Volume 14, Issue 3 (2022)
Iran J War Public Health 2022, 14(3): 295-302 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/04/30 | Accepted: 2022/06/20 | Published: 2022/08/13
Received: 2022/04/30 | Accepted: 2022/06/20 | Published: 2022/08/13
How to cite this article
Abed Hussein B, Fawzi M, Al-Saray S, Abed Hussein R. High-Fat-Low-Carbohydrate Diet Applied to Mice: a Model for Studying Physiological and Histological Changes in the Liver and Pancreas. Iran J War Public Health 2022; 14 (3) : 7
URL: http://ijwph.ir/article-1-1190-en.html
URL: http://ijwph.ir/article-1-1190-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Sciences, College of Basic Education, University of Misan, Maysan, Iraq
2- Department of Pharmacy, AL-Manara College for Medical Sciences, Maysan, Iraq
2- Department of Pharmacy, AL-Manara College for Medical Sciences, Maysan, Iraq
Full-Text (HTML) (1104 Views)
Introduction
Obesity has been considered a major health issue worldwide. Many diseases such as heart disease, diabetes mellitus, hypertension, and polycystic ovary syndrome are related to obesity and these diseases are usually caused by an unhealthy lifestyle and dietary habits. In this context, dietary interventions that give rise to weight loss can help in decreasing the burden and severity of obesity. For instance, the intake of a high-fat-low-carbohydrate diet (HFLCD) was found to be effective in slimming [1].
The HFLCD is an efficient diet for reducing body weight, with increased satiety and diminishing energy consumption, efficacious to improve glycaemic control and reduce hyperinsulinemia in diabetes mellitus [2]. De & Mukhopadhyay explained the mechanisms that lead to weight loss on a high-fat diet: an increase in satiety leads to less energy intake without starvation and a metabolic advantage [3]. Ketogenesis reduces appetite [4]; the mechanism of appetite reduction is not clear; probably occurs as a result of stopping the secretion of the hunger hormone (ghrelin) [5]. HFLCD commonly contains 20% of carbohydrates, more than 50% of fat, and insignificant amounts of protein [6]. Low-carbohydrate diets restrict the intake of legumes, fruits, sugars, starchy foods, and whole grains, these food groups are the foundation of the globally recognized food pyramid that promotes long-term health [7]. A previous study contradicts this diet and indicates that an HFLCD for a long-time lead to metabolic syndrome and an increased risk for diabetes in humans [8]. Some slimming diets are regular with healthy eating recommendations, while others diets promote illegitimate and sometimes unsafe practices.
The body is deprived of carbohydrates during the application of HFLCD, which leads to a decrease in insulin secretion and enters the body into a catabolic state [9, 10], leading to the use of stored glycogen which is all used up and will be decreased or be exhausted. The body will force to use an alternative strategy to provide energy by transforming stored fats; this process leads to increase fatty acid oxidation and produces ketone bodies in the liver [11]. In this essence, the adult liver can produce up to 185 g of ketone bodies per day. Ketogenesis includes β-oxidation of fatty acids to acetyl CoA, formation of acetoacetyl CoA, conversion of acetoacetyl CoA to 3-hydroxy-3-methylglutaryl CoA and then to acetoacetate; and finally reduction of acetoacetate to 3-β-hydroxybutyrate. This process depends on triglyceride lipase, which is found in peripheral adipocytes, and acetyl CoA carboxylase and hydroxymethylglutaryl-CoA synthase, which are found in the liver [12].
The purpose of the present study was to investigate the positive and negative histopathological effects of HFLCD on the liver and pancreas, lipid profiles, and physiological parameters including blood glucose, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels.
Materials and Methods
This experimental research was conducted in 2021 on 8-week-old (30 male & 30 female) mice obtained from Al-Manara College for Medical Sciences and maintained at 24°C on a 12:12-h light-dark cycle, with cleaning and sterilization of cages 2-3 times a week. Animals were allowed access to feed and water. All mice were harbored in well-ventilated cages and sanitary conditions and fed on an essential diet for 3 weeks for acclimation as described previously.
-Diet-induced fatness: The 8-week-old mice (except the control group) were given access to water and a high-fat- high carb diet (40% fat, 25% protein, 5% sucrose, 20% vitamin, 5% soybean oil, and 5% maltodextrin (Profoods nutrition, Mumbai, Maharashtra)) for 10 weeks before the start of the experimental diet as described previously [13]. To enhance gaining weight, mice were given water containing 20% fructose (w/v) (Profoods nutrition, Mumbai, Maharashtra) in addition to the high-fat diet. After 12 weeks, mice on the diet gained approximately 10g of excess body weight compared with body weight before starting weight gain procedures and were considered to get diet-induced fatness.
-HFLCD and Feeding Protocols: The basal diet of the control group consisted of 20% protein (Profoods nutrition, Mumbai, Maharashtra), 15% local Iraqi barley, 15% local Iraqi groats, 15% local Iraqi flour, 25% local Iraqi corn groats, and 10% vitamins (Hansal, Germany). The HFLCD for the treatment group contained 75% local Iraqi fat, 10% protein, 5% carbohydrate (wt/wt (Profoods nutrition, Mumbai, Maharashtra)), 5% vitamin, and 5% mineral (BioTech USA, Jordan). Foodstuffs were selected according to Noakes & Windt [2] who provided a recommended menu on the “Banting” diet similar to (HFLCD).
The animals were divided into 6 main groups, each group containing 10 mice. Four groups were subjected to the HFLCD and two groups were considered as control groups.
-Group I (group of male mice; n=10) this group was fed for 3 months on the HFLCD (MG1);
-Group II (group of males; n=10) this group was fed for 6 months on the HFLCD (MG2);
-Group III (group of males; n=10) this group was known as a control group fed on the basal diet (MCG);
-Group IV (group of females) was fed for 3 months on the HFLCD (FG1);
-Group V (group of females) was fed for 6 months on the HFLC diet (FG2);
-Group VI (group of females) was considered as a control group fed on the basal diet (FCG).
Bodyweight, water, and food intake were measured every three days throughout the intervention until the end of the experiment. During the experiment period, the behavior of mice such as movement, feeding, and breeding was monitored. On the other hand, groups have been allowed to mate; each female gave 4-12 newborns.
-Blood & Tissue Examination: For sample collection, animals were sacrificed by intraperitoneal anesthetizing them with a mixture of xylazine (Interchemie, Netherlands) and ketamine (Interchemie, Netherlands) at a concentration of 10% (1:1v/v). The blood was collected in a serum gel tube (Henso, China); the serum was separated by a centrifuge (10 min at 6000 RPM) and transferred to serum tubes (Henso, China) for refrigeration until biochemical analysis was performed. Tissue samples were taken for histopathological analysis. Liver and pancreas tissues were fixed in 10% formalin (Scharlau, Spain) before being embedded in wax (paraffin; Wollenweber, Germany). Deparaffinization of tissue was pursued using xylene and re-hydrated through a series of alcohols. Paraffin-embedded tissue samples were sectioned using a microtome (Histoline MRS3, Milano-Italy) into slices about 5μm in thickness. Staining for tissue was performed using hematoxylin (Quallcaic, India) and eosin stains (SIRC, China) to show the basic structures of the tissue. The slide was prepared for permanent preservation using Canada balsam, before being covered. Tissue samples were examined under an optical microscope (Meij with Omax Camera, Japan) at 125x, and 500x power. For biochemical analysis, blood AST, ALT, cholesterol, triglycerides (TG), and glucose were measured. All examinations were performed by Cobas c 111 auto analyzers (Roche Diagnostics GmbH, Sandhofer Strasse 116, Mannheim, Germany), according to the manufacturer's instructions. Absorbance was measured at the wavelength of 340nm (ALT, AST, and glucose), and 512nm (cholesterol and triglycerides) by UV-Spectrophotometer (Biotech, Germany).
