Volume 15, Issue 1 (2023)
Iran J War Public Health 2023, 15(1): 11-15 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/09/19 | Accepted: 2023/01/30 | Published: 2023/02/6
Received: 2022/09/19 | Accepted: 2023/01/30 | Published: 2023/02/6
How to cite this article
Mohi W, Shemran K, Alsaffar Y. Comparison of Visfatin and Leptin Levels in Type 2 Diabetic Patients with and without Atherosclerosis. Iran J War Public Health 2023; 15 (1) :11-15
URL: http://ijwph.ir/article-1-1178-en.html
URL: http://ijwph.ir/article-1-1178-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Biochemistry Department, College of Medicine, University of Babylon, Hilla, Iraq
Full-Text (HTML) (219 Views)
Introduction
Type 2 Diabetes Mellitus (T2DM) with overt chronic hyperglycemia typically represents the outcome of an imbalance between increased insulin resistance and the deterioration of insulin secretory function. A combination of potential contributing factors due to both aging and obesity can directly lead to this imbalance, which results in the development and progressive worsening of T2DM [1].
Diabetes mellitus is prevalent in Asia, and it is characterized by rapid progression over a short period, early onset, and high Body Mass Index (BMI) [2]. As Asian economies have developed and diets have shifted, so has the percentage of their populations that are overweight or obese. Central or abdominal obesity is especially dangerous for those with type 2 diabetes or Non-Insulin-Dependent Diabetes Mellitus (NIDDM). Obesity is associated with the increased production of pro-inflammatory cytokines from adipose tissue [3] and promotes metabolic dysregulation and inflammation [4].
Lack of adequate insulin secretion by the pancreas or insufficient production of insulin in the body leads to chronic diabetes [5]. Although there is currently no treatment for diabetes, previous medical evidence suggests that the disease's consequences can be effectively managed by preventative care and routine medical exams [6]. High blood sugar causes a person to urinate more often, thirst more often, and eat more often. Diabetic complications increase dramatically without treatment, such as chronic renal failure, heart disease, stroke, and long-term negative effects include vision loss and ulceration [7].
Accumulation of adipose tissue, particularly visceral adiposity, is the most common contributory factor in the development of Insulin Resistance (IR). Adipose tissue is an endocrine tissue that secretes many peptides that are adipocytokines including Leptin, adiponectin, resistin and visfatin. Adipokines are reported to affect glucose and lipid metabolism as well as food intake [8]. The serum and salivary levels of these adipokines were extensively assessed as biomarkers for early diagnosis of IR and its consequences for cardiovascular and renal diseases. Visfatin is an adipokine that has attracted the attention of researchers for its role in the pathogenesis of IR and the possibility of using its levels as a biomarker for IR detection [9].
Pre-B cell colony Enhancing Factor (PBEF) or Visfatin, which was first discovered as a substance that stimulates the growth of pre-B cells, has been related to both inflammation and the immunological response. Multiple metabolic problems, including obesity, type 2 diabetes, and cardiovascular disease, are linked to its role as an adipose hormone that promotes pro-inflammatory activities in peripheral tissues [10].
visfatin is regarded as a proinflammatory cytokine that has been shown to have multiple physiological functions related to cell metabolism [11], immunomodulation [12] and inflammation [11].
As the principal site of fat storage in the body's energy metabolism, it comes as no surprise that adipose tissue is the biggest endocrine organ ever discovered [13]. Adipokines are released in huge quantities by this organ; they control appetite, immunity, reproductive hormones, and neuroendocrine function [14]. Adipocytes are responsible for the majority of Leptin production. Brown adipose tissue is just one of several potential sources of Leptin, including the mammary epithelial cells of the bone marrow, the pituitary gland, the liver, and the placenta (the primary source of Leptin) [15]. It is a neuroendocrine mechanism controlled by adipose cells that send out a fullness signal and play a function in energy balance and body weight [16]. Subcutaneous adipose tissue is more insulin-secreting than visceral adipose tissue [17]. Increased hunger, decreased energy expenditure, and disturbed neuroendocrine function all result from a lack of Leptin, leading to weight gain [18].
