Volume 13, Issue 4 (2021)
Iran J War Public Health 2021, 13(4): 289-304 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/10/28 | Accepted: 2021/12/27 | Published: 2022/02/12
Received: 2021/10/28 | Accepted: 2021/12/27 | Published: 2022/02/12
How to cite this article
Hassani M. Histological and Histochemical Examination of Mucous Cells in Esophagus and Stomach of Rattus norvegicus. Iran J War Public Health 2021; 13 (4) :289-304
URL: http://ijwph.ir/article-1-1092-en.html
URL: http://ijwph.ir/article-1-1092-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
M.K. Hassani *
Department of Biology, College of Science, University of Misan, Amarah, Iraq
Full-Text (HTML) (1444 Views)
Introduction
Norway rats (Rattus norvegicus) are originally native to northern China. Today, Norway rats (also known as brown rats) can be found on every continent except Antarctica [1].
In Asia, R. norvegicus was native to forests and brushy areas. Today, however, Norway rats find their preferred habitat alongside the rapid expansion of the human population. Nearly every port city in the world has a substantial population of these rodents. They occupy various habitats, including garbage dumps, sewers, open fields and woodlands, basements, and anywhere else where food and shelter might be found. Anywhere that humans are located, R. norvegicus will most likely follow [2].
Norway rats are excellent foragers. Using their sense of smell and touch, they can survive quite easily, given that there is a steady supply of any food. In metropolitan areas, they survive mainly on discarded human food and anything else that can be eaten without negative consequences. Some Norway rats living near the sea have been observed catching fish with their paws. Chicks, mice, birds and small lizards are also preyed upon by Norway rats. They have even been known to attack infant human beings. Examination of a wild R. norvegicus stomach in Germany revealed 4000 items, most of which were plants, although studies have shown that Norway rats prefer meat when given the option [2].
The brown rat is a true omnivore and will consume almost anything, but cereals form a substantial part of its diet. Surplus animal feed, including the fallout from bird feeders, often attracts them. Foraging behavior is often population-specific and varies depending on the environment and food source. Examples have been found of rats eating birds and diving for mollusks where the food source is abundant. They feed on many things in urban environments, including food scraps from houses and restaurants [3].
Common rats occupy a wide range of lowland habitats, often associated with human sites, especially farms, industrial sites, rubbish tips, allotments, smallholdings, sewage farms, and sewers. This reflects their preference for sites that provide abundant food, especially
cereals and waste human food. Though they may live out in fields and on seashores in summer, they often move into farmyards and so on in winter [1].
Nowadays, the Norway rat as a laboratory animal is one of the most popular experimental models for research because it is easy to handle and inexpensive. Laboratory rats are the most used animal model in the liver and intestinal transplantation [4, 5] and gastrointestinal tract disease studies. Compared to humans, laboratory rats have a similar anatomical structure of the body organs [6].
The gastrointestinal tract includes the esophagus, stomach, small, and large intestine. The main function of the gastrointestinal tract is to digest food as it passes through it. In this process, nutrients and water are absorbed, and waste products are prepared for excretion from the body. Each section of the gastrointestinal tract has its unique histological features, which are closely associated with the function of that part of the gastrointestinal tract. The gastrointestinal tract organs are hollow and consist of four general tunic layers: mucosa, submucosa, muscularis externa and adventitia or serosa [7].
The esophagus is a narrow folded muscular tube whose main function is to transport food and fluids from the oral cavity to the stomach. It is the only part of the gastrointestinal tract that does not have metabolic, digest and absorb function. The esophagus is divided into three parts: cervical, thoracic, and abdominal [8, 9]. The entire gastrointestinal tract shows that the esophagus has four histological layers. The mucosa comprises three layers: a stratified squamous epithelium, a lamina propria and a lamina muscularis. Esophageal keratinization differs between species; some are keratinized, and others are non-keratinized [10].
The stomach is an enlarged portion of the gastrointestinal tube specializing in the enzymatic and hydrolytic breakdown of food into digestible nutrients. Its muscular wall helps to mix food. The stomach is covered by glandular mucosa in carnivores, while herbivores have a non-glandular area in addition to a glandular area covered by stratified squamous epithelium. There are noticeable differences between species in the gastric chamber manifested in the differences between monogastric and ruminant stomachs [11, 12]. Mucous cells secrete mucus, an important protective factor for preventing and repairing gastrointestinal ulcers. Mucous cells are mucous-producing cells that cover the inside of the stomach and protect it from gastric acid's corrosive nature. These cells line the gastric mucosal layer. Mucous cells can be divided according to surface mucous cells or foveolar cells, lining on the surface and gastric pits. Furthermore, the other is mucous neck cells, part of the neck of gastric glands and parietal cells [13, 14].
This study aimed at histological and histochemical examination of mucous cells in the esophagus and stomach of Norway rats (Rattus norvegicus).
Materials and Methods
In this experimental study, ten healthy adult male rats (R. norvegicus) with a mean age of 10-12 weeks and a mean weight of 200±25 g were prepared from the Animal House, College of Science, University of Misan.
Animals were housed under standard controlled conditions at a room temperature of 20-23°C in a 12:12 Light: Dark cycle. They were randomly isolated in plastic cages with hygienic beds and fed standard laboratory food.
At the end of the experiment, the rats were anesthetized using an overdose of chloroform and samples were taken from the esophagus and stomach (divided into three regions: cardia, fundus, and pylorus).
The tissues were then excised and fixed with 10% formalin for histological and histochemical studies.
Findings
Histological study
Light microscopic observations on sections of the esophagus (cervical, thoracic, abdominal) showed walls consisting of four layers: mucosa, submucosa, muscular and adventitia layer or serosa. Mucosa contains epithelium, lamina propria and muscular mucosa. The epithelium lining consists of a non-keratinized stratified squamous epithelium.
Also, the epithelium layer of the esophagus is formed of three layers. The basal layer consists of cuboidal or low columnar cells under the stratified epithelium. The middle layer of the epithelium is composed of polyhedral cells, and the superficial layer consists of flat squamous cells without keratin. Moreover, the lamina propria is composed of loose connective tissue containing elastic and collagen fibers, fibrocytes and blood vessels. Many dermal papillae appeared as finger-like extensions.
The muscularis mucosae consist of smooth muscle fibers arranged longitudinally between lamina propria and submucosa, also identifiable along the esophagus. The submucosa layer has loose connective tissue composed of intertwined collagen fiber, elastic fiber, fibrocytes, lymphocytes, and blood vessels in the presence of adipose tissue.
On the other hand, the muscular layer comprises two layers: the outer longitudinal layer and the inner circular layer. Collagen and reticular fibers separate the two muscle layers. Moreover, the muscular layer is composed of striated muscle throughout the cervical, thoracic and abdominal region.
The Adventitia layer is an external layer that covers the esophagus. It is composed of loose connective tissue and gradually transformed into a serosa layer in the abdominal region, composed of loose connective tissue and a mesothelium layer (Figures 1-6).

