Volume 13, Issue 4 (2021)
Iran J War Public Health 2021, 13(4): 247-253 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/09/4 | Accepted: 2021/10/4 | Published: 2022/01/24
Received: 2021/09/4 | Accepted: 2021/10/4 | Published: 2022/01/24
How to cite this article
Al-Sodani M, Wasfi Fadhil R, Al-Khayyad N, Dyab Allawi A. Acute Kidney Injury in Adult Iraqi Patients with COVID-19 Infection. Iran J War Public Health 2021; 13 (4) :247-253
URL: http://ijwph.ir/article-1-1027-en.html
URL: http://ijwph.ir/article-1-1027-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Medicine, College of Medicine, University of Baghdad, Baghdad, Iraq
2- Baghdad Teaching Hospital, Baghdad, Iraq
3- Kidney Transplant Center, Baghdad, Iraq
2- Baghdad Teaching Hospital, Baghdad, Iraq
3- Kidney Transplant Center, Baghdad, Iraq
Full-Text (HTML) (1040 Views)
Introduction
Severe acute respiratory coronavirus-2 (SARS-CoV-2) has recently emerged as a life-threatening virus causing COVID-19. However, the respiratory system is the major target of COVID-19. Reports indicate that kidney involvement is frequent and ranges from mild proteinuria to an advanced acute kidney injury (AKI) [1]. Epidemiology Data from China and the USA suggest that male sex, older age, black race, diabetic patients, CKD, hypertension, cardiovascular disease, congestive heart failure, and higher body mass index are associated with COVID-19 AKI [2]. AKI rates vary considerably between geographic regions and between different health systems. Data from China suggest that AKI is less common among patients in China [3] than among patients in the USA [4] and Europe [5].
This difference may be attributed to differences in the patient population studied; for example, patients in the Chinese studies had fewer comorbidities and were admitted to hospitals with less severe respiratory disease or acute respiratory distress syndrome (ARDS) than patients in other cohorts. Studies in Europe and the USA reveal that Covid-19 induces AKI in 20-40% of the patients admitted to the intensive care unit (ICU). AKI is deemed a negative prognostic factor and an indicator of disease severity [6]. Urinalysis and biomarkers of AKI are frequently abnormal in patients with COVID-19 and could be used to characterize AKI in these patients [7]. For example, one study reported that among the 32% of patients hospitalized with COVID-19 for whom urinalysis was available, 42.1% had significant proteinuria, with leukocyturia and haematuria in 36.5% and 40.9%, respectively [2]. Similarly, in a study of urinalysis data from 442 hospitalized Chinese patients with COVID-19, proteinuria was present in 43.9% (with 30% having ≥2+ on dipstick) with haematuria in 11.3% [7].
Patients with COVID-19 AKI have also been reported to have higher systemic markers of inflammation, particularly ferritin, C-reactive protein, procalcitonin, and lactate dehydrogenase, than patients with COVID-19 and normal kidney function [6].
The exact mechanism of kidney damage caused by COVID-19 is unclear [8]. It may be caused by many factors (figure 1-1). These factors include the Direct Effect of SARS-CoV-2 on kidney proximal tubule cells; it is assumed that the direct impact of the virus on the renal tubules reflects the kidney damage according to several findings [9]. First, the presence of viral fragments in urine either indicates a direct interaction with renal tubules or indicates a possible exposure of the tubules to the virus [10]. Second, the expression pattern of ACE2 is limited to proximal tubular cells [11]. Finally, between the second and third week of infection linked with the onset of AKI, SARS-CoV shedding was detected in the urine [12]. The possible hemodynamic, proinflammatory, and proapoptotic consequences of lung inflammation, cytokine release syndrome, and hypercoagulability on renal function and potential organ support options are shown [13].
The direct impact of SARS-CoV-2 on the kidney is mediated by an ACE2 pathway that leads to acute tubular necrosis, protein leakage in Bowman's capsule, collapsing glomerulopathy, and mitochondrial impairment [14]. Viral antigens or virus-induced immune responses may damage the kidneys. When viruses and other pathogens infect the body, they release pathogen-associated molecular patterns (PAMPs, including the nucleic acid, protein, and metabolic intermediates of pathogenic microorganisms. Activated immune cells in tissues and organs secrete many cytokines and chemokines, leading to cytokine storms [15]. Clinical studies have shown that the levels of inflammatory mediators interleukin-2 (IL-2), IL-7, and IL-10, interferon-inducible protein 10 (IP-10), granulocyte colony-stimulating factor (GCSF), macrophage inflammatory protein 1α (MIP1A), tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein1 (MCP1) and are significantly higher in patients with severe COVID-19 than in mild patients [16].
A report on 5700 patients with COVID-19 in the New York area also reported a significant increase in CRP, ferritin, and pro-calcitonin [7]. Hypoxemia, respiratory failure, shock, or hypotension can cause insufficient oxygen supply to the myocardium. After infection, the body's metabolism becomes more active, increasing the burden on the heart, leading to an unbalanced oxygen supply in the body [17].
Although there are no effective antiviral drugs for COVID-19, many patients in this outbreak have used antiviral drugs such as lopinavir, abide, and ritonavir. The kidney participates in the metabolism of antiviral drugs, which affects or aggravates kidney damage [18]. As outlined, renal involvement is common in patients with COVID-19 and may occur at any time before or during hospital admission. Initial assessment should include a full medical history and comorbidities, including factors that further increase the risk of acute kidney injury (chronic kidney disease, heart failure, liver disease, diabetes, previous history of acute kidney injury, age 65years or over) [19].
