Volume 14, Issue 3 (2022)
Iran J War Public Health 2022, 14(3): 351-357 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/06/9 | Accepted: 2022/08/11 | Published: 2022/10/12
Received: 2022/06/9 | Accepted: 2022/08/11 | Published: 2022/10/12
How to cite this article
Al-Mousawi A, Al-Amara S, Humoud M, Hattab S. Frequency of Inducible Clindamycin Resistance in Staphylococcus haemolyticus Isolated from Surgical Wounds Infections Using D-test and Molecular Methods in Al-Basrah, Iraq. Iran J War Public Health 2022; 14 (3) : 100
URL: http://ijwph.ir/article-1-1041-en.html
URL: http://ijwph.ir/article-1-1041-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Biology, College of Science, University of Basrah, Basrah, Iraq
2- Department of Pathological Analysis, College of Science, University of Basrah, Basrah, Iraq
3- Public Health Department, Basrah Health Directorate, Basrah, Iraq
2- Department of Pathological Analysis, College of Science, University of Basrah, Basrah, Iraq
3- Public Health Department, Basrah Health Directorate, Basrah, Iraq
Full-Text (HTML) (193 Views)
Introduction
Since the 1950s, Coagulase-Negative Staphylococci (CoNS) have been recognised as an important cause of human infection [1, 2]. CoNS are common skin commensals that start to colonize the body surfaces very early in life. After 48 h of birth, about 100% of infants acquire CoNS during passage through the birth canal or by contacting nursery personnel [3]. The most common colonizing species are Staphylococcus epidermidis, Staphylococcus warneri, and Staphylococcus haemolyticus [4]. S. haemolyticus, together with S. epidermidis and S. hominis, were the prevalent staphylococci species detected in surfaces that are touched at a high frequency in the community and hospitals in London [5]. Similarly, S. haemolyticus and S. epidermidis were the most common CoNS isolates (34% and 27%, respectively) detected in different hospital wards in Iran [6].
Coagulase negative staphylococcus is often underestimated as the etiological factor of human infections. One important specie in this group is S. haemolyticus. After Staphylococcus epidermidis, S. haemolyticus is the second most frequently isolated coagulase-negative staphylococcus from clinical cases, primarily from blood infections [7].
S. haemolyticus is one of the coagulase-negative staphylococci that is abundantly found as a common microbiota on the skin. S. haemolyticus is generally considred as the second leading opportunistic pathogen among CoNS after S. epidermidis that associated with immunocompromised individuals, especially those who are hospitalized or suffered from exposure to medical devices worldwide [7, 8].
S. haemolyticus causes severe infections in several body systems, including meningitis, endocarditis, prosthetic joint infections and bacteremia and is prevalent in the hospital environment and on the hands of healthcare workers. S. haemolyticus is also known to cause septicemia, peritonitis, otitis media and Diabetic Foot Ulcer (DFU) infections [9, 10].
Even though the virulence of S. haemolyticus is lesser than S. aureus, which means that it's potential to cause severe infections is lower, yet it has the ability to acquire resistance against multiple antimicrobial agents [11]. S. haemolyticus is notably more resistant to antibiotics than any other coagulase negative staphylococcus, and the widest spectrum of resistance was observed among strains isolated from the hospital environment [12, 13]. Taking into consideration its adaptability and the ability to survive in the hospital environment, especially on medical devices, S. haemolyticus has become one of the major agent in nosocomial infections caused by multi drug resistant Staphylococci [14]. The prolonged hospitalization, invasive procedures and exposure to multiple antibiotics can result in alteration of normal skin/mucous microbiota which leads to a highly adaptable Linezolid (LNZ) resistant MRSH (Methicillin Resistance Staphylococcus haemolyticus) [15].
S. haemolyticus, especially strains that cause nosocomial infections, are more resistant to antibiotics than other coagulase-negative staphylococci. There is clear evidence that the resistance genes can be acquired by other staphylococcus species through S. haemolyticus [16].
One of the characteristics of S. haemolyticus is its ability to form biofilm, which plays an essential role in causing infection. The produced exopolysaccharides can inhibit the growth of other bacteria and also decrease their ability to form biofilms [17, 18]. This species has gained an increased clinical significance due to its genome plasticity, which allowed a great adaptation and development of resistance to different antibiotics, including methicillin and its ability to survive in the hospital environment [19].
Antimicrobials Macrolide-Lincosamide- Streptogramin B (MLSB) family are commonly used to treat skin and soft tissue infections caused by CoNS [20], and also as a penicillin substitute in individuals who are allergic to penicillin [21]. Resistance to antibiotics in the MLSB family could be either constitutive (cMLSB) or inducible (iMLSB) [22, 23]. Although rRNA methylase is only produced in the presence of an inducing agent, which can also be another antibiotic from MLSB family, like erythromycin, or macrolide, and rRNA methylase is frequently created in the absence of an inducing agent in constitutive resistance [22, 23]. Since erythromycin generates iMLSB resistance, when using an erythromycin disc in relatively close proximity to a clindamycin disc (D-test) assists in capable of detecting this form of resistance in CoNS. Clindamycin is widely used to treat staphylococcal infections, particularly those of the skin and soft tissues, as well as to substitute penicillin in individuals who are allergic to penicillin [24, 25]. Clindamycin treatment could fail if iMLSB resistance isn't established [23, 26, 27].
Inducible or constitutive resistance to MLSB is conferred by erm genes. The structural genes can be produced inducibly or constitutively, and mutations in the regulatory area of genes are common, resulting in inducible resistance becoming constitutive resistance [25, 28].
The factors that affect the survival and spread of multi-drug resistant S. haemolyticus isolates in hospitals are not completely known. Bouchami et al. reported that the insertion sequence transposition (mainly IS1272) and chromosomal rearrangement and recombination processes in S. haemolyticus is one strategy that helps in the bacterial evolution, adaptation, pathogenesis, and survival in the hospitals, hence causing nosocomial infections [29].
Recently, several studies have focused on clinical isolates of S. haemolyticus due to their multidrug resistance characteristics among coagulase-negative staphylococci against various antibiotics, including penicillins, tetracyclines, cephalosporins, macrolides, aminoglycosides, and quinolones. S. haemolyticus may delete and add genes due to its ability to insert sequences, leading to frequent genetic rearrangement and drug resistance [28-30].