Figure 1. The section of cervical esophagus showing lumen (↔), stratified squamous epithelium layer (non-keratinized) (→), lamina propria (→), muscularis mucosa (→), submucosa muscularis layer (→), circular muscularis (→), longitudinal (→), serosa (→); H&E stain (10X)

Figure 2. The section of thoracic esophagus showing a stratified squamous epithelium layer (non-keratinized) consists of three-layer: surface layer (→), middle layer (→), basal layer (→); H&E stain (40X)

Figure 3. The section of thoracic esophagus showing lamina propria consists of loose connective tissue containing fibers (→), fat cells (→), blood vessels (→); H&E stain (40X)

Figure 4. The section of thoracic esophagus showing skeletal muscle: circular muscularis (→), connective tissue (→), longitudinal muscularis (→); H&E stain (40X)

Figure 5. The section of thoracic esophagus showing serosa consists of loose connective tissue containing blood vessels (→), fibers (→); H&E stain (40X)
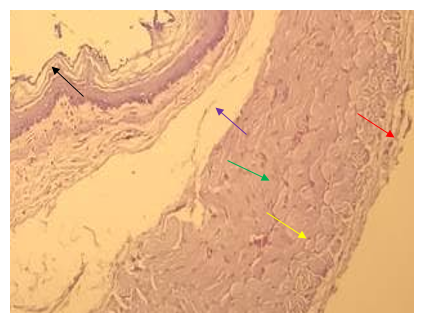
Figure 6. The section of the abdominal esophagus showing mucosa (→), submucosa (→), skeletal muscle including circular muscularis (→), longitudinal muscularis (→), serosa (→); H&E stain (40X)
It was observed that the stomach is composed of four layers: mucosa, submucosa, muscularis, and serosa. The mucosa layer consists of epithelium lamina propria and muscularis mucosae. The glandular stomach contains longitudinal rugae folds, and the epithelium lining consists of simple columnar epithelium. The epithelium is invaginated into the lamina propria to form the gastric pits. The depth of the gastric pit varies between regions of the stomach. The lamina propria comprises loose connective tissue containing blood vessels, lymphocytes, elastic, and collagen fibers. And glands. The muscularis mucosa consists of smooth muscle fibers arranged longitudinally. The submucosa layer has loose connective tissue and contains blood vessels. Lymphahe muscularis mucosa consists of smooth muscle fibers arranged longitudinally. The submucosa layer has loose connective tissue and contains blood vessels, lymphatic vessels, fat cells, tic vessels, and nerve fibers called Meissner's plexuses. Also, no submucosal glands were observed throughout the cardiac, fundus, and pylorus regions. The muscularis layer comprises two layers of smooth muscle fibers: a relatively thick inner circular layer and a thin longitudinal outer layer. A serosa layer between them consists of a loose connective tissue covered with mesothelium.
The stomach is divided into three areas: cardiac, fundus, and pylorus. The cardiac gland is simple, coiled and branched tubular. The gastric pits are deep in the cardiac region, and the glands in this region form mucous cells, which are shaped tall columnar that secrete mucous and lysozyme. Also, some parietal cells were observed in this region that secretes hydrochloric acid (HCL).
The fundus glands are simple, straight and branched tubular. These glands are composed of the parietal cells shaped pyramid with eosinophilic cytoplasm and circuit nucleus. They are distributed in the fundic glands full of strong eosinophilic granules in the cytoplasm. In addition, there are chief cells that have a circuit shape with a basophilic cytoplasm which secret pepsinogen. The pylorus glands are simple tubular. These glands, consisting of long-columnar mucus-secreting cells, also have a basophilic cytoplasm and contain smooth nuclei located at the base, mucus-secreting cells, and a small number of parietal cells (Figures 7-11).

Figure 7. The section of stomach-fundus showing layers: epithelium (simple columnar ) (→), lamina propria (→), muscularis mucosa (→), submucosa (→) muscularis (→), serosa (→); H&E stain (10X)

Figure 8. The section of stomach-cardiac showing gastric pit (→), gastric glands (→), muscularis mucosa (→); H&E stain (10X)

Figure 9. The section of the cardiac gland of the stomach (→) consisting of secret mucous cells (→), parietal cells (→); H&E stain (40X)

Figure 10. The section of the fundus gland of the stomach consists of parietal cells (→) chief cells (→) glands (→); H&E stain (40X)

