Volume 14, Issue 2 (2022)
Iran J War Public Health 2022, 14(2): 203-210 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/04/8 | Accepted: 2022/06/7 | Published: 2022/06/30
Received: 2022/04/8 | Accepted: 2022/06/7 | Published: 2022/06/30
How to cite this article
Abdul Jaleel A, Almulla A, Alnaji H, Mansor M, Abbas Abo Algon A. Diabetes Mellitus and Non-Proliferative Diabetic Retinopathy Are Accompanied by Increase Pro-Inflammatory Conditions Indicated by a High Blood-Derived Levels of Monocyte Chemoattractant Protein-1 and Interleukin-8. Iran J War Public Health 2022; 14 (2) :203-210
URL: http://ijwph.ir/article-1-1165-en.html
URL: http://ijwph.ir/article-1-1165-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Technology Department, College of Medical Technology, The Islamic University, Najaf, Iraq
2- Iraqi Education Ministry, Najaf, Iraq
2- Iraqi Education Ministry, Najaf, Iraq
Full-Text (HTML) (899 Views)
Introduction
Diabetes mellitus (DM) is a multifaceted illness of carbohydrate metabolism characterized by hyperglycemia and impaired glucose tolerance as the disease's main symptoms, resulting from resistance to the action of insulin [1, 2]. It is one of the major health crises of the 21st century and elevated mortality to be the eighth greatest cause of death in the USA in 2020 [3]. There are many types of DM, type 1 (T1DM) and type 2 DM (T2DM) represent 96% of all cases [4]. There are many differences between these two types, and the progression in the β-cell failure is common, although more obvious in the T1DM [5].
T2DM is identified through inflammatory disorders that provoke diminished metabolic compliance to insulin hormone known as insulin resistance (IR) in various human body tissues. The most affected organs are adipose tissues and skeletal muscles [2]. Consequently, β-cells of the pancreas will lower insulin production in response to the pronounced systemic inflammatory disease [3, 6]. T2DM is accompanied by several complications divided into macrovascular such as coronary heart disease and microvascular diseases like diabetes, namely diabetic neuropathy, nephropathy, and retinopathy [7].
Obesity is a potent predisposing factor for T2DM since it is considered a stressor for adipocytes and the whole metabolism [8, 9]. The link between obesity and DM emerged from IR and immune activation followed by releasing various cytokines and adipokines in obese patients [10, 11]. Bianca et al. revealed that long-term inflammation in adipose tissues and chronic stress of β-cells provoke adaptive immunity and contribute to the progression of inflammatory reaction [12]. However, several cohort studies reported that the onset of diabetes is explained in part by chronic low-grade inflammation [13-15].
In addition, there is an elevation in the levels of inflammatory markers, namely; acute phase proteins (ACPs) such as C-reactive protein (CRP), pro-inflammatory cytokines like interleukin-1β (IL)-1β, IL-6, IL-8, and IL-18 along with anti-inflammatory cytokines such as IL-1Ra all these substances were found as a pre-disease signal [16-18]. These biomarkers were also found to be high in patients with impaired fasting glucose [15, 19], thus another evidence for implicating them in the pathophysiology of DM.
DM is one of the causes of ocular dysfunction and visual loss consequent of diabetic retinopathy (DR), a major microvascular complication of DM [20]. DR is considered a major cause of blindness, where 18.5% of them result from DR, wherein macular edema is the main cause [21, 22]. In the middle east and north Africa, the percentage mean prevalence of DR is 32.90% [23]. Retinal microvascular induced by hyperglycemia, inflammation, and retinal neurodegeneration are the most common pathogenies of DR [21]. The researchers have considered inflammation since hyperleukocytosis was seen in the early stages of DR [21]. Miyamoto et al. revealed increased adherence of leukocytes in the retinal vasculature followed a short time of enhancing DM in an animal model [24]. Moreover, chemokines, which play an essential role in attracting and enticing white blood cells, were also found in DR patients such as monocytes chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1alpha (MIP-1α), and MIP-1β [25-27]. Furthermore, pro-inflammatory cytokines including TNF-α, and IL-6 were also detected in high concentrations and contributed to the progression of DR.
Previously, Hernandez et al. revealed vitreous fluid elevation of IL-8, and MCP-1 while the diminished concentration of IL-10 [28] in patients with proliferative diabetic retinopathy (PDR). Besides, they reported a significant correlation between these cytokine levels and PDR activity. Moreover, recent data from a review article showed increased MCP-1 levels in patients with different phases of DR measured in various specimens, namely, epiretinal membrane, cultured Muller cells, serum, vitreous and aqueous humor [27]. Thus, staging of DR was suggested to be associated with the levels of cytokine expression [27].
Therefore, there is an immune stimulation and inflammatory response during T2DM and contributed to the progression of DR confirmed by many former shreds of evidence. This study was aimed to investigate the levels of MCP-1 and IL-8 in the patients with DM and the early phase of DR and to discriminate their concentration between only DM patients and NDR to examine whether it can a predictor of the progression of DR.
Material and Methods
In our current study, 150 participants were involved which are categorized as 50 healthy control (HC) and 100 patients. Furthermore, the patients were divided into two groups namely fifty patients with diabetes mellitus and fifty non-proliferative diabetic retinopathies associated with diabetes mellitus. All the patients were diagnosed with type 2 diabetes mellitus. The healthy control group was from the same population of the patients which were gathered from the center of diabetes mellitus and endocrine, al-Sadar teaching hospital, Najaf, Iraq from December 2020 to February 2021. Healthy control was in part from the staff of the hospital and the friends of the authors. All the patients were diagnosed via a specialized ophthalmologist based on clinical diagnosis and laboratory assessment.
