Volume 14, Issue 2 (2022)
Iran J War Public Health 2022, 14(2): 221-224 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2021/09/5 | Accepted: 2022/04/23 | Published: 2022/05/30
Received: 2021/09/5 | Accepted: 2022/04/23 | Published: 2022/05/30
How to cite this article
AL-Kinani B, AL-Mashhedy L. The Association between Adiponectin, Adiponectin Receptor, and Obesity for Diabetic Female Type II. Iran J War Public Health 2022; 14 (2) :221-224
URL: http://ijwph.ir/article-1-1015-en.html
URL: http://ijwph.ir/article-1-1015-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
B.H. AL-Kinani *1, L.A. AL-Mashhedy1
1- Department of Chemistry, College of Science, University of Babylon, Hilla, Iraq
Full-Text (HTML) (636 Views)
Introduction
Metabolic syndrome is thought to be caused by obesity. Where obesity in adulthood is characterized by the occurrence of hypertrophy of adipocytes. In the regulation of energy homeostasis, adipose tissue is involved as an important endocrine organ and secretes a number of “bioactive lipoproteins [1].
Adiponectin and adiponectin receptors (AdipoRs) have been found to have important roles in chronic diseases and are a pathogen associated with obesity [2]. The functional and genetic studies confirm that adiponectin is a therapeutic target adipokine. Several interactions and roles with some other biomolecules (MCP-1) have been clearly identified to form a sinister adipokine network that is a cause of obesity-related insulin resistance and metabolic syndrome; PPARγ regulates HMW adiponectin and PPARα (Peroxisome proliferator-activated receptor α) regulates AdipoRs; dietary osmotin may act as a natural adiponectin receptor agonist [3].
Under starvation conditions, MMW (Low-molecular-weight adiponectin) activates AMPK (AMP-activated protein kinase). in the hypothalamus, food intake enhances, and at the same time, HMW adiponectin activates AMPK in peripheral tissues, such as skeletal muscle, and stimulates the combustion of fatty acids [4, 5].
Pathophysiological conditions, such as diabetes and obesity, have only decreased HMW adiponectin; Therefore, strategies to increase HMW adiponectin only lead to a logical approach that may provide a new treatment modality for obesity-related diseases, such as insulin resistance, type 2 diabetes, and atherosclerosis. It is hoped that this data will be useful in developing treatments to counteract the devastating, painful, and costly effects of obesity [6].
Hypertrophic adipocytes that have been associated with obesity with increased MCP-1, and decreased adiponectin action constitute a sinister adipokine network to causes insulin resistance, obesity-related diabetes, and metabolic syndrome. Strategies for activating the adiponectin/AdipoRs pathway may provide a new treatment modality for insulin resistance, metabolic syndrome, and atherosclerosis [7].
there are Multiple mechanisms linking obesity with CVDs [2, 8]. Many adipokines mediate the cross-talk between vasculature, heart, and adipose tissues in the “adipo-cardiovascular axis”; the altered release of adipokines promotes a prothrombotic state contributing to atherosclerosis disease [9]. Some studies indicate that adiponectin has a beneficial role in CVDs and atherosclerosis. Low serum adiponectin levels are predictors of myocardial infarction atherosclerosis [10].
The relationship between obesity and type 2 cardiovascular disease. It has now become a new concern. Adiponectin is a collagen plasma protein that is secreted by adipocytes that are responsible for the development of insulin resistance and hematological diseases. Studies have found that low protein leads to insulinomas, diabetes, atherosclerosis, and coronary artery disease. Up-regulation of adiponectin and its receptors is partly related to insulin sensitivity to antidiabetic drugs. We discuss the anti-hormone antagonism of adiponectin, its association with insulin resistance and obesity, and the use of adiponectin and its receptors as a therapeutic measure [11, 12].