Furthermore, antimicrobial resistance of S. haemolyticus colonizes in both hospitalized patient's skin and mucous membranes, acting as a reservoir for antibiotic resistance genes, which lead to limited options for treatment [28]. The existence of a large fragment of foreign DNA called the Staphylococcal Chromosomal Cassette mec (SCCmec) in the chromosome distinguishes methicillin-resistant S. haemolyticus isolates from methicillin-susceptible isolates.
A Penicillin-Binding transpeptidase PBP2A, with low affinity for b-lactams, is encoded by the mecA gene [21, 29]. The existence of two essential loci distinguishes the SCCmec element: the mec gene complex, which contains mecA and its regulation, and the ccr gene complex, which encodes recombinases and is responsible for SCCmec mobility [29].
Over recent years, different investigators have described an increasing frequency of multidrug-resistant strains of S. haemolyticus [12, 31, 32].
Current study aimed to investigate the frequency of inducible clindamycin resistance in S. haemolyticus isolated from surgical wounds infections using both D-test and molecular methods in Al-Basrah, Iraq.
Materials and Methods
Samples collection
200 surgical wound swabs were collected from September 2020 to November 2020 from Ports General Hospital in Basrah, Iraq.
Isolation and identification of bacterial strains
The conventional isolation and identification methods were used to identify CoNS strains according to the minimal standards recommended by Freney et al. [33]. Surgical wound swabs were cultured in blood agar, chocolate agar and heart infusion agar. As a preliminary scan to identify the isolates, the isolates were examined using gram staining and several conventional biochemical tests including oxidase, catalase, hemolysis types, and coagulase production tests. The identified strains were confirmed by the Vitek®2 system.
Detection of methicillin-resistant S. haemolyticus isolates
According to Clinical and Laboratory Standards Institute (CLSI) guidelines [34], cefoxitin (30 μg) disc (Bioanalyse; Tukey) was used to detect methicillin resistance S. haemolyticus isolates.
Detection of inducible clindamycin resistance in S. haemolyticus isolates
According to CLSI guidelines [34], clindamycin (2μg) and erythromycin (15μg) discs (Bioanalyse, Tukey) were used to detect inducible clindamycin resistance strains as the first step, and Vitek®2 system was used to confirm the results.
DNA extraction
Genomic DNA of S. haemolyticus isolates was extracted using Presto™ Mini gDNA Bacteria Kit (Geneaid, USA). The purity of the extracted DNA was measured and a purity ratio between 1.8 and 2.0 was accepted.
Detection of antibiotic resistance genes
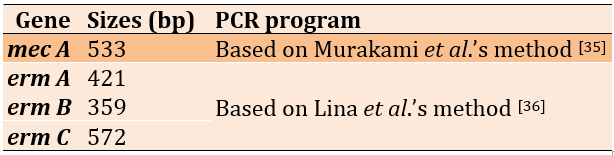
The emergence of antibiotic resistance genes of S. haemoliticus isolates was investigated using Polymerase Chain Reaction (PCR) method (Table 1).
Table 1) The resistant genes used in this study
Findings
Identification of the bacterial strains
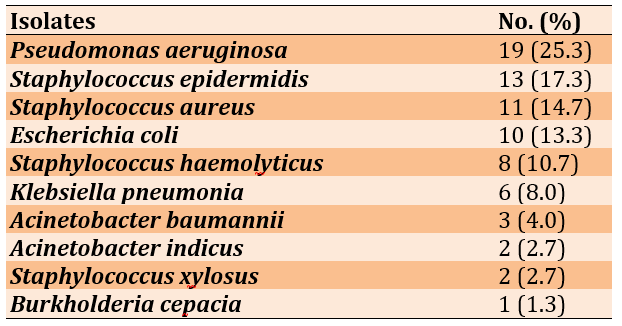
Out of 200 cases, 75 surgical wound swabs (37.5%) showed bacterial growth in blood agar, chocolate agar and heart brain infusion agar. The highest frequency of isolates belonged to Pseudomonas aeruginosa, followed by Staphylococcus epidermidis, Staphylococcus aureus and Escherichia coli, respectively (Table 2).
Table 2) Frequency distribution of bacterial isolated from surgical wound swabs
Antibiotic resistance patterns of S. haemoliticus isolates
Out of eight S. haemolyticus isolates, only 5 isolates (62.5%) showed inhibitory resistance criteria for both oxacillin and cefoxitin and were classified as MRSH (Methicillin Resistance Staphylococcus haemolyticus) isolates. While the rest of S. haemolyticus isolates (37.5%) showed sensitive criteria for oxacillin and cefoxitin and were classified as MSSH (Methicillin Sensitive Staphylococcus haemolyticu) isolates (Table 3).
On the other hand, out of eight isolates, 3 isolates (37.5%) were erythromycin-resistant and clindamycin sensitive with D-test positive. These isolates showed induced Macrolide-Lincosamide-Streptogramin B (iMLSB) resistance phenotype. While 2 isolates (25.0%) showed constitutive MLSB (cMLSB) resistance phenotype and 3 (37.5%) isolates were shown Macrolide-Streptogramin B (MSB) resistance phenotypes (Table 4).
Table 3) MRSH and MSSH patterns of S. haemolyticus isolates

Detection of antibiotic resistance genes
The most frequent resistance genes of S. haemolyticus strains were mecA (n=5, 62.5%), ermA (n=5, 62.5%), ermB (n=4, 50.0%), respectively, while ermC was found in 2 (25.0%) strains (Table 5; Figure 1).
Table 4) Frequency distribution of cMLSB, iMLSB, and MSB phenotypes and the result of D-test for S. haemolyticus isolates

Table 5) Patterns of antibiotic resistance genes in S. haemolyticus isolates
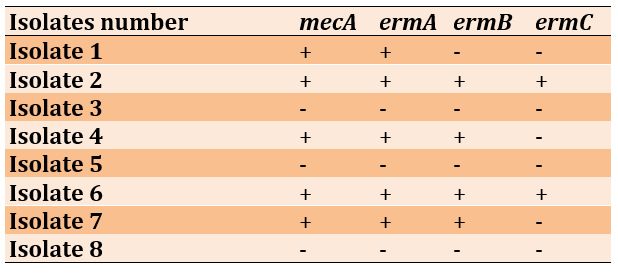

Figure 1) Gel electrophoresis profiles of PCR-amplified antibiotic resistance genes of S. haemolyticus: A) mecA gene, B) ermA gene, C) ermB gene (359 bp) and ermC (572bp); Gel electrophorphosis profiles with 2% concentration for 1hour at 75 vol
Discussion
Coagulase negative staphylococci are major nosocomial pathogens. Staphylococcus haemolyticus is the second opportunistic pathogen among CoNS after S. epidermidis and the third most common organism among clinical isolates of methicillin-resistant staphylococci [37]. S. haemolyticus, an emerging cause of nosocomial infection plays an important role in causing opportunistic infections related to medical devices [38]. The ability to form a biofilm is considered the most important virulence factor in CoNS associated infections [39].
