Volume 13, Issue 4 (2021)
Iran J War Public Health 2021, 13(4): 255-259 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/09/4 | Accepted: 2021/10/4 | Published: 2022/01/24
Received: 2021/09/4 | Accepted: 2021/10/4 | Published: 2022/01/24
How to cite this article
Jasim Abdullah Y, Hasan N, Zghair Jaber Alsaedi R. Correlation between Helicobacter pylori Infection and COVID-19. Iran J War Public Health 2021; 13 (4) :255-259
URL: http://ijwph.ir/article-1-1028-en.html
URL: http://ijwph.ir/article-1-1028-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Amara Medical Institute, Southern Technical University, Amara, Iraq
2- Department of Radiological Technique, Health and Medical Technical College, AL_Zahraa University for Women, Iraq
2- Department of Radiological Technique, Health and Medical Technical College, AL_Zahraa University for Women, Iraq
Full-Text (HTML) (1248 Views)
Introduction
The pandemic disease (COVID-19) caused by the Severe Respiratory Syndrome Coronavirus-2 (SARS-COV-2), a positive sense RNA enveloped virus, is responsible for causing the ongoing pandemic COVID-19 disease and poses a considerable challenge to global public health with extremely variable clinical outcomes ranging from a benign course to rapidly progressive disease that results in death within 2-3 weeks of symptom onset [1]. Fever, nausea, and respiratory disease are common symptoms of this viral infection, but some individuals also experience gastrointestinal symptoms, including abdominal pain, vomiting, and diarrhea. Intestinal cells have also been discovered to contain a small amount of viral nucleic acid [2]. Several proteins with distinct functions are found in the virus's lipid bilayer envelope. The spike, also known as S glycoprotein (SP), is important for invasion, adhesion, and entrance into human cells. It has two domains, S1 and S2. The main receptor for the virus on the human cell surface is angiotensin-converting enzyme-2 (ACE-2) which interacts with part of the S1protien called the receptor-binding domain. This is comparable to SARS-entry CoV's mechanism; however, the S2 domain is responsible for virus-cell membrane fusion and higher-affinity viral entrance [3]. In gastrointestinal (GI) cells, the (ACE-2) receptor is highly expressed. This means that the gastrointestinal system can be infected and serve as a replication site for COVID-19 [4].
Helicobacter pylori (HP) has been described as a gram-negative bacteria with spiral morphology and requires a microaerophilic condition. This pathogen has strongly linked to gastritis, peptic, and duodenal ulcer, as well as gastritis. It has also been linked to the onset of stomach carcinoma [5]. The transmission mode of HP is still unknown. The housefly has been identified as a possible carrier of the bacterium, particularly in places of the world where sanitation is low. Fecal–oral, iatrogenic, and oral–oral transmission pathways are also possible. Both gastrointestinal (GI) and extra-gastrointestinal (extra-GI) symptoms can be caused by HP. The pathogen's virulence actors and the immune system's corresponding response are associated with the duration and presentation of GI and extra-GI diseases caused by H. pylori [6]. H. pylori have several virulence factors that assist the colonization of the pathogen and evade the host's immune response. These virulence factors activate the host's immune system, causing elevated levels of cytokines including TNF-alpha, IL-6, IL-10, and IL-8 to be released, resulting in acute and chronic inflammation [7]. Moreover, HP has been linked to the increase in the expression of (ACE-2) receptors in the GI tract, directly linked to the infection progression and promoting immunological dysregulation through its virulent components [8].
The current study aimed to see if anti-HP IgG was present in COVID-19 individuals and associated with any clinical characteristics.
Materials and Methods
The current experimental study included COVID-19-infected patients from Karbalaa's COVID-19 Isolation Center (N=130) from 2021 Feb to 2021 Jul. A control group of (130) seemingly healthy people of similar ages and sexes. COVID-19-infected patients were confirmed by Reverse Transcription-Polymerase Chain Reaction device (Stratagene; USA).