Statistical significance was expressed using a one-way analysis of variance (ANOVA test) followed by the Tukey test. Data were expressed as the means± SD. Significant differences were considered at p<0.05. All experimental data were analyzed using Statistical Package for the Social Science for Windows (IBM Corp., version 26.0. Armonk, NY, US).
Findings
Throughout the experiments, the behavior of all studied groups was seemingly normal and the nutrition diet did not affect the activities of mice. To investigate the impact of HFLCD on liver physiology, serum levels of ALT, and AST were measured, which were the main markers of the liver function test. Plasma profile analyses for all experimental groups of mice were shown in Tables 1 & 2; Diagrams 1 & 2. Results of serum liver enzymes showed a significant increase in the level of AST and ALT in the FG1 compared with that of the FG2 and the control groups, which showed the highest recorded mean values (p<0.05).
As for the serum lipid concentration, cholesterol levels showed a significant increase after applying the dietary intervention to mice for 3 months as compared with the control group and the group of FG2. Moreover, the findings showed a highly significant decrease in TG after feeding the mice on the HFLCD with P<0.01 if compared with the control group. As for the analysis of the cumulative sugar, it showed a significant decrease in both groups of mice to which diet was applied compared with the control group, which had a higher glucose level with a significant difference (p<0.05; Table 1).
Regarding the male groups, the results of the experiment showed a significant increase in AST and ALT levels in the group of MG1 when compared with the group of MG2 and the control (p<0.05; Table 2). Cholesterol levels increased after applying the diet to MG1 compared to the control group. In this line, we found a decrease in the group of mice applied to the diet for 6 months compared with the control group with a highly significant difference (p<0.01). The TG level showed a significant decrease in the serum of both groups compared with its level in the control group (p<0.05; Table 2).
Table 1) Plasma profile in female control mice and female mice fed an HFLCD
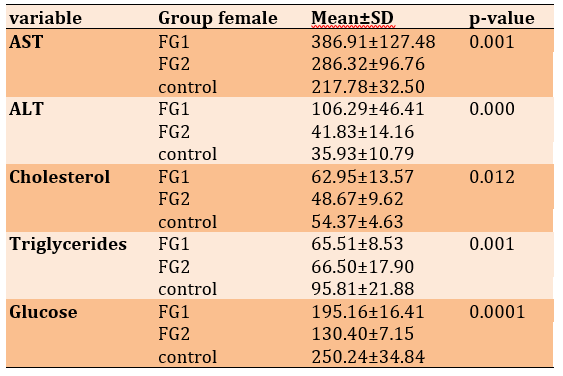
Table 2) Plasma profile in male control mice and male mice fed an HFLCD
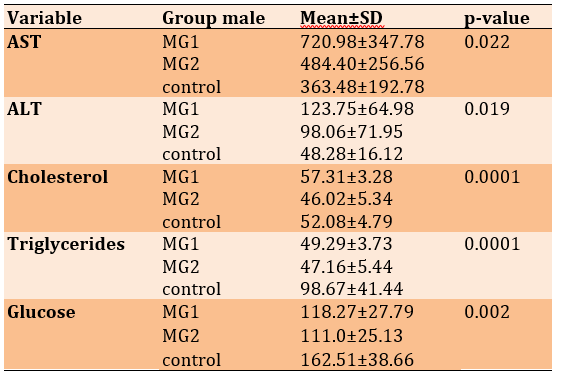
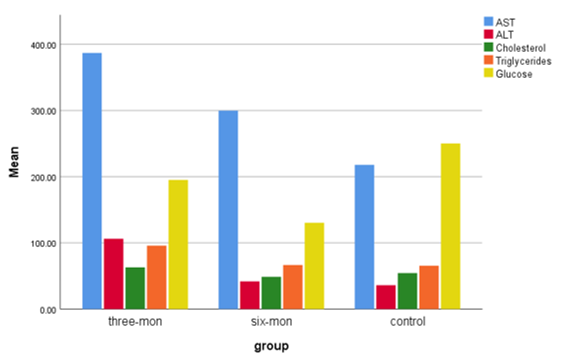
Diagram 1) showing the mean of AST, ALT, Cholesterol, Triglycerides, and Glucose of female control mice and female mice fed an HFLCD
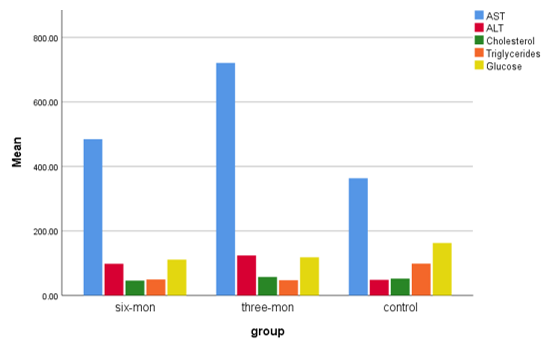
Diagram 2) Showing the mean of AST, ALT, Cholesterol, Triglycerides, and Glucose of male control mice and female mice fed an HFLCD
In control groups (Figures 1-A, 2-A & B), the histological structure of the liver and pancreas appeared with a normal systematic pattern of hepatocytes in the periportal area, normal pancreatic exocrine glands, and normal islands of Langerhans. The histological examination revealed the impact of HFLCD on liver tissue (Figure 3-A & B). The effect was more obvious on hepatocytes, where severe fatty changes were observed that led to edema of hepatocytes in the periportal area because of microvascular steatosis. After 6 months of feeding, the intensity of histological changes was detected, and appeared as degeneration of hepatocytes in the periportal area because of microvascular steatosis, bridging fibrosis between the centrilobular and periportal area (Figure 5-A & B). The results of the study showed that the application of the feeding regime upon mice had led to the presence of degenerated cells in the pancreatic exocrine glands with no change in the islands of Langerhans (Figure 4-A & B). In the group that fed the same system for 6 months, normal pancreatic exocrine glands and normal islands of Langerhans were shown (Figure 6).
The histological architecture of the liver in the group of females that fed the diet for 3 months showed microvascular steatosis (Figure 7-A), congestion of the central vein (Figure 7-B), and periportal inflammation (Figure 7-C). Histological examination of pancreatic tissue in mice with the diet for three- and 6 months showed normal histological structure as shown in (Figures 8 & 10), respectively. Hepatocytes of the female group that fed for 6 months contained a large lipid vacuole that filled the cell and dislodged its nuclei to the periphery, resulting in a degeneration of hepatocytes in the centrilobular area (Figure 9).
