Volume 14, Issue 2 (2022)
Iran J War Public Health 2022, 14(2): 231-242 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/04/1 | Accepted: 2022/05/25 | Published: 2022/05/30
Received: 2022/04/1 | Accepted: 2022/05/25 | Published: 2022/05/30
How to cite this article
Fadhil Kadhim E. Histological Evaluation of Vitronectin Protein/Angiopoietin-Like 4 Protein on Bone Healing in Rats. Iran J War Public Health 2022; 14 (2) :231-242
URL: http://ijwph.ir/article-1-1149-en.html
URL: http://ijwph.ir/article-1-1149-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
E. Fadhil Kadhim * 

College of Dentistry University Baghdad, Baghdad, Iraq
Full-Text (HTML) (702 Views)
Introduction
Bones are stiff organs that form part of a vertebrate's endoskeleton. They provide support and protection to the body's many organs, as well as the production of red and white blood cells and the storage of minerals [1].
There are three main stages of bone healing. Inflammation is the first stage of the bone healing process. During the early hours after an accident, tissue disruption causes a hematoma at the injury site. Local veins thrombose, resulting in bone necrosis at the fracture's margins. Increased capillary permeability creates a local inflammatory environment in which osteoinductive growth factors promote the proliferation and differentiation of mesenchymal stem cells. Repair: Periosteal callus forms along the periphery of the injury site (Intramembranous ossification initiated by preosteoblasts).
Intramedullary callus forms in the center of the fracture site (Endochondral ossification at the site of the fracture hematoma). Chemical and mechanical factors stimulate callus formation and mineralization. Woven bone is eventually transformed into the lamellar bone, and the Medullary cavity is reconstructed [2].
Vitronectin (VTN or VN) is a hemopexin family glycoprotein that is prevalent in serum, extracellular matrix, and bone. It is encoded by the VTN gene in humans [3].
Vitronectin enhances cell adhesion and spreading by binding to integrin alpha-V beta-3. It also binds to numerous serpins and reduces the membrane-damaging action of the terminal cytolytic complement pathway (serine protease inhibitors). It's a secreted protein that comes in two forms: a single chain and a clipped, two-chain form that's held together by a disulfide bond [4]. Vitronectin is thought to play a role in tumor malignancy and hemostasis [5].
Vitronectin is a 478-amino-acid glycoprotein with a molecular weight of 54kDa. Carbohydrates make up around a third of the protein's molecular mass. On rare occasions, the protein is cleaved after arginine 379, resulting in two-chain vitronectin with a disulfide bond connecting the two chains. Except for the N-terminal domain, no high-resolution structure has been established experimentally.
There are three domains in the protein:
Bones are stiff organs that form part of a vertebrate's endoskeleton. They provide support and protection to the body's many organs, as well as the production of red and white blood cells and the storage of minerals [1].
There are three main stages of bone healing. Inflammation is the first stage of the bone healing process. During the early hours after an accident, tissue disruption causes a hematoma at the injury site. Local veins thrombose, resulting in bone necrosis at the fracture's margins. Increased capillary permeability creates a local inflammatory environment in which osteoinductive growth factors promote the proliferation and differentiation of mesenchymal stem cells. Repair: Periosteal callus forms along the periphery of the injury site (Intramembranous ossification initiated by preosteoblasts).
Intramedullary callus forms in the center of the fracture site (Endochondral ossification at the site of the fracture hematoma). Chemical and mechanical factors stimulate callus formation and mineralization. Woven bone is eventually transformed into the lamellar bone, and the Medullary cavity is reconstructed [2].
Vitronectin (VTN or VN) is a hemopexin family glycoprotein that is prevalent in serum, extracellular matrix, and bone. It is encoded by the VTN gene in humans [3].
Vitronectin enhances cell adhesion and spreading by binding to integrin alpha-V beta-3. It also binds to numerous serpins and reduces the membrane-damaging action of the terminal cytolytic complement pathway (serine protease inhibitors). It's a secreted protein that comes in two forms: a single chain and a clipped, two-chain form that's held together by a disulfide bond [4]. Vitronectin is thought to play a role in tumor malignancy and hemostasis [5].
Vitronectin is a 478-amino-acid glycoprotein with a molecular weight of 54kDa. Carbohydrates make up around a third of the protein's molecular mass. On rare occasions, the protein is cleaved after arginine 379, resulting in two-chain vitronectin with a disulfide bond connecting the two chains. Except for the N-terminal domain, no high-resolution structure has been established experimentally.
There are three domains in the protein:
• Somatomedin B's N-terminal domain (1-39);
• A core domain that is homologous to hemopexin (131-342);
• A C-terminal domain (residues 347-459) that is homologous to hemopexin.
• A C-terminal domain (residues 347-459) that is homologous to hemopexin.
The Somatomedin B domain has been described in a variety of structures. The protein was first crystallized in a complex with one of its physiological binding partners, Plasminogen activator inhibitor-1 (PAI-1), and the structure of this complex was solved. Following that, two groups reported the domain's NMR structures [6]. With four disulfide connections within 35 residues, the somatomedin B domain is a close-knit disulfide knot. For this domain, many disulfide configurations have been reported, however, the crystal structure has clarified this problem [7]. For the middle and C-terminal domains, homology models have been developed [8].
Angiopoietin-like 4 is a protein encoded by the ANGPTL4 gene in humans [9]. It has been discovered that alternatively spliced transcript variants encoding various isoforms exist. ANGPTL2, HFARP, PGAR, or FIAF were the prior names for this gene, however, it has now been renamed ANGPTL4.
This gene is upregulated in numerous cell types under hypoxic (low oxygen) conditions and is a target of peroxisome proliferator-activated receptors. The encoded protein is a serum hormone that regulates lipid metabolism directly.
ANGPTL4 plays an important role in numerous cancers and is implicated in the metastatic process by modulating vascular permeability, cancer cell motility, and invasiveness [10].
The glycosylated, secreted protein with a coiled-coil N-terminal domain and a fibrinogen-like C-terminal domain is encoded by this gene, which belongs to the angiopoietin-like gene family [11].
This gene is upregulated in numerous cell types under hypoxic (low oxygen) conditions and is a target of peroxisome proliferator-activated receptors. The encoded protein is a serum hormone that regulates lipid metabolism directly. Intermolecular disulfide bonds allow full-length ANGPTL4 to form higher-order structures. The assembly of ANGPTL4 (nANGPTL4) is controlled by its N-terminal region. The linker region of full-length ANGPTL4 is proteolytically cleavage, releasing nANGPTL4 and the monomeric C-terminal part of ANGPTL4 (cANGPTL4). The biological functions of nANGPTL4 and cANGPTL4 are distinct [11].
To separate their functions, monoclonal antibodies targeting nANGPTL4 and cANGPTL4 have been produced [12].
ANGPTL4 is involved in the metastatic process by altering vascular permeability, cancer cell motility, and invasiveness in a variety of malignancies. ANGPTL4 promotes tumor growth while also protecting cells from anoikis, a type of programmed cell death that occurs when contact-dependent cells separate from the tissue matrix [13].
Absorbable Hemostatic sponge is made from highly purified neutral gelatin foam with a consistent fine porosity that ensures good hemostasis. When blood comes into touch with the sponge's matrix, the gelatin sponge activates the thrombocytes. They trigger the thrombocytes to secrete a chemical that promotes their aggregation while also changing their surface character, allowing them to act as a catalyst for the creation of fibrin. In 2-4 minutes, it becomes hemostatic, liquefies in 2-5 days when it comes into contact with mucosa, and absorbs at least 35 times its weight in blood and fluids. pH-neutral can be used dry or impregnated and is pharmaceutically compatible. It has a four-year shelf life [14].
The study aimed to see if the local application of vitronectin protein /angiopoietin-like 4 protein as an autoinducer could help with bone repair.
Material and Methods