Although S. haemolyticus is considered a common skin flora, the diseases caused by it have increased dramatically. The main reason is the multi-drug resistance of this bacterium, which subsequently poses serious risks to human health [28]. The worldwide spread of methicillin-resistant staphylococci alarmingly remains one of the most common hospital-acquired infections. The prevalence of hospitalized Methicillin Resistant Staphylococcus aureus (MRSA) and Methicillin-Resistant Coagulase Negative Staphylococci (MR-CoNS) has been recorded in various regions of the world [40].
The dramatic increment in the incidence and frequency of CoNS, as well as the ongoing health problems of methicillin resistance among staphylococci, have piqued our interest in trying to investigate the ideal treatment using clindamycin therapy. Clindamycin is considered an approperiate option, because of its tolerability, low cost, good permeability, and easy tissue entry [41].
Considerably, the common limitation of clindamycin therapy is its low ability to inhibit MR-CoNS through their inducible resistance phenotypes. Selective treatment cannot be performed without appropriate antibiotic susceptibility testing. Therefore, D-test has become a vital and crucial tool to achieve this goal [42]. Perez et al. [43] used the D-test method to detect inducible clindamycin resistance in CoNS and concluded that the D-test method is a simple and important technique in the detection of inducible clindamycin resistance. Jorgensen et al.'s study [44] demonstrated that inducible clindamycin resistance can be easily detected by disk induction testing on standard sheep blood agar plates used for verification of inoculum purity in conjunction with an automated susceptibility test system.
In Gatermann et al.’s study [25], out of 305 CoNS isolates, 155 (51%) isolates had constitutive clindamycin resistance, 78 (25.6%) had inducible clindamycin resistance, and 72 (23.6%) had non-inducible resistance. The prevalence of erythromycin resistance was almost 90% in S. haemolyticus. Also, most (63%) erythromycin-resistant isolates carried constitutively expressed ermC as the sole resistance determinant, with the notable exception of Staphylococcus hominis subsp. hominis, which carried inducible ermC.
S. haemolyticus has zoonotic character and is prevalent both in humans and animals. Ruzauskas et al. [11] determined the presence of MRSH in different groups of companion animals and to characterize isolates according their antimicrobial resistance. From a total of 754 samples tested, 12 MRSH isolates were obtained. The most frequent resistances of MRSH isolates demonstrated were to benzylpenicillin (91.7%) with the presence of the blaZ gene; erythromycin (91.7%) and clindamycin (41.7%) with the presence of ermA, ermC, and msrA genes; and gentamicin (75.0%) with the presence of aac(6′)-Ie-aph(2")-Ia and aph(3′)-IIIa genes.
Teeraputon et al. [45] determined antimicrobial resistance phenotypes and drug resistance genes of clinical coagulase-negative staphylococci isolates at Mae Sot Hospital in Tak province, Thailand. A total of 229 CoNS isolates were collected from clinical specimens during two periods in 2014 and in 2015. S. haemolyticus was the most prevalent species (37.55%). Methicillin-resistant CoNS (MRCoNS), containing the mecA gene, were detected in 145 of 229 isolates (63.32%), mostly found in S. haemolyticus and S. epidermidis. Among 125 erythromycin-resistant CoNS, the prevalence of constitutive type of MLSB, inducible clindamycin resistance and macrolide–streptogramin B resistance phenotypes were 72%, 13.60% and 14.40% respectively. These phenotypes were expressed in 80% of MRCoNS strains. In addition, the ermC gene (79.20%) was found to be more prevalent than the ermA gene (22.40%), especially among MRCoNS. S. haemolyticus appeared in nearly half of these isolates (n=69, 47.59%), followed by S. epidermidis, S. saprophyticus, and S. hominis.
Kitti et al. [40] examined antimicrobial susceptibility patterns, antimicrobial resistance genes, and SCCmec types of methicillin-resistant S. aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (MR-CoNS) isolated from patients in a hospital in Northern Thailand. They found that 82.6% of MR staphylococci (96.8% of MR-CoNS isolates) were resistant to 7–10 antibiotics. More than 70% of MRSA and MR-CoNS were resistant to cefoxitin, penicillin, oxacillin, erythromycin, clindamycin, gentamicin, and ciprofloxacin. In MRSA isolates, the prevalence of ermA (78.3%) and ermB (73.9%) genes was high compared to that of the ermC gene (4.3%). In contrast, ermC (87.1%) and qacA/B genes (70.9%) were predominant in MR-CoNS isolates. SCCmec type III was the dominant type of MRSA (13/23), whereas SCCmec type II was more present in S. haemolyticus (10/18). Ten MRSA isolates with SCCmec type III were ST239, which is the common type of MRSA in Asia.
Debnath et al. [41] detected inducible clindamycin resistance among the erythromycin resistant CoNS isolates. According their results, among 180 CoNS isolates, predominant isolated species were S. epidermidis (n=75, 41.67%) and S. haemolyticus (n=47, 26.11%). Out of 180 CoNS isolates, 108 (60%) showed erythromycin resistance, out of which, 29 (26.85%) isolates showed iMLSB. Among 180 CoNS isolates, 119 (66.11%) were MRCoNS isolates and 61 (33.89%) were MSCoNS isolates.
Barros et al. [30] used phenotypic and molecular methods to characterize the antibiotic resistance of 64 clinical isolates of S. haemolyticus. By PCR of the mecA gene, 87% were found to be methicillin resistant. Approximately 55% harbored SCCmec type V, and only one SCCmec type IV. Many isolates (75%) displayed multiresistance, and pulsotype analysis showed a high diversity.
Dziri et al. [46] evaluated the rate of detection of CoNS in environmental samples of 17 services in a Tunisian hospital and determined the antimicrobial resistance phenotypes and genotypes of recovered isolates. CoNS were obtained from 83 of the 200 tested samples (41.5%). S. haemolyticus was the most prevalent species (45.8%), followed by S. saprophyticus (36.1%). Methicillin-resistant CoNS were detected in 20 of the 200 tested samples (10%), and the mecA gene was demonstrated in 18 S. haemolyticus, one S. epidermidis and one S. saprophyticus isolates. Methicillin susceptible isolates were detected in 63 samples (31.5%). They reported that the high frequency of detection of multi-drug-resistant CoNS in the hospital environment, especially S. haemolyticus and S. saprophyticus, could be due to cross-transmission between patients, staff, and environment.