Clinical assessment should record fluid status by clinical examination (for example, peripheral perfusion, capillary refill, pulse rate, blood pressure, postural hypotension, jugular venous pressure, or pulmonary or peripheral edema) as well as fluid balance (fluid intake, urine output, and weight). Baseline investigations include complete blood count, blood urea, serum creatinine, and electrolytes (sodium, potassium, bicarbonate).
Medication review should be performed, and those that can cause or worsen the kidney injury should be stopped during the acute illness unless essential. This study aimed to evaluate the incidence, manifestations, association, and outcome of Acute Kidney Injury in Patients with COVID-19 infection during the first week of hospital admission.
Materials and Methods
This is a prospective cross-sectional study and includes 250 patients infected with SARS-CoV-2 (COVID-19), admitted and treated at the Emergency Department and isolation rooms of Baghdad Teaching Hospital, Iraq, from 1st October 2020 to 1st April 2021. The diagnosis of COVID-19 infection was based on clinical features, detection of viral RNA by using Reverse Transcription Polymerase Chain Reaction, and chest computed tomography (CT) scan. Inclusion Criteria were all adult patients confirmed with SARS-CoV-2 (COVID-19) infection. Exclusion criteria were age <18 years and patients known to have CKD (Chronic Kidney Disease) before admission.
The study was approved by the Iraqi Council of Medical Specializations. Written consent from each participant was obtained after informing them of the aim of the study. Each patient was given the complete unconditioned choice to withdraw anytime. The confidentiality of data throughout the study was guaranteed, and the patients were assured that data would be used for research purposes only. Patient demographics (age, gender), comorbidities and main complaints, vital signs (Blood pressure, Pulse Rate, Respiratory Rate, Temperature, O2 saturation with clinical manifestations) were collected through check-ups. Laboratory parameters included Total white blood cell count, absolute lymphocyte count, hemoglobin concentration, platelets count, serum creatinine, and blood urea. Urine analysis was the presence of proteinuria or hematuria. Also, we measured the inflammatory biomarkers (CRP, LDH, S. Ferritin, and D-dimer). Findings of Chest CT scan were collected from patients' recorded documents. AKI was defined according to both urine output and serum creatinine. According to Kidney Disease Improving Global Outcomes criteria [20], patients were categorized with and without acute kidney injury. (Table 1). Patients were stratified according to the highest AKI stage attained during the first seven days of ICU stay.
Patients were followed for one week after hospital admission. After one week of patient follow-up, we looked for the following outcomes: discharge well, ICU admission, need for dialysis & mortality.
The continuous variables with a normal distribution were presented as mean ± standard deviation. Categorical variables were presented as percentages. Comparisons between continuous variables were performed by the Student t-test. Comparisons between categorical variables were performed by the Chi-square test. All data were analyzed with Statistical processing of the data with SPSS 25 software.
Findings
The mean age of the patients was 57.31±14.05 years (range 22-98 years). Males represented 58.8% of the patients. Hypertension and diabetes mellitus were common comorbidities accounting for 44.81% and 41.49% of the patients, respectively (Table 2).
Of 250 patients, 58 patients (23.2%) developed AKI. The mean age of patients with AKI was higher than that of patients without AKI. Likewise, hypertension was more in patients with AKI than those without it (Table 2).
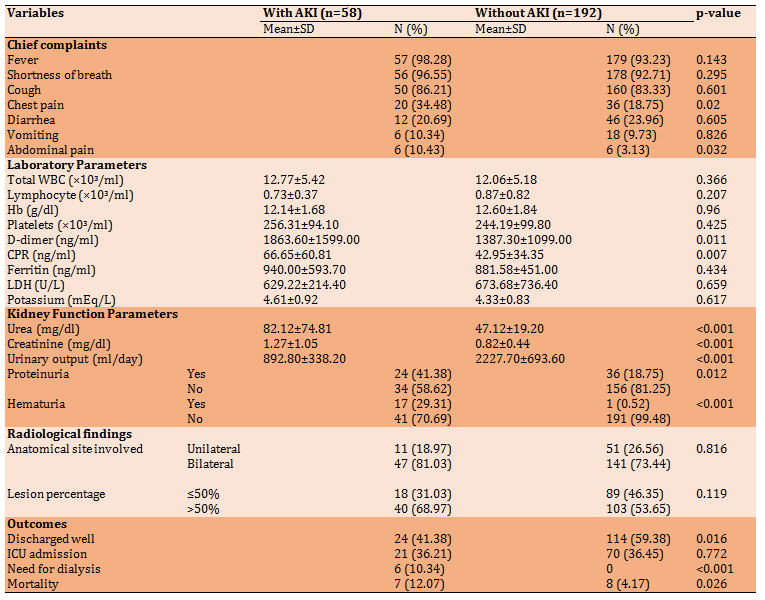
Based on results that showed in Table 3, the frequency of most complaints (fever, cough, and shortness of breath) had no statistical difference between patients with and without AKI (p>0.05). However, chest pain and abdominal pain were more frequent among the AKI group. Blood urea and serum creatinine showed a significant difference between patients with and without AKI (p<0.05). Serum concentrations of D-dimer and CRP in patients with AKI were significantly higher than those without AKI (p<0.05). Blood urea and serum creatinine were higher in patients with AKI. Although the involvement of more than 50% of the lung was more common among patients with AKI than those without AKI, the difference was not significant (p>0.05). The majority of patients without AKI were discharged well with better conditions than patients with AKI. On the other hand, the proportion of patients admitted to ICU was almost identical in the two groups with no significant difference. However, the death rate in patients with AKI was higher than in patients without AKI. Finally, none among patients without AKI needed for dialysis compared with 10.34% of patients with AKI required such intervention (Table 3).