Patients were excluded if they are chronic inflammatory diseases along with diabetes mellitus such as rheumatoid arthritis, ulcerative colitis, Crohn's disease, and inflammatory bowel disease as well as those with thyroid disorders. C-reactive protein levels in all of the participants were less than 6mg/L, indicating that there was no evidence of inflammation. Some of the patients were under the treatment (galvus 50/850 and 50/1000) as well as some of them were on glimepiride 4mg, for retinopathic patients eylea 2mg were prescribed. Iraqi and international ethics and privacy laws were followed during the study. In order to take part in this study, all participants and first-degree relatives of participants with schizophrenia provided their written informed consent (their legally authorized representatives are the father, mother, spouse, son, or brother). IRB approval was obtained from the College of Medicine at the University of Kufa in Iraq (347/2019), which is compliant with the Declaration of Helsinki's International Guideline for Human Research Protection.
On the same day of the clinical interview, 5 ml of venous blood sample was taken while the patients were fasting (> 8 h) as well as the same condition was applied with healthy control. EDTA tubes were utilized to store the blood for HbA1c along with a serum gel tube for the rest of the assays. Early morning 8:00 and 10:00 a.m. was the time for venipuncture via a plastic syringe provided with a disposable needle. Before 10 minutes and 3500 rpm centrifugation, the blood samples were left at room temperature for 15 minutes. A fasting blood sugar test was performed to assay the blood glucose concentration, and the rest of the serum were stored at -80°C until the day of ELISA analysis after it was transferred into separate Eppendorf. A kit supplied by Linear Cromatest, Spain, was utilized to measure serum CRP; this test was based on the latex agglutination principle. A specialized clinician recorded all required socio-demographic characteristics from patients and the author did that with healthy control. On the same day as the clinical interview, Body mass index (BMI) was calculated by dividing body weight (kg) by height (m2). Besides, the smoking state was identified. Tests within the diabetic panel were performed spectrophotometrically, fasting blood sugar by enzymatic colorimetric methods using linear Cromatest kit provided via LINEAR CHEMICALS, Spain, similar for HbA1c. Immune biomarkers namely MCP-1 and IL-8 were assayed using commercial ELISA sandwich kits provided (Elabscience, Inc., CA, USA), the assay procedure was performed by a microplate ELISA reader from a human company in Germany. We used sample dilution when necessary for samples containing highly concentrated biomarkers. In the data set, there were no missing values for any assays.
Kolmogorov–Smirnov test was used to test the normality of the data, all the variables followed non-normal distribution. Kruskal-Wallis one-way ANOVA was hired to delineate the differences in variables between groups as well as for the nominal variables (e.g., gender, smoking state), and analysis of contingency table (χ2 test) was employed to check the association. The association between biomarkers was identified through the computation of the correlation utilizing Spearman’s rank-order correlation coefficients. As part of our study, we recruited multivariate general linear model (GLM) analysis to examine how a diagnosis affects biomarkers and their composite scores while controlling for confounding variables such as nicotine dependence and gender. And hence, we conducted tests for between-subjects effects to identify the independent variables' effects on biomarkers.
Findings
Table 1 of the current study showed the results of the comparison study of the socio-demographic and clinical characteristics between the recruited patients and HC. Patients were divided into two groups namely; diabetic patients and diabetic patients with retinopathy determined by clinical examination along with the HC group. No significant differences were detected concerning; age, height, gender, and smoking whereas significant differences (p<0.001) emerged in each of weight, BMI, duration of illness (DOI), fasting blood sugar (FBS), glycated hemoglobin (HbA1c), MCP-1 and IL-8. All these variables were significantly different among patients and HC, as well as weight and MCP-1, were significantly different between the two groups of patients. MCP-1 was increased in DM patients to DM+RT patients and most of the variables were in the same direction.
Table 1) The data of clinical biomarkers and sociodemographic characteristics between patients and healthy control
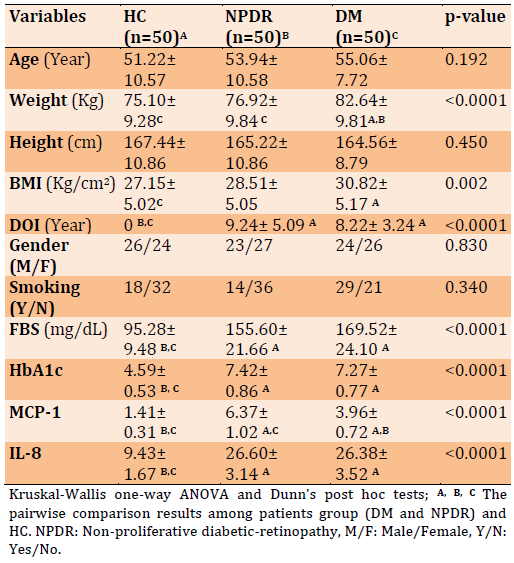
Herein the Table 2 data of the correlation study are presented, where the clinical biomarkers and some sociodemographic variables are correlated to each other. There is a significant positive correlation between DOI and each of MCP-1, IL-8, and HbA1c. Moreover, FBS significantly has a positive correlation among each MCP-1, IL-8, HbA1c, and DOI. While BMI showed a significant correlation with only DOI. To a lesser extent, some other variables are also correlated as shown in Table 2.
