermis, also known as the basal layer or stratum basale, was made up of cells with oval nuclei. A single layer of rounded-nucleated polyhedral cells was produced from these cells (stratum granulosum). The stratum granulosum cells were capable of undergoing a process that resulted in them flattening and changing into squamous cells, which had oval-shaped nuclei and eventually perished to produce the keratin layer (Figure 2). Small hocks and dome-shaped epidermal scales were developed on the dorsal surface of the animal, which was covered in a thin layer of keratinized skin (Figure 2).
Fibrous connective tissue, pigmentary cells, and blood vessels made up the dermal layer of the dorsal in H. frenatus along the length of the entire body (Figure 2).
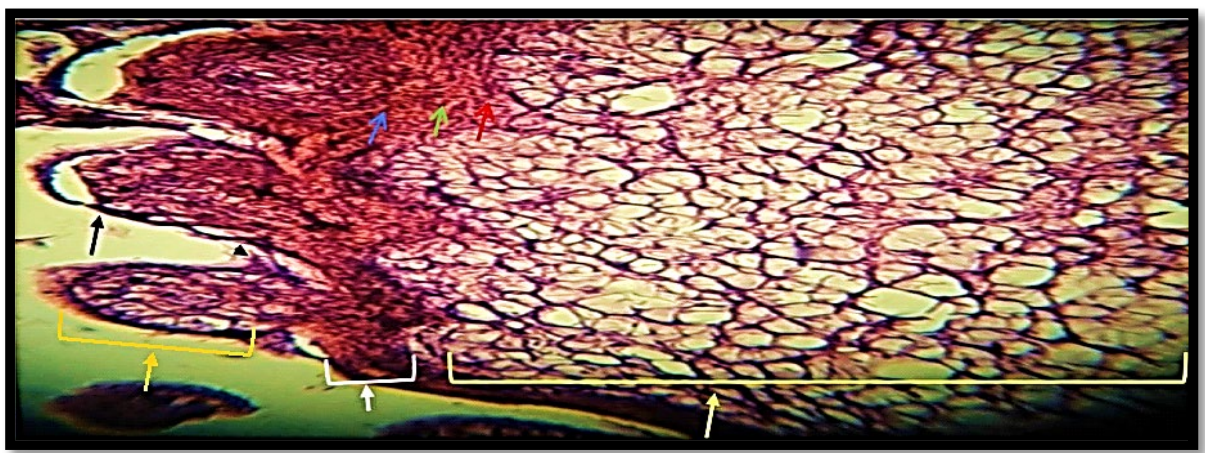
Figure 2) Dorsal surface of the H. frenatus, 100x, 400x. Dome-like shaped scale (arrow), inter-scale hinge (arrowhead), epidermal layer (white), stratum basale (red head), stratum granulosum (green arrow), stratum corneum (blue arrow), scale (orange arrow), and dermal layer (yellow arrow)
Melanophores and iridophores were visible as one layer of alternately arranged cells after H&E staining, located just below the basement membrane. The dermal layer of the dorsal skin had several copious melanophores with multi-dendritic structures. The dermis, which was located close to the iridophores, was filled with dark brown cells that were sometimes orientated along the basement membrane of the epidermis. These cells were the melanophores. The foundation membrane that the epidermis was situated next to was where the melanophores were positioned in other places (Figure 3, A, B, C, D, E, and F).
Xanthophores were more numerous than iridophores, which are light-reflecting cells, in H. frenatus (Figure 3, A, B, C and D). The melanophores, xanthophores, and iridophores types of chromatophores cells do not combine to form a functional “chromatophore unit”.
The dermis contains chromophores, cells of the extracellular matrix such as fibroblasts and fibrocytes, and collagen fibres (Figure 3, A, B, D, G and I). The orientation of collagen fibre bundles varies. The subcutaneous layer, which is situated beneath the dermis, is primarily composed of adipose cells and other cells (Figure 3, I).