The current study has been approved by the ethics committee of the Southern Technical University. Study procedures were supervised by the correspondence physicians of the COVID-19 isolation center in Karbala city. Venous blood samples (5mL) were obtained from the study group aseptic, then split into two portions (EDTA tubes and plane tubes). The first part from each collected blood sample was subjected to a complete blood count test, and the other part was used to estimate serum anti-HP IgG antibody test. Anti-HP IgG was diagnosed in the serum of the study groups using ELISA kits (California, USA). Biotech ELISA reader and washer (Biotech, USA) was used, and the procedure was applied, and results were calculated as indicated in the manufacturer's instructions. To estimate total counts of white blood cells (WBC), especially neutrophils, anticoagulated blood samples from both patients and the control group are run through a hematology autoanalyzer (Pentra 80, made by ABX-Horiba group, Minami-Ku Kyoto, Japan). The samples were processed by the equipment, and total WBCs and neutrophils are automatically computed.
Data were analyzed using SPSS 21 software by independent T and Chi-square tests. Statistical significance was defined as a p≤0.05.
Findings
The mean of Patients' age was 40.46±17.36 ranged from 15 to 75 years. The results of Table 1 illustrate the distribution of COVID-19 patients according to gender and age groups.
Table 1) Distribution of COVID-19 according to gender and age groups

Compared to the healthy control group, the mean of neutrophils was significantly increased (p<0.05), but lymphocytes were lower in patients than in control (Table 2).
Table 2) Mean±SD of total WBC counts, Lymphocytes, and neutrophils in COVID-19 patients and control group

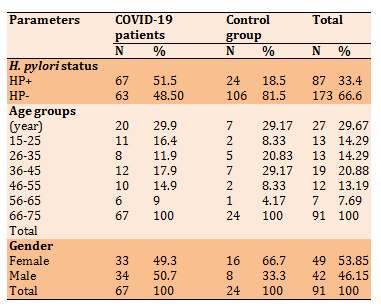
The correlation of H. pylori and COVID-19 infections was presented in Table 3. The existence of anti – H. pylori IgG antibodies was highly significant in COVID-19 patients than in healthy subjects (p<0.05). In all age groups listed in Table 3, anti – H. pylori IgG antibodies were higher in COVID-19 patients than in healthy persons. However, the highest co-occurrence of COVID-19 and H. pylori (29.9%) was detected in patients aged 15-25 years. The oldest patients in the current study (66-75 years) have the lowest coinfection of H. pylori and COVID-19. Females with COVID-19 infection were more affected by H. pylori than males, while the results were opposite in the healthy control group.
Table 3) Incidence of H. pylori and HP+ COVID-19 patients and control group according to the age groups and gender (n=260)
The current study also found that all lymphocytes, neutrophils, and total WBC counts were decreased in COVID-19 patients compared to COVID-19 patients with positive results to H. pylori serology (Table 4).
Table 4) Lymphocyte , neutrophils and total WBC counts in COVID-19 patients and HP+COVID-19 patients (Mean±SD)

Discussion
From the obtained results, COVID-19 was more frequent in males than females. The age rate of COVID-19 patients in this study was (40.46±17.36). The age-related differences showed that patients aged between (15-25 years) are the most influenced by the viral infection. In consistence with our results, Abate et al. [9] found that COVID-19 affected (55%) of males and (45%) of females, Kushwaha et al. [10] stated that (65%) of COVID-19 patients are men with a mean of age of (39.47±17.59) years for males and (36.85±18.51) years for females. Similar results were also found by Peckham et al. [11]. Immune system responses differ significantly between males and females, with females triggering higher immunity to infections. This variation in immunity could play a role in viral load, illness severity, and death.