Table 2) The correlation results between measured biomarkers and other sociodemographic characteristics
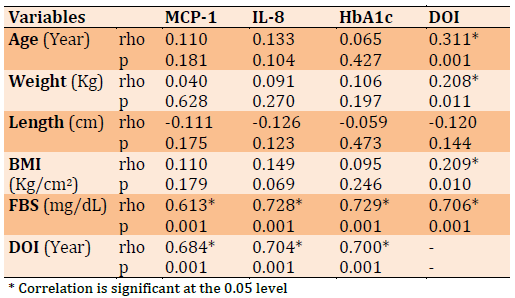
Figure 1 showed there is a significant positive strong correlation (rho=0.721, p<0.001) between MCP-1 and HbA1c, which means an increased level of HbA1c accompanied by elevation of MCP-1.
Figure 1) The correlation between MCP-1 and HbA1c
The results in Figure 2 display a significant positive strong correlation (rho=0.643, p<0.001) between IL-8 and HbA1c, which indicates that patients with poor control of diabetes (reflected with high HbA1c level) will also show an increased level of IL-8.
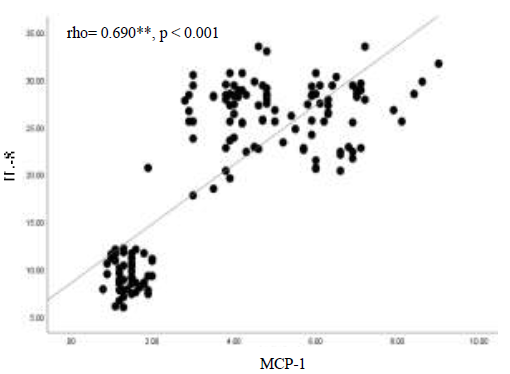
In Figure 3 there is evidence for a direct significant correlation (rho=0.690, p<0.001), as shown it is a strong positive association between those two chemokines in patients with DM, which means an increase in one will affect the other to elevate.
The data in Table 3 indicate the multiple regression analysis where the biomarkers were the dependent variables and diagnosis, age, gender, DOI and BMI are the explanatory variables. In Table 3 regression no. 1 displayed that the diagnosis is a highly significant effector on the biomarkers with a large effect size of 0.728, while no significant effect is detected from the rest of all confounding variables. Test between-subject effect demonstrates all of the biomarkers affect significantly with a huge effect size on the diagnosis, however, the highest effects come from MCP-1 and IL-8. Regression no. 2 of Table 3 showed the results of GLM as the composite score of biomarkers as a dependent variable and explanatory variable represented by diagnosis, age, DOI, BMI, and gender. The results indicate that the combined four biomarkers significantly affect the diagnosis with a large effect size of 0.664. Besides, the Test between-subject effect displayed all the computed composite scores effects on the diagnosis with a large effect size but the highest effect exerts through combined IL-8 and MCP-1.
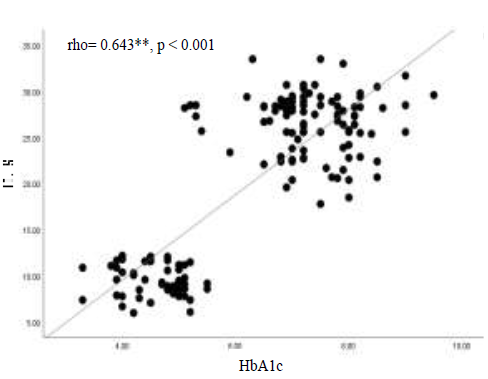
Figure 2) The correlation between IL-8 and HbA1c
Diabetes mellitus (DM) is a multifaceted illness of carbohydrate metabolism characterized by hyperglycemia and impaired glucose tolerance as the disease's main symptoms, resulting from resistance to the action of insulin [1, 2]. It is one of the major health crises of the 21st century and elevated mortality to be the eighth greatest cause of death in the USA in 2020 [3]. There are many types of DM, type 1 (T1DM) and type 2 DM (T2DM) represent 96% of all cases [4]. There are many differences between these two types, and the progression in the β-cell failure is common, although more obvious in the T1DM [5].
T2DM is identified through inflammatory disorders that provoke diminished metabolic compliance to insulin hormone known as insulin resistance (IR) in various human body tissues. The most affected organs are adipose tissues and skeletal muscles [2]. Consequently, β-cells of the pancreas will lower insulin production in response to the pronounced systemic inflammatory disease [3, 6]. T2DM is accompanied by several complications divided into macrovascular such as coronary heart disease and microvascular diseases like diabetes, namely diabetic neuropathy, nephropathy, and retinopathy [7].
Obesity is a potent predisposing factor for T2DM since it is considered a stressor for adipocytes and the whole metabolism [8, 9]. The link between obesity and DM emerged from IR and immune activation followed by releasing various cytokines and adipokines in obese patients [10, 11]. Bianca et al. revealed that long-term inflammation in adipose tissues and chronic stress of β-cells provoke adaptive immunity and contribute to the progression of inflammatory reaction [12]. However, several cohort studies reported that the onset of diabetes is explained in part by chronic low-grade inflammation [13-15].
In addition, there is an elevation in the levels of inflammatory markers, namely; acute phase proteins (ACPs) such as C-reactive protein (CRP), pro-inflammatory cytokines like interleukin-1β (IL)-1β, IL-6, IL-8, and IL-18 along with anti-inflammatory cytokines such as IL-1Ra all these substances were found as a pre-disease signal [16-18]. These biomarkers were also found to be high in patients with impaired fasting glucose [15, 19], thus another evidence for implicating them in the pathophysiology of DM.