Table 1) Kidney Disease Improving Global Outcomes criteria [20]

Table 2) results of demographic characteristics and association with AKI

Table 3) Association of chief complaints, laboratory parameters, kidney function parameters, radiological findings with AKI, and the outcomes of patients with and without AKI
Severe acute respiratory coronavirus-2 (SARS-CoV-2) has recently emerged as a life-threatening virus causing COVID-19. However, the respiratory system is the major target of COVID-19. Reports indicate that kidney involvement is frequent and ranges from mild proteinuria to an advanced acute kidney injury (AKI) [1]. Epidemiology Data from China and the USA suggest that male sex, older age, black race, diabetic patients, CKD, hypertension, cardiovascular disease, congestive heart failure, and higher body mass index are associated with COVID-19 AKI [2]. AKI rates vary considerably between geographic regions and between different health systems. Data from China suggest that AKI is less common among patients in China [3] than among patients in the USA [4] and Europe [5].
This difference may be attributed to differences in the patient population studied; for example, patients in the Chinese studies had fewer comorbidities and were admitted to hospitals with less severe respiratory disease or acute respiratory distress syndrome (ARDS) than patients in other cohorts. Studies in Europe and the USA reveal that Covid-19 induces AKI in 20-40% of the patients admitted to the intensive care unit (ICU). AKI is deemed a negative prognostic factor and an indicator of disease severity [6]. Urinalysis and biomarkers of AKI are frequently abnormal in patients with COVID-19 and could be used to characterize AKI in these patients [7]. For example, one study reported that among the 32% of patients hospitalized with COVID-19 for whom urinalysis was available, 42.1% had significant proteinuria, with leukocyturia and haematuria in 36.5% and 40.9%, respectively [2]. Similarly, in a study of urinalysis data from 442 hospitalized Chinese patients with COVID-19, proteinuria was present in 43.9% (with 30% having ≥2+ on dipstick) with haematuria in 11.3% [7].
Patients with COVID-19 AKI have also been reported to have higher systemic markers of inflammation, particularly ferritin, C-reactive protein, procalcitonin, and lactate dehydrogenase, than patients with COVID-19 and normal kidney function [6].
The exact mechanism of kidney damage caused by COVID-19 is unclear [8]. It may be caused by many factors (figure 1-1). These factors include the Direct Effect of SARS-CoV-2 on kidney proximal tubule cells; it is assumed that the direct impact of the virus on the renal tubules reflects the kidney damage according to several findings [9]. First, the presence of viral fragments in urine either indicates a direct interaction with renal tubules or indicates a possible exposure of the tubules to the virus [10]. Second, the expression pattern of ACE2 is limited to proximal tubular cells [11]. Finally, between the second and third week of infection linked with the onset of AKI, SARS-CoV shedding was detected in the urine [12]. The possible hemodynamic, proinflammatory, and proapoptotic consequences of lung inflammation, cytokine release syndrome, and hypercoagulability on renal function and potential organ support options are shown [13].
The direct impact of SARS-CoV-2 on the kidney is mediated by an ACE2 pathway that leads to acute tubular necrosis, protein leakage in Bowman's capsule, collapsing glomerulopathy, and mitochondrial impairment [14]. Viral antigens or virus-induced immune responses may damage the kidneys. When viruses and other pathogens infect the body, they release pathogen-associated molecular patterns (PAMPs, including the nucleic acid, protein, and metabolic intermediates of pathogenic microorganisms. Activated immune cells in tissues and organs secrete many cytokines and chemokines, leading to cytokine storms [15]. Clinical studies have shown that the levels of inflammatory mediators interleukin-2 (IL-2), IL-7, and IL-10, interferon-inducible protein 10 (IP-10), granulocyte colony-stimulating factor (GCSF), macrophage inflammatory protein 1α (MIP1A), tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein1 (MCP1) and are significantly higher in patients with severe COVID-19 than in mild patients [16].
A report on 5700 patients with COVID-19 in the New York area also reported a significant increase in CRP, ferritin, and pro-calcitonin [7]. Hypoxemia, respiratory failure, shock, or hypotension can cause insufficient oxygen supply to the myocardium. After infection, the body's metabolism becomes more active, increasing the burden on the heart, leading to an unbalanced oxygen supply in the body [17].
Although there are no effective antiviral drugs for COVID-19, many patients in this outbreak have used antiviral drugs such as lopinavir, abide, and ritonavir. The kidney participates in the metabolism of antiviral drugs, which affects or aggravates kidney damage [18]. As outlined, renal involvement is common in patients with COVID-19 and may occur at any time before or during hospital admission. Initial assessment should include a full medical history and comorbidities, including factors that further increase the risk of acute kidney injury (chronic kidney disease, heart failure, liver disease, diabetes, previous history of acute kidney injury, age 65years or over) [19].
Clinical assessment should record fluid status by clinical examination (for example, peripheral perfusion, capillary refill, pulse rate, blood pressure, postural hypotension, jugular venous pressure, or pulmonary or peripheral edema) as well as fluid balance (fluid intake, urine output, and weight). Baseline investigations include complete blood count, blood urea, serum creatinine, and electrolytes (sodium, potassium, bicarbonate).
Medication review should be performed, and those that can cause or worsen the kidney injury should be stopped during the acute illness unless essential. This study aimed to evaluate the incidence, manifestations, association, and outcome of Acute Kidney Injury in Patients with COVID-19 infection during the first week of hospital admission.