Leptin is a polypeptide hormone produced and secreted by White Adipose Tissue (WAT) [19] that circulates in proportion to body fat mass [20], enters the Central Nervous System (CNS) in proportion to its plasma level [21], and interacts with its receptor expressed in key brain areas that regulate food intake, energy expenditure, and autonomic function [22]. A large body of evidence suggests that Leptin plays a vital role in the regulation of energy homeostasis as conditions characterized by Leptin deficiency promote hyperphagia and weight gain [23], whereas administration of Leptin leads to reduced food intake, increased energy expenditure, and weight loss [24]. However, recent evidence implicates Leptin not only in the regulation of energy balance but glucose homeostasis as well [25].
While the effect of Leptin to reduce food intake and body adiposity can improve insulin sensitivity in peripheral tissues via indirect mechanisms, several observations suggest that Leptin can directly affect glucose metabolism independent of its effects on energy balance [26].
The present study aimed to assess Visfatin and Leptin levels in type 2 diabetic patients with and without atherosclerosis.
Instruments and Methods
In this descriptive study, 30 diabetic patients, 30 patients with diabetes mellitus and atherosclerosis, and 30 seemingly healthy persons (as a control group) were investigated. The Chemistry Department at the College of Medicine, the University of Babylon hosted this research. Diabetic patients had been referred to the Diabetes Clinic after experiencing symptoms of type 2 diabetes for more than three months. The Second group had been registered at the Iraqi Center for Cardiac Surgery in Baghdad Governorate. The control group had gone to Marjan Teaching Hospital in Hilla City, Babylon Governorate, to see the Babylon Center for Diabetes and Endocrinology. Inclusion criteria were no smoking, no history of cardiac illness, and no hypertension. Patients with type 1 diabetes and pregnant women were excluded from the study. Subjects were selected by available sampling method
Determination of Visfatin and Leptin concentration
Specific antibodies, antigens, and dilutions were utilized in the ELISA (Enzyme-Linked Immunosorbent Assay) procedure. Human Visfatin ELISA Test Kit (Cat. No. MBS723926; MyBioSource; San Diego, CA, USA) was used to determine Visfatin, and Human Leptin ELISA Test Kit (Cat. No. MBS169298; MyBioSource; San Diego, CA, USA) was used to determine Leptin.
Data analysis
The statistical analysis was done with SPSS 21 software. Frequency and percentage were used to describe categorical variables. To represent continuous variables, means and Standard Deviation (SD) were utilized. Comparison of means between the two groups were analyzed using a student t-test. P≤0.05 was considered significant.
Findings
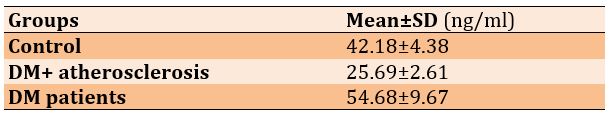
In DM patients and patients with DM and atherosclerosis, the level of Visfatin was significantly higher compared to the control group (p=0.0001). Also, the level of Visfatin in DM patients was higher than in those who had both diabetes and atherosclerosis (p=0.015; Table 1).
Table 1) Mean Visfatin levels in the studied groups

The frequency distribution of studied groups based on gender is shown in diagram 1.
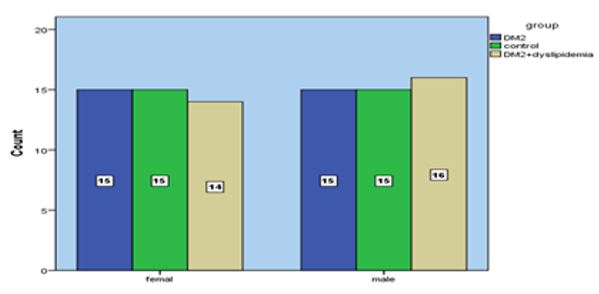
Diagram 1) Frequency distribution of studied groups based on gender
There was a significant increase in Leptin level in DM patients compared to the control group (p=0.0001), while Leptin level showed a significant decrease in patients with DM + atherosclerosis than in the control group (p=0.0001). The Leptin level of DM patients was much higher than that of patients with DM and atherosclerosis (p=0.0001; Table 2).