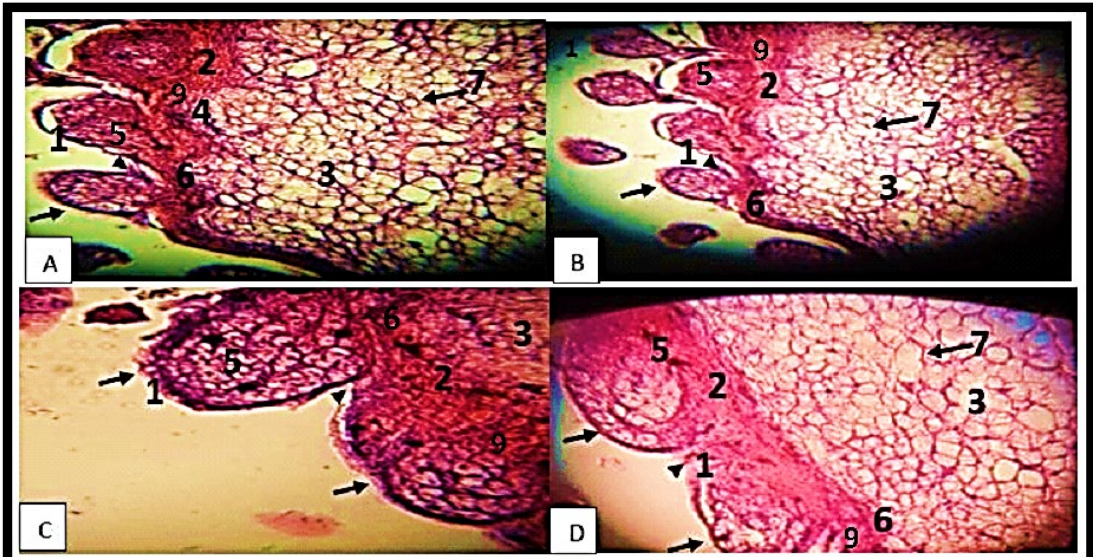
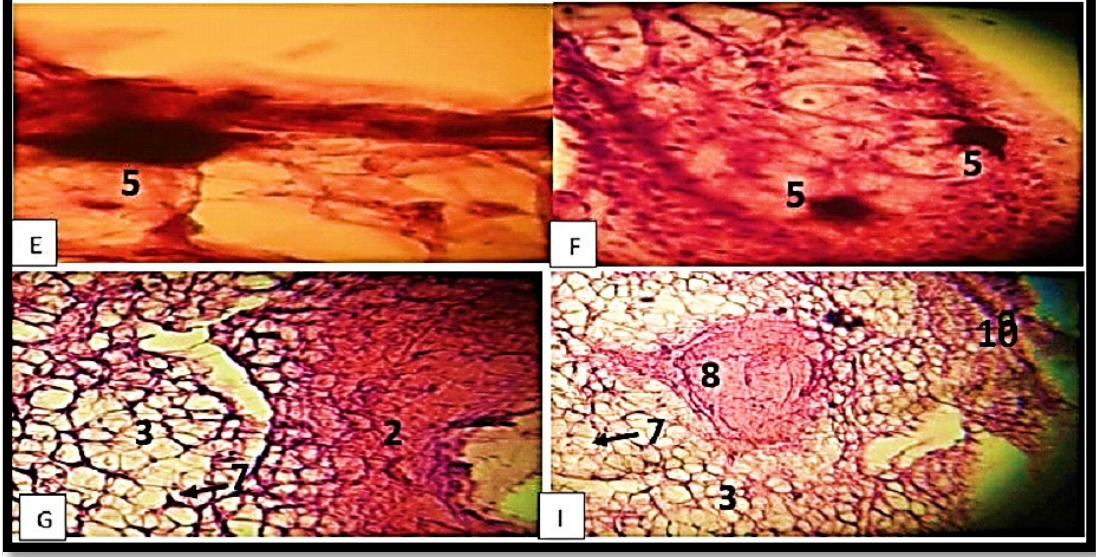
Figure 3) Dorsal surface of the H. frenatus, 100 x, 400 x. Dome-like shaped scale(arrow), inter-scale hinge (arrowhead), epidermal scale (1), epidermal layer (2), dermal layer (3), domelike shaped dermal (4), melanophores (5), iridophores (6), collagen fibers in dermis (7), blood vessel (8), Xanthophores (9) and Subcutaneous layer (10) (Haematoxylin and Eosin stain).
Discussion
The main line of defense against infection for the body is the skin. Reptiles have special characteristics that enable them to exist in harsh terrestrial habitats, despite the fact that it retains various functional tasks in avian and mammalian creatures. The skin of H. frenatus has been altered in a number of ways to help it withstand the harsh earthly circumstances, including mechanical barriers that kept moisture in and the sun out [2, 22].
H. frenatus is a nocturnal active species that can climb trees, rocks, and other vertical surfaces. It uses a "sit and wait" strategy to gather insects for sustenance. H. frenatus has an interior dermis and an exterior epidermis, which are both seen in the skin of other reptiles. H. frenatus possesses soft keratinized layers that cover its thin, readily visible epidermal layers. These findings concurred with those of Kardong [23] and Alibardi [24], who discovered that the firmness of a lizard's scales was provided by an epidermis that could be either thick or thin on the outside of the animal.