DM is one of the causes of ocular dysfunction and visual loss consequent of diabetic retinopathy (DR), a major microvascular complication of DM [20]. DR is considered a major cause of blindness, where 18.5% of them result from DR, wherein macular edema is the main cause [21, 22]. In the middle east and north Africa, the percentage mean prevalence of DR is 32.90% [23]. Retinal microvascular induced by hyperglycemia, inflammation, and retinal neurodegeneration are the most common pathogenies of DR [21]. The researchers have considered inflammation since hyperleukocytosis was seen in the early stages of DR [21]. Miyamoto et al. revealed increased adherence of leukocytes in the retinal vasculature followed a short time of enhancing DM in an animal model [24]. Moreover, chemokines, which play an essential role in attracting and enticing white blood cells, were also found in DR patients such as monocytes chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1alpha (MIP-1α), and MIP-1β [25-27]. Furthermore, pro-inflammatory cytokines including TNF-α, and IL-6 were also detected in high concentrations and contributed to the progression of DR.
Previously, Hernandez et al. revealed vitreous fluid elevation of IL-8, and MCP-1 while the diminished concentration of IL-10 [28] in patients with proliferative diabetic retinopathy (PDR). Besides, they reported a significant correlation between these cytokine levels and PDR activity. Moreover, recent data from a review article showed increased MCP-1 levels in patients with different phases of DR measured in various specimens, namely, epiretinal membrane, cultured Muller cells, serum, vitreous and aqueous humor [27]. Thus, staging of DR was suggested to be associated with the levels of cytokine expression [27].
Therefore, there is an immune stimulation and inflammatory response during T2DM and contributed to the progression of DR confirmed by many former shreds of evidence. This study was aimed to investigate the levels of MCP-1 and IL-8 in the patients with DM and the early phase of DR and to discriminate their concentration between only DM patients and NDR to examine whether it can a predictor of the progression of DR.
Material and Methods
In our current study, 150 participants were involved which are categorized as 50 healthy control (HC) and 100 patients. Furthermore, the patients were divided into two groups namely fifty patients with diabetes mellitus and fifty non-proliferative diabetic retinopathies associated with diabetes mellitus. All the patients were diagnosed with type 2 diabetes mellitus. The healthy control group was from the same population of the patients which were gathered from the center of diabetes mellitus and endocrine, al-Sadar teaching hospital, Najaf, Iraq from December 2020 to February 2021. Healthy control was in part from the staff of the hospital and the friends of the authors. All the patients were diagnosed via a specialized ophthalmologist based on clinical diagnosis and laboratory assessment.
Patients were excluded if they are chronic inflammatory diseases along with diabetes mellitus such as rheumatoid arthritis, ulcerative colitis, Crohn's disease, and inflammatory bowel disease as well as those with thyroid disorders. C-reactive protein levels in all of the participants were less than 6mg/L, indicating that there was no evidence of inflammation. Some of the patients were under the treatment (galvus 50/850 and 50/1000) as well as some of them were on glimepiride 4mg, for retinopathic patients eylea 2mg were prescribed. Iraqi and international ethics and privacy laws were followed during the study. In order to take part in this study, all participants and first-degree relatives of participants with schizophrenia provided their written informed consent (their legally authorized representatives are the father, mother, spouse, son, or brother). IRB approval was obtained from the College of Medicine at the University of Kufa in Iraq (347/2019), which is compliant with the Declaration of Helsinki's International Guideline for Human Research Protection.
On the same day of the clinical interview, 5 ml of venous blood sample was taken while the patients were fasting (> 8 h) as well as the same condition was applied with healthy control. EDTA tubes were utilized to store the blood for HbA1c along with a serum gel tube for the rest of the assays. Early morning 8:00 and 10:00 a.m. was the time for venipuncture via a plastic syringe provided with a disposable needle. Before 10 minutes and 3500 rpm centrifugation, the blood samples were left at room temperature for 15 minutes. A fasting blood sugar test was performed to assay the blood glucose concentration, and the rest of the serum were stored at -80°C until the day of ELISA analysis after it was transferred into separate Eppendorf. A kit supplied by Linear Cromatest, Spain, was utilized to measure serum CRP; this test was based on the latex agglutination principle. A specialized clinician recorded all required socio-demographic characteristics from patients and the author did that with healthy control. On the same day as the clinical interview, Body mass index (BMI) was calculated by dividing body weight (kg) by height (m2). Besides, the smoking state was identified. Tests within the diabetic panel were performed spectrophotometrically, fasting blood sugar by enzymatic colorimetric methods using linear Cromatest kit provided via LINEAR CHEMICALS, Spain, similar for HbA1c. Immune biomarkers namely MCP-1 and IL-8 were assayed using commercial ELISA sandwich kits provided (Elabscience, Inc., CA, USA), the assay procedure was performed by a microplate ELISA reader from a human company in Germany. We used sample dilution when necessary for samples containing highly concentrated biomarkers. In the data set, there were no missing values for any assays.
Kolmogorov–Smirnov test was used to test the normality of the data, all the variables followed non-normal distribution. Kruskal-Wallis one-way ANOVA was hired to delineate the differences in variables between groups as well as for the nominal variables (e.g., gender, smoking state), and analysis of contingency table (χ2 test) was employed to check the association. The association between biomarkers was identified through the computation of the correlation utilizing Spearman’s rank-order correlation coefficients. As part of our study, we recruited multivariate general linear model (GLM) analysis to examine how a diagnosis affects biomarkers and their composite scores while controlling for confounding variables such as nicotine dependence and gender. And hence, we conducted tests for between-subjects effects to identify the independent variables' effects on biomarkers.