Table 2) Mean Leptin levels in the studied groups
The mean BMI in DM patients and patients with DM and atherosclerosis was significantly higher than in the control group (p=0.0001). However, there was no significant difference between DM patients and patients with DM and atherosclerosis (p=0.15; Table 3).
Table 3) Mean BMI levels in the studied groups

Diagnosing diabetes mellitus with Visfatin
The cut-off point was 1.805 ng/ml (p=0.0001). The Receiver Operating Characteristic (ROC) curve showed the sensitivity and the specificity as 90% and 85%, respectively. Furthermore, the Positive predictive value (PPV) was 90%, while the negative predictive value (NPV) was 70% (Figure 1).
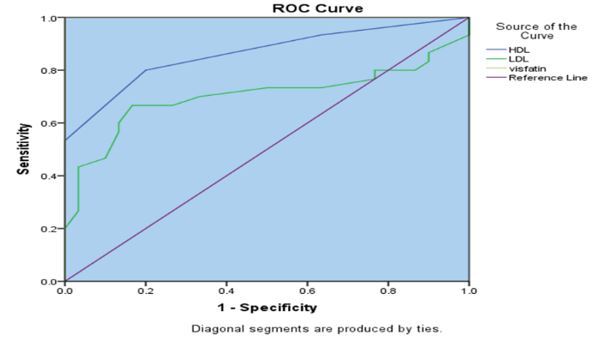
Figure 1) Visfatin ROC curve showing the sensitivity and specificity for diagnosing diabetes mellitus based on the concentration of the protein in the blood (in ng/ml).
Diagnosing diabetes mellitus with Leptin
The cut-off point was 30.667 ng/ml (p=0.0001). The ROC curve showed the sensitivity and the specificity as 97% and 100%, respectively. Furthermore, the PPV was 97%, while the NPV was 100% (Figure 2).
Figure 2) Leptin ROC curve showing the sensitivity and specificity for diagnosing diabetes mellitus based on the concentration of the protein in the blood (in ng/ml).
Discussion
The average age at diagnosis of type 2 diabetes in the United States decreased from 52 years in 1988-1994 to 46 years in 1999-2000. This ten-year decline is likely due to several factors. An early diagnosis, an earlier start of type 2 diabetes, or a mix of the two may be indicated by this shift. Statistics do not indicate whether the reduction in diagnostic age was brought on by a reduction in the actual age of onset for Americans or by earlier type 2 diabetes identification by medical professionals [27]. A prior study on diabetes in Iraq found results similar to the current study. Adaptation to the western lifestyle by reducing physical activity and a diet rich in fat and carbohydrates leads to the development of overweight and obesity in patients with diabetes mellitus type 2.
This is the first human study to show that food consumption may alter Visfatin levels. Our findings corroborate those of earlier research [28]. The effects of Visfatin were first documented by Adeghate in 2008. Visfatin binds to the insulin receptor at a site distinct from that of insulin and causes hypoglycaemia by reducing glucose release from liver cells and stimulating glucose utilization in adipocytes and myocytes. Visfatin is upregulated by hypoxia, inflammation and hyperglycaemia and downregulated by insulin, somatostatin and statins. This hormone is found in the cytoplasm as well as the nucleus of cells and has been identified in many tissues and organs including the brain, kidney, lung, spleen and testis but preferentially expressed in visceral adipose tissue and upregulated in some animal models of obesity. Visceral adipose tissue is regarded to be more pernicious than subcutaneous adipose tissue. Visfatin is an endocrine, autocrine as well as paracrine peptide with many functions including enhancement of cell proliferation, biosynthesis of nicotinamide mono- and dinucleotide and hypoglycaemic effect [29].
Visfatin plays a role in the development of cardiovascular complications associated with metabolic disorders. Increased serum Visfatin levels are linked to increased carotid plaque susceptibility in individuals with carotid stenosis, and serum Visfatin levels can be utilized as a predictor of cardiovascular mortality and morbidity in instances of acute ischemic stroke [30]. One of Visfatin's many functions is to control inflammation and atherosclerosis in the blood vessels [23]. Serum Visfatin concentration was similar in males and females across the whole cohort. In both sexes, individuals with atherosclerosis had greater blood levels of Visfatin than those without the disease.