H. frenatus developes its tough skin as it shelteres under rocks to evade predators. Moreover, H. frenatus's scales are non-overlapping and dome-shape along the entire length of the body. During food-based mobility, the arrangement of species' epidermal scales is helpful [14, 25]. There are many different complicated skin coloration patterns in different vertebrate species. The distribution of skin pigments has a significant impact on how a species will eventually be colored [26, 27].
Szydłowski et al.'s study described the relationship between the arrangement of dermal chromatophores in tokay gecko (Gekko gecko) skin and the formation of wild-type colouration, with emphasis on the ultrastructure of chromatophores. This study revealed that orange/red coloured skin of G. gecko containes erythrophores, which are located under basement membrane, and usually comprise deeper situated iridophores and melanophores which are form single layer with iridophores or are occupying the deepest region of dermis. In orange/red coloured skin, erythrophores were the predominant chromatophores. However, in blue areas, these cells occurred in small numbers or were not noticed at all. In blue pigmented areas, iridophores and melanophores were predominated. Iridophores were found just under basement membrane, but this superficial location of iridophores occurred only in areas without erythrophores. Distribution of erythrophores, melanophores, and iridophores determines the characteristic blue colour of the tokay gecko skin with orange/red dots on the whole body [14].
H. frenatus has a dermis made up of both adipose and collagenous tissue. Both water evaporation and UV radiation are blocked by the collagenous tissue. The adipose tissue in the skin of H. frenatus serves as a heat reservoir for the creature, which is most active at night [14, 25].
Three different types of chromatophores were discovered in the skin of H. frenatus, including melanophores, xanthophores, and iridophores. The findings of Bauer et al. [28] and Bagnara and Matsumoto [29] are similar to other species of reptiles and amphibians.
Bauer et al. investigated the mechanical properties of gekkonid lizard skin for the first time. Although the skin of certain geckos, such as Gekko gecko, behaves in typical vertebrate fashion, that of others, such as Ailuronyx seychellensis, exhibits unusual properties associated with identifiable morphological specializations. Light and scanning electron microscopy reveal that Ailuronyx dermis is functionally bilayered; the stratum compactum is divided into inner and outer layers by intervening loose connective tissue. The inner layer is strong and tough and does not differ significantly in its properties from the full Gekko gecko skin. However, the much thicker outer layer is only 1/20 as strong and 1/50 as tough as the inner layer and exhibits preformed zones of weakness. In nature, Ailuronyx has considerable portions of the outer components of the skin as an antipredator escape mechanism. Skin samples from 17 additional gecko species varied considerably in strength, stiffness, and toughness. None of the species with tough skin employs regional integumentary loss as a predator escape strategy. Weak skin alone is not sufficient to permit regional integumentary loss, as the capability to lose the skin involves not only inherent properties of the tissue, but also features of the mechanical interaction of skin layers with one another and with the underlying body wall [28].
Three different chromatophores, including melanophores, xanthophores, and iridophores, were found in the reported research to contribute to the H. frenatus skin's natural hue. The findings of Szydowski et al. [14], who discovered that the Gekko gecko's natural coloration contains four types of chromatophores with erythrophores, are distinct from this. It agrees with Szydowski et al. [14] and Dong et al. [30]. who found that the arrangement of the skin cells on H. frenatus does not create a "chromatophore unit", like some other reptiles and amphibians.
According to Saber et al. [31], the skin of H. frenatus has less iridophores, which are larger, blackish brown, and distributed inside the dermis beneath them, than other lizards like A. boskianus.
There were some iridophores or light-reflecting cells in H. frenatus, although they were few. This concurs with Saber et al.'s [31] and Broeckhoven et al.'s [32] theory that this form of pigmentation may reflect the ground's rays when some lizards are out throughout the day searching for food. A species of gecko known as H. frenatus is active both during the day and at night.
It is primarily active at night and requires its xanthophores, which reflect energy from the ground, to function (light-absorbing pigment cells). In accordance with the findings of Paray and Al- Sadoon [33] and Smith and Habersetzer [34], Xanthophores, pigments that absorb light, were more prevalent in H. frenatus.
Conclusion
In Hemidactylus frenatus, the epidermis is covered in overlapping, flattened scales all over the body. The epidermis, dermis, and subcutaneous layer make up the majority of the dorsal skin's three layers. In the basal layer of the epidermis, massive, dendritic black melanophores are observed. The horizontally arranged brownish granule-containing iridophores are adjacent to the basement membrane of the epidermis.
Acknowledgements: Nothing has been reported.
Ethical Permission: Nothing has been reported.