Findings
Table 1 of the current study showed the results of the comparison study of the socio-demographic and clinical characteristics between the recruited patients and HC. Patients were divided into two groups namely; diabetic patients and diabetic patients with retinopathy determined by clinical examination along with the HC group. No significant differences were detected concerning; age, height, gender, and smoking whereas significant differences (p<0.001) emerged in each of weight, BMI, duration of illness (DOI), fasting blood sugar (FBS), glycated hemoglobin (HbA1c), MCP-1 and IL-8. All these variables were significantly different among patients and HC, as well as weight and MCP-1, were significantly different between the two groups of patients. MCP-1 was increased in DM patients to DM+RT patients and most of the variables were in the same direction.
Table 1) The data of clinical biomarkers and sociodemographic characteristics between patients and healthy control

Herein the Table 2 data of the correlation study are presented, where the clinical biomarkers and some sociodemographic variables are correlated to each other. There is a significant positive correlation between DOI and each of MCP-1, IL-8, and HbA1c. Moreover, FBS significantly has a positive correlation among each MCP-1, IL-8, HbA1c, and DOI. While BMI showed a significant correlation with only DOI. To a lesser extent, some other variables are also correlated as shown in Table 2.
Table 2) The correlation results between measured biomarkers and other sociodemographic characteristics

Figure 1 showed there is a significant positive strong correlation (rho=0.721, p<0.001) between MCP-1 and HbA1c, which means an increased level of HbA1c accompanied by elevation of MCP-1.

Figure 1) The correlation between MCP-1 and HbA1c
The results in Figure 2 display a significant positive strong correlation (rho=0.643, p<0.001) between IL-8 and HbA1c, which indicates that patients with poor control of diabetes (reflected with high HbA1c level) will also show an increased level of IL-8.
In Figure 3 there is evidence for a direct significant correlation (rho=0.690, p<0.001), as shown it is a strong positive association between those two chemokines in patients with DM, which means an increase in one will affect the other to elevate.
The data in Table 3 indicate the multiple regression analysis where the biomarkers were the dependent variables and diagnosis, age, gender, DOI and BMI are the explanatory variables. In Table 3 regression no. 1 displayed that the diagnosis is a highly significant effector on the biomarkers with a large effect size of 0.728, while no significant effect is detected from the rest of all confounding variables. Test between-subject effect demonstrates all of the biomarkers affect significantly with a huge effect size on the diagnosis, however, the highest effects come from MCP-1 and IL-8. Regression no. 2 of Table 3 showed the results of GLM as the composite score of biomarkers as a dependent variable and explanatory variable represented by diagnosis, age, DOI, BMI, and gender. The results indicate that the combined four biomarkers significantly affect the diagnosis with a large effect size of 0.664. Besides, the Test between-subject effect displayed all the computed composite scores effects on the diagnosis with a large effect size but the highest effect exerts through combined IL-8 and MCP-1.

Figure 2) The correlation between IL-8 and HbA1c

Figure 3) The correlation between IL-8 and MCP-1
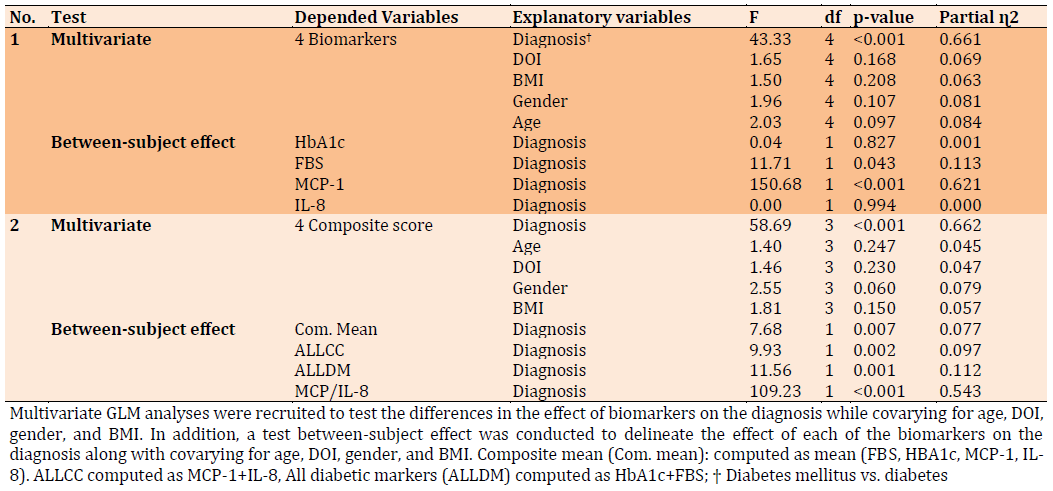
Table 4 showed the results of multivariate GLM analysis performed between the two groups of patients. In regression no. 1, diagnosis, DOI, BMI, age, and gender are the explanatory variable and four biomarkers represent the dependent variable in our model. The data indicate that four biomarkers significantly affect the diagnosis with a large effect size of 0.661 while no significant effects arise from other confounding variables. Test between-subject effect show MCP-1 is the only biomarker that significantly affects the discrimination between two groups of patients with a large effect size whereas no significant effect from other biomarkers.