Materials and Methods
This is a prospective cross-sectional study and includes 250 patients infected with SARS-CoV-2 (COVID-19), admitted and treated at the Emergency Department and isolation rooms of Baghdad Teaching Hospital, Iraq, from 1st October 2020 to 1st April 2021. The diagnosis of COVID-19 infection was based on clinical features, detection of viral RNA by using Reverse Transcription Polymerase Chain Reaction, and chest computed tomography (CT) scan. Inclusion Criteria were all adult patients confirmed with SARS-CoV-2 (COVID-19) infection. Exclusion criteria were age <18 years and patients known to have CKD (Chronic Kidney Disease) before admission.
The study was approved by the Iraqi Council of Medical Specializations. Written consent from each participant was obtained after informing them of the aim of the study. Each patient was given the complete unconditioned choice to withdraw anytime. The confidentiality of data throughout the study was guaranteed, and the patients were assured that data would be used for research purposes only. Patient demographics (age, gender), comorbidities and main complaints, vital signs (Blood pressure, Pulse Rate, Respiratory Rate, Temperature, O2 saturation with clinical manifestations) were collected through check-ups. Laboratory parameters included Total white blood cell count, absolute lymphocyte count, hemoglobin concentration, platelets count, serum creatinine, and blood urea. Urine analysis was the presence of proteinuria or hematuria. Also, we measured the inflammatory biomarkers (CRP, LDH, S. Ferritin, and D-dimer). Findings of Chest CT scan were collected from patients' recorded documents. AKI was defined according to both urine output and serum creatinine. According to Kidney Disease Improving Global Outcomes criteria [20], patients were categorized with and without acute kidney injury. (Table 1). Patients were stratified according to the highest AKI stage attained during the first seven days of ICU stay.
Patients were followed for one week after hospital admission. After one week of patient follow-up, we looked for the following outcomes: discharge well, ICU admission, need for dialysis & mortality.
The continuous variables with a normal distribution were presented as mean ± standard deviation. Categorical variables were presented as percentages. Comparisons between continuous variables were performed by the Student t-test. Comparisons between categorical variables were performed by the Chi-square test. All data were analyzed with Statistical processing of the data with SPSS 25 software.
Findings
The mean age of the patients was 57.31±14.05 years (range 22-98 years). Males represented 58.8% of the patients. Hypertension and diabetes mellitus were common comorbidities accounting for 44.81% and 41.49% of the patients, respectively (Table 2).
Of 250 patients, 58 patients (23.2%) developed AKI. The mean age of patients with AKI was higher than that of patients without AKI. Likewise, hypertension was more in patients with AKI than those without it (Table 2).
Based on results that showed in Table 3, the frequency of most complaints (fever, cough, and shortness of breath) had no statistical difference between patients with and without AKI (p>0.05). However, chest pain and abdominal pain were more frequent among the AKI group. Blood urea and serum creatinine showed a significant difference between patients with and without AKI (p<0.05). Serum concentrations of D-dimer and CRP in patients with AKI were significantly higher than those without AKI (p<0.05). Blood urea and serum creatinine were higher in patients with AKI. Although the involvement of more than 50% of the lung was more common among patients with AKI than those without AKI, the difference was not significant (p>0.05). The majority of patients without AKI were discharged well with better conditions than patients with AKI. On the other hand, the proportion of patients admitted to ICU was almost identical in the two groups with no significant difference. However, the death rate in patients with AKI was higher than in patients without AKI. Finally, none among patients without AKI needed for dialysis compared with 10.34% of patients with AKI required such intervention (Table 3).
Table 1) Kidney Disease Improving Global Outcomes criteria [20]

Table 2) results of demographic characteristics and association with AKI

Table 3) Association of chief complaints, laboratory parameters, kidney function parameters, radiological findings with AKI, and the outcomes of patients with and without AKI

Discussion
This study was designed to evaluate the incidence, manifestations, association, and outcome of AKI in patients with COVID-19 infection in the first week of hospital admission.
According to our study, the incidence of AKI was found in 58 (23.2%) patients with COVID-19 infection in the first week of hospital admission. The incidence of kidney involvement varies widely among different Studies. This rate is relatively high compared with other global studies. An early study of 138 COVID-19 patients reported that about 4% of them developed AKI [21]. In comparison, Huang et al. [16] found a 10% increase in serum Creatinine on admission and a 7% incidence of AKI in their series of 41 COVID-19 patients.
Recently published studies on COVID-19 worldwide reported AKI rates in hospitalized patients of 17.9% to 72.7% in Italy, [22, 23], 9.2% to 18.3% in Korea, [24, 25], 19.7% to 69.2% in Spain, [26, 27] 5.8% to 56.9% in the United States, [28, 29] 52.2% to 74.6% in Germany, [30, 21] and 4.7% to 55.9% in France and Belgium [32, 33].
This variation among different studies can be attributed to several factors, the most important of which are health system facilities, level of complexity of the center, epidemiologic strategy with viral testing, local protocols, available therapies, restrictive hospital admission policy or AKI definitions and time of hospital admission [34]. The relatively high rate in the present study compared to other studies is mainly due to the older ages included in this study, the high prevalence of comorbidities, especially DM and hypertension, and most cases were severe cases since mild or moderate cases usually are not admitted to hospital. In this regard, cumulative evidence suggests that the disease likely affects >20% of hospitalized patients and >50% of patients in the ICU [35-37].
In this study, AKI was found in patients 61 years versus 58 with a P-value of 0.006.
Hypertension was significantly associated with the development of AKI in patients with COVID-19. It is similar to a Chinese study on 394 patients. The authors revealed that HTN was significantly associated with AKI development [38].