Conflict of Interests: Nothing has been reported.
Authors’ Contribution: Al Kaabi ZSM (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer (100%)
Funding: Nothing has been reported.
The most diversity and the best skull movement can be seen in Squamaes compared to other groups. The only species that exhibits this unusual skull movement is the tuatara, which is almost extinct and only found on a few islands in New Zealand. A prominent parietal eye is present on top of the head of this reptile, which also possesses a non-joined skull, slow growth, and slow reproduction [3, 4].
Gecko is one of the lizards that belongs to the sub-order Gekkota, and prevails in warm environments all over the world [5]. It ranges from 1.6 cm to 60 cm in length, and it is unlike other lizards, because it is nocturnal and has huge potential for attachment. Geckos appear in multiple colors and shapes, and are one of the most common lizards in nature [6]. This genus includes approximately 20 species spread in Africa, the Middle East, and the Canary Islands, but it has few species in Europe and Equatorial America [7].
The gecko lizard (Hemidactylus frenatus), which is found in the family Gekkonidae, belongs there (infra-order Gekkota). It is regularly observed in several Asian countries, including Iraq, and is thought to have started in Southeast Asia. Even though “House Lizard” is a more precise name for this animal, its common name is “Asian House Gecko”. The Asian house gecko is an invading lizard that has thrived. It was first discovered in Asia and the Indo-Pacific region, and it has since spread to many tropical and subtropical areas across the world. It frequently develops itself in these situations due to its excellent adaptation to human settlements and heavily altered ecosystems [8, 9]. Hemidactylus frenatus is characterized by being nocturnal activity and has great ability to climb. It is widely found in human homes and feeds on insects, spiders and worms [7].
Reptiles represent the first amniotes. From stem reptiles, extant reptiles, birds and mammals have evolved. The early reptilian integument had to adapt to the challenges of terrestrial life, developing a multi-layered stratum corneum capable of barrier function and ultraviolet protection. For better mechanical protection, diverse reptilian scale types have evolved. The evolution of endothermy has driven the convergent evolution of hair and feather follicles: both form multiple localized growth units with stem cells and transient amplifying cells protected in the proximal follicle. This topological arrangement allows them to elongate, molt and regenerate without structural constraints. Another unique feature of reptile skin is the exquisite arrangement of scales and pigment patterns, making them testable models for mechanisms of pattern formation. Since they face the constant threat of damage on land, different strategies were developed to accommodate skin homeostasis and regeneration. Temporally, they can be under continuous renewal or sloughing cycles. Spatially, they can be diffuse or form discrete localized growth units (follicles) [2, 10-12].
Reptiles' skin is composed of a dermis on the inside and a typical epidermis on the surface. The epidermis is divided into stratum germinativum, which is germinative, and stratum corneum, which is keratinized. The stratum germinativum is composed of a single or double layer of epithelial cells above the basement membrane. According to Rödder et al. [13] and Szydowski et al. [14], the dermis is composed of chromatophores, connective tissue cells such fibrocytes, collagen fibers, and fibroblasts, which are located in the extracellular matrix.
This layer, which also prevents water loss from the epidermis distinguishes their skin from other people's skin in a significant way. This characteristic shows their greater commitment to terrestrial life. Even if there are scales, they fundamentally differ from fish dermal scales. Reptiles' scales are a permanent part of their skin, unlike fish, which can have their scales removed. The structural support of the bony understructure of the reptile scale is typically not greatly aided by the dermis. A surface epidermal scale is a fold in the epidermis. The articulation between adjacent epidermal scales serves as the flexible hinge of the epidermis [10, 15].
The term “scutes” refers to large, oblong epidermal scales that resemble plates. Some species' epidermal scales may overgrow, and numerous skin protrusions, including sensory receptors, plastron, micro ornamentation, spines, crests, scutes, horn-like processes, pits, and carapace, may form in various body regions. With no dermal involvement, these protrusions are primarily epidermal in origin. The stratum basale (germinativum), stratum granulosum, and stratum corneum, which are the three major layers of the epidermis in its optimal resting phase, are made up of a basal layer of living keratinocytes and four layers of dead yet fully differentiated keratinocytes [2, 16].
The dermis in reptiles is composed of lymphatic and blood vessels, pigmentary cells, nerves, and fibrous connective tissue. The subcutaneous layer is a tissue layer located beneath the dermis that primarily consists of macrophages, fat cells, and fibroblasts (hypoderm, hypodermis, and subcutis). Reptiles frequently have a weaker form of subcutaneous fat than mammals do. Although certain species of snakes and lizards have paired abdominal ‘fat bodies” (corpora adiposa) that serve as the central place for the storage of fat in adipose tissue, other snakes and lizards do not have this structure [17].