All experimental techniques were carried out in compliance with animal experimentation ethical norms. In this study, forty male Albino rats weighing 300-400 grams and aged 6-8 months were employed and kept under temperature, drinking, and food consumption control conditions. In this study, 48 albino male rats weighing 300-400 grams and aged 6-8 months were employed under temperature, drinking, and food consumption control settings. The animals underwent a surgical procedure on the medial side of the tibiae bone; in the control group, the bone defect will be treated with the local application of an absorbable hemostatic sponge; in the experimental group, for group I the bone defect was treated with local application of 1mg vitronectin protein, group II with local application of 1mg angiopoietin-like 4, and group III with local application of combination (0.5mg vitronectin protein and 0.5mg of angiopoietin-like 4). The absorbable hemostatic sponge was used to fix all of the experimental groups. 14 and 28 days following surgery, the rats were slaughtered (five rats for each period).
The following materials were used in this study:
Angiopoietin-like 4 is a protein encoded by the ANGPTL4 gene in humans [9]. It has been discovered that alternatively spliced transcript variants encoding various isoforms exist. ANGPTL2, HFARP, PGAR, or FIAF were the prior names for this gene, however, it has now been renamed ANGPTL4.
This gene is upregulated in numerous cell types under hypoxic (low oxygen) conditions and is a target of peroxisome proliferator-activated receptors. The encoded protein is a serum hormone that regulates lipid metabolism directly.
ANGPTL4 plays an important role in numerous cancers and is implicated in the metastatic process by modulating vascular permeability, cancer cell motility, and invasiveness [10].
The glycosylated, secreted protein with a coiled-coil N-terminal domain and a fibrinogen-like C-terminal domain is encoded by this gene, which belongs to the angiopoietin-like gene family [11].
This gene is upregulated in numerous cell types under hypoxic (low oxygen) conditions and is a target of peroxisome proliferator-activated receptors. The encoded protein is a serum hormone that regulates lipid metabolism directly. Intermolecular disulfide bonds allow full-length ANGPTL4 to form higher-order structures. The assembly of ANGPTL4 (nANGPTL4) is controlled by its N-terminal region. The linker region of full-length ANGPTL4 is proteolytically cleavage, releasing nANGPTL4 and the monomeric C-terminal part of ANGPTL4 (cANGPTL4). The biological functions of nANGPTL4 and cANGPTL4 are distinct [11].
To separate their functions, monoclonal antibodies targeting nANGPTL4 and cANGPTL4 have been produced [12].
ANGPTL4 is involved in the metastatic process by altering vascular permeability, cancer cell motility, and invasiveness in a variety of malignancies. ANGPTL4 promotes tumor growth while also protecting cells from anoikis, a type of programmed cell death that occurs when contact-dependent cells separate from the tissue matrix [13].
Absorbable Hemostatic sponge is made from highly purified neutral gelatin foam with a consistent fine porosity that ensures good hemostasis. When blood comes into touch with the sponge's matrix, the gelatin sponge activates the thrombocytes. They trigger the thrombocytes to secrete a chemical that promotes their aggregation while also changing their surface character, allowing them to act as a catalyst for the creation of fibrin. In 2-4 minutes, it becomes hemostatic, liquefies in 2-5 days when it comes into contact with mucosa, and absorbs at least 35 times its weight in blood and fluids. pH-neutral can be used dry or impregnated and is pharmaceutically compatible. It has a four-year shelf life [14].
The study aimed to see if the local application of vitronectin protein /angiopoietin-like 4 protein as an autoinducer could help with bone repair.
Material and Methods
All experimental techniques were carried out in compliance with animal experimentation ethical norms. In this study, forty male Albino rats weighing 300-400 grams and aged 6-8 months were employed and kept under temperature, drinking, and food consumption control conditions. In this study, 48 albino male rats weighing 300-400 grams and aged 6-8 months were employed under temperature, drinking, and food consumption control settings. The animals underwent a surgical procedure on the medial side of the tibiae bone; in the control group, the bone defect will be treated with the local application of an absorbable hemostatic sponge; in the experimental group, for group I the bone defect was treated with local application of 1mg vitronectin protein, group II with local application of 1mg angiopoietin-like 4, and group III with local application of combination (0.5mg vitronectin protein and 0.5mg of angiopoietin-like 4). The absorbable hemostatic sponge was used to fix all of the experimental groups. 14 and 28 days following surgery, the rats were slaughtered (five rats for each period).
The following materials were used in this study:
1. Vitronectin protein Abcam business
2. Abcam corporation Angiopoietin-like 4.
A surgical procedure was performed on the animals. The procedure was done in a sterilized environment with a gentle technique. The dose of general anesthesia given to each animal was calculated based on its weight. Intramuscular injections of xylazine 2% (0.4mg/kg B.W.) and ketamine HCL 50mg (40mg/kg B.W.) were used to induce general anesthesia. In addition, oxytetracycline 20% (0.7ml/kg) intramuscular injection was provided as an antibiotic cover.. Tibiae were shaved, and the skin was washed with a solution of ethanol and iodine, followed by an alcohol-soaked piece of cotton. The skin and fascia flap were mirrored after an incision was made. A hole of 1.8mm was drilled with a tiny spherical bur at a rotational speed of 1500rpm using instrument drilling and continuous cooling with irrigated saline. The operation site was cleaned with saline solution after the hole preparation to eliminate debris from the drilling site. After drying the area with air, the bone defect in the control group is treated with local application of absorbable hemostatic sponge, whereas the experimental group is divided into three groups: group I is treated with local application of 1mg vitronectin protein, group II is treated with local application of 1mg angiopoietin, and group III is treated with local application of 1mg angiopoietin. A local antibiotic was applied on the surgical site (tetracycline spray). The specimens were fixed in 10% formalin for 24 hours, then decalcified using a formic acid solution, and the bone tissue was dehydrated with alcohol before being embedded in paraffin. Hematoxylin and Eosin were used to stain 5 m sections, which were prepared as usual. A light microscope was used to do the histological evaluation.
Findings
In the control group, new trabeculated bone in the matrix of bone filled with a considerable number of osteocytes and osteoblasts visible at the peripheries of the trabecula after 14 days (Figure 1).
After 28 days, bone trabeculae almost completely fill the surgical site, with osteoblasts and osteocytes visible (Figure 2).
In the experimental group, for vitronectin, newly created bone trabeculae enclosing regions of bone marrow, with abundant osteocytes and osteoblasts were observed at the peripheries, after 14 days (Figure 3).
Mature bone fills the defect region after 28 days, defined by the presence of osteon (Haversian system) and regular distribution of osteocytes along the Haversian canal. The border of the Haversian canals was similarly lined by osteoblasts (Figure 4).
For angiopoietin-like 4 group: At 14 days, newly produced bone trabeculae surrounding patches of bone marrow, with numerous regularly distributed osteocytes, osteoblasts at peripheries, osteoclasts, and a reversal line between old and new bone (Figure 5).
Dense bone in the defect site after 28 days, with osteocytes grouped in a regular fashion around the Haversian canal. Osteoblasts at the bone's edges (Figure 6).
For combination group: Newly created bone trabeculae surrounding regions of bone marrow, containing many osteocytes and osteoblasts visible at peripheries at 14 days (Figure 7).
Mature bone fills the defect region after 28 days, defined by the presence of osteon (Haversian system) and regular distribution of osteocytes along the Haversian canal (Figure 8).
The creation of bone trabeculae in a bone defect location during 14 days is linked to stem cell differentiation into osteoblasts (Table 1).
The current study's histological findings demonstrated the creation of new irregular bone trabeculae in both the experimental and control groups, albeit at different rates of bone deposition and remodeling. Within the freshly produced bone trabeculae, lacunae containing osteocytes were distributed. Osteoblasts, on the other hand, surrounded their trabeculae by a rim. In between the freshly created trabeculae, fibrovascular marrow was detected (Table 2).
The results obtained from the vitronectin group showed numerous bone trabeculae with osteoblast rimmed on the surface and osteocyte scattered in the body of bone trabeculae in a significant value when compared to the control group (Table 3).