Figure 11. The section of the stomach-pylorus region shows four layers: mucosa (↔), gastric pit (→), lamina propria (→), muscularis mucosa (→), submucosa (→), muscularis (→), serosa (→); H&E stain (10X)
Norway rats (Rattus norvegicus) are originally native to northern China. Today, Norway rats (also known as brown rats) can be found on every continent except Antarctica [1].
In Asia, R. norvegicus was native to forests and brushy areas. Today, however, Norway rats find their preferred habitat alongside the rapid expansion of the human population. Nearly every port city in the world has a substantial population of these rodents. They occupy various habitats, including garbage dumps, sewers, open fields and woodlands, basements, and anywhere else where food and shelter might be found. Anywhere that humans are located, R. norvegicus will most likely follow [2].
Norway rats are excellent foragers. Using their sense of smell and touch, they can survive quite easily, given that there is a steady supply of any food. In metropolitan areas, they survive mainly on discarded human food and anything else that can be eaten without negative consequences. Some Norway rats living near the sea have been observed catching fish with their paws. Chicks, mice, birds and small lizards are also preyed upon by Norway rats. They have even been known to attack infant human beings. Examination of a wild R. norvegicus stomach in Germany revealed 4000 items, most of which were plants, although studies have shown that Norway rats prefer meat when given the option [2].
The brown rat is a true omnivore and will consume almost anything, but cereals form a substantial part of its diet. Surplus animal feed, including the fallout from bird feeders, often attracts them. Foraging behavior is often population-specific and varies depending on the environment and food source. Examples have been found of rats eating birds and diving for mollusks where the food source is abundant. They feed on many things in urban environments, including food scraps from houses and restaurants [3].
Common rats occupy a wide range of lowland habitats, often associated with human sites, especially farms, industrial sites, rubbish tips, allotments, smallholdings, sewage farms, and sewers. This reflects their preference for sites that provide abundant food, especially
cereals and waste human food. Though they may live out in fields and on seashores in summer, they often move into farmyards and so on in winter [1].
Nowadays, the Norway rat as a laboratory animal is one of the most popular experimental models for research because it is easy to handle and inexpensive. Laboratory rats are the most used animal model in the liver and intestinal transplantation [4, 5] and gastrointestinal tract disease studies. Compared to humans, laboratory rats have a similar anatomical structure of the body organs [6].
The gastrointestinal tract includes the esophagus, stomach, small, and large intestine. The main function of the gastrointestinal tract is to digest food as it passes through it. In this process, nutrients and water are absorbed, and waste products are prepared for excretion from the body. Each section of the gastrointestinal tract has its unique histological features, which are closely associated with the function of that part of the gastrointestinal tract. The gastrointestinal tract organs are hollow and consist of four general tunic layers: mucosa, submucosa, muscularis externa and adventitia or serosa [7].
The esophagus is a narrow folded muscular tube whose main function is to transport food and fluids from the oral cavity to the stomach. It is the only part of the gastrointestinal tract that does not have metabolic, digest and absorb function. The esophagus is divided into three parts: cervical, thoracic, and abdominal [8, 9]. The entire gastrointestinal tract shows that the esophagus has four histological layers. The mucosa comprises three layers: a stratified squamous epithelium, a lamina propria and a lamina muscularis. Esophageal keratinization differs between species; some are keratinized, and others are non-keratinized [10].
The stomach is an enlarged portion of the gastrointestinal tube specializing in the enzymatic and hydrolytic breakdown of food into digestible nutrients. Its muscular wall helps to mix food. The stomach is covered by glandular mucosa in carnivores, while herbivores have a non-glandular area in addition to a glandular area covered by stratified squamous epithelium. There are noticeable differences between species in the gastric chamber manifested in the differences between monogastric and ruminant stomachs [11, 12]. Mucous cells secrete mucus, an important protective factor for preventing and repairing gastrointestinal ulcers. Mucous cells are mucous-producing cells that cover the inside of the stomach and protect it from gastric acid's corrosive nature. These cells line the gastric mucosal layer. Mucous cells can be divided according to surface mucous cells or foveolar cells, lining on the surface and gastric pits. Furthermore, the other is mucous neck cells, part of the neck of gastric glands and parietal cells [13, 14].
This study aimed at histological and histochemical examination of mucous cells in the esophagus and stomach of Norway rats (Rattus norvegicus).
Materials and Methods
In this experimental study, ten healthy adult male rats (R. norvegicus) with a mean age of 10-12 weeks and a mean weight of 200±25 g were prepared from the Animal House, College of Science, University of Misan.
Animals were housed under standard controlled conditions at a room temperature of 20-23°C in a 12:12 Light: Dark cycle. They were randomly isolated in plastic cages with hygienic beds and fed standard laboratory food.
At the end of the experiment, the rats were anesthetized using an overdose of chloroform and samples were taken from the esophagus and stomach (divided into three regions: cardia, fundus, and pylorus).
The tissues were then excised and fixed with 10% formalin for histological and histochemical studies.
Findings
Histological study
Light microscopic observations on sections of the esophagus (cervical, thoracic, abdominal) showed walls consisting of four layers: mucosa, submucosa, muscular and adventitia layer or serosa. Mucosa contains epithelium, lamina propria and muscular mucosa. The epithelium lining consists of a non-keratinized stratified squamous epithelium.
Also, the epithelium layer of the esophagus is formed of three layers. The basal layer consists of cuboidal or low columnar cells under the stratified epithelium. The middle layer of the epithelium is composed of polyhedral cells, and the superficial layer consists of flat squamous cells without keratin. Moreover, the lamina propria is composed of loose connective tissue containing elastic and collagen fibers, fibrocytes and blood vessels. Many dermal papillae appeared as finger-like extensions.
The muscularis mucosae consist of smooth muscle fibers arranged longitudinally between lamina propria and submucosa, also identifiable along the esophagus. The submucosa layer has loose connective tissue composed of intertwined collagen fiber, elastic fiber, fibrocytes, lymphocytes, and blood vessels in the presence of adipose tissue.
On the other hand, the muscular layer comprises two layers: the outer longitudinal layer and the inner circular layer. Collagen and reticular fibers separate the two muscle layers. Moreover, the muscular layer is composed of striated muscle throughout the cervical, thoracic and abdominal region.
The Adventitia layer is an external layer that covers the esophagus. It is composed of loose connective tissue and gradually transformed into a serosa layer in the abdominal region, composed of loose connective tissue and a mesothelium layer (Figures 1-6).

Figure 1. The section of cervical esophagus showing lumen (↔), stratified squamous epithelium layer (non-keratinized) (→), lamina propria (→), muscularis mucosa (→), submucosa muscularis layer (→), circular muscularis (→), longitudinal (→), serosa (→); H&E stain (10X)

Figure 2. The section of thoracic esophagus showing a stratified squamous epithelium layer (non-keratinized) consists of three-layer: surface layer (→), middle layer (→), basal layer (→); H&E stain (40X)

Figure 3. The section of thoracic esophagus showing lamina propria consists of loose connective tissue containing fibers (→), fat cells (→), blood vessels (→); H&E stain (40X)

Figure 4. The section of thoracic esophagus showing skeletal muscle: circular muscularis (→), connective tissue (→), longitudinal muscularis (→); H&E stain (40X)

Figure 5. The section of thoracic esophagus showing serosa consists of loose connective tissue containing blood vessels (→), fibers (→); H&E stain (40X)

Figure 6. The section of the abdominal esophagus showing mucosa (→), submucosa (→), skeletal muscle including circular muscularis (→), longitudinal muscularis (→), serosa (→); H&E stain (40X)
It was observed that the stomach is composed of four layers: mucosa, submucosa, muscularis, and serosa. The mucosa layer consists of epithelium lamina propria and muscularis mucosae. The glandular stomach contains longitudinal rugae folds, and the epithelium lining consists of simple columnar epithelium. The epithelium is invaginated into the lamina propria to form the gastric pits. The depth of the gastric pit varies between regions of the stomach. The lamina propria comprises loose connective tissue containing blood vessels, lymphocytes, elastic, and collagen fibers. And glands. The muscularis mucosa consists of smooth muscle fibers arranged longitudinally. The submucosa layer has loose connective tissue and contains blood vessels. Lymphahe muscularis mucosa consists of smooth muscle fibers arranged longitudinally. The submucosa layer has loose connective tissue and contains blood vessels, lymphatic vessels, fat cells, tic vessels, and nerve fibers called Meissner's plexuses. Also, no submucosal glands were observed throughout the cardiac, fundus, and pylorus regions. The muscularis layer comprises two layers of smooth muscle fibers: a relatively thick inner circular layer and a thin longitudinal outer layer. A serosa layer between them consists of a loose connective tissue covered with mesothelium.
The stomach is divided into three areas: cardiac, fundus, and pylorus. The cardiac gland is simple, coiled and branched tubular. The gastric pits are deep in the cardiac region, and the glands in this region form mucous cells, which are shaped tall columnar that secrete mucous and lysozyme. Also, some parietal cells were observed in this region that secretes hydrochloric acid (HCL).
The fundus glands are simple, straight and branched tubular. These glands are composed of the parietal cells shaped pyramid with eosinophilic cytoplasm and circuit nucleus. They are distributed in the fundic glands full of strong eosinophilic granules in the cytoplasm. In addition, there are chief cells that have a circuit shape with a basophilic cytoplasm which secret pepsinogen. The pylorus glands are simple tubular. These glands, consisting of long-columnar mucus-secreting cells, also have a basophilic cytoplasm and contain smooth nuclei located at the base, mucus-secreting cells, and a small number of parietal cells (Figures 7-11).