Moreover, regression no. 2 in Table 4 manifests the results of GLM when the explanatory variables are the same in regression no. 1 while the dependent variable was computed as a composite score. The diagnosis is strongly explained with a composite score of biomarkers with a large effect size of 0.662 while no effect with other confounding variables. Test between subjects show all the computed composite scores affect the diagnosis, nevertheless, MCP-1/ IL-8 was the most effector on the diagnosis with a larger effect size of 0.543 in comparison with other biomarkers.
Table 4 showed the results of multivariate GLM analysis performed between the two groups of patients. In regression no. 1, diagnosis, DOI, BMI, age, and gender are the explanatory variable and four biomarkers represent the dependent variable in our model. The data indicate that four biomarkers significantly affect the diagnosis with a large effect size of 0.661 while no significant effects arise from other confounding variables. Test between-subject effect show MCP-1 is the only biomarker that significantly affects the discrimination between two groups of patients with a large effect size whereas no significant effect from other biomarkers.
Moreover, regression no. 2 in Table 4 manifests the results of GLM when the explanatory variables are the same in regression no. 1 while the dependent variable was computed as a composite score. The diagnosis is strongly explained with a composite score of biomarkers with a large effect size of 0.662 while no effect with other confounding variables. Test between subjects show all the computed composite scores affect the diagnosis, nevertheless, MCP-1/ IL-8 was the most effector on the diagnosis with a larger effect size of 0.543 in comparison with other biomarkers.
Table 3) Results of multivariate GLM analysis with the biomarkers as dependent variables and diagnosis with other demographic factors as an explanatory variable
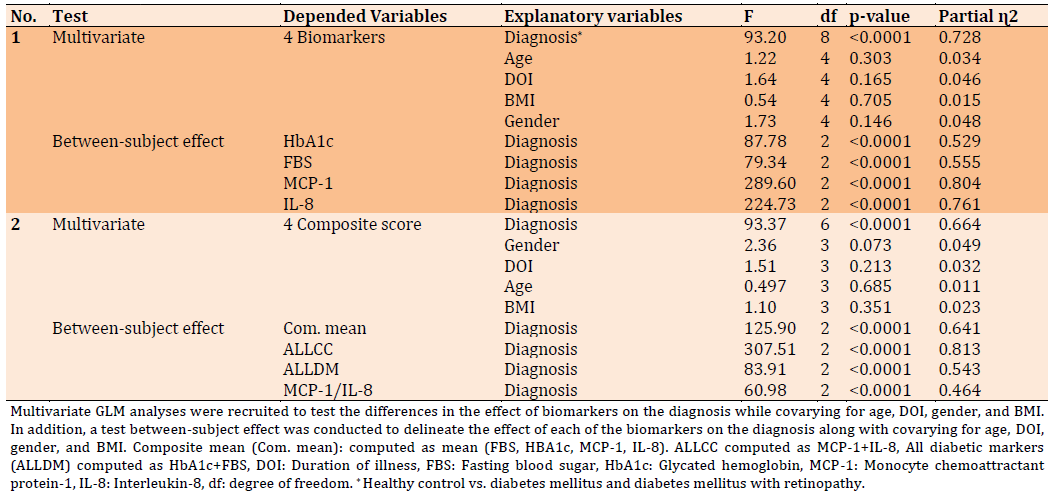
Table 4) Results of multivariate GLM analysis with the 4 biomarkers as dependent variables and diagnosis (DM and NPDR) with other demographic factors as the explanatory variable
Discussion
The first major findings of the comparison study revealed a significant difference in MCP-1 and IL-8 (p<0.0001) between patients in both groups (DM and NPDR) and healthy control besides the weight, DOI, and HBA1c as shown in Table 1 while no significant difference with the rest of variables either with control or within the groups of patients. Moreover, the comparison within two groups of patients showed no significant difference between the variables except MCP-1 (p<0.0001) significantly different between DM patients and NPDR. MCP-1 level was found in many studies elevated in patients with DR hence these studies confirm the present results [26, 29]. In patients with diabetic neuropathy, Mussa et al. recently reported results consistent with ours [30]. Although the frank role of these cytokines is still ambiguous in DM, a review article based on many studies reported improvement in the symptoms was noticed after diminished MCP-1 level [31]. Hence, MCP-1 and other pro-inflammatory cytokines may be implicated in the pathophysiology of DM as well as they may be the triggers for most of the complications.
The second major finding of the present study showed a significant positive correlation between DOI and MCP-1, IL-8 and HbA1c as presented in Table 2. Recently, results from a meta-analysis revealed the implication of many chemokines including MCP-1 and IL-8 in the progression of DM [32]. Moreover, the present results also found a significant strong positive correlation (p<0.0001) between HbA1c and the measured chemokines as shown in Figures 1 and 2. Therefore, these cytokines may be able to predict the staging and the worsening of the DM and even extend to the DR. IL-8 and MCP-1 were found to be released from adipose tissues which is increasing as the fat mass elevated in animals [33, 34], that may explain in part why obesity is a risk factor for DM. Our data didn’t comply with these results since there is no significant correlation between BMI and these cytokines. In the adipocytes, the induction of glucose uptake through insulin decreased in the presence of MCP-1 according to the results reported by Sartipy et al., which proves that insulin resistance may be triggered by chemokines [35]. The complication of DM like atherosclerosis may also explain in part by an elevated level of MCP-1 and to a lesser extent IL-8 since Kim et al. found a negative correlation of them with HDL-C while a direct positive correlation with C-reactive protein [36].