High BMI is one of the risk factors associated with T2DM And the unhealthy diet followed gives an inactive body Low socioeconomic status are factors that contribute to both obesity and T2DM where obesity was diagnosed when BMI measurements were used alone. People who are obese suffer T2DM and this leads to poor control of blood sugar when exacerbated by diabetes [13].
This study investigated the association between Adiponectin, Adiponectin Receptor, and Obesity for diabetic female Type II.
Material and Methods
Individuals were divided into two groups. The effect, the Tuck group was also divided into two parts. The research was conducted for a case-control study in the endocrinology center of Al-Murjan Al-Talafi Hospital in Al-Hilla, Babylon, Iraq. The following lists were of patients and Healthy people: age 26 to 70 years with type 2 DM who visited the diabetes study center there were interrogators and laws with both individuals and patients. The search was done using parameters to collect; pathological sex and. Weights and background. Body mass index (BMI) was measured as an average weight of 23.11-26.69kg/m2 or obesity >30kg/m2 according to the classification of the World Health Organization.
Blood samples for patients and observers were collected and drawn (5 ml) for each individual. And blood samples are allowed to coagulate and then use a centrifuge where it was centrifuged after 10 minutes at 4500rpm. The walk was separated and stored deeply Frozen until analysis.
For statistical analysis, statistics from the SPSS version (V.26.0) were used. The results were used as means±SD, ANOVA to find out the difference between groups two average semesters were compared with students' ratings. As for the relationships between variables, they were compared using Pearson's correlation coefficients. P-values two-tailed were scored considered significant at 0.05.
Findings
The research was including ninety women classified into two groups, 36 female monitors (18 obese and 18 non-obese) and 54 diabetic women (27 obesity, 27 non-obesity) patients. In Tables 1 and 2, the studied contributors were faced with biochemical and clinical variables. In the patient group, Adipo was significant compared with controls (p<0.05). AdipoR1 was not significant compared with controls (p>0.05). FBS was significant in patients relative to controls (p<0.05), and BMI was non-significant compared with controls (p>0.05).
Table 1) Clinical properties for DM2 patients and controls
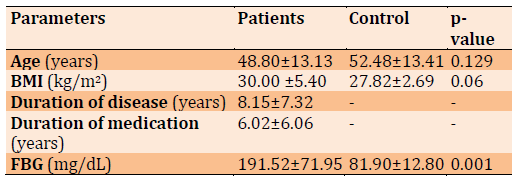
Table 2) The comparison of patient and control groups for Adipo (ng/mL)
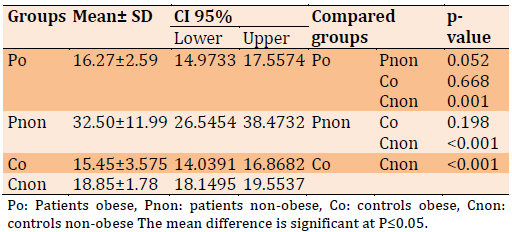
Adipo level comparison between different groups in Table 2. Adipo was significantly decreased in the patients obese compared to patients with non-obese (p=0.052) and controls (p<0.001); while Adipo levels were not significantly elevated in the Pnon compared to controls (p=0.001).
Table 3) The Comparison of Patient and Control Groups for AdipoR1 (ng/mL)
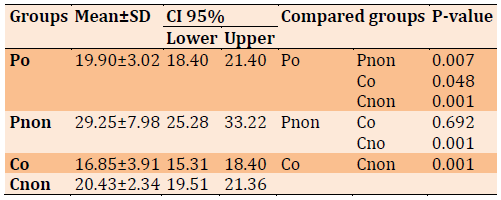
AdiporR1 level comparison between different groups in Table 3. AdipoR1 was significantly decreased in the patients obese compared to patients non-obese, (p=0.007). Similarly, the patients were obese compared to controls non-obese (p=0.048). while AdipoR1 levels were not significantly elevated in the Pnon compared to control obes, (p=0.692). and for Pnon compared with Cnon (p=0. 001).
