Volume 14, Issue 2 (2022)
Iran J War Public Health 2022, 14(2): 243-248 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/03/31 | Accepted: 2022/06/11 | Published: 2022/06/25
Received: 2022/03/31 | Accepted: 2022/06/11 | Published: 2022/06/25
How to cite this article
Abbas Tawil F, Awad Kadhim S, Kareem Hashim A. Comparing the Cadmium, Zinc, Lead, and Copper Concentrations between Lung Cancer Patients and Healthy Iraqi Individuals. Iran J War Public Health 2022; 14 (2) :243-248
URL: http://ijwph.ir/article-1-1189-en.html
URL: http://ijwph.ir/article-1-1189-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Physics, Faculty of Science, University of Kerbala, Kerbala, Iraq
Full-Text (HTML) (759 Views)
Introduction
Trace elements are necessary in small quantities for it continuation of humanity, and their loss from the organism results in death or severe dysfunction. Trace element concentrations in human tissues range from 0.01 to 100mg/kg [1]. All the essential trace elements can be toxic to humans and animals if their intake is large or lasts for a relatively long time [2]. The main distinction between trace minerals and heavy metals is that heavy metals are generally harmful at extremely low doses, whereas trace elements are not. Many trace elements have different roles based on their chemical composition or how they are synthesized and distributed throughout the body [3].
Copper is required for the functioning of numerous metalloproteinases and enzymes, as well as the control of gene expression. It is also required for growing, protection, bone health, and the formation of blood cells [3, 4]. Cadmium is released into the environment because of human activities, particularly industrial operations, and waste disposal [5]. Food is the primary source of cadmium exposure in humans, although tobacco smoke is also a significant source. Other issues caused by cadmium poisoning include cancer, cardiovascular disease, and hypertension. Cadmium is known to cause cancer in humans [5]. Copper deficiency leads to anemia and low blood cells leukemia, as well as a neurological disease, osteoporosis, and a disorder of the connective tissues. Excess copper poisoning is extremely rare and is usually caused by toxic water [5]. It leads to gastrointestinal issues. In the case of Wilson's illness, poisoning can also arise in some diseases, and copper poisoning from unknown causes, such as Wilson's illness Wilson inhibits copper excretion in the bile, causing it to build up in the kidney, liver, and brain [6]. Zinc is a mineral found in all human tissues [7], it is required for the metabolism of macromolecule nucleic acids and other metals [7]. It is a structural component of about 300 enzymes. Zinc does have a key function in the expression of genes. Approximately 10% of the proteins in the human genes have the binding zinc. Zinc deficiency is considered rare due to its frequent and near-ideal intake. Zinc is affecting health, growth, and reproductive function. Dermatitis of the extremities is caused by enteropathy [8]. It is a disease inherited from zinc deficiency. Pollution results in increased exposure to zinc. Headache, vomiting, diarrhea, coma, and fever are the most common symptoms of acute poisoning. Copper is mutually exclusive competing for absorption in the intestine [9]. Lead has long been recognized as a hazardous substance. It is brought about by a variety of human actions. Humans are exposed to it through the intake of food and water. Reproductive failure, encephalopathy, neurophysiological abnormalities, anemia, renal damage, hypertension blood, and poisoning are all symptoms of excessive lead exposure [10].
Researchers attempted to evaluate the link between serum concentration of copper (Cu), zinc (Zn), lead (Pb), cadmium (Cd), iron (Fe), manganese (Mn), cobalt (Co), and magnesium (Mg) and the lung cancer (LC). Cu, Pb, Zn, Fe, Mg, Co, Mn, and Cd have been confirmed to have a function in LC patients [10]. It was found in a meta-analysis in 2018, that there is a link between serum copper concentrations and LC risk. Serum copper concentrations were highest in patients with LC relative to healthy people, indicating that external copper exposure may be a major risk for LC development [11]. In a previous study, a relationship between serum and whole blood Zn and Cu level and clinical, socioeconomic variables, and nutritional data was confirmed. Also, there was a link between Cu and Zn and all-cause mortality in LC patients. Higher serum Cu levels, Cu/Zn ratios, and whole blood Zn levels were shown to be associated with a lower risk of death in LC patients [12].