This study found that patients with diabetes mellitus had lower levels of Leptin expression and higher levels of Visfatin expression. The progression of insulin resistance is connected with dysregulation of the adipoinsular axis, in which Leptin inhibits insulin synthesis via a negative feedback loop while insulin increases Leptin production. A considerable increased risk for developing insulin resistance and type 2 diabetes was seen in patients with low Leptin levels due to a lack of adipocytes or their breakdown, even after accounting for body mass index and waist circumference. Consistent with the results of earlier studies, this might be because type 2 diabetics with normal body mass index have a smaller proportional supply of insulin [29].
Leptin plasma levels were shown to be lower in the obese compared to the non-obese. As a result, it has been hypothesized that Leptin is crucial in maintaining healthy levels of insulin resistance, glucose, and lipids. We hypothesized that variations in blood Leptin levels might indicate certain pathophysiological anomalies that exist across individuals with T2DM because higher serum Leptin levels were associated with better glycemic control after one year of follow-up. Type 2 diabetes has been related to Leptin's effect on insulin sensitivity and inflammation. Traditional medicine holds that Leptin can combat diabetes and reduce inflammation. Although these adipocytokines have been identified and studied for quite some time, their roles in the development of type 2 diabetes remain controversial [31].
The satiety hormone Leptin has been proposed as a potential marker for both excess weight and type 2 diabetes. Serum Leptin levels in people with type 2 diabetes and their associations with other clinical markers (e.g., BMI, insulin concentration, and waist circumference) are more than a mystery [32].
Diabetic patients who are neither obese nor overweight have different results compared to healthy subjects in studies comparing Leptin concentrations. Despite this, some publications suggest that serum Leptin levels in DM2 patients have either declined or remained steady. The findings of the current study are in excellent accord with those of earlier research addressing the role of Leptin in DM2 [33].
Conclusion
Visfatin serum levels are significantly higher in atherosclerosis patients compared to healthy people, while low Leptin and high Visfatin expression are seen in patients with diabetes.
Acknowledgements: None declared by authors.
Ethical Permission: The study followed the Declaration of Helsinki's tenets on how to conduct research responsibly. Before any samples were obtained, we made sure to get the patient's verbal and analytical consent. To receive this permission, a local ethics committee read and stamped Document #1013 on February 10, 2021, which included the research protocol, subject information, and consent form.
Conflict of Interests: None declared by authors.
Authors’ Contribution: Mohi WZ (First Author), Introduction Writer/Main Researcher/Discussion Writer (50%); Shemran KA (Second Author), Methodologist/Assistant Researcher (25%); Alsaffar Y (Third Author), Assistant Researcher/Statistical Analyst (25%)
Funding: None declared by authors.
Type 2 Diabetes Mellitus (T2DM) with overt chronic hyperglycemia typically represents the outcome of an imbalance between increased insulin resistance and the deterioration of insulin secretory function. A combination of potential contributing factors due to both aging and obesity can directly lead to this imbalance, which results in the development and progressive worsening of T2DM [1].
Diabetes mellitus is prevalent in Asia, and it is characterized by rapid progression over a short period, early onset, and high Body Mass Index (BMI) [2]. As Asian economies have developed and diets have shifted, so has the percentage of their populations that are overweight or obese. Central or abdominal obesity is especially dangerous for those with type 2 diabetes or Non-Insulin-Dependent Diabetes Mellitus (NIDDM). Obesity is associated with the increased production of pro-inflammatory cytokines from adipose tissue [3] and promotes metabolic dysregulation and inflammation [4].
Lack of adequate insulin secretion by the pancreas or insufficient production of insulin in the body leads to chronic diabetes [5]. Although there is currently no treatment for diabetes, previous medical evidence suggests that the disease's consequences can be effectively managed by preventative care and routine medical exams [6]. High blood sugar causes a person to urinate more often, thirst more often, and eat more often. Diabetic complications increase dramatically without treatment, such as chronic renal failure, heart disease, stroke, and long-term negative effects include vision loss and ulceration [7].