Conclusion
D-test and molecular technique are appropriate for detection of inducible clindamycin resistance in S. haemolyticus strains and should be routinely used in antibiotic susceptibility testing to obtain a more accurate result about the appropriate antibiotic and avoid poor treatment.
Acknowledgments: The authors acknowledge the Department of Biology, College of Science, University of Basrah for providing their necessary laboratories to complete the research. Also, the authors would like to thank the patients and staff of Port General Hospital, Al-Basrah.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: There was no conflicts
Authors’ Contribution: Adnan A. Al-Mousawi (First Author), Introduction Writer/Main Researcher (30%); Saad S. Al-Amara (Second Author), Methodologist/Discussion Writer (30%); Majid Noori Humoud (Third Author), Statistical Analyst (20%); Sadiq Oda Hattab (Fourth Author), Assistant Researcher (20%)
Funding/Support: None declared by the authors.
Since the 1950s, Coagulase-Negative Staphylococci (CoNS) have been recognised as an important cause of human infection [1, 2]. CoNS are common skin commensals that start to colonize the body surfaces very early in life. After 48 h of birth, about 100% of infants acquire CoNS during passage through the birth canal or by contacting nursery personnel [3]. The most common colonizing species are Staphylococcus epidermidis, Staphylococcus warneri, and Staphylococcus haemolyticus [4]. S. haemolyticus, together with S. epidermidis and S. hominis, were the prevalent staphylococci species detected in surfaces that are touched at a high frequency in the community and hospitals in London [5]. Similarly, S. haemolyticus and S. epidermidis were the most common CoNS isolates (34% and 27%, respectively) detected in different hospital wards in Iran [6].
Coagulase negative staphylococcus is often underestimated as the etiological factor of human infections. One important specie in this group is S. haemolyticus. After Staphylococcus epidermidis, S. haemolyticus is the second most frequently isolated coagulase-negative staphylococcus from clinical cases, primarily from blood infections [7].
S. haemolyticus is one of the coagulase-negative staphylococci that is abundantly found as a common microbiota on the skin. S. haemolyticus is generally considred as the second leading opportunistic pathogen among CoNS after S. epidermidis that associated with immunocompromised individuals, especially those who are hospitalized or suffered from exposure to medical devices worldwide [7, 8].
S. haemolyticus causes severe infections in several body systems, including meningitis, endocarditis, prosthetic joint infections and bacteremia and is prevalent in the hospital environment and on the hands of healthcare workers. S. haemolyticus is also known to cause septicemia, peritonitis, otitis media and Diabetic Foot Ulcer (DFU) infections [9, 10].
Even though the virulence of S. haemolyticus is lesser than S. aureus, which means that it's potential to cause severe infections is lower, yet it has the ability to acquire resistance against multiple antimicrobial agents [11]. S. haemolyticus is notably more resistant to antibiotics than any other coagulase negative staphylococcus, and the widest spectrum of resistance was observed among strains isolated from the hospital environment [12, 13]. Taking into consideration its adaptability and the ability to survive in the hospital environment, especially on medical devices, S. haemolyticus has become one of the major agent in nosocomial infections caused by multi drug resistant Staphylococci [14]. The prolonged hospitalization, invasive procedures and exposure to multiple antibiotics can result in alteration of normal skin/mucous microbiota which leads to a highly adaptable Linezolid (LNZ) resistant MRSH (Methicillin Resistance Staphylococcus haemolyticus) [15].
S. haemolyticus, especially strains that cause nosocomial infections, are more resistant to antibiotics than other coagulase-negative staphylococci. There is clear evidence that the resistance genes can be acquired by other staphylococcus species through S. haemolyticus [16].
One of the characteristics of S. haemolyticus is its ability to form biofilm, which plays an essential role in causing infection. The produced exopolysaccharides can inhibit the growth of other bacteria and also decrease their ability to form biofilms [17, 18]. This species has gained an increased clinical significance due to its genome plasticity, which allowed a great adaptation and development of resistance to different antibiotics, including methicillin and its ability to survive in the hospital environment [19].
Antimicrobials Macrolide-Lincosamide- Streptogramin B (MLSB) family are commonly used to treat skin and soft tissue infections caused by CoNS [20], and also as a penicillin substitute in individuals who are allergic to penicillin [21]. Resistance to antibiotics in the MLSB family could be either constitutive (cMLSB) or inducible (iMLSB) [22, 23]. Although rRNA methylase is only produced in the presence of an inducing agent, which can also be another antibiotic from MLSB family, like erythromycin, or macrolide, and rRNA methylase is frequently created in the absence of an inducing agent in constitutive resistance [22, 23]. Since erythromycin generates iMLSB resistance, when using an erythromycin disc in relatively close proximity to a clindamycin disc (D-test) assists in capable of detecting this form of resistance in CoNS. Clindamycin is widely used to treat staphylococcal infections, particularly those of the skin and soft tissues, as well as to substitute penicillin in individuals who are allergic to penicillin [24, 25]. Clindamycin treatment could fail if iMLSB resistance isn't established [23, 26, 27].
Inducible or constitutive resistance to MLSB is conferred by erm genes. The structural genes can be produced inducibly or constitutively, and mutations in the regulatory area of genes are common, resulting in inducible resistance becoming constitutive resistance [25, 28].
The factors that affect the survival and spread of multi-drug resistant S. haemolyticus isolates in hospitals are not completely known. Bouchami et al. reported that the insertion sequence transposition (mainly IS1272) and chromosomal rearrangement and recombination processes in S. haemolyticus is one strategy that helps in the bacterial evolution, adaptation, pathogenesis, and survival in the hospitals, hence causing nosocomial infections [29].
Recently, several studies have focused on clinical isolates of S. haemolyticus due to their multidrug resistance characteristics among coagulase-negative staphylococci against various antibiotics, including penicillins, tetracyclines, cephalosporins, macrolides, aminoglycosides, and quinolones. S. haemolyticus may delete and add genes due to its ability to insert sequences, leading to frequent genetic rearrangement and drug resistance [28-30].