Furthermore, because estrogen has immune-enhancing properties while testosterone has immunosuppressive properties, variations in sex-related hormone milieus could be a predictor of viral infections [12]. However, age-related differences in COVID-19 incidence could be attributable to age-related changes in adaptive immunity, which plays an important role in the immunity to viruses and falls after a certain age, leaving us exposed to different infections. Adaptive immunity differs between males and females as they get older [13]. Males have higher IgA, IgM, and Treg cells throughout the childhood or newborn stage and an equivalent number of helper/cytotoxic T-cells ratio, CD8+T cell, and B cells. Females' CD4/CD8 ratio, B cells, immunoglobulins, and T cell proliferation/activation increase as they become older (after puberty/adulthood). The outcomes may be significant and explained based on the evidence that; the male adaptive immune system weakens with increasing age compared to females [14].
Hematologic data of this study revealed that COVID-19 patients have considerably elevated numbers of WBC, especially neutrophils, and a lower number of lymphocytes than the healthy group. Several pieces of research have shown similar outcomes [15-17]. Regarding WBC counts, lymphopenia and eosinopenia were frequently seen in COVID-19 patients, and their severity correlated with illness severity. This helps identify this illness from typical viral infections, in which the counts of lymphocytes are usually higher, and eosinopenia is rare [18]. The fundamental processes of COVID-19 lymphopenia are unknown. Possible explanations include atypical hematopoiesis caused by direct infection of bone marrow progenitors, CD4 and CD8 cells, or an autoimmune reaction against blood cells [19]. The ability to replenish lymphocytes, particularly CD4, which are destroyed by the virus, may also be necessary for survival [20].
Concerning the incidence and correlation of COVID-19 with H. pylori infection, the current study, the first one in Iraq, found that the H. pylori infection rate was higher in COVID-19 patients (p≤0.001) compared to the healthy persons. Furthermore, the coexistence of anti-H. pylori IgG and COVID-19 infection were higher in all age groups and females more than males. COVID-19 with positive tests for H. pylori have higher counts of total WBC, neutrophils, and lymphocytes than patients without H. pylori infection. It is hypothesized that H. pylori increase the expression of (ACE-2) receptors in the GI tract cell, making it another susceptible organ for the replication of COVID-19 [8, 21]. Globally, there is no data available to compare our results, but Lansbury et al. [22] found that about (7%) of the hospitalized COVID-19 patients have a bacterial coinfection Mycoplasma pneumonia, Pseudomonas aeruginosa, and Haemophilus influenzae. Rawson et al. [23] stated that COIVD-19 patients may suffering from bacterial and/or fungal coinfection such as blood stream bacteremia and urinary tract infection. On the other hand, Langford et al. [24], concluded that in COVID-19-infected hospitalized patients, bacterial coinfection is uncommon. The majority of these people may not need to be treated with antibacterials.
More detailed molecular studies are required to confirm the correlation between H. pylori and COVID-19 infections.
Conclusion
There is a correlation between H. pylori IgG antibodies and COVID-19, suggesting that H. pylori-infected patients may be more susceptible to COVID-19 infection than other people.
Acknowledgments: The researchers would like to extend their thanks and appreciation to all the workers in the COVID-19 Isolation Center in the holy city of Karbala.
Ethical Permissions: The current study has been approved by the ethics committee of the southern technical university, Amara medical institute, by ethic code 7/18/5 in 23 January 2021. Study procedures were supervised by the correspondence doctors of the COVID-19 isolation center in Karbala city.
Conflicts of Interests: All authors declare that there is no conflict of interest in the study.
Authors’ Contributions: Jasim Abdullah Y. (First Author), Introduction Writer/Main Researcher (40%); Hasan N.F. (Second Author), Assistant Researcher/Statistical Analyst/Discussion Writer (30%); Zghair Jaber Alsaedi R. (Third Author), Assistant Researcher/Statistical Analyst/Discussion Writer (30%).
Funding/Sources: This is self-funded study. All authors participated in the costs.