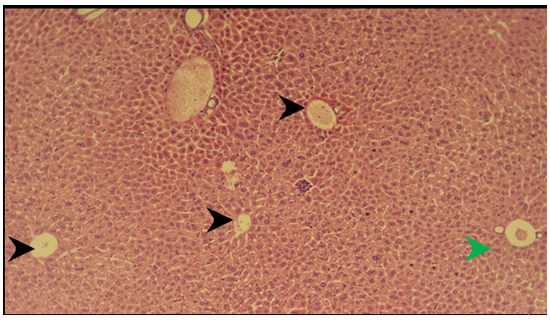
Figure 1) Section of liver of the control group showed normal hepatocytes in the centrilobular area (black arrow), normal hepatocytes in the periportal area (green arrow) H&E; 125X
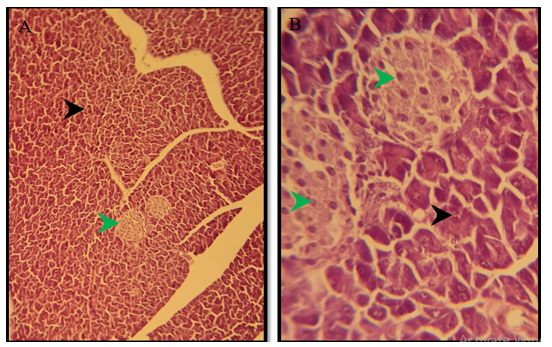
Figure 2) Section of the pancreas of the control group showed normal pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; A) 125X, and B) 500X
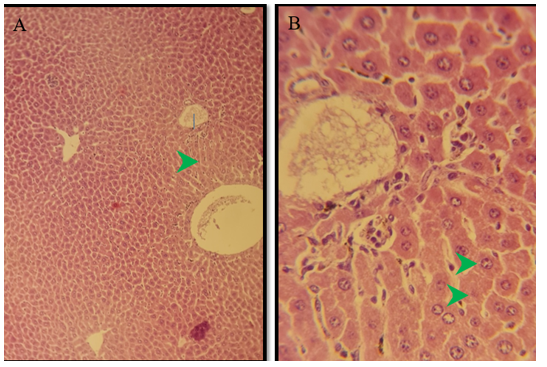
Figure 3) Section of liver of treated group of males after 3 months of treatment show swelling of hepatocytes in the periportal area (green arrow) because of microvascular steatosis H&E; 125X
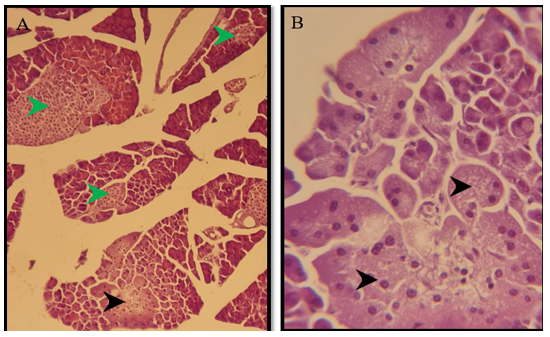
Figure 4) Section of the pancreas of the treated group of males after 3 months of treatment show degenerated cells in the pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; A) 125X, and B) 500X

Figure 5) Section of liver of treated group of males after 6 months of treatment showed marked degeneration of hepatocytes in the periportal area (green arrow) because of microvascular steatosis, bridging fibrosis between the centrilobular and periportal area (black arrow) H&E; A) 125X, and B) 500X, and C) 125X
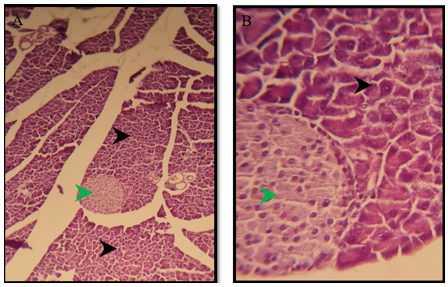
Figure 6) Section of a pancreas of the treated group of males after 6 months show normal pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; A) 125X, and B) 500X
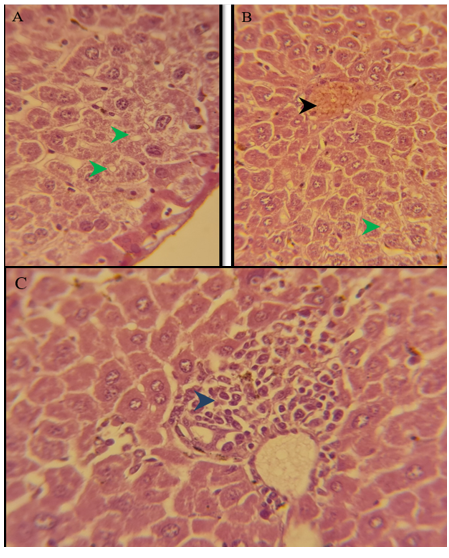
Figure 7) Section of liver of treated group of females after 3 months of treatment showed marked degeneration of hepatocytes in the periportal area (green arrow) because of microvascular steatosis, congestion of the central vein (black arrow), periportal inflammation (blue arrow) H&E; A) 125X, B) 500X, and C) 125X
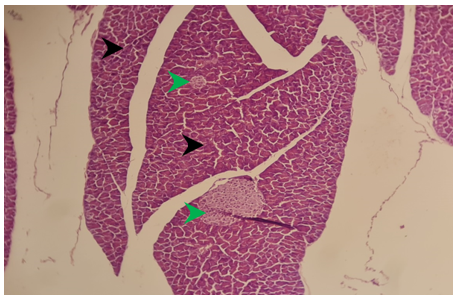
Figure 8) Section of the pancreas of the treated group of females after 3 months showed normal pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; 125X
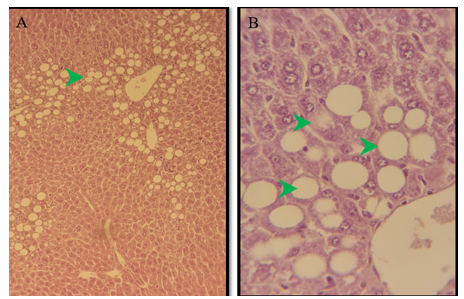
Figure 9) Section of the liver of the treated group of females after 6 months of treatment showed marked degeneration of hepatocytes in the centrilobular area (green arrow) because of macrovascular steatosis. H&E; A) 125X, and B) 500X
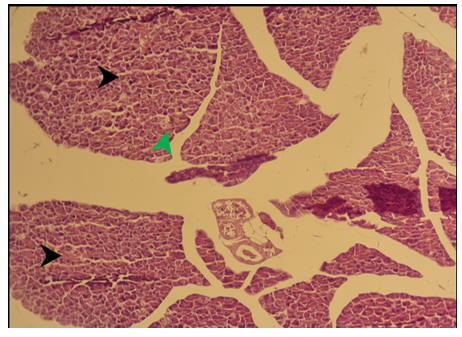
Figure 10) Section of the pancreas of the treated group of females after 6 months showed normal pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; 125X
Discussion
Dietary patterns that depend on the low level of carbohydrates and a high level of fat harm the body causing a deficit in energy intake, and it leads to a state called ketosis because of the lowering of carbohydrates. Ketosis is a physiological metabolic case; it allows the generation of energy from the fat of the liver to be utilized by other organs. The body uses glucose to provide energy, part of it is used as fuel in vital processes and the other part is stored as glycogen in the liver and muscles.