Furthermore, antimicrobial resistance of S. haemolyticus colonizes in both hospitalized patient's skin and mucous membranes, acting as a reservoir for antibiotic resistance genes, which lead to limited options for treatment [28]. The existence of a large fragment of foreign DNA called the Staphylococcal Chromosomal Cassette mec (SCCmec) in the chromosome distinguishes methicillin-resistant S. haemolyticus isolates from methicillin-susceptible isolates.
A Penicillin-Binding transpeptidase PBP2A, with low affinity for b-lactams, is encoded by the mecA gene [21, 29]. The existence of two essential loci distinguishes the SCCmec element: the mec gene complex, which contains mecA and its regulation, and the ccr gene complex, which encodes recombinases and is responsible for SCCmec mobility [29].
Over recent years, different investigators have described an increasing frequency of multidrug-resistant strains of S. haemolyticus [12, 31, 32].
Current study aimed to investigate the frequency of inducible clindamycin resistance in S. haemolyticus isolated from surgical wounds infections using both D-test and molecular methods in Al-Basrah, Iraq.
Materials and Methods
Samples collection
200 surgical wound swabs were collected from September 2020 to November 2020 from Ports General Hospital in Basrah, Iraq.
Isolation and identification of bacterial strains
The conventional isolation and identification methods were used to identify CoNS strains according to the minimal standards recommended by Freney et al. [33]. Surgical wound swabs were cultured in blood agar, chocolate agar and heart infusion agar. As a preliminary scan to identify the isolates, the isolates were examined using gram staining and several conventional biochemical tests including oxidase, catalase, hemolysis types, and coagulase production tests. The identified strains were confirmed by the Vitek®2 system.
Detection of methicillin-resistant S. haemolyticus isolates
According to Clinical and Laboratory Standards Institute (CLSI) guidelines [34], cefoxitin (30 μg) disc (Bioanalyse; Tukey) was used to detect methicillin resistance S. haemolyticus isolates.
Detection of inducible clindamycin resistance in S. haemolyticus isolates
According to CLSI guidelines [34], clindamycin (2μg) and erythromycin (15μg) discs (Bioanalyse, Tukey) were used to detect inducible clindamycin resistance strains as the first step, and Vitek®2 system was used to confirm the results.
DNA extraction
Genomic DNA of S. haemolyticus isolates was extracted using Presto™ Mini gDNA Bacteria Kit (Geneaid, USA). The purity of the extracted DNA was measured and a purity ratio between 1.8 and 2.0 was accepted.
Detection of antibiotic resistance genes
The emergence of antibiotic resistance genes of S. haemoliticus isolates was investigated using Polymerase Chain Reaction (PCR) method (Table 1).
Table 1) The resistant genes used in this study

Findings
Identification of the bacterial strains
Out of 200 cases, 75 surgical wound swabs (37.5%) showed bacterial growth in blood agar, chocolate agar and heart brain infusion agar. The highest frequency of isolates belonged to Pseudomonas aeruginosa, followed by Staphylococcus epidermidis, Staphylococcus aureus and Escherichia coli, respectively (Table 2).
Table 2) Frequency distribution of bacterial isolated from surgical wound swabs

Antibiotic resistance patterns of S. haemoliticus isolates
Out of eight S. haemolyticus isolates, only 5 isolates (62.5%) showed inhibitory resistance criteria for both oxacillin and cefoxitin and were classified as MRSH (Methicillin Resistance Staphylococcus haemolyticus) isolates. While the rest of S. haemolyticus isolates (37.5%) showed sensitive criteria for oxacillin and cefoxitin and were classified as MSSH (Methicillin Sensitive Staphylococcus haemolyticu) isolates (Table 3).
On the other hand, out of eight isolates, 3 isolates (37.5%) were erythromycin-resistant and clindamycin sensitive with D-test positive. These isolates showed induced Macrolide-Lincosamide-Streptogramin B (iMLSB) resistance phenotype. While 2 isolates (25.0%) showed constitutive MLSB (cMLSB) resistance phenotype and 3 (37.5%) isolates were shown Macrolide-Streptogramin B (MSB) resistance phenotypes (Table 4).
Table 3) MRSH and MSSH patterns of S. haemolyticus isolates

Detection of antibiotic resistance genes
The most frequent resistance genes of S. haemolyticus strains were mecA (n=5, 62.5%), ermA (n=5, 62.5%), ermB (n=4, 50.0%), respectively, while ermC was found in 2 (25.0%) strains (Table 5; Figure 1).
Table 4) Frequency distribution of cMLSB, iMLSB, and MSB phenotypes and the result of D-test for S. haemolyticus isolates

Table 5) Patterns of antibiotic resistance genes in S. haemolyticus isolates


Figure 1) Gel electrophoresis profiles of PCR-amplified antibiotic resistance genes of S. haemolyticus: A) mecA gene, B) ermA gene, C) ermB gene (359 bp) and ermC (572bp); Gel electrophorphosis profiles with 2% concentration for 1hour at 75 vol
Discussion
Coagulase negative staphylococci are major nosocomial pathogens. Staphylococcus haemolyticus is the second opportunistic pathogen among CoNS after S. epidermidis and the third most common organism among clinical isolates of methicillin-resistant staphylococci [37]. S. haemolyticus, an emerging cause of nosocomial infection plays an important role in causing opportunistic infections related to medical devices [38]. The ability to form a biofilm is considered the most important virulence factor in CoNS associated infections [39].
Although S. haemolyticus is considered a common skin flora, the diseases caused by it have increased dramatically. The main reason is the multi-drug resistance of this bacterium, which subsequently poses serious risks to human health [28]. The worldwide spread of methicillin-resistant staphylococci alarmingly remains one of the most common hospital-acquired infections. The prevalence of hospitalized Methicillin Resistant Staphylococcus aureus (MRSA) and Methicillin-Resistant Coagulase Negative Staphylococci (MR-CoNS) has been recorded in various regions of the world [40].
The dramatic increment in the incidence and frequency of CoNS, as well as the ongoing health problems of methicillin resistance among staphylococci, have piqued our interest in trying to investigate the ideal treatment using clindamycin therapy. Clindamycin is considered an approperiate option, because of its tolerability, low cost, good permeability, and easy tissue entry [41].