The pandemic disease (COVID-19) caused by the Severe Respiratory Syndrome Coronavirus-2 (SARS-COV-2), a positive sense RNA enveloped virus, is responsible for causing the ongoing pandemic COVID-19 disease and poses a considerable challenge to global public health with extremely variable clinical outcomes ranging from a benign course to rapidly progressive disease that results in death within 2-3 weeks of symptom onset [1]. Fever, nausea, and respiratory disease are common symptoms of this viral infection, but some individuals also experience gastrointestinal symptoms, including abdominal pain, vomiting, and diarrhea. Intestinal cells have also been discovered to contain a small amount of viral nucleic acid [2]. Several proteins with distinct functions are found in the virus's lipid bilayer envelope. The spike, also known as S glycoprotein (SP), is important for invasion, adhesion, and entrance into human cells. It has two domains, S1 and S2. The main receptor for the virus on the human cell surface is angiotensin-converting enzyme-2 (ACE-2) which interacts with part of the S1protien called the receptor-binding domain. This is comparable to SARS-entry CoV's mechanism; however, the S2 domain is responsible for virus-cell membrane fusion and higher-affinity viral entrance [3]. In gastrointestinal (GI) cells, the (ACE-2) receptor is highly expressed. This means that the gastrointestinal system can be infected and serve as a replication site for COVID-19 [4].
Helicobacter pylori (HP) has been described as a gram-negative bacteria with spiral morphology and requires a microaerophilic condition. This pathogen has strongly linked to gastritis, peptic, and duodenal ulcer, as well as gastritis. It has also been linked to the onset of stomach carcinoma [5]. The transmission mode of HP is still unknown. The housefly has been identified as a possible carrier of the bacterium, particularly in places of the world where sanitation is low. Fecal–oral, iatrogenic, and oral–oral transmission pathways are also possible. Both gastrointestinal (GI) and extra-gastrointestinal (extra-GI) symptoms can be caused by HP. The pathogen's virulence actors and the immune system's corresponding response are associated with the duration and presentation of GI and extra-GI diseases caused by H. pylori [6]. H. pylori have several virulence factors that assist the colonization of the pathogen and evade the host's immune response. These virulence factors activate the host's immune system, causing elevated levels of cytokines including TNF-alpha, IL-6, IL-10, and IL-8 to be released, resulting in acute and chronic inflammation [7]. Moreover, HP has been linked to the increase in the expression of (ACE-2) receptors in the GI tract, directly linked to the infection progression and promoting immunological dysregulation through its virulent components [8].
The current study aimed to see if anti-HP IgG was present in COVID-19 individuals and associated with any clinical characteristics.
Materials and Methods
The current experimental study included COVID-19-infected patients from Karbalaa's COVID-19 Isolation Center (N=130) from 2021 Feb to 2021 Jul. A control group of (130) seemingly healthy people of similar ages and sexes. COVID-19-infected patients were confirmed by Reverse Transcription-Polymerase Chain Reaction device (Stratagene; USA).
The current study has been approved by the ethics committee of the Southern Technical University. Study procedures were supervised by the correspondence physicians of the COVID-19 isolation center in Karbala city. Venous blood samples (5mL) were obtained from the study group aseptic, then split into two portions (EDTA tubes and plane tubes). The first part from each collected blood sample was subjected to a complete blood count test, and the other part was used to estimate serum anti-HP IgG antibody test. Anti-HP IgG was diagnosed in the serum of the study groups using ELISA kits (California, USA). Biotech ELISA reader and washer (Biotech, USA) was used, and the procedure was applied, and results were calculated as indicated in the manufacturer's instructions. To estimate total counts of white blood cells (WBC), especially neutrophils, anticoagulated blood samples from both patients and the control group are run through a hematology autoanalyzer (Pentra 80, made by ABX-Horiba group, Minami-Ku Kyoto, Japan). The samples were processed by the equipment, and total WBCs and neutrophils are automatically computed.
Data were analyzed using SPSS 21 software by independent T and Chi-square tests. Statistical significance was defined as a p≤0.05.