This study aimed to identify certain trace minerals in the serum of Iraqi LC patients using the atomic absorption spectroscopy technique and investigate the distribution of these elements compared to healthy people.
Instrument and Methods
Blood samples were taken from the Imam Hussein Center for Cancerous Tumors in Karbala Governorate in 2021. The study was carried out on 100 subjects (55 males and 45 females), including the healthy (n=35) and the lung cancer (n=65) groups. The samples were selected using a simple random sampling method. Inclusion criteria were non-smokers, no family history of cancer, living in Karbala governorate, and tendency to participate in the study. The blood samples were taken by syringe. Then, the dye was separated from the plasma by a centrifuge, which was in an Abendouf tube and stored in a refrigerator prepared for digestion.
The serum samples were digested according to the procedures by mixing 2ml of nitric acid with 1ml of perchloric acid with 1ml of serum in a conical flask with shaking the beaker and were placed on a heater in a special hood to withdraw all the gases until it becomes salt (ash). Then, we filtered the sample with filter paper using a mixture of 5ml of distilled water and kept it in a glass container to prepare for measurement with the device [13].
The blood serum was also digested to obtain the concentrations of copper, zinc, lead, and cadmium [14]. After preparing the samples, they were sent to the laboratory for measuring zinc, copper lead, and cadmium using the Flame Atomic Absorption Spectrophotometer (FAAS) 6300 (Shimadzu; Japan). Before the measurement, the FAAS was calibrated by Dilution of global standards for copper, zinc, lead, and cadmium, where four diluted concentrations were made to calibrate the device for each element separately (Figure 1).

Figure 1) Calibration of the atomic absorption system for A: copper, B: zinc, C: lead, and D: cadmium
The data was analyzed using SPSS 20 software through independent T, ANOVA, Pearson, and Tukey HSD tests. The normality of parameters was also confirmed using the Kolmogorov-Smirnov test.
Findings
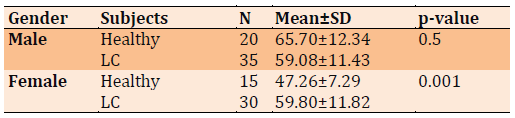
The average age for healthy and lung cancer subjects in female were 65.70±12.34 and 59.08±11.43, respectively and for male were 47.26±7.29, and 59.80±11.82, respectively (Table 1).
Table 1) Descriptive characteristics of patient and healthy subjects
There was a significant difference in concentration of Zinc between the healthy and LC males and females. There was a significant difference in Cu concentration between healthy and LC males while there was no significant difference between the healthy and LC females. Also, a significant difference in Pb concentration was revealed between the healthy and LC males, whereas there was no significant difference in Pb concentration between the healthy and LC females (p>0.05). The findings showed a significant difference in Cd concentration between the healthy females and males with LC females and males, respectively (Table 2).
Table 2) Results of comparison of serum concentrations (μg/L) of zinc, copper, lead, and cadmium between the subjects by independent T-test

A significance difference of serum concentrations was observed between the healthy females and female with lung cancer (p<0.05). The concentration of Pb, Cd, and Cu was higher in the female with lung cancer, whereas, the Zn concentration was higher in healthy female than female with lung cancer. There was a significance difference of the serum concentrations between the two groups of healthy males and male with lung cancer (p=0.001). The findings showed a high concentration of Pb, Cd, and Cu in LC patients in the male subjects, while, the concentration of Zn was higher on the group of healthy males (Table 3).
Table 3) Results of intergroup comparison of serum concentrations (μg/L) of zinc, copper, lead, and cadmium between the subjects by ANOVA

A positive correlation was observed between Pb with Zn level, and between Cu with Zn level in healthy females. There was a negative correlation between Cd with Pb level and Cd with Zn level in healthy females. Also, there was a negative correlation between Cu with Pb and Cd with Pb level in healthy females. There was a positive correlation between Cu with Pb level, and Cu with Cd level in LC females. Also, a negative correlation was found between Zn with Pb levels, Cd with Pb, Cd with Zn, and Cu with Zn levels in LC females (Table 4).