The intake of HFLCD by mice has led to weight loss in a few weeks, which is in agreement with previous studies conducted on rodents [14, 15]. In other studies, the ketogenic diet was reported to enhance elevated glucose levels, body weight, and hyperlipidemia [16, 17]. Intake of HFLCD for long-term has not resulted in weight loss in mice, but caloric restriction which raised insulin sensitivity, reduce glucose and increase ketone bodies, also restricting total energy intake and rectifying weight gain or hyperlipidemia [18]. In this regard, Rosenbaum et al. [19] showed that raised cholesterol, symptoms of inflammation, reduced triglycerides, and decreased insulin-mediated anti-lipolysis are correlated with HFLCD.
Extrapolation of the laboratory study to humans and studying the histopathological features resulting from the application of the diet is difficult, so mice were used as an experimental model for the development of obesity and the application of the diet to obese mice. After consuming the HFHCD for 12 weeks, the mice were weighed to confirm weight gain. The weights of mice before applying the HFHC diet were 27-30g for males and 20-24g for females. While, after applying the HFHCD, the weights ranged from 40-45g and 39-45g for males and females, respectively.
During the experiment period, sexual behavior in mice was observed; all mice were seen as sexually active. We spotted new births raising the number of males more than that of females. The total number of recent births was 114. The number of males was 74 while the number of females was 40. Contrasting results with our study have been reported; Samuel et al. [20] revealed that a high-fat diet causes apoptosis of testicular cells, which alters fertility in male mice.
In the present study, mice fed HFLCD showed signs of steatohepatitis and increased levels of AST and ALT. These findings correspond with the results of previous studies conducted on mice that observed metabolic changes as a result of feeding via a ketogenic diet 8, and this perhaps marks an early phase of non-alcoholic fatty liver disease. Another study showed that supplementation of methionine and choline with a KETO diet could reduce hepatic steatosis and the pro-inflammatory state of the liver [21]. Lipid aggregation in hepatocytes occurs by mechanisms that are not clearly understood but probably this is due to the overproduction of fatty acids, increased uptake of fatty acids, and decreased hepatic lipid metabolism [22]. An increase in ALT or AST activities is considered a marker of damage in hepatocytes with advanced stages of steatosis (fatty change) and cirrhosis (fibrosis). This rise is attributed to an abundance of free radicals that causes lipid peroxidation of the cell membrane, which leads to the loss of the cellular membrane and hepatocyte damage [23].
The level of cholesterol in the HFLCD group increased significantly when compared with the control. A previous study reported that serum levels of cholesterol increased in mice fed a ketogenic diet [24]. Another study reported that HFLCD causes hypercholesterolemia [25]. High cholesterol levels lead to an increase in the risk of liver fibrosis [26]. Results of some other studies, conducted on humans and rodents with a ketogenic diet, contradict the results of our study. Cholesterol levels are decreased or increased [27], or not affected by the diet in adults with a ketogenic diet. High levels of cholesterol contribute to heart disease by increasing the fat in the arteries, this leads to the narrowing of the arteries and the development of atherosclerosis. Atherosclerosis causes myocardial infarction and apoplexy [28]. The high level of cholesterol in the blood is due to an increased intake of saturated fats or the production of excessive ketones, which can be exploited in the process of energy production [29].
Decreasing serum triglycerides and glucose levels were detected in the HFLCD groups when compared to those of the control. This was in agreement with Yang et al. [30] who expressed that the KETO diet was effective in lowering blood glucose, improving glucose tolerance, and raising insulin sensitivity. Badman et al. [31] stated that the ketogenic diet gives rise to high plasma cholesterol levels whilst, that plasma TG levels were decreased; this finding is essentially consistent with the results of our study. A low TG value is probably a precocious marker of autoimmunity or immune system hyper-reactivity [32]. Reduced TG levels are perhaps the consequence of associated factors such as congestive heart failure, advanced states of congestion in the gastrointestinal system and liver, decreased food intake, increased cachexia, and increased levels of inflammation [33]. Low TG is probably a marker of liver damage due to diminished oxygen supply or increased levels of inflammation [34]. A recent study showed that an increased TG level was detected in children with epilepsy during the treatment classic ketogenic diet [35]. Hyperlipidemia is a known side effect of ketogenic dietary therapy [36]. Although, long-term application of the KETO diet can cause hepatic lipid accumulation and hepatic steatosis. According to the results of Zhang et al. [37], the KETO diet is considered a new and possible approach to the therapy of diabetes.
Histopathological results in mice fed the diet showed the appearance of lipid changes in hepatocytes, which led to edema of hepatocytes in the periportal area because of microvascular steatosis; this degeneration was more prominent in mice groups for 6 months of the diet. Appeared degeneration of hepatocytes in the periportal area is because by macrovascular and microvascular steatosis, as well as the formation of fibrous bridges between the centrilobular and periportal area causes, bridging fibrosis consider the main stage in the advancement of liver fibrosis. Hepatic histological results coincide with the biochemical analysis findings where the signs of deterioration in liver tissue appeared in H&E stained sections. The present results are in agreement with those of Garbow et al. [38] who found that mice fed HFLCD had hepatocellular damage, inflammatory response, severe hepatic steatosis, apoptosis, and autophagy. Macrovesicular steatosis is characterized by large fat globules aggregated in the cytoplasm of the hepatocytes. The nucleus of hepatocytes is pressed and dislocated into the rim of the cytoplasm by the fat vacuole. Microvesicular steatosis is used as a mark of rigorous hepatic dysfunction and is described via abundant tiny collections of lipid-laden macrophages and Lipid droplets in the hepatocytes. Macrovesicular and microvesicular steatosis may contribute to causing intense diseases like nonalcoholic steatohepatitis and fibrosis [39]. Although exocytosis of the exocrine pancreatic glands occurred in the 3-months-fed male, it did not appear in the other groups. This indicates that diet has no side effects on the tissues of the organ itself.
Conclusion
The present study has provided evidence for explaining the defects in the liver and pancreatic function caused by a high-fat-low-carb diet, and their associated pathological defects in mice models. The plasma profile of the HFLCD group pointed out that the diet is unhealthy. The plasma profile of the HFLCD group showed, that ALT, AST, and cholesterol were higher in the HFLCD group. However, the level of TG and glucose have decreased. The HFLCD group showed severe fatty changes that led to edema of hepatocytes in the periportal area and degeneration of hepatocytes in the periportal area because of microvascular steatosis, bridging fibrosis between the centrilobular and periportal area. Some limitations of the study need to be considered. Further long-term follow-up studies are required to validate our results. Moreover, more focus is needed on the effectiveness of the diet on the reproductive system, fertilization, and sex determination.
Acknowledgments: We thank all research participants who contributed to completing this search.
Ethical Permissions: Ethical Clearance: The project was approved by the local ethical committee in AL-Manara College for Medical Sciences.
Conflicts of Interests: None declared.
Authors’ Contributions: Abed Hussein B (First Author), Introduction Writer/Main Researcher/Statistical Analyst/Discussion Writer (50%); Fawzi M (Second Author), Methodologist/Assistant Researcher/Statistical Analyst/Discussion Writer (30%); Al-Saray ST (Third Author), Assistant Researcher (10%); Abed Hussein R (Forth Author), Assistant Researcher (10%)
Funding/Support: None declared.
Obesity has been considered a major health issue worldwide. Many diseases such as heart disease, diabetes mellitus, hypertension, and polycystic ovary syndrome are related to obesity and these diseases are usually caused by an unhealthy lifestyle and dietary habits. In this context, dietary interventions that give rise to weight loss can help in decreasing the burden and severity of obesity. For instance, the intake of a high-fat-low-carbohydrate diet (HFLCD) was found to be effective in slimming [1].