FBS levels and BMI were compared between different groups of patients and controls as illustrated in Table 4. Obese patients have low FBS (80.59±14.90) with BMI (33.16±2.81) compared to non-obese patients with FBS (83.11±10.76) with BMI (22.47±2.57), according to criteria compared to obese control FBS (177.68±64.74) with BMI (33.64±2.86) compared another non-obese control FBS (207.02±71.50) with BMI (25.39±2.62).
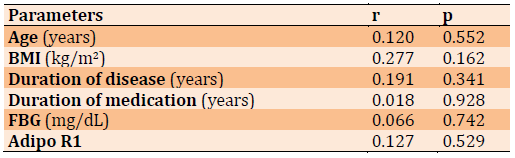
Adipo in Table 5 shows a positive correlation with diabetes duration FBG, BMI Duration of medication, Duration of medication, Age, and Adipo R1.
Adipo and FBS in Table 6 show, weak associated with Duration of medication and positive correlation for parameter.
Table 4) The FBS Levels (mg/dL) and BMI (kg/m2) for Patient Groups Compared to Control Groups 2

Table 5) The correlation between Adipo and variables for obese diabetic patients

Table 6) The correlation between Adipo and variables for non-obese diabetic patients
Discussion
Kawano and Arora, are found a negative correlation was found between plasma adiponectin levels and body mass index (BMI) in men and women. Plasma adiponectin concentration was negatively correlated with percentage body fat and waist-high ratio, while also having an inverse relationship with fasting plasma insulin concentration. The study helped to confirm a link between obesity and type 2 diabetes in association with low plasma adiponectin concentration [11].
The result of Cnop et al also shows that plasma adiponectin concentration is more closely related to insulin sensitivity and fasting insulinemia than to glycemia and adiposity. The results suggested that type 2 diabetes and obesity in patients with low adiponectin levels were in large part attributable to insulin resistance and/or hyperinsulinemia [14, 15].
Researchers have found that the adiposity associated with low circulating levels of adiponectin contains the expansion of visceral and abdominal adipose tissue rather than total body adiposity [7, 8] The importance of this is related to the fact abdominal fat expansion has a greater effect on insulin resistance than total obesity [16].
The study associated a lower adiponectin level with a higher risk of type 2 diabetes The relationship was found through fasting and post-load glucose levels. Increased adiponectin levels were also associated with a lower risk of impaired glucose metabolism in women. The results are shown in this study propose that levels of adiponectin are in some way complicated in the pathophysiology linking adiposity and type 2 diabetes [17].
Conclusion
The conclusion of our study suggested that adipo levels, which consider a pro-inflammatory marker for obesity enhance complications such as metabolic syndrome, insulin resistance, ischemic heart disease, and diabetic renal disease for women obese diabetes patients compared with nonobese diabetes patients.
Acknowledgments: None declared.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contributions: AL-Kinani BH (First Author), Introduction Writer/Methodologist/Main Researcher/
Discussion Writer (50%); AL-Mashhedy LA (Second Author), Assistant Researcher/Statistical Analyst/
Discussion Writer (50%)
Funding/Support: None declared.
Metabolic syndrome is thought to be caused by obesity. Where obesity in adulthood is characterized by the occurrence of hypertrophy of adipocytes. In the regulation of energy homeostasis, adipose tissue is involved as an important endocrine organ and secretes a number of “bioactive lipoproteins [1].
Adiponectin and adiponectin receptors (AdipoRs) have been found to have important roles in chronic diseases and are a pathogen associated with obesity [2]. The functional and genetic studies confirm that adiponectin is a therapeutic target adipokine. Several interactions and roles with some other biomolecules (MCP-1) have been clearly identified to form a sinister adipokine network that is a cause of obesity-related insulin resistance and metabolic syndrome; PPARγ regulates HMW adiponectin and PPARα (Peroxisome proliferator-activated receptor α) regulates AdipoRs; dietary osmotin may act as a natural adiponectin receptor agonist [3].
