Volume 14, Issue 2 (2022)
Iran J War Public Health 2022, 14(2): 217-220 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/03/1 | Accepted: 2022/04/28 | Published: 2022/05/30
Received: 2022/03/1 | Accepted: 2022/04/28 | Published: 2022/05/30
How to cite this article
Umran Z, Abbas Hadi A, Mohammad A. Assessment of β-catenin as a Potential Marker for Human Colorectal Carcinoma. Iran J War Public Health 2022; 14 (2) :217-220
URL: http://ijwph.ir/article-1-1122-en.html
URL: http://ijwph.ir/article-1-1122-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Laboratory Investigations, Faculty of Science, University of Kufa, Najaf, Iraq
2- Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa, Najaf, Iraq
2- Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa, Najaf, Iraq
Full-Text (HTML) (829 Views)
Introduction
Cancer is a term used to describe a group of disorders in which aberrant cells proliferate and spread uncontrollably. Colorectal cancer (CRC) is a type of cancer that begins in the colon or rectum. Colon and rectal cancers are frequently lumped together because they share many characteristics [1].
It is currently the most frequent malignant cancer of the gastrointestinal system, accounting for 13% of all malignant tumors, and it is the second most common cause of death from cancer worldwide, affecting men and women equally [2]. There was a steady rise in numbers of CRC in Iraq in the last years. According to Iraq Cancer Board (ICB), CRC contributes about 6.15% for both genders, from all other cancer recorded in Iraq in 2018, in males was 7.52% and in females was 5.13% [3].
Biomarkers are molecular patterns which can be used to detect early-stage cancer and to tailor CRC treatment. They are classified as diagnostic, prognostic, or predictive. Thus, biomarkers are useful at various stages of the disease in determining disease progression and recurrence, as well as serving as a personalized indicator of therapeutic efficacy [4].
β-catenin is 90 kD multifunctional protein encoded by CTNNB gene. It is involved in cell adhesion and is also a component of the Wnt signaling pathway [5]. The Wnt/β-catenin signaling pathway is a highly conserved evolutionary route that regulates the embryonic pattern of bodily axes, stem cell fate, and tissue homeostasis [6].
Numerous studies indicate a link between β-catenin and cancer in a variety of tumour types including breast, hepatocellular, renal, CRC, and acute myeloid leukemia [7-13]. The aim of this study was to clarify the relationship between β-catenin levels in sera and some clinical and pathological variables in CRC patients, as well as explore the link between this biomarker and disease diagnosis and progression.
Material and Methods
The current research was carried out in the laboratory of advanced research of the Department of Laboratory Investigations, Faculty of Science, University of Kufa. The patient samples were obtained at the AL-Furat Al-Awsat Center for Tumors in AL-Najaf province between 1-12-2020 and 1-3-2021. The study included (55) patients (men and women) diagnosed with CRC and (35) healthy subjects. The patients were examined and diagnosed by specialist physicians. The participants were informed about this study and their agreement was obtained. Also, the scientific ethical committee permitted the project. The patients were divided into subgroups according to gender, age, histopathological type, stages, grades, tumor location, and body mass index (BMI). Only healthy volunteers who had no history of chronic diseases or acute infections and who were not smokers were chosen for the study.
Five milliliters of blood were drawn from the cubital vein of patients and the healthy group. The samples were isolated at room temperature by centrifuge at 3000 revolutions per minute (rpm) for 15 minutes and transported into Eppendorf tubes and stored in freezing conditions at -20ᴼC until they were examined [12]. The levels of β-catenin in the serum were determined by the enzyme-linked immunosorbent assay (ELISA) method.
The Statistical Package for the Social Sciences (SPSS, ver. 23) was used for statistical analysis of the research results. The t-test and the one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test were employed to compare the several groups in this study. The probability of <0.05 was considered a significant value in the statistical tests.
Findings
The results indicated a significant elevation in β-catenin levels (p<0.05) in CRC patients compared to healthy subjects (Figure 1).