Considerably, the common limitation of clindamycin therapy is its low ability to inhibit MR-CoNS through their inducible resistance phenotypes. Selective treatment cannot be performed without appropriate antibiotic susceptibility testing. Therefore, D-test has become a vital and crucial tool to achieve this goal [42]. Perez et al. [43] used the D-test method to detect inducible clindamycin resistance in CoNS and concluded that the D-test method is a simple and important technique in the detection of inducible clindamycin resistance. Jorgensen et al.'s study [44] demonstrated that inducible clindamycin resistance can be easily detected by disk induction testing on standard sheep blood agar plates used for verification of inoculum purity in conjunction with an automated susceptibility test system.
In Gatermann et al.’s study [25], out of 305 CoNS isolates, 155 (51%) isolates had constitutive clindamycin resistance, 78 (25.6%) had inducible clindamycin resistance, and 72 (23.6%) had non-inducible resistance. The prevalence of erythromycin resistance was almost 90% in S. haemolyticus. Also, most (63%) erythromycin-resistant isolates carried constitutively expressed ermC as the sole resistance determinant, with the notable exception of Staphylococcus hominis subsp. hominis, which carried inducible ermC.
S. haemolyticus has zoonotic character and is prevalent both in humans and animals. Ruzauskas et al. [11] determined the presence of MRSH in different groups of companion animals and to characterize isolates according their antimicrobial resistance. From a total of 754 samples tested, 12 MRSH isolates were obtained. The most frequent resistances of MRSH isolates demonstrated were to benzylpenicillin (91.7%) with the presence of the blaZ gene; erythromycin (91.7%) and clindamycin (41.7%) with the presence of ermA, ermC, and msrA genes; and gentamicin (75.0%) with the presence of aac(6′)-Ie-aph(2")-Ia and aph(3′)-IIIa genes.
Teeraputon et al. [45] determined antimicrobial resistance phenotypes and drug resistance genes of clinical coagulase-negative staphylococci isolates at Mae Sot Hospital in Tak province, Thailand. A total of 229 CoNS isolates were collected from clinical specimens during two periods in 2014 and in 2015. S. haemolyticus was the most prevalent species (37.55%). Methicillin-resistant CoNS (MRCoNS), containing the mecA gene, were detected in 145 of 229 isolates (63.32%), mostly found in S. haemolyticus and S. epidermidis. Among 125 erythromycin-resistant CoNS, the prevalence of constitutive type of MLSB, inducible clindamycin resistance and macrolide–streptogramin B resistance phenotypes were 72%, 13.60% and 14.40% respectively. These phenotypes were expressed in 80% of MRCoNS strains. In addition, the ermC gene (79.20%) was found to be more prevalent than the ermA gene (22.40%), especially among MRCoNS. S. haemolyticus appeared in nearly half of these isolates (n=69, 47.59%), followed by S. epidermidis, S. saprophyticus, and S. hominis.
Kitti et al. [40] examined antimicrobial susceptibility patterns, antimicrobial resistance genes, and SCCmec types of methicillin-resistant S. aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (MR-CoNS) isolated from patients in a hospital in Northern Thailand. They found that 82.6% of MR staphylococci (96.8% of MR-CoNS isolates) were resistant to 7–10 antibiotics. More than 70% of MRSA and MR-CoNS were resistant to cefoxitin, penicillin, oxacillin, erythromycin, clindamycin, gentamicin, and ciprofloxacin. In MRSA isolates, the prevalence of ermA (78.3%) and ermB (73.9%) genes was high compared to that of the ermC gene (4.3%). In contrast, ermC (87.1%) and qacA/B genes (70.9%) were predominant in MR-CoNS isolates. SCCmec type III was the dominant type of MRSA (13/23), whereas SCCmec type II was more present in S. haemolyticus (10/18). Ten MRSA isolates with SCCmec type III were ST239, which is the common type of MRSA in Asia.
Debnath et al. [41] detected inducible clindamycin resistance among the erythromycin resistant CoNS isolates. According their results, among 180 CoNS isolates, predominant isolated species were S. epidermidis (n=75, 41.67%) and S. haemolyticus (n=47, 26.11%). Out of 180 CoNS isolates, 108 (60%) showed erythromycin resistance, out of which, 29 (26.85%) isolates showed iMLSB. Among 180 CoNS isolates, 119 (66.11%) were MRCoNS isolates and 61 (33.89%) were MSCoNS isolates.
Barros et al. [30] used phenotypic and molecular methods to characterize the antibiotic resistance of 64 clinical isolates of S. haemolyticus. By PCR of the mecA gene, 87% were found to be methicillin resistant. Approximately 55% harbored SCCmec type V, and only one SCCmec type IV. Many isolates (75%) displayed multiresistance, and pulsotype analysis showed a high diversity.
Dziri et al. [46] evaluated the rate of detection of CoNS in environmental samples of 17 services in a Tunisian hospital and determined the antimicrobial resistance phenotypes and genotypes of recovered isolates. CoNS were obtained from 83 of the 200 tested samples (41.5%). S. haemolyticus was the most prevalent species (45.8%), followed by S. saprophyticus (36.1%). Methicillin-resistant CoNS were detected in 20 of the 200 tested samples (10%), and the mecA gene was demonstrated in 18 S. haemolyticus, one S. epidermidis and one S. saprophyticus isolates. Methicillin susceptible isolates were detected in 63 samples (31.5%). They reported that the high frequency of detection of multi-drug-resistant CoNS in the hospital environment, especially S. haemolyticus and S. saprophyticus, could be due to cross-transmission between patients, staff, and environment.
Conclusion
D-test and molecular technique are appropriate for detection of inducible clindamycin resistance in S. haemolyticus strains and should be routinely used in antibiotic susceptibility testing to obtain a more accurate result about the appropriate antibiotic and avoid poor treatment.
Acknowledgments: The authors acknowledge the Department of Biology, College of Science, University of Basrah for providing their necessary laboratories to complete the research. Also, the authors would like to thank the patients and staff of Port General Hospital, Al-Basrah.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: There was no conflicts
Authors’ Contribution: Adnan A. Al-Mousawi (First Author), Introduction Writer/Main Researcher (30%); Saad S. Al-Amara (Second Author), Methodologist/Discussion Writer (30%); Majid Noori Humoud (Third Author), Statistical Analyst (20%); Sadiq Oda Hattab (Fourth Author), Assistant Researcher (20%)
Funding/Support: None declared by the authors.