Figure 7. The section of stomach-fundus showing layers: epithelium (simple columnar ) (→), lamina propria (→), muscularis mucosa (→), submucosa (→) muscularis (→), serosa (→); H&E stain (10X)

Figure 8. The section of stomach-cardiac showing gastric pit (→), gastric glands (→), muscularis mucosa (→); H&E stain (10X)

Figure 9. The section of the cardiac gland of the stomach (→) consisting of secret mucous cells (→), parietal cells (→); H&E stain (40X)

Figure 10. The section of the fundus gland of the stomach consists of parietal cells (→) chief cells (→) glands (→); H&E stain (40X)

Figure 11. The section of the stomach-pylorus region shows four layers: mucosa (↔), gastric pit (→), lamina propria (→), muscularis mucosa (→), submucosa (→), muscularis (→), serosa (→); H&E stain (10X)
Histochemical study
The surface layer of the epithelium reacted strongly to Periodic Acid-Schiff (PAS) staining. Still, the rest of the epithelium had a moderate reaction to PAS in all regions of the esophagus. The submucosa showed a weak reaction to PAS in all regions of the esophagus (cervical, thoracic, and abdominal). The muscularis layer in the cervical, thoracic, and abdominal esophagus regions reacted moderately to PAS; however, the serosa showed a weakly reaction to PAS in cervical, thoracic and abdominal regions (Figures 12-15).
The mucosa layer of the cardiac region of the stomach showed a strong reaction to PAS. Distributed polysaccharides were concentrated in surface cells and body glands and showed a magenta red, purple color. In the fundus region, the PAS staining reacted strongly to surface cells in the upper area of the mucosa layer, while PAS staining showed a weakly reaction to parietal cells and chief cells. In the pylorus region, the mucous layer reacted strongly to PAS. The distribution of neutral polysaccharides in the pylorus glands is approximately equal. However, the submucosa and serosa layers showed a weak reaction to PAS, but the muscularis reaction to PAS was moderate in cardiac, fundus and pylorus regions (Figures 16-19).
The surface layer of the epithelium reacted strongly to Periodic Acid-Schiff (PAS) staining. Still, the rest of the epithelium had a moderate reaction to PAS in all regions of the esophagus. The submucosa showed a weak reaction to PAS in all regions of the esophagus (cervical, thoracic, and abdominal). The muscularis layer in the cervical, thoracic, and abdominal esophagus regions reacted moderately to PAS; however, the serosa showed a weakly reaction to PAS in cervical, thoracic and abdominal regions (Figures 12-15).
The mucosa layer of the cardiac region of the stomach showed a strong reaction to PAS. Distributed polysaccharides were concentrated in surface cells and body glands and showed a magenta red, purple color. In the fundus region, the PAS staining reacted strongly to surface cells in the upper area of the mucosa layer, while PAS staining showed a weakly reaction to parietal cells and chief cells. In the pylorus region, the mucous layer reacted strongly to PAS. The distribution of neutral polysaccharides in the pylorus glands is approximately equal. However, the submucosa and serosa layers showed a weak reaction to PAS, but the muscularis reaction to PAS was moderate in cardiac, fundus and pylorus regions (Figures 16-19).

Figure 12. The section of esophagus showing a squamous layer of non-keratinized stratified squamous epithelium layer with a strong reaction to PAS (→); PAS stain (10X)

Figure 13. The section of the cervical esophagus region showing squamous cells with a strong reaction to PAS (→), mucosa layer with a moderate reaction to PAS (→), submucosa layer with a weak reaction to PAS (→); PAS stain (10X)

Figure 14. The section of thoracic esophagus region showing surface layer with a strong reaction to PAS (→), mucosa layer with a moderate reaction to PAS (→), submucosa with a weak reaction to PAS (→); PAS stain (40X)

Figure 15. The section of the abdominal esophagus region shows surface layer with a strong reaction to PAS (→), mucosa layer with a moderate reaction to PAS (→), submucosa and serosa with a weak reaction to PAS (→), muscularis with a moderate reaction to PAS (→); PAS stain (40X)
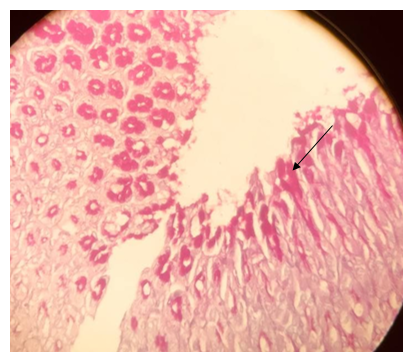
Figure 16. The section of the stomach-cardiac region showing mucosa layer with a strong reaction to PAS (→); PAS stain (40X)

Figure 17. The section of the stomach- fundus region showing surface cells of mucosa layer with a strong reaction to PAS (→); PAS stain (40X)
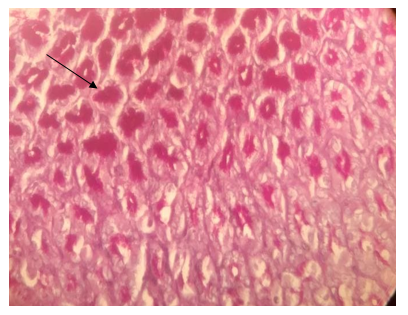
Figure 18. The section of the stomach –pylorus region showing mucosa layer with a strong reaction to PAS (→); PAS stain (40X)