Table 4) Correlation among samples of serum concentrations (μg/L) of zinc, copper, lead, and cadmium minerals in healthy and LC females

Table 5) Results of correlation between serum concentrations of (μg/L) zinc, copper, lead and cadmium minerals in healthy and LC male group

Positive correlations were observed between Zn with Pb levels and Cu with Pb levels in the group of healthy males. There was a negative correlation between Cd with Pb, and Cd with Zn, Cu with Zn, and Cu with Cd levels in the healthy males. A positive correlation was released between Cd with Pb, Cu with Pb, and Cu with Cd levels. A negative correlation was observed between Zn with Pb, Cd with Zn, and Cu with Zn levels (Table 5).
Discussion
This study aimed to identify certain trace minerals in serum in Iraqi patients with LC using the atomic absorption spectroscopy technique and investigate the distribution of these elements compared to healthy people.
Zhang et al. [15] performed a meta-analysis using 33 articles to explore the association between serum copper levels and the risk of lung cancer. They suggested that serum copper levels were higher in lung cancer than that in controls. Gómez et al. [16] and had studied the association of zinc and its role in lung cancer and concluded that zinc deficiency has a significant role to prevent lung cancer, because Zinc has a key role in oxidative stress, apoptosis, and/or cell signaling alterations [16], and this may play a role in preventing lung cancer. Wang et al. [17] investigated the concentration of Zinc in lung cancer patients by a meta-analysis of 32 articles through the PubMed, Wanfang, Cochrane, ScienceDirect website, CNKI, and SinoMed databases. They concluded that serum Zinc was significantly lower in lung cancer patients than in controls. Shen et al., through a meta-analysis, suggested that patients with thyroid cancer had higher copper levels than healthy controls. Zhao et al. [18] assessed the Cu, Zn, Fe, Mn, and Ca in 300 patients with lung cancer and 100 healthy subjects and concluded that Cu, Mn, and Cu/Zn ratios were significantly higher in lung cancer patients but Zn, and Ca were significantly lower in lung cancer patients than in healthy people. Emre et al. [19] found a higher level of Cu, Mg, Pb, Cr, Zn, Mn, and Cd in patients with metastatic colon cancer compared to healthy subjects. Also, the results of our study showed a higher level of Cu/Zn in the subjects with lung cancer than in healthy subjects.
Demir et al. [20] found a low level of Zn, Fe, Mn, Mg, and Cu in patients with cancers of the lip and oral cavity than in healthy control groups and a higher level of Cd, Co, and Pb l in the patients than healthy control groups.
Diez et al. [21] investigated the concentration of Serum zinc (Zn), copper (Cu), and the Cu/Zn ratio in 20 patients with benign and 64 with malignant lung tumors before surgery and in 100 healthy normal controls and found the higher Cu/Zn ratio in malignant tumors than in benign tissue and the lower ratio in the normal group. Their findings suggest that Cu/Zn ratio may be used as a diagnostic test in lung cancer patients. Jin et al. [22] also suggested the higher ratio of Cu/Zn in patients with lung cancer to the pathogenesis of lung cancer. Zowczak et al. [23] assessed the relationship between the Cp oxidase activity and concentrations of Cu and Zn in serum of 62 patients with breast (BCA), lung (LCA), gastrointestinal (GICA), and gynecological (GYNCA) cancer, and found a significant increase in the mean serum Cp oxidase activity and total Cu concentrations in all patient groups compared with the control one. Moreover, Wu et al. [24] investigated the serum concentration of 13 elements such as Cd, Mn, Fe, Cr, and Zn in breast tumor patients and indicated a significant difference in concentration of all elements in serum between breast cancer patients and controls.
Our findings are consistent with the above studies. Indeed, Copper and zinc are closely related trace elements involved in cell proliferation, growth, gene expression, apoptosis, and other processes. These two trace elements are necessary for superoxide dismutase's proper activity due to their integral role as cofactors or ions stabilizing the molecular structure.