Under starvation conditions, MMW (Low-molecular-weight adiponectin) activates AMPK (AMP-activated protein kinase). in the hypothalamus, food intake enhances, and at the same time, HMW adiponectin activates AMPK in peripheral tissues, such as skeletal muscle, and stimulates the combustion of fatty acids [4, 5].
Pathophysiological conditions, such as diabetes and obesity, have only decreased HMW adiponectin; Therefore, strategies to increase HMW adiponectin only lead to a logical approach that may provide a new treatment modality for obesity-related diseases, such as insulin resistance, type 2 diabetes, and atherosclerosis. It is hoped that this data will be useful in developing treatments to counteract the devastating, painful, and costly effects of obesity [6].
Hypertrophic adipocytes that have been associated with obesity with increased MCP-1, and decreased adiponectin action constitute a sinister adipokine network to causes insulin resistance, obesity-related diabetes, and metabolic syndrome. Strategies for activating the adiponectin/AdipoRs pathway may provide a new treatment modality for insulin resistance, metabolic syndrome, and atherosclerosis [7].
there are Multiple mechanisms linking obesity with CVDs [2, 8]. Many adipokines mediate the cross-talk between vasculature, heart, and adipose tissues in the “adipo-cardiovascular axis”; the altered release of adipokines promotes a prothrombotic state contributing to atherosclerosis disease [9]. Some studies indicate that adiponectin has a beneficial role in CVDs and atherosclerosis. Low serum adiponectin levels are predictors of myocardial infarction atherosclerosis [10].
The relationship between obesity and type 2 cardiovascular disease. It has now become a new concern. Adiponectin is a collagen plasma protein that is secreted by adipocytes that are responsible for the development of insulin resistance and hematological diseases. Studies have found that low protein leads to insulinomas, diabetes, atherosclerosis, and coronary artery disease. Up-regulation of adiponectin and its receptors is partly related to insulin sensitivity to antidiabetic drugs. We discuss the anti-hormone antagonism of adiponectin, its association with insulin resistance and obesity, and the use of adiponectin and its receptors as a therapeutic measure [11, 12].
High BMI is one of the risk factors associated with T2DM And the unhealthy diet followed gives an inactive body Low socioeconomic status are factors that contribute to both obesity and T2DM where obesity was diagnosed when BMI measurements were used alone. People who are obese suffer T2DM and this leads to poor control of blood sugar when exacerbated by diabetes [13].
This study investigated the association between Adiponectin, Adiponectin Receptor, and Obesity for diabetic female Type II.
Material and Methods
Individuals were divided into two groups. The effect, the Tuck group was also divided into two parts. The research was conducted for a case-control study in the endocrinology center of Al-Murjan Al-Talafi Hospital in Al-Hilla, Babylon, Iraq. The following lists were of patients and Healthy people: age 26 to 70 years with type 2 DM who visited the diabetes study center there were interrogators and laws with both individuals and patients. The search was done using parameters to collect; pathological sex and. Weights and background. Body mass index (BMI) was measured as an average weight of 23.11-26.69kg/m2 or obesity >30kg/m2 according to the classification of the World Health Organization.
Blood samples for patients and observers were collected and drawn (5 ml) for each individual. And blood samples are allowed to coagulate and then use a centrifuge where it was centrifuged after 10 minutes at 4500rpm. The walk was separated and stored deeply Frozen until analysis.
For statistical analysis, statistics from the SPSS version (V.26.0) were used. The results were used as means±SD, ANOVA to find out the difference between groups two average semesters were compared with students' ratings. As for the relationships between variables, they were compared using Pearson's correlation coefficients. P-values two-tailed were scored considered significant at 0.05.