Figure 1) At 14 days, a view of the control group reveals fresh trabeculated bone BT within a matrix of bone BM filled with a substantial number of osteocytes OC and osteoblasts OB at the trabecula's peripheries (H & E) 40X.
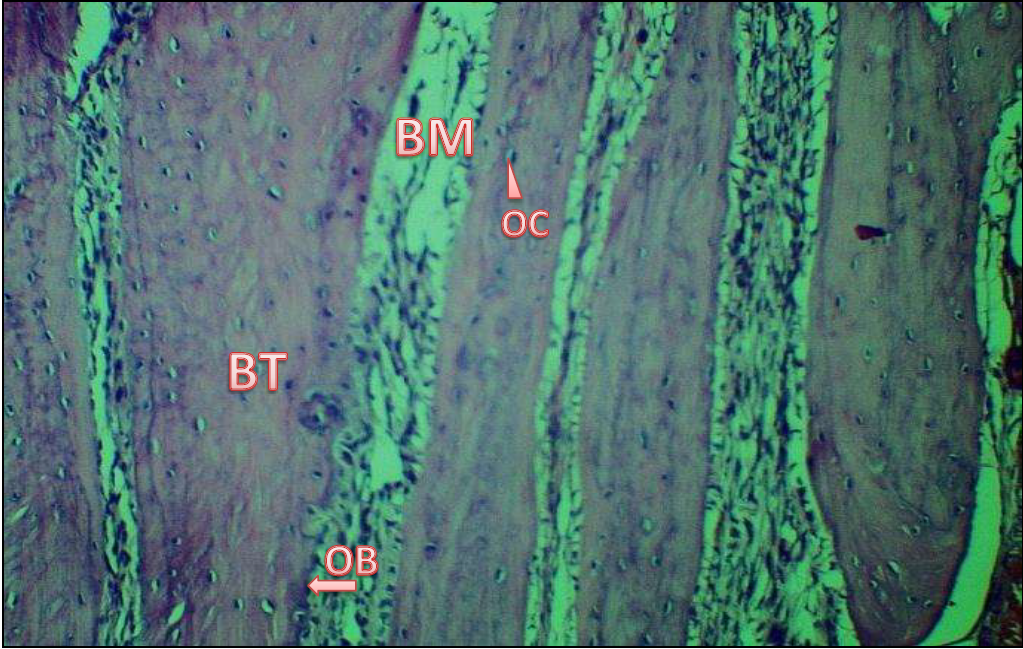
Figure 2) View of the control group after 28 days shows a large number of trabeculae BT of bone almost completely filling the operative site within bone marrow BM, as well as osteoblasts OB can be seen at the bone edges and osteocytes OC, infiltrated newly bone trabeculae (H & E) 40X.

Figure 3) View of 14-day-old vitronectin protein, newly formed bone trabeculae BT enclosing sections of bone marrow and including numerous regularly ordered osteocytes OC and osteoblasts OB at the periphery (H & E) 40X.
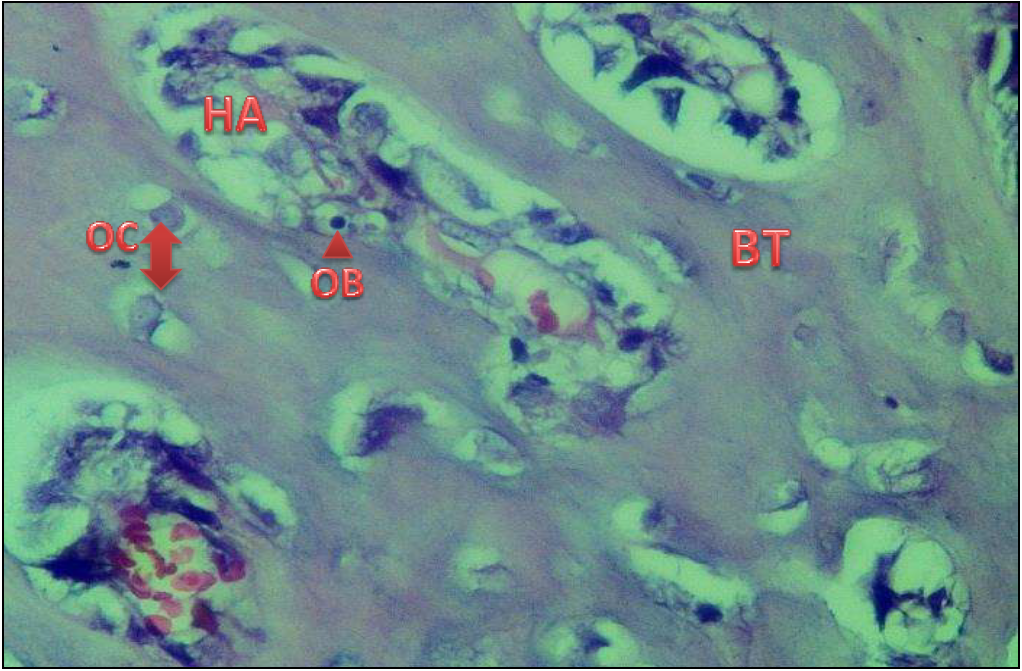
Figure 4) A 28-day view of vitronectin protein reveals thick bone at the defect region and osteocytes OC grouped in a consistent fashion around the Haversian canal HA. At the edges of the Haversian Canal, osteoblasts OB be detected in H & E 40X

Figure 5) At 14 days, the 4 groups of angiopoietin were seen. Many osteocytes OC inside bone trabeculae BT and osteoblast at the perimeter of bone trabeculae with a reversal line RLappearing between old OLD and new developing bone NB (H & E) 40X.
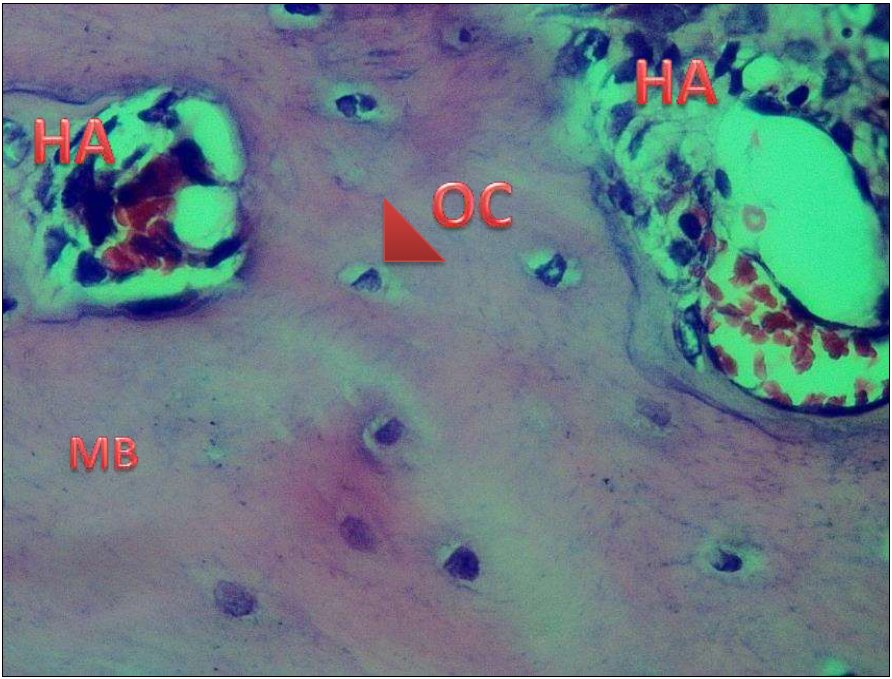
Figure 6) At 28 days, the mature bone MB with numerous Haversian canals HA with osteoblasts OB at their periphery and numerous osteocyte OC can be seen in the angiopoietin-like 4 group (H & E) 40X.