Accumulation of adipose tissue, particularly visceral adiposity, is the most common contributory factor in the development of Insulin Resistance (IR). Adipose tissue is an endocrine tissue that secretes many peptides that are adipocytokines including Leptin, adiponectin, resistin and visfatin. Adipokines are reported to affect glucose and lipid metabolism as well as food intake [8]. The serum and salivary levels of these adipokines were extensively assessed as biomarkers for early diagnosis of IR and its consequences for cardiovascular and renal diseases. Visfatin is an adipokine that has attracted the attention of researchers for its role in the pathogenesis of IR and the possibility of using its levels as a biomarker for IR detection [9].
Pre-B cell colony Enhancing Factor (PBEF) or Visfatin, which was first discovered as a substance that stimulates the growth of pre-B cells, has been related to both inflammation and the immunological response. Multiple metabolic problems, including obesity, type 2 diabetes, and cardiovascular disease, are linked to its role as an adipose hormone that promotes pro-inflammatory activities in peripheral tissues [10].
visfatin is regarded as a proinflammatory cytokine that has been shown to have multiple physiological functions related to cell metabolism [11], immunomodulation [12] and inflammation [11].
As the principal site of fat storage in the body's energy metabolism, it comes as no surprise that adipose tissue is the biggest endocrine organ ever discovered [13]. Adipokines are released in huge quantities by this organ; they control appetite, immunity, reproductive hormones, and neuroendocrine function [14]. Adipocytes are responsible for the majority of Leptin production. Brown adipose tissue is just one of several potential sources of Leptin, including the mammary epithelial cells of the bone marrow, the pituitary gland, the liver, and the placenta (the primary source of Leptin) [15]. It is a neuroendocrine mechanism controlled by adipose cells that send out a fullness signal and play a function in energy balance and body weight [16]. Subcutaneous adipose tissue is more insulin-secreting than visceral adipose tissue [17]. Increased hunger, decreased energy expenditure, and disturbed neuroendocrine function all result from a lack of Leptin, leading to weight gain [18].
Leptin is a polypeptide hormone produced and secreted by White Adipose Tissue (WAT) [19] that circulates in proportion to body fat mass [20], enters the Central Nervous System (CNS) in proportion to its plasma level [21], and interacts with its receptor expressed in key brain areas that regulate food intake, energy expenditure, and autonomic function [22]. A large body of evidence suggests that Leptin plays a vital role in the regulation of energy homeostasis as conditions characterized by Leptin deficiency promote hyperphagia and weight gain [23], whereas administration of Leptin leads to reduced food intake, increased energy expenditure, and weight loss [24]. However, recent evidence implicates Leptin not only in the regulation of energy balance but glucose homeostasis as well [25].
While the effect of Leptin to reduce food intake and body adiposity can improve insulin sensitivity in peripheral tissues via indirect mechanisms, several observations suggest that Leptin can directly affect glucose metabolism independent of its effects on energy balance [26].
The present study aimed to assess Visfatin and Leptin levels in type 2 diabetic patients with and without atherosclerosis.
Instruments and Methods
In this descriptive study, 30 diabetic patients, 30 patients with diabetes mellitus and atherosclerosis, and 30 seemingly healthy persons (as a control group) were investigated. The Chemistry Department at the College of Medicine, the University of Babylon hosted this research. Diabetic patients had been referred to the Diabetes Clinic after experiencing symptoms of type 2 diabetes for more than three months. The Second group had been registered at the Iraqi Center for Cardiac Surgery in Baghdad Governorate. The control group had gone to Marjan Teaching Hospital in Hilla City, Babylon Governorate, to see the Babylon Center for Diabetes and Endocrinology. Inclusion criteria were no smoking, no history of cardiac illness, and no hypertension. Patients with type 1 diabetes and pregnant women were excluded from the study. Subjects were selected by available sampling method
Determination of Visfatin and Leptin concentration
Specific antibodies, antigens, and dilutions were utilized in the ELISA (Enzyme-Linked Immunosorbent Assay) procedure. Human Visfatin ELISA Test Kit (Cat. No. MBS723926; MyBioSource; San Diego, CA, USA) was used to determine Visfatin, and Human Leptin ELISA Test Kit (Cat. No. MBS169298; MyBioSource; San Diego, CA, USA) was used to determine Leptin.
Data analysis
The statistical analysis was done with SPSS 21 software. Frequency and percentage were used to describe categorical variables. To represent continuous variables, means and Standard Deviation (SD) were utilized. Comparison of means between the two groups were analyzed using a student t-test. P≤0.05 was considered significant.