Cobanoglu et al. [25] determined the relationship between some mineral, trace element, and heavy metal levels in patients with lung cancer by measuring serum levels of copper (Cu), lead (Pb), zinc (Zn), iron (Fe), cobalt (Co), cadmium (Cd), manganese (Mn), magnesium (Mg). Mg, Cu, and Zn were lower in patients with lung cancer than the controls, whereas Pb, Mn, and Co were higher in those with lung cancer than controls. At the same time, our findings showed more levels of Cu in the subjects with lung cancer than in healthy subjects. Yari et al. [26] investigated the serum levels of Zn, Cd, Cu, and Pb metals in prostate cancer patients and compared them in a control group. Serum levels of Cd and Cu were significantly higher in patients with prostate cancer than in controls (p<0.05). In contrast, the mean concentrations of Zn and Pb were higher in patients, which is different from our findings based on the low level of Zn in lung cancer patients. Serum et al. [27] assessed the serum concentration of Ag, Pb, and Cd in the female with breast cancer in Iraq and found no significant difference between the serum concentration in the breast cancer females with healthy females, which is not consistent with our findings. The possible reasons for the difference between our findings and the mentioned studies are the differences in the geographical factors, nutrition, use of nutritional or therapeutic supplements, the nature of people's occupations, and the different nature of cancers.
Conclusion
Serum zinc levels are lower in lung cancer patients than in healthy subjects, whereas serum lead, cadmium, and copper are higher in lung cancer patients than in healthy subjects.
Acknowledgments The authors thank all the staff working at the Imam Husain Center for Oncology and Hematology, Karbala, Iraq, for the facilities provided to accomplish the work of this search.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contributions: Abbas Tawil F (First Author), Introduction Writer/Methodologist/Main Researcher/
Discussion Writer (40%); Awad Kadhim Sh (Second Author), Statistical Analyst/Discussion Writer (35%); Kareem Hashim A (Third Author), Methodologist (25%)
Funding/Support: None declared.
Trace elements are necessary in small quantities for it continuation of humanity, and their loss from the organism results in death or severe dysfunction. Trace element concentrations in human tissues range from 0.01 to 100mg/kg [1]. All the essential trace elements can be toxic to humans and animals if their intake is large or lasts for a relatively long time [2]. The main distinction between trace minerals and heavy metals is that heavy metals are generally harmful at extremely low doses, whereas trace elements are not. Many trace elements have different roles based on their chemical composition or how they are synthesized and distributed throughout the body [3].
Copper is required for the functioning of numerous metalloproteinases and enzymes, as well as the control of gene expression. It is also required for growing, protection, bone health, and the formation of blood cells [3, 4]. Cadmium is released into the environment because of human activities, particularly industrial operations, and waste disposal [5]. Food is the primary source of cadmium exposure in humans, although tobacco smoke is also a significant source. Other issues caused by cadmium poisoning include cancer, cardiovascular disease, and hypertension. Cadmium is known to cause cancer in humans [5]. Copper deficiency leads to anemia and low blood cells leukemia, as well as a neurological disease, osteoporosis, and a disorder of the connective tissues. Excess copper poisoning is extremely rare and is usually caused by toxic water [5]. It leads to gastrointestinal issues. In the case of Wilson's illness, poisoning can also arise in some diseases, and copper poisoning from unknown causes, such as Wilson's illness Wilson inhibits copper excretion in the bile, causing it to build up in the kidney, liver, and brain [6]. Zinc is a mineral found in all human tissues [7], it is required for the metabolism of macromolecule nucleic acids and other metals [7]. It is a structural component of about 300 enzymes. Zinc does have a key function in the expression of genes. Approximately 10% of the proteins in the human genes have the binding zinc. Zinc deficiency is considered rare due to its frequent and near-ideal intake. Zinc is affecting health, growth, and reproductive function. Dermatitis of the extremities is caused by enteropathy [8]. It is a disease inherited from zinc deficiency. Pollution results in increased exposure to zinc. Headache, vomiting, diarrhea, coma, and fever are the most common symptoms of acute poisoning. Copper is mutually exclusive competing for absorption in the intestine [9]. Lead has long been recognized as a hazardous substance. It is brought about by a variety of human actions. Humans are exposed to it through the intake of food and water. Reproductive failure, encephalopathy, neurophysiological abnormalities, anemia, renal damage, hypertension blood, and poisoning are all symptoms of excessive lead exposure [10].