Findings
The mean of Patients' age was 40.46±17.36 ranged from 15 to 75 years. The results of Table 1 illustrate the distribution of COVID-19 patients according to gender and age groups.
Table 1) Distribution of COVID-19 according to gender and age groups

Compared to the healthy control group, the mean of neutrophils was significantly increased (p<0.05), but lymphocytes were lower in patients than in control (Table 2).
Table 2) Mean±SD of total WBC counts, Lymphocytes, and neutrophils in COVID-19 patients and control group

The correlation of H. pylori and COVID-19 infections was presented in Table 3. The existence of anti – H. pylori IgG antibodies was highly significant in COVID-19 patients than in healthy subjects (p<0.05). In all age groups listed in Table 3, anti – H. pylori IgG antibodies were higher in COVID-19 patients than in healthy persons. However, the highest co-occurrence of COVID-19 and H. pylori (29.9%) was detected in patients aged 15-25 years. The oldest patients in the current study (66-75 years) have the lowest coinfection of H. pylori and COVID-19. Females with COVID-19 infection were more affected by H. pylori than males, while the results were opposite in the healthy control group.
Table 3) Incidence of H. pylori and HP+ COVID-19 patients and control group according to the age groups and gender (n=260)

The current study also found that all lymphocytes, neutrophils, and total WBC counts were decreased in COVID-19 patients compared to COVID-19 patients with positive results to H. pylori serology (Table 4).
Table 4) Lymphocyte , neutrophils and total WBC counts in COVID-19 patients and HP+COVID-19 patients (Mean±SD)

Discussion
From the obtained results, COVID-19 was more frequent in males than females. The age rate of COVID-19 patients in this study was (40.46±17.36). The age-related differences showed that patients aged between (15-25 years) are the most influenced by the viral infection. In consistence with our results, Abate et al. [9] found that COVID-19 affected (55%) of males and (45%) of females, Kushwaha et al. [10] stated that (65%) of COVID-19 patients are men with a mean of age of (39.47±17.59) years for males and (36.85±18.51) years for females. Similar results were also found by Peckham et al. [11]. Immune system responses differ significantly between males and females, with females triggering higher immunity to infections. This variation in immunity could play a role in viral load, illness severity, and death.
Furthermore, because estrogen has immune-enhancing properties while testosterone has immunosuppressive properties, variations in sex-related hormone milieus could be a predictor of viral infections [12]. However, age-related differences in COVID-19 incidence could be attributable to age-related changes in adaptive immunity, which plays an important role in the immunity to viruses and falls after a certain age, leaving us exposed to different infections. Adaptive immunity differs between males and females as they get older [13]. Males have higher IgA, IgM, and Treg cells throughout the childhood or newborn stage and an equivalent number of helper/cytotoxic T-cells ratio, CD8+T cell, and B cells. Females' CD4/CD8 ratio, B cells, immunoglobulins, and T cell proliferation/activation increase as they become older (after puberty/adulthood). The outcomes may be significant and explained based on the evidence that; the male adaptive immune system weakens with increasing age compared to females [14].
Hematologic data of this study revealed that COVID-19 patients have considerably elevated numbers of WBC, especially neutrophils, and a lower number of lymphocytes than the healthy group. Several pieces of research have shown similar outcomes [15-17]. Regarding WBC counts, lymphopenia and eosinopenia were frequently seen in COVID-19 patients, and their severity correlated with illness severity. This helps identify this illness from typical viral infections, in which the counts of lymphocytes are usually higher, and eosinopenia is rare [18]. The fundamental processes of COVID-19 lymphopenia are unknown. Possible explanations include atypical hematopoiesis caused by direct infection of bone marrow progenitors, CD4 and CD8 cells, or an autoimmune reaction against blood cells [19]. The ability to replenish lymphocytes, particularly CD4, which are destroyed by the virus, may also be necessary for survival [20].