This association may be because HTN and AKI may be explained based on two facts: firstly, hypertension is associated with more severe COVI-19. Supporting this fact are many studies worldwide. In a cohort of 1389 patients, a history of hypertension was more common among those who had severe than COVID-19 [39]. Similarly, in a separate cohort of 1590 hospitalized patients in China, underlying hypertension was independently associated with severe COVID-19 [40]. The second most interesting fact is that medications based on RAAS inhibition, such as ACE inhibitors and ARBs, can upregulate the expression of ACE2 in the kidney tubules [41] and could hypothetically increase the targeting of kidney virus. Chest pain and abdominal were more in patients with AKI. Regarding abdominal pain, it mostly results rather than risk factors for AKI. On the other hand, chest pain may reflect the severity of lung involvement. In the same context, Pei et al. [35] investigated 333 patients for AKI and reported that the severity of pneumonia was the most common risk factor for AKI. Hirsch et al. [42] found that 90% of patients who developed AKI needed invasive mechanical ventilation and that AKI developed in temporal association with respiratory failure. This study also showed that D-dimer and CRP serum concentration in patients with AKI were significantly higher than those without (p-value 0.011, 0.007 respectively. Other authors reported that CRP >10 mg/L was significantly associated with AKI. In another study, Zheng et al. [43] retrospectively studied 555 patients, which demonstrated that AKI patients had a higher level of D-dimer and CRP. The high D-dimer level in AKI may reflect the severity of the disease, reduce its elimination by the kidneys, and the activation of coagulation in patients with renal diseases. Indeed, decreasing renal function has also been shown to be associated with increasing levels of other hemostatic markers, such as soluble thrombomodulin, soluble tissue factor, von Willebrand factor, factor VIII levels, fibrinogen, and thrombin-antithrombin complex [44]. The association of CRP with AKI is rather complex. Some evidence indicated that CRP activates the mitogen-activated protein kinase (MAPK) pathway and upregulates T cell expressed and secreted (RANTES) expression, which plays a key role in recruiting leukocytes into inflammatory sites human renal distal tubular cells in a dose-dependent manner [45].
All kidney function-related variables in the current study were, per se, more in patients with AKI and statistically significant than those without AKI. In a retrospective observational study of 3993 hospitalized adult patients aged> 18 years, the urine analysis of acute kidney injury patients shows proteinuria in 84%, hematuria in 81%, and 60% had leukocyturia [46]. Leukocyturia was not investigated in the present study, while K level did not differ significantly between patients with and without AKI.
Kidney damage associated with COVID-19 typically exhibited tubular damage with noticeable urinalysis abnormalities [47]. Proteinuria is common in patients with kidney damage caused by COVID-19, while proteinuria is often mild. The same study revealed that 27% of the 59 COVID-19 patients (including 28 severe patients) had elevated urea nitrogen levels, and the serum creatinine level was elevated in 19% of the patients [48]. Another research displayed that 4.3% of 173 patients with severe infection and only 1% of 926 patients with mild infection had serum creatinine >133μmol/L [2]. A study on 99 patients showed that 6% elevated serum urea nitrogen, 3% presented with elevated serum creatinine, while the incidence of AKI was only 3% [49]. A Chinese study including 710 COVID-19 patients indicated that proteinuria is present in slightly less than half of patients. At the same time, hematuria was reported in 26.9% of the patients. on the other hand, 15.5% presented with raised serum creatinine, and 14.1% of the patients had raised urea nitrogen levels.
This study shows that AKI was more common in COVID-19 patients with bilateral lung involvement by chest CT scan 80% versus 73%, and more common in patients with more than 50% of lung involvement, 68% versus 53%. However, both of these findings are statistically not significant.
This study showed the outcomes of a patient with AKI, more need for ICU admission, need dialysis with higher mortality. Kidney involvement should be investigated in patients with COVID-19 infection, especially those presented with old age, hypertension, chest pain, high serum level of D-dimer, and CRP. Further studies are needed in the future with a large sample and a specific one for AKI in pediatric patients with COVID-19 infection.
Conclusion
In the first week, the incidence of AKI among Iraqi patients with COVID-19 infection was 23.2%. Old-Age, Hypertension, chest pain, abdominal pain, high D-dimer, and CRP may be markers for possible association with AKI in COVID-19 infection. COVID-19 patients with AKI have a low rate of discharging well & a higher mortality rate, and about 10% of them need dialysis.
Acknowledgments: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: Al-Sodani M.H. (First Author), Introduction Writer/Discussion Writer (25%); Wasfi Fadhil R. (Second Author), Methodologist (25%); Al-Khayyad N. (Third Author), Assistant Researcher (25%); Dyab Allawi A.A.M. (Fourth author), Assistant Researcher (25%)
Funding/Support: None declared by the authors.
This study was designed to evaluate the incidence, manifestations, association, and outcome of AKI in patients with COVID-19 infection in the first week of hospital admission.
According to our study, the incidence of AKI was found in 58 (23.2%) patients with COVID-19 infection in the first week of hospital admission. The incidence of kidney involvement varies widely among different Studies. This rate is relatively high compared with other global studies. An early study of 138 COVID-19 patients reported that about 4% of them developed AKI [21]. In comparison, Huang et al. [16] found a 10% increase in serum Creatinine on admission and a 7% incidence of AKI in their series of 41 COVID-19 patients.
Recently published studies on COVID-19 worldwide reported AKI rates in hospitalized patients of 17.9% to 72.7% in Italy, [22, 23], 9.2% to 18.3% in Korea, [24, 25], 19.7% to 69.2% in Spain, [26, 27] 5.8% to 56.9% in the United States, [28, 29] 52.2% to 74.6% in Germany, [30, 21] and 4.7% to 55.9% in France and Belgium [32, 33].