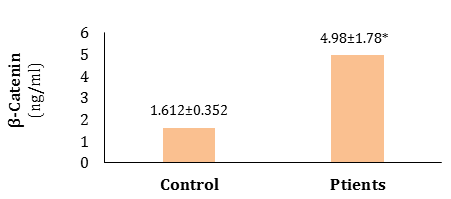
Figure 1) β-catenin levels in serum of CRC patient group and control group. (*)=Significant differences exist at the p<0.05.
Table 1) Mean±SD comparison between β-catenin levels in sera of CRC patients according to clinical features
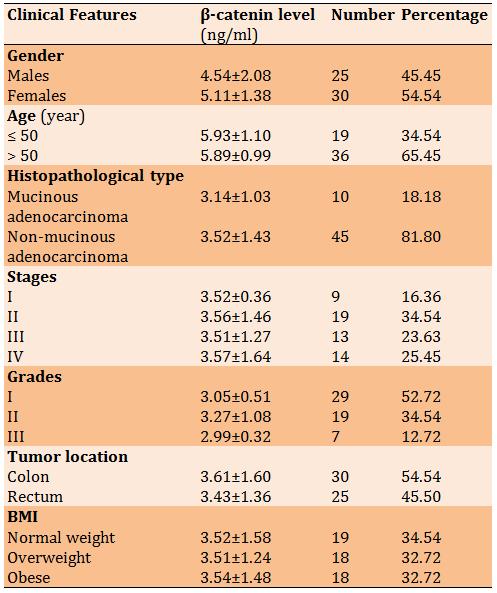
The results of the relationship between the levels of β-catenin in the serum and some clinical features in CRC patients were presented in Table 1. Our results showed no significant differences in β-catenin levels in the sera of patients with CRC disease for gender, age, histopathological type, stages, grades, tumor location, and BMI (p>0.05).
Discussion
The result in Figure 1 is consistent with the findings of Li et al. [11] who obtained that serum β-catenin concentrations were significantly greater in colorectal polyp (CRP) and CRC patients than in the healthy group. Similar results were noted in other types of cancer. Liu et al. [9] demonstrated that individuals with renal cancer had significant increases in β-catenin levels in their peripheral blood. In parallel, this result agrees with the previously reported finding that showed a significant increase in β-catenin levels in hepatocellular carcinoma [14].
Wnt/β-catenin signaling is expected to play a significant role in the advancement of CRC. Elevated nuclear catenin expression is considered an indication of abnormal signaling pathway activation and is believed to enhance cancer progression [15]. Moreover, by comparing the levels of β-catenin in patient serum following surgery, it was revealed that increased serum β-catenin levels were associated with a worse survival rate. This could be due to the stimulated signaling pathway's ability to promote cancer tissue growth and metastasis [9].
On the other hand, there was no statistically significant difference in serum β-catenin levels between the male and female groups, according to the results of the study. This result is consistent with a previous study that showed there was no significant difference in serum β-catenin values according to gender [11].
In one of the immunohistochemistry studies, Wangefjord et al. [16] demonstrated that β-catenin overexpression had no gender-specific prognostic or predictive value. On the contrary, Abdulrahman et al. [10] showed a significant increase in β-catenin immunohistochemistry expression in CRC in females compared to males.
The findings showed no significant difference in serum β-catenin levels in the group ˃50 years in comparison with the ≤ 50-year group. Similar findings were noted by Li et al. who recorded that serum levels of β-catenin were not significantly different between groups younger than 60 years old from the group older than 60 years. On the other hand, Abdulrahman et al. observed that nuclear and cytoplasmic β-catenin expression was higher in patients ≥50 years old [10].
The results of this study indicated no significant difference in β-catenin levels between mucinous adenocarcinoma and non-mucinous adenocarcinoma. We did not find another study in the literature regarding the serum expression of β-catenin in patients with CRC disease, but the available information on nuclear expression was mentioned in one of the studies which indicated that non-mucinous tumors were more intense than mucinous tumors [10].