Figure 7) View of the combination group after 14 days shows newly created bone trabeculae BT with numerous newly Haversian canal HA formation, numerous osteocytes OC and osteoblasts OB visible at the periphery (H & E) 40X.

Figure 8) Combination group's perspective mature bone MA filled the defect region after 28 days, defined from old bone OLD by the presence of the Haversian HA system and regular distribution of osteocytes OC around the Haversian canal (H & E) 40X.
Table 1) Descriptive statistics of the bone cells count (H & E) and groups’ differences in each duration
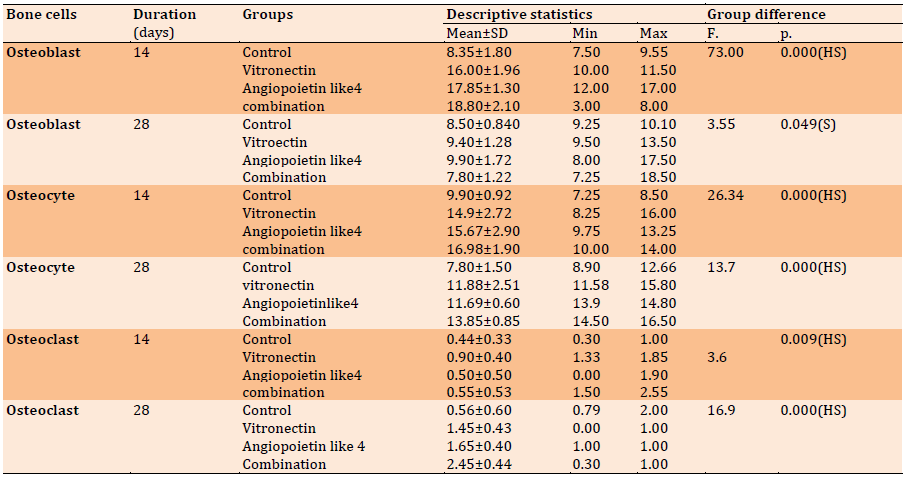
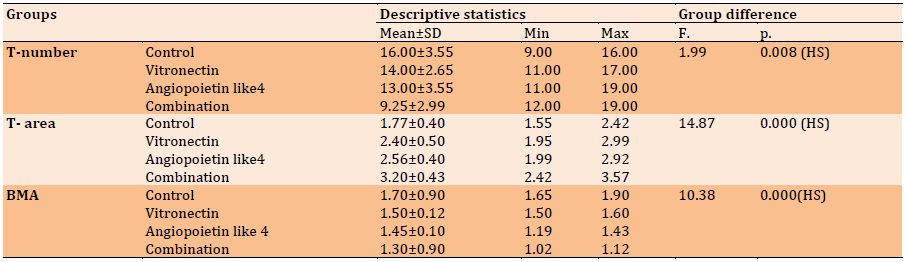
Table 2) Descriptive statistics of T-number, T-area, and BMA (H & E) and groups’ difference in each duration regarding 14 days
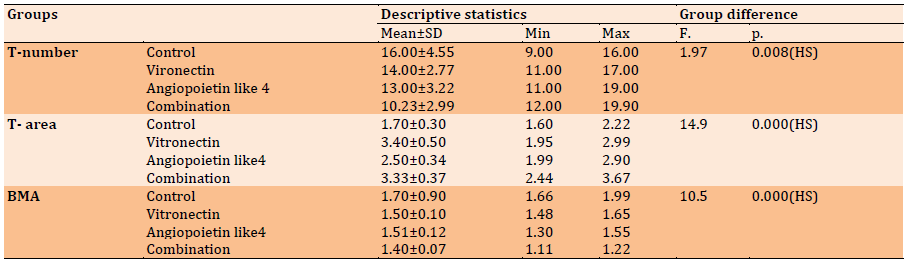
Table 3) Descriptive statistics of T-number, T-area, and BMA (H & E) and groups’ difference in each duration regarding 28 days
Findings
In the control group, new trabeculated bone in the matrix of bone filled with a considerable number of osteocytes and osteoblasts visible at the peripheries of the trabecula after 14 days (Figure 1).
After 28 days, bone trabeculae almost completely fill the surgical site, with osteoblasts and osteocytes visible (Figure 2).
In the experimental group, for vitronectin, newly created bone trabeculae enclosing regions of bone marrow, with abundant osteocytes and osteoblasts were observed at the peripheries, after 14 days (Figure 3).
Mature bone fills the defect region after 28 days, defined by the presence of osteon (Haversian system) and regular distribution of osteocytes along the Haversian canal. The border of the Haversian canals was similarly lined by osteoblasts (Figure 4).
For angiopoietin-like 4 group: At 14 days, newly produced bone trabeculae surrounding patches of bone marrow, with numerous regularly distributed osteocytes, osteoblasts at peripheries, osteoclasts, and a reversal line between old and new bone (Figure 5).
Dense bone in the defect site after 28 days, with osteocytes grouped in a regular fashion around the Haversian canal. Osteoblasts at the bone's edges (Figure 6).
For combination group: Newly created bone trabeculae surrounding regions of bone marrow, containing many osteocytes and osteoblasts visible at peripheries at 14 days (Figure 7).
Mature bone fills the defect region after 28 days, defined by the presence of osteon (Haversian system) and regular distribution of osteocytes along the Haversian canal (Figure 8).
The creation of bone trabeculae in a bone defect location during 14 days is linked to stem cell differentiation into osteoblasts (Table 1).
The current study's histological findings demonstrated the creation of new irregular bone trabeculae in both the experimental and control groups, albeit at different rates of bone deposition and remodeling. Within the freshly produced bone trabeculae, lacunae containing osteocytes were distributed. Osteoblasts, on the other hand, surrounded their trabeculae by a rim. In between the freshly created trabeculae, fibrovascular marrow was detected (Table 2).
The results obtained from the vitronectin group showed numerous bone trabeculae with osteoblast rimmed on the surface and osteocyte scattered in the body of bone trabeculae in a significant value when compared to the control group (Table 3).

Figure 1) At 14 days, a view of the control group reveals fresh trabeculated bone BT within a matrix of bone BM filled with a substantial number of osteocytes OC and osteoblasts OB at the trabecula's peripheries (H & E) 40X.

Figure 2) View of the control group after 28 days shows a large number of trabeculae BT of bone almost completely filling the operative site within bone marrow BM, as well as osteoblasts OB can be seen at the bone edges and osteocytes OC, infiltrated newly bone trabeculae (H & E) 40X.

Figure 3) View of 14-day-old vitronectin protein, newly formed bone trabeculae BT enclosing sections of bone marrow and including numerous regularly ordered osteocytes OC and osteoblasts OB at the periphery (H & E) 40X.