Findings
In DM patients and patients with DM and atherosclerosis, the level of Visfatin was significantly higher compared to the control group (p=0.0001). Also, the level of Visfatin in DM patients was higher than in those who had both diabetes and atherosclerosis (p=0.015; Table 1).
Table 1) Mean Visfatin levels in the studied groups

The frequency distribution of studied groups based on gender is shown in diagram 1.

Diagram 1) Frequency distribution of studied groups based on gender
There was a significant increase in Leptin level in DM patients compared to the control group (p=0.0001), while Leptin level showed a significant decrease in patients with DM + atherosclerosis than in the control group (p=0.0001). The Leptin level of DM patients was much higher than that of patients with DM and atherosclerosis (p=0.0001; Table 2).
Table 2) Mean Leptin levels in the studied groups

The mean BMI in DM patients and patients with DM and atherosclerosis was significantly higher than in the control group (p=0.0001). However, there was no significant difference between DM patients and patients with DM and atherosclerosis (p=0.15; Table 3).
Table 3) Mean BMI levels in the studied groups

Diagnosing diabetes mellitus with Visfatin
The cut-off point was 1.805 ng/ml (p=0.0001). The Receiver Operating Characteristic (ROC) curve showed the sensitivity and the specificity as 90% and 85%, respectively. Furthermore, the Positive predictive value (PPV) was 90%, while the negative predictive value (NPV) was 70% (Figure 1).

Figure 1) Visfatin ROC curve showing the sensitivity and specificity for diagnosing diabetes mellitus based on the concentration of the protein in the blood (in ng/ml).
Diagnosing diabetes mellitus with Leptin
The cut-off point was 30.667 ng/ml (p=0.0001). The ROC curve showed the sensitivity and the specificity as 97% and 100%, respectively. Furthermore, the PPV was 97%, while the NPV was 100% (Figure 2).

Figure 2) Leptin ROC curve showing the sensitivity and specificity for diagnosing diabetes mellitus based on the concentration of the protein in the blood (in ng/ml).
Discussion
The average age at diagnosis of type 2 diabetes in the United States decreased from 52 years in 1988-1994 to 46 years in 1999-2000. This ten-year decline is likely due to several factors. An early diagnosis, an earlier start of type 2 diabetes, or a mix of the two may be indicated by this shift. Statistics do not indicate whether the reduction in diagnostic age was brought on by a reduction in the actual age of onset for Americans or by earlier type 2 diabetes identification by medical professionals [27]. A prior study on diabetes in Iraq found results similar to the current study. Adaptation to the western lifestyle by reducing physical activity and a diet rich in fat and carbohydrates leads to the development of overweight and obesity in patients with diabetes mellitus type 2.
This is the first human study to show that food consumption may alter Visfatin levels. Our findings corroborate those of earlier research [28]. The effects of Visfatin were first documented by Adeghate in 2008. Visfatin binds to the insulin receptor at a site distinct from that of insulin and causes hypoglycaemia by reducing glucose release from liver cells and stimulating glucose utilization in adipocytes and myocytes. Visfatin is upregulated by hypoxia, inflammation and hyperglycaemia and downregulated by insulin, somatostatin and statins. This hormone is found in the cytoplasm as well as the nucleus of cells and has been identified in many tissues and organs including the brain, kidney, lung, spleen and testis but preferentially expressed in visceral adipose tissue and upregulated in some animal models of obesity. Visceral adipose tissue is regarded to be more pernicious than subcutaneous adipose tissue. Visfatin is an endocrine, autocrine as well as paracrine peptide with many functions including enhancement of cell proliferation, biosynthesis of nicotinamide mono- and dinucleotide and hypoglycaemic effect [29].
Visfatin plays a role in the development of cardiovascular complications associated with metabolic disorders. Increased serum Visfatin levels are linked to increased carotid plaque susceptibility in individuals with carotid stenosis, and serum Visfatin levels can be utilized as a predictor of cardiovascular mortality and morbidity in instances of acute ischemic stroke [30]. One of Visfatin's many functions is to control inflammation and atherosclerosis in the blood vessels [23]. Serum Visfatin concentration was similar in males and females across the whole cohort. In both sexes, individuals with atherosclerosis had greater blood levels of Visfatin than those without the disease.