Our results showed no significant differences in serum β-catenin levels at the different stages and grades of patients with CRC disease. According to Abdulrahman et al., they found no significant difference in the serum levels of β-catenin between Dukes stages (I+II) and Dukes stages (III+IV). Notably, associations between microsatellite instability (MSI) screening status and beta-catenin expression in the entire population and individuals with stages III-IV, β-catenin overexpression was related to a favorable prognosis [16]. Furthermore, immunostaining and cytoplasmic expression were higher in patients with tumor stages III-IV than in cases with tumor stages I-II, and high-grade β-catenin expression was linked with lymph node metastases [10].
In a previous study, Suzuki et al. suggested that a nuclear increase in β-catenin in invasive frontal cells and vessels is the most predictive factor of liver invasion in CRC. This may be a critical marker for adjuvant therapy or other treatment methods' selection [17]. According to Wangefjord et al., the histologic β-catenin overexpression was significantly associated with well-differentiated tumors [16]. Abdulrahman et al. found that tumors that were well-moderately differentiated had a greater staining intensity than tumors that were poorly differentiated [10]. Also, Yoshida et al. confirmed that elevated nuclear β-catenin expression was related to a worse outcome in advanced CRC [15].
The results of the current research indicated that there was no significant difference in levels of β-catenin with colon carcinoma compared with rectum carcinoma. Another study by Li et al. found a little statistical difference in serum β-catenin levels between the colon tumor and rectal tumor groups [11]. Concerning the immunohistochemical analysis, overexpression of β-catenin was significantly related to the distal colon tumor site [16]. A considerable relationship between β-catenin expression and left-sided colonic tumors was observed in the study of Abdulrahman et al., who reported that left-sided colonic tumors were more prevalent (85.7%) than right-sided tumors (14.3%) [10].
Finally, in this study, the statistical analysis showed no significant changes in β-catenin levels in the sera of patients with CRC disease for BMI. As it is widely known, a sizable proportion of the population in developed nations is overweight or obese, and there is a strong link between obesity and CRC disease [18]. However, the serum expression of β-catenin and its relationship to BMI has not been researched and we need further studies in this regard.
Conclusion
The data showed a significant increase in serum β-catenin level in patients and there was no significant difference in its level and the clinical parameters of CRC. Thus, β-catenin may be involved in the progression of colorectal tumors and this biomarker may be of interest as an independent prognostic factor of the malignant development of this disease.
Acknowledgments: The researchers would like to extend their thanks and appreciation to all the workers in the Department of Laboratory Investigations, Faculty of Science, University of Kufa.
Ethical Permissions: The current study has been approved by the ethics committee of the Faculty of Sciences, University of Kufa.
Conflicts of Interests: All authors declare that there is no conflict of interest in the study.
Authors’ Contributions: Umran ZA (First Author), Introduction Writer/Main Researcher (40%); Abbas Hadi AH (Second Author), Assistant Researcher/Statistical Analyst/Discussion Writer (30%); Mohammad AR (Third Author), Assistant Researcher/Statistical Analyst/Discussion Writer (30%)
Funding/Sources: This is a self-funded study. All authors participated in the costs.
Cancer is a term used to describe a group of disorders in which aberrant cells proliferate and spread uncontrollably. Colorectal cancer (CRC) is a type of cancer that begins in the colon or rectum. Colon and rectal cancers are frequently lumped together because they share many characteristics [1].
It is currently the most frequent malignant cancer of the gastrointestinal system, accounting for 13% of all malignant tumors, and it is the second most common cause of death from cancer worldwide, affecting men and women equally [2]. There was a steady rise in numbers of CRC in Iraq in the last years. According to Iraq Cancer Board (ICB), CRC contributes about 6.15% for both genders, from all other cancer recorded in Iraq in 2018, in males was 7.52% and in females was 5.13% [3].
Biomarkers are molecular patterns which can be used to detect early-stage cancer and to tailor CRC treatment. They are classified as diagnostic, prognostic, or predictive. Thus, biomarkers are useful at various stages of the disease in determining disease progression and recurrence, as well as serving as a personalized indicator of therapeutic efficacy [4].