Concerning the incidence and correlation of COVID-19 with H. pylori infection, the current study, the first one in Iraq, found that the H. pylori infection rate was higher in COVID-19 patients (p≤0.001) compared to the healthy persons. Furthermore, the coexistence of anti-H. pylori IgG and COVID-19 infection were higher in all age groups and females more than males. COVID-19 with positive tests for H. pylori have higher counts of total WBC, neutrophils, and lymphocytes than patients without H. pylori infection. It is hypothesized that H. pylori increase the expression of (ACE-2) receptors in the GI tract cell, making it another susceptible organ for the replication of COVID-19 [8, 21]. Globally, there is no data available to compare our results, but Lansbury et al. [22] found that about (7%) of the hospitalized COVID-19 patients have a bacterial coinfection Mycoplasma pneumonia, Pseudomonas aeruginosa, and Haemophilus influenzae. Rawson et al. [23] stated that COIVD-19 patients may suffering from bacterial and/or fungal coinfection such as blood stream bacteremia and urinary tract infection. On the other hand, Langford et al. [24], concluded that in COVID-19-infected hospitalized patients, bacterial coinfection is uncommon. The majority of these people may not need to be treated with antibacterials.
More detailed molecular studies are required to confirm the correlation between H. pylori and COVID-19 infections.
Conclusion
There is a correlation between H. pylori IgG antibodies and COVID-19, suggesting that H. pylori-infected patients may be more susceptible to COVID-19 infection than other people.
Acknowledgments: The researchers would like to extend their thanks and appreciation to all the workers in the COVID-19 Isolation Center in the holy city of Karbala.
Ethical Permissions: The current study has been approved by the ethics committee of the southern technical university, Amara medical institute, by ethic code 7/18/5 in 23 January 2021. Study procedures were supervised by the correspondence doctors of the COVID-19 isolation center in Karbala city.
Conflicts of Interests: All authors declare that there is no conflict of interest in the study.
Authors’ Contributions: Jasim Abdullah Y. (First Author), Introduction Writer/Main Researcher (40%); Hasan N.F. (Second Author), Assistant Researcher/Statistical Analyst/Discussion Writer (30%); Zghair Jaber Alsaedi R. (Third Author), Assistant Researcher/Statistical Analyst/Discussion Writer (30%).
Funding/Sources: This is self-funded study. All authors participated in the costs.
Keywords:
References
1. Sun Y, Dong Y, Wang L, Xie H, Li B, Chang C, et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. J Autoimmun. 2020;112:102473. [Link] [DOI:10.1016/j.jaut.2020.102473] [PMID] [PMCID]
2. Vetter P, Vu DL, L'Huillier AG, Schibler M, Kaiser L, Jacquerioz F. Clinical features of covid-19. BMJ. 2020;369:m1470. [Link] [DOI:10.1136/bmj.m1470] [PMID]
3. Rodriguez-Morales AJ, Bonilla-Aldana DK, Balbin-Ramon GJ, Rabaan AA, Sah R, Paniz-Mondolfi A, et al. History is repeating itself: Probable zoonotic spillover as the cause of the 2019 novel Coronavirus Epidemic. Infez Med. 2020;28(1):3-5. [Link]
4. Sonkar C, Kashyap D, Varshney N, Baral B, Jha HC. Impact of gastrointestinal symptoms in COVID-19: A molecular approach. SN Compr Clin Med.2020;4:1-12. [Link] [DOI:10.1007/s42399-020-00619-z] [PMID] [PMCID]
5. Al Sulami AA, Al Taee AMR, Juma'a MG. Isolation and identification of Helicobacter pylori from drinking water in Basra governorate, Iraq. East Mediterr Health J. 2020;16(9):920-5. [Link] [DOI:10.26719/2010.16.9.920]