The Mediterranean house gecko, or Hemidactylus turcicus, is one illustration of this. It is distinguished by having enormous subcutaneous fat pads. A considerable deposit of subcutaneous tissue may also be present in the tail, particularly in the case of geckos [18].
The more common overlapping variants of gecko scales are only noticeable on the ventral surface and the animal's foot. Modified tuberculate scales are virtually always present. There are numerous color variations for gecko scales. In spite of this, the pattern of keratinization on the exterior of the scales is constant throughout [19, 20].
The present study was conducted with the aim of histomorphological examination of the dorsal skin of Hemidactylus farnatus in Iraq.
Materials and Methods
In this exprimental study, 10 Hemidactylus frenatus (Asian House Gecko) were examined, which were gathered from various parts of the Najaf Province, Iraq. They were fixed by adhesive and were killed by chloroform. The length of the animal's body, including the tail, was between 3cm and 5cm.
Histo-morphological examination
A light microscopic analysis of the skin was done. In the following 48 hours, 10% formalin was used to heal the dorsal skin. Another histological technique was carried out. At first, the tissues were washed by tap water, then dehydrated with graduated alcohol concentrations (50%, 70%, 90%, and 100%). After that, the tissues were cleared with xylol, embedded in paraffin wax, and a paraffin block was cut with a rotary microtome using Mayer's albumin at a thickness of 5 micrometres. In the next stage, the sections were stained with hematoxylin and eosin (H & E) to adhere to the glass slides. Sections were mounted with DPX, covered with a cover slip. Then, they were examined with a light microscope at magnifications between 10x and 40x and took pictures with a camera [21].
Findings
The epidermis and dermis of the skin were examined in vertical sections of the skin of the Asian House Gecko (Hemidactylus frenatus), a species of reptile. In comparison to the ventral region, the dorsal region's skin was more keratinized and rougher (Figure 1).

Figure 1) An adult Common House Gecko (Hemidactylus frenatus)
Hemidactylus frenatus had a thick epidermis made of several cell layers. The base of the epidermis, also known as the basal layer or stratum basale, was made up of cells with oval nuclei. A single layer of rounded-nucleated polyhedral cells was produced from these cells (stratum granulosum). The stratum granulosum cells were capable of undergoing a process that resulted in them flattening and changing into squamous cells, which had oval-shaped nuclei and eventually perished to produce the keratin layer (Figure 2). Small hocks and dome-shaped epidermal scales were developed on the dorsal surface of the animal, which was covered in a thin layer of keratinized skin (Figure 2).
Fibrous connective tissue, pigmentary cells, and blood vessels made up the dermal layer of the dorsal in H. frenatus along the length of the entire body (Figure 2).

Figure 2) Dorsal surface of the H. frenatus, 100x, 400x. Dome-like shaped scale (arrow), inter-scale hinge (arrowhead), epidermal layer (white), stratum basale (red head), stratum granulosum (green arrow), stratum corneum (blue arrow), scale (orange arrow), and dermal layer (yellow arrow)
Melanophores and iridophores were visible as one layer of alternately arranged cells after H&E staining, located just below the basement membrane. The dermal layer of the dorsal skin had several copious melanophores with multi-dendritic structures. The dermis, which was located close to the iridophores, was filled with dark brown cells that were sometimes orientated along the basement membrane of the epidermis. These cells were the melanophores. The foundation membrane that the epidermis was situated next to was where the melanophores were positioned in other places (Figure 3, A, B, C, D, E, and F).
Xanthophores were more numerous than iridophores, which are light-reflecting cells, in H. frenatus (Figure 3, A, B, C and D). The melanophores, xanthophores, and iridophores types of chromatophores cells do not combine to form a functional “chromatophore unit”.
The dermis contains chromophores, cells of the extracellular matrix such as fibroblasts and fibrocytes, and collagen fibres (Figure 3, A, B, D, G and I). The orientation of collagen fibre bundles varies. The subcutaneous layer, which is situated beneath the dermis, is primarily composed of adipose cells and other cells (Figure 3, I).


Figure 3) Dorsal surface of the H. frenatus, 100 x, 400 x. Dome-like shaped scale(arrow), inter-scale hinge (arrowhead), epidermal scale (1), epidermal layer (2), dermal layer (3), domelike shaped dermal (4), melanophores (5), iridophores (6), collagen fibers in dermis (7), blood vessel (8), Xanthophores (9) and Subcutaneous layer (10) (Haematoxylin and Eosin stain).