This study found that patients with diabetes mellitus had lower levels of Leptin expression and higher levels of Visfatin expression. The progression of insulin resistance is connected with dysregulation of the adipoinsular axis, in which Leptin inhibits insulin synthesis via a negative feedback loop while insulin increases Leptin production. A considerable increased risk for developing insulin resistance and type 2 diabetes was seen in patients with low Leptin levels due to a lack of adipocytes or their breakdown, even after accounting for body mass index and waist circumference. Consistent with the results of earlier studies, this might be because type 2 diabetics with normal body mass index have a smaller proportional supply of insulin [29].
Leptin plasma levels were shown to be lower in the obese compared to the non-obese. As a result, it has been hypothesized that Leptin is crucial in maintaining healthy levels of insulin resistance, glucose, and lipids. We hypothesized that variations in blood Leptin levels might indicate certain pathophysiological anomalies that exist across individuals with T2DM because higher serum Leptin levels were associated with better glycemic control after one year of follow-up. Type 2 diabetes has been related to Leptin's effect on insulin sensitivity and inflammation. Traditional medicine holds that Leptin can combat diabetes and reduce inflammation. Although these adipocytokines have been identified and studied for quite some time, their roles in the development of type 2 diabetes remain controversial [31].
The satiety hormone Leptin has been proposed as a potential marker for both excess weight and type 2 diabetes. Serum Leptin levels in people with type 2 diabetes and their associations with other clinical markers (e.g., BMI, insulin concentration, and waist circumference) are more than a mystery [32].
Diabetic patients who are neither obese nor overweight have different results compared to healthy subjects in studies comparing Leptin concentrations. Despite this, some publications suggest that serum Leptin levels in DM2 patients have either declined or remained steady. The findings of the current study are in excellent accord with those of earlier research addressing the role of Leptin in DM2 [33].
Conclusion
Visfatin serum levels are significantly higher in atherosclerosis patients compared to healthy people, while low Leptin and high Visfatin expression are seen in patients with diabetes.
Acknowledgements: None declared by authors.
Ethical Permission: The study followed the Declaration of Helsinki's tenets on how to conduct research responsibly. Before any samples were obtained, we made sure to get the patient's verbal and analytical consent. To receive this permission, a local ethics committee read and stamped Document #1013 on February 10, 2021, which included the research protocol, subject information, and consent form.
Conflict of Interests: None declared by authors.
Authors’ Contribution: Mohi WZ (First Author), Introduction Writer/Main Researcher/Discussion Writer (50%); Shemran KA (Second Author), Methodologist/Assistant Researcher (25%); Alsaffar Y (Third Author), Assistant Researcher/Statistical Analyst (25%)
Funding: None declared by authors.
Keywords:
Type 2 Diabetes Mellitus [MeSH], Atherosclerosis [MeSH], Leptin [MeSH], Visfatin [MeSH], Body Mass Index [MeSH], Iraq [MeSH]
References
1. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021;17(9):534-48. [Link] [DOI:10.1038/s41574-021-00512-2]
2. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377-90. [Link] [DOI:10.1038/s41581-020-0278-5]
3. Mancuso P, Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front Endocrinol. 2019;10:137. [Link] [DOI:10.3389/fendo.2019.00137]
4. Martyniak K, Masternak MM. Changes in adipose tissue cellular composition during obesity and aging as a cause of metabolic dysregulation. Exp Gerontol. 2017;94:59-63. [Link] [DOI:10.1016/j.exger.2016.12.007]
5. De Gaetano A, Gaz C, Palumbo P, Panunzi S. A unifying organ model of pancreatic insulin secretion. PLoS One. 2015;10(11):e0142344. [Link] [DOI:10.1371/journal.pone.0142344]
6. Rout M, Kaur A. Prediction of diabetes based on data mining techniques. Think India J. 2019;22(16):3743-50. [Link]