Keywords:
References
1. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nature Rev Microbiol. 2019;17(4):203-18. [Link] [DOI:10.1038/s41579-018-0147-4]
2. Abdullah-Al-Shoeb M, Huq S, Abul Kalam Azad M, Rahman N. Assessment of antibacterial efficacy of Lugol's iodine compared with commercial hand sanitizers of Bangladesh. J World Poultry Res. 2019;9(5):130-7. [Link] [DOI:10.36380/scil.2019.jlsb21]
3. Brook I. Bacteriology of neonatal omphalitis. J Infect. 1982;5(2):127-31. [Link] [DOI:10.1016/S0163-4453(82)91656-5]
4. Bjorkqvist M, Liljedahl M, Zimmermann J, Schollin J, Soderquist B. Colonization pattern of coagulase-negative staphylococci in preterm neonates and the relation to bacteremia. Eur J Clin Microbiol Infect Dis. 2010;29(9):1085-93. [Link] [DOI:10.1007/s10096-010-0966-3]
5. Cave R, Misra R, Chen J, Wang S, Mkrtchyan HV. Whole genome sequencing revealed new molecular characteristics in multidrug resistant staphylococci recovered from high frequency touched surfaces in London. Sci Rep. 2019;9:9637. [Link] [DOI:10.1038/s41598-019-45886-6]
6. Mehri H, Jahanbakhsh R, Shakeri F, Ardebili A, Behnampour N, Khodabakhshi B, Ghaemi EA. Investigation of glycopeptide susceptibility of coagulase-negative staphylococci (CoNS) from a tertiary care hospital in Gorgan, Northern Iran. Arch Pediatr Infect. 2017;5(1):e37264. [Link] [DOI:10.5812/pedinfect.37264]
7. Czekaj T, Ciszewski M, and Szewczyk EM. Staphylococcus haemolyticus-an emerging threat in the twilight of the antibiotics age. Microbiology 2015;161(11):2061-8. [Link] [DOI:10.1099/mic.0.000178]
8. Eltwisy HO, Abdel-Fattah M, Elsisi AM, Omar MM, Abdelmoteleb AA, El-Mokhtar MA. Pathogenesis of Staphylococcus haemolyticus on primary human skin fibroblast cells. Virulence. 2020;11(1):1142-57. [Link] [DOI:10.1080/21505594.2020.1809962]
9. do Carmo Ferreira N, Schuenck RP, Dos Santos KRN, de Freire Bastos M, Giambiagi-deMarval M. Diversity of plasmids and transmission of high-level mupirocin mupA resistance gene in Staphylococcus haemolyticus. FEMS Immunol Med Microbiol. 2011;61(2):147-52. [Link] [DOI:10.1111/j.1574-695X.2010.00756.x]
10. Schuenck RP, Pereira EM, Iorio NLP, Dos Santos KRN. Multiplex PCR assay to identify methicillin-resistant Staphylococcus haemolyticus. FEMS Immunol Med Microbiol. 2008;52(3):431-5. [Link] [DOI:10.1111/j.1574-695X.2008.00387.x]
11. Ruzauskas M, Siugzdiniene R, Klimiene I, Virgailis M, Mockeliunas R, et al. Prevalence of methicillin-resistant Staphylococcus haemolyticus in companion animals: A cross-sectional study. Ann Clin Microbiol Antimicrob. 2014;13:56. [Link] [DOI:10.1186/s12941-014-0056-y]
12. Bouchami O, Achour W, Mekni MA, Rolo J, Ben Hassen A. Antibiotic resistance and molecular characterization of clinical isolates of methicillin-resistant coagulase-negative staphylococci isolated from bacteremic patients in oncohematology. Folia Microbiol. 2011;56(2):122-30. [Link] [DOI:10.1007/s12223-011-0017-1]
13. Rodriguez-Aranda A, Daskalaki M, Villar J, Sanz F, Otero JR, Chaves F. Nosocomial spread of linezolid-resistant Staphylococcus haemolyticus infections in an intensive care unit. Diagn Microbiol Infect Dis. 2009;63(4):398-402. [Link] [DOI:10.1016/j.diagmicrobio.2008.12.008]
14. Froggatt JW, Johnston JL, Galetto DW, Archer GL. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother 1989;33(4):460-6. [Link] [DOI:10.1128/AAC.33.4.460]
15. Ahmed A, Satti L, Zaman G, Gardezi A, Sabir N, et al. Catheter related recurrent blood stream infection caused by linezolid-resistant, methicillin resistant Staphylococcus haemolyticus; an emerging super bug. J Pak Med Assoc. 2019;69(2):261-3. [Link]
16. Eltwisy HO, Twisy HO, Hafez MH, Sayed IM, El-Mokhtar MA. Clinical infections, antibiotic resistance, and pathogenesis of Staphylococcus haemolyticus. Microorganisms. 2022;10(6):1130. [Link] [DOI:10.3390/microorganisms10061130]
17. Silva PV, Cruz RS, Keim LS, de Paula GR, Carvalho BTF, et al. The antimicrobial susceptibility, biofilm formation and genotypic profiles of Staphylococcus haemolyticus from bloodstream infections. Mem Inst Oswaldo Cruz. 2013;108(6):812-3. [Link] [DOI:10.1590/0074-0276108062013022]
18. Rossi CC, Santos-Gandelman JF, Barros EM, Alvarez VM, Laport MS, et al. Staphylococcus haemolyticus as a potential producer of biosurfactants with antimicrobial, anti-adhesive and synergistic properties. Lett Appl Microbiol. 2016;63(3):215-21. [Link] [DOI:10.1111/lam.12611]
19. Takeuchi F, Watanabe S, Baba T, Yuzawa H, Ito T, et al. Whole-genome sequencing of staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol. 2005;187(21):7292-308. [Link] [DOI:10.1128/JB.187.21.7292-7308.2005]
20. Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol. 2010;5(2):183-95. [Link] [DOI:10.1586/edm.10.6]
21. Bora P, Datta P, Gupta V, Singhal L, Chander J. Characterization and antimicrobial susceptibility of coagulase-negative staphylococci isolated from clinical samples. J Lab Physicians. 2018;10(4):414-9. [Link] [DOI:10.4103/JLP.JLP_55_18]
22. Khatoon R, Jahan N. Evaluation of prevalence of inducible clindamycin resistance among Coagulase Negative Staphylococci (CoNS) isolated from various clinical samples in a tertiary care hospital of North India. Int J Curr Microbiol Appl Sci. 2018;7(2):513-22. [Link] [DOI:10.20546/ijcmas.2018.702.065]
23. Chika E, Joseph NF, Chijioke E, Peter E. Detection of constitutive- and inducible-clindamycin-resistance in clinical isolates of Staphylococcus aureus from a federal teaching hospital in Abakaliki, Nigeria. J Bacteriol Infect Dis. 2018;2(1):31-4. [Link]