Figure 19. The section of the stomach-cardiac region showing layers: submucosa showed a weak reaction to PAS, muscularis (→), inner circular (→), outer longitudinal reacted moderately to PAS (→), serosa reacted weakly to PAS (→); PAS stain (10X)
Discussion
The results show that the esophageal region (cervical, thoracic, abdominal) has a wall consisting of four layers: mucosa, submucosa, muscular and adventitia layer or serosa. The mucosa contains epithelium, lamina propria, and muscular mucosa. The epithelium lining consisted of a non-keratinized stratified squamous epithelium.
These results are consistent with Mukaddes et al. [15], who reported that the esophagus is composed of mucosa, submucosa, muscularis and adventitia/serosa; The mucosa consists of the epithelium, the lamina propria, and the muscularis mucosa. The surface of the epithelium is non-keratinized stratified squamous. The lamina propria, like a loose connective tissue, has some mucous glands called "esophageal cardiac glands" at the proximal and distal ends of the esophagus.
Nzalak [11] reported that the esophagus is divided into the cervical, thoracic, and abdominal portions. He also mentioned the esophageal lining is observed as a non-keratinized stratified squamous epithelium.
Sarosiek et al. [16] stated that the esophagus is a highly specialized organ designed to prop food from the mouth to the stomach. As a result of its position in the gastrointestinal tract, it may be exposed to various noxious stimuli. The African giant rat being omnivorous, needs this mucous to increase the viscosity of the esophagus, which is important for lubricating the mucosa and allowing the passage of large food particles, as well as immobilizing hunted food such as arthropods and grasshopper.
Mahmoud et al. [17] also observed in rabbits that the epithelium lining of the esophagus is non-keratinized stratified squamous. The epithelium of the esophagus is formed of three layers: the stratum basal, which is cuboidal or low columnar and is located under the stratified epithelium; the cells in the middle layer of the epithelium, which are polyhedral; and the surface layer, which are squamous cells without keratin.
Eroschenko [18] stated that the non-keratinized stratified squamous epithelium layer of the esophagus consists of squamous cells, polyhedral cells, and stratum basale.
The muscularis mucosa consists of smooth muscle fibers arranged longitudinally. The submucosa layer has loose connective tissue composed of interwoven collagen fibers, elastic fibers, fibrocytes, lymphocytes, and blood vessels denser than the lamina propria, which contains mucous tubuloalveolar esophageal glands proper. These results are consistent with Mukaddes et al. [15], who reported that the muscularis is a longitudinally arranged smooth muscle layer, and the submucosa contains mucous tubuloalveolar esophageal glands. The same was observed for the buffalo calves by Gupta et al. [19] and detected seromucous tubuloalveolar glands in the initial portion of the esophagus. Mahmoud et al. [17] also observed this gland in the rabbit's esophagus.
The muscular layer consists of two layers; the outer longitudinal layer and the inner circular layer. The collagen and reticular fibers separate the two muscle layers. The muscular layer is also composed of striated muscle throughout the cervical, thoracic, and abdominal region. These results are consistent with Banks [20], who reported that striated skeletal muscle might allow regurgitation to chew and allow any foreign body to be pushed toward the rumen more rapidly. At the same time, adventitia is composed of loose connective tissue that gradually transforms into serosa in the abdominal region, composed of loose connective tissue and a mesothelium layer. These findings are consistent with the study of Hussein et al. [21].
The results showed that the stomach comprises four layers: mucosa, submucosa, muscularis, and serosa. The mucosa layer consists of epithelium lamina propria and muscularis mucosae. The glandular stomach contains longitudinal rugae folds, and the epithelium lining consists of simple columnar epithelium. The epithelium is invaginated into the lamina propria to form the gastric pits. The depth of the gastric pit varies between regions of the stomach. The lamina propria comprises loose connective tissue containing blood vessels, lymphocytes, elastic fibers, collagen, and glands. Muscularis mucosa consists of smooth muscle fibers that are arranged longitudinally. The submucosa layer has loose connective tissue and contains blood vessels. Lymphahe muscularis mucosa consists of smooth muscle fibers arranged longitudinally. The submucosa layer has loose connective tissue and contains blood vessels, lymphatic vessels, fat cells, tic vessels, and nerve fibers called Meissner's plexuses. Also, no submucosal glands were observed throughout the cardiac, fundus, and pylorus regions. These results are consistent with Mukaddes et al. [15], who reported that the stomach contains mucosa, submucosa muscularis (muscularis externa), and serosa. The mucosa is composed of epithelium, lamina propria, and muscularis mucosa. A simple columnar epithelium functions as both lining and secretory epithelium. The covering epithelium also protects the mucosa against the acidic content of the stomach by producing mucus. The mucosa and submucosa together make longitudinal folds and ridges called rugae.
The tissue of simple columnar epithelium secretes mucous that softens food and protects the mucosa from the effects of rough foods, as well as prevents microbes from entering the tissue below it [18]. The lamina propria comprises loose connective tissue containing blood vessels, lymphocytes, elastic fibers, collagen, and glands. This is consistent with a study by Mahesh et al. [22], which reinforces these results. Depending on the type of glands, the stomach is divided into three areas: the cardiac, the fundus, and the pylorus. Some animals, such as cows, do not have a cardiac region or horse; this region is small. The gastric glands vary according to the species of animals [23, 24].
The cardiac glands are simple, branched tubular, and coiled. The cardiac region also contains many mucus-secreting cells and small cells, which are the same findings of previous studies [7, 25].
The fundus glands are simple, straight, and branched tubular. In addition, parietal cells in the fundus gland are eosinophilic because they contain large amounts of mitochondria that are metabolically activated to secrete HCL. These results are reinforced by Dyce et al. [23], who observed in a horse that the glands in this region are simple tubular and by Al-Neamy [26], who confirms the histological characteristics of parietal cells are the same in the stomach of humans, ruminant and mono-gastric animals. The chief cells in the fundus gland have basophilic cytoplasm because they have a large rough endoplasmic reticulum in the cytoplasm that contains ribosomes; these cells have been described by Junqueira & Mescher [27].
Pylorus glands are simple tubular. This difference in the type of glands indicates the function of the entire stomach region. On the other hand, Sujana [28] described that the pyloric glands in pigs are devoid of parietal cells but have mucous cells.
Muscularis mucosa consists of longitudinal smooth-muscle fibers and helps the glands to secrete by contracting these muscles [27]. Furthermore, the submucosa layer consists of connective tissue containing reticular fiber, blood vessels, nerve plexus, elastic fibers and collagen fibers in the cardiac, fundus and pyloric region [10].