Researchers attempted to evaluate the link between serum concentration of copper (Cu), zinc (Zn), lead (Pb), cadmium (Cd), iron (Fe), manganese (Mn), cobalt (Co), and magnesium (Mg) and the lung cancer (LC). Cu, Pb, Zn, Fe, Mg, Co, Mn, and Cd have been confirmed to have a function in LC patients [10]. It was found in a meta-analysis in 2018, that there is a link between serum copper concentrations and LC risk. Serum copper concentrations were highest in patients with LC relative to healthy people, indicating that external copper exposure may be a major risk for LC development [11]. In a previous study, a relationship between serum and whole blood Zn and Cu level and clinical, socioeconomic variables, and nutritional data was confirmed. Also, there was a link between Cu and Zn and all-cause mortality in LC patients. Higher serum Cu levels, Cu/Zn ratios, and whole blood Zn levels were shown to be associated with a lower risk of death in LC patients [12].
This study aimed to identify certain trace minerals in the serum of Iraqi LC patients using the atomic absorption spectroscopy technique and investigate the distribution of these elements compared to healthy people.
Instrument and Methods
Blood samples were taken from the Imam Hussein Center for Cancerous Tumors in Karbala Governorate in 2021. The study was carried out on 100 subjects (55 males and 45 females), including the healthy (n=35) and the lung cancer (n=65) groups. The samples were selected using a simple random sampling method. Inclusion criteria were non-smokers, no family history of cancer, living in Karbala governorate, and tendency to participate in the study. The blood samples were taken by syringe. Then, the dye was separated from the plasma by a centrifuge, which was in an Abendouf tube and stored in a refrigerator prepared for digestion.
The serum samples were digested according to the procedures by mixing 2ml of nitric acid with 1ml of perchloric acid with 1ml of serum in a conical flask with shaking the beaker and were placed on a heater in a special hood to withdraw all the gases until it becomes salt (ash). Then, we filtered the sample with filter paper using a mixture of 5ml of distilled water and kept it in a glass container to prepare for measurement with the device [13].
The blood serum was also digested to obtain the concentrations of copper, zinc, lead, and cadmium [14]. After preparing the samples, they were sent to the laboratory for measuring zinc, copper lead, and cadmium using the Flame Atomic Absorption Spectrophotometer (FAAS) 6300 (Shimadzu; Japan). Before the measurement, the FAAS was calibrated by Dilution of global standards for copper, zinc, lead, and cadmium, where four diluted concentrations were made to calibrate the device for each element separately (Figure 1).

Figure 1) Calibration of the atomic absorption system for A: copper, B: zinc, C: lead, and D: cadmium
The data was analyzed using SPSS 20 software through independent T, ANOVA, Pearson, and Tukey HSD tests. The normality of parameters was also confirmed using the Kolmogorov-Smirnov test.
Findings
The average age for healthy and lung cancer subjects in female were 65.70±12.34 and 59.08±11.43, respectively and for male were 47.26±7.29, and 59.80±11.82, respectively (Table 1).
Table 1) Descriptive characteristics of patient and healthy subjects

There was a significant difference in concentration of Zinc between the healthy and LC males and females. There was a significant difference in Cu concentration between healthy and LC males while there was no significant difference between the healthy and LC females. Also, a significant difference in Pb concentration was revealed between the healthy and LC males, whereas there was no significant difference in Pb concentration between the healthy and LC females (p>0.05). The findings showed a significant difference in Cd concentration between the healthy females and males with LC females and males, respectively (Table 2).
Table 2) Results of comparison of serum concentrations (μg/L) of zinc, copper, lead, and cadmium between the subjects by independent T-test

A significance difference of serum concentrations was observed between the healthy females and female with lung cancer (p<0.05). The concentration of Pb, Cd, and Cu was higher in the female with lung cancer, whereas, the Zn concentration was higher in healthy female than female with lung cancer. There was a significance difference of the serum concentrations between the two groups of healthy males and male with lung cancer (p=0.001). The findings showed a high concentration of Pb, Cd, and Cu in LC patients in the male subjects, while, the concentration of Zn was higher on the group of healthy males (Table 3).
Table 3) Results of intergroup comparison of serum concentrations (μg/L) of zinc, copper, lead, and cadmium between the subjects by ANOVA

A positive correlation was observed between Pb with Zn level, and between Cu with Zn level in healthy females. There was a negative correlation between Cd with Pb level and Cd with Zn level in healthy females. Also, there was a negative correlation between Cu with Pb and Cd with Pb level in healthy females. There was a positive correlation between Cu with Pb level, and Cu with Cd level in LC females. Also, a negative correlation was found between Zn with Pb levels, Cd with Pb, Cd with Zn, and Cu with Zn levels in LC females (Table 4).