Figure 4) A 28-day view of vitronectin protein reveals thick bone at the defect region and osteocytes OC grouped in a consistent fashion around the Haversian canal HA. At the edges of the Haversian Canal, osteoblasts OB be detected in H & E 40X

Figure 5) At 14 days, the 4 groups of angiopoietin were seen. Many osteocytes OC inside bone trabeculae BT and osteoblast at the perimeter of bone trabeculae with a reversal line RLappearing between old OLD and new developing bone NB (H & E) 40X.

Figure 6) At 28 days, the mature bone MB with numerous Haversian canals HA with osteoblasts OB at their periphery and numerous osteocyte OC can be seen in the angiopoietin-like 4 group (H & E) 40X.

Figure 7) View of the combination group after 14 days shows newly created bone trabeculae BT with numerous newly Haversian canal HA formation, numerous osteocytes OC and osteoblasts OB visible at the periphery (H & E) 40X.

Figure 8) Combination group's perspective mature bone MA filled the defect region after 28 days, defined from old bone OLD by the presence of the Haversian HA system and regular distribution of osteocytes OC around the Haversian canal (H & E) 40X.
Table 1) Descriptive statistics of the bone cells count (H & E) and groups’ differences in each duration

Table 2) Descriptive statistics of T-number, T-area, and BMA (H & E) and groups’ difference in each duration regarding 14 days

Table 3) Descriptive statistics of T-number, T-area, and BMA (H & E) and groups’ difference in each duration regarding 28 days