Nevertheless, the muscularis layer comprises two smooth muscle layers: a thick inner circular layer and a thin outer longitudinal layer. The circular muscle layer prevents food from moving backward, while the longitudinal layer shortens the tract. An Auerbach's plexus between the two muscularis layers serves as a mechanical sensory serosa layer consisting of a loose connective tissue covered with mesothelium [7].
Ahmed et al. [29] reported that the stomach of Varanus niloticus is divided into two distinct parts: the fundus or body and the pylorus. The stomach was lined through its length with mucus-secreting columnar epithelium that showed numerous invaginations, gastric pits, which led to glandular structures, the gastric glands, the gastric surface epithelium showed the same histological features in both fundic and pyloric regions. The results of a histochemical study on esophageal regions show that the surface layer of the epithelial layer reacts strongly to PAS. Still, the other layers of the epithelial layer have a moderate reaction to PAS in all regions of the esophagus. These results are consistent with previous studies [30-32], which show that the PAS reacts to the esophagus due to mucus-secreting cells.
Selim et al. [33] also observed that the response with PAS is a strong reaction to the inner layer of the mucosa and a moderate reaction to the lamina propria in the rabbit esophagus.
Ahmed et al. [29] observed that the esophagus of Varanus niloticus contains mucus-secreting cells which stained positive with PAS and AB, suggesting that they secrete both neutral and acidic mucosubstance. They further observed that this mucous, especially the acidic one, which increases the viscosity of the mucous content, may be important for lubricating the mucosa for the passage of large food particles and immobilization of overcome hunted food such as arthropods.
The submucosa showed a weak reaction to PAS in each esophagus region, which may be because of the absence of the glands. Nzalak [11] reported that the esophagus of the African giant rat does not have glands in the submucosa. The mucus produced by the salivary glands may help protect the esophageal mucosal surface from sharp objects. The mucosal barrier is also an important factor in protecting the esophagus from damage.
The muscularis layer reacted moderately to PAS, which may be due to the presence of a small amount of glycogen in the skeletal muscle. Listrat et al. [34] detected that skeletal muscle contains 1% glycogen.
The results of a histochemical study of the stomach regions show that the mucosa layer in the cardiac region reacts strongly to PAS, which appears as pink or magenta in the surface cell and body of the gland, and this indicates that these cells secrete mucous to counteract the acid of the stomach wall, but neutral polysaccharides differ in distribution. This study is similar to the Khalel study [35] in rabbits and Al-Saffar and Eyhab study [36], which reported that neutral polysaccharides are important to protect the stomach wall against acid digestion.
In the fundus region, the mucosa layer also reacted strongly to PAS, indicating that the mucosa layer cell secretes mucous, but the neutral polysaccharides are distributed only in the superficial cells of the mucosa layer. While the basal fundus showed a negative reaction to PAS, this may be due to the parietal cells and primary cells that react negatively to PAS because the function of these cells is limited and parietal cells secret HCL and chief cells secret pepsin. This result is consistent with the Khalel study [35] in rabbits.
In the pylorus region, the mucosa reacted strongly to PAS, showing that neutral polysaccharides' distribution is almost equal in all glands. Still, this region was more intense than in other regions because these glands are mostly made of cells secret mucous, which appear as magenta color and consider mucus-producing cells. Few parietal cells and
this finding are consistent with previous studies [27, 37].
Conclusion
Recognizing the anatomical and histochemical features of different small mammalians as the models of laboratory animals for testing drug absorbance can help understand the probable results in human patients.
Acknowledgments: -
Ethical Permissions: -
Conflicts of Interests: -
Authors’ Contributions: Hassani M.K. (First Author), Introduction Writer/Methodologist/ Main or Assistant Researcher /Statistical Analyst/Discussion Writer (100%).
Funding/Sources: -
The results show that the esophageal region (cervical, thoracic, abdominal) has a wall consisting of four layers: mucosa, submucosa, muscular and adventitia layer or serosa. The mucosa contains epithelium, lamina propria, and muscular mucosa. The epithelium lining consisted of a non-keratinized stratified squamous epithelium.
These results are consistent with Mukaddes et al. [15], who reported that the esophagus is composed of mucosa, submucosa, muscularis and adventitia/serosa; The mucosa consists of the epithelium, the lamina propria, and the muscularis mucosa. The surface of the epithelium is non-keratinized stratified squamous. The lamina propria, like a loose connective tissue, has some mucous glands called "esophageal cardiac glands" at the proximal and distal ends of the esophagus.
Nzalak [11] reported that the esophagus is divided into the cervical, thoracic, and abdominal portions. He also mentioned the esophageal lining is observed as a non-keratinized stratified squamous epithelium.
Sarosiek et al. [16] stated that the esophagus is a highly specialized organ designed to prop food from the mouth to the stomach. As a result of its position in the gastrointestinal tract, it may be exposed to various noxious stimuli. The African giant rat being omnivorous, needs this mucous to increase the viscosity of the esophagus, which is important for lubricating the mucosa and allowing the passage of large food particles, as well as immobilizing hunted food such as arthropods and grasshopper.
Mahmoud et al. [17] also observed in rabbits that the epithelium lining of the esophagus is non-keratinized stratified squamous. The epithelium of the esophagus is formed of three layers: the stratum basal, which is cuboidal or low columnar and is located under the stratified epithelium; the cells in the middle layer of the epithelium, which are polyhedral; and the surface layer, which are squamous cells without keratin.
Eroschenko [18] stated that the non-keratinized stratified squamous epithelium layer of the esophagus consists of squamous cells, polyhedral cells, and stratum basale.
The muscularis mucosa consists of smooth muscle fibers arranged longitudinally. The submucosa layer has loose connective tissue composed of interwoven collagen fibers, elastic fibers, fibrocytes, lymphocytes, and blood vessels denser than the lamina propria, which contains mucous tubuloalveolar esophageal glands proper. These results are consistent with Mukaddes et al. [15], who reported that the muscularis is a longitudinally arranged smooth muscle layer, and the submucosa contains mucous tubuloalveolar esophageal glands. The same was observed for the buffalo calves by Gupta et al. [19] and detected seromucous tubuloalveolar glands in the initial portion of the esophagus. Mahmoud et al. [17] also observed this gland in the rabbit's esophagus.