The HFLCD is an efficient diet for reducing body weight, with increased satiety and diminishing energy consumption, efficacious to improve glycaemic control and reduce hyperinsulinemia in diabetes mellitus [2]. De & Mukhopadhyay explained the mechanisms that lead to weight loss on a high-fat diet: an increase in satiety leads to less energy intake without starvation and a metabolic advantage [3]. Ketogenesis reduces appetite [4]; the mechanism of appetite reduction is not clear; probably occurs as a result of stopping the secretion of the hunger hormone (ghrelin) [5]. HFLCD commonly contains 20% of carbohydrates, more than 50% of fat, and insignificant amounts of protein [6]. Low-carbohydrate diets restrict the intake of legumes, fruits, sugars, starchy foods, and whole grains, these food groups are the foundation of the globally recognized food pyramid that promotes long-term health [7]. A previous study contradicts this diet and indicates that an HFLCD for a long-time lead to metabolic syndrome and an increased risk for diabetes in humans [8]. Some slimming diets are regular with healthy eating recommendations, while others diets promote illegitimate and sometimes unsafe practices.
The body is deprived of carbohydrates during the application of HFLCD, which leads to a decrease in insulin secretion and enters the body into a catabolic state [9, 10], leading to the use of stored glycogen which is all used up and will be decreased or be exhausted. The body will force to use an alternative strategy to provide energy by transforming stored fats; this process leads to increase fatty acid oxidation and produces ketone bodies in the liver [11]. In this essence, the adult liver can produce up to 185 g of ketone bodies per day. Ketogenesis includes β-oxidation of fatty acids to acetyl CoA, formation of acetoacetyl CoA, conversion of acetoacetyl CoA to 3-hydroxy-3-methylglutaryl CoA and then to acetoacetate; and finally reduction of acetoacetate to 3-β-hydroxybutyrate. This process depends on triglyceride lipase, which is found in peripheral adipocytes, and acetyl CoA carboxylase and hydroxymethylglutaryl-CoA synthase, which are found in the liver [12].
The purpose of the present study was to investigate the positive and negative histopathological effects of HFLCD on the liver and pancreas, lipid profiles, and physiological parameters including blood glucose, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels.
Materials and Methods
This experimental research was conducted in 2021 on 8-week-old (30 male & 30 female) mice obtained from Al-Manara College for Medical Sciences and maintained at 24°C on a 12:12-h light-dark cycle, with cleaning and sterilization of cages 2-3 times a week. Animals were allowed access to feed and water. All mice were harbored in well-ventilated cages and sanitary conditions and fed on an essential diet for 3 weeks for acclimation as described previously.
-Diet-induced fatness: The 8-week-old mice (except the control group) were given access to water and a high-fat- high carb diet (40% fat, 25% protein, 5% sucrose, 20% vitamin, 5% soybean oil, and 5% maltodextrin (Profoods nutrition, Mumbai, Maharashtra)) for 10 weeks before the start of the experimental diet as described previously [13]. To enhance gaining weight, mice were given water containing 20% fructose (w/v) (Profoods nutrition, Mumbai, Maharashtra) in addition to the high-fat diet. After 12 weeks, mice on the diet gained approximately 10g of excess body weight compared with body weight before starting weight gain procedures and were considered to get diet-induced fatness.
-HFLCD and Feeding Protocols: The basal diet of the control group consisted of 20% protein (Profoods nutrition, Mumbai, Maharashtra), 15% local Iraqi barley, 15% local Iraqi groats, 15% local Iraqi flour, 25% local Iraqi corn groats, and 10% vitamins (Hansal, Germany). The HFLCD for the treatment group contained 75% local Iraqi fat, 10% protein, 5% carbohydrate (wt/wt (Profoods nutrition, Mumbai, Maharashtra)), 5% vitamin, and 5% mineral (BioTech USA, Jordan). Foodstuffs were selected according to Noakes & Windt [2] who provided a recommended menu on the “Banting” diet similar to (HFLCD).
The animals were divided into 6 main groups, each group containing 10 mice. Four groups were subjected to the HFLCD and two groups were considered as control groups.
-Group I (group of male mice; n=10) this group was fed for 3 months on the HFLCD (MG1);
-Group II (group of males; n=10) this group was fed for 6 months on the HFLCD (MG2);
-Group III (group of males; n=10) this group was known as a control group fed on the basal diet (MCG);
-Group IV (group of females) was fed for 3 months on the HFLCD (FG1);
-Group V (group of females) was fed for 6 months on the HFLC diet (FG2);
-Group VI (group of females) was considered as a control group fed on the basal diet (FCG).
Bodyweight, water, and food intake were measured every three days throughout the intervention until the end of the experiment. During the experiment period, the behavior of mice such as movement, feeding, and breeding was monitored. On the other hand, groups have been allowed to mate; each female gave 4-12 newborns.
-Blood & Tissue Examination: For sample collection, animals were sacrificed by intraperitoneal anesthetizing them with a mixture of xylazine (Interchemie, Netherlands) and ketamine (Interchemie, Netherlands) at a concentration of 10% (1:1v/v). The blood was collected in a serum gel tube (Henso, China); the serum was separated by a centrifuge (10 min at 6000 RPM) and transferred to serum tubes (Henso, China) for refrigeration until biochemical analysis was performed. Tissue samples were taken for histopathological analysis. Liver and pancreas tissues were fixed in 10% formalin (Scharlau, Spain) before being embedded in wax (paraffin; Wollenweber, Germany). Deparaffinization of tissue was pursued using xylene and re-hydrated through a series of alcohols. Paraffin-embedded tissue samples were sectioned using a microtome (Histoline MRS3, Milano-Italy) into slices about 5μm in thickness. Staining for tissue was performed using hematoxylin (Quallcaic, India) and eosin stains (SIRC, China) to show the basic structures of the tissue. The slide was prepared for permanent preservation using Canada balsam, before being covered. Tissue samples were examined under an optical microscope (Meij with Omax Camera, Japan) at 125x, and 500x power. For biochemical analysis, blood AST, ALT, cholesterol, triglycerides (TG), and glucose were measured. All examinations were performed by Cobas c 111 auto analyzers (Roche Diagnostics GmbH, Sandhofer Strasse 116, Mannheim, Germany), according to the manufacturer's instructions. Absorbance was measured at the wavelength of 340nm (ALT, AST, and glucose), and 512nm (cholesterol and triglycerides) by UV-Spectrophotometer (Biotech, Germany).
Statistical significance was expressed using a one-way analysis of variance (ANOVA test) followed by the Tukey test. Data were expressed as the means± SD. Significant differences were considered at p<0.05. All experimental data were analyzed using Statistical Package for the Social Science for Windows (IBM Corp., version 26.0. Armonk, NY, US).
Findings
Throughout the experiments, the behavior of all studied groups was seemingly normal and the nutrition diet did not affect the activities of mice. To investigate the impact of HFLCD on liver physiology, serum levels of ALT, and AST were measured, which were the main markers of the liver function test. Plasma profile analyses for all experimental groups of mice were shown in Tables 1 & 2; Diagrams 1 & 2. Results of serum liver enzymes showed a significant increase in the level of AST and ALT in the FG1 compared with that of the FG2 and the control groups, which showed the highest recorded mean values (p<0.05).