β-catenin is 90 kD multifunctional protein encoded by CTNNB gene. It is involved in cell adhesion and is also a component of the Wnt signaling pathway [5]. The Wnt/β-catenin signaling pathway is a highly conserved evolutionary route that regulates the embryonic pattern of bodily axes, stem cell fate, and tissue homeostasis [6].
Numerous studies indicate a link between β-catenin and cancer in a variety of tumour types including breast, hepatocellular, renal, CRC, and acute myeloid leukemia [7-13]. The aim of this study was to clarify the relationship between β-catenin levels in sera and some clinical and pathological variables in CRC patients, as well as explore the link between this biomarker and disease diagnosis and progression.
Material and Methods
The current research was carried out in the laboratory of advanced research of the Department of Laboratory Investigations, Faculty of Science, University of Kufa. The patient samples were obtained at the AL-Furat Al-Awsat Center for Tumors in AL-Najaf province between 1-12-2020 and 1-3-2021. The study included (55) patients (men and women) diagnosed with CRC and (35) healthy subjects. The patients were examined and diagnosed by specialist physicians. The participants were informed about this study and their agreement was obtained. Also, the scientific ethical committee permitted the project. The patients were divided into subgroups according to gender, age, histopathological type, stages, grades, tumor location, and body mass index (BMI). Only healthy volunteers who had no history of chronic diseases or acute infections and who were not smokers were chosen for the study.
Five milliliters of blood were drawn from the cubital vein of patients and the healthy group. The samples were isolated at room temperature by centrifuge at 3000 revolutions per minute (rpm) for 15 minutes and transported into Eppendorf tubes and stored in freezing conditions at -20ᴼC until they were examined [12]. The levels of β-catenin in the serum were determined by the enzyme-linked immunosorbent assay (ELISA) method.
The Statistical Package for the Social Sciences (SPSS, ver. 23) was used for statistical analysis of the research results. The t-test and the one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test were employed to compare the several groups in this study. The probability of <0.05 was considered a significant value in the statistical tests.
Findings
The results indicated a significant elevation in β-catenin levels (p<0.05) in CRC patients compared to healthy subjects (Figure 1).

Figure 1) β-catenin levels in serum of CRC patient group and control group. (*)=Significant differences exist at the p<0.05.
Table 1) Mean±SD comparison between β-catenin levels in sera of CRC patients according to clinical features

The results of the relationship between the levels of β-catenin in the serum and some clinical features in CRC patients were presented in Table 1. Our results showed no significant differences in β-catenin levels in the sera of patients with CRC disease for gender, age, histopathological type, stages, grades, tumor location, and BMI (p>0.05).
Discussion
The result in Figure 1 is consistent with the findings of Li et al. [11] who obtained that serum β-catenin concentrations were significantly greater in colorectal polyp (CRP) and CRC patients than in the healthy group. Similar results were noted in other types of cancer. Liu et al. [9] demonstrated that individuals with renal cancer had significant increases in β-catenin levels in their peripheral blood. In parallel, this result agrees with the previously reported finding that showed a significant increase in β-catenin levels in hepatocellular carcinoma [14].
Wnt/β-catenin signaling is expected to play a significant role in the advancement of CRC. Elevated nuclear catenin expression is considered an indication of abnormal signaling pathway activation and is believed to enhance cancer progression [15]. Moreover, by comparing the levels of β-catenin in patient serum following surgery, it was revealed that increased serum β-catenin levels were associated with a worse survival rate. This could be due to the stimulated signaling pathway's ability to promote cancer tissue growth and metastasis [9].
On the other hand, there was no statistically significant difference in serum β-catenin levels between the male and female groups, according to the results of the study. This result is consistent with a previous study that showed there was no significant difference in serum β-catenin values according to gender [11].
In one of the immunohistochemistry studies, Wangefjord et al. [16] demonstrated that β-catenin overexpression had no gender-specific prognostic or predictive value. On the contrary, Abdulrahman et al. [10] showed a significant increase in β-catenin immunohistochemistry expression in CRC in females compared to males.