This variation among different studies can be attributed to several factors, the most important of which are health system facilities, level of complexity of the center, epidemiologic strategy with viral testing, local protocols, available therapies, restrictive hospital admission policy or AKI definitions and time of hospital admission [34]. The relatively high rate in the present study compared to other studies is mainly due to the older ages included in this study, the high prevalence of comorbidities, especially DM and hypertension, and most cases were severe cases since mild or moderate cases usually are not admitted to hospital. In this regard, cumulative evidence suggests that the disease likely affects >20% of hospitalized patients and >50% of patients in the ICU [35-37].
In this study, AKI was found in patients 61 years versus 58 with a P-value of 0.006.
Hypertension was significantly associated with the development of AKI in patients with COVID-19. It is similar to a Chinese study on 394 patients. The authors revealed that HTN was significantly associated with AKI development [38].
This association may be because HTN and AKI may be explained based on two facts: firstly, hypertension is associated with more severe COVI-19. Supporting this fact are many studies worldwide. In a cohort of 1389 patients, a history of hypertension was more common among those who had severe than COVID-19 [39]. Similarly, in a separate cohort of 1590 hospitalized patients in China, underlying hypertension was independently associated with severe COVID-19 [40]. The second most interesting fact is that medications based on RAAS inhibition, such as ACE inhibitors and ARBs, can upregulate the expression of ACE2 in the kidney tubules [41] and could hypothetically increase the targeting of kidney virus. Chest pain and abdominal were more in patients with AKI. Regarding abdominal pain, it mostly results rather than risk factors for AKI. On the other hand, chest pain may reflect the severity of lung involvement. In the same context, Pei et al. [35] investigated 333 patients for AKI and reported that the severity of pneumonia was the most common risk factor for AKI. Hirsch et al. [42] found that 90% of patients who developed AKI needed invasive mechanical ventilation and that AKI developed in temporal association with respiratory failure. This study also showed that D-dimer and CRP serum concentration in patients with AKI were significantly higher than those without (p-value 0.011, 0.007 respectively. Other authors reported that CRP >10 mg/L was significantly associated with AKI. In another study, Zheng et al. [43] retrospectively studied 555 patients, which demonstrated that AKI patients had a higher level of D-dimer and CRP. The high D-dimer level in AKI may reflect the severity of the disease, reduce its elimination by the kidneys, and the activation of coagulation in patients with renal diseases. Indeed, decreasing renal function has also been shown to be associated with increasing levels of other hemostatic markers, such as soluble thrombomodulin, soluble tissue factor, von Willebrand factor, factor VIII levels, fibrinogen, and thrombin-antithrombin complex [44]. The association of CRP with AKI is rather complex. Some evidence indicated that CRP activates the mitogen-activated protein kinase (MAPK) pathway and upregulates T cell expressed and secreted (RANTES) expression, which plays a key role in recruiting leukocytes into inflammatory sites human renal distal tubular cells in a dose-dependent manner [45].
All kidney function-related variables in the current study were, per se, more in patients with AKI and statistically significant than those without AKI. In a retrospective observational study of 3993 hospitalized adult patients aged> 18 years, the urine analysis of acute kidney injury patients shows proteinuria in 84%, hematuria in 81%, and 60% had leukocyturia [46]. Leukocyturia was not investigated in the present study, while K level did not differ significantly between patients with and without AKI.
Kidney damage associated with COVID-19 typically exhibited tubular damage with noticeable urinalysis abnormalities [47]. Proteinuria is common in patients with kidney damage caused by COVID-19, while proteinuria is often mild. The same study revealed that 27% of the 59 COVID-19 patients (including 28 severe patients) had elevated urea nitrogen levels, and the serum creatinine level was elevated in 19% of the patients [48]. Another research displayed that 4.3% of 173 patients with severe infection and only 1% of 926 patients with mild infection had serum creatinine >133μmol/L [2]. A study on 99 patients showed that 6% elevated serum urea nitrogen, 3% presented with elevated serum creatinine, while the incidence of AKI was only 3% [49]. A Chinese study including 710 COVID-19 patients indicated that proteinuria is present in slightly less than half of patients. At the same time, hematuria was reported in 26.9% of the patients. on the other hand, 15.5% presented with raised serum creatinine, and 14.1% of the patients had raised urea nitrogen levels.
This study shows that AKI was more common in COVID-19 patients with bilateral lung involvement by chest CT scan 80% versus 73%, and more common in patients with more than 50% of lung involvement, 68% versus 53%. However, both of these findings are statistically not significant.
This study showed the outcomes of a patient with AKI, more need for ICU admission, need dialysis with higher mortality. Kidney involvement should be investigated in patients with COVID-19 infection, especially those presented with old age, hypertension, chest pain, high serum level of D-dimer, and CRP. Further studies are needed in the future with a large sample and a specific one for AKI in pediatric patients with COVID-19 infection.
Conclusion
In the first week, the incidence of AKI among Iraqi patients with COVID-19 infection was 23.2%. Old-Age, Hypertension, chest pain, abdominal pain, high D-dimer, and CRP may be markers for possible association with AKI in COVID-19 infection. COVID-19 patients with AKI have a low rate of discharging well & a higher mortality rate, and about 10% of them need dialysis.
Acknowledgments: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: Al-Sodani M.H. (First Author), Introduction Writer/Discussion Writer (25%); Wasfi Fadhil R. (Second Author), Methodologist (25%); Al-Khayyad N. (Third Author), Assistant Researcher (25%); Dyab Allawi A.A.M. (Fourth author), Assistant Researcher (25%)
Funding/Support: None declared by the authors.