As for the serum lipid concentration, cholesterol levels showed a significant increase after applying the dietary intervention to mice for 3 months as compared with the control group and the group of FG2. Moreover, the findings showed a highly significant decrease in TG after feeding the mice on the HFLCD with P<0.01 if compared with the control group. As for the analysis of the cumulative sugar, it showed a significant decrease in both groups of mice to which diet was applied compared with the control group, which had a higher glucose level with a significant difference (p<0.05; Table 1).
Regarding the male groups, the results of the experiment showed a significant increase in AST and ALT levels in the group of MG1 when compared with the group of MG2 and the control (p<0.05; Table 2). Cholesterol levels increased after applying the diet to MG1 compared to the control group. In this line, we found a decrease in the group of mice applied to the diet for 6 months compared with the control group with a highly significant difference (p<0.01). The TG level showed a significant decrease in the serum of both groups compared with its level in the control group (p<0.05; Table 2).
Table 1) Plasma profile in female control mice and female mice fed an HFLCD

Table 2) Plasma profile in male control mice and male mice fed an HFLCD


Diagram 1) showing the mean of AST, ALT, Cholesterol, Triglycerides, and Glucose of female control mice and female mice fed an HFLCD

Diagram 2) Showing the mean of AST, ALT, Cholesterol, Triglycerides, and Glucose of male control mice and female mice fed an HFLCD
In control groups (Figures 1-A, 2-A & B), the histological structure of the liver and pancreas appeared with a normal systematic pattern of hepatocytes in the periportal area, normal pancreatic exocrine glands, and normal islands of Langerhans. The histological examination revealed the impact of HFLCD on liver tissue (Figure 3-A & B). The effect was more obvious on hepatocytes, where severe fatty changes were observed that led to edema of hepatocytes in the periportal area because of microvascular steatosis. After 6 months of feeding, the intensity of histological changes was detected, and appeared as degeneration of hepatocytes in the periportal area because of microvascular steatosis, bridging fibrosis between the centrilobular and periportal area (Figure 5-A & B). The results of the study showed that the application of the feeding regime upon mice had led to the presence of degenerated cells in the pancreatic exocrine glands with no change in the islands of Langerhans (Figure 4-A & B). In the group that fed the same system for 6 months, normal pancreatic exocrine glands and normal islands of Langerhans were shown (Figure 6).
The histological architecture of the liver in the group of females that fed the diet for 3 months showed microvascular steatosis (Figure 7-A), congestion of the central vein (Figure 7-B), and periportal inflammation (Figure 7-C). Histological examination of pancreatic tissue in mice with the diet for three- and 6 months showed normal histological structure as shown in (Figures 8 & 10), respectively. Hepatocytes of the female group that fed for 6 months contained a large lipid vacuole that filled the cell and dislodged its nuclei to the periphery, resulting in a degeneration of hepatocytes in the centrilobular area (Figure 9).

Figure 1) Section of liver of the control group showed normal hepatocytes in the centrilobular area (black arrow), normal hepatocytes in the periportal area (green arrow) H&E; 125X

Figure 2) Section of the pancreas of the control group showed normal pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; A) 125X, and B) 500X

Figure 3) Section of liver of treated group of males after 3 months of treatment show swelling of hepatocytes in the periportal area (green arrow) because of microvascular steatosis H&E; 125X

Figure 4) Section of the pancreas of the treated group of males after 3 months of treatment show degenerated cells in the pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; A) 125X, and B) 500X

Figure 5) Section of liver of treated group of males after 6 months of treatment showed marked degeneration of hepatocytes in the periportal area (green arrow) because of microvascular steatosis, bridging fibrosis between the centrilobular and periportal area (black arrow) H&E; A) 125X, and B) 500X, and C) 125X

Figure 6) Section of a pancreas of the treated group of males after 6 months show normal pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; A) 125X, and B) 500X

Figure 7) Section of liver of treated group of females after 3 months of treatment showed marked degeneration of hepatocytes in the periportal area (green arrow) because of microvascular steatosis, congestion of the central vein (black arrow), periportal inflammation (blue arrow) H&E; A) 125X, B) 500X, and C) 125X

Figure 8) Section of the pancreas of the treated group of females after 3 months showed normal pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; 125X

Figure 9) Section of the liver of the treated group of females after 6 months of treatment showed marked degeneration of hepatocytes in the centrilobular area (green arrow) because of macrovascular steatosis. H&E; A) 125X, and B) 500X

Figure 10) Section of the pancreas of the treated group of females after 6 months showed normal pancreatic exocrine glands (black arrow), normal islands of Langerhans (green arrow) H&E; 125X
Discussion
Dietary patterns that depend on the low level of carbohydrates and a high level of fat harm the body causing a deficit in energy intake, and it leads to a state called ketosis because of the lowering of carbohydrates. Ketosis is a physiological metabolic case; it allows the generation of energy from the fat of the liver to be utilized by other organs. The body uses glucose to provide energy, part of it is used as fuel in vital processes and the other part is stored as glycogen in the liver and muscles.
The intake of HFLCD by mice has led to weight loss in a few weeks, which is in agreement with previous studies conducted on rodents [14, 15]. In other studies, the ketogenic diet was reported to enhance elevated glucose levels, body weight, and hyperlipidemia [16, 17]. Intake of HFLCD for long-term has not resulted in weight loss in mice, but caloric restriction which raised insulin sensitivity, reduce glucose and increase ketone bodies, also restricting total energy intake and rectifying weight gain or hyperlipidemia [18]. In this regard, Rosenbaum et al. [19] showed that raised cholesterol, symptoms of inflammation, reduced triglycerides, and decreased insulin-mediated anti-lipolysis are correlated with HFLCD.
Extrapolation of the laboratory study to humans and studying the histopathological features resulting from the application of the diet is difficult, so mice were used as an experimental model for the development of obesity and the application of the diet to obese mice. After consuming the HFHCD for 12 weeks, the mice were weighed to confirm weight gain. The weights of mice before applying the HFHC diet were 27-30g for males and 20-24g for females. While, after applying the HFHCD, the weights ranged from 40-45g and 39-45g for males and females, respectively.
During the experiment period, sexual behavior in mice was observed; all mice were seen as sexually active. We spotted new births raising the number of males more than that of females. The total number of recent births was 114. The number of males was 74 while the number of females was 40. Contrasting results with our study have been reported; Samuel et al. [20] revealed that a high-fat diet causes apoptosis of testicular cells, which alters fertility in male mice.
In the present study, mice fed HFLCD showed signs of steatohepatitis and increased levels of AST and ALT. These findings correspond with the results of previous studies conducted on mice that observed metabolic changes as a result of feeding via a ketogenic diet 8, and this perhaps marks an early phase of non-alcoholic fatty liver disease. Another study showed that supplementation of methionine and choline with a KETO diet could reduce hepatic steatosis and the pro-inflammatory state of the liver [21]. Lipid aggregation in hepatocytes occurs by mechanisms that are not clearly understood but probably this is due to the overproduction of fatty acids, increased uptake of fatty acids, and decreased hepatic lipid metabolism [22]. An increase in ALT or AST activities is considered a marker of damage in hepatocytes with advanced stages of steatosis (fatty change) and cirrhosis (fibrosis). This rise is attributed to an abundance of free radicals that causes lipid peroxidation of the cell membrane, which leads to the loss of the cellular membrane and hepatocyte damage [23].