The muscular layer consists of two layers; the outer longitudinal layer and the inner circular layer. The collagen and reticular fibers separate the two muscle layers. The muscular layer is also composed of striated muscle throughout the cervical, thoracic, and abdominal region. These results are consistent with Banks [20], who reported that striated skeletal muscle might allow regurgitation to chew and allow any foreign body to be pushed toward the rumen more rapidly. At the same time, adventitia is composed of loose connective tissue that gradually transforms into serosa in the abdominal region, composed of loose connective tissue and a mesothelium layer. These findings are consistent with the study of Hussein et al. [21].
The results showed that the stomach comprises four layers: mucosa, submucosa, muscularis, and serosa. The mucosa layer consists of epithelium lamina propria and muscularis mucosae. The glandular stomach contains longitudinal rugae folds, and the epithelium lining consists of simple columnar epithelium. The epithelium is invaginated into the lamina propria to form the gastric pits. The depth of the gastric pit varies between regions of the stomach. The lamina propria comprises loose connective tissue containing blood vessels, lymphocytes, elastic fibers, collagen, and glands. Muscularis mucosa consists of smooth muscle fibers that are arranged longitudinally. The submucosa layer has loose connective tissue and contains blood vessels. Lymphahe muscularis mucosa consists of smooth muscle fibers arranged longitudinally. The submucosa layer has loose connective tissue and contains blood vessels, lymphatic vessels, fat cells, tic vessels, and nerve fibers called Meissner's plexuses. Also, no submucosal glands were observed throughout the cardiac, fundus, and pylorus regions. These results are consistent with Mukaddes et al. [15], who reported that the stomach contains mucosa, submucosa muscularis (muscularis externa), and serosa. The mucosa is composed of epithelium, lamina propria, and muscularis mucosa. A simple columnar epithelium functions as both lining and secretory epithelium. The covering epithelium also protects the mucosa against the acidic content of the stomach by producing mucus. The mucosa and submucosa together make longitudinal folds and ridges called rugae.
The tissue of simple columnar epithelium secretes mucous that softens food and protects the mucosa from the effects of rough foods, as well as prevents microbes from entering the tissue below it [18]. The lamina propria comprises loose connective tissue containing blood vessels, lymphocytes, elastic fibers, collagen, and glands. This is consistent with a study by Mahesh et al. [22], which reinforces these results. Depending on the type of glands, the stomach is divided into three areas: the cardiac, the fundus, and the pylorus. Some animals, such as cows, do not have a cardiac region or horse; this region is small. The gastric glands vary according to the species of animals [23, 24].
The cardiac glands are simple, branched tubular, and coiled. The cardiac region also contains many mucus-secreting cells and small cells, which are the same findings of previous studies [7, 25].
The fundus glands are simple, straight, and branched tubular. In addition, parietal cells in the fundus gland are eosinophilic because they contain large amounts of mitochondria that are metabolically activated to secrete HCL. These results are reinforced by Dyce et al. [23], who observed in a horse that the glands in this region are simple tubular and by Al-Neamy [26], who confirms the histological characteristics of parietal cells are the same in the stomach of humans, ruminant and mono-gastric animals. The chief cells in the fundus gland have basophilic cytoplasm because they have a large rough endoplasmic reticulum in the cytoplasm that contains ribosomes; these cells have been described by Junqueira & Mescher [27].
Pylorus glands are simple tubular. This difference in the type of glands indicates the function of the entire stomach region. On the other hand, Sujana [28] described that the pyloric glands in pigs are devoid of parietal cells but have mucous cells.
Muscularis mucosa consists of longitudinal smooth-muscle fibers and helps the glands to secrete by contracting these muscles [27]. Furthermore, the submucosa layer consists of connective tissue containing reticular fiber, blood vessels, nerve plexus, elastic fibers and collagen fibers in the cardiac, fundus and pyloric region [10].
Nevertheless, the muscularis layer comprises two smooth muscle layers: a thick inner circular layer and a thin outer longitudinal layer. The circular muscle layer prevents food from moving backward, while the longitudinal layer shortens the tract. An Auerbach's plexus between the two muscularis layers serves as a mechanical sensory serosa layer consisting of a loose connective tissue covered with mesothelium [7].
Ahmed et al. [29] reported that the stomach of Varanus niloticus is divided into two distinct parts: the fundus or body and the pylorus. The stomach was lined through its length with mucus-secreting columnar epithelium that showed numerous invaginations, gastric pits, which led to glandular structures, the gastric glands, the gastric surface epithelium showed the same histological features in both fundic and pyloric regions. The results of a histochemical study on esophageal regions show that the surface layer of the epithelial layer reacts strongly to PAS. Still, the other layers of the epithelial layer have a moderate reaction to PAS in all regions of the esophagus. These results are consistent with previous studies [30-32], which show that the PAS reacts to the esophagus due to mucus-secreting cells.
Selim et al. [33] also observed that the response with PAS is a strong reaction to the inner layer of the mucosa and a moderate reaction to the lamina propria in the rabbit esophagus.
Ahmed et al. [29] observed that the esophagus of Varanus niloticus contains mucus-secreting cells which stained positive with PAS and AB, suggesting that they secrete both neutral and acidic mucosubstance. They further observed that this mucous, especially the acidic one, which increases the viscosity of the mucous content, may be important for lubricating the mucosa for the passage of large food particles and immobilization of overcome hunted food such as arthropods.
The submucosa showed a weak reaction to PAS in each esophagus region, which may be because of the absence of the glands. Nzalak [11] reported that the esophagus of the African giant rat does not have glands in the submucosa. The mucus produced by the salivary glands may help protect the esophageal mucosal surface from sharp objects. The mucosal barrier is also an important factor in protecting the esophagus from damage.
The muscularis layer reacted moderately to PAS, which may be due to the presence of a small amount of glycogen in the skeletal muscle. Listrat et al. [34] detected that skeletal muscle contains 1% glycogen.
The results of a histochemical study of the stomach regions show that the mucosa layer in the cardiac region reacts strongly to PAS, which appears as pink or magenta in the surface cell and body of the gland, and this indicates that these cells secrete mucous to counteract the acid of the stomach wall, but neutral polysaccharides differ in distribution. This study is similar to the Khalel study [35] in rabbits and Al-Saffar and Eyhab study [36], which reported that neutral polysaccharides are important to protect the stomach wall against acid digestion.