Discussion
The main line of defense against infection for the body is the skin. Reptiles have special characteristics that enable them to exist in harsh terrestrial habitats, despite the fact that it retains various functional tasks in avian and mammalian creatures. The skin of H. frenatus has been altered in a number of ways to help it withstand the harsh earthly circumstances, including mechanical barriers that kept moisture in and the sun out [2, 22].
H. frenatus is a nocturnal active species that can climb trees, rocks, and other vertical surfaces. It uses a "sit and wait" strategy to gather insects for sustenance. H. frenatus has an interior dermis and an exterior epidermis, which are both seen in the skin of other reptiles. H. frenatus possesses soft keratinized layers that cover its thin, readily visible epidermal layers. These findings concurred with those of Kardong [23] and Alibardi [24], who discovered that the firmness of a lizard's scales was provided by an epidermis that could be either thick or thin on the outside of the animal.
H. frenatus developes its tough skin as it shelteres under rocks to evade predators. Moreover, H. frenatus's scales are non-overlapping and dome-shape along the entire length of the body. During food-based mobility, the arrangement of species' epidermal scales is helpful [14, 25]. There are many different complicated skin coloration patterns in different vertebrate species. The distribution of skin pigments has a significant impact on how a species will eventually be colored [26, 27].
Szydłowski et al.'s study described the relationship between the arrangement of dermal chromatophores in tokay gecko (Gekko gecko) skin and the formation of wild-type colouration, with emphasis on the ultrastructure of chromatophores. This study revealed that orange/red coloured skin of G. gecko containes erythrophores, which are located under basement membrane, and usually comprise deeper situated iridophores and melanophores which are form single layer with iridophores or are occupying the deepest region of dermis. In orange/red coloured skin, erythrophores were the predominant chromatophores. However, in blue areas, these cells occurred in small numbers or were not noticed at all. In blue pigmented areas, iridophores and melanophores were predominated. Iridophores were found just under basement membrane, but this superficial location of iridophores occurred only in areas without erythrophores. Distribution of erythrophores, melanophores, and iridophores determines the characteristic blue colour of the tokay gecko skin with orange/red dots on the whole body [14].
H. frenatus has a dermis made up of both adipose and collagenous tissue. Both water evaporation and UV radiation are blocked by the collagenous tissue. The adipose tissue in the skin of H. frenatus serves as a heat reservoir for the creature, which is most active at night [14, 25].
Three different types of chromatophores were discovered in the skin of H. frenatus, including melanophores, xanthophores, and iridophores. The findings of Bauer et al. [28] and Bagnara and Matsumoto [29] are similar to other species of reptiles and amphibians.
Bauer et al. investigated the mechanical properties of gekkonid lizard skin for the first time. Although the skin of certain geckos, such as Gekko gecko, behaves in typical vertebrate fashion, that of others, such as Ailuronyx seychellensis, exhibits unusual properties associated with identifiable morphological specializations. Light and scanning electron microscopy reveal that Ailuronyx dermis is functionally bilayered; the stratum compactum is divided into inner and outer layers by intervening loose connective tissue. The inner layer is strong and tough and does not differ significantly in its properties from the full Gekko gecko skin. However, the much thicker outer layer is only 1/20 as strong and 1/50 as tough as the inner layer and exhibits preformed zones of weakness. In nature, Ailuronyx has considerable portions of the outer components of the skin as an antipredator escape mechanism. Skin samples from 17 additional gecko species varied considerably in strength, stiffness, and toughness. None of the species with tough skin employs regional integumentary loss as a predator escape strategy. Weak skin alone is not sufficient to permit regional integumentary loss, as the capability to lose the skin involves not only inherent properties of the tissue, but also features of the mechanical interaction of skin layers with one another and with the underlying body wall [28].
Three different chromatophores, including melanophores, xanthophores, and iridophores, were found in the reported research to contribute to the H. frenatus skin's natural hue. The findings of Szydowski et al. [14], who discovered that the Gekko gecko's natural coloration contains four types of chromatophores with erythrophores, are distinct from this. It agrees with Szydowski et al. [14] and Dong et al. [30]. who found that the arrangement of the skin cells on H. frenatus does not create a "chromatophore unit", like some other reptiles and amphibians.
According to Saber et al. [31], the skin of H. frenatus has less iridophores, which are larger, blackish brown, and distributed inside the dermis beneath them, than other lizards like A. boskianus.
There were some iridophores or light-reflecting cells in H. frenatus, although they were few. This concurs with Saber et al.'s [31] and Broeckhoven et al.'s [32] theory that this form of pigmentation may reflect the ground's rays when some lizards are out throughout the day searching for food. A species of gecko known as H. frenatus is active both during the day and at night.