References
1. Ronco C, Reis T, Husain‐Syed F. Management of acute kidney injury in patients with COVID‐19. Lancet Respir Med. 2020;8:738‐42. [Link] [DOI:10.1016/S2213-2600(20)30229-0]
2. Li Z, Wu M, Yao J, Guo J, Liao X, Song S, et al. Caution on kidney dysfunctions of COVID‐19 patients. medRxiv. 2020 Mar. [Link] [DOI:10.1101/2020.02.08.20021212]
3. Chaomin W, Xiaoyan Ch, Yanping C, Jia'an X, Xing Z, Sha X, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-43. [Link] [DOI:10.1001/jamainternmed.2020.0994] [PMID] [PMCID]
4. Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. Coronavirus disease 19 infection does not result in acute kidney injury: An analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343‐8. [Link] [DOI:10.1159/000507471] [PMID] [PMCID]
5. Rabi FA, Al Zoubi NS, Kasasbeh GhA, Salameh DM, Al-Nasser AD. SARS‐CoV‐2 and coronavirus disease 2019: What we know so far. Pathogens. 2020;9(3):231. [Link] [DOI:10.3390/pathogens9030231] [PMID] [PMCID]
6. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in hospital death of patients with COVID‐19. Kidney Int. 2020;97(5):829‐38. [Link] [DOI:10.1016/j.kint.2020.03.005] [PMID] [PMCID]
7. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052-9. [Link] [DOI:10.1001/jama.2020.6775] [PMID] [PMCID]
8. Bo Diao, Chenhui W, Rongshuai W, Feng Z, Zhang J, Yang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2020;12:2506. [Link] [DOI:10.1038/s41467-021-22781-1] [PMID] [PMCID]
9. Soleimani M. Acute kidney injury in SARS‐CoV‐2 infection: Direct effect of virus on kidney proximal tubule cells. Int J Mol Sci. 2020;21(9):3275. [Link] [DOI:10.3390/ijms21093275] [PMID] [PMCID]
10. Diao B, Wen K, Chen J, Liu Y, Yuan Z, Han Ch, et al. Diagnosis of acute respiratory syndrome Coronavirus 2 infection by detection of nucleocapsid protein. medRxiv. 2020 Mar. [Link] [DOI:10.1101/2020.03.07.20032524]
11. Soler MJ, Wysocki J, Batlle D. ACE2 alterations in kidney disease. Nephrol Dial Transpl. 2013;28(11):2687‐97. [Link] [DOI:10.1093/ndt/gft320] [PMID] [PMCID]
12. Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, Fai To K, et al. Acute renal impairment in coronavirus‐associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698‐705. [Link] [DOI:10.1111/j.1523-1755.2005.67130.x] [PMID] [PMCID]
13. Ronco, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8(7):738-42. [Link] [DOI:10.1016/S2213-2600(20)30229-0]
14. Wang K, Chen W, Zhou Y‐S, Lian JQ, Zhang Z, Du P, et al. SARS‐CoV‐2 invades host cells via a novel route: CD147‐spike protein. bioRxiv. 2020 Mar. [Link] [DOI:10.1101/2020.03.14.988345]
15. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect. 2020;80(6):607-13. [Link] [DOI:10.1016/j.jinf.2020.03.037] [PMID] [PMCID]
16. Huang C, Wang Y, Li X, Jiaping J, Hu Y, et al. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet Respir Med. 2020;395(10223):497-506. [Link] [DOI:10.1016/S0140-6736(20)30183-5]
17. Werns SW, Lucchesi BR. Myocardial ischemia and reperfusion: The role of oxygen radicals in tissue injury. Cardiovasc Drugs Ther. 1989;2:761-69. [Link] [DOI:10.1007/BF00133206] [PMID]
18. Jiang Hua, Deng Hongfei, Wang Yu. Lopinavir / ritonavir (LPV / r) for 2019 new crown the possibility of treatment of viral pneumonia: A rapid systematic review based on previous coronavirus pneumonia research. China J Emerg Med. 2020;29(2):182. [Link]
19. Alvarez-Belon L, Sarnowski A, Forni LG. COVID-19 infection and the kidney. Br J Hospital Med (Lond). 2020;81(10):1-8. [Link] [DOI:10.12968/hmed.2020.0574] [PMID]
20. Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):138. [Link]
21. Silva Borba MG, Almeida Val FF, Sampaio VS, Araújo Alexandre MA, Cardoso Melo G, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: A randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. [Link] [DOI:10.1001/jamanetworkopen.2020.8857] [PMID]
22. Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, Gambino CG, et al. Abnormal liver function testspredict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40(10):2394-406. [Link] [DOI:10.1111/liv.14565] [PMID] [PMCID]
23. Fominskiy EV, Scandroglio AM, Monti G, Calabrò MG, Landoni G, Dell'Acqua A, et al. Prevalence, characteristics, risk factors, and outcomes of invasively ventilated COVID-19 patients with acute kidney injury and renal replacement therapy. Blood Purif. 2021;50(1):102-9. [Link] [DOI:10.1159/000508657] [PMID] [PMCID]
24. Hong KS, Lee KH, Chung JH, Cheol Shin K, Young Choi E, Jung Jin H, et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: A brief descriptive study. Yonsei Med J. 2020;61(5):431-7. [Link] [DOI:10.3349/ymj.2020.61.5.431] [PMID] [PMCID]
25. Lim J-H, Park S-H, Jeon Y, Cho JH, Jung HY, Choi JY, et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med. 2020;9(6):1718. [Link] [DOI:10.3390/jcm9061718] [PMID] [PMCID]
26. Uribarri A, Nez-Gil InJ, Aparisi A, Becerra-Muñoz VM, Feltes G, Trabattoni D, et al. Impact of renal functionon admission in COVID-19 patients: An analysis of the international HOPE COVID-19 (Health outcome predictive evaluationfor COVID 19) registry. J Nephrol. 2020;29:1-9. [Link] [DOI:10.1007/s40620-020-00790-5] [PMID] [PMCID]