Table 4) Correlation among samples of serum concentrations (μg/L) of zinc, copper, lead, and cadmium minerals in healthy and LC females

Table 5) Results of correlation between serum concentrations of (μg/L) zinc, copper, lead and cadmium minerals in healthy and LC male group

Positive correlations were observed between Zn with Pb levels and Cu with Pb levels in the group of healthy males. There was a negative correlation between Cd with Pb, and Cd with Zn, Cu with Zn, and Cu with Cd levels in the healthy males. A positive correlation was released between Cd with Pb, Cu with Pb, and Cu with Cd levels. A negative correlation was observed between Zn with Pb, Cd with Zn, and Cu with Zn levels (Table 5).
Discussion
This study aimed to identify certain trace minerals in serum in Iraqi patients with LC using the atomic absorption spectroscopy technique and investigate the distribution of these elements compared to healthy people.
Zhang et al. [15] performed a meta-analysis using 33 articles to explore the association between serum copper levels and the risk of lung cancer. They suggested that serum copper levels were higher in lung cancer than that in controls. Gómez et al. [16] and had studied the association of zinc and its role in lung cancer and concluded that zinc deficiency has a significant role to prevent lung cancer, because Zinc has a key role in oxidative stress, apoptosis, and/or cell signaling alterations [16], and this may play a role in preventing lung cancer. Wang et al. [17] investigated the concentration of Zinc in lung cancer patients by a meta-analysis of 32 articles through the PubMed, Wanfang, Cochrane, ScienceDirect website, CNKI, and SinoMed databases. They concluded that serum Zinc was significantly lower in lung cancer patients than in controls. Shen et al., through a meta-analysis, suggested that patients with thyroid cancer had higher copper levels than healthy controls. Zhao et al. [18] assessed the Cu, Zn, Fe, Mn, and Ca in 300 patients with lung cancer and 100 healthy subjects and concluded that Cu, Mn, and Cu/Zn ratios were significantly higher in lung cancer patients but Zn, and Ca were significantly lower in lung cancer patients than in healthy people. Emre et al. [19] found a higher level of Cu, Mg, Pb, Cr, Zn, Mn, and Cd in patients with metastatic colon cancer compared to healthy subjects. Also, the results of our study showed a higher level of Cu/Zn in the subjects with lung cancer than in healthy subjects.
Demir et al. [20] found a low level of Zn, Fe, Mn, Mg, and Cu in patients with cancers of the lip and oral cavity than in healthy control groups and a higher level of Cd, Co, and Pb l in the patients than healthy control groups.
Diez et al. [21] investigated the concentration of Serum zinc (Zn), copper (Cu), and the Cu/Zn ratio in 20 patients with benign and 64 with malignant lung tumors before surgery and in 100 healthy normal controls and found the higher Cu/Zn ratio in malignant tumors than in benign tissue and the lower ratio in the normal group. Their findings suggest that Cu/Zn ratio may be used as a diagnostic test in lung cancer patients. Jin et al. [22] also suggested the higher ratio of Cu/Zn in patients with lung cancer to the pathogenesis of lung cancer. Zowczak et al. [23] assessed the relationship between the Cp oxidase activity and concentrations of Cu and Zn in serum of 62 patients with breast (BCA), lung (LCA), gastrointestinal (GICA), and gynecological (GYNCA) cancer, and found a significant increase in the mean serum Cp oxidase activity and total Cu concentrations in all patient groups compared with the control one. Moreover, Wu et al. [24] investigated the serum concentration of 13 elements such as Cd, Mn, Fe, Cr, and Zn in breast tumor patients and indicated a significant difference in concentration of all elements in serum between breast cancer patients and controls.
Our findings are consistent with the above studies. Indeed, Copper and zinc are closely related trace elements involved in cell proliferation, growth, gene expression, apoptosis, and other processes. These two trace elements are necessary for superoxide dismutase's proper activity due to their integral role as cofactors or ions stabilizing the molecular structure.