It is primarily active at night and requires its xanthophores, which reflect energy from the ground, to function (light-absorbing pigment cells). In accordance with the findings of Paray and Al- Sadoon [33] and Smith and Habersetzer [34], Xanthophores, pigments that absorb light, were more prevalent in H. frenatus.
Conclusion
In Hemidactylus frenatus, the epidermis is covered in overlapping, flattened scales all over the body. The epidermis, dermis, and subcutaneous layer make up the majority of the dorsal skin's three layers. In the basal layer of the epidermis, massive, dendritic black melanophores are observed. The horizontally arranged brownish granule-containing iridophores are adjacent to the basement membrane of the epidermis.
Acknowledgements: Nothing has been reported.
Ethical Permission: Nothing has been reported.
Conflict of Interests: Nothing has been reported.
Authors’ Contribution: Al Kaabi ZSM (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer (100%)
Funding: Nothing has been reported.
References
1. Canei J, Nonclercq D. Morphological study of the integument and corporal skeletal muscles of two psammophilous members of Scincidae (Scincus scincus and Eumeces schneideri). J Morphol. 2021;282(2):230-46. [Link] [DOI:10.1002/jmor.21298]
2. Chang C, Wu P, Baker RE, Maini PK, Alibardi L, Chuong CM. Reptile scale paradigm: Evo-Devo, pattern formation and regeneration. Int J Dev Biol. 2009;53(5-6):813-26. [Link] [DOI:10.1387/ijdb.072556cc]
3. Vitt LJ, Pianka ER, Cooper Jr WE, Schwenk K. History and the global ecology of squamate reptiles. Am Nat. 2003;162(1):44-60. [Link] [DOI:10.1086/375172]
4. Williams C, Kirby A, Marghoub A, Kéver L, Ostashevskaya‐Gohstand S, Bertazzo S, et al. A review of the osteoderms of lizards (Reptilia: Squamata). Biol Rev. 2022;97(1):1-19. [Link] [DOI:10.1111/brv.12788]
5. Borsuk-Bianlynicka M. Gobekko cretacicus gen. et sp. n., a new gekkonid lizard from the Cretaceous of the Gobi Desert. Acta Palaeontol Polonica. 1990;35(1-2):67-76. [Link]
6. Piper R. Extraordinary animals: an encyclopedia of curious and unusual animals. London: Greenwood Press; 2007. [Link]
7. Abbas AM, Habeeb IN. Prevalence of Parasites in House Gecko (Hemidactylus frenatus) in Al-Hilla City, Babylon Province. J Med Chem Sci. 2022;5(2):270-4. [Link]
8. Hoskin CJ. The invasion and potential impact of the Asian House Gecko (Hemidactylus frenatus) in Australia. Austral Ecol. 2011;36(3):240-51. [Link] [DOI:10.1111/j.1442-9993.2010.02143.x]
9. Joseph MR, Al Bshabshe A, Al-Hakami AM, Assiry MM, Mathew A, Hamid ME. Identification of Basidiobolus species from the common house gecko (Hemidactylus frenatus) and their association with isolates from human basidiobolomycosis. Afr J Microbiol Res. 2022;16(5):178-83. [Link] [DOI:10.5897/AJMR2022.9616]
10. Alibardi L. Adaptation to the land: the skin of reptiles in comparison to that of amphibians and endotherm amniotes. J Exp Zool B Mol Dev Evol. 2003;298(1):12-41. [Link] [DOI:10.1002/jez.b.24]
11. Kligman AM. What is the 'true' function of skin. Exp Dermatol. 2002;11(2):159-87. [Link]
12. Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, et al. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48(2-3):249-70. [Link] [DOI:10.1387/ijdb.15272390]
13. Rödder D, Solé M, Böhme W. Predicting the potential distributions of two alien invasive Housegeckos (Gekkonidae: Hemidactylus frenatus, Hemidactylus mabouia). North West J Zool. 2008;4(2):236-46. [Link]
14. Szydłowski P, Madej JP, Mazurkiewicz-Kania M. Histology and ultrastructure of the integumental chromatophores in tokay gecko (Gekko gecko) (Linnaeus, 1758) skin. Zoomorphology. 2017;136(2):233-40. [Link] [DOI:10.1007/s00435-017-0348-9]
15. Xue J, Marghoub A, Bertazzo S, Evans SE, Moazen M. Biomechanics of osteoderms in a lizard skull-a preliminary finite element study. In: Anatomical Society Winter Meeting; London, 2016, [Link]