Discussion
Recruitment of mesenchymal cells, proliferation, differentiation, and finally extracellular matrix secretion and biomineralization are all steps in the formation of hard tissues [15].
Repairing bone fractures is one of the most common orthopedic treatments in the United States, with an estimated 7 million visits per year. While the majority of fractures respond to treatment and return to their original form and function without leaving scars, fracture healing can sometimes be delayed or result in a non-:union:. Fracture repair that is delayed or fails raises the expense of treatment, needs further surgeries, and leads to a longer duration of convalescence, all of which are linked to higher mortality in the elderly [16].
The current study investigated the ability of exogenous vitronectin / angiopoietin-like 4 proteins in the faulty bone to associate angiogenesis with bone growth and remodeling. Furthermore, vitronectin plays a role in regulating proteolysis triggered by plasminogen activation. Furthermore, because vitronectin is a component of platelets, it plays a role in hemostasis. The RGD (45-47) sequence found in vitronectin serves as a binding site for membrane-bound integrins, such as the vitronectin receptor, that serve to anchor cells to the extracellular matrix. The proteolysis triggered by plasminogen activation is regulated by vitronectin Furthermore, vitronectin is a component of platelets and so plays a role in hemostasis. The RGD (45-47) sequence found in vitronectin serves as a binding site for membrane-bound integrins, such as the vitronectin receptor, that serve to anchor cells to the extracellular matrix. The proteolysis triggered by plasminogen activation is regulated by vitronectin. Furthermore, because vitronectin is a component of platelets, it plays a role in hemostasis. As a result, it could serve as a primary mediator for other elements involved in bone healing. The current study found that early bone deposition in the Vitronectin group at 14 days is linked to the creation of bone trabeculae in the bone defect location, which comprises stem cells transforming into osteoblasts, which is aided by the vitronectin protein, which has been linked to angiogenesis. As a result, bone accumulates as a mineralized bone 14 and 28 days after damage by intramembranous ossification [17]. Angiogenesis is required for both normal and pathological bone physiology, according to the findings. The creation of bone trabeculae in a bone defect location during 14 days is linked to stem cell differentiation into osteoblasts, which is aided by the angiopoietin-like 4 protein, which has been linked to angiogenesis and altered inflammatory response [18].
In comparison to the histologic view for the control, the angiopoietin-like 4 group showed mature bone trabeculae filled approximately the whole defect after 28 days. This conclusion can be attributable to the fact that the angiopoietin-like 4 protein promotes primary osteoblast development directly. In comparison to the control group, the present statistical analysis for the mean value of osteoblast, active osteocyte, and osteoclast in the combination group revealed a highly significant difference. The following are some of the findings:
Exogenous Vitronectin and angiopoietins like 4 proteins have a mechanism of action in bone repair. Endothelial cells and osteoblasts communicate with each other during bone production and fracture repair. Angptl4 levels in differentiating MC3T3-E1 osteoblastic cells were highly increased at day 14 in previous work [19]. Because osteoblast-like cells produce this angiogenic factor in a differentiation-dependent way, angiopoietin protein 4 could be a key player in this interaction. Exogenously administered angiopoietin, such as 4, also improves osteoblast differentiation, bone formation, and remodeling [20].
The general histology data revealed that both the control and experimental groups had a strong healing pathway, although there were differences in the rate of bone deposition and remodeling, as well as their durations.
The current study's histological findings demonstrated the creation of new irregular bone trabeculae in both the experimental and control groups, albeit at different rates of bone deposition and remodeling. Within the freshly produced bone trabeculae, lacunae containing osteocytes were distributed. Osteoblasts, on the other hand, surrounded their trabeculae by a rim. In between the freshly created trabeculae, fibrovascular marrow was detected. This finding is consistent with recent research [21, 22]. that showed the deformity zone was filled with fragile and interwoven bone trabeculae.
Our study found that bone formation from the cortical bone surfaces in marrow cavities was evident at 14 days, and medullary cavities were filled with marrow cells, which agrees with Chang et al. [23] who found no antigenic activities at the bone-implant interfaces and excellent bone-to-implant contact ratios. Furthermore, emerging evidence indicates that, besides its osteogenic activity, The biological functions of vitronectin include stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism. The biological functions of vitronectin include stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism.
Our findings for angiopoietin-like4 indicated an increase in the number of trabeculae as compared to the control group, enclosing newly created bone marrow with an increase in the number of osteoblast and osteocyte, as well as a decrease in bone marrow gaps, indicating bone repair aging. Angptl4 levels were highly elevated at day 14 in differentiating MC3T3-E1 osteoblastic cells, according to Sabrina et al. [19]. These latter activities imply that therapeutic use may necessitate measures to reduce the risk of potentially harmful side effects while maximizing vessel-protective effects.
When compared to the control group, the combination group with vitronectin and angiopoietin-like 4 showed a significant decrease in the number of bone trabeculae enclosing Haversian canals as well as a significant decrease in bone marrow area, indicating new bone formation. This was due to the strong effectiveness of both vitronectins as well as angiopoietin-like 4 in angiogenesis and remodeling of BMSC.
As reported by Amera, greater regions of bone growth were found at 4 weeks, with thin and tiny bony trabeculae, but the current data indicated thick mature bone at defect sites in all tested groups after 4 weeks.
The results obtained from the vitronectin group showed numerous bone trabeculae with osteoblast rimmed on the surface and osteocyte scattered in the body of bone trabeculae in a significant value when compared to the control group. These findings agree with those of Min et al. [24]. who demonstrated that vitronectin regulates osteoblast and osteoclast differentiation. Numerous bone trabeculae with osteoblast rimmed on the surface of bone and scattering of osteocytes within newly created bone were seen in the angiopoietin like4 group. This result is consistent with Helene [25]. who indicated that angiopoietin-like 4 promotes angiogenesis and vascular permeability. Furthermore, when compared to other study groups, histological evaluation of vitronectin combination of angiopoietin like4 and angiopoietin 2 at 4 weeks postoperatively demonstrated an increase in osteocytes mean number with an increase in newly produced bone. Because there is greater creation and maturation of woven bone later in 4 weeks, most osteoblasts generating bone become imprisoned inside the matrix and named osteocytes cells, this outcome can be explained by the fact that most surviving osteoblasts cells are situated on the bony defect location.
The histomorphometric analysis was performed to determine whether or not there was any bone in the deficiency. The importance of this form of study was validated in the study [26], which stated that quantitative analysis was required for studies evaluating the efficacy of new treatment modalities for bone neogenesis. Trabecular area (TA), trabecular number (TN), and bone marrow area (BMA).
Mean values of TA recorded in this study were higher in experimental groups, more clearly observed in 4 weeks duration which may seem to be in line with histomorphometric results of Majeed et al., 2018 who stated that the increase in osteogenesis seen during the transition from the 30 to 60 days of observation and the total areas of the newly created bone trabeculae showed that the groups submitted to bone filling biomaterial (osteoconduction and autogenous bone graft) showed bone trabeculate area values higher than the control group of the same animal.
It has been proposed that the bone repair process occurs within the degree of bone maturing progression and that the observation period progression does not produce an increase in the area size of the bone trabeculae, but rather in the maturation degree of the bone trabeculae [27]. Morphometric comparison dramatically reduced trabecular bone volume (BV/TV) and bone mass density (BMD) in transgenic versus WT mice [28]. The mineral density of the mending bone increased with time [29]. In the healing bone matrix, the Ca/P ratio increased, whereas the C/Ca and C/P ratios dropped. According to the findings, as the bone heals, the mineral content improves in density and quality while the organic components decrease.
A study by Prodinger et al. [30] discovered that when a bone is implanted within the cortical gap between 14 and 21 days, the density of primary bone increases dramatically as secondary bone matures.
According to Chang et al. [23], vitronectin showed superior histomorphometric osseointegration, which is consistent with our findings, which show that vitronectin improves the bone healing process. Our findings support Helene's finding [25] that angiopoietin-like 4 promotes angiogenesis.
As a population of stem cells capable of differentiating into hematological, chondrogenic, or osteogenic cell populations, bone marrow stromal cells (BMSCs), which contain cells of the fibroblastic, reticular, adipogenic, and osteogenic lineages, have been a key focus of research. In animal and clinical investigations, bone marrow-derived stromal cells have been shown to speed up the healing of bone abnormalities. It has a high proliferation ability and does not differentiate into terminal osteoblasts in vitro without external stimulation [31].
Osteoprogenitor cells were thought to be the primary source of osteoblasts, which are required for the mineralization of the bone matrix. They were bone tissue-derived autologous progenitor cells that took part in the normal remodeling and healing process [32].
According to Otto et al. [33], mesenchymal cells in the regenerating bone marrow are involved in the creation of the medullary callus, which does not require endochondral ossification.