27. Trujillo H, Caravaca-Font F, Sevillano N, Gutiérrez E, Caro J, Gutiérrez E, et al. SARS-CoV-2 infection in hospitalized patients with kidney disease. Kidney Int Rep. 2020;5(6):905-9. [Link] [DOI:10.1016/j.ekir.2020.04.024] [PMID] [PMCID]
28. Imam Z, Odish F, Gill I, O'Connor D, Armstrong J, Vanood A, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469-76. [Link] [DOI:10.1111/joim.13119] [PMID] [PMCID]
29. Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TJ, et al. AKI in hospitalized patientswith and without COVID-19: A comparison study. J Am Soc Nephrol. 2020;31(9):2145-57. [Link] [DOI:10.1681/ASN.2020040509] [PMID] [PMCID]
30. Arnold F, Westermann L, Rieg S, Neumann-Haefelin E, Biever PM, Walz G, et al. Superior anticoagulationstrategies for renal replacement therapy in critically ill patientswith COVID-19: A cohort study. BMC Nephrology. 2020 Jul. [Link] [DOI:10.1101/2020.06.26.20140699]
31. Husain-Syed F, Wilhelm J, Kassoumeh S, Birk HW, Herold S, Vadasz I, et al. Acute kidneyinjury and urinary biomarkers in hospitalized patients with Coronavirus disease 2019. Nephrol Dial Transplant. 2020;35(7):1271-4. [Link] [DOI:10.1093/ndt/gfaa162] [PMID] [PMCID]
32. Oussalah A, Gleye S, Urmes IC, Laugel E, Barbé F, Orlowski S, et al. Follow-up of multi-organdysfunction and inflammation using biomarker kinetics in patientswith severe COVID-19 disease and association with disease outcomes:Results from a referral center cohort in the north east of France. SSRN. 2020 Jun. [Link] [DOI:10.2139/ssrn.3590489]
33. Grimaldi D, Aissaoui N, Blonz G, Carbutti G, Courcelle R, Gaudry S, et al. Characteristics and outcomesof acute respiratory distress syndrome related to COVID-19 in Belgian and French intensive care units according to antiviralstrategies: The COVADIS multicenter observational study. Annals of Intensive Care. 2020;10:131. [Link] [DOI:10.1186/s13613-020-00751-y] [PMID] [PMCID]
34. Oksanen A, Kaakinen M, Latikka R, Savolainen L, Savela N, Koivula A. Regulation and trust: 3-month follow-up study on COVID-19 mortality in 25 European countries. JMIR Public Health Surveill. 2020;6(2):e19218. [Link] [DOI:10.2196/19218] [PMID] [PMCID]
35. Pei G, Zhang Z, Peng J, Liu L, Zhang Ch, Yu Ch, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157-65. [Link] [DOI:10.1681/ASN.2020030276] [PMID] [PMCID]
36. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:m1295. [Link] [DOI:10.1136/bmj.m1295] [PMID]
37. Zhou F, Wu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054-62. [Link] [DOI:10.1016/S0140-6736(20)30566-3]
38. Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: A retrospective cohort study. PLoS Med. 2020;17(10):e1003406. [Link] [DOI:10.1371/journal.pmed.1003406] [PMID] [PMCID]
39. Zhang J, Li J, Su L, Yung J, Jiang X, Jiang N, et al. Clinical characteristics and risk factors of acute kidney injury in coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32(4):407-11. [Chinese] [Link]
40. Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52(6):1193-4. [Link] [DOI:10.1007/s11255-020-02451-9] [PMID] [PMCID]
41. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur Respir J. 2020;55(5):2000547. [Link] [DOI:10.1183/13993003.00547-2020] [PMID] [PMCID]
42. Angel-Korman A, Brosh T, Glick K, Leiba A. COVID-19, the kidney and hypertension. Harefuah. 2020;159(4):231-4. [Link]
43. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett R, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209-18. [Link] [DOI:10.1016/j.kint.2020.05.006] [PMID] [PMCID]
44. Zheng X, Yang H, Li X, Li H, Xu L, Yu Q, et al. Prevalence of kidney injury and associations with critical illness and death in patients with COVID-19. Clin J Am Soc Nephrol. 2020;15(11):1549-56. [Link] [DOI:10.2215/CJN.04780420] [PMID] [PMCID]
45. Robert-Ebadi H, Bertoletti L, Combescure C, Le Gal G, Bounameaux H, Righini M. Effects of impaired renal function on levels and performance of D-dimer in patients with suspected pulmonary embolism. Thromb Haemost. 2014;112:614-20. [Link] [DOI:10.1160/TH13-12-1024] [PMID]
46. Baer PC, Gauer S, Wegner B, Schubert R, Geiger H. C‐reactive protein induced activation of MAP‐K and RANTES in human renal distal tubular epithelial cells in vitro. Clin. Nephrol. 2006;66(3):177-83. [Link] [DOI:10.5414/CNP66177] [PMID]
47. Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao Sh, et al. AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151-60. [Link] [DOI:10.1681/ASN.2020050615] [PMID] [PMCID]
48. Hui D, Zumla A. Severe acute respiratory syndrome: Historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33(4):869-89. [Link] [DOI:10.1016/j.idc.2019.07.001] [PMID] [PMCID]
49. Guan WJ, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of Coronavirus disease 2019 in China. New Engl J Med. 2020;382(18):1708-20. [Link] [DOI:10.1056/NEJMoa2002032] [PMID] [PMCID]