16. Brisbane JLK, Dewynter M, Angin B, Questel K, van den Burg MP. Importation of ornamental plants facilitates establishment of the Common House Gecko, Hemidactylus frenatus Duméril & Bibron, in the Lesser Antilles. Caribbean Herpetol. 2021;77:1-5. [Link] [DOI:10.31611/ch.77]
17. Savage JM. The amphibians and reptiles of Costa Rica: a herpetofauna between two continents, between two seas. University of Chicago Press; 2002. [Link]
18. Rutland CS, Cigler P, Kubale V. Reptilian skin and its special histological structures. In Rutland CS, Kubale V, editors. Veterinary anatomy and physiology. IntechOpen.; 2019. [Link]
19. Lapwong Y, Dejtaradol A, Webb JK. Shifts in thermal preference of introduced Asian house geckos (Hemidactylus frenatus) in temperate regions of southeastern Australia. J Therm Biol. 2020;91:102625. [Link] [DOI:10.1016/j.jtherbio.2020.102625]
20. Maderson PFA. Keratinized epidermal derivatives as an aid to climbing in gekkonid lizards. Nature. 1964;203(4946):780-1. [Link] [DOI:10.1038/203780a0]
21. Suvarna KS, Layton C, Bancroft JD. Bancroft's theory and practice of histological techniques. Elsevier Health Sciences; 2018. [Link]
22. Nagumantri SP, Banu S, Idris MM. Transcriptomic and proteomic analysis of Hemidactylus frenatus during initial stages of tail regeneration. Sci Rep. 2021;11(1):3675. [Link] [DOI:10.1038/s41598-021-83283-0]
23. Kardong KV. Vertebrates: comparative anatomy, function, evolution. 2nd Edition. WCB McGraw-Hill; 1997. [Link]
24. Kardong KV. Vertebrates: comparative anatomy, function, evolution. 8th Edition. McGraw Hill; 2018. [Link]
25. Darwish ST. Comparative light and ultrastructural studies of skin in Stenodactylus petrii and Ptyodactylus guttatus (Reptilia: Gekkonidae). Egypt J Exp Biol. 2012;8(1):9-14. [Link]
26. Bagnara JT, Hadley ME. Chromatophores and color change. The comparative physiology of animal pigmentation. New Jersey: Prentice-Hall; 1973. [Link]
27. Broeckhoven C, Le Fras Nortier Mouton PL, Hui C. Proximatecauses of variation in dermal armour: insights from armadillo lizards. Oikos. 2018;127(10):1449-58. [Link] [DOI:10.1111/oik.05401]
28. Bauer AM, Russell AP. Shadwick RE. Mechanical properties and morphological correlates of fragile skin in gekkonid lizards. J Exp Biol. 1989;145(1):79-102. [Link] [DOI:10.1242/jeb.145.1.79]
29. Bagnara JT, Matsumoto J. Comparative anatomy and physiology of pigment cells in nonmammalian tissues. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, editors. The pigmentary system: Physiology and pathophysiology. 2nd Edition. Wiley: 2006. Pp: 11-59. [Link] [DOI:10.1002/9780470987100.ch2]
30. Dong LP, Wang Y, Evans SE. A new lizard (Reptilia: Squamata) fromthe Early Cretaceous Yixian Formation of China, with a taxonomic revision of Yabeinosaurus. Cretaceous Res. 2017;72:161-71. [Link] [DOI:10.1016/j.cretres.2016.12.017]
31. Saber SA, ElSalkh BA, Gadel-Rab AG, Mahmoud FA, El-Dahshan AA, Gewily DI. Comparative and functional study of integumentary system of two different reptiles: adaptation to their different modes of life. Egyp J Hosp Med. 2018;73(6):6802-11. [Link] [DOI:10.21608/ejhm.2018.16723]
32. Broeckhoven C, El Adak Y, Hui C, Van Damme R, Stankowich T. On dangerous ground: the evolution of body armour in cordyline lizards. Proc Biol Sci. 2018;285(1880):20180513. [Link] [DOI:10.1098/rspb.2018.0513]
33. Paray BA, Al-Sadoon MK. Ultrastructure of the dermal chromatophores in the Fringe-toed lizard, Acanthodactylus orientalis. Zoologia. 2017;34:1-7. [Link] [DOI:10.3897/zoologia.34.e11923]
34. Smith KT, Habersetzer J. The anatomy, phylogenetic relationships, and autecology of the carnivorous lizard "Saniwa" feisti Stritzke, 1983 from the Eocene of Messel, Germany. Comptes Rendus Palevol. 2021;20(23);441-506. [Link] [DOI:10.5852/cr-palevol2021v20a23]