The level of cholesterol in the HFLCD group increased significantly when compared with the control. A previous study reported that serum levels of cholesterol increased in mice fed a ketogenic diet [24]. Another study reported that HFLCD causes hypercholesterolemia [25]. High cholesterol levels lead to an increase in the risk of liver fibrosis [26]. Results of some other studies, conducted on humans and rodents with a ketogenic diet, contradict the results of our study. Cholesterol levels are decreased or increased [27], or not affected by the diet in adults with a ketogenic diet. High levels of cholesterol contribute to heart disease by increasing the fat in the arteries, this leads to the narrowing of the arteries and the development of atherosclerosis. Atherosclerosis causes myocardial infarction and apoplexy [28]. The high level of cholesterol in the blood is due to an increased intake of saturated fats or the production of excessive ketones, which can be exploited in the process of energy production [29].
Decreasing serum triglycerides and glucose levels were detected in the HFLCD groups when compared to those of the control. This was in agreement with Yang et al. [30] who expressed that the KETO diet was effective in lowering blood glucose, improving glucose tolerance, and raising insulin sensitivity. Badman et al. [31] stated that the ketogenic diet gives rise to high plasma cholesterol levels whilst, that plasma TG levels were decreased; this finding is essentially consistent with the results of our study. A low TG value is probably a precocious marker of autoimmunity or immune system hyper-reactivity [32]. Reduced TG levels are perhaps the consequence of associated factors such as congestive heart failure, advanced states of congestion in the gastrointestinal system and liver, decreased food intake, increased cachexia, and increased levels of inflammation [33]. Low TG is probably a marker of liver damage due to diminished oxygen supply or increased levels of inflammation [34]. A recent study showed that an increased TG level was detected in children with epilepsy during the treatment classic ketogenic diet [35]. Hyperlipidemia is a known side effect of ketogenic dietary therapy [36]. Although, long-term application of the KETO diet can cause hepatic lipid accumulation and hepatic steatosis. According to the results of Zhang et al. [37], the KETO diet is considered a new and possible approach to the therapy of diabetes.
Histopathological results in mice fed the diet showed the appearance of lipid changes in hepatocytes, which led to edema of hepatocytes in the periportal area because of microvascular steatosis; this degeneration was more prominent in mice groups for 6 months of the diet. Appeared degeneration of hepatocytes in the periportal area is because by macrovascular and microvascular steatosis, as well as the formation of fibrous bridges between the centrilobular and periportal area causes, bridging fibrosis consider the main stage in the advancement of liver fibrosis. Hepatic histological results coincide with the biochemical analysis findings where the signs of deterioration in liver tissue appeared in H&E stained sections. The present results are in agreement with those of Garbow et al. [38] who found that mice fed HFLCD had hepatocellular damage, inflammatory response, severe hepatic steatosis, apoptosis, and autophagy. Macrovesicular steatosis is characterized by large fat globules aggregated in the cytoplasm of the hepatocytes. The nucleus of hepatocytes is pressed and dislocated into the rim of the cytoplasm by the fat vacuole. Microvesicular steatosis is used as a mark of rigorous hepatic dysfunction and is described via abundant tiny collections of lipid-laden macrophages and Lipid droplets in the hepatocytes. Macrovesicular and microvesicular steatosis may contribute to causing intense diseases like nonalcoholic steatohepatitis and fibrosis [39]. Although exocytosis of the exocrine pancreatic glands occurred in the 3-months-fed male, it did not appear in the other groups. This indicates that diet has no side effects on the tissues of the organ itself.
Conclusion
The present study has provided evidence for explaining the defects in the liver and pancreatic function caused by a high-fat-low-carb diet, and their associated pathological defects in mice models. The plasma profile of the HFLCD group pointed out that the diet is unhealthy. The plasma profile of the HFLCD group showed, that ALT, AST, and cholesterol were higher in the HFLCD group. However, the level of TG and glucose have decreased. The HFLCD group showed severe fatty changes that led to edema of hepatocytes in the periportal area and degeneration of hepatocytes in the periportal area because of microvascular steatosis, bridging fibrosis between the centrilobular and periportal area. Some limitations of the study need to be considered. Further long-term follow-up studies are required to validate our results. Moreover, more focus is needed on the effectiveness of the diet on the reproductive system, fertilization, and sex determination.
Acknowledgments: We thank all research participants who contributed to completing this search.
Ethical Permissions: Ethical Clearance: The project was approved by the local ethical committee in AL-Manara College for Medical Sciences.
Conflicts of Interests: None declared.
Authors’ Contributions: Abed Hussein B (First Author), Introduction Writer/Main Researcher/Statistical Analyst/Discussion Writer (50%); Fawzi M (Second Author), Methodologist/Assistant Researcher/Statistical Analyst/Discussion Writer (30%); Al-Saray ST (Third Author), Assistant Researcher (10%); Abed Hussein R (Forth Author), Assistant Researcher (10%)
Funding/Support: None declared.
Keywords:
Body Fat Distribution [MeSH], Carbohydrate [MeSH], Diet [MeSH], Steatohepatitis [MeSH], Fibrosis [MeSH]
References
1. Yang Q, Lang X, Li W, Liang Y. The effects of low-fat, high-carbohydrate diets vs. low-carbohydrate, high-fat diets on weight, blood pressure, serum liquids and blood glucose: a systematic review and meta-analysis. Eur J Clin Nutr. 2022;76:16-27. [Link] [DOI:10.1038/s41430-021-00927-0]
2. Noakes TD, Windt J. Evidence that supports the prescription of low-carbohydrate high-fat diets: a narrative review. Br J Sports Med. 2017;51(2):133-9. [Link] [DOI:10.1136/bjsports-2016-096491]
3. De P, Mukhopadhya S. Low-carbohydrate high-fat (LCHF) diet: evidence of its benefits. In: Waisundara V, editor. Diabetes food plan. London: IntechOpen; 2018. [Link] [DOI:10.5772/intechopen.73138]
4. Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82(1):41-8. [Link] [DOI:10.1093/ajcn.82.1.41]
5. Roekenes J, Martins C. Ketogenic diets and appetite regulation. Curr Opin Clin Nutr Metab Care. 2021;24(4):359-63. [Link] [DOI:10.1097/MCO.0000000000000760]
6. Volek JS, Freidenreich DJ, Saenz C, Kunces LJ, Creighton BC, Bartley JM, et al. Metabolic characteristics of KETO-adapted ultra-endurance runners. Metabolism. 2016;65(3):100-10. [Link] [DOI:10.1016/j.metabol.2015.10.028]
7. Dyson PA, Kelly T, Deakin T, Duncan A, Frost G, Harrison Z, et al. Diabetes UK evidence‐based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2011;28(11):1282-8. [Link] [DOI:10.1111/j.1464-5491.2011.03371.x]
8. Ellenbroek JH, van Dijck L, Töns HA, Rabelink TJ, Carlotti F, Ballieux BE, et al. Long-term ketogenic diet causes glucose intolerance and reduced β-and α-cell mass but no weight loss in mice. Am J Physiol Endocrinol Metab. 2014;306(5):552-8. [Link] [DOI:10.1152/ajpendo.00453.2013]
9. Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53(9):2375-82. [Link] [DOI:10.2337/diabetes.53.9.2375]
10. Nuttall FQ, Almokayyad RM, Gannon MC. Comparison of a carbohydrate-free diet vs. fasting on plasma glucose, insulin and glucagon in type 2 diabetes. Metabolism. 2015;64(2):253-62. [Link] [DOI:10.1016/j.metabol.2014.10.004]
11. Jagadish S, Payne ET, Wong-Kisiel L, Nickels KC, Eckert S, Wirrell EC. The ketogenic and modified Atkins diet therapy for children with refractory epilepsy of genetic etiology. Pediatr Neurol. 2019;94:32-7. [Linkvv] [DOI:10.1016/j.pediatrneurol.2018.12.012]
12. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412-26.