Cobanoglu et al. [25] determined the relationship between some mineral, trace element, and heavy metal levels in patients with lung cancer by measuring serum levels of copper (Cu), lead (Pb), zinc (Zn), iron (Fe), cobalt (Co), cadmium (Cd), manganese (Mn), magnesium (Mg). Mg, Cu, and Zn were lower in patients with lung cancer than the controls, whereas Pb, Mn, and Co were higher in those with lung cancer than controls. At the same time, our findings showed more levels of Cu in the subjects with lung cancer than in healthy subjects. Yari et al. [26] investigated the serum levels of Zn, Cd, Cu, and Pb metals in prostate cancer patients and compared them in a control group. Serum levels of Cd and Cu were significantly higher in patients with prostate cancer than in controls (p<0.05). In contrast, the mean concentrations of Zn and Pb were higher in patients, which is different from our findings based on the low level of Zn in lung cancer patients. Serum et al. [27] assessed the serum concentration of Ag, Pb, and Cd in the female with breast cancer in Iraq and found no significant difference between the serum concentration in the breast cancer females with healthy females, which is not consistent with our findings. The possible reasons for the difference between our findings and the mentioned studies are the differences in the geographical factors, nutrition, use of nutritional or therapeutic supplements, the nature of people's occupations, and the different nature of cancers.
Conclusion
Serum zinc levels are lower in lung cancer patients than in healthy subjects, whereas serum lead, cadmium, and copper are higher in lung cancer patients than in healthy subjects.
Acknowledgments The authors thank all the staff working at the Imam Husain Center for Oncology and Hematology, Karbala, Iraq, for the facilities provided to accomplish the work of this search.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contributions: Abbas Tawil F (First Author), Introduction Writer/Methodologist/Main Researcher/
Discussion Writer (40%); Awad Kadhim Sh (Second Author), Statistical Analyst/Discussion Writer (35%); Kareem Hashim A (Third Author), Methodologist (25%)
Funding/Support: None declared.
Keywords:
References
1. Gupta UC, Gupta SC. Trace element toxicity relationships to crop production and livestock and human health: implications for management. Commun Soil Sci Plant Analysis. 1998;29(11-14):1491-522. [Link] [DOI:10.1080/00103629809370045]
2. Underwood E. Trace elements in human and animal nutrition. New York: Elsevier; 2012. [Link]
3. Cai L, Li XK, Song Y, Cherian MG. Essentiality, toxicology and chelation therapy of zinc and copper. Curr Med Chem. 2005;12(23):2753-63. [Link] [DOI:10.2174/092986705774462950]
4. Oliver MA. Soil and human health: a review. Eur J Soil Sci. 1997;48(4):573-92. [Link] [DOI:10.1111/j.1365-2389.1997.tb00558.x]
5. Angelova M, Asenova S, Nedkova V, Koleva-Kolarova R. Copper in the human organism. Trakia J Sci. 2011;9(1):88-98. [Link]
6. Huster D. Wilson disease. Best Pract Res Clin Gastroenterol. 2010;24(5):531-9. [Link] [DOI:10.1016/j.bpg.2010.07.014]
7. Osredkar J, Sustar N. Copper and zinc, biological role and significance of copper/zinc imbalance. J Clinic Toxicol S. 2011;3(2161):0495. [Link] [DOI:10.4172/2161-0495.S3-001]
8. Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013. 4(2):176-90. [Link] [DOI:10.3945/an.112.003210]
9. Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons, seventh edition. New York: McGraw-Hill Medical. 2008. [Link]
10. Arslan M, Demir H, Arslan H, Gokalp AS, Demir C. Trace elements, heavy metals and other biochemical parameters in malignant glioma patients. Asian Pac J Cancer Prev. 2011;12(2):447-51. [Link]