Research by Ode et al. [34] found that bone bridge development is also influenced by bone marrow-derived mesenchymal cells. The mesenchymal cell population decreased and the amount of primitive mesenchymal cells decreased on day 14 when the bone bridge was well established and on subsequent days when the bone bridge solidified.
Bone bridge creation after growth plate injury happens directly via intramembranous ossification by recruitment of marrow-derived osteoprogenitor cells, according to Tsai et al. [35].
The current findings revealed that as time passed, the mean values of positively stained cells in BMSCs decreased. The results of this study agreed with those of Zhaoguo et al. [36] who discovered a decrease in the BMSC score mean value of positively stained cells in the fracture site of rats after 1, 2, and 6 weeks of healing.
Conclusion
The findings of this investigation revealed that a combination of vitronectin and angiopoietin-like proteins exhibited potential efficacy in boosting bone defect healing.
Acknowledgments: I would like to thank, Prof Dr. Raghad A Alhashimi Dean of the college of the dentistry university of Baghdad who never saved efforts to help others, my sincere thanks to Prof. Dr. Ali I. Al-bustani assistant dean for scientific affairs and post-graduate students for his help and my respect to Prof Dr. Bashar Hamid Chairman of the department of oral diagnosis which was very cooperative and helpful.
Ethical Permissions: All experimental techniques were carried out in compliance with animal experimentation ethical norms according to ethical approval of the college of the dentistry university of Baghdad with the number 005718. This study was also registered in the college of the dentistry university of Baghdad.
Conflicts of Interests: None declared.
Authors’ Contributions: Fadhil Kadhim E was the only author of the article (100%)
Funding/Support: It was done by author.
Recruitment of mesenchymal cells, proliferation, differentiation, and finally extracellular matrix secretion and biomineralization are all steps in the formation of hard tissues [15].
Repairing bone fractures is one of the most common orthopedic treatments in the United States, with an estimated 7 million visits per year. While the majority of fractures respond to treatment and return to their original form and function without leaving scars, fracture healing can sometimes be delayed or result in a non-:union:. Fracture repair that is delayed or fails raises the expense of treatment, needs further surgeries, and leads to a longer duration of convalescence, all of which are linked to higher mortality in the elderly [16].
The current study investigated the ability of exogenous vitronectin / angiopoietin-like 4 proteins in the faulty bone to associate angiogenesis with bone growth and remodeling. Furthermore, vitronectin plays a role in regulating proteolysis triggered by plasminogen activation. Furthermore, because vitronectin is a component of platelets, it plays a role in hemostasis. The RGD (45-47) sequence found in vitronectin serves as a binding site for membrane-bound integrins, such as the vitronectin receptor, that serve to anchor cells to the extracellular matrix. The proteolysis triggered by plasminogen activation is regulated by vitronectin Furthermore, vitronectin is a component of platelets and so plays a role in hemostasis. The RGD (45-47) sequence found in vitronectin serves as a binding site for membrane-bound integrins, such as the vitronectin receptor, that serve to anchor cells to the extracellular matrix. The proteolysis triggered by plasminogen activation is regulated by vitronectin. Furthermore, because vitronectin is a component of platelets, it plays a role in hemostasis. As a result, it could serve as a primary mediator for other elements involved in bone healing. The current study found that early bone deposition in the Vitronectin group at 14 days is linked to the creation of bone trabeculae in the bone defect location, which comprises stem cells transforming into osteoblasts, which is aided by the vitronectin protein, which has been linked to angiogenesis. As a result, bone accumulates as a mineralized bone 14 and 28 days after damage by intramembranous ossification [17]. Angiogenesis is required for both normal and pathological bone physiology, according to the findings. The creation of bone trabeculae in a bone defect location during 14 days is linked to stem cell differentiation into osteoblasts, which is aided by the angiopoietin-like 4 protein, which has been linked to angiogenesis and altered inflammatory response [18].
In comparison to the histologic view for the control, the angiopoietin-like 4 group showed mature bone trabeculae filled approximately the whole defect after 28 days. This conclusion can be attributable to the fact that the angiopoietin-like 4 protein promotes primary osteoblast development directly. In comparison to the control group, the present statistical analysis for the mean value of osteoblast, active osteocyte, and osteoclast in the combination group revealed a highly significant difference. The following are some of the findings:
Exogenous Vitronectin and angiopoietins like 4 proteins have a mechanism of action in bone repair. Endothelial cells and osteoblasts communicate with each other during bone production and fracture repair. Angptl4 levels in differentiating MC3T3-E1 osteoblastic cells were highly increased at day 14 in previous work [19]. Because osteoblast-like cells produce this angiogenic factor in a differentiation-dependent way, angiopoietin protein 4 could be a key player in this interaction. Exogenously administered angiopoietin, such as 4, also improves osteoblast differentiation, bone formation, and remodeling [20].
The general histology data revealed that both the control and experimental groups had a strong healing pathway, although there were differences in the rate of bone deposition and remodeling, as well as their durations.
The current study's histological findings demonstrated the creation of new irregular bone trabeculae in both the experimental and control groups, albeit at different rates of bone deposition and remodeling. Within the freshly produced bone trabeculae, lacunae containing osteocytes were distributed. Osteoblasts, on the other hand, surrounded their trabeculae by a rim. In between the freshly created trabeculae, fibrovascular marrow was detected. This finding is consistent with recent research [21, 22]. that showed the deformity zone was filled with fragile and interwoven bone trabeculae.
Our study found that bone formation from the cortical bone surfaces in marrow cavities was evident at 14 days, and medullary cavities were filled with marrow cells, which agrees with Chang et al. [23] who found no antigenic activities at the bone-implant interfaces and excellent bone-to-implant contact ratios. Furthermore, emerging evidence indicates that, besides its osteogenic activity, The biological functions of vitronectin include stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism. The biological functions of vitronectin include stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism.
Our findings for angiopoietin-like4 indicated an increase in the number of trabeculae as compared to the control group, enclosing newly created bone marrow with an increase in the number of osteoblast and osteocyte, as well as a decrease in bone marrow gaps, indicating bone repair aging. Angptl4 levels were highly elevated at day 14 in differentiating MC3T3-E1 osteoblastic cells, according to Sabrina et al. [19]. These latter activities imply that therapeutic use may necessitate measures to reduce the risk of potentially harmful side effects while maximizing vessel-protective effects.
When compared to the control group, the combination group with vitronectin and angiopoietin-like 4 showed a significant decrease in the number of bone trabeculae enclosing Haversian canals as well as a significant decrease in bone marrow area, indicating new bone formation. This was due to the strong effectiveness of both vitronectins as well as angiopoietin-like 4 in angiogenesis and remodeling of BMSC.
As reported by Amera, greater regions of bone growth were found at 4 weeks, with thin and tiny bony trabeculae, but the current data indicated thick mature bone at defect sites in all tested groups after 4 weeks.
The results obtained from the vitronectin group showed numerous bone trabeculae with osteoblast rimmed on the surface and osteocyte scattered in the body of bone trabeculae in a significant value when compared to the control group. These findings agree with those of Min et al. [24]. who demonstrated that vitronectin regulates osteoblast and osteoclast differentiation. Numerous bone trabeculae with osteoblast rimmed on the surface of bone and scattering of osteocytes within newly created bone were seen in the angiopoietin like4 group. This result is consistent with Helene [25]. who indicated that angiopoietin-like 4 promotes angiogenesis and vascular permeability. Furthermore, when compared to other study groups, histological evaluation of vitronectin combination of angiopoietin like4 and angiopoietin 2 at 4 weeks postoperatively demonstrated an increase in osteocytes mean number with an increase in newly produced bone. Because there is greater creation and maturation of woven bone later in 4 weeks, most osteoblasts generating bone become imprisoned inside the matrix and named osteocytes cells, this outcome can be explained by the fact that most surviving osteoblasts cells are situated on the bony defect location.
The histomorphometric analysis was performed to determine whether or not there was any bone in the deficiency. The importance of this form of study was validated in the study [26], which stated that quantitative analysis was required for studies evaluating the efficacy of new treatment modalities for bone neogenesis. Trabecular area (TA), trabecular number (TN), and bone marrow area (BMA).
Mean values of TA recorded in this study were higher in experimental groups, more clearly observed in 4 weeks duration which may seem to be in line with histomorphometric results of Majeed et al., 2018 who stated that the increase in osteogenesis seen during the transition from the 30 to 60 days of observation and the total areas of the newly created bone trabeculae showed that the groups submitted to bone filling biomaterial (osteoconduction and autogenous bone graft) showed bone trabeculate area values higher than the control group of the same animal.