The third major finding revealed through GLM, where Table 3 shows the results as the biomarkers are dependent variables and diagnosis along with other variables are explanatory. The results indicate that MCP-1 and IL-8 could significantly (p<0.0001) predicts the diagnosis even if the effect size is much more than HbA1c and FBS, but the latter is more reliable as they are directly related to the concentration of glucose in the blood, but the MCP-1 and IL-8 reflects the severity of inflammatory conditions in the DM patients. Furthermore, the effect size represented by (ɳ2) will increase when the dependent variables are calculated as composite scores as shown in Table 3. In agreement with our results, many previous articles were published, and their data shows high levels of IL-8 and MCP-1 in patients with DM [26, 37]. Several pro-inflammatory chemokines begin to release by the islet cells of the pancreas beside the visceral fat followed by exposure of these tissues to an early injury [32]. The chemotaxis effect of these chemokines invites many other cytokines to be inside islets and visceral cells for preparing for immune invasion [32]. Thus, the dysfunction of beta cells of the pancreas after a while of onset of the disease could be attributed to the activation of pro-inflammatory conditions.
In addition, Table 4 showed the GLM results within the groups of patients namely DM and NPDR where the biomarkers are the dependent variables and diagnosis, age, gender, BMI, and DOI are the explanatory variables. Our findings revealed only MCP-1 displayed a significant difference (p<0.0001) among the two groups with diagnosis, hence it may be the part that discriminates the patients with NPDR. While no significant difference with other explanatory variables. However, taking the biomarkers as a composite score make more effectors of diagnosis, but also no effect upon other explanatory variables. Previous studies showed a high level of MCP-1 and IL-8 along with other pro-inflammatory cytokines in the vitreous fluid of patients with PDR [28, 38-40]. Thus, the progression of the DR may be partially attributed to an increase in these cytokines as long as the presence of a pronounced pro-inflammatory condition. Nonetheless, it should be considered the differences between diverse media of assessment of these cytokines, where Almulla et al., revealed with a recent meta-analysis that levels of blood-derived metabolites are not consistent with those in the brain tissue or cerebrospinal fluids [41]. All in all, the inflammatory conditions and increased blood levels of cytokines should be taken into account during the monitoring of the DM patients because dysregulated levels may aggravate the complications not limited to DR.
This study should be addressed along with some limitations; first, the medication profile of all DM patients was not considered. Second, the small sample size within the groups of patients. Third, the history of COVID-19 infection was unknown during the time of sampling. Fourth, the exact time of the initial diagnosis with DR was ambiguous.
Conclusion
From the results, we could conclude the following; first, MCP-1 and IL-8 were increased in DM patients which is clear evidence for the probable implication of those chemokines in the pathophysiology of DM and developing NPDR. Second, an increasing level of MCP-1 is more likely to predict developing NPDR. Hence, the pro-inflammatory conditions are a part of the pathological factors of DM and its complications and should be addressed with further research to figure out the whole frame of its role in those patients.
Acknowledgments: Our sincere thanks to the Islamic university for their support.
Ethical Permissions: This work was performed after IRB approval obtained from the College of Medicine at the University of Kufa in Iraq (347/2019), which is compliant with the Declaration of Helsinki's International Guideline for Human Research Protection. All of the participants provide written consent to confirm their acceptance to enroll in this research.
Conflicts of Interests: The authors announce that the work in this article is free from any competing financial interests or other effects that emerged from personal relationships.
Authors’ Contributions: Abdul Jaleel AK (First Author), Introduction Writer/Methodologist/Main Researcher (40%); Almulla AF (Second Author), Main Researcher/Statistical Analyst (30%); Alnaji HA (Third Author), Discussion Writer (10%); Mansor MR (Fourth Author), Assistant Researcher (10%); Abbas Abo Algon A (Fifth Author), Assistant Researcher (10%)
Funding/Support: This study was conducted with self-funding by the authors.
Keywords:
References
1. Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14(1):50-9. [Link] [DOI:10.15420/ecr.2018.33.1]
2. Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol. 2020;11:1582. [Link] [DOI:10.3389/fimmu.2020.01582]
3. Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829-30. [Link] [DOI:10.1001/jama.2021.5469]
4. Menke A, Orchard TJ, Imperatore G, Bullard KM, Mayer-Davis E, Cowie CC. The prevalence of type 1 diabetes in the United States. Epidemiology. 2013;24(5):773-4. [Link] [DOI:10.1097/EDE.0b013e31829ef01a]
5. Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 Suppl 2: S97-107. [Link] [DOI:10.2337/diabetes.54.suppl_2.S97]
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-33. [Link] [DOI:10.2337/dc21-S002]
7. Baynest HW. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J Diabetes Metab. 2015;6(5). [Link] [DOI:10.4172/2155-6156.1000541]
8. Grossmann V, Schmitt VH, Zeller T, Panova-Noeva M, Schulz A, Laubert-Reh D, et al. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care. 2015;38(7):1356-64. [Link] [DOI:10.2337/dc14-3008]