6. Mezmale L, Coelho LG, Bordin D, Leja M. Review: Epidemiology of helicobacter pylori. Helicobacter. 2020;25(51):e12734. [Link] [DOI:10.1111/hel.12734] [PMID]
7. Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, et al. Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells. 2020;10(1):27. [Link] [DOI:10.3390/cells10010027] [PMID] [PMCID]
8. Balamtekin N, Artuk C, Arslan M, Gülşen M. The effect of helicobacter pylori on the presentation and clinical course of coronavirus disease 2019 infection. J Pediatr Gastroenterol Nutr. 2021;72(4):511-3. [Link] [DOI:10.1097/MPG.0000000000003005] [PMID] [PMCID]
9. Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA. Sex difference in coronavirus disease (COVID-19): A systematic review and meta-analysis. BMJ Open. 2020;10(10):e040129. [Link] [DOI:10.1136/bmjopen-2020-040129] [PMID] [PMCID]
10. Kushwaha S, Khanna P, Rajagopal V, Kiran T. Biological attributes of age and gender variations in Indian COVID-19 cases: A retrospective data analysis. Clin Epidemiol Glob Health. 2021;11:100788. [Link] [DOI:10.1016/j.cegh.2021.100788] [PMID] [PMCID]
11. Peckham H, De Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. [Link] [DOI:10.1038/s41467-020-19741-6] [PMID] [PMCID]
12. Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID-19: Are males more vulnerable? Biol Sex Differ. 2020;11(1):53. [Link] [DOI:10.1186/s13293-020-00330-7] [PMID] [PMCID]
13. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626-38. [Link] [DOI:10.1038/nri.2016.90] [PMID]
14. Cai KC, Van Mil S, Murray E, Mallet JF, Matar C, Ismail N. Age and sex differences in immune response following LPS treatment in mice. Brain Behav Immun. 2016;58:327-37. [Link] [DOI:10.1016/j.bbi.2016.08.002] [PMID]
15. Naoum FA, Ruiz ALZ, Martin FHDO, Brito THG, Hassem V, Oliveira MGDL. Diagnostic and prognostic utility of WBC counts and cell population data in patients with COVID‐19. Int J Lab Hematol. 2021;43(S1):124-8. [Link] [DOI:10.1111/ijlh.13395] [PMID] [PMCID]
16. Wang X, Fang J, Zhu Y, Chen L, Ding F, Zhou R, et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 202;26(8):1063-8. [Link] [DOI:10.1016/j.cmi.2020.03.032] [PMID] [PMCID]
17. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non‐ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6):1321-6. [Link] [DOI:10.1111/liv.14449] [PMID] [PMCID]
18. Tanni F, Akker E, Zaman MM, Figueroa N, Tharian B, Hupart KH. Eosinopenia and COVID-19. J Osteopath Assoc Med. 2020;120(8):504-8. [Link] [DOI:10.7556/jaoa.2020.091] [PMID]
19. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389-99. [Link] [DOI:10.1080/10408363.2020.1770685] [PMID] [PMCID]
20. Henry BM. COVID-19, ECMO, and lymphopenia: A word of caution. Lancet Respir Med. 2020;8(4):e24. [Link] [DOI:10.1016/S2213-2600(20)30119-3]
21. Zhang M, Feng C, Zhang X, Hu S, Zhang Y, Min M, et al. Susceptibility factors of stomach for SARS-CoV-2 and treatment implication of mucosal protective agent in COVID-19. Front Med (Lausanne). 2021;7:597967. [Link] [DOI:10.3389/fmed.2020.597967] [PMID] [PMCID]
22. Lansbury L, Lim B, Baskaran V, Lim WS. Coinfections in people with COVID-19: A systematic review and meta-analysis. J Infect. 2020;81(2):266-75. [Link] [DOI:10.1016/j.jinf.2020.05.046] [PMID] [PMCID]
23. Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459-68. [Link] [DOI:10.1093/cid/ciaa530] [PMID] [PMCID]
24. Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial coinfection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-9. [Link] [DOI:10.1016/j.cmi.2020.07.016] [PMID] [PMCID]