It has been proposed that the bone repair process occurs within the degree of bone maturing progression and that the observation period progression does not produce an increase in the area size of the bone trabeculae, but rather in the maturation degree of the bone trabeculae [27]. Morphometric comparison dramatically reduced trabecular bone volume (BV/TV) and bone mass density (BMD) in transgenic versus WT mice [28]. The mineral density of the mending bone increased with time [29]. In the healing bone matrix, the Ca/P ratio increased, whereas the C/Ca and C/P ratios dropped. According to the findings, as the bone heals, the mineral content improves in density and quality while the organic components decrease.
A study by Prodinger et al. [30] discovered that when a bone is implanted within the cortical gap between 14 and 21 days, the density of primary bone increases dramatically as secondary bone matures.
According to Chang et al. [23], vitronectin showed superior histomorphometric osseointegration, which is consistent with our findings, which show that vitronectin improves the bone healing process. Our findings support Helene's finding [25] that angiopoietin-like 4 promotes angiogenesis.
As a population of stem cells capable of differentiating into hematological, chondrogenic, or osteogenic cell populations, bone marrow stromal cells (BMSCs), which contain cells of the fibroblastic, reticular, adipogenic, and osteogenic lineages, have been a key focus of research. In animal and clinical investigations, bone marrow-derived stromal cells have been shown to speed up the healing of bone abnormalities. It has a high proliferation ability and does not differentiate into terminal osteoblasts in vitro without external stimulation [31].
Osteoprogenitor cells were thought to be the primary source of osteoblasts, which are required for the mineralization of the bone matrix. They were bone tissue-derived autologous progenitor cells that took part in the normal remodeling and healing process [32].
According to Otto et al. [33], mesenchymal cells in the regenerating bone marrow are involved in the creation of the medullary callus, which does not require endochondral ossification.
Research by Ode et al. [34] found that bone bridge development is also influenced by bone marrow-derived mesenchymal cells. The mesenchymal cell population decreased and the amount of primitive mesenchymal cells decreased on day 14 when the bone bridge was well established and on subsequent days when the bone bridge solidified.
Bone bridge creation after growth plate injury happens directly via intramembranous ossification by recruitment of marrow-derived osteoprogenitor cells, according to Tsai et al. [35].
The current findings revealed that as time passed, the mean values of positively stained cells in BMSCs decreased. The results of this study agreed with those of Zhaoguo et al. [36] who discovered a decrease in the BMSC score mean value of positively stained cells in the fracture site of rats after 1, 2, and 6 weeks of healing.
Conclusion
The findings of this investigation revealed that a combination of vitronectin and angiopoietin-like proteins exhibited potential efficacy in boosting bone defect healing.
Acknowledgments: I would like to thank, Prof Dr. Raghad A Alhashimi Dean of the college of the dentistry university of Baghdad who never saved efforts to help others, my sincere thanks to Prof. Dr. Ali I. Al-bustani assistant dean for scientific affairs and post-graduate students for his help and my respect to Prof Dr. Bashar Hamid Chairman of the department of oral diagnosis which was very cooperative and helpful.
Ethical Permissions: All experimental techniques were carried out in compliance with animal experimentation ethical norms according to ethical approval of the college of the dentistry university of Baghdad with the number 005718. This study was also registered in the college of the dentistry university of Baghdad.
Conflicts of Interests: None declared.
Authors’ Contributions: Fadhil Kadhim E was the only author of the article (100%)
Funding/Support: It was done by author.
Keywords:
References
1. Fadhil E, Alhijazi A. Histological and immunohistochemical evaluation of the effect of local exogenous application of VEGF on bone healing (experimental study in rat). J Baghdad Coll Dentistry. 2014;26. [Link] [DOI:10.12816/0015148]
2. Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285(33):25103-8. [Link] [DOI:10.1074/jbc.R109.041087]
3. Boron WF, Boulpaep EL. Medical physiology. 2nd Edition. Saunders; 2012. p. 1097. [Link]
4. Preissner KT, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thrombos Res. 1998;89(1):1-21. [Link] [DOI:10.1016/S0049-3848(97)00298-3]
5. Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5(5):864-8. [Link] [DOI:10.1016/0955-0674(93)90036-P]
6. Zhou A, Huntington JA, Pannu NS, Carrell RW, Read RJ. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat Struct Biol. 2003;10(7):541-4. [Link] [DOI:10.1038/nsb943]
7. Horn NA, Hurst GB, Mayasundari A, Whittemore NA, Serpersu EH, Peterson CB. Assignment of the four disulfides in the N-terminal somatomedin B domain of native vitronectin isolated from human plasma. J Biol Chem. 2004;279(34):35867-78. [Link] [DOI:10.1074/jbc.M405716200]
8. Xu D, Baburaj K, Peterson CB, Xu Y. Model for the three-dimensional structure of vitronectin: predictions for the multi-domain protein from threading and docking. Proteins. 2001;44(3):312-20. [Link] [DOI:10.1002/prot.1096]
9. Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, et al. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol. 2000;20(14):5343-9. [Link] [DOI:10.1128/MCB.20.14.5343-5349.2000]
10. Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66-77. [Link] [DOI:10.1016/j.cell.2008.01.046]
11. Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin-like 4: a decade of research. Biosci Rep. 2012;32(3):211-9. [Link] [DOI:10.1042/BSR20110102]
12. Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Nat Acad Sci U S A. 2007;104(28):11766-71. [Link] [DOI:10.1073/pnas.0705041104]
13. Adhikary T, Brandt DT, Kaddatz K, Stockert J, Naruhn S, Meissner W, et al. Inverse PPARβ/δ agonists suppress oncogenic signaling to the ANGPTL4 gene and inhibit cancer cell invasion. Oncogene. 32(44):5241-52. [Link] [DOI:10.1038/onc.2012.549]
14. Brindle NPJ, Saharinen P, Alitalo K. signalling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98(8):1014-23. [Link] [DOI:10.1161/01.RES.0000218275.54089.12]
15. Fong CD, Cerny R, Hammarstrom L, Slaby I. Sequential expression of an amelin gene in mesenchymal and epithelial cells during odontogenesis in rats. Eur J Oral Sci. 1998;106 Suppl 1:324-30. [Link] [DOI:10.1111/j.1600-0722.1998.tb02193.x]
16. American Academy of Professional Coders. Official CPC certification study guide. New York: Delmar Cengage Learning; 2012. [Link]
17. Sandberg OH, Aspenberg P. Inter-trabecular bone formation: a specific mechanism for healing of cancellous bone. Acta Orthop. 2016;87(5):459-65. [Link] [DOI:10.1080/17453674.2016.1205172]
18. Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285(43):32999-3009. [Link] [DOI:10.1074/jbc.M110.108175]
19. Wilson SS, Wong A, Toupadakis CA, Yellowley CE. Expression of angiopoietin-like protein 4 at the fracture site: Regulation by hypoxia and osteoblastic differentiation. J Orthop Res. 2015;33(9):1364-73. [Link] [DOI:10.1002/jor.22898]
20. Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10(2):118-25. [Link] [DOI:10.1007/s11914-012-0105-4]
21. Bastos MF, Brilhante FV, Bezerra JP, Silva CA, Duarte PM. Trabecular bone area and bone healing in spontaneously hypertensive rats: a histometric study. Braz Oral Res. 2010;24(2):170-6. [Link] [DOI:10.1590/S1806-83242010000200008]
22. Albertazzi P. Purified phytoestrogens in postmenopausal bone health: Is there a role for genistein?. Climacteric. 2002;5(2):190-6. [Link] [DOI:10.1080/cmt.5.2.190.196]
23. Monfoulet L, Rabier B, Chassande O, Fricain JC. Drilled hole defects in mouse femur as models of intramembranous cortical and cancellous bone regeneration. Calcif Tissue. 2010;86(1):72-81. [Link] [DOI:10.1007/s00223-009-9314-y]
24. Min SK, Kang HK, Jung SY, Jang DH, Min BM. A vitronectin-derived peptide reverses ovariectomy-induced bone loss via regulation of osteoblast and osteoclast differentiation. Cell Death Differ. 2018;25(2):268-81. [Link] [DOI:10.1038/cdd.2017.153]
25. Knowles HJ. Multiple roles of angiopoietin-like 4 in osteolytic disease. Front Endocrinol. 2017:8:80. [Link] [DOI:10.3389/fendo.2017.00080]
26. Guimarãe KB, do Egito Vasconcelos BC, de Assis Limeira Júnior F, de Sousa FB, de Souza Andrade ES, de Holanda Vasconcello RJ. Histomorphometric evaluation of calcium phosphate bone grafts on bone repair. Braz J Ortorhinolarynol. 2011;77(4):447-54. [Portuguese] [Link] [DOI:10.1590/S1808-86942011000400007]
27. Majeed SS, Ghani BA. Effect of topical application of flavanoids extract of Hibiscus sabdariffa on experimentally induced bone defect. J Baghdad Coll Dentistry. 2018;30(1):33-8. [Link] [DOI:10.12816/0046309]
28. Lu X, Li W, Fukumoto S, Yamada Y, Evans CA, Diekwisch T, et al. The ameloblastin extracellular matrix molecule enhances bone fracture resistance and promotes rapid bone fracture healing. Matrix Biol. 2016;52-54:113-26. [Link] [DOI:10.1016/j.matbio.2016.02.007]
29. Karpouzos A, Diamantis E, Farmaki P, Savvanis S, Troupis T. Nutritional aspects of bone health and fracture healing. J Osteoporos. 2017;2017:4218472. [Link] [DOI:10.1155/2017/4218472]
30. Prodinger PM, Bürklein D, Foehr P, Kreutzer K, Pilge H, Schmitt A, et al. Improving results in rat fracture models: enhancing the efficacy of biomechanical testing by a modification of the experimental setup. BMC Musculoskelet Disord. 2018;19(1):243. [Link] [DOI:10.1186/s12891-018-2155-y]
31. Li Q, Geng Y, Lu L, Yang T, Zhang M, Zhou Y. Platelet-rich fibrin-induced bone marrow mesenchymal stem cell differentiation into osteoblast-like cells and neural cells. Neural Rege Res. 2011;6(31):2419-23. [Link]
32. Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37(12):2491-8. [Link] [DOI:10.1007/s00264-013-2059-2]
33. Otto WR, Rao J. Tomorrow's skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 2004;37(1):97-110. [Link] [DOI:10.1111/j.1365-2184.2004.00303.x]
34. Ode A, Kopf J, Kurtz A, Schmidt-Bleek K, Schrade P, Kolar P, et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur Cell Mater. 2011;22:26-42. [Link] [DOI:10.22203/eCM.v022a03]
35. Tsai MT, Lin DJ, Huang S, Lin HT, Chang WH. Osteogenic differentiation is synergistically influenced by osteoinductive treatment and direct cell-cell contact between murine osteoblasts and mesenchymal stem cells. Int Orthop. 2012;36:199-205. [Link] [DOI:10.1007/s00264-011-1259-x]
36. Zhaoguo Liu, Song Zhou, Ya Zhang, Ming Zhao. Rat bone marrow mesenchymal stem cells(BMSCs) inhibit liver fibrosis by activating GSK3β and inhibiting the Wnt3a/β-catenin pathway. Infecti Agent Cancer. 2022;17(1):17. [Link] [DOI:10.1186/s13027-022-00432-4]