https://doi.org/10.1002/(SICI)1520-7560(199911/12)15:6<412::AID-DMRR72>3.0.CO;2-8 [Link] [DOI:10.1002/(SICI)1520-7560(199911/12)15:63.0.CO;2-8]
13. Ayer A, Borel F, Moreau F, Prieur X, Neunlist M, Cariou B, et al. Techniques of sleeve gastrectomy and modified Roux-en-Y gastric bypass in mice. J Vis Exp. 2017;121:54905. [Link] [DOI:10.3791/54905]
14. Hansen SL, Nielsen AH, Knudsen KE, Artmann A, Petersen G, Kristiansen U, et al. Ketogenic diet is antiepileptogenic in pentylenetetrazole kindled mice and decrease levels of N-acylethanolamines in hippocampus. Neurochem Int. 2009;54(3-4):199-204. [Link] [DOI:10.1016/j.neuint.2008.10.012]
15. Kennedy AR, Pissios P, Otu H, Xue B, Asakura K, Furukawa N, et al. High-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab,. 2007;292(6):E1724-39. [Link] [DOI:10.1152/ajpendo.00717.2006]
16. Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep. 2018;8:6670. [Link] [DOI:10.1038/s41598-018-25190-5]
17. Martuscello RT, Vedam-Mai V, McCarthy DJ, Schmoll ME, Jundi MA, Louviere CD, et al. A supplemented high-fat low-carbohydrate diet for the treatment of glioblastoma. Clin Cancer Res. 2016;22(10):2482-95. [Link] [DOI:10.1158/1078-0432.CCR-15-0916]
18. Meidenbauer JJ, Ta N, Seyfried TN. Influence of a ketogenic diet, fish-oil, and calorie restriction on plasma metabolites and lipids in C57BL/6J mice. Nutr Metab. 2014;11:23. [Link] [DOI:10.1186/1743-7075-11-23]
19. Rosenbaum M, Hall KD, Guo J, Ravussin E, Mayer LS, Reitman ML, et al. Glucose and lipid homeostasis and inflammation in humans following an isocaloric ketogenic diet. Obesity. 2019;27(6):971-81. [Link] [DOI:10.1002/oby.22468]
20. Samuel RJ, Joy O, KrishnaMurthy P, Iyer S, Ramachandra SG, Thamaraiselvi K. Effect of feeding high fat diet on reproductive parameters in male mice. Indian J Anim Res. 2019;53(4):505-9. [Link] [DOI:10.18805/ijar.B-3528]
21. Pissios P, Hong S, Kennedy AR, Prasad D, Liu FF, Maratos-Flier E. Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Mol Metab. 2013;2(3):306-13. [Link] [DOI:10.1016/j.molmet.2013.07.003]
22. Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. 2017;234(1):R1-21. [Link] [DOI:10.1530/JOE-16-0513]
23. Ingawale DK, Mandlik SK, Naik SR. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): a critical discussion. Environ Toxicol Pharmacol. 2014;37(1):118-33. [Link] [DOI:10.1016/j.etap.2013.08.015]
24. Douris N, Melman T, Pecherer JM, Pissios P, Flier JS, Cantley LC, et al. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim Biophys Acta. 2015;1852(10):2056-65. [Link] [DOI:10.1016/j.bbadis.2015.07.009]
25. Goldberg IJ, Ibrahim N, Bredefeld C, Foo S, Lim V, Gutman D, et al. Ketogenic diets, not for everyone. J Clin Lipidol. 2021;15(1):61-7. [Link] [DOI:10.1016/j.jacl.2020.10.005]
26. Twu YC, Lee TS, Lin YL, Hsu SM, Wang YH, Liao CY, et al. Niemann-Pick type C2 protein mediates hepatic stellate cells activation by regulating free cholesterol accumulation. Int J Mol Sci. 2016;17(7):1122. [Link] [DOI:10.3390/ijms17071122]
27. Westman EC, Yancy WS, Edman JS, Tomlin KF, Perkins CE. Effect of 6-month adherence to a very low carbohydrate diet program. Am J Med. 2002;113(1):30-6. [Link] [DOI:10.1016/S0002-9343(02)01129-4]
28. Guo P. Protective effect of aerobic exercise combined with Paeonol PLGA nanoparticles on obese rats with hyperlipidemia. Revista Científica de la Facultad de Ciencias Veterinarias. 2020;30(2):766-75. [Link]
29. Diamond DM, O'Neill BJ, Volek JS. Low carbohydrate diet: Are concerns with saturated fat, lipids, and cardiovascular disease risk justified?. Curr Opin Endocrinol Diabetes Obes. 2020;27(5):291-300. [Link] [DOI:10.1097/MED.0000000000000568]
30. Yang Z, Mi J, Wang Y, Xue L, Liu J, Fan M, et al. Effects of low-carbohydrate diet and ketogenic diet on glucose and lipid metabolism in type 2 diabetic mice. Nutrition. 2021;89:111230. [Link] [DOI:10.1016/j.nut.2021.111230]
31. Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab. 2009;297(5):E1197-204. [Link] [DOI:10.1152/ajpendo.00357.2009]
32. Iannello S, Cavaleri A, Milazzo P, Cantarella S, Belfiore F. Low fasting serum triglyceride level as a precocious marker of autoimmune disorders. Med Gen Med. 2003;5(3):20. [Link]
33. Kozdag G, Ertas G, Emre E, Akay Y, Celikyurt U, Sahin T, et al. Low serum triglyceride levels as predictors of cardiac death in heart failure patients. Tex Heart Inst J. 2013;40(5):521-8. [Link]
34. Perna S, Ferraris C, Guglielmetti M, Alalwan TA, Mahdi AM, Guido D, et al. Effects of classic ketogenic diet in children with refractory epilepsy: a retrospective cohort study in Kingdom of Bahrain. Nutrients. 2022;14(9):1744. [Link] [DOI:10.3390/nu14091744]
35. Nizamuddin J, Turner Z, Rubenstein JE, Pyzik PL, Kossoff EH. Management and risk factors for dyslipidemia with the ketogenic diet. J Child Neurol. 2008;23:758-61. [Link] [DOI:10.1177/0883073808318061]
36. Kwiterovich Jr PO, Vining EP, Pyzik P, Skolasky Jr R, Freeman JM. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA. 2003;290(7):912-20. [Link] [DOI:10.1001/jama.290.7.912]
37. Zhang X, Qin J, Zhao Y, Shi J, Lan R, Gan Y, et al. Long-term ketogenic diet contributes to glycemic control but promotes lipid accumulation and hepatic steatosis in type 2 diabetic mice. Nutr Res. 2016;36(4):349-58. [Link] [DOI:10.1016/j.nutres.2015.12.002]
38. Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G956-67. [Link] [DOI:10.1152/ajpgi.00539.2010]
39. Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(42):15539-48. [Link] [DOI:10.3748/wjg.v20.i42.15539]