The findings showed no significant difference in serum β-catenin levels in the group ˃50 years in comparison with the ≤ 50-year group. Similar findings were noted by Li et al. who recorded that serum levels of β-catenin were not significantly different between groups younger than 60 years old from the group older than 60 years. On the other hand, Abdulrahman et al. observed that nuclear and cytoplasmic β-catenin expression was higher in patients ≥50 years old [10].
The results of this study indicated no significant difference in β-catenin levels between mucinous adenocarcinoma and non-mucinous adenocarcinoma. We did not find another study in the literature regarding the serum expression of β-catenin in patients with CRC disease, but the available information on nuclear expression was mentioned in one of the studies which indicated that non-mucinous tumors were more intense than mucinous tumors [10].
Our results showed no significant differences in serum β-catenin levels at the different stages and grades of patients with CRC disease. According to Abdulrahman et al., they found no significant difference in the serum levels of β-catenin between Dukes stages (I+II) and Dukes stages (III+IV). Notably, associations between microsatellite instability (MSI) screening status and beta-catenin expression in the entire population and individuals with stages III-IV, β-catenin overexpression was related to a favorable prognosis [16]. Furthermore, immunostaining and cytoplasmic expression were higher in patients with tumor stages III-IV than in cases with tumor stages I-II, and high-grade β-catenin expression was linked with lymph node metastases [10].
In a previous study, Suzuki et al. suggested that a nuclear increase in β-catenin in invasive frontal cells and vessels is the most predictive factor of liver invasion in CRC. This may be a critical marker for adjuvant therapy or other treatment methods' selection [17]. According to Wangefjord et al., the histologic β-catenin overexpression was significantly associated with well-differentiated tumors [16]. Abdulrahman et al. found that tumors that were well-moderately differentiated had a greater staining intensity than tumors that were poorly differentiated [10]. Also, Yoshida et al. confirmed that elevated nuclear β-catenin expression was related to a worse outcome in advanced CRC [15].
The results of the current research indicated that there was no significant difference in levels of β-catenin with colon carcinoma compared with rectum carcinoma. Another study by Li et al. found a little statistical difference in serum β-catenin levels between the colon tumor and rectal tumor groups [11]. Concerning the immunohistochemical analysis, overexpression of β-catenin was significantly related to the distal colon tumor site [16]. A considerable relationship between β-catenin expression and left-sided colonic tumors was observed in the study of Abdulrahman et al., who reported that left-sided colonic tumors were more prevalent (85.7%) than right-sided tumors (14.3%) [10].
Finally, in this study, the statistical analysis showed no significant changes in β-catenin levels in the sera of patients with CRC disease for BMI. As it is widely known, a sizable proportion of the population in developed nations is overweight or obese, and there is a strong link between obesity and CRC disease [18]. However, the serum expression of β-catenin and its relationship to BMI has not been researched and we need further studies in this regard.
Conclusion
The data showed a significant increase in serum β-catenin level in patients and there was no significant difference in its level and the clinical parameters of CRC. Thus, β-catenin may be involved in the progression of colorectal tumors and this biomarker may be of interest as an independent prognostic factor of the malignant development of this disease.
Acknowledgments: The researchers would like to extend their thanks and appreciation to all the workers in the Department of Laboratory Investigations, Faculty of Science, University of Kufa.
Ethical Permissions: The current study has been approved by the ethics committee of the Faculty of Sciences, University of Kufa.
Conflicts of Interests: All authors declare that there is no conflict of interest in the study.
Authors’ Contributions: Umran ZA (First Author), Introduction Writer/Main Researcher (40%); Abbas Hadi AH (Second Author), Assistant Researcher/Statistical Analyst/Discussion Writer (30%); Mohammad AR (Third Author), Assistant Researcher/Statistical Analyst/Discussion Writer (30%)
Funding/Sources: This is a self-funded study. All authors participated in the costs.