In the fundus region, the mucosa layer also reacted strongly to PAS, indicating that the mucosa layer cell secretes mucous, but the neutral polysaccharides are distributed only in the superficial cells of the mucosa layer. While the basal fundus showed a negative reaction to PAS, this may be due to the parietal cells and primary cells that react negatively to PAS because the function of these cells is limited and parietal cells secret HCL and chief cells secret pepsin. This result is consistent with the Khalel study [35] in rabbits.
In the pylorus region, the mucosa reacted strongly to PAS, showing that neutral polysaccharides' distribution is almost equal in all glands. Still, this region was more intense than in other regions because these glands are mostly made of cells secret mucous, which appear as magenta color and consider mucus-producing cells. Few parietal cells and
this finding are consistent with previous studies [27, 37].
Conclusion
Recognizing the anatomical and histochemical features of different small mammalians as the models of laboratory animals for testing drug absorbance can help understand the probable results in human patients.
Acknowledgments: -
Ethical Permissions: -
Conflicts of Interests: -
Authors’ Contributions: Hassani M.K. (First Author), Introduction Writer/Methodologist/ Main or Assistant Researcher /Statistical Analyst/Discussion Writer (100%).
Funding/Sources: -
References
1. Silver J. The introduction and spread of house rats in the United States. J Mammal. 1927;8(1):58-60. [Link] [DOI:10.1093/jmammal/8.1.58a]
2. Whitaker JO, Hamilton WJ. Mammals of Eastern United States. 3rd Edition. Ithaca, NY: Cornell University Press; 1998. [Link]
3. Mammals Society. Brown rat - Rattus norvegicus [Internet]. Southampton: Mammals Society; 2018 [Cited 2022 Jan 30]. Available from: https://www.mammal.org.uk/species-hub/full-species-hub/discover-mammals/species-brown-rat/ [Link]
4. Lopes MF, Cartucho DJ, Cabrita AM, Patrício JA. Techniques of intestinal transplantation in rat. Microsurgery. 1998;18(7):424-9.
https://doi.org/10.1002/(SICI)1098-2752(1998)18:7<424::AID-MICR7>3.0.CO;2-N [Link] [DOI:10.1002/(SICI)1098-2752(1998)18:73.0.CO;2-N]
5. Galvão FHF, Santos RMN, Machado MAC, Bacchella T, Machado MCC. Simplified rat model of intestinal transplantation. Transplantation. 2005;80(10):1522-3. [Link] [DOI:10.1097/01.tp.0000184448.86704.c8] [PMID]
6. Vdoviaková K, Petrovová E, Maloveská M, Krešáková L, Teleky J, Elias MZJ, et al. Surgical anatomy of the gastrointestinal tract and its vasculature in the laboratory rat. Gastroenterol Res Pract. 2016;2016:2632368. [Link] [DOI:10.1155/2016/2632368] [PMID] [PMCID]
7. Cui D, Daley WP, Fratkin JD, Haines DE, Lynch JC, Naftel JP, et al. Atlas of histology: with functional and clinical correlations. Wolters Kluwer /Lippincott Williams & Wilkins; 2011 [Link]
8. Kumar P, Mahesh R, Kumar P. Histological architecture of esophagus of goat (Capra hircus). Haryana Veterinary. 2009;48:29-32. [Link]
9. Kadhim KK. Histomorphology and Histochemical study of esophagus and stomach in grey mongoose (herpestes edwardsii ) in Iraq. Indian J Natural Sci. 2019;9(52):16458-75. [Link]
10. Frye FL, Aughey E. Digestive system. In: Frye FL, Aughey E. comparative veterinary histology with clinical correlates. CRC Press; 2001 [Link] [DOI:10.1201/b15184]
11. Nzalak JO. Anatomical and histochemical studies of the digestive system of the African giant rat (cricetomys gambianus-waterhouse) [dissertation]. Zaria: Ahmadu Bello University; 2010. [Link] [DOI:10.21608/jva.2010.44900]
12. Jennings R, Premanandan C, Cianciolo R, Wilkle D, Wong A, Kendziorski J. Veterinary histology. Ohio State University; 2017. [Link]
13. Niv Y, Boltin D. Secreted and membrane-bound mucins and idiopathic peptic ulcer diseas. Digestion. 2012;86(3):258-63. [Link] [DOI:10.1159/000341423] [PMID]
14. Young B, Woodford P, O'Dowd G. Wheater's functional histology: a text and colour atlas. 6th ed. Elsevier; 2013. [Link]
15. Mukaddes E, Elif T, Aslı C. Development of the esophagus and stomach. Bezmialem Sci. 2017;5:175-82. [Link] [DOI:10.14235/bs.2017.811]
16. Saroseik J, McCallum RW. Mechanism of esophageal mucosal defense. Pract Res clin Gastroenterol. 2000;14:701-17. [Link] [DOI:10.1053/bega.2000.0119] [PMID]
17. Mahmood HB, Al-aameli MH, Obead WF. Histological study of esophagus in dogs and rabbits. J Kerbala Univ. 2017;15(3):55-62. [Arabic] [Link]
18. Eroschenko VP. Difiore's atlas of histology with functional correlations. 11th ed. Lippincott Williams & Wilkins; 2008 [Link]
19. Gupta SK, Sharma DN. Regional histology of the oesophagus of buffalo calves. Indian J Anim Sci. 1991;61(7):722-4. [Link]
20. Banks WJ. Applied veterinary histology. 3rded. Baltimore: Mosby year Book; 1986 [Link]
21. Hussein AJ, Cani MM, Hussein DM. Anatomical and histological studies of esophagus of one-humped camel (Camelus dromedaries). Mirror Res Veterinary Sci Anim. 2016;5:8-11. [Link]
22. Mahesh R, Singh G, Kumar P. Light microscopic studies on the abomasum of goat (Capra hircus). Veterinary Res. 2017;5(1):21-7. [Link] [DOI:10.5455/ijlr.20170312042215]
23. Dyce KM, Sack WO, Wensing CJ. Text book of veterinary anatomy. 3rd ed. Elsevier Science; 2002 [Link]
24. Reece WO. Functional anatomy and physiology of domestic animals. 4th ed. Wiley - Blackwell; 2009. [Link]
25. Samuelson DA. Textbook of veterinary histology. 1st ed. Printed in China; 2007. [Link]
26. Al-Neamy EMKA. Anatomical, Histological and ultrastructural study of Abomasum and its glands development in Iraq male goat [dissertation]. Baghdad University; 2007. [Link]
27. Junqueira LC, Mescher AL. Junqueira's basic histology. 13th ed. Text atlas /Anthony L.Mescher; 2013. [Link]
28. Sujana K. Gross,Histological and Histochemical studies on stomach of the PIG (Sus scrofa domesticus) [dissertation]. Rajendranagar P.V. Narasimha RAO Telangana Veterinary University Hyderabao; 2017. [Link]
29. Ahmed YA, El-Hafez AAE, Zayed AE. Histological and histochemical studies on the esophagus, stomach and small intestines of Varanus niloticus. J Veterinary Anatomy. 2009;2(1):35-48. [Link] [DOI:10.21608/jva.2009.45136]
30. Parillo, F, Gargiulo, A. M, Fagioli, O. Complex carbohydrates occurring in the digestive apparatus of Umbrina cirosa (L). Veterinary Res Commun. 2005;28:267-78. [Link] [DOI:10.1023/B:VERC.0000026671.88218.08] [PMID]
31. Elliott JR. Overview of reptile biology, anatomy and histology. New York: Taylor and Francis Groud; 2007. [Link]
32. Selvan PS, Ushakumary S, Ramesh G. Studies on the histochemistry of proventriclus and gizzard of post hatched Guinea fowl (Numida meleagris). Int J Poultry Sci. 2008;7:1-4. [Link] [DOI:10.3923/ijps.2008.1112.1116]
33. Selim A, Hazaa E, Goda W. Comparative histological studies of the esophagus wall of Oryctolagus cuniculus rabbit adult, young and lactating using light microscope. J Cytol Histol. 2017;8(2):1-4. [Link] [DOI:10.4172/2157-7099.1000456]
34. Listrat A, Lebret B, Louveau I, Astruc T, Bonnet M, Lefaucheur L, et al. How muscle structure and composition influence meat and flesh quality. Sci World J. 2016 [Link] [DOI:10.1155/2016/3182746] [PMID] [PMCID]
35. Khalel EM. Anatomical and histological study of stomach in adult local rabbits Oryctolagus cuniculus. Al-Mustansiriyah J Sci. 2012;23(7):1-22. [Link]
36. Al-Saffar FJ, Eyhab RM. Histomorphological and histochemical study of stomach of domestic pigeon (Columba liviadomestica ). Iraq J Veterinary Med. 2016;40(1):89-96. [Link] [DOI:10.30539/iraqijvm.v40i1.144]
37. Wang J, Zhang R, Zhang L, Wang C, Shao B, Wang J. Histomorphometric Adaptation of Yak (Bos grunniens) Abomasum to the Qinghai-Tibetan Plateau Environment. Int J Morphol. 2015;33(2):764-76. [Link] [DOI:10.4067/S0717-95022015000200055]