9. Algon AAA, Almulla A, Najm AH, Keshwan RA. Role of glucagon-like peptide-1 in appetite regulation in patients with morbid obesity and leptin resistance. Int J Peptide Res Therapeutics. 2019;26(1):579-83. [Link] [DOI:10.1007/s10989-019-09864-w]
10. Donath MY. Inflammation as a sensor of metabolic stress in obesity and type 2 diabetes. Endocrinology. 2011;152(11):4005-6. [Link] [DOI:10.1210/en.2011-1691]
11. Velloso LA, Eizirik DL, Cnop M. Type 2 diabetes mellitus--an autoimmune disease?. Nat Rev Endocrinol. 2013;9(12):750-5. [Link] [DOI:10.1038/nrendo.2013.131]
12. Itariu BK, Stulnig TM. Autoimmune aspects of type 2 diabetes mellitus - a mini-review. Gerontology. 2014;60(3):189-96. [Link] [DOI:10.1159/000356747]
13. Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system?. Diabetologia. 1998;41(10):1241-8. [Link] [DOI:10.1007/s001250051058]
14. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98-107. [Link] [DOI:10.1038/nri2925]
15. Luotola K, Pietilä A, Zeller T, Moilanen L, Kähönen M, Nieminen MS, et al. Associations between interleukin-1 (IL-1) gene variations or IL-1 receptor antagonist levels and the development of type 2 diabetes. J Intern Med. 2011;269(3):322-32. [Link] [DOI:10.1111/j.1365-2796.2010.02294.x]
16. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327-34. [Link] [DOI:10.1001/jama.286.3.327]
17. Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabák AG, Schloot NC, et al. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care. 2009;32(3):421-3. [Link] [DOI:10.2337/dc08-1161]
18. Cruz NG, Sousa LP, Sousa MO, Pietrani NT, Fernandes AP, Gomes KB. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013;99(2):85-92. [Link] [DOI:10.1016/j.diabres.2012.09.003]
19. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813-23. [Link] [DOI:10.2337/diacare.27.3.813]
20. Tsegaw A, Alemu S, Dessie A, Patterson CC, Parry EHO, Phillips DIW, et al. Diabetic retinopathy in type 2 diabetes mellitus patients attending the diabetic clinic of the University of Gondar Hospital, Northwest Ethiopia. J Ophthalmol. 2021;2021:6696548. [Link] [DOI:10.1155/2021/6696548]
21. Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19(6):1816. [Link] [DOI:10.3390/ijms19061816]
22. GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-60. [Link]
23. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580-91. [Link] [DOI:10.1016/j.ophtha.2021.04.027]
24. Miyamoto K, Hiroshiba N, Tsujikawa A, Ogura Y. In vivo demonstration of increased leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci. 1998;39(11):2190-4. [Link]
25. Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. 2011;55(3):256-63. [Link] [DOI:10.1007/s10384-011-0004-8]
26. Reddy S, Amutha A, Rajalakshmi R, Bhaskaran R, Monickaraj F, Rangasamy S, et al. Association of increased levels of MCP-1 and cathepsin-D in young onset type 2 diabetes patients (T2DM-Y) with severity of diabetic retinopathy. J Diabetes Complications. 2017;31(5):804-9. [Link] [DOI:10.1016/j.jdiacomp.2017.02.017]
27. Taghavi Y, Hassanshahi G, Kounis NG, Koniari I, Khorramdelazad H. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in diabetic retinopathy: latest evidence and clinical considerations. J Cell Commun Signal. 2019;13(4):451-62. [Link] [DOI:10.1007/s12079-018-00500-8]
28. Hernandez C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simo R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22(6):719-722. [Link] [DOI:10.1111/j.1464-5491.2005.01538.x]
29. Ozturk BT, Bozkurt B, Kerimoglu H, Okka M, Kamis U, Gunduz K. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol Vision. 2009;15:1906-14. [Link]
30. Mussa BM, Srivastava A, Al-Habshi A, Mohammed AK, Halwani R, Abusnana S. Inflammatory biomarkers levels in T2DM Emirati patients with diabetic neuropathy. Diabetes Metab Syndr Obes. 2021;14:3389-97. [Link] [DOI:10.2147/DMSO.S319863]
31. Panee J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60(1):1-12. [Link] [DOI:10.1016/j.cyto.2012.06.018]
32. Pan X, Kaminga AC, Wen SW, Liu A. Chemokines in prediabetes and type 2 diabetes: a meta-analysis. Front Immunol. 2021;12: 622438. [Link] [DOI:10.3389/fimmu.2021.622438]
33. Bruun JM, Lihn AS, Madan AK, Pedersen SB, Schiøtt KM, Fain JN, Richelsen B. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286(1):E8-13. [Link] [DOI:10.1152/ajpendo.00269.2003]
34. Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes. 2005;29(1):146-50. [Link] [DOI:10.1038/sj.ijo.0802839]
35. Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100(12):7265-70. [Link] [DOI:10.1073/pnas.1133870100]
36. Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30(9):1347-55. [Link] [DOI:10.1038/sj.ijo.0803259]
37. Borilova Linhartova P, Kavrikova D, Tomandlova M, Poskerova H, Rehka V, Dušek L, et al. Differences in interleukin-8 plasma levels between diabetic patients and healthy individuals independently on their periodontal status. Int J Mol Sci. 2018;19(10):3214. [Link] [DOI:10.3390/ijms19103214]
38. Funatsu H, Yamashita H, Noma H, Mimura T, Nakamura S, Sakata K, Hori S. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243(1):3-8. [Link] [DOI:10.1007/s00417-004-0950-7]
39. Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye. 2006;20(12):1366-9. [Link] [DOI:10.1038/sj.eye.6702138]
40. Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73-9. [Link] [DOI:10.1016/j.ophtha.2008.09.037]
41. Almulla AF, Vasupanrajit A, Tunvirachaisakul C, Al-Hakeim HK, Solmi M, Verkerk R, et al. The tryptophan catabolite or kynurenine pathway in schizophrenia: meta-analysis reveals dissociations between central, serum and plasma compartments. Mol Psychiatry. 2022 Apr. [Link] [DOI:10.1038/s41380-022-01552-4]