Keywords:
References
1. American Cancer Society. Global cancer: facts and figures. Atlanta: American Cancer Society Report. 4th Edition; 2018. [Link]
2. Granados-Romero JJ, Valderrama-Treviño AI, Contreras-Flores EH, Barrera-Mera B, Enríquez MH, Uriarte-Ruíz K, et al. Colorectal cancer: a review. Int J Res Med Sci. 2017;5:4667-76. [Link] [DOI:10.18203/2320-6012.ijrms20174914]
3. Iraqi Cancer Board. Iraq cancer registry for 2018 report. Iraq: Ministry of Health and Environment; 2018. [Arabic] [Link]
4. Ogunwobi OO, Mahmood F, Akingboye A. Biomarkers in colorectal cancer: current research and future prospects. Int J Mol Sci. 2020;21(15):5311. [Link] [DOI:10.3390/ijms21155311]
5. Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8(1):95-102. [Link] [DOI:10.1016/S0959-437X(98)80068-3]
6. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192-205. [Link] [DOI:10.1016/j.cell.2012.05.012]
7. Lee WA. Prognostic significance of abnormal β-catenin expression in breast carcinoma. Korean J Pathol. 2005;39:114-9. [Link]
8. Lai TY, Su CC, Kuo WW, Yeh YL, Kuo WH, Tsai FJ, et al. β-catenin plays a key role in metastasis of human hepatocellular carcinoma. Oncol Rep. 2011;26(2):415-22. [Link]
9. Liu Z, Liu XW, Liu SA, Lv JJ, Fu Q. Clinical significance of changes of expression of the Wnt/β-catenin signaling pathway in renal clear cell carcinoma. Eur Rev Med Pharmacol Sci. 2016;20(23):4840-5. [Link]
10. Abdulrahman ZA, Ismael AT, Jalal JA, Alnuaimy WMT. Significance of B-catenin immunohistochemical expression in colorectal carcinoma. J Kurdistan Board of Med Specialties. 2018;4(1):51-6. [Link]
11. Li S, Huang M, Liu Q, Wang D, Wu R, Zhang X, et al. Serum expression of β-catenin is a potential detection marker in patients with colorectal cancer. Dis Mark. 2019;5070524. [Link] [DOI:10.1155/2019/5070524]
12. Yadav N, Ghalaut VS, Kumar S. Study of serum beta catenin levels in acute myeloid leukemia (AML) patients. Int J Adv Res. 2020;8(03):188-94. [Link] [DOI:10.21474/IJAR01/10615]
13. Gunes BA, Ozkan T, Gurel AK, Hekmatshoar Y, Beksac M, Sunguroglu A. β-catenin mutations in acute myeloid leukemia. Egyp J Haematol. 2020;45(2):105-10. [Link] [DOI:10.4103/ejh.ejh_54_19]
14. Zekri AR, Bahnassy AA, Alam El-Din HM, Morsy HM, Shaarawy S, et al. Serum levels of β-catenin as a potential marker for genotype 4/hepatitis C-associated hepatocellular carcinoma. Oncol Rep. 2011;26(4):825-31. [Link]
15. Yoshida N, Kinugasa T, Ohshima K, Yuge K, Ohchi T, Fujino S, et al. Analysis of Wnt and β-catenin expression in advanced colorectal cancer. Anticancer Res. 2015;35(8):4403-10. [Link]
16. Wangefjord S, Brändstedt J, Lindquist KE, Nodin B, Jirström K, Eberhard J. Associations of beta-catenin alterations and MSI screening status with expression of key cell cycle regulating proteins and survival from colorectal cancer. Diagn Pathol. 2013;8:10. [Link] [DOI:10.1186/1746-1596-8-10]
17. Suzuki H, Masuda N, Shimura, T, Araki K, Kobayashi T, Tsutsumi S, et al. Nuclear beta-catenin expression at the invasive front and in the vessels predicts liver metastasis in colorectal carcinoma. Anticancer Res. 2008;28(3B):1821-30. [Link]
18. Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med. 2016;14(21):1-12. [Link] [DOI:10.1186/s12967-016-0772-5]