11. Zhang X, Yang Q. Association between serum copper levels and lung cancer risk: A meta-analysis. J Int Med Res. 2018;46(12):4863-73. [Link] [DOI:10.1177/0300060518798507]
12. Martínez-Peinado M, Rueda-Robles A, Nogueras-López F, Villalón-Mir M, Oliveras-López MJ, Navarro-Alarcón M. Serum zinc and copper concentrations and ratios in cirrhotic patients: correlation with severity index. Nutr Hosp. 2018;35(3):627-32. [Link] [DOI:10.20960/nh.1579]
13. Alshebly SAK. et al. Serum levels of lead, cadmium and silver in patients with breast cancer compared with healthy females in Iraq. in AIP Conf Proc. 2019;2086:030048. [Link] [DOI:10.1063/1.5095133]
14. SilvestrebM MD, Lagarda J, Farré R, Martı́nez-CostaC, Brines J. Copper, iron and zinc determinations in human milk using FAAS with microwave digestion. Food Chem. 2000;68(1):95-9. [Link] [DOI:10.1016/S0308-8146(99)00160-0]
15. Zhang X, Yang Q. Association between serum copper levels and lung cancer risk: A meta-analysis. Journal of International Medical Research. 2018;46(12):4863-4873. [Link] [DOI:10.1177/0300060518798507]
16. Gómez NN, Biaggio VS, Ciminari ME, Pérez Chaca ME, Álvarez SM. Zinc: What is your role in lung cancer?. Nutritional Deficiency. 2016; Chapter 3:47-53. [Link] [DOI:10.5772/63209]
17. Wang Y, Sun Z, Li A, Zhang Y. Association between serum zinc levels and lung cancer: a meta-analysis of observational studies. World J Surg Oncol. 2019;17(1):1-8. [Link] [DOI:10.1186/s12957-019-1617-5]
18. Zhao HW, Lin J, Wang XB, Cheng X, Wang JY, Yong J, et al. Assessing Plasma Levels of Selenium, Copper, Iron and Zinc in Patients of Parkinson's Disease. PLOS ONE. 2013;8(12):e83060. [Link] [DOI:10.1371/journal.pone.0083060]
19. Emre O, Demir H, Dogan E, Esen R, Gur T, Demir C, et al. Plasma concentrations of some trace element and heavy metals in patients with metastatic colon cancer. J Cancer Ther. 2013;4:1085. [Link] [DOI:10.4236/jct.2013.46124]
20. Demir DC. Determining the levels of some trace elements and heavy metals (Cu, Mn, Mg, Fe, Zn, CO, Pb and CD) in the cancers of lip and oral cavity. Res Sq. 2021 Mar. [Link] [DOI:10.21203/rs.3.rs-309806/v1]
21. Diez M. Cerdan FJ, Arroyo M, Balibrea JL. Use of the copper/zinc ratio in the diagnosis of lung cancer. Cancer. 1989;63(4):726-730
https://doi.org/10.1002/1097-0142(19890215)63:4<726::AID-CNCR2820630421>3.0.CO;2-P [Link] [DOI:10.1002/1097-0142(19890215)63:43.0.CO;2-P]
22. Jin Y, Zhang C, Xu H, Xue S, Wang Y, Hou Y, Kong Y, Xu Y. Combined effects of serum trace metals and polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: a hospital-based case-control study in China. Cancer Epidemiol. 2011;35(2):182-7. [Link] [DOI:10.1016/j.canep.2010.06.004]
23. Zowczak M, Iskra M, Paszkowski J, Mańczak M, Torliński L, Wysocka E. Oxidase activity of ceruloplasmin and concentrations of copper and zinc in serum of cancer patients. J Trace Elements Med Biol. 2001;15(2-3):193-6. [Link] [DOI:10.1016/S0946-672X(01)80066-3]
24. Wu HDI, Chou SY, Chen DR, Kuo HW. Differentiation of serum levels of trace elements in normal and malignant breast patients. Biological Trace Element Research. 2006;113(1):9-18. [Link] [DOI:10.1385/BTER:113:1:9]
25. Cobanoglu U, Demir H, Sayir F, Duran M, Mergan D. Some mineral, trace element and heavy metal concentrations in lung cancer. Asian Pac J Cancer Preve. 2010;11(5):1383-8. [Link]
26. Yari H, Mohseni M, Vardi R, Mirza Alizadeh A, Mazloomzadeh S. Copper, Lead, Zinc and Cadmium levels in serum of prostate cancer patients by polarography in Iran. J Chem Pharmaceut Res. 2015;7(2):403-8. [Link]
27. Serum levels of lead, cadmium and silver in patients with breast cancer compared with healthy females in Iraq. AIP Conference Proceedings. 2019;2086(1):030048 [Link]