24. Fiebelkorn KR, Crawford SA, McElmeel ML, Jorgensen JH. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J Clin Microbiol. 2003;41(10):4740-4. [Link] [DOI:10.1128/JCM.41.10.4740-4744.2003]
25. Gatermann SG, Koschinski T, Friedrich S. Distribution and expression of macrolide resistance genes in coagulase-negative staphylococci. Clin Microbiol Infect. 2007;13(8):777-81. [Link] [DOI:10.1111/j.1469-0691.2007.01749.x]
26. Malek-Jafarian M, Hosseini F, Ahmadi A. Pattern of infection and antibiotic activity among Streptococcus agalactiae isolates from adults in Mashhad, Iran. Rep Biochem Mol Biol. 2015;3(2):89-93. [Link]
27. Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27(4):870-926. [Link] [DOI:10.1128/CMR.00109-13]
28. Manoharan M, Sistla S, Ray P. Prevalence and molecular determinants of antimicrobial resistance in clinical isolates of Staphylococcus haemolyticus from India. Microb Drug Resist. 2021;27(4) : 501-8. [Link] [DOI:10.1089/mdr.2019.0395]
29. Bouchami O, de Lencastre H, and Miragaia M. Impact of insertion sequences and recombination on the population structure of Staphylococcus haemolyticus. PLoS One. 2016;11(6):e0156653. [Link] [DOI:10.1371/journal.pone.0156653]
30. Barros EM, Ceotto H, Bastos MCF, Dos Santos KRN, Giambiagi-Demarval M. Staphylococcus haemolyticus as an important hospital pathogen and carrier of methicillin resistance genes. J Clin Microbiol. 2012;50(1):166-8. [Link] [DOI:10.1128/JCM.05563-11]
31. Cavanagh JP, Hjerde E, Holden MTG, Kahlke T, Klingenberg C, et al. Whole-genome sequencing reveals clonal expansion of multiresistant Staphylococcus haemolyticus in European hospitals. J Antimicrob Chemother. 2014;69(11):2920-7. [Link] [DOI:10.1093/jac/dku271]
32. Hope R, Livermore DM, Brick G, Lillie M, Reynolds R, et al. Non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001-2006. J Antimicrob Chemother. 2008;62 Suppl 2:ii65-74. [Link] [DOI:10.1093/jac/dkn353]
33. Freney J, Kioos WE, Hajek V, Webster JA, Bes M, et al. Recommended minimal standards for description of new staphylococcal species. Int J Syst Bacteriol. 1999;49 Pt 2: 489-502. [Link] [DOI:10.1099/00207713-49-2-489]
34. Clinical & Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 28th Edition. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Link]
35. Murakami K, Minamide W, Wada K, Nakamura, E, Teraoka H, Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29(10):2240-4. [Link] [DOI:10.1128/jcm.29.10.2240-2244.1991]
36. Lina G, Quaglia A, Reverdy M, Leclercq R, Vandenesch F, Etienne J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43(5):1062-6. [Link] [DOI:10.1128/AAC.43.5.1062]
37. Sanches IS, Mato R, de Lancastre H, Tomsaz A. Patterns of multidrug resistance among methicillin-resistant hospital isolates of coagulase-positive and coagulase-negative Staphylococci collected in the international multicenter study RESIST in 1997 and 1998. Microb Drug Resist. 2000;6(3):199-211. [Link] [DOI:10.1089/mdr.2000.6.199]
38. Gaudioso de Allori MC, Jure MA, Romero C, de Castillo ME. Antimicrobial resistance and production of biofilms in clinical isolates of coagulase-negative Staphylococcus strains. Biol Pharm Bull. 2006;29(8):1592-6. [Link] [DOI:10.1248/bpb.29.1592]
39. Mack D, Rohde H, Harris LG, Davies AP , Horstkotte MA, Knobloch JK. Biofilm formation in medical device-related infection. Int J Artif Organs. 2006;29(4):343-59. [Link] [DOI:10.1177/039139880602900404]
40. Kitti T, Seng R, Saiprom N, Thummeepak R, Chantratita N, et al. Molecular characteristics of methicillinresistant staphylococci clinical isolates from a tertiary Hospital in Northern Thailand. Can J Infect Dis Med Microbiol. 2018;2018:8457012. [Link] [DOI:10.1155/2018/8457012]
41. Debnath A, Ghosh R , Ghosh D. Detection of inducible clindamycin resistance (iMLSB) among the erythromycin resistant CoNS isolates in a rural tertiary care hospital- need of time. Int JHealth Sci Res. 2020;10(6):12-18. [Link]
42. Bansal N, Chaudhary U, and Gupta V. Prevalence of inducible clindamycin resistance in clinical isolates of coagulase negative staphylococci at a tertiary care hospital. Ann Trop Med Public Health. 2012;5(5):427-30. [Link] [DOI:10.4103/1755-6783.105124]
43. Perez LRR, Caierão J, Antunes ALS, d'Azevedo PA. Use of the D test method to detect inducible clindamycin resistance in coagulase negative staphylococci (CoNS). Braz J Infect Dis. 2007;11(2):186-8. [Link] [DOI:10.1590/S1413-86702007000200002]
44. Jorgensen JH, Crawford SA, McElmeel ML, Fiebelkorn KR. Detection of inducible clindamycin resistance of staphylococci in conjunction with performance of automated broth susceptibility testing. J Clin Microbiol. 2004;42(4):1800-2. [Link] [DOI:10.1128/JCM.42.4.1800-1802.2004]
45. Teeraputon S, Santanirand P, Wongchai T, Songjang1 W, Lapsomthob N, et al. Prevalence of methicillin resistance and macrolide-lincosamide- streptogramin B resistance in Staphylococcus haemolyticus among clinical strains at a tertiary-care hospital in Thailand. New Microbes New Infect. 2017;19:28-33. [Link] [DOI:10.1016/j.nmni.2017.05.007]
46. Dziri R, Klibi N, Lozano C, Ben Said L, Bellaaj R, Tenorio C, Boudabous A, et al. High prevalence of Staphylococcus haemolyticus and Staphylococcus saprophyticus in environmental samples of a Tunisian hospital. Diagn Microbiol Infect Dis. 2016;85(2):136-40. [Link] [DOI:10.1016/j.diagmicrobio.2016.03.006]