Findings
The research was including ninety women classified into two groups, 36 female monitors (18 obese and 18 non-obese) and 54 diabetic women (27 obesity, 27 non-obesity) patients. In Tables 1 and 2, the studied contributors were faced with biochemical and clinical variables. In the patient group, Adipo was significant compared with controls (p<0.05). AdipoR1 was not significant compared with controls (p>0.05). FBS was significant in patients relative to controls (p<0.05), and BMI was non-significant compared with controls (p>0.05).
Table 1) Clinical properties for DM2 patients and controls

Table 2) The comparison of patient and control groups for Adipo (ng/mL)

Adipo level comparison between different groups in Table 2. Adipo was significantly decreased in the patients obese compared to patients with non-obese (p=0.052) and controls (p<0.001); while Adipo levels were not significantly elevated in the Pnon compared to controls (p=0.001).
Table 3) The Comparison of Patient and Control Groups for AdipoR1 (ng/mL)

AdiporR1 level comparison between different groups in Table 3. AdipoR1 was significantly decreased in the patients obese compared to patients non-obese, (p=0.007). Similarly, the patients were obese compared to controls non-obese (p=0.048). while AdipoR1 levels were not significantly elevated in the Pnon compared to control obes, (p=0.692). and for Pnon compared with Cnon (p=0. 001).
FBS levels and BMI were compared between different groups of patients and controls as illustrated in Table 4. Obese patients have low FBS (80.59±14.90) with BMI (33.16±2.81) compared to non-obese patients with FBS (83.11±10.76) with BMI (22.47±2.57), according to criteria compared to obese control FBS (177.68±64.74) with BMI (33.64±2.86) compared another non-obese control FBS (207.02±71.50) with BMI (25.39±2.62).
Adipo in Table 5 shows a positive correlation with diabetes duration FBG, BMI Duration of medication, Duration of medication, Age, and Adipo R1.
Adipo and FBS in Table 6 show, weak associated with Duration of medication and positive correlation for parameter.
Table 4) The FBS Levels (mg/dL) and BMI (kg/m2) for Patient Groups Compared to Control Groups 2

Table 5) The correlation between Adipo and variables for obese diabetic patients

Table 6) The correlation between Adipo and variables for non-obese diabetic patients

Discussion
Kawano and Arora, are found a negative correlation was found between plasma adiponectin levels and body mass index (BMI) in men and women. Plasma adiponectin concentration was negatively correlated with percentage body fat and waist-high ratio, while also having an inverse relationship with fasting plasma insulin concentration. The study helped to confirm a link between obesity and type 2 diabetes in association with low plasma adiponectin concentration [11].
The result of Cnop et al also shows that plasma adiponectin concentration is more closely related to insulin sensitivity and fasting insulinemia than to glycemia and adiposity. The results suggested that type 2 diabetes and obesity in patients with low adiponectin levels were in large part attributable to insulin resistance and/or hyperinsulinemia [14, 15].
Researchers have found that the adiposity associated with low circulating levels of adiponectin contains the expansion of visceral and abdominal adipose tissue rather than total body adiposity [7, 8] The importance of this is related to the fact abdominal fat expansion has a greater effect on insulin resistance than total obesity [16].
The study associated a lower adiponectin level with a higher risk of type 2 diabetes The relationship was found through fasting and post-load glucose levels. Increased adiponectin levels were also associated with a lower risk of impaired glucose metabolism in women. The results are shown in this study propose that levels of adiponectin are in some way complicated in the pathophysiology linking adiposity and type 2 diabetes [17].
Conclusion
The conclusion of our study suggested that adipo levels, which consider a pro-inflammatory marker for obesity enhance complications such as metabolic syndrome, insulin resistance, ischemic heart disease, and diabetic renal disease for women obese diabetes patients compared with nonobese diabetes patients.
Acknowledgments: None declared.
Ethical Permissions: None declared.
Conflicts of Interests: None declared.