7. Preethikaa S, Brundha MP. Awareness of diabetes mellitus among general population. Res J Pharm Technol. 2018;11(5):1825-9. [Link] [DOI:10.5958/0974-360X.2018.00339.6]
8. Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016;11(4):e0154003. [Link] [DOI:10.1371/journal.pone.0154003]
9. Zhang Y, Huo Y, He W, Liu S, Li H, Li L. Visfatin is regulated by interleukin6 and affected by the PPARγ pathway in BeWo cells. Mol Med Rep. 2019;19(1):400-6. [Link] [DOI:10.3892/mmr.2018.9671]
10. Rahman A, Babar B, Sebtain A, Gul H, Qasim M, Mustafa SH. Frequency of proteinuria in newly diagnosed diabetic patients. Pakistan J Med Heal Sci. 2022;16(06):935. [Link] [DOI:10.53350/pjmhs22166935]
11. Sun Z, Lei H, Zhang Z. Pre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functions. Cytokine Growth Factor Rev. 2013;24(5):433-42. [Link] [DOI:10.1016/j.cytogfr.2013.05.006]
12. Ognjanovic S, Ku TL, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium. Am J Obstet Gynecol. 2005;193(1):273-82. [Link] [DOI:10.1016/j.ajog.2004.11.003]
13. World Health Organization. Classification of diabetes mellitus [Internet]. Geneva: World Health Organization; 2019 [cited 2022 Feb 23]. Available from: https://www.who.int/publications-detail-redirect/classification-of-diabetes-mellitus [Link]
14. Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42 Suppl 1:S10-5. [Link] [DOI:10.1016/j.jcjd.2017.10.003]
15. Gonzalez-Abuin N, Pinent M, Casanova-Martí À, Arola L, Blay M, Ardévol A. Procyanidins and their healthy protective effects against type 2 diabetes. Curr Med Chem. 2015;22(1):39-50. [Link] [DOI:10.2174/0929867321666140916115519]
16. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45-63. [Link]
17. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. [Link] [DOI:10.3390/ijms21176275]
18. Abusaib M, Ahmed M, Nwayyir HA, Alidrisi HA, Al-Abbood M, Al-Bayati A, et al. Iraqi experts consensus on the management of type 2 diabetes/prediabetes in adults. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420942232. [Link] [DOI:10.1177/1179551420942232]
19. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-32. [Link] [DOI:10.1038/372425a0]
20. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292-5. [Link] [DOI:10.1056/NEJM199602013340503]
21. Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D. Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589-93. [Link] [DOI:10.1038/nm0596-589]
22. Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661-71. [Link] [DOI:10.1038/35007534]
23. Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus-Implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16(6):321-31. [Link] [DOI:10.1038/s41574-020-0334-z]
24. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540-3. [Link] [DOI:10.1126/science.7624776]
25. Schwartz MW, Porte D. Diabetes, obesity, and the brain. Science. 2005;307(5708):375-9. [Link] [DOI:10.1126/science.1104344]
26. Meek TH, Morton GJ. Leptin, diabetes, and the brain. Indian J Endocrinol Metab. 2012;16(Suppl 3): S534-42. [Link] [DOI:10.4103/2230-8210.105568]
27. Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708. [Link] [DOI:10.1016/j.biopha.2020.110708]
28. Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus-mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599-612. [Link] [DOI:10.1038/s41575-021-00448-y]
29. Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15(18):1851-62. [Link] [DOI:10.2174/092986708785133004]
30. Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25(8):1987. [Link] [DOI:10.3390/molecules25081987]
31. Mambiya M, Shang M, Wang Y, Li Q, Liu S, Yang L, et al. The play of genes and non-genetic factors on type 2 diabetes. Front public Heal. 2019;7:349. [Link] [DOI:10.3389/fpubh.2019.00349]
32. Tripathy D, Merovci A, Basu R, Abdul-Ghani M, DeFronzo RA. Mild physiologic hyperglycemia induces hepatic insulin resistance in healthy normal glucose-tolerant participants. J Clin Endocrinol Metab. 2019;104(7):2842-50. [Link] [DOI:10.1210/jc.2018-02304]
33. Gillen JB, Estafanos S, Govette A. Exercise-nutrient interactions for improved postprandial glycemic control and insulin sensitivity. Appl Physiol Nutr Metab. 2021;46(8):856-65. [Link] [DOI:10.1139/apnm-2021-0168]