Authors’ Contributions: AL-Kinani BH (First Author), Introduction Writer/Methodologist/Main Researcher/
Discussion Writer (50%); AL-Mashhedy LA (Second Author), Assistant Researcher/Statistical Analyst/
Discussion Writer (50%)
Funding/Support: None declared.
Keywords:
References
1. Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. 2022;185(3):419-46. [Link] [DOI:10.1016/j.cell.2021.12.016]
2. Zhang Z, Du J, Shi H, Wang S, Yan Y, Xu Q, et al. Adiponectin suppresses tumor growth of nasopharyngeal carcinoma through activating AMPK signaling pathway. J Transl Med. 2022;20:89. [Link] [DOI:10.1186/s12967-022-03283-0]
3. Natalucci V, Virgili E, Calcagnoli F, Valli G, Agostini D, Zeppa SD, et al. Cancer related anemia: an integrated multitarget approach and lifestyle interventions. Nutrients. 2021;13(2):482. [Link] [DOI:10.3390/nu13020482]
4. Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LβT2 gonadotropes. Mol Endocrinol. 2008;22(3):760-71. [Link] [DOI:10.1210/me.2007-0330]
5. Abou-Samra M, Selvais CM, Dubuisson N, Brichard SM. Adiponectin and its mimics on skeletal muscle: insulin sensitizers, fat burners, exercise mimickers, muscling pills … or everything together?. Int J Mol Sci. 2020;21(7):2620. [Link] [DOI:10.3390/ijms21072620]
6. Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes. 2008;32 Suppl 7:S13-8. [Link] [DOI:10.1038/ijo.2008.233]
7. Gonzalez LL, Garrie K, Turner MD. Type 2 diabetes-an autoinflammatory disease driven by metabolic stress. Biochim Biophys Acta Mol Basis Dis. 2018;1864(11):3805-23. [Link] [DOI:10.1016/j.bbadis.2018.08.034]
8. Choi SH, Hong ES, Lim S. Clinical implications of adipocytokines and newly emerging metabolic factors with relation to insulin resistance and cardiovascular health. Front Endocrinol (Lausanne). 2013;4:97. [Link] [DOI:10.3389/fendo.2013.00097]
9. Rega-Kaun G, Kaun C, Wojta J. More than a simple storage organ: adipose tissue as a source of adipokines involved in cardiovascular disease. Thromb Haemost. 2013;110(4):641-50. [Link] [DOI:10.1160/TH13-03-0212]
10. Lindber S, Mogelvang R, Pedersen SH, Bjerre M, Frystyk J, Flyvbjerg A, et al. Relation of serum adiponectin levels to number of traditional atherosclerotic risk factors and all-cause mortality and major adverse cardiovascular events (from the Copenhagen City Heart Study). Am J Cardiol. 2013;111(8):1139-45. [Link] [DOI:10.1016/j.amjcard.2012.12.043]
11. Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr. 2009;4(1):44-9. [Link] [DOI:10.1111/j.1559-4572.2008.00030.x]
12. Al-Ameri AA, AM AL-Mashhedy L. The Association between Lipocalin 2 and obesity for diabetic Female Type II. Nveo-Nat Volatiles Essential Oils J. 2021;8805-14. [Link]
13. Almubarak F. The association between known risk factors for type 2 diabetes, and the body mass index of diabetic adults [dissertation]. University of Arkansas; 2016 [Link]
14. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459-69. [Link] [DOI:10.1007/s00125-003-1074-z]
15. Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, et al. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63(2):135-42. [Link] [DOI:10.1016/j.diabres.2003.09.010]
16. Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47(5):699-713. [Link] [DOI:10.2337/diabetes.47.5.699]
17. Snijder MB, Heine RJ, Seidell JC, Bouter LM, Stehouwer CD, Nijpels, G, et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the hoorn study. Diabetes care. 2006;29(11):2498-503. [Link] [DOI:10.2337/dc06-0952]








